Abstract
In this study, we optimized the process for extracting lipids and proteins from wet biomasses of Spirulina sp. using a 4-kHz ultrasonic osmotic shock method with ultrasound enhancement at a constant frequency of 40 kHz. Optimization was conducted using a response surface methodology (RSM) at an osmotic NaCl concentration of 10–30%, solvent:biomass ratio of 5–15 v/w, and extraction times of 20–50 min. The present osmotic shock method with ultrasound irradiation increased lipid yields to 6.65% in the presence of 11.9% NaCl, a solvent:biomass ratio of 12:1 v/w, and a 22-min extraction time, and protein yields to 43.96% with 15.12% NaCl, a solvent:biomass ratio of 10:1 v/w, and a 30-min extraction time.
Keywords: Extraction, Lipid, Microalgae, Osmotic shock, Ultrasound
Introduction
Microalgae Spirulina sp. has been identified as a potential natural dietary supplement because of its hypocholesterolemic properties, and because of its biliprotein, which have anti-oxidant and anti-cancer activities (Sanchez et al., 2003). Spirulina sp. proteins comprise 60–70% of the dry weight and are rich in the essential amino acids methionine, cysteine, tryptophan, and lysine (Lupatini et al., 2016; Saranraj and Sivasakthi, 2014). This algae is also widely used as a functional food and contains various active ingredients that influence human health positively (Tan et al., 2016). In particular, the high lipid contents (20–50%) of microalgae predominantly comprise essential fatty acids, including the polyunsaturated fatty acids linoleic acid (18:2ω6; LA) and α-linolenic acid (18:3ω3; ALA; Solana et al., 2014; Yazar and Sevket, 2006).
Specific extraction processes are crucial to isolating lipids and proteins from microalgae (Lupatini et al., 2016), and essential microalgae compounds have been traditionally extracted using heat separation methods such as steam- and hydro-distillation. However, thermal degradation of some compounds is a major barrier to product quality (Solana et al., 2014). Moreover, conventional extractions of algal biomasses are performed on the basis of dry weights, with the high energy demands of removing water (Yoo et al., 2012). Therefore, drying steps have been eliminated in direct extractions of wet algae biomasses. Water in algal biomasses leads to swelling of the centers of cell matrixes and acts as co-polar solvent that facilitates extraction (Du et al., 2016). Among wet extraction techniques (Yoo et al., 2012), osmotic shocks are applied with sudden changes in solute concentrations around cells following the addition of osmotic agents that cause rapid changes in water movement across semipermeable cell membranes (Montazeri-Najafabady et al., 2016). Under these conditions, pressure differences lead to removal of certain substances from cells. This method has also been effective for protein extraction from bacteria, and high yields have been achieved (Chang et al., 2014; Cheung and Lee, 2009; Rathore et al., 2003). However, osmotic shock methods have not been optimized for microalgal applications, and are influenced by factors such as particle sizes, solute, and solvent types, temperature, solvent:biomass ratios, and extraction times (Yoo et al., 2012). Therefore, we optimized these factors to achieve high lipid and protein yields from algae biomasses. In particular, we used osmotic shock methods with ultrasound irradiation to maximize lipid and protein yields from wet Spirulina sp. biomasses. Ultrasound‐assisted extraction eliminates the issues associated with conventional mechanical disruption, and other advantageous include low set‐up costs, fast operational times, and high purity of final products (Phong et al. 2017). During extraction, ultrasound waves accelerate reactions by exerting heat and causing structural changes that facilitate penetration of solutes into cells (Moraes et al., 2011). In a recent study, Phong et al. (2017) demonstrated the efficiencies of ultrasound for microalgal cell disruption and extraction of protein in two phase systems. Thus, we identified optimal osmotic agents, solvent:biomass ratios, and extraction times for extraction of lipids and proteins using the response surface optimization method. Subsequently, we characterized the fatty acids and amino acids from the highest-yield extractions.
Materials and methods
Materials
Spirulina platensis wet biomass was provided by CV. Neoalgae (Sukoharjo, Indonesia). Sodium chloride (CV Indrasari) or sorbitol (Sigma–Aldrich) were used as osmotic shock treatments. All organic solvents (n-hexane, ethanol) were of ACS reagent grade (Merck, Germany).
Osmotic shock treatments
NaCl solutions of 10, 20, and 30% were used as osmotic agents, and 20-g samples of wet Spirulina sp. biomass were incubated in 50 ml NaCl solutions for 1 h and were then filtered to isolate extracts.
Ultrasound assisted extraction of lipids and proteins
Lipids were extracted using n-hexane:ethanol solvent with an ultrasound device (Brnson 2510) at 40 kHz and 40 °C (Wiyarno et al., 2010). In these procedures, samples were added to n-hexane at solvent:biomass ratios of 5:1, 10:1, and 15:1 (v/w), and were stirred for 1 h. Mixtures were then sonicated for 20, 35, and 50 min, and residues and supernatants were separated using filter paper (0.2 μm) and a vacuum pump. Supernatants were then evaporated using a rotary vacuum evaporator (Rotavapor® R-100, Switzerland) at 60 °C and the remaining lipids were then weighed to calculate yields.
Proteins were extracted in separate experiments that were performed using an ultrasonic bath (Branson 2510) with solvent:biomass ratios of 5:1, 10:1, and 15:1 (v/wat) 25 °C, and methanol and the mixture 2:1 ratio of methanol and ethanol (v/v) as described previously (Nee et al., 2016). Ultrasound assisted extractions were performed for 20, 35, and 50 min with 15-s ultrasonication cycles separated by 10-s rests to prevent heat damage to proteins (Safi et al. 2014). Supernatants were then separated from precipitates for analyses of protein contents using the Kjeldahl method (Persson et al., 2008).
Determination of fatty acid and amino acid contents
Lipid compositions were determined using transesterification methods as described by Lepage and Roy (Chen et al., 2013). Extracted microalgal lipids were analyzed using a gas chromatograph (GC-2014, Shimadzu) equipped with a flame ionization detector. Samples were injected into a capillary column with an internal diameter of 0.32 mm, and were eluted with Helium(He) at a flow rate of 1.25 mL/min. The injector temperature was set at 250 °C and the detector temperature was 280 °C. The temperature of oven was initially set at 115 °C and was increased from 150 to 180 °C at a rate of 10 °C/min, and from 180 to 260 °C at a rate of 30 °C/min, and was then held at 260 °C for 5 min.
Amino acid contents were analyzed using ion-exchange chromatography with column ninhydrin detection (Hitachi 8900). Before analyses, extracted biomasses were transferred to basic or acid hydrolysis conditions using 6-M sodium hydroxide or 5-M hydrogen chloride to quantify tryptophan and other amino acids, respectively.
Response surface optimization
Extraction process were optimized for lipid and protein extraction using response surface methods and Statistica10 software. Specifically, the effects of NaCl solutions, solvent:biomass ratios, and extraction times on lipid yields from Spirulina sp. biomasses were investigated using a central composite design (CCD; Table 1). This CCD comprised a full 8 factorial design with 2 replicates at the center of the domain and two star points of each factor for a total of 16 runs (Table 2). A second-order polynomial equation (Eq 1) was used to correlate experimental data in multiple regression analyses (Zhang et al., 2018). Independent variables were NaCl solution concentrations (X1), solvent:biomass ratios (X2), and times of extraction (X3), as shown in the following polynomial equation:
| 1 |
where Y represents response variables of lipid and protein yields, βo is the intercept constant, β1, β2, and β3 are linear coefficients, β11, β22, and β33 are quadratic coefficients, and β12, β13, and β23 are interaction coefficients for each variable.
Table 1.
Actual and coded levels of the factors used in the experimental design
| Variables | Factor level | ||||
|---|---|---|---|---|---|
| − α (− 1.68) |
− 1 | 0 | 1 | + α (+ 1.68) |
|
| NaCl (%) | 2.36 | 10 | 20 | 30 | 37.64 |
| Solvent: biomass (v/w) | 1.18 | 5 | 10 | 15 | 18.82 |
| Extraction time (min) | 8.54 | 20 | 35 | 50 | 61.46 |
Table 2.
Central composite design for 3 variables over 16 runs; experimental and predicted %lipid and %protein values are presented
| Run | Factors | Responses | |||||
|---|---|---|---|---|---|---|---|
| NaCl (%) | Solvent: biomass (v/w) | Time (min) | Experimental values | Predicted values | |||
| (X1) | (X2) | (X3) | Yield lipid (%) | Protein (%) | Yield lipid (%) | Protein (%) | |
| 1 | 10 | 5 | 20 | 4.89 | 29.22 | 4.65 | 29.37 |
| 2 | 10 | 5 | 50 | 3.25 | 38.34 | 3.62 | 40.90 |
| 3 | 10 | 15 | 20 | 6.09 | 43.17 | 6.39 | 46.43 |
| 4 | 10 | 15 | 50 | 3.92 | 19.86 | 3.67 | 18.14 |
| 5 | 30 | 5 | 20 | 2.52 | 22.61 | 2.91 | 26.91 |
| 6 | 30 | 5 | 50 | 3.54 | 46.94 | 3.37 | 46.26 |
| 7 | 30 | 15 | 20 | 2.67 | 31.25 | 2.44 | 31.26 |
| 8 | 30 | 15 | 50 | 0.82 | 8.38 | 1.19 | 10.81 |
| 9 | 20 | 10 | 35 | 6.01 | 42.38 | 6.02 | 43.45 |
| 10 | 2.36 | 10 | 35 | 4.85 | 37.07 | 4.81 | 35.92 |
| 11 | 37.64 | 10 | 35 | 1.23 | 29.46 | 1.09 | 27.28 |
| 12 | 20 | 1.18 | 35 | 3.59 | 52.17 | 3.45 | 49.84 |
| 13 | 20 | 18.82 | 35 | 3.12 | 34.59 | 3.08 | 33.60 |
| 14 | 20 | 10 | 8.54 | 5.17 | 26.1 | 5.10 | 23.04 |
| 15 | 20 | 10 | 61.46 | 3.21 | 15.37 | 3.09 | 15.17 |
| 16 | 20 | 10 | 35 | 6.06 | 44.89 | 6.02 | 43.45 |
Results and discussion
Effects of solvent types on extraction yields of lipid and protein
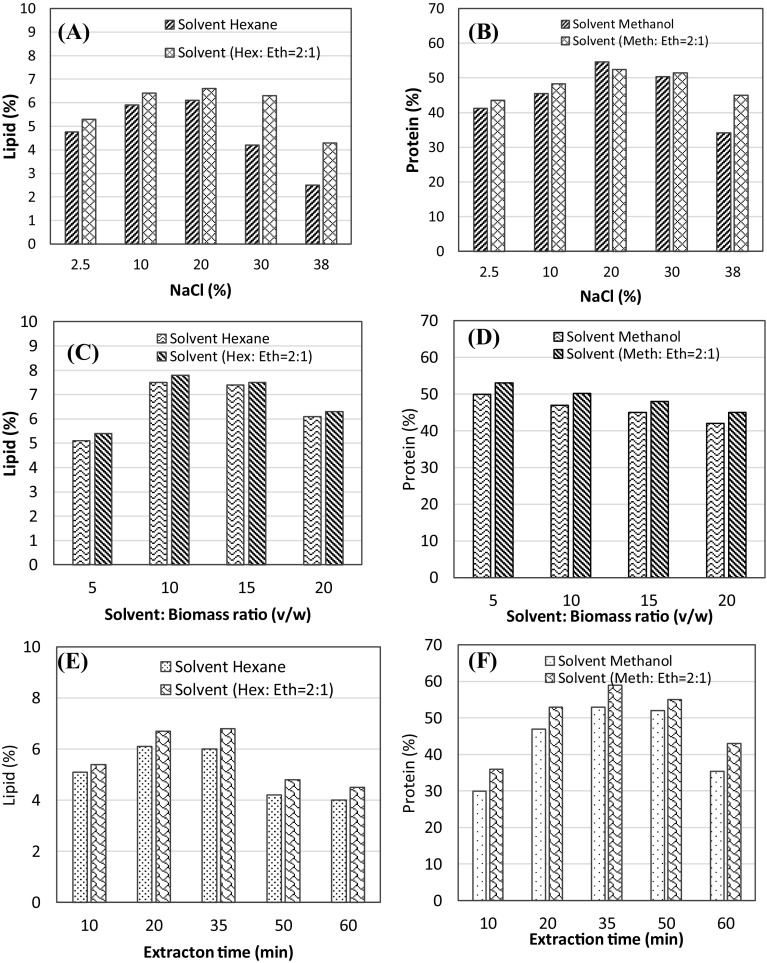
Suitable solvent systems are critical to the extraction of oils and lipids from microalgae, and lipid yields and qualities also depend on algal strains, solvent systems, and cell disruption techniques (Anshari et al., 2017). Herein, lipids were extracted using hexane and hexane–ethanol mixtures, whereas proteins were extracted using methanol and methanol–ethanol mixtures. Microalgal lipid yields were lower with hexane than with hexane and ethanol at a ratio of 2:1 [Fig. 1(A), (B), (C)]. Because ethanol is a polar solvent, it favors the extraction of polar lipids, leading to greater availability of triacylglycerides for extraction by the nonpolar solvent hexane (Li et al., 2014). In agreement, Lewis et al. (2000) showed that extraction mixtures of polar and nonpolar solvents led to greater lipid yields, and similar results were observed during protein extraction from microalgae [Fig. 1(B), (D), (F)]. Taken together, these data indicate that appropriate proportions of polar and nonpolar solvents achieve higher yields of lipids compared with single-solvent extraction methods (Li et al., 2014).
Fig. 1.
Effects of hexane and mixtures of hexane and ethanol on lipid yields, and methanol and mixtures of methanol and ethanol on protein yields; (A, B) NaCl concentrations; (C, D) solvent:biomass ratios; (E, F) extraction times
The effects of osmotic agents on lipid and protein yields
The osmotic shock method exploits changes in osmotic pressures following additions of NaCl to drive the transfer of cellular molecules through cell membranes. Although lipids and proteins are not optimally extracted from cells by low pressure differences, excessive NaCl concentrations gradients have also been associated with poor release of cell contents (Yoo et al., 2012). Accordingly, yields of lipid and protein were increased with NaCl concentrations until optimal concentrations were reached [Fig. 1(A), (B)], whereupon yields decreased (15–20% NaCl). The optimum lipid yield (6.09%) was achieved at 15% NaCl and the optimal protein yield (52.17%) was achieved at 20% NaCl.
Effects of solvent:biomass ratios on lipid and protein yields
Solvent volumes and ratios with biomasses are critical factors during extraction processes (Hadiyanto et al. 2014). Herein, we investigating the effects of varying solvent:biomass ratios on yields and extracted lipids using n-hexane and proteins using methanol, as shown in Fig. 1(B). In a previous study, greater volumes of nonpolar solvents improved solubility of analytes, reflecting greater contact areas between molecules and solvents (Wijanarko and Putri, 2012). In the present study, the highest lipid yield (6.09%) was achieved at a solvent:biomass ratio of 15:1, whereas the highest protein and lipid yields were achieved at a solvent:biomass ratio of 5:1 [v/w; Fig. 1(C), (D)]. However, excess solvent decreased protein yields, potentially indicating altered solute solubility (Zeb et al., 2017) and disturbed chemical equilibriums (Cavonius et al., 2014).
Effects of ultrasonic assisted extraction times on lipid and protein yields
Ultrasound waves facilitate cell swelling, leading to improved dissolution and mass transfer of lipids and proteins through enlarged pores, and reduced extraction times (Soares et al., 2006).
Figure 1(E, F) show significant relationships between extraction times and lipid and protein yields. Specifically, increasing extraction times to 20 min increased the yield of lipid to 5.27%, and a protein yield of 26% was achieved after 35 min. However, extraction times of > 35 min did not improve yields, likely because the lipids and proteins were already released via bubble cavitation from ultrasound irradiation (Safi et al. 2014).
Optimally, the yield of lipid from ultrasound assisted extractions was 6% over 30 min, and this was almost twice that (3.5%) from conventional 2-h extraction procedures (El-Shimi et al., 2013). These data also show that ultrasound irradiation increases cell lysis during the extraction process. In particular, micro-bubbles that form due to cavitation deliver large amounts of energy to surrounding biomasses, leading to disruption of algal cell walls and increased lipid release (Wang et al., 2014). Although greater extraction times can also increase transfer rates, continuous ultrasound radiation may lead to saturation of solutes with solvents and decreased lipid yields (El-Shimi et al., 2013).
Optimization using response surface methodology
To define optimal process conditions for lipid and protein extractions using osmotic shock, we used the response surface methodology of Statistica10 software (Table 3) and calculated regression coefficients for polynomial equations that predict lipid and protein yields.
Table 3.
Estimated regression coefficients for the quadratic model of lipid and protein yields
| Parameters | Regression Coeff. | Standard error | p value | |||
|---|---|---|---|---|---|---|
| Lipid | Protein | Lipid | Protein | Lipid | Protein | |
| β0 | 3.657 | 47.309 | 0.256 | 0.256 | 0.000001 | 0.000002 |
| β1 | 0.314 | 1.456 | 0.192 | 0.192 | 0.000340 | 0.035764 |
| β2 | 1.108 | 5.439 | 0.192 | 0.192 | 0.000306 | 0.002296 |
| β3 | 1.161 | 3.352 | 0.192 | 0.192 | 0.001036 | 0.049230 |
| β12 | − 0.011 | 0.064 | 0.256 | 0.256 | 0.004941 | 0.039574 |
| β13 | 0.003 | 0.013 | 0.256 | 0.256 | 0.027172 | 0.157424 |
| β23 | − 0.006 | − 0.133 | 0.256 | 0.256 | 0.016125 | 0.000175 |
| β11 | − 0.010 | − 0.038 | 0.224 | 0.224 | 0.000119 | 0.011497 |
| β22 | − 0.035 | − 0.022 | 0.224 | 0.224 | 0.000218 | 0.618783 |
| β33 | − 0.003 | − 0.035 | 0.224 | 0.224 | 0.001503 | 0.000318 |
| Lack of feet | 0.067499 | 0.351072 | ||||
| R2 | 0.97978 | 0.96819 | ||||
| Adj R2 | 0.94945 | 0.92048 | ||||
These regression analyses showed R2 values of 97.9% and 96.8% for lipids and proteins, respectively. Moreover, variables X1, X2, and X3 had significant (> 95%, p < 0.05) effects on the model, indicating acceptability of these second-order polynomial equations (Singgih, 2001). However, regression coefficients for β13 and β22 in protein predictions were not significant (p > 0.05), although lack of fit values of 0.067 and 0.351 for lipid and protein, respectively, indicated good agreement between the model and the present data.
| 2 |
| 3 |
These equations relate lipid and protein responses (Y) with NaCl concentrations (X1), solvent:biomass ratios (X2), and extraction times (X3). NaCl concentrations (X1) and solvent:biomass ratios (X3) influenced yields, whereas solvent:biomass (X2) ratios were not significantly predictive. Moreover, quadratic factors for NaCl concentrations (X11), solvent:biomass ratios (X22), and quadratic times (X33) had negative effects on lipid and protein yields.
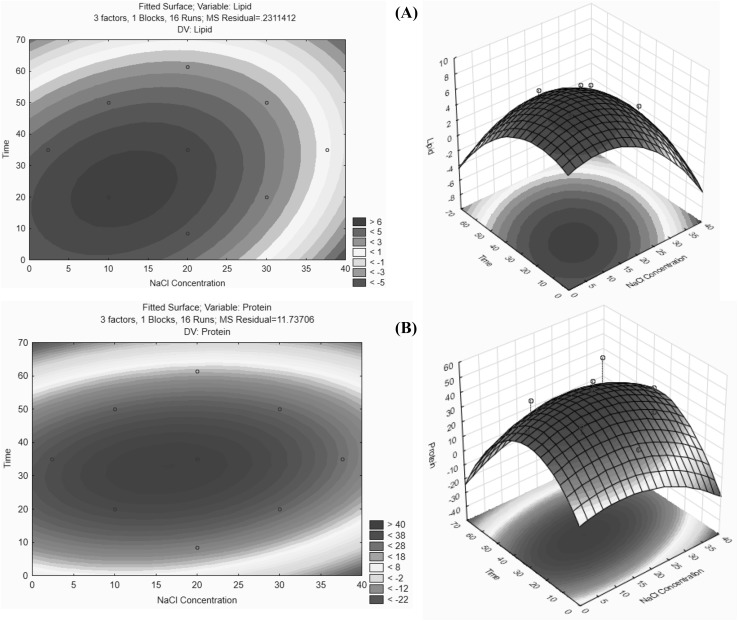
Although no significant interactions of factors were identified in previous analyses (Hadiyanto and Suttrisnorhadi, 2016), the present 3D surface and 2D contour plots of interactions of NaCl concentrations (X1) and extraction times (X3) with protein and lipid yields (Fig. 2) indicate optimal solvent:biomass ratios (X2) and optimal extraction times of 22 min for lipids and 31 min for proteins.
Fig. 2.
Contour and surface plots of interactions between NaCl concentrations (X1), extraction times (X3), and solvent:biomass ratios (X3); 12:1 for (A) lipid and (B) protein yields
Moreover, relationships of NaCl concentrations and extraction times with lipid yields had maximum stationary areas, whereas those with protein contents had saddle-shaped optimal regions. In subsequent experiments, optimum conditions for lipid extraction were X1 = 11.9%, X2 = 12:1 (v/w), and X3 = 22 min, and produced a lipid yield of 6.09%. Similarly, optimal conditions for protein extraction were X1 = 15.12%, X2 = 10:1 (v/w), and X3 = 30 min, and produced a protein yield of 43.96%.
Fatty acid and amino acids profiles
Based on our measurements of fatty acid profiles (Table 4), fatty acid compounds comprised 0.02–34.65% of dry weight, and the major fatty acid component comprised the saturated fatty acid (SFA) palmitic acid. We also identified several unsaturated fatty acids, including mono unsaturated fatty acids (MUFA) and poly unsaturated fatty acids (PUFA). Among PUFA in Spirulina platensis, linoleic acid (omega 6) was the most abundant, as shown previously in Spirulina platensis and Isocrysis galbana (Tokusoglu and Unal, 2003).
Table 4.
Fatty acid and amino acid profiles of lipid and protein extracts from Spirulina platensis
| Fatty acids | Class of fatty acids | (%) | Amino acids | Class of amino acids | (%) |
|---|---|---|---|---|---|
| Capric acid, C10:0 | SFA* | 0.02 | Aspartic acid | Non-essential | 5.14 |
| Lauric acid, C12:0 | SFA | 0.34 | Glutamic acid | Non-essential | 8.65 |
| Myristic acid, C14:0 | SFA | 0.06 | Serine | Non-essential | 2.42 |
| Myristoleic acid, C14:1 | MUFA* | 0.13 | Histidine | Essential | 0.62 |
| Palmitic acid, C16:0 | SFA | 34.65 | Glycine | Non-essential | 1.67 |
| Palmitoleic acid, C16:1 | MUFA | 1.20 | Threonine | Essential | 2.15 |
| Heptadecanoic acid, C17:0 | SFA | 0.10 | Arginine | Non-essential | 3.70 |
| Cis-10-Heptadecanoic acid, C17:1 | MUFA | 0.06 | Alanine | Non-essential | 3.81 |
| Stearic acid, C18:0 | SFA | 0.87 | Tyrosine | Non-essential | 2.00 |
| Elaidic acid, C18:1n9t | PUFA* | 0.03 | Methionine | Essential | 0.77 |
| Oleic acid, C18:1n9c | PUFA | 0.94 | Valine | Essential | 2.94 |
| Linoleic acid, C18:2n6c | PUFA | 9.34 | Phenylalanine | Essential | 3.22 |
| Arachidic acid, C20:0 | SFA | 0.03 | Isoleucine | Essential | 2.87 |
| γ-Linoleic acid, C18:3n6c | PUFA | 15.21 | Leucine | Essential | 4.32 |
| Cis-11,14-Eicosedienoic acid, C20:2 | PUFA | 0.17 | Lysine | Essential | 1.42 |
| Cis-8,11,14-Eicosetnenoic acid, C20:3n6c | PUFA | 0.22 | Amino acid total | 45.69 | |
| Arachidonic acid, C20:4n:6c | PUFA | 0.05 | |||
| Fatty acid total | 63.41 |
SFA saturated fatty acid; MUFA mono unsaturated fatty acid; PUFA poly unsaturated fatty acid
MUFA of Spirulina sp. were present at 1.39% and included myristoleic acid, palmitoleic acid, and cis-10-heptadecanoic acid. Diraman et al. (2010) also found that Spirulina sp. contain MUFA such as palmitoleic acid in the range of 1–8%.
In further analyses, we determined amino acid contents of extracted proteins using HPLC as described previously (Rathore et al., 2003), and revealed the presence of 7 non-essential amino acids and 8 essential amino acids in the present samples (Table 4). Among these, glutamic and aspartic acids, which are non-essential, were the most abundant, whereas histidine was present at the lowest levels. Glutamic acid plays roles in fat and sugar metabolism and is often used as a food seasoning (Bashir et al., 2016). Accordingly, the high amino acid contents of Spirulina spp. have led to consideration as a supplement for cereal based foods (WHO, 2007).
In summary, the present data demonstrate optimal parameters for osmotic shock and ultrasound extraction processes. We also revealed advantages of wet microalgal biomasses, including more rapid lipid and protein extraction. However, we only optimized conditions for certain ranges of variables and a constant ultrasound frequency, in particular warranting further optimization studies at multiple ultrasound frequencies. In addition, further studies are required to investigate the efficacy of osmotic shock and ultrasound irradiation for extractions of other added value products of microalgae, such as phycocyanin and astaxanthin. Finally, alternative osmotic agents may facilitate algal cell lysis more effectively than NaCl.
Acknowledgement
The authors would like to thank Ministry of Research, Technology and Higher Education for their grants through Hibah Kompetensi of Year 2018. All staffs of Center of Biomass and Renewable Energy (CBIORE) are highly appreciated.
References
- Anshari FA, Gupta SK, Shriwastav A, Guldhe A, Rawat I, Bux F. Evaluation of various solvent systems for lipid extraction from wet microalgal biomass and its effects on primary metabolites of lipid-extracted biomass. Environ. Sci. Pollut. Res. 2017;24:15299–15307. doi: 10.1007/s11356-017-9040-3. [DOI] [PubMed] [Google Scholar]
- Bashir S, Sharif MK, Butt MS, Shahid M. Functional properties and amino acid profile of Spirulina platensis protein isolates. Pak. J. Sci. Ind. Res. Ser. B: Biol. Sci. 2016;59:12–19. [Google Scholar]
- Cavonius LR, Carlsson N, Undeland I. Quantification of total fatty acids in microalgae: comparison of extraction and transesterification methods. Anal. Bioanal. Chem. 2014;406:7313–7322. doi: 10.1007/s00216-014-8155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Coleman S, Lee YE, Schellenberg-Beaver T. Differential effects of sucrose or NaCl osmotic shock on β-galactosidase activity in Escherichia coli BW25993 is dependent on normalization method by total viable count or total protein content. J. Exp. Microbiol. Immunol. 2014;18:54–59. [Google Scholar]
- Chen C-Y, Chang J-S, Chang H-Y, Chen T-Y, Wu J-H, Lee W-L. Enhancing microalgal oil/lipid production from Chlorella sorokiniana CY1 using deep-sea water supplemented cultivation medium. Biochem. Eng. J. 2013;77:74–81. doi: 10.1016/j.bej.2013.05.009. [DOI] [Google Scholar]
- Cheung C, Lee J. (NaCl) and non-ionic (Sucrose) osmotic stress on the expression of β-galactosidase in wild type E. coli BW25993 and in the isogenic BW25993ΔlacI mutant. J. Exp. Microbiol. Immunol. 2009;13:1–6. [Google Scholar]
- Diraman H, Koru E, Dibeklioglu H. Fatty acid profile of Spirulina platensis used as a food supplement. Isr. J. Aquacult. 2010;61:134–142. [Google Scholar]
- Du Y, Schuur B, Kersten SRA, Brilman DWF. Microalgae wet extraction using N-ethyl butylamine for fatty acid production. Green Energy Environ. 2016;1:79–83. doi: 10.1016/j.gee.2016.07.001. [DOI] [Google Scholar]
- El-Shimi H, Attia NK, El-Sheltawy ST, El-Diwani GI. Biodiesel production from spirulina-platensis microalgae by in-situ transesterification process. J. Sustain. Bioenergy Syst. 2013;3:224–233. doi: 10.4236/jsbs.2013.33031. [DOI] [Google Scholar]
- Hadiyanto H, Sutanto AA, Suharto Y. Ultrasound assisted extraction of antioxidant from Coleus tuberosus peels. Carpath. J. Food Sci. Technol. 2014;6:58–65. [Google Scholar]
- Hadiyanto H, Suttrisnorhadi S. Response surface optimization of ultrasound assisted extraction (UAE) of phycocyanin from microalgae Spirulina platensis. Emir. J. Food Agric. 2016;28(4):227–234. doi: 10.9755/ejfa.2015-05-193. [DOI] [Google Scholar]
- Li Y, Naghdi FG, Garg S, Adarme-Vega TC, Thurecht KJ, Ghafor WA, Tannock S, Schenk PM. A comparative study: the impact of different lipid extraction methods on current microalgal lipid research. Microbial. Cell Fact. 2014;2014(13):1–9. doi: 10.1186/1475-2859-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T, Nichols PD, McMeekin TA. Evaluation of extraction methods for recovery of fatty acids from lipid-producing microheterotrophs. J Microbiol. Methods. 2000;43:107–116. doi: 10.1016/S0167-7012(00)00217-7. [DOI] [PubMed] [Google Scholar]
- Lupatini AL, Bispo LO, Colla LM, Costa JAV, Canan C, Colla E. Protein and carbohydrate extraction from S. platensis biomass by ultrasound and mechanical agitation. Food Res. Int. 2016;99:1–8. doi: 10.1016/j.foodres.2016.11.036. [DOI] [PubMed] [Google Scholar]
- Montazeri-Najafabady N, Negahdaripour M, Salehi MH. Morowvat MH, Shaker S. Ghasemi, Y. Effects of osmotic shock on production of carotene and glycerol in a naturally isolated strain of Dunaliella salina. Journal of Applied Pharmaceutical. Science. 2016;6(8):160–163. [Google Scholar]
- Moraes CC, Sala L, Cerveira GP, Kali SJ. C-Phycocyanin extraction from Spirulina platensis wet biomass. Brazilian Journal of Chemical Engineering. 2011;28(1):45–49. doi: 10.1590/S0104-66322011000100006. [DOI] [Google Scholar]
- Nee W, Phonga WN, Leb CF, Show PL, Lam HL, Linga TC. Evaluation of different solvent types on the extraction of proteins from microalgae. Chem. Eng. Trans. 2016;52:1063–1068. [Google Scholar]
- Persson JA, Wennerholm M, O’Halloran S. Handbook for kjeldahl digestion, FOSS, DK-3400 Hilleroed, Denmark (2008)
- Phong WN, Le CF, Show PL, Chang JS, Ling TC. Extractive disruption process integration using ultrasonication and an aqueous two-phase system for protein recovery from Chlorella sorokiniana. Eng. Life Sci. 2017;17:357–369. doi: 10.1002/elsc.201600133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore AS, Bilbrey RE, Steinmeyer DE. Optimization of an osmotic shock procedure for isolation of a protein product expressed in E. coli. Biotechnology Progress. 2003;19(5):1541–1546. doi: 10.1021/bp034030s. [DOI] [PubMed] [Google Scholar]
- Safi C, Ursu AV, Laroche C, Zebib B, Merah O, Pontalier PY, Garcia CV. Aqueous extraction of proteins from microalgae: Effect of different cell disruption methods. Algal Research. 2014;3:1–5. doi: 10.1016/j.algal.2013.11.012. [DOI] [Google Scholar]
- Sanchez M, Castillo JB, Rozo C, Rodríguez I. Spirulina (Arthospira): An Edible Microorganisme: A Review. Universitas Scientiarium. 2003;8(1):7–24. [Google Scholar]
- Saranraj P, Sivasakthi S. Spirulina platensis-food for future: a review. Asian J. Pharm. Sci. Technol. 2014;4(1):26–33. [Google Scholar]
- Soares MIS., Péres VF, Dariva C, Zini CA, Abad FC, Martinez MM, Caramão EB. Optimization of the sonication extraction method of Hibiscus tiliaceus L. flowers. Ultrason. Sonochemistry. 13: 242–250 (2006) [DOI] [PubMed]
- Solana M, Rizza CS, Bertucco A. Exploiting microalgae as a source of essential fatty acids bysupercritical fluid extraction of lipids: Comparison between Scenedesmus obliquus, Chlorella protothecoides and Nannochloropsis salina. J. Supercrit. Fluids. 2014;92:311–318. doi: 10.1016/j.supflu.2014.06.013. [DOI] [Google Scholar]
- Tan CH, Chen CY, Show PL, Ling TC, Lam HL, Lee DJ, Chang JS. Strategies for enhancing lipid production from indigenous microalgae isolates. Journal of the Taiwan Institute of Chemical Engineers. 2016;63:189–194. doi: 10.1016/j.jtice.2016.02.034. [DOI] [Google Scholar]
- Wang M, Yuan W, Jiang X, Jing Y, Wang Z. Disruption of microalgal cells using high-frequency focused ultrasound. Bioresource Technology. 2014;153:315–321. doi: 10.1016/j.biortech.2013.11.054. [DOI] [PubMed] [Google Scholar]
- WHO . Protein and Amino Acid Requirements in Human Nutrition. Geneva: Switzerland; 2007. [PubMed] [Google Scholar]
- Wijanarko B, Putri LD. Ekstraksi Lipid dari Mikroalga (Nanochloropsis sp.) dengan Solven Methanol dan Chloroform. Jurnal Teknologi Kimia dan Industri. 1: 130–138 (2012)
- Wiyarno B, Yunus R, Mel M. Oil Algae Extraction from Nannochloropsis sp: a study of soxhlet extraction and ultrasonic assisted extraction. Int. J. Sci. Eng. 1–5 (2010)
- Yazar D, Sevket G. α-Tocopherol and Fatty acids of Spirulina platensis Biomass in Glass Planel Bioreactor. Pak. J. Biol. Sci. 2006;9(15):2901–2904. doi: 10.3923/pjbs.2006.2901.2904. [DOI] [Google Scholar]
- Yoo G, Park WK, Kim CW, Choi YE, Yang JW. Direct lipid extraction from wet Chlamydomonas reinhardtii biomass using osmotic shock. Bioresource Technology. 2012;123:717–722. doi: 10.1016/j.biortech.2012.07.102. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kong X, Wang Z, Sun Y, Zhu S, Li L, Lv P. Optimization of enzymatic hydrolysis for effective lipid extraction from microalgae Scenedesmus sp. Renew. Energy. 2018;125:1049–1057. doi: 10.1016/j.renene.2018.01.078. [DOI] [Google Scholar]
- Zeb H, Riaz A, Kim J. The Journal of Supercritical Fluids Understanding the effect of biomass-to-solvent ratio on macroalgae (Saccharina japonica) liquefaction in supercritical ethanol. The Journal of Supercritical Fluids. 2017;120:65–74. doi: 10.1016/j.supflu.2016.10.013. [DOI] [Google Scholar]