Abstract
Introduction
Glypican-3 (GPC3) is involved in regulation of cell proliferation and morphogenesis. It is abundant in embryonic tissue, but limited in most adult tissues. GPC3 deletion or mutation can disturb the balance between cell apoptosis and proliferation, which may result in tumorigenesis. This study aimed to investigate the GPC3 expression in salivary gland tumors (SGTs) and the adjacent non-neoplastic tissues.
Methods
This study reviewed 50 samples of salivary tumors from the archive of Khalili Hospital, Shiraz, Iran, including 17 cases of pleomorphic adenoma (PA), 16 cases of mucoepidermoid carcinoma (MEC), and 17 cases of adenoid cystic carcinoma (ACC); as well as a control group of 23 cases of normal salivary gland tissues. GPC3 expression was investigated through immunohistochemistry.
Results
GPC3 expression was significantly higher in malignant tumors (MEC and ACC) than in PA, and higher in PA than in the normal salivary glands (P < 0.001). The expression intensity was moderate to strong in malignant tumors and weak to moderate in benign tumors. No strong positivity was observed in normal salivary gland tissues (P < 0.001). Nor was any association detected between the GPC3 expression and intensity with the clinicopathologic parameters.
Conclusion
Although GPC3 overexpression was observed at the protein level in SGTs, and its expression was not related with the clinicopathologic factors, the potential use of GPC3 for diagnostic, therapeutic, and prognostic purposes requires further investigations.
Keywords: Salivary gland tumor, PA, ACC, MEC, GPC3
1. Introduction
Salivary gland tumors are one of the important neoplasms in maxillofacial pathology, and constitute 3–6% of all head and neck tumors. These tumors demonstrate a wide spectrum of pathologic and clinical variants that lead to hard management and diagnosis. Mucoepidermoid carcinoma (MEC), adenoid cystic carcinoma (AdCC) and Pleomorphic adenoma (PA) are the most common malignant and benign tumors and have histopathologic similarities in many cases1. Although, hematoxylin and eosin-stained (H&E) tissue sections are used usually for diagnosis in many situation, the definite diagnosis is sometimes hard.
Glypicans (GPC) are a family of heparin sulfate proteoglycans which are bound to cell membrane via a glycosyl-phosphatidylinositol anchor. The Glypican gene family consists of six members in mammals.2 GPC can reveal from the cell membrane by a lipase called Notum. It is involved in regulating different signaling pathways consisting of Wnt, Hedgehogs, fibroblast growth factors, and bone morphogenetic proteins.3,4
GPC3 is involved in regulation of cell proliferation and morphogenesis. Although it is abundant in embryonic tissue, GPC3 is limited found in most adult tissues.5,6 GPC3 deletion or mutation can disturb the balance between cell apoptosis and proliferation which may result in tumorigenesis.6 GPC3 upregulation and overexpression have been observed in hepatocellular carcinoma, malignant melanoma, neuroblastoma, and colon cancer.7, 8, 9, 10
The aim of the present study was to investigate the GPC3 expression in the malignant and benign salivary gland tumors (SGT) and the adjacent non-neoplastic tissue by using immunohistochemical methods and to define its expression in relation to clinicopathologic features.
2. Methods
This study reviewed 50 samples of salivary gland tumors from Khalili Hospital archive, Shiraz, Iran, including 17 cases of pleomorphic adenoma (PA), 16 cases of mucoepidermoid carcinoma (MEC) and 17 cases of adenoid cystic carcinoma (ACC). A control group was considered consisting of 23 cases of normal salivary gland tissues adjacent to the previous biopsy of the oral cavity or SGT.
Having reviewed the H & E slides, the blocks with definite diagnosis and enough cellular tissue were selected and subjected to immunohistochemical staining (IHC) by using EnvsionLabled Peroxides System (DAKO, Carpentaria, CA, USA). The stained samples were fixed in 10% buffered formalin and embedded in paraffin. Then, 4-μm sections were cut, deparaffinized in xylene, rehydrated in graded alcohol, and rinsed with distilled water. DAKO cytomation target retrieval solution (pH = 9) was used for 20 min for antigen retrieval, and 3% H2O2 was used to inhibit the internal peroxidase activity. Incubation of the tissue sections was done for 30 min by using the anti-glypican-3 antibody (Abcam, ab66596) at 1/100 dilution.
Normal samples were stained with the same amount of antibody used for staining the tumoral tissues. Omission of the primary antibody was employed as negative control, while gastric epithelium was used as positive control for GPC311 stages collected by the American Joint Committee on Cancer (AJCC) TNM stage.12 Tumor grade in ADCC, grade I is referred to as a tubular growth pattern, grade II as a cribriform growth pattern, and grade III as a solid growth pattern. MEC was classified as grade I, if it showed a well demarcated border, macrocystic spaces and a bland cyst lining; grade II, if it showed a more solid pattern with only few microcysts, and focal infiltration; and grade III, with no cystic spaces and a highly infiltrative growth pattern, and cleared nuclear atypia.13
Brown membranous and cytoplasmic staining for GPC3 was considered as positive. A tumor was considered positive for GPC3 if more than 10% of the neoplastic cells showed strong cytoplasmic and/or membranous reactivity. GPC3 expression was assessed as negative and positive. GPC3 immunoreactivity was evaluated by using a semiquantitative scoring system for the staining intensity (0: negative staining, 1: weak staining, 2: moderate staining, 3: intense staining). Chi-square test was used to compare the results between the two groups and the relationship with clinicopathologic features.
3. Results
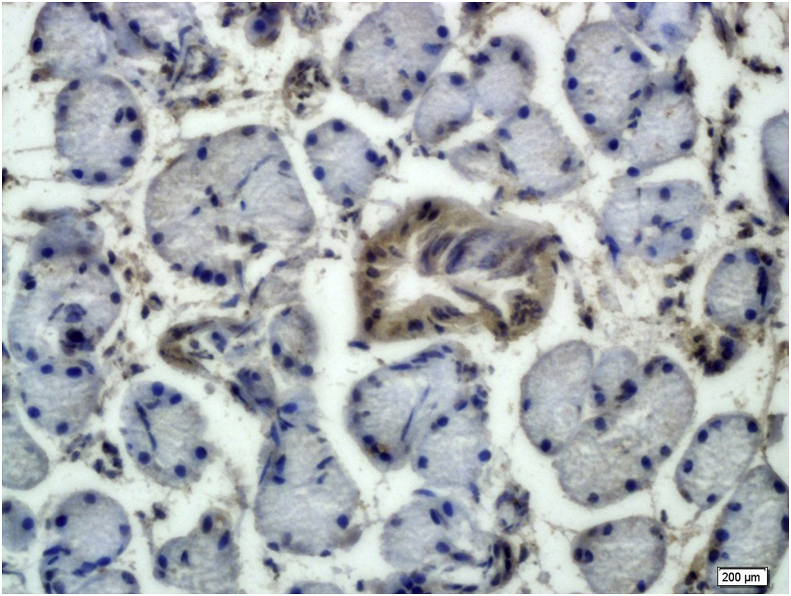
Of the 50 patients included in this study, 19 were male (38%) and 31 were female (62%). The patients’ mean age was 47.3 ± 10.2 (ranging 7–79). Most tumors (64%) involved the major salivary glands. GPC3 expression was mostly both membranous and cytoplasmic in SGT, but only cytoplasmic pattern was seen in normal salivary gland tissue. GPC3 immunoreactivity was seen in 4 cases of normal salivary gland tissues (17.40%). GPC3 expression was only seen in the epithelial lining of the salivary duct (Fig. 1, Table 1).
Fig. 1.
Moderate Glypican-3 expression in the duct of normal salivary gland ( × 400).
Table 1.
Glypican-3 expression in benign and malignant salivary gland tumors in comparison with normal salivary gland tissues.
| Types of lesion | Number of patient | Glypican-3 expression |
|
|---|---|---|---|
| Negative N (%) | Positive N (%) | ||
| Mucoepidermoid carcinoma | 16 | 2 (12.5) | 14 (87.5) |
| Adenoid cystic carcinoma | 17 | 3 (17.6) | 14 (82.4) |
| Pleomrphic adenoma | 17 | 6 (35.3) | 11 (64.7) |
| Normal salivary gland tissue | 23 | 19 (82.6) | 4 (17.40) |
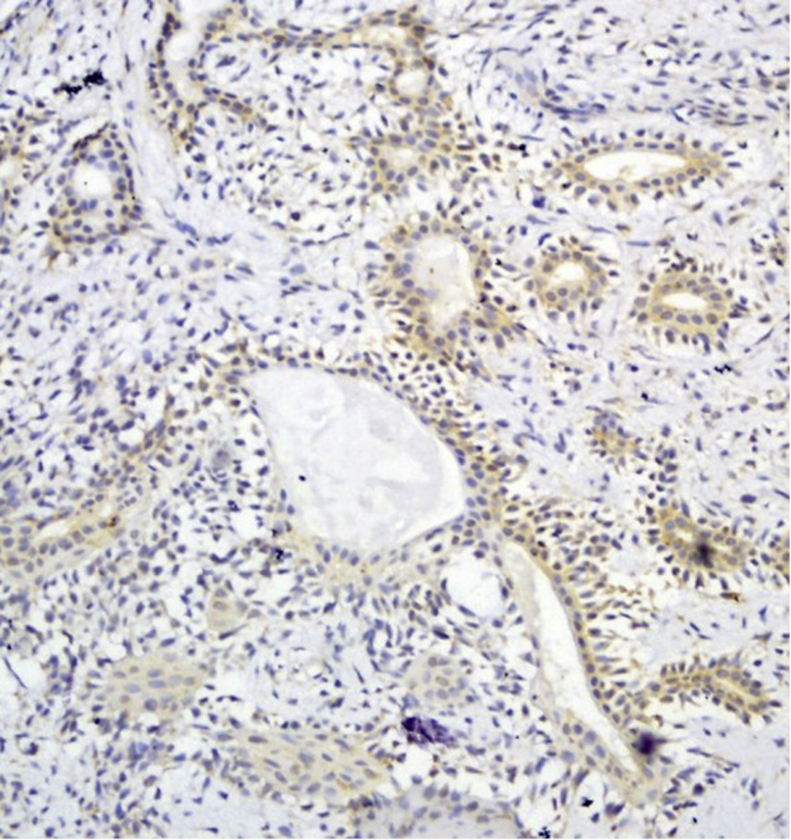
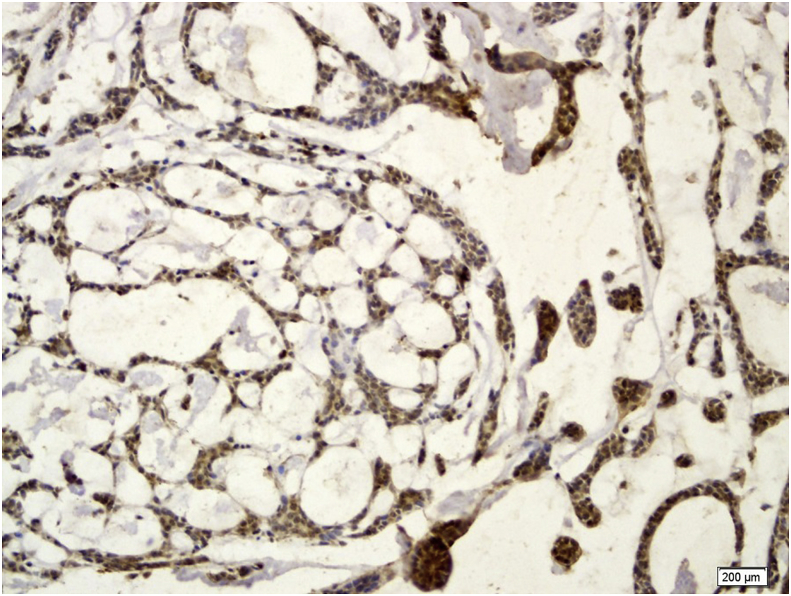
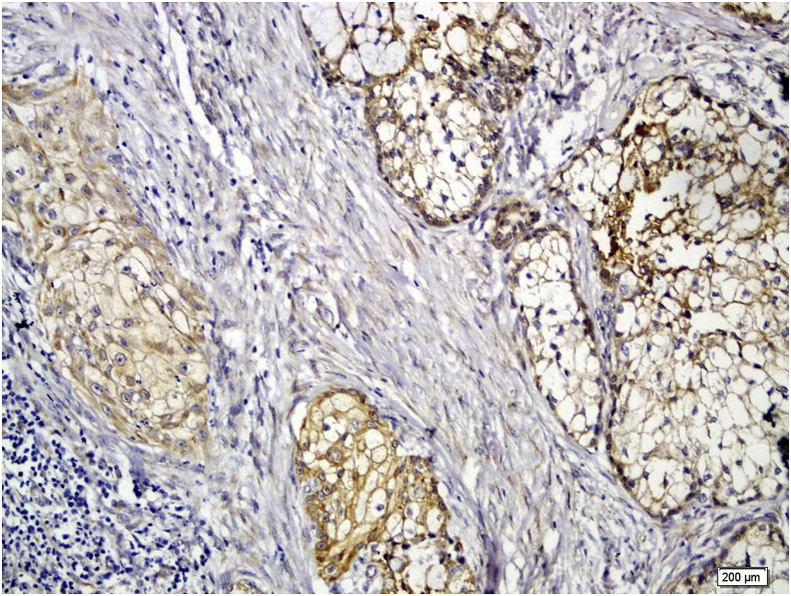
GPC3 positive staining was seen in 11 cases (64.7%) of the PA. In PA, both ductal and myoepithelial cells showed GPC3 staining (Fig. 2). GPC3 immunostaining was seen in 14 cases of ACC (82.4%) and 14 cases of MEC (87.5%) (Fig. 3, Fig. 4). All histologic subtypes of ACC showed GPC3 immunoreactivity. The Kruskal-Wallis test showed a significant difference between the mean expression of GPC3 in the tumoral groups (p = 0.001). Mann-Whitney test revealed that normal salivary gland were significantly different from both PA (p = 0.039) and malignant SGT(MEC and ACC) (p = 0.000) in GPC3 expression, and also significantly different among the malignant SGT and PA (p = 0.047).
Fig. 2.
Moderate Glypican-3 expression in pleomorphic adenoma ( × 200).
Fig. 3.
Severe Glypican-3 expression in cribriform adenoid cystic carcinoma ( × 200).
Fig. 4.
Severe membranous Glypican-3 expression in mucoepidermoid carcinoma ( × 200).
Moreover, GPC3 expression intensity was moderate to strong in malignant SGT and weak to moderate in benign SGT; but, no strong positivity was seen in normal salivary gland tissue (Table 2).
Table 2.
Glypican-3 intensity in benign and malignant salivary gland tumors in comparison with normal salivary gland tissues.
| Types of lesion | Number of patient | Glypican-3 Intensity |
|||
|---|---|---|---|---|---|
| Negative N (%) | Weak N (%) | Moderate N (%) | Strong N (%) | ||
| Mucoepidermoid carcinoma | 16 | 2 (12.5) | 1 (6.3) | 7 (43.8) | 6 (37.5) |
| Adenoid cystic carcinoma | 17 | 3 (17.6) | 2 (11.8) | 5 (29.4) | 7 (41.2) |
| Pleomrphic adenoma | 17 | 6 (35.3) | 4 (23.5) | 5 (29.4) | 2 (11.8) |
| Normal salivary gland tissue | 23 | 19 (82.6) | 2 (8.7) | 2 (8.7) | 0 (0) |
Using the Kruskal-Wallis test, the intensity of staining was significantly different among the groups (p = 0.000) and by Mann-Whitney test, the intensity of GPC3 was higher in malignant SGT(MEC and ACC) than those of PA (p = 0.013) and normal salivary gland (p = 0.000), and significant difference was seen between normal salivary gland (P = 0.007) and PA. No significant difference was seen between MEC and ACC (P = 0.87).
The percentage and intensity of GPC3 expression in tumoral were not statistically different among the groups as regards tumor size, stage, and grade (Kruskal- Wallis and Dunn's test, all p > 0.05) (Table 3).
Table 3.
Glypican-3 expression and intensity in relation to clinicopathologic parameters in patients with malignant salivary gland tumor.
| Variable | N (%) | Glypican-3 expression |
P Value | Glypican-3 Intensity |
P Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Negative N (%) | Positive N (%) | Negative N (%) | Weak N (%) | Moderate N (%) | Strong N (%) | ||||
| T status | |||||||||
| T1+T2 | 18 (54.5) | 4 (22.2) | 14 (77.8) | 4 (22.2) | 1 (5.6) | 6 (33.3) | 7 (38.9) | ||
| T3+T4 | 15 (45.5) | 1 (6.7) | 14 (93.3) | 0.3 | 1 (6.7) | 2 (13.3) | 6 (40) | 6 (40) | 0.7 |
| N Status | |||||||||
| N0 | 27 (81.8) | 5 (18.5) | 22 (81.5) | 5 (18.5) | 2 (7.5) | 10 (37) | 10 (37) | ||
| N1 | 6 (18.2) | 0 (0) | 6 (100) | 0.5 | 0 ( ) | 1 (16.7) | 2 (33.3) | 3 (50) | 0.5 |
| M Status | |||||||||
| M0 | 30 (40.9) | 5 (16.7) | 25 (83.3) | 5 (16.6) | 3 (10) | 10 (33.3) | 12 (40.1) | ||
| M1 | 3 (9.1) | 0 (0) | 3 (100) | – | 0 ( ) | 0 ( ) | 2 (66.6) | 1 (33.4) | – |
| Stage | |||||||||
| I + II | 15 (45.4) | 4 (26.7) | 11 (73.3) | 4 (26.7) | 0 (0) | 5 (33.3) | 6 (40) | ||
| III + IV | 18 (54.6) | 1 (5.6) | 17 (94.4) | 0.1 | 1 (5.6) | 3 (16.7) | 7 (38.9) | 7 (38.9) | 0.7 |
| Histiologic grade | |||||||||
| I | 12 (36.3) | 3 (25) | 9 (75) | 3 (25) | 0 (0) | 4 (333) | 5 (41.4) | ||
| II | 11 (33.3) | 1 (9.1) | 10 (90.9) | 1 (9.09) | 2 (18.1) | 3 (27.2) | 5 (45.6) | ||
| III | 10 (30.4) | 1 (10) | 9 (90) | 0.2 | 1 (10) | 1 (10) | 5 (50) | 3 (30) | 0.9 |
4. Discussion
GPC3, a marker of the glypican family, is a one of the cell surface heparan sulfate proteoglycans which is bind to the plasma membrane via glycosyl-phosphatidylinositol anchor.14 Heparan sulfate proteoglycans can interact with the growth factor via heparan sulfate chains, so they act as co-receptor for heparin binding growth factors.14 Different expressions of GPC3 in normal and neoplastic tissues revealed that it can be used as a diagnostic tool for the distinction of various tumor entities and for non-neoplastic, pre-neoplastic and neoplastic disorders.6
Downregulation of GPC3 due to hypermethylation of GPC3 promoter was seen in different malignant tumors such as lung adenocarcinoma, clear cell renal cell carcinoma, ovarian carcinoma, and breast cancer.15, 16, 17, 18 In contrast, overexpression of GPC3 was seen in hepatocellular carcinoma, colon cancer, melanoma, and neuroblastoma.7, 8, 9, 10
In the present study, GPC3 expression was higher in benign SGTs than in normal tissues, and higher in malignant SGTs than in benign tumors; confirming the GPC3 role in the tumorigenesis and carcinogenesis of SGTs. It was in agreement with those of the previous studies which showed overexpression of GPC3 in colon cancer, melanoma and hepatocellular carcinoma.7,8,10 However, it was in contrast with the findings of the studies that reported GPC3 downregulation in lung adenocarcinoma, ovarian carcinoma and breast carcinoma revealing the difference in the GPC3 function in a tissue-dependent manner.15,17,18
Downregulation of GPC3 in tumoral tissues compared with the adjacent non-tumoral tissues in some tumors suggested an inhibitory effect on cell proliferation and tumor suppressor function.17,18 But, its overexpression in tumoral tissues compared with the corresponding normal tissue displayed oncofetal protein-like characteristics.6 Accordingly, the present study can suggest oncofetal protein-like characteristics for GPC3 in SGTs.
Currently, the biological functions and the role of GPC3 in tumorigenesis are poorly understood and many possible mechanisms regulated by GPC3 during tumorigenesis and tumor progression can be suggested.17 GPC3 is involved in several signaling pathways consisting of IGF, Hh, Wnts and regulating the apoptosis of protein-like Bax and Bcl2.4,19, 20, 21, 22
Many studies revealed that GPC3 can regulate the proliferation, differentiation, and adhesion of tumoral cells; so, it could moderate tumor growth and metastasis.23,24 Moreover, Shirakwa et al. found no correlation between the GPC3 expression and any of the clinicopathologic parameters, except for the histological grade of hepatocellular carcinoma.25 In a study conducted by Castillo et al. on human breast tissues, no association was found between the GPC3 expression and clinicopathologic parameters.26 The present study detected no association between the GPC3 expression and clinicopathologic parameters, suggesting that GPC3 might be an independent marker. Yet, further studies with larger sample size are recommended.
In benign and malignant SGTs, both cytoplasmic and membranous GPC3 expressions were seen; but in the normal tissues, only cytoplasmic protein was observed. The functional difference between two different GPC3 expression pattern (membranous and cytoplasmic) is unknown,25 so additional studies are recommended to elucidate the significance of different localization patterns.
The higher GPC3 expression in SGT compared with the normal tissues may suggest GPC3 as a potential target for antibody-based therapy, as recently investigated.4 Nakatsura et al. showed that GPC3 particle vaccine increased the immune response and overall survival in advanced hepatocellular carcinoma.27
In conclusion, the current study showed the GPC3 overexpression at the protein level in SGTs. However, no association was detected between GPC3 and the clinicopathologic factors. Therefore, its potential use for diagnostic, therapeutic and prognostic proposes requires further investigations.
In the present study, we showed no statistically significant difference between GPC3 expression and tumor stage, grade, or size. According to the different roles of GPC3in tumor progression, it is possible that the limited number of our patients with complete clinical data have resulted in these investigations. Also, future studies are recommended to assess the relationship between GPC3 overexpression with clinical act of salivary gland tumors. In conclusion, the overexpression of GPC3 in salivary gland tumors in comparison with carcinomas and also in normal glands in comparison with benign tumors may show the role of this protein in the malignant transformation of salivary glands and also in the tumorigenesis and tumor invasion. In this regard, we offer further researches to evaluate the exact mechanism of GPC3 protein and its possible use as a therapeutic target.
Declarations of interest
We wish to confirm that there are no known conflicts of interest associated with this publication that could have influenced its outcome.
Acknowledgments
The authors thank the Vice-Chancellery of Shiraz University of Medical Science for supporting this research (Grant# 13186). This manuscript is based on the thesis of Ali Gudarzi for partial fulfillment of DDS degree. The authors are grateful to Dr. M. Vossoughi from the Dental Research Development Center of the Dental School for the statistical analysis.
References
- 1.Jaafari-Ashkavandi Z., Ashraf M.-J., Moshaverinia M. Salivary gland tumors: a clinicopathologic study of 366 cases in southern Iran. Asian Pac J Cancer Prev APJCP. 2013;14(1):27–30. doi: 10.7314/apjcp.2013.14.1.27. [DOI] [PubMed] [Google Scholar]
- 2.Filmus J., Selleck S.B. Glypicans: proteoglycans with a surprise. J Clin Invest. 2001 Aug;108(4):497–501. doi: 10.1172/JCI13712. PubMed PMID: 11518720. Pubmed Central PMCID: PMC209407. Epub 2001/08/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torisu Y., Watanabe A., Nonaka A. Human homolog of NOTUM, overexpressed in hepatocellular carcinoma, is regulated transcriptionally by β‐catenin/TCF. Canc Sci. 2008;99(6):1139–1146. doi: 10.1111/j.1349-7006.2008.00814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Cat B., Muyldermans S.-Y., Coomans C. Processing by proprotein convertases is required for glypican-3 modulation of cell survival, Wnt signaling, and gastrulation movements. J Cell Biol. 2003;163(3):625–635. doi: 10.1083/jcb.200302152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grozdanov P.N., Yovchev M.I., Dabeva M.D. The oncofetal protein glypican-3 is a novel marker of hepatic progenitor/oval cells. Lab Invest. 2006;86(12):1272–1284. doi: 10.1038/labinvest.3700479. [DOI] [PubMed] [Google Scholar]
- 6.Baumhoer D., Tornillo L., Stadlmann S., Roncalli M., Diamantis E.K., Terracciano L.M. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4,387 tissue samples. Am J Clin Pathol. 2008;129(6):899–906. doi: 10.1309/HCQWPWD50XHD2DW6. [DOI] [PubMed] [Google Scholar]
- 7.Hsu H.-C., Cheng W., Lai P.-L. Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma: biological significance and temporospatial distribution. Canc Res. 1997;57(22):5179–5184. [PubMed] [Google Scholar]
- 8.Ikuta Y., Nakatsura T., Kageshita T. Highly sensitive detection of melanoma at an early stage based on the increased serum secreted protein acidic and rich in cysteine and glypican-3 levels. Clin Canc Res. 2005;11(22):8079–8088. doi: 10.1158/1078-0432.CCR-05-1074. [DOI] [PubMed] [Google Scholar]
- 9.Saikali Z., Sinnett D. Expression of glypican 3 (GPC3) in embryonal tumors. Int J Canc. 2000;89(5):418–422. [PubMed] [Google Scholar]
- 10.Lage H., Dietel M., Fröschle G., Reymann A. Expression of the novel mitoxantrone resistance associated gene MXR7 in colorectal malignancies. Int J Clin Pharmacol Therapeut. 1998;36(1):58–60. [PubMed] [Google Scholar]
- 11.Jeiroodi N., Bagherpour M., Zare R., Torabi A.S., Andisheh A.T. Evaluation of midkine expression in dentigerous cysts, odontogenic keratocysts and different types of ameloblastoma. Turk J Pathol. 2018;1(1):001–007. doi: 10.5146/tjpath.2017.01421. [DOI] [PubMed] [Google Scholar]
- 12.Patel S.G., Shah J.P. TNM staging of cancers of the head and neck: striving for uniformity among diversity. CA A Cancer J Clin. 2005;55(4):242–258. doi: 10.3322/canjclin.55.4.242. [DOI] [PubMed] [Google Scholar]
- 13.Seethala R.R. An update on grading of salivary gland carcinomas. Head and neck pathology. 2009;3(1):69–77. doi: 10.1007/s12105-009-0102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang F., Jing X., Wang T. Differential diagnostic value of GPC3-CD34 combined staining in small liver nodules with diameter less than 3 cm. Am J Clin Pathol. 2012;137(6):937–945. doi: 10.1309/AJCP0KZZ5DSIGMNY. [DOI] [PubMed] [Google Scholar]
- 15.Kim H., Xu G.-L., Borczuk A.C. The heparan sulfate proteoglycan GPC3 is a potential lung tumor suppressor. Am J Respir Cell Mol Biol. 2003;29(6):694–701. doi: 10.1165/rcmb.2003-0061OC. [DOI] [PubMed] [Google Scholar]
- 16.Valsechi M.C., Oliveira A.B.B., Conceição A.L.G. GPC3 reduces cell proliferation in renal carcinoma cell lines. BMC Canc. 2014;14(1):631. doi: 10.1186/1471-2407-14-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin H., Huber R., Schlessinger D., Morin P.J. Frequent silencing of the GPC3 gene in ovarian cancer cell lines. Canc Res. 1999;59(4):807–810. [PubMed] [Google Scholar]
- 18.Xiang Y.-Y., Ladeda V., Filmus J. Glypican-3 expression is silenced in human breast cancer. Oncogene. 2001;20(50):7408–7412. doi: 10.1038/sj.onc.1204925. [DOI] [PubMed] [Google Scholar]
- 19.Yao N., Yao D., Wang L. Inhibition of autocrine IGF-II on effect of human HepG2 cell proliferation and angiogenesis factor expression. Tumor Biol. 2012;33(5):1767–1776. doi: 10.1007/s13277-012-0436-x. [DOI] [PubMed] [Google Scholar]
- 20.Wang J., He X.D., Yao N., Liang W.J., Zhang Y.C. A meta-analysis of adjuvant therapy after potentially curative treatment for hepatocellular carcinoma. Canadian Journal of Gastroenterology and Hepatology. 2013;27(6):351–363. doi: 10.1155/2013/417894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song H.H., Shi W., Xiang Y.Y., Filmus J. The loss of glypican-3 induces alterations in Wnt signaling. J Biol Chem. 2005 Jan 21;280(3):2116–2125. doi: 10.1074/jbc.M410090200. PubMed PMID: 15537637. Epub 2004/11/13. eng. [DOI] [PubMed] [Google Scholar]
- 22.Srivastava K., Srivastava A., Mittal B. Potential biomarkers in gallbladder cancer: present status and future directions. Biomarkers. 2013;18(1):1–9. doi: 10.3109/1354750X.2012.717105. [DOI] [PubMed] [Google Scholar]
- 23.Peters M., Farias E., Colombo L., Filmus J., Puricelli L., de Kier Joffe E.B. Inhibition of invasion and metastasis by glypican-3 in a syngeneic breast cancer model. Breast Canc Res Treat. 2003;80(2):221–232. doi: 10.1023/A:1024549729256. [DOI] [PubMed] [Google Scholar]
- 24.Foda A.A.-R.M., Mohammad M.A., Abdel-Aziz A., El-Hawary A.K. Relation of glypican-3 and E-cadherin expressions to clinicopathological features and prognosis of mucinous and non-mucinous colorectal adenocarcinoma. Tumor Biol. 2015;36(6):4671–4679. doi: 10.1007/s13277-015-3115-x. [DOI] [PubMed] [Google Scholar]
- 25.Shirakawa H., Suzuki H., Shimomura M. Glypican‐3 expression is correlated with poor prognosis in hepatocellular carcinoma. Canc Sci. 2009;100(8):1403–1407. doi: 10.1111/j.1349-7006.2009.01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castillo L., Huvelle M.A.L., Fujita A. Expression of Glypican-3 (GPC3) in malignant and non-malignant human breast tissues. Open Canc J. 2015;8(1) [Google Scholar]
- 27.Nakatsura T., Kageshita T., Ito S. Identification of glypican-3 as a novel tumor marker for melanoma. Clin Canc Res. 2004;10(19):6612–6621. doi: 10.1158/1078-0432.CCR-04-0348. [DOI] [PubMed] [Google Scholar]