Abstract
BACKGROUND: Patient-derived xenografts (PDX) provide histologically accurate cancer models that recapitulate patient malignant phenotype and allow for highly correlative oncologic in-vivo downstream translational studies. Primary PDX engraftment failure has significant negative consequences on programmatic efficiency and resource utilization and is due to either no tumor growth or development of lymphoproliferative tumors. We aimed to determine if secondary engraftment of previously cryopreserved patient tumor tissues would allow salvage of PDX models that failed previous primary engraftment and increase overall engraftment efficiency. METHODS: Patient hepatobiliary and pancreatic cancers that failed primary engraftment were identified. Previously cryopreserved primary patient cancerous tissues were implanted into immunodeficient mice (NOD/SCID). Mice were monitored, growth metrics calculated, and secondary engraftment outcomes were recorded. Established PDX were verified and compared to original patient tissue through multiple generations by a GI pathologist. RESULTS: We identified 55 patient tumors that previously failed primary engraftment: no tumor growth (n = 46, 84%) or lymphoproliferative tumor (LT) (n = 9, 16%). After secondary implantation using cryopreserved patient tissues, 29 new histologically validated PDX models were generated with an overall secondary engraftment rate of 53% for all tumor types with greatest yield in pancreatic and biliary tract cancers. Of the secondary engraftment failures (n = 26), 21 (38%) were due to no growth and 5 (9%) developed LT. CONCLUSION: Secondary PDX engraftment using cryopreserved primary cancerous is feasible after previous failed engraftment attempts and can result in a 50% increase in overall engraftment efficiency with decreases in LT formation. This technique allows for salvage of critical patient PDX models that would otherwise not exist. SYNOPSIS: Patient-derived xenografts have many important translational applications however can be limited by engraftment failure. We demonstrate optimized methodology utilizing cryopreservation of primary tumor tissue that allows for subsequent successful secondary engraftment and creation of PDX models that failed previous primary engraftment and allowed salvage of patient PDX models that would otherwise not exist.
Background
Patient-derived xenografts (PDXs) are clinically relevant, high fidelity models that allow for amplification of precious primary patient cancer tissue [1]. PDX models are most commonly used for preclinical in-vivo evaluation of therapeutic sensitivities to guide individualized medicine or in identifying diagnostic or predictive biomarkers [2] as these PDX recapitulate patient biological phenotype more accurately than traditional cell line or transgenic models and PDX tumor responses correlate with clinical response [3]. One of the measures of a successful PDX program is dependent on development of numerous unique patient models for each histologic subtype in order to capture and represent the biological and molecular heterogeneity present in these tumors as well as assuring high engraftment rates to minimize resource utilization that is significant for maintaining such PDX models [4], [5].
Primary engraftment failure occurs by two primary etiologies: (1) failed tumor growth or (2) tumor degradation into a lymphoproliferative tumor (LT) [5], [6], [7], [8], [9]. Failed tumor growth can be attributed to both technical issues at time of engraftment (i.e. ischemia/viability) or biological (i.e. proliferative rate/aggressiveness) and is dependent on individual patient and tumor types as well as tumor treatment status prior to engraftment [10]. Warm tissue ischemia is likely the largest single preventable technical factor responsible for failed tumor growth [11]. As ischemia begins immediately after the blood supply to the tumor is ligated in the operating room or at time of radiologic biopsy, our programmatic goals are to implant tumors in mice within 60 minutes from patient acquisition. Several authors have characterized LT development in different tumor types suggesting the etiology is related to host-derived lymphocytes within an immunocompromised mouse model [6], [8], [9]. This underscores the critical need to histologically confirm and validate any derived PDX tumors routinely to exclude LT which can obfuscate downstream applications and contaminate inventories and subsequent experimental results. Recent work has demonstrated that administration of rituxumab (anti-CD20) as a potential method for reducing LT development [12]. Thus in order optimize our PDX engraftment success and minimize LT formation, we evaluated the role of secondary engraftment attempts using cryopreserved primary patient cancer tissues that had failed primary engraftment attempts. We hypothesized that this methodology could be utilized to potentially increase overall PDX model engraftment efficiency by salvaging unique PDX tumors that have failed previously, and potentially reduce the development of LT.
Methods
With informed consent and institutional IRB approval, we maintain a large volume hepatobiliary and pancreatic malignancy prospective tumor registry, cryopreserved biobank, and PDX repository. This research was conducted in accordance to the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Determination of Primary Engraftment Failures
The prospectively maintained PDX registry was queried for patient samples that demonstrated primary engraftment failure due to (1) no tumor growth or (2) LT. Primary engraftment refers to the initial direct PDX engraftment attempts utilizing freshly obtained patient tissue obtained either at surgical resection or biopsy. Secondary engraftment refers to any subsequent attempt at direct PDX engraftment after failure of initial primary engraftment procedures utilizing original patient cancerous tissue that had been previously cryopreserved at time of initial tissue collection. This is to differentiate from reanimation engraftment, in which previously successful PDX tumor tissues were cryopreserved and subsequently reanimated at a later date.
Engraftment failure due to no tumor growth is defined in our program as no visible or palpable tumor growth after 180 days from implantation. At 180 days, mice are euthanized and bilateral flank implantation sites are examined to either confirm no tumor growth, or if a small viable tumor is present, it is subsequently cryopreserved and passed into the next generation. Although successful engraftment after this extended time period has been observed, it is markedly infrequent (<2%) and usually only associated with a handful of low grade, indolent tumors. Maintenance of large murine colonies for prolonged duration significantly increases lab costs and resources as well as results in worsening health of these animals over time thus our IACUC protocols have supported this practice. Engraftment failure due to LT is evaluated by histologic confirmation of lymphoproliferative histology and immunohistochemistry for immune cell markers for any questionable cases. Figure 1 demonstrates a histologic example of LT surrounding xenografted tumor tissue. Clinical suspicion for LT is determined by: (1) rapid tumor growth - within weeks of engraftment, (2) soft, malleable and easily fractured tumor specimen, (3) as well as poor murine clinical status (hunched appearance, rapid weight loss, balding or unexpected death) suggestive of LT induced cachexia. PDX models that demonstrated primary engraftment failure for either of these reasons were included in this analysis. Other etiologies of primary engraftment failure can potentially include murine host mortality due to health decline; however, this has not been an issue in our engraftment failures given that we routinely implant 5–10 mice for each unique patient model thus allowing redundancy.
Figure 1.
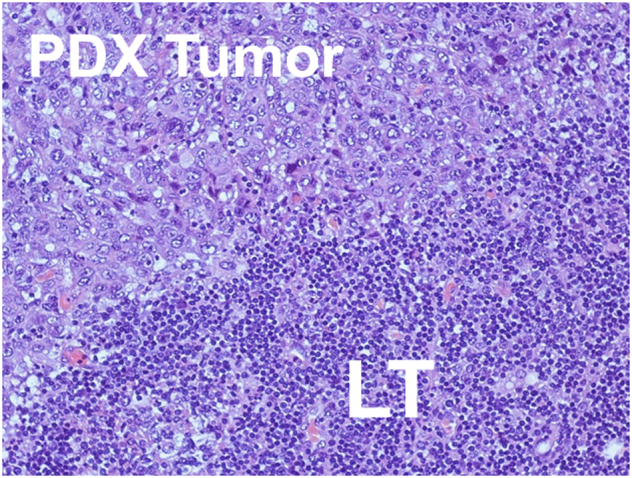
Example of lymphoproliferative tumor and patient-derived xenograft.
PDX Primary Engraftment and Cryopreservation Technique
Using an established institutional protocol with optimized processing workflow (operating room, radiologic suite, frozen section pathology, animal facilities), at the time of surgical resection or biopsy, patient cancerous tissue is obtained and immediately assessed using frozen section pathologic analysis to evaluate for viability of tumor samples. Once viable tumor is confirmed, a portion of this cancerous tissue is provided to our laboratory personnel. These specimens are immediately placed into transport tubes containing cooled tissue culture media (Roswell Park Memorial Institute, RPMI, Invivogen, Carlsbad, CA) with 2% penicillin and streptomycin for immediate transport into our animal engraftment facilities which are located in the immediate vicinity to minimize tissue ischemia time. We routinely cryopreserve all excess primary tumor tissue as well as any derived PDX tumor tissue using specialized cryoprotectant solutions that has been previously demonstrated to allow for high reanimation efficiency [13].
The specific size of samples obtained varies by the clinical circumstances from large resected tumors to small 18G core needle biopsy samples; this is entirely dependent on the clinical case. Transported tumor samples are immediately processed with fragmentation into approximately 1x2 mm pieces. These minced fragments are then coated with Matrigel (Corning, Corning, NY) and immediately engrafted into NOD/SCID mice (Primary Engraftment). Concurrently excess patient tumor tissue is placed into cryovials (Corning, Corning, NY) with specialized cryopreservation fluid (Cryostor, Biolife Solutions, Bothell, WA) [13] and then immediately cooled at optimal temperatures and cooling rates and into long term storage (Thermo Fisher Scientific, Waltham, MA) per our previous published methods [13]. As the engraftment and cryopreservation take place concurrently there is no additional tissue ischemia time according to our protocol. Our programmatic goal of less than 60 minutes from patient tumor tissue acquisition to murine engraftment or cryopreservation is based on our previous experience with high inverse correlation with tissue ischemia time and engraftment outcomes. Cryopreserved vials are catalogued in a PDX registry for future use. Care is taken to minimize temperature fluctuations and prevent thaw.
PDX Secondary Engraftment Technique of Cryopreserved Samples
Patient cancer tissue was obtained from our cryopreserved tissue biobank and vials were warmed in 37°C water bath for 10 minutes. Using a sterile petri dish, the cryopreserved tissues were emptied and five milliliters of phosphate buffered saline (PBS, Invitrogen, Carlsbad, CA) wash was applied twice to rinse away any residual cryoprotectant solution. Tumor fragments were then arrayed, sectioned into 1×2 millimeter pieces, and placed onto an ice cold sterile petri dish containing 300 microliters of Matrigel (Corning, Corning, NY) with care to minimize exposure to air and prevent desiccation.
Immunocompromised mice (NOD/SCID) were anesthetized using 2% isoflurane. A 70% ethanol wash is applied to the murine dorsum. Using scissors, bilateral subcutaneous flank pockets are created. Matrigel (Corning, Corning, NY) coated tumor fragments are implanted in the flank subcutaneous space and the wounds are closed using Vetbond (3 M, Maplewood, MN). Mice were treated with 0.1 (mg/mL) of rituximab (anti-CD20, Genentech, South San Francisco, CA) via intraperitoneal injection to assist in minimization of LT formation. Mice were monitored biweekly for the development of tumor growth using both visual and manual palpation assessments. Once PDX tumors are confirmed to be growing they are measured with calipers in 3 dimensions biweekly until subsequent harvest.
PDX Growth Metrics
A variety of PDX metrics were utilized to characterize tumor growth kinetics and overall PDX engraftment efficiency. Ischemic time was defined as the time in minutes from patient tumor acquisition (resection or biopsy) to murine implantation. Time to tumor formation (TTF) was defined as the number of days from implantation to the first confirmed palpable tumor growth (approximately 3-4 mm). Time to tumor harvest (TTH) was defined as the number of days from implantation to tumor harvest when the tumor has reached approximately 10 mm in diameter and ready for harvest based on our IACUC approved protocols. All generated tumors were evaluated for histomorphology using hematoxylin and eosin (H&E) comparing PDX tumors with original patient tumor slides by a Mayo Clinic GI pathologist and any questionable LT tumors were further assessed via immunohistochemistry for lymphocyte markers.
Conditional Tumor Tissue Viability
To demonstrate differences in tissue viability between freshly acquired, ischemic, and cryopreserved tissue, we assessed for apoptosis via TUNEL assay. The following tissue types and conditions were utilized for several patient models: 1) Fresh resected primary patient tissue, 2) freshly thawed primary patient tissue after previous long duration cryopreservation, and 3) freshly obtained but ischemic (6 hours) primary patient tissue incubated in 3 ml of Roswell Park Memorial Institute 1640 media (Invitrogen, Carlsbad, CA) at 4 °C. Histology and fluorescent TUNEL assay (in situ cell death detection kit, Roche, Basel, Switzerland) was utilized on tissue sections. Sections were paraformaldehyde-fixed and hydrated. The TUNEL assay was performed using the manufacturer's protocol. Slides were mounted with ProLong Antifade (Invitrogen-Molecular Probes) containing DAPI. The slides were analyzed by fluorescent confocal microscopy (LSM 780, Zeiss). Dead cells were quantified by counting TUNEL-positive nuclei in 20 random microscopic fields (20×). This experiment was performed on 5 different tumor types and repeated three times.
Results
Salvage of Previous Failed PDX Models Utilizing Secondary Engraftment of Cryopreserved Tumor Tissue
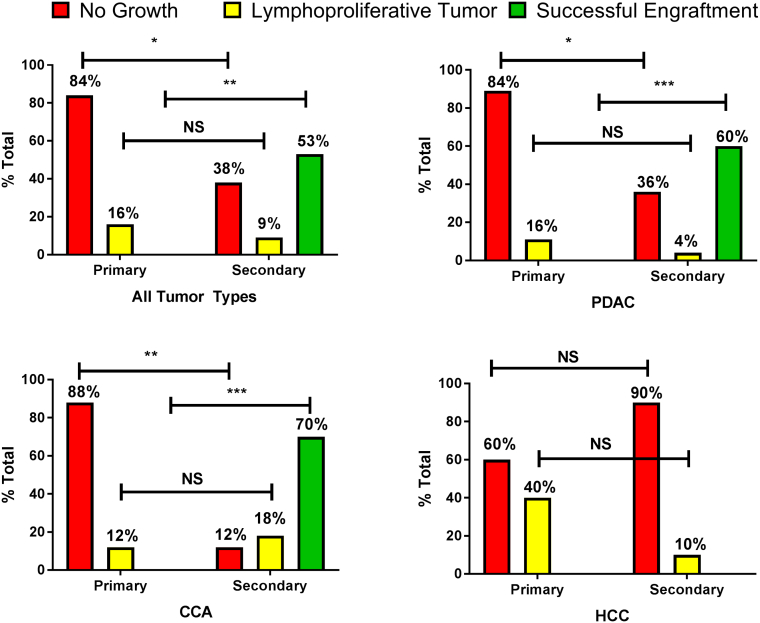
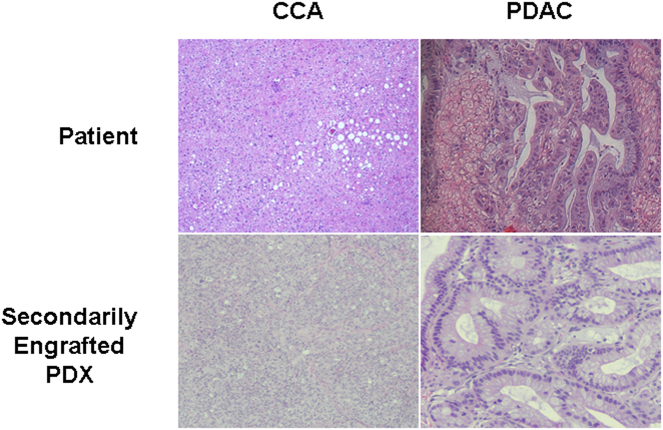
During the study period, a total of 811 generations of PDX have been generated with 338 individual patient tumors. Of these, 55 (16%) unique patient tumors failed to develop validated PDX models after previous primary engraftment. The etiologies for failed primary PDX engraftment were no tumor growth (n = 46, 84%) and lymphoproliferative tumor (n = 9, 16%). These patient tumors comprised of pancreatic ductal carcinoma (n = 28), cholangiocarcinoma (n = 17), and hepatocellular carcinoma (n = 10). Utilizing previous cryopreserved original patient tumor samples, 29 new validated PDX models were successfully generated after having previously failed primary engraftment, resulting in a secondary engraftment efficiency of 53%. The secondary engraftment efficiency varied amongst tumor types with CCA demonstrating the highest rates (70%) followed by PDAC (60%) with no secondary engraftment success in HCC tumors (0%), Table 1 and Figure 2. Histologic recapitulation of selected successfully generated secondarily engrafted tumors is presented in Figure 3. The secondary engraftment failure rate was (n = 26, 47%) and was due to no tumor growth (n = 21, 38%) and lymphoproliferative tumors (n = 5, 5%). Overall, there was a 44% decrease in the lymphoproliferative tumor formation rate after secondary engraftment (n = 5) compared to primary engraftment (n = 9) using cryopreserved primary patient tissue in combination with routine rituximab administration. While this is a considerable decrease in LT formation, it was not statistically significant.
Table 1.
Primary Engraftment Failure Types and Secondary Engraftment Outcomes for All Tumor Types and Selected PDX Models
| Tumor Histologic Type | ||||
|---|---|---|---|---|
| All Tumor Types (n = 55) | PDAC (n = 28) |
CCA (n = 17) |
HCC (n = 10) |
|
| Primary Engraftment Failure Types | ||||
| No growth | 46 (84%) | 25 (89%) | 15 (88%) | 6 (60%) |
| LT | 9 (16%) | 3 (11%) | 2 (12%) | 4 (40%) |
| Secondary Engraftment Outcomes | ||||
| No Growth | 21 (38%) | 10 (36%) | 2 (12%) | 9 (90%) |
| LT | 5 (9%) | 1 (3.6%) | 3 (18%) | 1 (10%) |
| Successful PDX (OPEE) | 29 (53%) | 17 (60.4%) | 12(70%) | 0 (0%) |
| TTF, days | 55 (26–96) | 55 (26–100) | 45 (23–85) | 100 (57–149) |
| TTH, days | 119 (66–161) | 129 (80–171) | 70 (62–153) | 128 (88–160) |
LT – lymphoproliferative tumor, PDAC – pancreatic ductal adenocarcinoma, CCA – cholangiocarcinoma, HCC – hepatocellular carcinoma, TTF – time to tumor formation, TTH – time to tumor harvest, OPEE – overall patient engraftment efficiency.
Figure 2.
Engraftment outcomes by tumor type.
Figure 3.
Histologic similarity between patient and secondarily engrafted tissues for cholangiocarcinoma and pancreatic ductal adenocarcinoma.
Cryopreservation Model
In order to model tissue ischemia and demonstrate its impact on reduced cellular viability and subsequent successful PDX engraftment as well as support the viability benefits of utilizing adequately cryopreserved tissue specimens, we assessed apoptosis of several tumor tissues under various conditions. Figure 4 demonstrates the differences between freshly acquired and implanted cancer tissues, tissues immediately thawed after extended cryopreservation, and fresh cancer tissue specimens after 6 hours of cold incubation. TUNEL assay demonstrated similar low levels of apoptosis in both fresh acquired and thawed cryopreserved tissues suggesting that with appropriate cryopreservation techniques there is minimal loss of viable cells between freshly cryopreserved and freshly resected tissues; however in the delayed implantation model there was significantly increased apoptotic cells, further supporting the routine immediate engraftment of either fresh or freshly cryopreserved tissues to maximize successful engraftment by minimizing tissue ischemia and decreased cellular viability.
Figure 4.
Comparative analysis of fresh, frozen, and thawed cancerous tissues in five separate patient tumors.
Discussion
The study demonstrates the feasibility of high salvage rate of precious unique PDX models for pancreatic ductal adenocarcinoma and cholangiocarcinoma but not for hepatocellular carcinoma. In models that failed primary engraftment, secondary engraftment of previously cryopreserved patient tissue appeared to improve programmatic efficiency. As a result of these techniques, numerous PDX models that otherwise would not have existed were recovered. This underscores the importance of maintaining cryopreserved biobanks of original patient tissue using appropriate techniques. We have shown that subsequent secondary engraftment of cryopreserved patient tumor tissue results in a high proportion (53%) of unique PDX models that can be successfully salvaged. Successful secondary engraftments appear to be tumor type dependent with biliary and pancreas cancers demonstrating the highest rates of success. Our rates of successful engraftment are comparable to other groups assessing the yield of cryopreserved tissues, however with different tumor types [14]. Furthermore, these results also highlight that LT rates appeared to be reduced by using a combination of cryopreserved primary patient tissue implantation and pre-treatment with anti-CD20 antibody.
The need for long-term storage of cancerous tissues, both reserve samples of original patient specimens and subsequent derived PDX tissue is apparent [15], [16]. A cryopreserved tissue biobank has implications for several downstream investigations [17]. Cryopreservation is a practical and necessary method for (1) high volume storage of original patient and xenograft derived tissue, (2) rapid access and shipment of tissues for collaborative studies, and (3) availability to rapidly reanimate cryopreserved PDX tumors for in-vivo experimentation [14], [18], [19]. Recently, the efficacy of primary engraftment using cryopreserved tissue for colorectal tumor types demonstrated an improvement in PDX engraftment after a period of cyropreservation [20]. We are unaware of other data specifically evaluating secondary engraftment after previous failed engraftment and this has limited the understanding and important role that cryopreservation has for generation of primary PDX tumor models, specifically in hepatobiliary and pancreatic malignancies [21]. The present study further extends our previous work showing the benefits of utilizing optimal cryoprotectant solutions and techniques in order to maximize PDX program metrics [13].
Using cryopreserved primary patient tissue, multiple unique patient PDX models were salvaged and subsequently expanded resulting in an overall increase in our PDX efficiency metrics as well as providing the critically needed additional models in order to better represent the molecular heterogeneity present in any given malignant histologic subtype. For example, cholangiocarcinoma is a difficult tumor to successfully engraft and previous reports have demonstrated low PDX engraftment rates. [22], [23], [24]. Our program has enjoyed a high rate of primary engraftment success given our institutions significant clinical and surgical volume of these cancers, however there have still been multiple primary engraftment failures. After a period of cryopreservation, cholangiocarcinoma demonstrated the highest rate (70%) of successful secondary PDX generation. This result is similar to that reported by Zeng et al. wherein cryopreservation techniques for cholangiocarcinoma were analyzed [25]. We also demonstrated similar secondary engraftment salvage (60%) for pancreatic cancers, another malignant tumor type of which we have our largest proportion in our PDX catalog. Overall these demonstrated methods could be utilized on various primary tumor tissues currently cryopreserved, thus potentially expanding the repertoire of available PDX models for downstream research applications in any significant PDX program.
Primary engraftment failure is very common with hepatocellular carcinoma (HCC) and most often a result of LT formation due to high proportions of tumor-associated lymphocytes within primary tumor specimens, and thus only a few validated PDX models have been reported for HCC [26], [27]. This current study was unable to demonstrate secondary engraftment success for these tumors. Hepatocellular carcinoma represents a specific challenging histologic type wherein the etiologies for PDX engraftment success are obviously multifactorial. The study results highlight that other methods may need further research to improve secondary engraftment rates in HCC such as generation of an indirect xenograft. While the attempts at secondary engraftment were not successful in HCC, significantly lower rates LT were observed. This significantly assists in limiting false-positive tumors and subsequent inventory contamination and excess resource utilization. The overall decreased rate of LT suggests that engraftment using cryopreserved primary patient tissue and pre-treatment with anti-CD 20 antibodies may have utility. The utilization of anti-CD20 antibody had previously demonstrated reduction in LT rates for ovarian carcinoma PDX models [12]. It is possible that the combination of a period of cryopreservation and anti-CD20 antibody administration could display synergism in reducing LT. This has not been previously explored and would require more formal analyses beyond the scope of this current study.
We also assessed levels of tissue apoptosis under various conditions. The purpose of this was to show that primary tumor tissue, when optimally cryopreserved and stored, have similar viability to freshly acquired tissue, this has not been shown before, and supports the utility of having a cryopreserved primary tissue bank. Minimal apoptosis after thawing suggests cellular integrity despite long-term cryopreservation when utilizing optimal freezing methods and appropriate cryoprotective media as we had previous demonstrated [13]. Conversely, the model of fresh but partially ischemic tissue results in significant apoptotic cellular death. This highlights the critical need to immediately engraft or cryopreserve primary patient or patient-derived tissue expeditiously to minimize cellular ischemic stress and its potential detrimental impact on PDX engraftment outcomes [28]. It is possible that increased rates of cancer cellular apoptosis may occur due to prolonged ischemia and exposure to desiccation, minimizing the opportunity for successful engraftment [28]. Nevertheless, the changes induced by ischemia time may even impact on genomic expression signatures and efforts to reduce the ischemia time in order to complete engraftment as well as successful cryopreservation should not be underestimated [28].
Despite the important findings of this study for any program utilizing PDX, there are several limitations. It is possible that originally implanted tumor fragments lacked viable cells at the time of implantation and this was responsible for both primary and secondary engraftment failure. To address this, we histologically validate viable tumor cells in patient primary tissues with frozen section at the time of engraftment. As to the mechanism responsible for successful secondary engraftment compared to primary engraftment within the same patient tumor tissue is not currently understood and beyond the scope of this current observational report. Although we can provide assumption and hypotheses such as variable malignant potential in certain populations of cells engrafted, unmeasured technical issues during primary engraftment, proportion of LT-associated host lymphocytes, health of initial mice, etc. none of these can be definitively proven. However, the primary purpose of this current work is to provide feasibility data that secondary engraftment success is “possible,” this is the novelty in these results that make it critical for any PDX program in that a significant proportion of failed primary engraftments can be salvaged. A PDX program is completely dependent on the repertoire of its catalog in terms of overall unique tumor types but also the variety of individual patients in order to better represent the heterogeneity of malignant phenotypes. Thus any failed PDX engraftment is a major loss for downstream subsequent experimentation and translation research. This is the largest series that demonstrates that secondary engraftment of previously cryopreserved patient tumor tissue is not only feasible but leads to the critical salvage of many PDX models that would otherwise not exit if a secondary engraftment had not been attempted. We also did not account for freezer temperature shifts and alterations that potentially could have occurred and affected tissue viability. Moreover, we have not evaluated the DNA and RNA integrity after periods of cryopreservation although with optimal media, methods, and thawing practices this should be minimal. The fact that the derived secondary engrafted PDX tumor tissue is consistent with the primary patient tissue supports our hypothesis, yet, we were unable to account for why specifically some models were successful and others were not.
Conclusions
Minimizing PDX engraftment failure is critical to any PDX program. We demonstrate that secondary engraftment techniques using cryopreserved cancerous patient tissue is feasible after previous failed primary engraftment and these methods are critical in salvaging unique PDX models. Furthermore, the ability to appropriately cryopreserve, store, and rapidly access viable original patient tumor tissue is crucial and allows for a high degree of successful secondary engraftment in order to improve PDX programmatic efficiency.
Author Contribution
MCH, LY, TS, JRB performed the literature search, study design, and data collection. MCH, LY, JLL, TI, RG, JRB, MJT contributed to the data analysis, interpretation, writing and critical revisions.
Acknowledgements
This work was funded in part by the Mayo Clinic Clinician Investigator Program and Roger Thrun. The authors have read and agreed to this journal's authorship agreement. The authors do not have any financial or personal relationships that could potentially influence this work.
Footnotes
Disclosures: None of the authors have any conflicts of interest (internal or external).
Contributor Information
Matthew C. Hernandez, Email: Hernandez.matthew@mayo.edu.
Lin Yang, Email: yang.lin@mayo.edu.
Jennifer L. Leiting, Email: leiting.jennifer@mayo.edu.
Takaaki Sugihara, Email: Sugihara.takaaki@mayo.edu.
John R. Bergquist, Email: Bergquist.john@mayo.edu.
Tommy Ivanics, Email: tommy.ivanics@gmail.com.
Rondell Graham, Email: graham.rondell@mayo.edu.
Mark J. Truty, Email: truty.mark@mayo.edu.
References
- 1.Hidalgo M, Amant F, Biankin a V, Budinska E, Byrne a T, Caldas C, Clarke RB, de Jong S, Jonkers J, Mælandsmo GM. Patient-Derived Xenograft Models: An Emerging Platform for Translational Cancer Research. Cancer Discov. 2014:998–1013. doi: 10.1158/2159-8290.CD-14-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tentler JJ, Tan AC, Weekes CD, Jimeno A, Leong S, Pitts TM, Arcaroli JJ, Messersmith WA, Eckhardt SG. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9(6):338–350. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Pham N-A, Tong J, Sakashita S, Allo G, Kim L, Yanagawa N, Raghavan V, Wei Y, To C. Molecular heterogeneity of non-small cell lung carcinoma patient-derived xenografts closely reflect their primary tumors. Int J Cancer. 2017;140(3):662–673. doi: 10.1002/ijc.30472. [DOI] [PubMed] [Google Scholar]
- 4.Bruna A, Rueda OM, Greenwood W, Batra AS, Callari M, Batra RN, Pogrebniak K, Sandoval J, Cassidy JW, Tufeqdzic-Vidakovic A. A Biobank of Breast Cancer Explants with Preserved Intra-tumor Heterogeneity to Screen Anticancer Compounds. Cell. 2016;167(1):260–274.e22. doi: 10.1016/j.cell.2016.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukohyama J, Iwakiri D, Zen Y, Mukohara T, Minami H, Kakeji Y, Shimono Y. Evaluation of the risk of lymphomagenesis in xenografts by the PCR-based detection of EBV BamHI W region in patient cancer specimens. Oncotarget. 2016;7(31) doi: 10.18632/oncotarget.10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen K, Ahmed S, Adeyi O, Dick JE, Ghanekar A. Human solid tumor xenografts in immunodeficient mice are vulnerable to lymphomagenesis associated with Epstein-Barr virus. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0039294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.John T, Yanagawa N, Kohler D, Craddock KJ, Bandarchi-Chamkaleh B, Pintillie M, Sykes J, To C, Li M, Panchal D. Characterization of Lymphomas Developing in Immunodeficient Mice Implanted With Primary. JTO Acquis. 2012;7(7):1101–1108. doi: 10.1097/JTO.0b013e3182519d4d. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Liu Y, Wang X, Tang Z, Li S, Hu Y, Zong X, Wu X, Bu Z, Wu A. The extent of inflammatory infiltration in primary cancer tissues is associated with lymphomagenesis in immunodeficient mice. Sci Rep. 2015;5:9447. doi: 10.1038/srep09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bondarenko G, Ugolkov A, Rohan S, Kulesza P, Dubrovskyi O, Gursel D, Matthew J, O'Halloran TV, Wei JJ, Mazar P. Patient-Derived Tumor Xenografts Are Susceptible to Formation of Human Lymphocytic Tumors. Neoplasia (New York) 2015;17(9):735–741. doi: 10.1016/j.neo.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim MP, Truty MJ, Choi W, Kang Y, Chopin-Lally X, Gallick GE, Wang H, McConkey DJ, Hwang R, Logsdon C. Molecular profiling of direct xenograft tumors established from human pancreatic adenocarcinoma after neoadjuvant therapy. Ann Surg Oncol. 2011;2012(19 Suppl. 3):S395–S403. doi: 10.1245/s10434-011-1839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudloff U, Bhanot U, Gerald W, Klimstra DS, Jarnagin WR, Brennan MF, Allen PJ. Biobanking of human pancreas cancer tissue: Impact of ex-vivo procurement times on RNA quality. Ann Surg Oncol. 2010;17(8):2229–2236. doi: 10.1245/s10434-010-0959-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler KA, Hou X, Becker MA, Zanfagnin V, Enderica-Gonzalez S, Visscher D, Kalli KR, Tienchaianada P, Haluska P, Weroha SJ. Prevention of Human Lymphoproliferative Tumor Formation in Ovarian Cancer Patient-Derived Xenografts. Neoplasia (United States) 2017;19(8):628–636. doi: 10.1016/j.neo.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivanics T, Bergquist JR, Liu G, Kim MP, Kang Y, Katz MH, Perez MVR, Thomas RM, Fleming JB, Truty MJ. Patient-derived xenograft cryopreservation and reanimation outcomes are dependent on cryoprotectant type. Lab Investig. 2018:1–10. doi: 10.1038/s41374-018-0042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smeds S, Trulsson L, Garovoy M, Gumbert M, Clark OH. Survival of human parathyroid tissue xenotransplanted in nude mice after 9 to 55 months’ cryopreservation. APMIS. 1999;107(4):445–450. doi: 10.1111/j.1699-0463.1999.tb01579.x. [DOI] [PubMed] [Google Scholar]
- 15.Caixeiro NJ, Lai K, Lee CS. Quality assessment and preservation of RNA from biobank tissue specimens: a systematic review. J Clin Pathol. 2016;69(3):260–265. doi: 10.1136/jclinpath-2015-203384. [DOI] [PubMed] [Google Scholar]
- 16.Castillo-Pelayo T, Babinszky S, LeBlanc JWP. The importance of biobanking in cancer research. Biopreserv Biobank. 2015;13(3):172–177. doi: 10.1089/bio.2014.0061. [DOI] [PubMed] [Google Scholar]
- 17.Luo J, Guo XR, Tang XJ, Sun XY, Yang ZS, Zhang Y, Dai LJ, Warnock GL. Intravital biobank and personalized cancer therapy: The correlation with omics. Int J Cancer. 2014;135(7):1511–1516. doi: 10.1002/ijc.28632. [DOI] [PubMed] [Google Scholar]
- 18.Ota J, Giuliano EA, Mullen SF, Turk JR, Lewis MR, Cohn LA, Moore CP, Critser J. Xenotransplantation of cryopreserved equine squamous cell carcinoma to athymic nude and SCID mice. Res Vet Sci. 2007;83(3):355–359. doi: 10.1016/j.rvsc.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Nisolle M, Casanas-Roux F, Qu J, Motta P, Donnez J. Histologic and ultrastructural evaluation of fresh and frozen-thawed human ovarian xenografts in nude mice. Fertil Steril. 2000;74(1):122–129. doi: 10.1016/s0015-0282(00)00548-3. [DOI] [PubMed] [Google Scholar]
- 20.Linnebacher M, Maletzki C, Ostwald C, Klier U, Krohn M, Klar E, Prall F. Cryopreservation of human colorectal carcinomas prior to xenografting. BMC Cancer. 2010;10:362. doi: 10.1186/1471-2407-10-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorio C, Bonora A, Orlandini S, Moore PS, Capelli P, Cristofori P, Dal Negro G, Marchiori P, Gavirhagi G, Falconi M. Successful xenografting of cryopreserved primary pancreatic cancers. Virchows Arch. 2001;438(2):154–158. doi: 10.1007/s004280000343. [DOI] [PubMed] [Google Scholar]
- 22.Jang SY, Bae HI, Lee IK, Park HK, Cho C-M. Successful Xenograft of Endoscopic Ultrasound-Guided Fine-Needle Aspiration Specimen from Human Extrahepatic Cholangiocarcinoma into an Immunodeficient Mouse. Gut Liver. 2015;9(6):805–808. doi: 10.5009/gnl14279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavalloni G, Peraldo-Neia C, Sassi F, Chiorino G, Sarotto I, Aglietta M, Leone F. Establishment of a patient-derived intrahepatic cholangiocarcinoma xenograft model with KRAS mutation. BMC Cancer. 2016;16:90. doi: 10.1186/s12885-016-2136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavalloni G, Peraldo-Neia C, Varamo C, Casorzo L, Dell’Aglio C, Bernabei P, Chiorino G, Aglietta M, Leone F. Establishment and characterization of a human intrahepatic cholangiocarcinoma cell line derived from an Italian patient. Tumor Biol. 2016;37(3):4041–4052. doi: 10.1007/s13277-015-4215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng M, Yang QR, Fu GB, Zhang Y, Zhou X, Huang WJ, Zhang HD, Li WJ, Wang ZY, Yan HX. Maintaining viability and characteristics of cholangiocarcinoma tissue by vitrification-based cryopreservation. Cryobiology. 2017;78:41–46. doi: 10.1016/j.cryobiol.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Cheung PFY, Yip CW, Ng LWC, Lo KW, Chow C, Chan KF, Cheung TT, Cheung ST. Comprehensive characterization of the patient-derived xenograft and the paralleled primary hepatocellular carcinoma cell line. Cancer Cell Int. 2016;16:41. doi: 10.1186/s12935-016-0322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Q, Zhou H, Liu Q, Cao Y, Wang G, Hu A, Ruan L, Wang S, Bo Q, Chen W. Prognostic value of the expression of cancer stem cell-related markers CD133 and CD44 in hepatocellular carcinoma: From patients to patient-derived tumor xenograft models. Oncotarget. 2016;7(30):47431–47443. doi: 10.18632/oncotarget.10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caboux E, Paciencia M, Durand G, Robinot N, Wozniak MB, Galateau-Salle F, Byrnes G, Hainaut P, Le Calvez-Kelm F. Impact of delay to cryopreservation on RNA integrity and genome-wide expression profiles in resected tumor samples. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0079826. [DOI] [PMC free article] [PubMed] [Google Scholar]