Abstract
Background
Considering the microbial etiology of the periodontal disease, the periodontal therapy aims to control or abolish the pathogenic microbes. The gold standard scaling and root planing procedure has been used since time immemorial but the drawbacks associated with it have led to the development of various adjunctive means. The current study was therefore, performed to comparatively assess the efficacy of local delivery of chlorhexidine and 808-nm diode LASER as an appurtenance to scaling and root planing in patients with chronic periodontitis.
Methods
In a randomized split mouth evaluation, 30 patients having probing depth of ≥5 mm which bled on probing at least at 3 different sites were included. At baseline, the evaluation of plaque index, bleeding index, probing pocket depth and clinical attachment level was done and the microbial samples were collected for the assessment of spirochetes, motile rods and coccoid cells. The 3 selected sites of each subject were allocated to 3 different groups A, B, C viz; Scaling and root planing (SRP) + chlorhexidine chip, SRP + diode LASER and SRP respectively. The patients were recalled after 4 weeks to re-evaluate the clinical and microbiological parameters.
Results
All the parameters significantly reduced from baseline to 4 weeks in all the 3 groups. Intergroup comparisons revealed remarkable difference between group A and C and group B and C, respectively; no notably significant difference was found between group A and B.
Conclusion
The additional use of LASER and chlorhexidine chip assures anti-inflammatory effect and anti-microbial effect that allows reduction in bacterial counts and promotes healing. The use of adjuncts have been found to be efficacious in controlling disease and promoting periodontal health and thereby reducing the need for surgical procedures to be undertaken.
Keywords: Soft tissue laser, Chlorhexidine chip, Scaling and root planing
1. Introduction
The periodontal therapy aims to keep the pathogenic microbial flora subdued to allow for maintenance of periodontal health. During the 19th century, this goal was achieved by means of mechanical debridement whether that be surgical or non-surgical. Non-surgical therapy included scaling and root planing (SRP) that restored health to periodontal tissues by removing plaque, calculus and endotoxins tenaciously stuck to the root surface. Even after evolution over the past so many years, this method of mechanical debridement is still the gold standard that forms the basis for other interventions being discovered. The only obstruction to its efficacy is the difficulty to access the deep and complex pockets. Further, the use of curettes is technically overous and time consuming. In addition, ingress to areas such as furcations, concavities and grooves is limited.1 These issues provided the limiting factors to long term reliability of the treatment outcomes of non-surgical technique and became the basis for the need of adjunctive therapies with antibiotics, antiseptics as well as non-chemical modalities along with mechanical debridement.2
The periodontal disease is not merely a profusion of bacteria but also a shift in the microbial species from gram positive to gram negative. This has led to the utilization of antimicrobials (systemic or local) as an additional modality in intervening periodontal disease. The customary use of systemic antibiotics over long stretch of time is contraindicated due to evolution of resistant strains and possible systemic side effects. The local application of various antimicrobial agents in various forms has gained popularity as they overcome the drawbacks of the systemic antimicrobials. The excessive concentration of antimicrobial agent at the diseased sites by local delivery provides a major advantage over systemic drug administration.
A local drug delivery device comprises of a drug reservoir and a limiting element that steers the rate of medicament release. This maintains the concentration of active agents at the site of activity for long periods, despite loss of drug from crevicular fluid clearance.3 Various agents like tetracycline, chlorhexidine, metronidazole etc. have been formulated into local delivery devices. Chlorhexidine is considered among the most effective antiplaque agents in use today. It has a property of substantivity and broad spectrum antimicrobial action. A biodegradeable platform based on a cross linked hydrolyzed gelatin matrix controlled delivery device for chlorhexidine has been developed.
The word “LASER” stands for “light amplification by stimulated emission of radiation”. It system uses devices that transfigure light of varying frequencies into a single, intense, non-divergent beam.4 The application of LASER as an adjunct to conventional SRP is based on the premise that it allows the subgingival debridement and eradication of pathogenic microorganisms which provides new loci for attachment of connective tissue.5 The use of LASERs provide additional advantages of minimal pain and better haemostasis.
A vast array of studies has been conducted revealing the efficacy of LASERs and chlorhexidine separately. The motive of the present study was to comparatively measure the competence of diode LASER and chlorhexidine chip as adjuncts to the scaling and root planing procedure.
2. Materials and method
The present study was a randomized clinical trial conducted on 30 patients at 90 sites.
2.1. Armamentarium
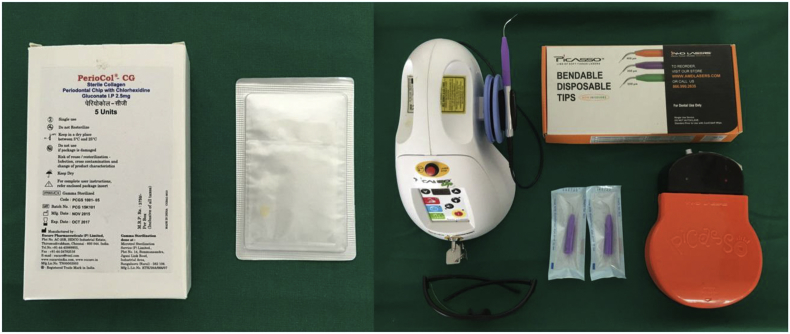
Materials used for specific treatment included chlorhexidine chip and diode LASER (Fig. 1).
Fig. 1.
Chlorhexidine chip and diode LASER used.
2.2. Chlorhexidine
Periocol- CG ™ - it is manufactured by Eucare Pharmaceuticals (P) Ltd. It is an orange-brown colored small rectangular chip form, rounded at one end for uncomplicated insertion into periodontal pockets. Each Periocol CG™ contains 2.5 mg of chlorhexidine gluconate in a biodegradeable matrix of type I collagen derived from fish sources. It is gamma sterilized and supplied in individual packing. It releases chlorhexidine in vitro with a release profile of about 40–45% within 24 h and afterward in linear fashion for 7–8 days.6
2.3. Diode LASER
A GaAlAs Diode LASER which had a wavelength of 808 nm, a power range of 0.1–7 Watts and pulse duration range of 20 ms - 9.9 s was used for the study. It consisted of 3 modes, the speed mode, comfort mode and the decontamination mode. It was used in the decontamination mode. The parameters used for bacterial reduction were 0.4 W, continuous wave, 20 s per site and for coagulation were 0.8 W, continuous wave, 10 s per site.7
3. Study design
The subjects with moderate to severe periodontal disease, ageing between 30 and 65 years that had a pocket depth of ≥5 mm which bled on probing at least at 3 different sites who were systemically healthy, not received any antibiotic/periodontal therapy for the last 6 months were included in the study.
At these 3 different sites, different treatment protocols were followed:
-
•
GROUP A - Scaling and Root planing with Local delivery of Chlorhexidine Chip
-
•
GROUP B - Scaling and Root planing with Diode LASER therapy
-
•
GROUP C - Scaling and Root planing alone.
On the first day the clinical parameters were taken down for the patient and subgingival samples were collected for microbiological assessment under dark field microscope. A full mouth oral prophylaxis i.e. scaling and root planing (SRP) was done by deploying ultrasonic scalers and curettes. 2 days after SRP the patient was recalled and on 2 different sites LASER treatment and chlorhexidine chip placement was done. Randomization was done by blind selection of one of two pieces of paper, each one containing one of the treatments. After a span of 4 weeks, patient was recalled for the follow up and examined clinically and microbiologically for improvement.
3.1. Clinical examination
The following parameters were evaluated at baseline and 4 weeks after specific treatment.
-
•
Plaque Index (PI) – Silness and Loe (1964) plaque index was measured to verify oral hygiene status.8
-
•
Modified Sulcular Bleeding Index (mSBI)9 – Mombelli et al. (1987) assessed the severity of gingival bleeding along the periodontally affected sites.9
-
•
Probing Pocket Depth (PPD) – distance between gingival margin and bottom of probable pocket was recorded.
-
•
Clinical Attachment Level (CAL) – distance from cemento-enamel junction (CEJ) to base of the pocket.
All the clinical measurements were made using a UNC-15 probe that has markings from 1 to 15 at 1 mm interval. It has colour coding at markings 5, 10 and 15. The apical margin of the acrylic splint was used as the fixed reference point.
3.2. Microbiological assessment
3.2.1. Bacterial sampling
The supragingival plaque accretions may have affected the bacterial samples, so the sample was procured from subgingival sulcus. Moreover, the periodontal status is better reflected by the composition of subgingival flora.10].
The supragingival plaque was therefore, removed using ultrasonic scaler. The subgingival sample was then collected using a sterile curette extending it as apically as possible. If the amount of bacteria in pocket was large, only a portion was removed. The sample was then suspended in 0.85% sodium chloride solution alongwith 1% gelatin by roiling instrument tip in the solution. Small eppendorf tubes are sufficient to suspend 0.1–0.3 ml of sample containing saline solution.10
The whole process of sample collection and evaluation was to be completed in 1 h to minimize agglomeration and loss of motility of bacteria. A single drop of solution was placed onto the microscopic slide and cover slipped. The slide was then inverted over an absorbent surface to remove excess fluid. The slide was then inspected by dark field microscopy at a magnification of 1200×. The microbes were counted and categorized according to shapes into coccoid cells, motile rods, spirochetes and others.11 The microbiological examination was done at the baseline visit and 4 weeks after specific treatment.
4. Observations and results
4.1. Clinical parameters
The plaque index for group A changed from 2.60 ± 0.37 at baseline to 0.95 ± 0.30 at 4 weeks. For group B, it changed from 2.25 ± 0.42 at baseline to 1.02 ± 0.36 at 4 weeks. A change from 2.56 ± 0.38 at baseline to 1.62 ± 0.32 at 4 weeks for the scaling and root planing group was observed. The reduction in plaque index for group A was 1.65 ± 0.41 and group B was 1.51 ± 0.46 which was significantly more compared to group C (0.93 ± 0.36) (Table 1).
Table 1.
Comparison of plaque index and modified sulcular bleeding index at different observation periods in different groups.
| Clinical Parameter | Group | Baseline | 4 weeks | Change | P value |
|---|---|---|---|---|---|
| PI | CHX + SRP | 2.60 ± 0.37 | 0.95 ± 0.30 | 1.65 ± 0.41 | <0.001** |
| LASER + SRP | 2.25 ± 0.42 | 1.02 ± 0.36 | 1.51 ± 0.46 | <0.001** | |
| SRP (Control) | 2.56 ± 0.38 | 1.62 ± 0.32 | 0.93 ± 0.36 | <0.001** | |
| CHX + SRP vs SRP p < 0.001** | LASER + SRP vs SRP p < 0.001** | CHX + SRP vs LASER + SRP p = 0.245 | |||
| mSBI | CHX + SRP | 2.74 ± 0.43 | 0.33 ± 0.40 | 2.41 ± 0.51 | <0.001** |
| LASER + SRP | 2.65 ± 0.47 | 0.42 ± 0.44 | 2.23 ± 0.51 | <0.001** | |
| SRP (Control) | 2.61 ± 0.45 | 1.10 ± 0.32 | 1.51 ± 0.39 | <0.001** | |
| CHX + SRP vs SRP p < 0.001** | LASER + SRP vs SRP p < 0.001** | CHX + SRP vs LASER + SRP p = 0.189 | |||
The bleeding index scores also reduced in all the 3 groups. The reduction of bleeding scores occurred from 2.74 ± 0.43 to 0.33 ± 0.40 for group A, 2.65 ± 0.47 to 0.42 ± 0.44 for group B and 2.61 ± 0.45 to 1.10 ± 0.32 for group C respectively. The reduction for group A, B and C was 2.41 ± 0.51, 2.23 ± 0.51 and 1.51 ± 0.39 respectively (Table 1).
The probing depth reduced from 6.03 ± 0.81 to 3.87 ± 0.63, 6.13 ± 0.90 to 4.17 ± 0.83 and 5.87 ± 0.68 to 4.80 ± 0.71 from baseline to 4 weeks for groups A, B and C respectively. A notable reduction in pocket depth was seen in group A (2.17 ± 0.38) as compared to group C (1.07 ± 0.25) (Table 2).
Table 2.
Comparison of probing pocket depth and clinical attachment level at different observation periods in different groups.
| PPD | CHX + SRP | 6.03 ± 0.81 | 3.87 ± 0.63 | 2.17 ± 0.38 | <0.001** |
| LASER + SRP | 6.13 ± 0.90 | 4.17 ± 0.83 | 1.97 ± 0.41 | <0.001** | |
| SRP (Control) | 5.87 ± 0.68 | 4.80 ± 0.71 | 1.07 ± 0.25 | <0.001** | |
| CHX + SRP vs SRP p < 0.001** | LASER + SRP vs SRP p < 0.001** | CHX + SRP vs LASER + SRP p = 0.060 | |||
| CAL | CHX + SRP | 6.23 ± 0.90 | 4.97 ± 0.85 | 1.27 ± 0.45 | <0.001** |
| LASER + SRP | 6.33 ± 0.90 | 5.20 ± 1.45 | 1.13 ± 0.35 | <0.001** | |
| SRP (Control) | 6.00 ± 1.02 | 5.37 ± 0.93 | 0.63 ± 0.49 | <0.001** | |
| CHX + SRP vs SRP p < 0.001** | LASER + SRP vs SRP p < 0.001** | CHX + SRP vs LASER + SRP p = 0.200 | |||
The loss in the clinical attachment level for group A, B and C was from 6.23 ± 0.90 to 4.97 ± 0.85, 6.33 ± 0.90 to 5.20 ± 1.45 and 6.00 ± 1.02 to 5.37 ± 0.93, respectively (Table 2).
The change in clinical parameters from baseline to 4 weeks in all the three groups has been clearly depicted in Fig. 2.
Fig. 2.
Bar Diagram showing reduction in clinical parameters in all three groups.
4.2. Bacterial counts
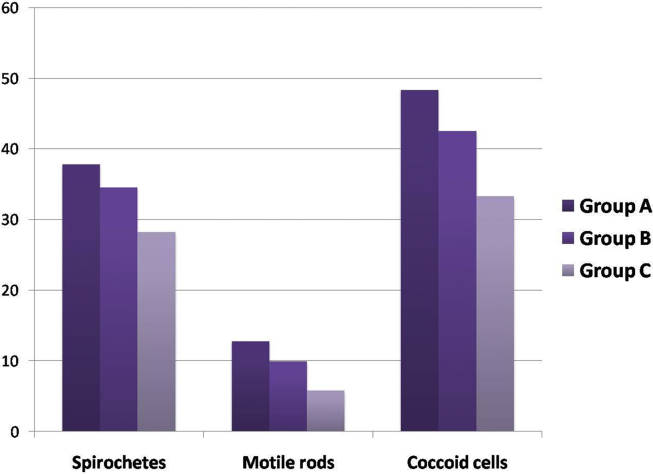
In group A (chlorhexidine), the levels of spirochetes changed from 47.74 ± 4.47 to 9.88 ± 2.97. The reduction in motile rods occurred from 30.14 ± 3.69 to 17.36 ± 4.66. The level of coccoid cells raised from 17.47 ± 3.02 to 65.76 ± 7.15. A change of 37.82 ± 5.29, 12.79 ± 5.03 and 48.29 ± 7.40 occurred in spirochetes, motile rods and coccoid cells respectively from baseline to 4 weeks.
In group B, the spirochetes reduced from 47.57 ± 4.07 at baseline to 13.03 ± 3.57 at 4 weeks, the motile rods reduced from 29.91 ± 3.89 to 19.95 ± 4.96 and the coccoid cells rose from 18.03 ± 3.04 to 60.52 ± 7.73. A statistically significant change of 34.54 ± 5.68, 9.96 ± 4.48 and 42.49 ± 7.08 was seen in spirochetes, motile rods and coccoid cells respectively.
In group C, a change from 47.93 ± 4.72 to 19.71 ± 3.13 was observed in spirochetes, the motile rods reduced from 29.89 ± 3.62 to 24.11 ± 3.15 and the coccoid cells increased from 17.12 ± 3.40 to 50.41 ± 5.74. A reduction of 28.22 ± 5.64 in spirochetes, 5.78 ± 4.14 in motile rods and a hike of 33.31 ± 7.00 was observed.
The improvements in the bacterial counts have been depicted in Table 3 and Fig. 3.
Table 3.
Comparison of microbiological parameters at different observation periods in different groups.
| Parameter | Group | Baseline | 4 weeks | Change | P value |
|---|---|---|---|---|---|
| Spirochetes | CHX + SRP | 47.74 ± 4.47 | 9.88 ± 2.97 | 37.82 ± 5.29 | <0.001** |
| LASER + SRP | 47.57 ± 4.07 | 13.03 ± 3.57 | 34.54 ± 5.68 | <0.001** | |
| SRP (Control) | 47.93 ± 4.72 | 19.71 ± 3.13 | 28.22 ± 5.64 | <0.001** | |
| CHX + SRP vs SRP - p < 0.001** | LASER + SRP vs SRP - p < 0.001** | CHX + SRP vs LASER + SRP - p = 0.061 | |||
| Motile rods | CHX + SRP | 30.14 ± 3.69 | 17.36 ± 4.66 | 12.79 ± 5.03 | <0.001** |
| LASER + SRP | 29.91 ± 3.89 | 19.95 ± 4.96 | 9.96 ± 4.48 | <0.001** | |
| SRP (Control) | 29.89 ± 3.62 | 24.11 ± 3.15 | 5.78 ± 4.14 | <0.001** | |
| CHX + SRP vs SRP p < 0.001** | LASER + SRP vs SRP p < 0.001** | CHX + SRP vs LASER + SRP p = 0.014 | |||
| Coccoid cells | CHX + SRP | 17.47 ± 3.02 | 65.76 ± 7.15 | 48.29 ± 7.40 | <0.001** |
| LASER + SRP | 18.03 ± 3.04 | 60.52 ± 7.73 | 42.49 ± 7.08 | <0.001** | |
| SRP (Control) | 17.12 ± 3.40 | 50.41 ± 5.74 | 33.31 ± 7.00 | <0.001** | |
| CHX + SRP vs SRP p < 0.001** | LASER + SRP vs SRP p < 0.001** | CHX + SRP vs LASER + SRP p = 0.007** | |||
Fig. 3.
Bar Diagram showing change in microbiological parameters in all three groups.
5. Discussion
Mechanical debridement consisting of scaling and root planing are the initial steps of the anti-infective therapy. They are geared towards removing gingival inflammation, eliminating/shifting the bacterial microbes from gram negative anaerobes to gram positive facultative bacteria to establish health However, complete elimination of subgingival calculus may not be possible following subgingival instrumentation. This may be due to the complex anatomy of the root and contour of the lesion that may encumber the treatment and prevent sufficient reduction. Listgarten (1985) stated that the rate of reinfection of the pocket increases with poor plaque control.12 Scaling and root planing in combination with optimal oral hygiene maintenance, alters the subgingival plaque sufficiently to stop periodontal destruction.
These problems associated with the conventional mechanical debridement form the basis for the use of various adjuvant methods in non-surgical periodontal therapy. Various methods that have been tried as adjuncts include antimicrobial agents – systemic and local, use of various host modulating agents, application of LASERs for sulcular bacterial reduction, etc.13
Gary Greenstein in his review compared different forms of antimicrobial agents like mouthwashes, subgingival irrigation, systemic delivery and controlled delivery devices and suggested that controlled delivery had advantage over other forms in terms of reaching the site of disease activity, adequate concentration of the drug and adequate duration of the therapy.14 Chlorhexidine had been used as an antiseptic in various forms but it has been associated with certain side effects like staining, altered taste sensation, etc. However, the chip incorporates small amount of drug (2.5 mg) that induces minimal side effects.15
Further, diode LASERs offer a good choice for curtailment of bacteria and coagulation in the sulcus. The water transmits and hydroxyapatite poorly absorbs the LASER energy but it is well absorbed by pigmented cells. Therefore, proves highly beneficial when used in periodontal pocket filled with dark inflamed tissue and pigmented bacteria. For better delivery to the diseased tissue, flexible fibre optic system of 300–400 μm is used.7
The present study was accomplished with the objective to compare and evaluate the locally delivered chlorhexidine chip containing 2.5 mg chlorhexidine – Periocol CG™ and diode LASER (808 nm) as adjunctive treatment options to scaling and root planing in the treatment of chronic periodontitis.
Overman (2000) reviewed the basic properties of a biofilm and suggested that the microorganisms in the biofilm are resistant to antibiotics, antimicrobials and host responses.16 Hence, full-mouth scaling and root planing was done initially to allow disruption of the plaque biofilm as done in previously conducted study by Birang R et al. (2011).17 The removal of biofilm favours effectiveness of adjuncts against subgingival pathogens.
The specific treatments were carried out 2 days after the session of scaling and root planning as it had been stated by Borrajo that the presence of blood in gingival sulcus acts as an interfering factor which can elevate the peril of thermal damage due to LASER therapy.18 The thin biofilm of blood covering the root surfaces of periodontal pocket can considerably elevate the absorption of energy and may lead to thermal damage to the dental pulp.
All three groups were evaluated for clinical parameters namely, plaque index, bleeding index, pocket depth and clinical attachment level, 4 weeks after the specific treatment had been done as done by Lin J et al. (2011).19 It has been stated by Divya PV et al. (2006), that the complete resorption of the chlorhexidine chip occurs in about 30 days.6 Moreover, Morrison and colleagues have demonstrated that the reduction in inflammation is seen 4 weeks after the hygienic phase.20
The microscopic evaluation of the flora aids in diagnosis and helps to evaluate the progress of treatment during initial periodontal therapy. Total motile organisms including the spirochetes and motile rods are predominantly seen in diseased sites and the coccoid cells -dominate the healthy sites. In the present study the microbiological assessment of 3 morphologic forms was done that included spirochetes, motile rods and cocci. This was done in accordance with the study conducted by Mizrak et al. (2006).21
All clinical and microbiological parameters showed no significant difference in all the groups at baseline.
All patients showed statistically and clinically significant improvements in plaque index at the follow-up visit, when compared to the baseline level. The reduction in plaque index could be due to proper oral hygiene maintenance and thoroughness of scaling and root planing. The reduction in plaque index for group A and group B was significantly more compared to group C. The significant reduction in plaque index between the chlorhexidine group and control group has been shown in the study conducted by Mizrak et al. (2006).21 The reduction in plaque index was more for group B (LASER) compared to group C (control) and the difference was statistically significant. These results are close to those shown in the study carried out by Ugo Caruso et al. (2008).22
The bleeding index scores also reduced in all the 3 groups. A significant reduction occurred in group A at 4 weeks. Similar results have been shown by study of Azmak N et al. (2002).23 The reduction was also significant in group B in accordance with the study done by Lin J et al. (2011).19 The reduction in the bleeding scores represents reduced inflammation that may be attributed to lowered prostaglandin E2 levels due to the effects of LASER treatment.22 The bleeding index scores in chlorhexidine and LASER therapy groups had no significant difference after treatment, although the difference between control and experimental groups was significant. These results were similar to the study conducted by Reza Birang et al. (2011).17
The measurement of probing pocket depth (PPD) and clinical attachment level (CAL) forms the basis for the diagnosis of periodontitis. The pocket depth reduction and clinical attachment level gain post treatment are, thus, important means to evaluate the efficacy of treatment interventions. A remarkable reduction in pocket depth was seen in group A (2.17 ± 0.38) as compared to group C (1.07 ± 0.25). This is in confirmity with the study conducted by Grover V et al. (2011).24 The amount of pocket reduction is due to the soft tissue shrinkage following scaling and root planing as well as resolution of gingival inflammation due to the action of the antimicrobial agent. Reduction in probing depth for group B (1.97 ± 0.41) was more evident compared to group C. This is in accordance with the study conducted by Crispino A et al. (2015).25 The remarkable difference between LASER and control group in improving pocket depth is attributable to the advantages from the use of diode LASER which includes the bactericidal effect, the curettage effect and the bio-stimulating effect.18 No significant difference in reduction of probing pocket was seen in group A and B. This is similar to the results presented by Crispino et al. (2015).25
A statistically significant CAL gain has occurred in group A (1.27 ± 0.45) as compared to group C (0.63 ± 0.49). Similar results have been shown in the study conducted by Grover V et al. (2011).24 The greater gain in clinical attachment level in group A could be due to the absence of bacterial challenge, caused by retained antimicrobial agent, during the critical initial phase of healing following scaling and root planing. The gain in CAL in group B (1.13 ± 0.35) was significantly more than that in group C. Conlan (1996) found that the proliferation and differentiation of fibroblasts and collagen synthesis within the periodontal ligament, increased by almost 50% after LASER therapy.26 According to Choi (2010) this proliferation and differentiation begins to expresses between the next 24–48 h to LASER treatment and intensifies especially after 72 h.27 These reactions might have accelerated the healing process and encouraged a rapid recovery in clinical attachment in the LASER group. No statistically significant difference was seen in CAL gain in group A and B. This is in accordance with the study conducted by Birang R et al. (2011).17
The subgingival plaque flora has been known to be associated with periodontitis. Lindhe et al. found that spirochetes deemed for 57.2% of the subgingival flora in diseased periodontal sites.28 Out of the main four species of spirochetes such as Treponema denticola, T. socranskii, T. vincentii, T. pectinovorum, Moore et al. (1985)29 suggested that T. denticola has been significantly associated with diseased sites. The reduction in the bacterial counts like spirochetes and motile rods are thus, considered an important aspect of treatment interventions. Therefore, in the present study, the reduction in the number of spirochetes and motile rods and increase in the levels of coccoid cells has been assessed.
In group A, a change of 37.82 ± 5.29, 12.79 ± 5.03 and 48.29 ± 7.40 occurred in spirochetes, motile rods and coccoid cells respectively from baseline to 4 weeks. All these differences from baseline to 4 weeks were statistically significant. Similar results were shown in the study conducted by Mizrak et al. (2006).21 Chlorhexidine hinders the growth of large variety of bacterial species secluded from the subgingival plaque at a concentration of 125 μg/ml which can be obtained in sulcular fluid even after 7 days of chip placement.29 A sufficient concentration of drug in the sulcular fluid might have led to the reduction in the counts of pathogenic species of bacteria.
In group B, a statistically significant change of 34.54 ± 5.68, 9.96 ± 4.48 and 42.49 ± 7.08 was seen in spirochetes, motile rods and coccoid cells respectively. This reduction in the bacterial counts could be due to the disinfecting thermal effect on bacteria which is based on the absorption of radiation by tissue and subsequent conversion of LASER energy into heat.30 A reduction of 28.22 ± 5.64 in spirochetes, 5.78 ± 4.14 in motile rods and a hike of 33.31 ± 7.00 was observed in group C and these results are similar to those given by Lavanchy DL et al. (1987).31 The changes in the bacterial levels are significantly more for group A and group B as compared to group C whereas no significant difference was found when the comparison was made between the two test groups. Similar results have been shown by Birang R et al. (2011).17
All the clinical and microbiological parameters reduced significantly from baseline to 4 weeks from group A, B and C. But the results were better for group A and B. Higher improvements in the clinical parameters in chlorhexidine group as compared to group C can be attributed to chlorhexidine, which is known to inhibit microbial proteases from potent periodontal pathogens that destroy periodontal tissues during the progression of disease. This is in accordance with the results obtained by Grisi et al. (2002).32 The use of chlorhexidine chip seems to reduce PGE2 levels (a known inflammatory mediator) that might have led to the improvement in the clinical parameters in accordance with Mizrak T et al. (2006).21
Combining LASER therapy with conventional procedures has been shown to achieve a more effective decontamination of the pocket, along with slower recolonization than sites treated only mechanically.30 Further, due to the curettage effect, the LASER removes the sulcular epithelium in a total and complete way than conventional methods of treatment with manual tools. According to Kriesler et al. (2005), the greatest reduction in the degree of tooth mobility and probing depth in the group of patients who underwent scaling and root planing + LASER therapy can be mainly attributed to the killing of bacteria in the periodontal pocket as well as to the removal of infected sulcular epithelium which jeopardizes periodontal healing.33
Adjunctive therapies using antimicrobials like chlorhexidine chip or LASER therapy, appreciably improve the benefits of scaling and root planing. The results of this study bring to the forefront that most periodontal cases can be managed non-surgically with the use of adjunctive therapies and the threshold for surgical therapy might be shifted towards the deeper pockets. In the present study, we have evaluated the short-term effects of the adjunctive therapies. Further long-term studies are needed to evaluate the efficiency of chlorhexidine and diode LASER in non-surgical periodontal therapy.
6. Summary and conclusion
Within the limitations of the present study, the clinical and microbiological parameters improved significantly in all the three groups. The diode LASER prevents repopulation of the gingival sulcus, promotes healing due its biostimulation effect and has thus, been considerd to be a useful adjunctive tool in non-surgical periodontal therapy.
Funding source
Nil.
Conflicts of interest
Nil.
Ethical guidelines
Informed consent was taken from all the subjects.
Contributor Information
Vrishti Bansal, Email: bvrishti@gmail.com.
Rajan Gupta, Email: drrajan68@gmail.com.
Parveen Dahiya, Email: parveen_132@yahoo.com.
Mukesh Kumar, Email: drmks78@gmail.com.
Japnit Kaur Samlok, Email: jsamlok@gmail.com.
References
- 1.Aoki A., Sasaki K.M., Watanabe H., Ishkawa I. Lasers in non-surgical periodontal therapy. Periodontol. 2000;36:59–97. doi: 10.1111/j.1600-0757.2004.03679.x. 2004. [DOI] [PubMed] [Google Scholar]
- 2.Saglam M., Kantarci A., Dundar N., Hakki S. Clinical and biochemical effects of diode LASER as an adjunct to non-surgical treatment of chronic periodontitis: a randomized controlled clinical trial. Laser Med Sci. 2014;29:37–46. doi: 10.1007/s10103-012-1230-0. [DOI] [PubMed] [Google Scholar]
- 3.report Academy. The role of controlled drug delivery for periodontits. J Periodontol. 2000;71:125–140. doi: 10.1902/jop.2000.71.1.125. [DOI] [PubMed] [Google Scholar]
- 4.Karlsson M.R., Lofgren C.I.D., Jansson H.M. The effect of LASER therapy as an adjunct to non-surgical periodontal treatment in subjects with chronic periodontitis: a systematic review. J Periodontol. 2008;79:2021–2028. doi: 10.1902/jop.2008.080197. [DOI] [PubMed] [Google Scholar]
- 5.Dukic W., Bago I., Aurer A., Rogulgic M. Clinical effectiveness of diode LASER therapy as an adjunct to non-surgical periodontal treatment: a randomized clinical study. J Periodontol. 2013;84:11117. doi: 10.1902/jop.2012.110708. [DOI] [PubMed] [Google Scholar]
- 6.Divya P.V., Nandkumar K. Local drug delivery – Periocol in periodontics. Trends Biomater Artif Organs. 2006;19:74–80. [Google Scholar]
- 7.Raffetto N. LASERs for initial periodontal therapy. Dent Clin. 2004;48:923–936. doi: 10.1016/j.cden.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Silness P., Loe H. Periodontal disease in pregnancy. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 9.Mombelli A., Van Osten M.A.C., Schruch E., Jr., Lang N.P. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987;2:145–151. doi: 10.1111/j.1399-302x.1987.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 10.Listgarten M.A., Hellden L. Relative distribution of bacteria at clinically healthy and periodontally diseased sites in humans. J Clin Periodontol. 1978;5:115–132. doi: 10.1111/j.1600-051x.1978.tb01913.x. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg E.S., Evian C.I., Listgarten M.A. The composition of subgingival microbiota after periodontal therapy. J Periodontol. 1981;52:435–441. doi: 10.1902/jop.1981.52.8.435. [DOI] [PubMed] [Google Scholar]
- 12.Listgarten M.A., Schifter C.C., Laster L. Three year longitudinal study of the periodontal status of an adult population with gingivitis. J Clin Periodontol. 1985;12:225–238. doi: 10.1111/j.1600-051x.1985.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 13.Coluzzi D.J. LASERs and soft tissue curettage: an update. Comp Cont Educ Dent. 2002;23:1104–1111. [PubMed] [Google Scholar]
- 14.Greenstein G., Polson A. The Role of local drug delivery in the management of periodontal diseases: a comprehensive review. J Periodontol. 1998;69:507–520. doi: 10.1902/jop.1998.69.5.507. [DOI] [PubMed] [Google Scholar]
- 15.Soskolne W.A., Chajek T., Flashner M. An in vivo study of the chlorhexidine release profile of the periochip in the gingival crevicular fluid, plasma and urine. J Clin Periodontol. 1998;25:1017–1021. doi: 10.1111/j.1600-051x.1998.tb02407.x. [DOI] [PubMed] [Google Scholar]
- 16.Overman P.R. Biofilm: a new view of plaque. J Contemp Dent Pract. 2000;1:18–29. [PubMed] [Google Scholar]
- 17.Birang R., Yaghini J., Adibrad M., Kiany S., Mohammadi Z., Birang E. The effects of diode LASER (980nm) and chlorhexidine gel in the treatment of chronic periodontitis. J Laser Med Sci. 2011;2:131–138. [Google Scholar]
- 18.Borrajo J.L., Varela L., Castro G., Nunez I., Torreira M.G. Diode LASER as an adjunct to scaling and root planing. Photo Med Laser Surg. 2004;22:509–512. doi: 10.1089/pho.2004.22.509. [DOI] [PubMed] [Google Scholar]
- 19.Lin J., Bi L., Wang L. Gingival curettage study comparing a LASER treatment to hand instruments. Laser Med Sci. 2011;26:7–11. doi: 10.1007/s10103-009-0732-x. [DOI] [PubMed] [Google Scholar]
- 20.Morrison E.C., Ramfjord S.P., Hill R.W. Short-term effects of initial, non-surgical periodontal treatment (hygienic phase) J Clin Periodontol. 1980;7:199–211. doi: 10.1111/j.1600-051x.1980.tb01963.x. [DOI] [PubMed] [Google Scholar]
- 21.Mizrak T., Guncu N.G., Caglayan F., Balci T.A., Aktar G.S., Ipek F. Effect of a controlled release chlorhexidine chip on clinical and microbiological parameters and prostaglandin E2 levels in gingival crevicular fluid. J Periodontol. 2006;77:437–443. doi: 10.1902/jop.2006.050105. [DOI] [PubMed] [Google Scholar]
- 22.Caruso U., Nastri L., Piccolomini R., d'Ercole S., Mazza C., Guida L. Use of diode LASER 980 nm as adjunctive therapy in the treatment of chronic periodontitis: a randomized controlled clinical trial. N Microbiol. 2008;31:513–518. [PubMed] [Google Scholar]
- 23.Azmak N., Atilla G., Luoto H., Sorsa T. The effect of subgingival controlled release delivery of chlorhexidine chip on clinical parameters and matrix metalloproteinase-8 levels in gingival crevicular fluid. J Periodontol. 2002;73:608–615. doi: 10.1902/jop.2002.73.6.608. [DOI] [PubMed] [Google Scholar]
- 24.Grover V., Kapoor A., Malhotra R., Battu V.S., Bhatia A., Sachdeva S. To assess the effectiveness of a chlorhexidine chip in the treatment of chronic periodontitis: a clinical and radiographic study. J Indian Soc Periodontol. 2011;15:139–146. doi: 10.4103/0972-124X.84383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crispino A., Figliuzzi M.M., Lovane C. Effectiveness of a diode LASER in addition to non-surgical periodontal therapy: study of intervention. Ann Stomatol. 2015;6:15–20. [PMC free article] [PubMed] [Google Scholar]
- 26.Conlan M.J., Rapley J.W., Cobb C.M. Biostimulation of wound healing by low-energy LASER irradiation. A review. J Clin Periodontol. 1996;23:492–496. doi: 10.1111/j.1600-051x.1996.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 27.Choi E.J., Yim J.Y., Koo K.T. Biological effects of a semiconductor diode LASER on human periodontal ligament fibroblasts. J Periodontol Implant Sci. 2010;40:105–110. doi: 10.5051/jpis.2010.40.3.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindhe J., Liljenberg B., Listgarten M.A. Some microbiological and histopathological features of periodontal disease in man. J Periodontol. 1980;51:264. doi: 10.1902/jop.1980.51.5.264. [DOI] [PubMed] [Google Scholar]
- 29.Moore W.E.C., Holdeman L.V., Cato E.P. Comparative bacteriology of juvenile periodontitis. Infect Immun. 1985;48:507–519. doi: 10.1128/iai.48.2.507-519.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moritz A., Schoop U., Goharkhay K. Treatment of periodontal pockets with a diode LASER. Laser Surg Med. 1998;22(5):302–311. doi: 10.1002/(sici)1096-9101(1998)22:5<302::aid-lsm7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 31.Lavanchy D.L., Bickel M., Baehni P.C. The effect of plaque control after scaling and root planing on subgingival microflora in human periodontitis. J Clin Periodontol. 1987;14:295–299. doi: 10.1111/j.1600-051x.1987.tb01536.x. [DOI] [PubMed] [Google Scholar]
- 32.Grisi D.C., Salvador S.L., Figueiredo L.C., Souza S.L.S., Novaes A.B., Jr., Grisi M.F.M. Effect of a controlled release chlorhexidine chip on clinical and microbiological parameters of periodontal syndrome. J Clin Periodontol. 2002;29:875–881. doi: 10.1034/j.1600-051x.2002.291001.x. [DOI] [PubMed] [Google Scholar]
- 33.Kriesler M., Al Haj H., d'Hoedt B. Clinical efficacy of semiconductor LASER application as an adjunct to conventional scaling and root planing. Laser Surg Med. 2005;37:350–355. doi: 10.1002/lsm.20252. [DOI] [PubMed] [Google Scholar]