Abstract
The problem of pesticides is not new and its exposure to human due to indiscriminate use is largely associated with the health related problems including neurotoxicological alterations. High levels of pesticide residues and their metabolites in the dietary constituents, food materials, maternal blood, cord blood, placenta breast milk have been reported and linked to alterations in birth weight, crown heel length, head circumference, mid-arm circumference and ponderal index of the neonates. Epidemiological studies have suggested that exposure of pesticide to human could be a significant risk factor for neurological disorders, including Parkinson’s disease, Alzheimer’s disease and multiple sclerosis. Cholinergic and non-cholinergic dysfunctions in pesticide exposed population, especially in children have also been frequently reported in recent years. Developmental neurotoxicity is another concern in the area where pregnant are more prone towards its exposure and which results in the abnormalities in the fetus. In view of the increasing risk of human health through pesticide exposure, the present review has been focused on the studies pertaining to pesticide induced neurochemical alterations and associated behavioral abnormalities in farm workers which could establish a possible link between the its exposure and associated health hazards.
Keywords: Pesticides, Neurotoxicity, Neurobehavior, Suicidal ideation, Farm workers, Neurological disorders
Introduction
The incidences of pesticide poisoning and suicides are very high in developing countries due to poverty, lack of awareness, education and other factors. WHO has reported around 3 million cases of pesticide poisoning with more than 2,50,000 deaths every year world over [1]. Cases of pesticide poisoning from India and many other countries have been frequently reported [1]. In India, most of the population is dependent on traditional agricultural methods, therefore the country is the second largest producer of pesticides in Asia and ranks fourth position in the world for the use of pesticides [2]. However, India, predominantly use insecticide instead of other pesticide (different class of pesticide, i.e. herbicide, weedicide etc.) in contrast to developing countries which enforce higher acute risk of poisoning. The organophosphate pesticide such as monocrotophos and others are expected to involve the highest incidences of suicidal poisoning from different regions and States of India. Recently, Zhang et al. [3] also suggested the association of occupational pesticide poisoning with the decreased neurobehavioral function and enhanced psychiatric morbidity in Chinese farm workers. Pesticides are chemical or mixture of chemical substances widely used to repel unwanted pests including insects, weeds, fungi and rodents which are classified into—insecticide, weedicides, fungicides, rodenticides and fumigants. Insecticides, kill insects by targeting their nervous system, are further divided into organophosphates, pyrethroids, organochlorine and carbamates insecticides. The risk of exposure enhanced many times due to heavy use of pesticides and it could be accidental or occupational during manufacturing, applying, harvesting, handling of crops and public health uses [4]. Human exposure to pesticides is quite imminent due to its indiscriminate and injudicious use in households, agriculture, veterinary practices, occupational and non-occupational settings. Individuals attempting suicides has been frequently reported to take the pesticide intentionally for the purpose [1]. The contamination of food by means of crops routinely sprayed with pesticides is an important source of exposure due to its accumulation in animal’s tissue, milk and food products [5]. At the same time high levels of residues of organophosphate, pyrethroids and their metabolites detected in the dietary products and biological tissues of exposed individuals associated with adverse health effects are again a matter of concern. Dewan et al. [6] demonstrated that a significant amount of organophosphates levels in the maternal blood, cord blood, placenta breast milk alters several parameters like birth weight, crown heel length, head circumference, mid-arm circumference and ponderal index of the neonates. The metabolites of pyrethroid have been detected in the urine of pregnant women and children of preschool [7]. The toxic effects of pesticides could be determined by the dose, route, time of exposure, and rate of metabolism. Some long-term health impacts are delayed or not immediately apparent, such as infertility, birth defects, endocrine disruption, neurological disorders and cancer [8]. Incidences of both acute and chronic exposure have been found in farm workers during agricultural activities including spraying, mixing, loading of pesticides. The exposure pattern become quite high due to unawareness of toxic effects of pesticides and no use of suitable protective equipment during the pesticide application.
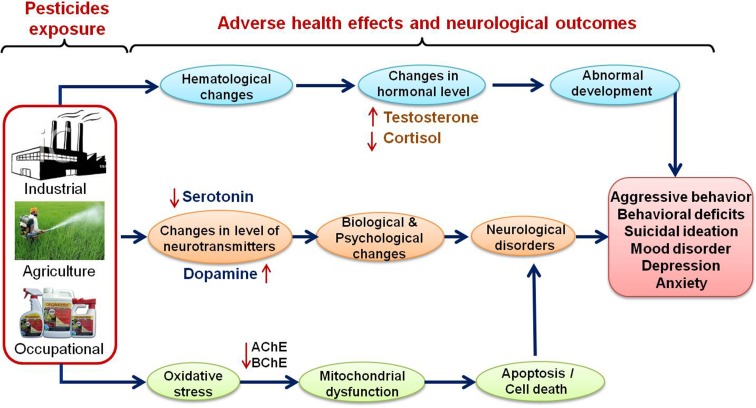
Several epidemiological studies have identified pesticide exposure as a significant risk factor for neurological disorders, including Parkinson’s disease, Alzheimer’s disease and multiple sclerosis [2, 9]. The other negative outcome of pesticide exposure includes birth defects, fetal death, still, pre-term birth, adverse pregnancy outcomes and neurodevelopmental disorder [10, 11]. Association of pesticide exposure with the incidence of chronic diseases, genetic damages, epigenetic modifications, endocrine disruption, mitochondrial dysfunction, oxidative stress, impairment of the ubiquitin proteasome system and defective autophagy has also been frequently reported [12]. Developing fetus and children are at high risk of exposure and allied adverse consequences due to immature blood brain barrier and detoxification mechanism. In the real life situation the use of mixtures of pesticides is commonly observed and it is also related to higher incidence of pesticide poisonings and deaths [13]. The present review has therefore been focused on pesticide induced neurochemical alterations and associated behavioral abnormalities in farm workers to establish a possible link between the pesticide exposure and behavioral change in human with possible clinical implications (Fig. 1).
Fig. 1.
Pesticides and their exposure to human beings through agricultural activities, industrial and occupational sources linked with various adverse health effects, altered biochemical and neurotransmitter levels and neurobehavioral outcomes
Biochemical Modifications and Adverse Clinical Outcomes
Occupational exposure to pesticides resulted in alterations of some hematological parameters including decrease size of red blood cells, higher platelet and WBC counts (increase in lymphocyte and monocyte count) [14, 15]. Increased activities of GGT, ALP bilirubin has also been shown to be linked to the hepatic cell damage in pesticide exposed condition [14]. Elevated levels of plasma urea and creatinine suggested nephrotoxic changes in workers occupationally exposed to pesticides have also been reported [16]. Studies have been demonstrated that the long term exposure of pesticides in individuals may lead to the development of vascular diseases, decrease in the activity of butylcholinesterase and acetylcholinesterase and alterations in hematological parameters [14, 17, 18]. Organophosphate induced biochemical alteration associated with the adverse health effects, including dizziness, headache and anxiety has been reported in the farm workers [19, 20]. Impaired motor coordination, reduced motor conduction velocity, verbal memory and other neurological and neuropsychiatric effects has been shown in farm workers [21–23]. The detailed symptomatic effects followed by organophosphate exposure have been represented in the Table 1 [24–34]. Besides organophosphates, organochlorine and pyrethroids has also been frequently used in the agriculture and found to be linked with the adverse neurological consequences. Autism spectrum disorders in the children of female farm workers and risk of Alzheimer’s disease and genetic disorders have been reported in Table 2 [35–43]. Limited studied have been published which suggested the multiple pesticide exposure at a time in humans than that of single toxicant. Mixtures of organophosphates, carbamates and pyrethroids has reported to cause synergistic effects and is a matter of great concern. It has been shown to cause depression, anxiety, obsessive compulsiveness and other central nervous system problems as given in Table 3 [44–49]. Synergistic interaction can also be expected in mixtures of pesticides, as or may act on totally different systems and thus not interact. Furthermore, even a single chemical may have multiple effects and affect more than one organ system. Effects may vary with age and metabolites may have totally different actions from the parent compound. In a study on French general population, Crepet et al. [50] conducted a research through PERICLES research program to assess the potential combined effects of pesticide mixtures. They suggested that in pesticide mixtures exposed population, the toxic effects cannot be predicted based on the toxic potential of each compound as observed in human cells.
Table 1.
Chronic exposure of organophosphate to farm workers and its association with the exposure pattern, adverse health effects, neurochemical and behavioral dysfunctions
| Type of pesticide and place | Subject (N) |
Types of exposure | Common health effects | Neurochemical and behavioral alterations | References |
|---|---|---|---|---|---|
| Organophosphates (OPs) South Africa |
Farm workers (N = 247) |
Occupational exposure | Dizziness and headache | Dizziness, sleepiness and headache had a significantly higher overall neurological symptom score (p < 0.05) | London et al. [19] |
| OPs USA |
Farm workers (N = 44) |
Occupational and residential exposure | Anxiety | Anxiety score of the pesticide applicators was significantly higher (p < 0.05) than that of the farmers. |
Levin et al. [20] |
| OPs India |
Farm workers (N = 24) | Occupational exposure |
Headache (59%), giddiness (50%), ocular symptoms (27%) and Paraesthesia (18%) |
Reduced motor conduction velocity and serum AChE levels |
Misra et al. [21] |
| OPs (Iowa and North Carolina) |
Pesticide applicators (N = 701) |
Occupational and residential exposure | Not examined | Reduce in the verbal memory, motor speed and motor coordination | Starks et al. [22] |
| OPs (W. Cape province of SA) |
Farm workers (N = 57) |
Occupational and residential exposure | Not examined | Some neurological and neuropsychiatric effects on adults like ADHD, anxiety, depression disorder | London et al. [23] |
| OPs Italy |
Pesticide applicators (N = 216) | Occupational exposure | Muscle pain in the lower limbs, distal numbness and paraesthesiae occur, followed by progressive weakness, depression of deep tendon reflexes in the lower limb | Organophosphate-induced delayed polyneuropathy | Lotti and Moretto [24] |
| OPs Southeastern Spain |
Pesticide applicators (N = 66) | Occupational exposure | Not examined | Worse performance in neuropsychological functions attention, reasoning, memory, perception, visuomotor skills, expressive language and motor performance |
Roldan-Tapia et al. [25] |
| OPs southern Brazil |
Farm workers (N = 37) | Occupational and residential exposure | Dermatitis, diarrhea, abdominal pain, and sialorrhea | Headache, hypertension, anxiety and depression disorders higher in exposed population than controls | Salvi et al. [26] |
| OPs Asia, Africa, America |
Pesticide applicators as well as suicide attempters (N = 1838) |
Occupational as well as accidental exposure | Acute and chronic OP exposure is associated with affective disorders |
Suicide rates are high in farming populations. | London et al. [27] |
| OPs (Oregon, USA) |
Agricultural workers (N = 119) |
Occupational exposure | Not examined | Neurological impairment as measured by the attention, remembrance, reaction time and deficits in neurobehavioral performance | Rohlman et al. [28] |
| OPs (Northeastern Colorado) |
Farm workers (N = 479) | Occupational and residential exposure | Eye irritation, nausea or vomiting, skin irritation |
High depressive symptoms, and headache dizziness |
Stallones and Beseler [29] |
| OPs (London) |
Farm workers (N = 37) | Occupational and residential exposure | Not examined | Slower response on neuropsychological performance like processing speed, attention |
Stephens and Sreenivasan [30] |
| OPs (London, UK) |
Sheep dipping farmers (N = 612) | Occupational and residential exposure | Not examined | Sensory and vibration thresholds were higher, neurological symptoms | Pilkington et al. [31] |
| OPs (Egypt) |
Farm workers (N = 52) | Occupational and residential exposure | Not examined | Serum acetylcholinesterase was significantly lower, neurological abnormalities | Farahat et al. [32] |
| OPs Oregon and Columbia, USA |
Farm worker (N = 46) |
Occupational and residential exposure | Not examined | Lower neurobehavioral performance like learning test, symbol-digit, reaction time | Rothlein et al. [33] |
| OPs N. and S. West regions of England |
Farm workers (N = 127) | Occupational and residential exposure | Not examined | Significantly higher anxiety and depression in exposed population | Mackenzie Rossa et al. [34] |
Table 2.
Chronic exposure of organochlorine and pyrethroid to farm workers and their association with the exposure pattern, adverse health effects, neurochemical and behavioral dysfunctions
| Type of pesticide and place | Subject (N) |
Types of exposure | Common health effects | Neurochemical and behavioral alterations | References |
|---|---|---|---|---|---|
| Organochlorine (OC) (p,p′-DDE) Finland |
Females farmwokers (N = 150) |
Occupational and residential exposure | Maternal serum sample prior pregnancy | Autism spectrum disorders (ASD), neurodevelopmental disorders |
Cheslack-Postava et al. [35] and Roberts et al. [36] |
| OC (Texas) |
Farm residents (N = 165) |
Occupational and residential exposure | Not examined | Exposure of human neuroblastoma cells to DDT or DDE increased levels of amyloid precursor proteins, alzheimer’s disease | Richardson et al. [37] |
| OC (Dieldrin, DDT, PCBs, HCB) |
Farm workers (N = 145) |
Occupational and residential exposure | Not examined | Risk of Alzheimer’s disease (AD) in the north Indian population AD | Singh et al. [38] and Kim et al. [39] |
| OC Mexico Community |
Farm Workers (N = 41) | Occupational and residential exposure | Acute poisoning (20% of the cases) and diverse alterations of the digestive, neurological, respiratory, circulatory, dermatological, renal, and reproductive system | Free DNA fragments in plasma (90.8 vs 49.05 ng/mL) as well as a higher level of lipid peroxidation | Payan-Rentería et al. [40] |
| OC (DDT and DDE) USA |
Mexican farm-workers (N = 360) |
Occupational and residential exposure | Not examined | No association with mental development at 6 months but a 2- to 3-point decrease in Mental Developmental Index scores for p,p’-DDT and o,p’-DDT at 12 and 24 months, corresponding to 7- to 10-point decreases across the exposure rang | Eskenazi et al. [41] |
| PYR Phillipines |
Farmers (N = 542) | Occupational and residential exposure | Alteration in haematological parameters | Abnormal cranial nerve function, and motor strength | Lu et al. [42] |
| PYR Bolivian |
Farm sparayers (N- = 120) |
Occupational exposure | Not examined | Deterioration in neurocognitive performance | Hansen et al. [43] |
Table 3.
Chronic exposure of mixture of pesticides (Organophosphate, carbamate, organochlorine and pyrethroid) to farm workers and their association with the exposure pattern, adverse health effects, neurochemical and behavioral dysfunctions
| Type of pesticide and place | Subject (N) |
Types of exposure | Common health effects | Neurochemical and behavioral alterations | References |
|---|---|---|---|---|---|
| OPs and Carbamates, PYR China |
Farm workers (N = 121) | Occupational and residential exposure | Not examined | Higher anger-hostility, depression-dejection, tension-anxiety and lower for vigor-activity compared to controls | Zhang et al. [3] |
| OPs and Carbamates Sri Lanka |
Farmers (N = 260) |
Occupational and residential exposure | Not examined | More inhibition of cholinesterase activity in exposed population |
Smit et al. [44] |
| OPs and Carbamates | Banana farm workers (N = 78) |
Occupational and residential exposure | Not examined | Somatisation, obsessive- compulsiveness, interpersonal sensitivity, depression and anxiety |
Wesseling et al. [45] |
| OPs and Carbamates Kenya, East African |
Pesticide applicators (N = 256) | Occupational and residential exposure | Respiratory, eye disorders | Inhibition of cholinesterase activity and central nervous system problems | Ohayo-Mitoko et al. [46] |
| OP and OCs (Iowa and North Carolina) |
Pesticides used by applicators (N = 21,208) |
Occupational and residential exposure | Not examined | Positive association between pesticide exposure and depression | Beard et al. [47] |
| OPs and others Northeastern Colorado, USA |
Agricultural workers and spouse (N = 684) |
Occupational and residential exposure | Not examined | Poor health, financial difficulties and a history of pesticide poisoning significantly explained the depressive symptoms | Beseler et al. [48] |
| PYR and Ops Western Cape South Africa | Farm workers (n = 121) |
Occupational and residential exposure | Not examined | Problems with buttoning, reading and notes | Motsoeneng and Dalvie [49] |
The role of oxidative stress in pesticide induced neurotoxiciy has been well established and associated with various neurological disorders including Alzheimer’s and Parkinson’s diseases. Exposure to pesticide in humans is mainly associated with enhanced generation of free radical species which caused an increased level of lipid peroxidation and decrease antioxidant capacity, including reduced glutathione, superoxide dismutase, catalase and subsequently turns into the diseased conditions [51, 52]. These free radicals act on the mitochondria to trigger the apoptotic cascade and involves in the process of neurodegeneration. Further, the oxidative damages cause alterations in the mitochondria permeability transition and disrupts calcium homeostasis, leading to apoptosis and cell death. Due to lipophilic nature, pyrethroids and other class of pesticides accumulate in biological membranes and tissues leading to oxidative insult. The toxic products produced as a result of oxidative stress including malondialdehyde, 4-hydroxynonenal (HNE) and acrolein have been found to disturb the homeostasis of cell in the body and alter the cellular structure and physiological functions [53].
Neurochemical Alterations and Behavioral Abnormalities
Several pesticides such as endosulfan, acephate, chlorpyrifos, dichlorvos and methamidophos are found to be involved in the endocrine disruption and reproductive toxicity hence, cause adverse health effects on the human and fetus [54]. Leon-Olea et al. [55] demonstrated that behavioral features affected by some of pesticide include cognitive deficits, heightened anxiety or anxiety-like, socio-sexual, locomotor and appetitive behaviors. The inhibition of AChE and BChE activities in the pesticide exposed group has been frequently reported [14, 56]. Therefore, the cholinesterases activities could be useful biomarkers in the monitoring of populations exposed to pesticides [57]. Pyrethroids have been found to alter the levels of neurotransmitters and metabolites of monoamine neurotransmitters in the brain. Organophosphate pesticides inhibited the enzyme AChE leading to the production of an excessive amount of ROS, which is due to the inhibition of oxidative phosphorylation [58]. Srivastava et al. [59] showed that workers exposed to quinalphos, an organophosphate pesticide had altered plantar and ankle reflexes and also learning and memory impairment. A range of cognitive, psychomotor and emotional behavior, including impaired memory, attention, alertness, depression, anxiety and irritability impairments have also been reported to result from chronic and low level organophosphorus exposure studies [60]. The relevance of a serotonin and dopamine model of aggression in the major risk factor for suicide has been demonstrated [61]. Also, the role of serotonin and dopamine in depressed mood and possibly the individual’s ability to cope with imminent suicidality have been suggested [62]. The serotonergic system have rejuvenated its role in depression and identified additional associations with suicidal behavior, impulsive aggression, eating disorders, obsessive–compulsive disorder and anxiety [62]. Exposure of pyrethroids and other insecticide to human could decrease brain serotonin levels [63] which may be linked to the decreased synthesis of serotonin and loss of serotonergic neurons. Chronic exposure to pyrethroids and other group of pesticides have been found to involve multiple sites within the central nervous system, leading to neurobehavioral and neurochemical alterations.
The postmortem studies to find out the role of dopamine and serotonin in aggressive behavior and development of suicidal tendency in human have been carried out, but have variable results due to differences in age, presence of psychotropic medications and also have unique pathobiology [64]. Altered levels of homovanillic acid (HVA) have been found in the frontal cortex and basal ganglia of suicide victims [65]. Concentration of HVA in cerebro-spinal fluid has been found to be lower in suicide attempters than controls [66]. Behavioral and functional changes have been found to be associated with the alterations in dopaminergic system that further linked with delayed neurotoxicity [67]. Pesticide poisoning is found to be one of the risk factors for depression, which, if addressed timely, may reduce risk of suicides among agriculture workers [68, 69]. The studies have been reported that specific depressive symptoms occur more often in those with a pesticide poisoning and these symptoms lead to an increased risk of farm injuries [45, 70]. Studies have been carried out to explore possible causal pathways between pesticide exposure and depression, impulsivity, suicide ideation and eventual suicide [71]. Saravi and Dehpour [72] suggested the correlation of environmental as well as genetic factors to the pathophysiology of neurodevelopmental and neurobehavioral defects. They further demonstrated that maternal exposure to organochlorine pesticides results in impaired motor and cognitive development in newborns and infants. Also, occupational exposure to the pesticides in farmers and chemical workers leads to brain damage, cognitive deficits, behavioral and neurodegenerative disorders.
Molecular Targets and Mechanism of Action
Inhibition of cholinesterase activity in case of organophosphates, carbamates and prolonged opening of voltage sensitive sodium channels in case of pyrethroids and organochlorine have been identified as the most common causes of severe acute pesticide poisonings [73]. The primary mechanism of organophosphate toxicity is the inhibition of AChE in the nervous system and the resultant over activation of cholinergic tone via acetylcholine accumulation in synapses and neuromuscular junctions [74]. Pesticide exposure also found to be associated with the mitochondrial dysfunction and cell death through the initiation of apoptotic pathways [73]. Dysfunction in the mitochondrial complexes I and IV due to disruption in oxidative phosphorylation have been reported following exposure to pesticides [75]. Assessment of pesticide induced toxicity and its interaction with the body could be done by analysis between the genetic polymorphisms studied (PON1192, PON155, PON1-108, PON1-909, GSTM1, GSTT1, BCHE), cholinesterase activities (AChE and BChE) and erythrocyte enzyme levels, only a few studies have addressed gene–environment interactions in populations exposed to pesticides [76]. In addition to this, a number of signaling cascade has been found to be involved in the neurotoxicity of pesticides. Decreased expression of BCl2 and increased expression of Bax, Caspases-3, 9, JNK1, 2, TrkA/p75 have been reported in pesticide exposed individuals [77, 78]. Although a number of epidemiological studies have been carried out on pesticide exposed farm workers, but the exact mechanism of toxicity in human is still not fully understand. For this, various experimental studies were performed by health scientist to explore the pathways involved in the neurobehavioral toxicity and could be a possible tool for the preventive approach. Studies have been demonstrated that low level repeated exposure of organophosphates in cultured neural cells induces inflammatory responses, which could be linked with the imapaired learning and memory [79]. Liu et al. [80] suggested that exposure to PCB153 and p,p’-DDE in rats and human thyroid follicular epithelial cell lines activated the PI3 K/Akt and MAPK pathways which finally disrupt the hypothalamic-pituitary-thyroid (HPT) axis via TRβ1 and TRHr and then decrease TH levels and hence disturb TH homeostasis.
Conclusions
Exposure to pesticides in humans has been found to alter the biochemical levels and influence the hematological profile, liver and kidney functions and lipid profile which could be used as suitable biomarkers for the development of therapeutic approaches. Cholinergic and non-cholinergic systems linked to depression, impulsivity and mood disturbances has also found to be affected following pesticide exposure in humans, which could explain an elevated association of pesticide exposure with suicidal ideation and other behavioral alterations. The modulation in serotoninergic and dopaminergic transmission affects the emotional state of a person and aggravate aggression which might lead to fatal consequences. The use of pesticides and its associated neurochemical alterations leading to behavioral deficits in both children and adults become a serious concern among the health scientists. There is a strong need of translational research to understand the detailed mechanism of neurobehavioral toxicity in individuals chronically exposed to pesticides. The present review may help to understand the detailed mechanism of pesticide induce behavioral changes and to find out preventive measures accordingly. The study may provide new insights into neurobehavioral toxicity and open new vistas for regulatory agencies to draw suitable guide maps for its use, risk assessments and reviews the threshold limit values.
Acknowledgement
The authors are thankful to Dr. Harisingh Gour Vishwavidyalaya (A Central University), Sagar (MP), India for providing the opportunity to work and their support and interest. Authors are also thankful to the SERB-Department of Science and Technology (DST), New Delhi, India for providing the SERB-DST Young Scientist startup research grant. Authors are also thankful to the University Grants Commission (UGC), New Delhi for providing research fellowship.
Conflict of interest
Authors declares no conflict of interest.
Ethical Approval
This is a review article and does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.World Health Organization . Health implications from monocrotophos use: a review of the evidence in India. Geneva: World Health Organization; 2009. [Google Scholar]
- 2.Yadav RS, Tiwari NK. Lipid integration in neurodegeneration: an overview of Alzheimer’s disease. Mol Neurobiol. 2014;50(1):168–176. doi: 10.1007/s12035-014-8661-5. [DOI] [PubMed] [Google Scholar]
- 3.Zhang C, Hu R, Huang J, Huang X, Li Y, Yin Y, Chen Z. Health effect of agricultural pesticide use in China: implications for the development of GM crops. Sci Rep. 2016;6:34918. doi: 10.1038/srep34918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa LG, Gennaro G, Guizzetti M, Vitalone A. Neurotoxicity of pesticides: a brief review. Front Biosci. 2008;13:1240–1249. doi: 10.2741/2758. [DOI] [PubMed] [Google Scholar]
- 5.Lazartigues A, Thomas M, Banas D, Brun-Bellut J, Cren-Oliv C, Feidt C. Accumulation and half-lives of 13 pesticides in muscle tissue of freshwater fishes through food exposure. Chemosphere. 2013;91(4):530–535. doi: 10.1016/j.chemosphere.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 6.Dewan P, Jain V, Gupta P, Banerjee BD. Organochlorine pesticide residues in maternal blood, cord blood, placenta, and breast milk and their relation to birth size. Chemosphere. 2013;90(5):1704–1710. doi: 10.1016/j.chemosphere.2012.09.083. [DOI] [PubMed] [Google Scholar]
- 7.Morgan MK, Sheldon LS, Croghan CW, Jones PA, Chuang JC, Wilson NK. An observational study of 127 preschool children at their homes and daycare centers in Ohio: environmental pathways to cis- and trans-permethrin exposure. Environ Res. 2007;104:266–274. doi: 10.1016/j.envres.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Clementi M, Tiboni GM, Causin R, La Rocca C, Maranghi F, Raffagnato F, Tenconi R. Pesticides and fertility: an epidemiological study in Northeast Italy and review of the literature. Reprod Toxicol. 2008;26(1):13–18. doi: 10.1016/j.reprotox.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 9.Yadav SS, Singh MK, Yadav RS. Organophosphates induced Alzheimer’s disease: an epigenetic aspect. J Clin Epigenetics. 2016;2(1:10):1–8. [Google Scholar]
- 10.Fenster L, Eskenazi B, Anderson M, Bradman A, Harley K, Hernandez H, Hubbard A, Barr DB. Association of in utero organochlorine pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ Health Perspect. 2006;114(4):597–602. doi: 10.1289/ehp.8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell EM, Hertz-Picciotto I, Beaumout JJ. A case-control study of pesticides and fetal death due to congenital anomalies. Epidemiology. 2001;12:148–156. doi: 10.1097/00001648-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Mostafalou S, Abdollahi M. Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol Appl Pharmacol. 2013;268(2):157–177. doi: 10.1016/j.taap.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 13.Rizzati V, Briand O, Guillou H, Gamet-Payrastre L. Effects of pesticide mixtures in human and animal models: an update of the recent literature. Chem Biol Interact. 2016;25(254):231–246. doi: 10.1016/j.cbi.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Wafa T, Nadia K, Amel N, Ikbal C, Insaf T, Asma K, Hedi MA, Mohamed H. Oxidative stress, hematological and biochemical alterations in farmers exposed to pesticides. J Environ Sci Health B. 2013;48(12):1058–1069. doi: 10.1080/03601234.2013.824285. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Garcia CR, Parron T, Requena M, Alarcon R, et al. Occupational pesticide exposure and adverse health effects at the clinical, hematological and biochemical level. Life Sci. 2016;15(145):274–283. doi: 10.1016/j.lfs.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Attia AM. Risk assessment of occupational exposure to pesticides. In: Linkov I, Ramadan AB, editors. Comparative risk assessment and environmental decision making. Nato science series: IV: earth and environmental sciences. Dordrecht: Springer; 2004. p. 38. [Google Scholar]
- 17.Arnal N, Astiz M, de Alaniz MJT, Marra CA. Clinical parameters and biomarkers of oxidative stress in agricultural workers who applied copper-based pesticides. Ecotoxicol Environ Saf. 2011;74:1779–1786. doi: 10.1016/j.ecoenv.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Lozano-Paniagua D, Gomez-Martin A, Gil F, Parron T, Alarcon R, Requena M, Lacasana M, Hernandez AF. Activity and determinants of cholinesterases and paraoxonase-1 in blood of workers exposed to non-cholinesterase inhibiting pesticides. Chem Biol Interact. 2016;25,259(Pt B):160–167. doi: 10.1016/j.cbi.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 19.London L, Nell V, Thompson ML, Myers JE. Effects of long-term organophosphate exposures on neurological symptoms, vibration sense and tremor amongst South African farm workers. JE Scand J Work Environ Health. 1998;24(1):18–29. doi: 10.5271/sjweh.274. [DOI] [PubMed] [Google Scholar]
- 20.Levin HS, Rodnitzky RL, Mick DL. Anxiety associated with exposure to organophosphate compounds. Arch Gen Psychiat. 1976;33:225–228. doi: 10.1001/archpsyc.1976.01770020061010. [DOI] [PubMed] [Google Scholar]
- 21.Misra UK, Nag D, Khan WA, Ray PK. A study of nerve conduction velocity, late responses and neuromuscular synapse functions in organophosphate workers in India. Arch Toxicol. 1988;61:496–500. doi: 10.1007/BF00293697. [DOI] [PubMed] [Google Scholar]
- 22.Starks SE, Gerr F, Kamel F, Lynch CF, Jones MP, Alavanja MC, et al. Neurobehavioral function and organophosphate insecticide use among pesticide applicators in the Agricultural Health Study. Neurotoxicol Teratol. 2012;34(1):168–176. doi: 10.1016/j.ntt.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.London L, Beseler C, Bouchard MF, Bellinger DC, Colosio C, Grandjean P, et al. Neurobehavioral and neurodevelopmental effects of pesticide exposures. Neurotoxicology. 2012;33:887–896. doi: 10.1016/j.neuro.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lotti M, Moretto A. Organophosphate-induced delayed polyneuropathy. Toxicol Rev. 2005;24(1):37–49. doi: 10.2165/00139709-200524010-00003. [DOI] [PubMed] [Google Scholar]
- 25.Roldan-Tapia L, Parron T, Sanchez-Santed F. Neuropsychological effects of long-term exposure to organophosphate pesticides. Neurotoxicol Teratol. 2005;27:259–266. doi: 10.1016/j.ntt.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Salvi RM, Lara DR, Ghisolfi ES, Portela LV, Dias RD, Souza DO. Neuropsychiatric evaluation in subjects chronically exposed to organophosphate pesticides. Toxicol Sci. 2003;72:267–271. doi: 10.1093/toxsci/kfg034. [DOI] [PubMed] [Google Scholar]
- 27.London L, Flisher AJ, Wesseling C, Mergler D, Kromhout H. Suicide and exposure to organophosphate insecticides: cause or effect? Am J Ind Med. 2005;47:308–321. doi: 10.1002/ajim.20147. [DOI] [PubMed] [Google Scholar]
- 28.Rohlman DS, Lasarev M, Anger WK, Scherer J, Stupfel J, McCauley L. Neurobehavioral performance of adult and adolescent agricultural workers. Neurotoxicology. 2007;28(2):374–380. doi: 10.1016/j.neuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Stallones L, Beseler C. Pesticide poisoning and depressive symptoms among farm residents. Ann Epidemiol. 2002;12:389–394. doi: 10.1016/S1047-2797(01)00298-8. [DOI] [PubMed] [Google Scholar]
- 30.Stephens R, Sreenivasan B. Neuropsychological effects of long-term low-level organophosphate exposure in orchard sprayers in England. Arch Environ Health. 2004;59:566–574. doi: 10.1080/00039890409603435. [DOI] [PubMed] [Google Scholar]
- 31.Pilkington A, Buchanan D, Jamal GA, Gillham R, Hansen S, Kidd M, et al. An epidemiological study of the relations between exposure to organophosphate pesticides and indices of chronic peripheral neuropathy and neuropsychological abnormalities in sheep farmers and dippers. Occup Environ Med. 2001;58:702–710. doi: 10.1136/oem.58.11.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farahat TM, Abdelrasoul GM, Amr MM, Shebl MM, Farahat FM, Anger WK. Neurobehavioural effects among workers occupationally exposed to organophosphorous pesticides. Occup Environ Med. 2003;60:279–286. doi: 10.1136/oem.60.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothlein J, Rohlman D, Lasarev M, Phillips J, Muniz J, McCauley L. Organophosphate pesticide exposure and neurobehavioral performance in agricultural and nonagricultural Hispanic workers. Environ Health Perspect. 2006;114:691–696. doi: 10.1289/ehp.8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MackenzieRoss SJ, Brewin CR, Curran HV, Furlong CE, Abraham-Smith KM, Harrison V. Neuropsychological and psychiatric functioning in sheep farmers exposed to low levels of organophosphate pesticides. Neurotoxicol Teratol. 2010;32:452–459. doi: 10.1016/j.ntt.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheslack-Postava K, Rantakokko PV, Hinkka-Yli-Salomäki S, Surcel HM, McKeague IW, Kiviranta HA. Maternal serum persistent organic pollutants in the Finnish prenatal study of autism: a pilot study. Neurotoxicol Teratol. 2013;38:1–5. doi: 10.1016/j.ntt.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California central valley. Environ Health Perspect. 2007;115:1482–1489. doi: 10.1289/ehp.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richardson JR, Roy A, Shalat SL, Buckley B, Winnik B, Gearing M, et al. β-Hexachlorocyclohexane levels in serum and risk of Parkinson’s disease. NeuroToxicol. 2011;32:640–645. doi: 10.1016/j.neuro.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh NK, Chhillar N, Banerjee BD, Bala K, Basu M, Mustafa M. Organochlorine pesticide levels anrisk of Alzheimer’s disease in north Indian population. Hum Exp Toxicol. 2012;32(1):24–30. doi: 10.1177/0960327112456315. [DOI] [PubMed] [Google Scholar]
- 39.Kim KS, Lee YM, Lee HW, Jacobs JDR, Lee DH. Associations between organochlorines pesticides and cognition in U.S. elders: national health and nutrition examination survey 1999–2002. Environ Int. 2015;75:87–92. doi: 10.1016/j.envint.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Payan-Renteria R, Garibay-Chavez G, Rangel-Ascencio R, Preciado-Martinez V, Munoz-Islas L, Beltran-Miranda C, et al. Effect of chronic pesticide exposure in farm workers of a Mexico community. Arch Environ Occup Health. 2012;67(1):22–30. doi: 10.1080/19338244.2011.564230. [DOI] [PubMed] [Google Scholar]
- 41.Eskenaz B, Marks AR, Bradman A, Fenster L, Johnson C, Barr DB, et al. In utero exposure to dichlorodiphenyltrichloroethane (DDT) and dichlorodiphenyldichloroethylene (DDE) and neurodevelopment among young Mexican American children. Pediatrics. 2006;118:233–241. doi: 10.1542/peds.2005-3117. [DOI] [PubMed] [Google Scholar]
- 42.Lu JL. Comparison of pesticide exposure and physical examination, neurological assessment, and laboratory findings between full-time and part-time vegetable farmers in the Philippines. Environ Health Prev Med. 2009;14:345–352. doi: 10.1007/s12199-009-0105-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hansen MRH, Jors E, Lander F, Condarco G, Debes F, Bustillos NT, Schlünssen V. Neurological deficits after long-term pyrethroid exposure. Environ Health Insights. 2017. https://doi.org/1178630217700628. [DOI] [PMC free article] [PubMed]
- 44.Smit LA, van-Wendel-de-Joode BN, Heederik D, Peiris-John RJ, van der Hoek W. Neurological symptoms among Sri Lankan farmers occupation- ally exposed to acetylcholinesterase-inhibiting insecticides. Am J Ind Med. 2003;44(3):254–264. doi: 10.1002/ajim.10271. [DOI] [PubMed] [Google Scholar]
- 45.Wesseling C, Joode BW, Keifer M, London L, Mergler D, Stallones L. Symptoms of psychological distress andsuicidal ideation among banana workers witha history of poisoning by organophosphateor n-methyl carbamate pesticides. Occup Environ Med. 2010;67:778–784. doi: 10.1136/oem.2009.047266. [DOI] [PubMed] [Google Scholar]
- 46.Ohayo-Mitoko GJ, Kromhout H, Simwa JM, Boleij JS, Heederik D. Self reported symptoms and inhibition of acetylcholinesterase activity among Kenyan agricultural workers. Occup Environ Med. 2000;57(3):195–200. doi: 10.1136/oem.57.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beard JD, Hoppin JA, Richards M, Alavanja MCR, Blair A, Sandler DP, et al. Pesticide exposure and self-reported incident depression among wives in the agricultural health study. Environ Res. 2013;126:31–42. doi: 10.1016/j.envres.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beseler CL, Stallones L. Structural equation modeling of the relationships between pesticide poisoning, depressive symptoms and safety behaviors among Colorado farm residents. J Agromed. 2006;11:35–46. doi: 10.1300/J096v11n03_05. [DOI] [PubMed] [Google Scholar]
- 49.Motsoeneng PM, Dalvie MA. Relationship between urinary pesticide residue levels and neurotoxic symptoms among women on farms in the Western Cape, South Africa Int. J Environ Res Public Health. 2015;12:6281–6299. doi: 10.3390/ijerph120606281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crepet A, Heraud F, Bechaux C, Gouze ME, Pierlot S, Fastier A, et al. The PERICLES research program: an integrated approach to characterize the combined effects of mixtures of pesticide residues to which the French population is exposed. Toxicology. 2013;16,313(2–3):83–93. doi: 10.1016/j.tox.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Rohlman DS, Anger WK, Lein PJ. Correlating neurobehavioral performance with biomarkers of organophosphorous pesticide exposure. Neurotoxicology. 2011;32(2):268–276. doi: 10.1016/j.neuro.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muniz JF, McCauley L, Scherer J, Lasarev M, Koshy M, Kow Y. Biomarkers of oxidative stress and DNA damage in agricultural workers: a pilot study. Toxicol Appl Pharmacol. 2008;227:97–107. doi: 10.1016/j.taap.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 53.Hardas SS, Sultana R, Clark AM, et al. Oxidative modification of lipoic acid by HNE in Alzheimer’s disease brain. Redox Biol. 2013;1:80–85. doi: 10.1016/j.redox.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Environ Health Perspect. 2011;119(6):878. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leon-Olea M, Martyniuk CJ, Orlando EF, Ottinger MA, Rosenfeld CS, Wolstenholme JT, Trudeau VL. Current concepts in neuroendocrine disruption. Gen Comp Endocrinol. 2014;203:158–173. doi: 10.1016/j.ygcen.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strelitz J, Engel LS, Keifer MC. Blood acetylcholinesterase and butyrylcholinesterase as biomarkers of cholinesterase depression among pesticide handlers. Occup Environ Med. 2014;71(12):842–847. doi: 10.1136/oemed-2014-102315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kori RK, Jain AK, Yadav RS. Biomarkers: an essential gizmo in pesticide toxicity. Biomark J. 2016;2(1:9):1–5. [Google Scholar]
- 58.Milatovic D, Gupta RC, Aschner M. Anticholinesterase toxicity and oxidative stress. Sci World J. 2006;6:295–310. doi: 10.1100/tsw.2006.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Srivastava AK, Gupta BN, Bihari V, Mathur N, Srivastava LP, Pangtey BS, Bharti RS, Kumar P. Clinical, biochemical and neurobehavioral studies of workers engaged in the manufacture of quinalphos. Food Chem Toxicol. 2000;38:65–69. doi: 10.1016/S0278-6915(99)00123-4. [DOI] [PubMed] [Google Scholar]
- 60.Ismail AA, Bodner TE, Rohlman DS. Neurobehavioral performance among agricultural workers and pesticide applicators: a meta-analytic study. Occup Environ Med. 2012;69(7):457–464. doi: 10.1136/oemed-2011-100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ryding E, Lindstrom M, Traskman-Bendz L. The role of dopamine and serotonin in suicidal behaviour and aggression serotonin-dopamine interaction. Exp Evid Ther Relevance Prog Brain Res. 2008;72:307–315. doi: 10.1016/S0079-6123(08)00915-1. [DOI] [PubMed] [Google Scholar]
- 62.Seo D, Patrick CJ, Kennealy PJ. The role of serotonin and dopamine system interactions in the neurobiology of impulsive aggression and its comorbidity with other clinical disorders. Aggress Violent Beh. 2008;13(5):383–395. doi: 10.1016/j.avb.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinez-Larranaga MR, Anadon A, Martinez MA, Martinez M, Castellano VJ, Diaz MJ. 5-HT loss in rat brain by type II pyrethroid insecticides. Toxicol Ind Health. 2003;19:147–155. doi: 10.1191/0748233703th184oa. [DOI] [PubMed] [Google Scholar]
- 64.Oquendo MA, Mann JJ. The biology of impulsivity and suicidality. Psychiatr Clin North Am. 2000;23:11–25. doi: 10.1016/S0193-953X(05)70140-4. [DOI] [PubMed] [Google Scholar]
- 65.Ohmori T, Arora RC, Meltzer HY. Serotonergic measures in suicide brain: the concentration of 5-HIAA, HVA and tryptophan in frontal cortex of suicide victims. Biol Psychiat. 1992;32:57–71. doi: 10.1016/0006-3223(92)90142-M. [DOI] [PubMed] [Google Scholar]
- 66.Engstrom G, Alling C, Blennow K, Regnell G, Tanskman-Bendz L. Reduced cerebrospinal HVA concentration and HVA/5-HIAA ratios in suicide attempters. Eur Neuropsychopharmacol. 1999;9:399–405. doi: 10.1016/S0924-977X(99)00016-4. [DOI] [PubMed] [Google Scholar]
- 67.Choudhary S, Raheja G, Gupta V, Gill KD. Possible involvement of dopaminergic neurotransmitter system in dichlorvos induced delayed neurotoxicity. J Biochem Mol Biol Biophys. 2002;6(1):29–36. doi: 10.1080/10258140290010197. [DOI] [PubMed] [Google Scholar]
- 68.Beseler CL, Stallones L. Safety knowledge, safety behaviors depression and injuries in Colorado farm residents. Am J Industrial Med. 2010;53:47–54. doi: 10.1002/ajim.20779. [DOI] [PubMed] [Google Scholar]
- 69.Joo Y, Roh S. Risk factors associated with depression and suicidal ideation in a rural population. Environ Health Toxicol. 2016;26(31):e2016018. doi: 10.5620/eht.e2016018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beard JD, Umbach DM, Hoppin JA, Richards M, Alavanja MC, Blair A, Sandler DP, Kamel F. Pesticide exposure and depression among male private pesticide applicators in the agricultural health study. Environ Health Perspect. 2014;122(9):984–991. doi: 10.1289/ehp.1307450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang J, Stewart R, Phillips M, Shi Q, Prince M. Pesticide exposure and suicidal ideation in rural communities in Zhejiang province, China. Bull World Health Organ. 2009;87(10):745–753. doi: 10.2471/BLT.08.054122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saravi SSS, Dehpour AR. Potential role of organochlorine pesticides in the pathogenesis of neurodevelopmental, neurodegenerative, and neurobehavioral disorders: a review. Life Sci. 2016;145:255–264. doi: 10.1016/j.lfs.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 73.Hossain MM, Richardson JR. Mechanism of pyrethroid pesticide-induced apoptosis: role of Calpain and the ER stress pathway. Toxicol Sci. 2011;122(2):512–525. doi: 10.1093/toxsci/kfr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pearson JN, Patel M. The role of oxidative stress in organophosphate and nerve agent toxicity. Ann NY Acad Sci. 2016;1378:17–24. doi: 10.1111/nyas.13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chan JY, Chan SH, Dai KY, et al. Cholinergic-receptor-independent dysfunction of mitochondrial respiratory chain enzymes, reduced mitochondrial transmembrane potential and ATP depletion underlie necrotic cell death induced by the organophosphate poison mevinphos. Neuropharmacology. 2006;51:1109–1119. doi: 10.1016/j.neuropharm.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 76.Harley KG, Huen K, Aguilar Schall R, Holland NT, et al. Association of organophosphate pesticide exposure and paraoxonase with birth outcome in Mexican-American women. PLoS ONE. 2011;6(8):e23923. doi: 10.1371/journal.pone.0023923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bloom SE, Lemley AT, Muscarella DE. Potentiation of apoptosis by heat stress plus pesticide exposure in stress resistant human B-lymphoma cells and its attenuation through interaction with follicular dendritic cells: role for c-Jun N-terminal kinase signaling. Toxicol Sci. 2006;89(1):214–223. doi: 10.1093/toxsci/kfj021. [DOI] [PubMed] [Google Scholar]
- 78.Kumar V, Gupta AK, Shukla RK, Tripathi VK, Jahan S, Pandey A, Srivastava A, Agrawal M, Yadav S, Khanna VK, Pant AB. Molecular Mechanism of Switching of TrkA/p75(NTR) Signaling in Monocrotophos Induced Neurotoxicity. Sci Rep. 2015;15(5):14038. doi: 10.1038/srep14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Monnet-Tschudi F, Zurich MG, Honegger P. Neurotoxicant-induced inflammatory response in threedimensional brain cell cultures. Hum Exp Toxicol. 2007;26:339–346. doi: 10.1177/0960327107074589. [DOI] [PubMed] [Google Scholar]
- 80.Liu C, Li L, Ha M, Qi S, Duan P, Yang K. The PI3 K/Akt and ERK pathways elevate thyroid hormone receptor β1 and TRH receptor to decrease thyroid hormones after exposure to PCB153 and p, p’-DDE. Chemosphere. 2015;118:229–238. doi: 10.1016/j.chemosphere.2014.09.023. [DOI] [PubMed] [Google Scholar]