Abstract
Kiwifruit is known to contain considerable amount of antioxidative phenolics. The objective of this study was to evaluate the antioxidative, anti-inflammatory and immunomodulatory effects of Actinidia eriantha cv. Bidan and A. deliciosa cv. Hayward kiwifruits. The antioxidant capacity of kiwifruit was measured with the DPPH, ABTS and ORAC assays, and was significantly (p < 0.05) higher in cv. Bidan than in cv. Hayward. The production of proinflammatory cytokines interleukin-6, interleukin-12 and tumor necrosis factor-α by peritoneal macrophages from male BALB/c mice was significantly (p < 0.05) lower following treatment of cv. Bidan extracts than after treatment with lipopolysaccharide alone. Cv. Bidan extracts significantly (p < 0.05) increased the proliferation of splenocytes stimulated with an anti-CD3 antibody and significantly (p < 0.05) reduced their interferon-γ secretion. Taken together, these findings suggest that cv. Bidan kiwifruit is rich in antioxidants and may be a source of anti-inflammatory and immunomodulatory agents.
Keywords: Cytokines, Green kiwifruit, Interferon-γ, Vitamin C equivalents, White kiwifruit
Introduction
Frequent intake of fruits and vegetables can reduce the risk of various chronic diseases, including hypertension, stroke, dementia and cancer [1], although its effects on cognition depend on the group of fruits or vegetables and the type of cognitive function [2]. Kiwifruit is one of the more popular fruits today. Kiwifruit is known as a good source of minerals, carotenoids, vitamin C and phenolics [3, 4]. Kiwifruit has a considerable antioxidant capacity [5, 6] and thus can reduce oxidative stress [7]. As an edible fruit grown from deciduous woody fruiting vines, kiwifruit belongs to the genus Actinidia, which includes a diversity of species and cultivars. The phenolic content and antioxidant capacity of kiwifruit depend on the cultivar and maturation stage [3, 8]; thus, different varieties may have different biological effects.
Reactive oxygen species (ROS) such as hydroxyl radical, hydrogen peroxide, singlet oxygen and superoxide are naturally present in living systems and are continuously produced during normal physiologic events [9]. A variety of conditions and factors can increase cellular ROS production, such as inflammation, infection, UV exposure and drug intake, initiating a sequence of inflammatory processes [9]. Excessive ROS induce oxidative damage of crucial biomolecules in the human body, resulting in degenerative disorders such as chronic inflammation, neurodegenerative diseases, atherosclerosis, and aging. Thus, preventing excessive ROS generation is critical for preventing the deterioration of various diseases. In humans, antioxidant defense systems including endogenous antioxidant enzymes and antioxidant food constituents remove or repair the molecules damaged by ROS and maintain normal cellular function and health [10, 11]. The immune system is influenced by oxidative stress because immune cells rely on cell–cell communication, particularly via membrane-bound receptors, to function well [10]. Phenolics in fruit have been reported to have anti-inflammatory and immunomodulatory effects [12].
Kiwifruit of Actinidia eriantha cv. Bidan has been reported to contain phenolics such as chlorogenic acid, cinnamoyl glucose, feruloylquinic acid and cirsimaritin [13]. A. deliciosa cv. Hayward kiwifruit contains various phenolic acids and flavonoids, including chlorogenic acid, catechin, quercetin and rutin [13, 14]. The flavonoid cirsimaritin was reported to inhibit interleukin (IL)-6 and tumor necrosis factor-α (TNF-α), regulated by transcription factors such as c-fos and signal transducer and activator of transcription 3 in lipopolysaccharide-stimulated RAW 264.7 cells [15]. The phenolic acid chlorogenic acid was found to reduce the levels of pro-inflammatory cytokines (IL-1β, TNF-α and IL-6) and to inhibit the translocation of nuclear factor-κB [16]. Kiwifruit of cv. Hayward increased cytokine production and reduced oxidative stress in male ICR mice [17]. Due to their hydroxyl group, phenolics serve as potent antioxidants, although this varies according to their structure [18]. Antioxidants in kiwifruit can effectively modulate immune and inflammatory reactions in the body. However, there is limited information on the antioxidative, anti-inflammatory and immunomodulatory effects of kiwifruit, especially A. eriantha cv. Bidan kiwifruit.
In the current study, we comparatively evaluated the total phenolic contents, antioxidant capacities, anti-inflammatory effects and immunomodulatory effects of A. eriantha cv. Bidan and A. deliciosa cv. Hayward kiwifruits. The antioxidant capacities of both types of kiwifruit were measured with the ABTS, DPPH and ORAC assays. Peritoneal macrophages and splenocytes isolated from male BALB/c mice were used to investigate the anti-inflammatory and immunomodulatory effects of both types of kiwifruit extracts in vitro.
Materials and methods
Reagents
Ascorbic acid, ABTS, catechin, DPPH, 2,2′-azobis-(2-methylpropionamidine) dihydrochloride (AAPH), Folin-Ciocalteu’s phenol reagent, gallic acid, lipopolysaccharide (LPS) and phosphate-buffered saline (PBS) were purchased from Sigma Chemical Co. LLC. (St. Louis, MO, USA). Thioglycollate solution was purchased from BD Diagnostic Systems (Sparks, MD, USA). BD OptEIA sets for measurements of IL-6, IL-12, TNF-α, and interferon (IFN)-γ, along with an anti-CD3 antibody, were purchased from BD Biosciences (San Diego, CA, USA). Dulbecco’s Modified Eagle’s Medium (DMEM), Roswell Park Memorial Institute (RPMI) 1640 medium, and fetal bovine serum (FBS) were obtained from HyClone (Logan, Utah, USA). A WST cell proliferation assay kit was purchased from Enzo Life Sciences (Farmingdale, NY, USA). Dulbecco’s phosphate-buffered saline (DPBS) was purchased from Welgene Inc. (Daegu, Republic of Korea). All other reagents were of analytical or HPLC grade.
Kiwifruit
Kiwifruit of A. eriantha cv. Bidan (white kiwifruit) was harvested at the Jeonnam Agricultural Research and Extension Services in September, 2013 (Jeollanam-do, Republic of Korea) and then ripened by ethylene gas. Kiwifruit of A. deliciosa cv. Hayward (green kiwifruit) globally marketed under the ZESPRI™ brand was purchased from a local market in Suwon, Republic of Korea.
Extraction of phenolics from fresh kiwifruit
A mixture of kiwifruit flesh and absolute methanol (100 mL) was homogenized with Polytron homogenizer (PT 10/35, Kinematica, Kriens-Luzern, Switzerland) at 15,000 rpm for 3 min. Homogenized samples were filtered through Whatman #1 filter paper (Whatman International Ltd., Kent, England) by means of a chilled Büchner funnel. The residue was re-extracted using the process with the homogenization for 2 min instead of 3 min described above. The filtrate was evaporated on a rotary evaporator at 40 °C. The phenolic extracts of kiwifruit were obtained in triplicate, freeze-dried and stored at − 80 °C.
Animals
Eight-week-old male BALB/c mice were purchased from the Korean branch of Taconic, SamTaco (Osan, Republic of Korea) and fed rodent chow and water ad libitum in a temperature- and humidity-controlled pathogen-free facility at Kyung Hee University. Mice were maintained in accordance with the Guide for the Care and Use of Laboratory Animals issued by the US National Research Council, and the animal protocol (KHMC-LACUC12-006) was approved by the Kyung Hee University Medical Center Institutional Animal Care and Use Committee (Yongin, Republic of Korea).
Determination of total phenolic content
The total phenolic content of kiwifruit was evaluated by colorimetric analysis with Folin–Ciocalteu’s phenol reagent according to the method of Singleton and Rossi [19] with some modifications [20]. Each diluted extract (0.2 mL) was mixed with 2.6 mL of deionized water, and then 0.2 mL of Folin–Ciocalteu’s phenol reagent was added to the mixture. After 6 min, 2.0 mL of 7% (w/v) Na2CO3 solution was added to the reaction mixture. At 90 min, the absorbance at 750 nm was measured with a spectrophotometer (Spectronic 200, Thermo Fisher Scientific Inc., Waltham, MA, USA). Deionized water was used as a blank instead of the sample. The total phenolic content in kiwifruit was described as mg gallic acid equivalents (GAE)/100 g fresh weight.
Measurements of antioxidant capacity with the ABTS assay
ABTS radicals were used to measure the antioxidant capacity of kiwifruit extracts [21]. A mixture of 1 mM AAPH and 2.5 mM ABTS in PBS was heated in a water bath at 70 °C to create ABTS radicals. The solution of ABTS radicals was adjusted to an absorbance of 0.650 ± 0.020 at 734 nm. The reactions between the ABTS radical solution (980 μL) and the appropriately diluted extracts (20 μL) were conducted at 37 °C for 10 min. The absorbance at 734 nm was immediately measured with a spectrophotometer (Spectronic 200, Thermo Fisher Scientific Inc.). Vitamin C was used as an antioxidant standard to quantify the antioxidant capacity. The antioxidant capacity of kiwifruit was expressed as mg vitamin C equivalents (VCE)/100 g fresh weight.
Measurements of antioxidant capacity with the DPPH assay
The antioxidant capacity of kiwifruit was determined from its DPPH radical scavenging capacity [21]. The absorbance of fresh deep-purple DPPH radicals in 80% (v/v) aqueous methanol was set to 0.650 ± 0.020 at 517 nm. The reactions between the appropriately diluted extracts (50 μL) and the DPPH radical solution (2.95 mL) took place at 23 °C for 30 min. The reduction of absorbance at 517 nm was immediately measured with a spectrophotometer (Spectronic 200, Thermo Fisher Scientific Inc.). Vitamin C was used as an antioxidant standard to build a calibration curve. The antioxidant capacity of kiwifruit was expressed as mg VCE/100 g fresh weight.
Measurements of antioxidant capacity with the oxygen radical absorbance capacity (ORAC) assay
The ORAC assay was used to measure the antioxidant capacity using the method of Huang et al. [22] with some modifications [23]. The extract (25 μL) and 150 μL of a fluorescein solution (81.6 nM) were added to a 96-well plate and incubated at 37 °C for 10 min. Then, 25 μL of AAPH solution (153 mM) was added. The fluorescence was detected every minute for 90 min on a microplate reader (Infinite M200, Tecan Austria GmbH, Grödig, Austria) with 485 nm excitation and 520 nm emission wavelengths. The antioxidant capacity of kiwifruit was expressed as mg VCE/100 g fresh weight.
Isolation and culture of mouse peritoneal macrophages
Mice were intraperitoneally injected with 2 mL of 3.5% sterile thioglycollate medium. Three days later, the mice were sacrificed by cervical dislocation. Macrophages were collected by peritoneal lavage with cold DMEM. After centrifugation, the cells were resuspended in DMEM with 10% FBS and incubated for 2 h in a humidified atmosphere of 5% CO2 at 37 °C. Non-adherent cells were removed, and the resulting adherent cell population consisted of 95% macrophages, as determined by morphology and non-specific esterase staining.
Preparation of mouse splenocytes
Spleens were aseptically isolated from male BALB/c mice, and were disrupted between glass slides in complete medium (RPMI 1640 with 10% FBS and 1% antibiotic–antimycotic) to prepare splenocytes. After centrifugation at 800×g for 10 min to separate the splenocytes from debris, the splenocytes were washed in RPMI 1640 medium and the erythrocytes were lysed. The cells were then counted, and viability was determined by the trypan blue exclusion assay.
Determination of splenocyte proliferation
Splenocytes were seeded in 96-well plates and stimulated with an anti-CD3 monoclonal antibody (mAb; 2 μg/mL). The cells were treated with various concentrations of kiwifruit extracts for 48 h at 37 °C in 5% CO2. The proliferative effects of the kiwifruit extracts on the splenocytes were determined with the WST assay. The absorbance was read at 450 nm with a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Measurements of cytokines
The levels of IL-6, IL-12, TNF-α and IFN-γ were measured with an enzyme-linked immunosorbent assay (ELISA) by means of BD OptEIA sets. Flat-bottomed 96-well plates were coated overnight at 4 °C with coating mAbs. The primary mAbs were discarded and then the plates were blocked with assay diluent for 1 h at ambient temperature. The plates were washed three times with wash buffer (0.05% Tween 20 in PBS) and then the diluted samples were added. The plates were incubated for 2 h at ambient temperature. The supernatant was discarded and the wells were washed five times with wash buffer. Detecting mAbs plus avidin horseradish peroxidase were added and incubated for 1 h at ambient temperature. After a washing step, tetramethylbenzidine substrate solution was added. The color was allowed to develop for 30 min in the dark before the reaction was quenched with 0.2 M H2SO4. The plates were then read at 450 nm with the reference wavelength of 570 nm using a microplate reader (Molecular Devices). The sample concentrations were determined with a standard curve.
Statistical analysis
The data are presented as the mean ± SD of three replicate determinations. Tests for statistical significance were performed with IBM SPSS software Version 21 (IBM SPSS Statistics Inc., Chicago, IL, USA). Significance of differences in average values was determined using Student’s t test and Duncan’s multiple range test (p < 0.05).
Results and discussion
Total phenolic content and antioxidant capacity
The total phenolic levels of fresh A. eriantha cv. Bidan and A. deliciosa cv. Hayward kiwifruits were 380.9 and 91.4 mg GAE/100 g, respectively (Table 1). The phenolic content of cv. Bidan was approximately 4.2-fold higher than that of cv. Hayward. A previous study also found that the total phenolic content of cv. Bidan was higher than that of cv. Hayward [7, 24]. Wild A. eriantha species have significantly (p < 0.05) higher total phenolic contents than cultivars of A. deliciosa, including cv. Hayward [13, 25]. The total phenolic content of cv. Hayward was previously reported to range from 115 to 164 mg GAE/100 g fresh weight [26], which was higher than that determined in this study. Different levels of total phenolics may be ascribed to the genotype, cultivar, harvest season and growing location [8, 24–27].
Table 1.
Total phenolic content and antioxidant capacity of flesh of fresh Actinidia eriantha cv. Bidan and A. deliciosa cv. Hayward kiwifruit
| Cultivar | Total phenolic content (mg gallic acid equiv./100 g FW1) | Antioxidant capacity (mg vitamin C equiv./100 g FW) | ||
|---|---|---|---|---|
| ABTS | DPPH | ORAC | ||
| Bidan | 380.9 ± 5.0*2 | 608.9 ± 2.3* | 620.9 ± 6.7* | 1016.8 ± 11.7b* |
| Hayward | 91.4 ± 1.6 | 143.5 ± 3.6 | 116.0 ± 1.6 | 423.1 ± 13.2 |
1FW stands for fresh weight
2All data are presented as the mean ± SD (n = 3). The asterisk indicates the means in the same column considered to be significantly different by Student’s t test (p < 0.05)
The antioxidant capacities of cv. Bidan and cv. Hayward kiwifruits as measured with the ABTS, DPPH and ORAC assays are presented in Table 1. The antioxidant capacities of cv. Bidan kiwifruit as measured with the ABTS, DPPH and ORAC assays were 608.9, 620.9 and 1016.8 mg VCE/100 g fresh weight, respectively, whereas those of cv. Hayward kiwifruit were 143.5, 116.0 and 423.1 mg VCE/100 g fresh weight, respectively. In all three different assays measuring the antioxidant capacity of fresh kiwifruit, the antioxidant capacity of cv. Bidan kiwifruit was approximately 2.4- to 5.4-fold higher than that of cv. Hayward kiwifruit. Similarly, it was previously reported that the white kiwifruit cv. Bidan had a higher antioxidant capacity than the green kiwifruit cv. Hayward [8, 13, 24].
Effects of kiwifruit on pro-inflammatory cytokines in primary macrophages
The overproduction of mediators, including IL-6 and TNF-α, has been implicated in some inflammatory diseases, including Alzheimer’s disease, rheumatoid arthritis and cancer [28, 29]. Inflammation is regulated by various immune cells and by mediator molecules such as proinflammatory cytokines. Pharmacological inhibition of these mediators seems to be an effective therapeutic strategy for reducing inflammatory reactions and the risk of inflammation [28]. Macrophages play a critical role in the immune system and express a wide range of cytokines in inflammatory and immune responses to harmful stimuli (including LPS) from pathogenic bacteria [9]. Hence, the inhibitory effects of kiwifruit phenolics on proinflammatory cytokines such as IL-6, IL-12 and TNF-α were investigated in mouse peritoneal macrophages stimulated with LPS.
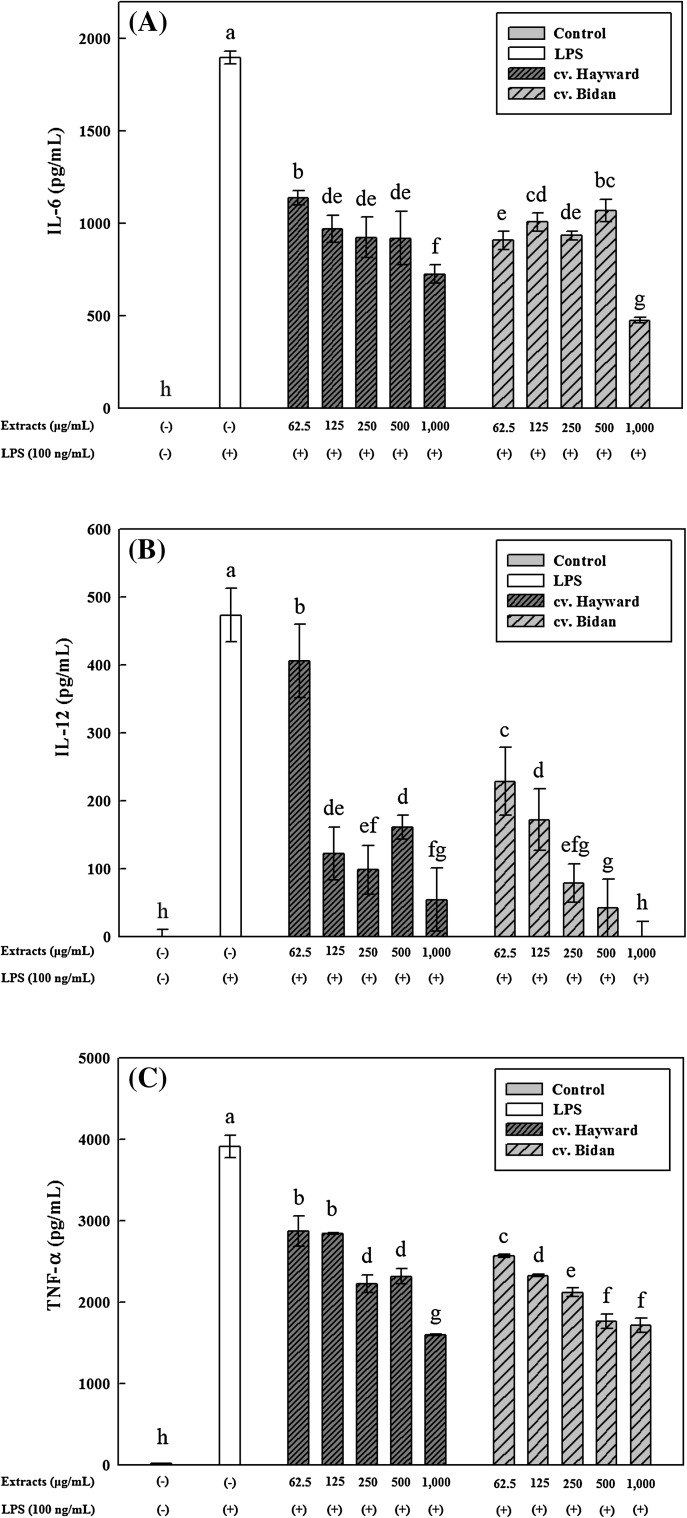
The inhibitory effects of kiwifruit phenolic extracts on the production of the pro-inflammatory cytokines IL-6, IL-12 and TNF-α in the culture supernatants of primary macrophages isolated from male BALB/c mice are shown in Fig. 1. Cells treated with phenolic extracts of both A. eriantha cv. Bidan and A. deliciosa cv. Hayward generated significantly (p < 0.05) less IL-6 than negative controls treated with LPS only [Fig. 1(A)]. LPS-stimulated macrophages produced 1897.0 pg/mL of IL-6 [Fig. 1(A)]. Phenolic extracts of A. deliciosa cv. Hayward reduced IL-6 production to 1139.2 pg/mL at the lowest dose, and to 726.3 pg/mL at the highest dose [Fig. 1(A)]. Phenolic extracts of A. eriantha cv. Bidan reduced IL-6 production to 1070.8 pg/mL at the lowest dose and to 475.9 pg/mL at the highest dose [Fig. 1(A)]. Thus, IL-6 expression was reduced by approximately 75% when A. eriantha cv. Bidan phenolic extracts were applied at a concentration of 1000 μg/mL.
Fig. 1.
Effects of Actinidia eriantha cv. Bidan and A. deliciosa cv. Hayward phenolic extracts on interleukin (IL)-6 (A), IL-12 (B) and tumor necrosis factor (TNF)-α (C) production in lipopolysaccharide (LPS)-stimulated peritoneal macrophages from male BALB/c mice. The data are displayed as the mean ± SD (bars) of three replicates. Different letters on the bars indicate significant differences by Duncan’s multiple range test (p < 0.05)
IL-12 is produced by activated macrophages and is specifically associated with the development of type I T helper (Th1) cells, which further stimulates the activation of macrophages [30]. Peritoneal macrophages treated with all the concentrations of both phenolic extracts used in this study produced significantly (p < 0.05) less IL-12 than those stimulated with LPS only [Fig. 1(B)]. LPS-stimulated primary macrophages produced 473.2 pg/mL of IL-12 [Fig. 1(B)]. The phenolic extracts of A. eriantha cv. Bidan reduced IL-12 production in a dose-dependent manner. When the cv. Bidan phenolic extracts were applied at a concentration of 1000 μg/mL, macrophages produced IL-12 at a similar level to control cells not treated with LPS or extracts [Fig. 1(B)].
TNF-α is a cytokine that stimulates endothelial cells, recruits neutrophils and promotes coagulation [31]. LPS-stimulated macrophages produced 3912.3 pg/mL of TNF-α [Fig. 1(C)]. Macrophages treated with all the tested concentrations of both A. eriantha cv. Bidan and A. deliciosa cv. Hayward phenolic extracts produced significantly (p < 0.05) less TNF-α than negative control cells treated with LPS only. The phenolic extracts of A. eriantha cv. Bidan reduced TNF-α production in a dose-dependent manner. At 1000 μg/mL, the A. eriantha cv. Bidan phenolic extracts reduced TNF-α production by approximately 56% compared with the negative control [Fig. 1(C)].
This inhibition of the production of the proinflammatory cytokines IL-6, IL-12 and TNF-α suggests that both cv. Bidan and cv. Hayward kiwifruits may modulate cell-mediated inflammatory reactions, although different concentrations of the kiwifruit phenolic extracts had different effects on cytokine production (Fig. 1). Similar to our study, a previous study demonstrated that green kiwifruit (cv. Hayward) exerted anti-inflammatory effects in LPS-stimulated primary macrophages [32]. Oral administration of kiwifruit to lead-exposed Sprague–Dawley rat pups significantly (p < 0.05) reduced the mRNA expression of the inflammatory cytokines IL-1ß and TNF-α in the hippocampus [33]. Cv. Bidan has been reported to have higher phenolic acid and vitamin C contents as well as antioxidant capacity than cv. Hayward [3, 7, 13]. It was previously reported that phenolic acids such as protocatechuic, vanillic, caffeic, syringic, ferulic and p-coumaric acids were identified in cv. Bidan and cv. Hayward [3]. Protocatechuic and vanillic acids have been reported to exert anti-inflammatory effects by suppressing the production of the proinflammatory cytokines such as IL-6 and TNF-α [34, 35]. Therefore, the phenolic acids in cv. Bidan and cv. Hayward kiwifruit flesh extracts were likely responsible for the observed anti-inflammatory effects.
Effects of kiwifruit on splenocyte proliferation and IFN-γ production in response to anti-CD3
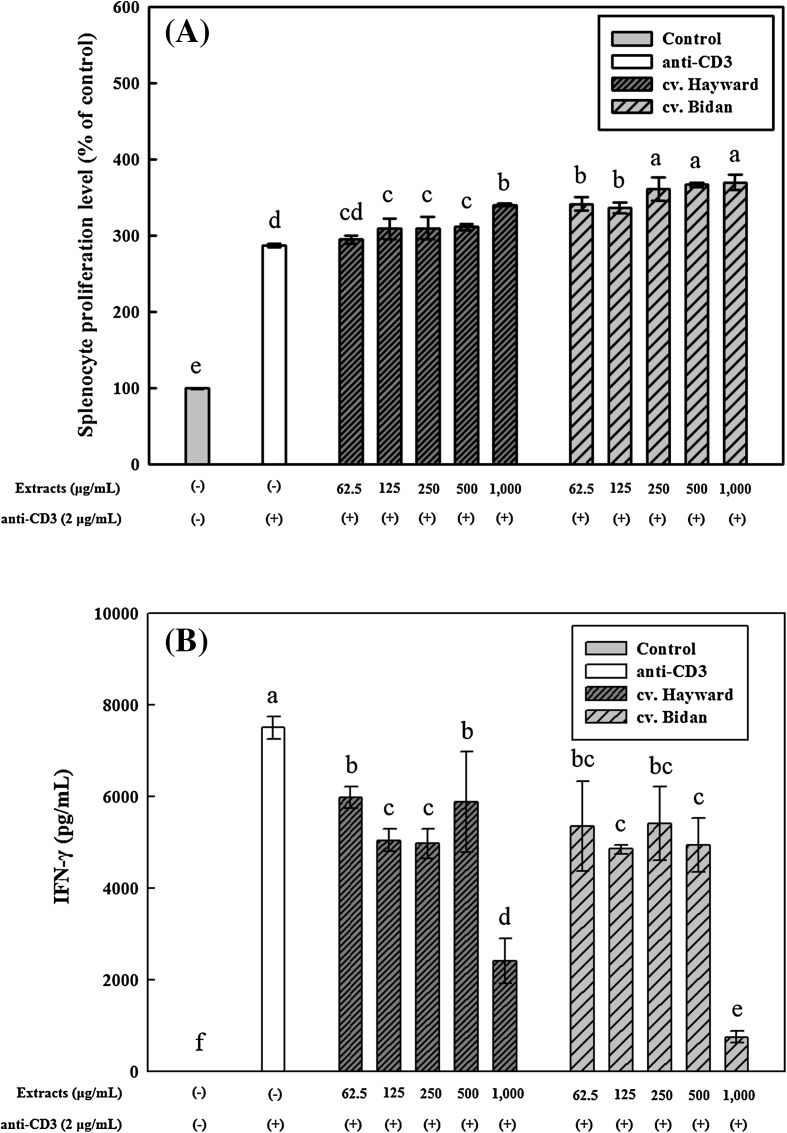
Anti-CD3 antibody is a polyclonal mitogen and acts to induce mitogenic response in all T cells. Proliferation is one of the key responses in T cell immune response. The proliferation of anti-CD3-induced splenocytes was significantly enhanced by cv. Bidan and cv. Hayward kiwifruit extract treatments [p < 0.05; Fig. 2(A)]. Splenocytes treated with 1000 μg/mL of cv. Bidan extracts displayed the highest proliferation level (369.8%), whereas controls treated only with anti-CD3 displayed 286.7% proliferation. At the same treatment concentrations, cv. Bidan kiwifruit extracts induced splenocyte proliferation to a significantly (p < 0.05) greater extent than cv. Hayward kiwifruit extracts. Similarly, it was previously reported that fruits such as strawberry and mulberry exerted immunomodulatory effects by stimulating splenocyte proliferation, and that fruit phenolics as immunomodulatory components highly induced the proliferation of primary splenocytes [36]. Kiwifruit extracts in this study were found to stimulate splenocyte proliferation [Fig. 2(A)], partly due to their content of immunomodulatory phenolics like flavonoids.
Fig. 2.
Effects of Actinidia eriantha cv. Bidan and A. deliciosa cv. Hayward kiwifruit extracts on the proliferation (A) and interferon (IFN)-γ production (B) of splenocytes from male BALB/c mice. Splenocytes were stimulated with anti-CD3 (2 μg/mL) for 48 h, and simultaneously treated with various concentrations of kiwifruit extracts. The data are displayed as the mean ± SD (bars) of three replicates. Different letters on the bars indicate significant differences by Duncan’s multiple range test (p < 0.05)
IFN-γ is produced by natural killer cells and Th1 cells. Because Th1 cell-derived IFN-γ activates macrophages, it is important to regulate this cytokine for the control of chronic inflammation. The effects of phenolic extracts of both A. eriantha cv. Bidan and A. deliciosa cv. Hayward kiwifruits on IFN-γ production were also investigated in splenocytes from male BALB/c mice. Splenocytes stimulated only with anti-CD3 produced 7500.2 pg/mL of IFN-γ [Fig. 2(B)]. Although the inhibition of IFN-γ release was not dose-dependent, A. eriantha cv. Bidan phenolic extracts reduced IFN-γ expression by approximately 89.9% at a concentration of 1000 μg/mL. A. eriantha cv. Bidan phenolic extracts reduced IFN-γ production to a significantly (p < 0.05) greater extent than A. deliciosa cv. Hayward phenolic extracts.
Pretreatment of splenocytes with kiwifruit extracts significantly (p < 0.05) induced their proliferation compared with the group exposed to anti-CD3 only [Fig. 2(A)]. Activated T cells, in particular, CD4 T cells, produce IFN-γ and other cytokines such as IL-2 and IL-4. It was previously reported that kiwifruit increased IFN-γ production in allergic models [37]. In this study, kiwifruit extract suppressed IFN-γ production in splenocytes under neutral conditions [Fig. 2(B)]. Since kiwifruit extract did not decrease the number of splenocytes [Fig. 2(A)], the decrease in IFN-γ production did not happen in a state of decreasing cell numbers. Therefore, we assume that some active components such as phenolics in kiwifruit may interfere with IFN-γ production signaling pathway in splenocytes.
In conclusion, cv. Bidan kiwifruit had a significantly (p < 0.05) higher antioxidant capacity than cv. Hayward kiwifruit. Phenolic extracts of A. eriantha cv. Bidan kiwifruit significantly (p < 0.05) reduced the production of pro-inflammatory cytokines TNF-α, IL-6 and IL-12 in LPS-stimulated peritoneal macrophages of male BALB/c mice. Along with inducing the proliferation of splenocytes, the cv. Bidan extracts significantly (p < 0.05) attenuated INF-γ production in splenocytes compared with anti-CD3 mAb-stimulated controls. Thus, as a good source of antioxidants, cv. Bidan kiwifruit may serve as an inflammatory modulator. The identification of phenolics in cv. Bidan kiwifruit warrants further study. Also, the phenolics that function as potential anti-inflammatory compounds in cv. Bidan kiwifruit and their mechanisms based on molecular levels remain to be further elucidated.
Acknowledgements
This research was supported by the Agricultural Biotechnology Development Program (Project No. 114076-3), Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Boeing H, Bechthold A, Bub A, Ellinger S, Haller D, Kroke A, Leschik-Bonnet E, Müller MJ, Oberritter H, Schulze M, Stehle P, Watzl B. Critical review: vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012;51:637–663. doi: 10.1007/s00394-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Péneau S, Galan P, Jeandel C, Ferry M, Andreeva V, Hercberg S. Kesse-Guyot E, the SU.VI.MAX 2 Research Group. Fruit and vegetable intake and cognitive function in the SU.VI.MAX 2 prospective study. Am. J. Clin. Nutr. 2011;94:1295–1303. doi: 10.3945/ajcn.111.014712. [DOI] [PubMed] [Google Scholar]
- 3.Park Y-S, Leontowicz H, Leontowicz M, Namiesnik J, Suhaj M. Milena Cvikrová, Martincová O, Weisz M, Gorinstein S. Comparison of the contents of bioactive compounds and the level of antioxidant activity in different kiwifruit cultivars. J. Food Compos. Anal. 2011;24:963–970. doi: 10.1016/j.jfca.2010.08.010. [DOI] [Google Scholar]
- 4.Lim YJ, Oh C-S, Park Y-D, Kim D-O, Kim U-J, Cho Y-S, Eom SH. Physiological components of kiwifruits with in vitro antioxidant and acetylcholinesterase inhibitory activities. Food Sci. Biotechnol. 2014;23:943–949. doi: 10.1007/s10068-014-0127-z. [DOI] [Google Scholar]
- 5.Chun OK, Kim D-O, Smith N, Schroeder D, Han JT, Lee CY. Daily consumption of phenolics and total antioxidant capacity from fruit and vegetables in the American diet. J. Sci. Food. Agric. 2005;85:1715–1724. doi: 10.1002/jsfa.2176. [DOI] [Google Scholar]
- 6.Hwang J-S, Cho CH, Baik M-Y, Park S-K, Heo HJ, Cho Y-S, Kim D-O. Effects of freeze-drying on antioxidant and anticholinesterase activities in various cultivars of kiwifruit (Actinidia spp.) Food Sci. Biotechnol. 2017;26:221–228. doi: 10.1007/s10068-017-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee I, Lee BH, Eom SH, Oh C-S, Kang H, Cho Y-S, Kim D-O. Antioxidant capacity and protective effects on neuronal PC-12 cells of domestic bred kiwifruit. Korean J. Hort. Sci. Technol. 2015;33:259–267. doi: 10.7235/hort.2015.14123. [DOI] [Google Scholar]
- 8.Lee I, Im S, Jin C-R, Heo HJ, Cho Y-S, Baik M-Y, Kim D-O. Effect of maturity stage at harvest on antioxidant capacity and total phenolics in kiwifruits (Actinidia spp.) grown in Korea. Hort. Environ. Biotechnol. 2015;56:841–848. [Google Scholar]
- 9.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes DA. Effects of dietary antioxidants on the immune function of middle-aged adults. Proc. Nutr. Soc. 1999;58:79–84. doi: 10.1079/PNS19990012. [DOI] [PubMed] [Google Scholar]
- 11.Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr. J. 2016;15:71. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González-Gallego J, García-Mediavilla MV, Sánchez-Campos S, Tuñón MJ. Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 2010;104:S15–S27. doi: 10.1017/S0007114510003910. [DOI] [PubMed] [Google Scholar]
- 13.Park Y-S, Namiesnik J, Vearasilp K, Leontowicz H, Leontowicz M, Barasch D, Nemirovski A, Trakhtenberg S, Gorinstein S. Bioactive compounds and the antioxidant capacity in new kiwi fruit cultivars. Food Chem. 2014;165:354–361. doi: 10.1016/j.foodchem.2014.05.114. [DOI] [PubMed] [Google Scholar]
- 14.Fiorentino A, D’Abrosca B, Pacifico S, Mastellone C, Scognamiglio M, Monaco P. Identification and assessment of antioxidant capacity of phytochemicals from kiwi fruits. J. Agric. Food Chem. 2009;57:4148–4155. doi: 10.1021/jf900210z. [DOI] [PubMed] [Google Scholar]
- 15.Shin M-S, Park JY, Lee J, Yoo HH, Hahm D-H, Lee SC, Lee S, Hwang GS, Jung K, Kang KS. Anti-inflammatory effects and corresponding mechanisms of cirsimaritin extracted from Cirsium japonicum var. maackii Maxim. Bioorg. Med. Chem. Lett. 2017;27:3076–3080. doi: 10.1016/j.bmcl.2017.05.051. [DOI] [PubMed] [Google Scholar]
- 16.Hwang SJ, Kim Y-W, Park Y, Lee H-J, Kim K-W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014;63:81–90. doi: 10.1007/s00011-013-0674-4. [DOI] [PubMed] [Google Scholar]
- 17.Iwasawa H, Morita E, Ueda H, Yamazaki M. Influence of kiwi fruit on immunity and its anti-oxidant effects in mice. Food Sci. Technol. Res. 2010;16:135–142. doi: 10.3136/fstr.16.135. [DOI] [Google Scholar]
- 18.Kim D-O, Lee CY. Comprehensive study of vitamin C equivalent antioxidant capacity (VCEAC) of various polyphenolics in scavenging a free radical and its structural relationship. Crit. Rev. Food Sci. Nutr. 2004;44:253–273. doi: 10.1080/10408690490464960. [DOI] [PubMed] [Google Scholar]
- 19.Singleton VL, Rossi JA., Jr Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- 20.Kim D-O, Padilla-Zakour OI, Griffiths PD. Flavonoids and antioxidant capacity of various cabbage genotypes at juvenile stage. J. Food Sci. 2004;69:C685–C689. doi: 10.1111/j.1365-2621.2004.tb09916.x. [DOI] [Google Scholar]
- 21.Kim D-O, Lee KW, Lee HJ, Lee CY. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002;50:3713–3717. doi: 10.1021/jf020071c. [DOI] [PubMed] [Google Scholar]
- 22.Huang D, Ou B, Hampsch-Woodill M, Flanagan JA, Prior RL. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002;50:4437–4444. doi: 10.1021/jf0201529. [DOI] [PubMed] [Google Scholar]
- 23.Lim D, Kim W, Lee M-G, Heo HJ, Chun OK, Kim D-O. Evidence for protective effects of coffees on oxidative stress-induced apoptosis through antioxidant capacity of phenolics. Food Sci. Biotechnol. 2012;21:1735–1744. doi: 10.1007/s10068-012-0231-x. [DOI] [Google Scholar]
- 24.Park YS, Kim BW, Kim T-C, Jang HG, Chon SU, Cho JY, Jiang SH, Heo BG. Physiological activity of methanol extracts from Korean kiwifruits. Korean J. Hort. Sci. Technol. 2008;26:495–500. [Google Scholar]
- 25.Du G, Li M, Ma F, Liang D. Antioxidant capacity and the relationship with polyphenol and vitamin C in Actinidia fruits. Food Chem. 2009;113:557–562. doi: 10.1016/j.foodchem.2008.08.025. [DOI] [Google Scholar]
- 26.Pal RS, Kumar VA, Arora S, Sharma AK, Kumar V, Agrawal S. Physicochemical and antioxidant properties of kiwifruit as a function of cultivar and fruit harvested month. Braz. Arch. Biol. Technol. 2015;58:262–271. doi: 10.1590/s1516-8913201500371. [DOI] [Google Scholar]
- 27.Scalzo J, Politi A, Pellegrini N, Mezzetti B, Battino M. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition. 2005;21:207–213. doi: 10.1016/j.nut.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 28.Laveti D, Kumar M, Hemalatha R, Sistla R, Naidu VGM, Talla V, Verma V, Kaur N, Nagpal R. Anti-inflammatory treatments for chronic diseases: a review. Inflamm. Allergy Drug Targets. 2013;12:349–361. doi: 10.2174/18715281113129990053. [DOI] [PubMed] [Google Scholar]
- 29.Leyva-López N, Gutierrez-Grijalva EP, Ambriz-Perez DL, Heredia JB. Flavonoids as cytokine modulators: A possible therapy for inflammation-related diseases. Int. J. Mol. Sci. 2016;17:921. doi: 10.3390/ijms17060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cosmi L, Maggi L, Santarlasci V, Liotta F, Annunziato F. T helper cells plasticity in inflammation. Cytometry A. 2014;85A:36–42. doi: 10.1002/cyto.a.22348. [DOI] [PubMed] [Google Scholar]
- 31.Semenzato G. Tumour necrosis factor:a cytokine with multiple biological activities. Br. J. Cancer. 1990;61:354–361. doi: 10.1038/bjc.1990.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edmunds SJ, Roy NC, Love DR, Laing WA. Kiwifruit extracts inhibit cytokine production by lipopolysaccharide-activated macrophages, and intestinal epithelial cells isolated from IL10 gene deficient mice. Cell. Immunol. 2011;270:70–79. doi: 10.1016/j.cellimm.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Xue W-Z, Yang Q-Q, Chen Y, Zou R-X, Xing D, Xu Y, Liu Y-S, Wang H-L. Kiwifruit alleviates learning and memory deficits induced by Pb through antioxidation and inhibition of microglia activation in vitro and in vivo. Oxid. Med. Cell. Longev. 2017;2017:5645324. doi: 10.1155/2017/5645324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Min S-W, Ryu S-N, Kim D-H. Anti-inflammatory effects of black rice, cyanidin-3-O-β-D-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int. Immunopharmacol. 2010;10:959–966. doi: 10.1016/j.intimp.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Kim M-C, Kim S-J, Kim D-S, Jeon Y-D, Park SJ, Lee HS, Um J-Y, Hong S-H. Vanillic acid inhibits inflammatory mediators by suppressing NF-κB in lipopolysaccharide-stimulated mouse peritoneal macrophages. Immunopharmacol. Immunotoxicol. 2011;33:525–532. doi: 10.3109/08923973.2010.547500. [DOI] [PubMed] [Google Scholar]
- 36.Lin J-Y, Tang C-Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007;101:140–147. doi: 10.1016/j.foodchem.2006.01.014. [DOI] [Google Scholar]
- 37.Park E-J, Kim B, Eo H, Park K, Kim Y, Lee HJ, Son M, Chang Y-S, Cho S-H, Kim S, Jin M. Control of IgE and selective TH1 and TH2 cytokines by PG102 isolated from Actinidia arguta. J. Allergy Clin. Immunol. 2005;116:1151–1157. doi: 10.1016/j.jaci.2005.07.024. [DOI] [PubMed] [Google Scholar]