Abstract
This study was carried out to determine the antimicrobial activities of leptospermone isolated from Leptospermum scoparium and its derivatives against six foodborne bacteria (Listeria monocytogenes, Salmonella typhimurium, Shigella flexneri, Shigella sonnei, Staphylococcus intermedius and Staphylococcus aureus), with a view to developing safer antimicrobial agents. The essential oil of L. scoparium seeds possessed potent antimicrobial activity against six bacterial strains. The antimicrobial compound of L. scoparium was isolated by chromatographic analyses and identified as leptospermone. To investigate the structure–activity relationships, the antimicrobial activities of leptospermone and its derivatives (2-acetyl-1,3-cyclohexanedione, 1,3-cyclohexanedione, 1,2,3-cyclohexanetrione-1,3-dioxime, 5,5-dimethyl-1,3-cyclohexanedione and 2,2,4,4,6,6-hexamethyl-1,3,5-cyclohexanetrione) were examined against six foodborne bacteria. Based on the MIC values, leptospermone (MIC 23.6–69.7 μg/mL), 1,2,3-cyclohexanetrione-1,3-dioxime (MIC 43.9–88.5 μg/mL) and 2,2,4,4,6,6-hexamethyl-1,3,5-cyclohexanetrione (MIC 43.9–88.5 μg/mL) exhibited antimicrobial activities against the six foodborne bacteria. These results indicated that leptospermone and its derivatives could potentially be developed as natural food preservatives, rather than using hazardous synthetic preservatives.
Keywords: Antimicrobial activity, Foodborne bacteria, Leptospermone, Structure–activity relationship, Leptospermum scoparium
Introduction
Foodborne diseases are one of the most important public health problems, which globally threatens millions of people (Kim et al., 2016; Law et al., 2015). According to a study from Scallan et al. (2011), 9.4 million cases of foodborne diseases, 1351 deaths and 55,961 hospitalizations caused by foodborne pathogens have been reported annually in the United States. Escherichia coli, Listeria monocytogenes, Salmonella enterica and Staphylococcus aureus were among the most prevalent foodborne bacteria involved in foodborne disease outbreaks (Bhargava et al., 2015; Law et al., 2015). Generally, foodborne disease can be attributed to consumption of food or water polluted with pathogens or their toxins (Law et al., 2015). To prevent foodborne disease outbreaks, artificial food additives and preservatives are used. However, the use of several food processing and preservation methods have raised concerns about failures to control foodborne bacteria, because of the increase in antibiotic resistance of foodborne pathogens (Gyawali and Ibrahim, 2014). In addition, consumers are concerned about the safety of foods with artificial preservatives (Smith-Palmer et al., 1998; Wilcock et al., 2004). Increasing consumer perception of the potential side-effects of artificial preservatives on health has increased interest in new types of natural additives or preservatives, such as naturally derived chemicals, plant extracts and their essential oils (Gyawali and Ibrahim, 2014; Wilcock et al., 2004).
Essential oils are secondary metabolites that are generally extracted from various parts of plants. These products are used to control a wide spectrum of insect pests, viruses, fungi, and other pathogens, because many essential oils are known to exhibit antimicrobial, antioxidant and insecticidal activities (Cho et al., 2016; Kim and Lee, 2016; Sanyacharernkul et al., 2016). Since ancient times, essential oils have been used worldwide as food additives to prolong the shelf-life of foods and to prevent food spoilage (Shan et al., 2007). Leptospermum scoparium (Myrtaceae), commonly called the Manuka myrtle, is an indigenous ‘tea-tree’ to eastern Australia and New Zealand (Christoph et al., 2000; Douglas et al., 2004). L. scoparium oil is commercially used as an antimicrobial agent and in natural remedies for infections and other diseases throughout the world (Christoph et al., 2000; Lis-Balchin et al., 2000; Song et al., 2013). L. scoparium oil contains separate chemotypes such as monoterpenes, sesquiterpenes and triketones (Perry et al., 1997). Especially, triketones are known to have antimicrobial properties in the essential oil of one chemotype of L. scoparium (Lis-Balchin et al., 2000; Porter and Wilkins, 1999). This study was conducted to identify the active components of L. scoparium oil and to examine the antimicrobial activities of L. scoparium oil, leptospermone, and its derivatives against six foodborne bacteria.
Materials and methods
Chemicals
1,2,3-Cyclohexanetrione-1,3-dioxime, 1,3-cyclohexanedione, 2,2,4,4,6,6-hexamethyl-1,3,5-cyclohexanetrione and 5,5-dimethyl-1,3-cyclohexanedione were supplied from Sigma (St. Louis, MO, USA).
Isolation and identification
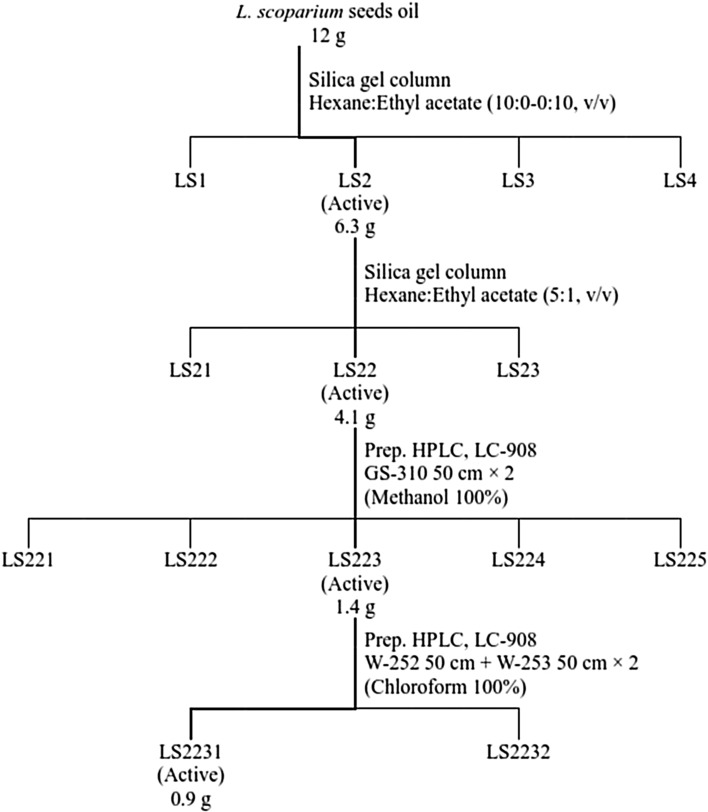
Essential oil of L. scoparium seeds was supplied from Tairawhiti Pharmaceuticals Co. Ltd., and originally produced in New Zealand. Essential oil (12 g) was purified by column chromatography (diameter, 8 × 93 cm; 70-230 mesh), using silica gel (620 g) from Merck (Rahway, NJ, USA) and eluted with hexane:ethyl acetate (10:0 to 0:10, v/v). Each fraction was loaded on a thin layer chromatography (TLC) plate to identify similar fraction patterns. Four fractions (LS1 to LS4) were obtained and then bio-assayed at a dose of 5.0 mg/disc. Active fractions (LS2, 6.3 g) were separated on a silica gel column using solvent gradients composed of hexane:ethyl acetate (5:1, v/v). Bioassay-guided fractionation (LS22, 4.1 g) was performed and then the fractions were further isolated by prep. HPLC (LC908C-W60, recycling prep. HPLC, JAI Co., LTD., Tokyo, Japan). In this step, the fraction was uploaded on a Jai gel GS Series column (GS 310 column 50 cm plus GS 310 column 50 cm), with a mobile phase composed of methanol (100%, v/v) at a flow rate of 2.8 mL/min. This procedure produced 5 fractions (LS221-225), which were each bioassayed. Active fraction (LS223, 1.4 g) was then additionally separated using a Jaigel W Series Column (W-252 50 cm plus W-253 50 cm, inside diameter 20.0 mm, JAI Co., Ltd., Tokyo, Japan), using chloroform (100%. v/v) delivered at a rate of 4.5 mL/min. Finally, active compound (LS2231, 0.9 g) was isolated as a single peak (Fig. 1). The structure of LS2231 was determined using NMR spectroscopy. The 1H and 13C NMR spectroscopy were measured in CDCl3 using a JNM-LA 400F7 spectrometer (JEOL Co., Tokyo, Japan) at 150 and 600 MHz. Additionally, DEPT, COSY and HMQC experiments were carried out.
Fig. 1.
Procedure of leptospermone isolation from the essential oil of L. scoparium seeds
Bacterial strains and preparation of cultures
Three Gram-positive bacteria: Listeria monocytogenes ATCC 15313, Staphylococcus aureus ATCC 25923 and Staphylococcus intermedius ATCC 29663, and three Gram-negative bacteria: Salmonella typhimurium IFO 14193, Shigella flexneri ATCC 29903 and Shigella sonnei ATCC 25931 were prepared by the Korean Culture Center of Microorganisms (Seoul, South Korea). Bacterial strains were grown in nutrient broth (NB; Difco, USA) and incubated overnight at 37 °C, excluding S. aureus, which was grown in Tryptic Soy broth (TSB; Difco, USA).
Agar diffusion method
To assess the antimicrobial activity of L. scoparium seed oil, leptospermone and its derivatives were tested against six foodborne bacteria, by using an agar diffusion method. The foodborne bacteria were maintained in NB at 37 °C for 24 h, except for S. aureus, which was grown in TSB, to yield roughly 1.0 × 107 colony-forming units (CFU)/mL, that corresponded to the turbidity of the McFarland turbidity standard. Tested bacterial suspensions (0.1 mL with 1.0 × 107 CFU/mL) were spread on Muller Hinton agar (MHA; Difco, USA) plates using a sterile glass spreader. The concentration tested were 20, 10, 5, 2, 1, 0.5, 0.25 and 0.125 mg/disc. Appropriate amount of each sample was dissolved to methanol as a solvent (40 μL) to obtain desired concentrations. The sample (40 μL) was injected on sterilized paper discs (8 mm in diameter, Advantec Co., Tokyo, Japan). The negative control was prepared using the same solvent. After drying in a fume hood for 20 min, the loaded paper discs were placed in the center of each MHA plate. These plates were maintained aerobically and incubated at 37 °C for 24 h. Triplicate sets of plates were prepared for each experiment.
Minimum inhibitory concentration (MIC)
A two-fold serial dilution assay was used to determine the MIC values of L. scoparium seed oil, leptospermone and its derivatives. Each sample (10 mg) was dissolved in methanol (10 mL) and then was consecutively diluted using the same solvent, ranging from 100 to 1 μg/mL. Each dilution (50 μL) was prepared in a 96-well microtiter plate which contained 100 μL of Mueller–Hinton Broth (MHB). A 50 µL bacterial suspension (107 CFU/mL) was inoculated into each well. After incubation at 37 °C for 24 h, MIC values of six foodborne bacteria in microdilution wells were confirmed through turbidity readings at 600 nm.
Results and discussion
The antimicrobial activity of the essential oil extracted from L. scoparium seeds was determined using an agar diffusion method (Table 1). Based on the diameter of inhibition zone values, the essential oil of L. scoparium seeds had excellent antimicrobial activity against six foodborne bacteria, S. typhimurium, S. flexneri, S. sonnei, S. aureus, S. intermedius and L. monocytogenes, at concentrations of 10–2 mg/disc. The negative control did not exhibit antimicrobial activity against all the tested bacteria. According to previous studies, the natural products derived from L. scoparium have an antimicrobial activity against a variety of bacterial species (Henriques et al., 2010; Lis-Balchin et al., 2000) and have virucidal activity against Herpes virus (Reichling et al., 2005).
Table 1.
Antimicrobial activities of essential oil of L. scoparium seeds against foodborne bacteria
| Sample | Conc. (mg/disc) | Bacterial species | |||||
|---|---|---|---|---|---|---|---|
| Gram positive bacteria | Gram negative bacteria | ||||||
| L. monocytogenes | S. aureus | S. intermedius | S. typhimurium | S. flexneri | S. sonnei | ||
| L. scoparium oil | 20 | ++++a | ++++ | ++++ | ++++ | +++ | +++ |
| 10 | ++++ | ++++ | ++++ | ++++ | +++ | +++ | |
| 5 | +++ | ++ | ++ | +++ | ++ | ++ | |
| 2 | + | + | + | ++ | + | + | |
| Negative control (only solvent) | 20 | − | − | − | − | − | − |
Cultured on Mueller–Hinton agar at 37 °C for 24 h in an incubator
aDiameter of inhibition zone > 30 mm; ++++ ; 21–30 mm, +++; 16–20 mm, ++; 10–15 mm, +; and < 10 mm, −
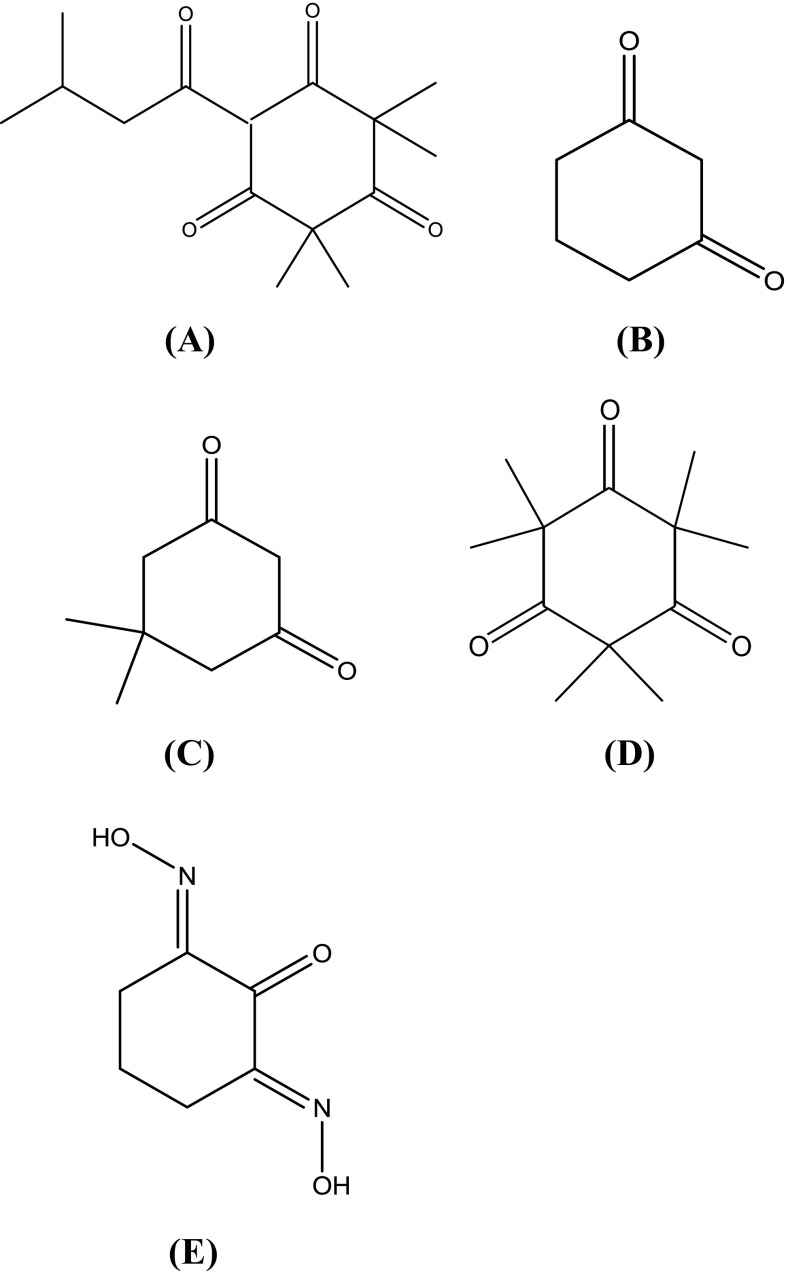
Due to the notable antimicrobial activity of L. scoparium oil, active component from L. scoparium oil was purified by various analytical techniques such as silica gel column chromatography, TLC, and prep. HPLC. Identification of the active component (LS2231) was achieved by spectroscopic analyses, such as EI-MS, 13C NMR and 1H NMR (Table 2). LS2231 was characterized as leptospermone (Fig. 2(A)). Leptospermone (6-isovaleryl-2,2,4,4-tetramethyl-1,3,5-cyclohexanetrione) has an oily liquid with a characteristic light-yellow appearance (C15H22O4, molecular weight 266.0): EI-MS (70 eV) m/z M+ 266, 251, 238, 223, 209, 196, 181, 163, 150, 139, 126, 111, 96, 81, 69, 57, 41; 1H NMR (CD3OD, 600 MHz) δ 3.63 (1H, d, J = 7.2 Hz), 2.88 (2H, d, J = 6.6 Hz), 2.17 (1H, m, J = 26.2 Hz), 1.34 (3H, m, J = 18.0 Hz), 1.34 (3H, m, J = 18.0 Hz), 1.25 (3H, d, J = 49.8 Hz), 1.25 (3H, d, J = 49.8 Hz), 0.92 (3H, m, J = 92.4 Hz), 0.92 (3H, m, J = 92.4 Hz); 13C NMR (CDCl3, 150 MHz) δ 209.9 (C), 209.9 (C), 203.6 (C), 199.5 (C), 109.5 (CH), 56.9 (C), 52.4 (C), 47.2 (CH2), 26.9 (CH), 26.1 (CH3), 24.2 (CH3), 23.8 (CH3), 22.6 (CH3), 16.9 (CH3), 11.8 (CH3). The analytical data of leptospermone was consistent with those of previously reported triketones (Kashman et al., 1974). Triketones were found mainly in the essential oils of various species of Myrtaceae, including Eucalyptus, Leptospermum, and others (Hellyer, 1968; Porter and Wilkins, 1999).
Table 2.
1H NMR, 13C NMR, and DEPT spectral dataa of LS2231
| Carbon | Partial structure | δc (ppm) | δH (ppm) |
|---|---|---|---|
| 1 | C | 209.9 | |
| 2 | C | 209.9 | |
| 3 | C | 203.6 | |
| 4 | C | 199.5 | |
| 5 | CH | 109.5 | 3.63 (1H, db, J = 7.2 Hz) |
| 6 | C | 56.9 | |
| 7 | C | 52.4 | |
| 8 | CH2 | 47.2 | 2.88 (2H, d, J = 6.6 Hz) |
| 9 | CH | 26.9 | 2.17 (1H, m, J = 26.2 Hz) |
| 10 | CH3 | 26.1 | 0.92 (3H, m, J = 92.4 Hz) |
| 11 | CH3 | 24.2 | 0.92 (3H, m, J = 92.4 Hz) |
| 12 | CH3 | 23.8 | 1.25 (3H, d, J = 49.8 Hz) |
| 13 | CH3 | 22.6 | 1.25 (3H, d, J = 49.8 Hz) |
| 14 | CH3 | 16.9 | 1.34 (3H, m, J = 18.0 Hz) |
| 15 | CH3 | 11.8 | 1.34 (3H, m, J = 18.0 Hz) |
a1H NMR (600 MHz), 13C NMR and DEPT (150 MHz), TMS, δ ppm, J in Hz
bd doublet, m multiplet
Fig. 2.
Structures of leptospermone and its derivatives (A) leptospermone; (B) 1,3-cyclohexanedione; (C) 5,5-dimethyl-1,3-cyclohexanedione; (D) 2,2,4,4,6,6-hexamethyl-1,3,5-cyclohexanetrione; (E) 1,2,3-Cyclohexanetrione-1,3-dioxime
The antimicrobial activity of leptospermone isolated from L. scoparium seeds was evaluated by an agar diffusion method against six foodborne bacteria (Table 3). To establish the structure–activity relationships of leptospermone and its derivatives, 1,3-cyclohexanedione, 1,2,3-cyclohexanetrione-1,3-dioxime, 2-acetyl-1,3-cyclohexanedione, 2,2,4,4,6,6-hexamethyl-1,3,5-cyclohexanetrione and 5,5-dimethyl-1,3-cyclohexanedione were selected as derivatives (Fig. 2). Leptospermone had excellent antimicrobial activity against six foodborne bacteria at 2.0–0.125 mg/disc. At a concentration of 2.0 mg/disc, 1,2,3-cyclohexanetrione-1,3-dioxime had strong antimicrobial activity against L. monocytogenes and S. typhimurium and moderate antimicrobial activity against S. aureus, S. flexneri, S. intermedius and S. sonnei. In addition, 2,2,4,4,6,6-hexamethyl-1,3,5-cyclohexanetrione showed strong antimicrobial activity against S. aureus and moderate activity against the other five foodborne bacteria. However, 1,3-cyclohexanedione and 5,5-dimethyl-1,3-cyclohexanedione did not exhibit antimicrobial activities against six foodborne bacteria at 2.0 mg/disc. The MIC values of leptospermone and its derivatives were comparable to the positive control (tetracycline) for six foodborne bacteria using a broth-dilution method (Table 4). Leptospermone possessed the strongest inhibitory activity (MIC 23.63-69.7 μg/mL) against six foodborne bacteria. 1,2,3-Cyclohexanetrione-1,3-dioxime and 2,2,4,4,6,6-hexamethyl-1,3,5-cyclohexanetrione possessed inhibitory activities at 43.9-88.5 μg/mL and 48.1-75.8 μg/mL, respectively. However, 1,3-cyclohexanedione and 5,5-dimethyl-1,3-cyclohexanedione did not show inhibitory activities against six foodborne bacteria. The MIC values of tetracycline were determined at 5.85-25.4 μg/mL. The antimicrobial activity of leptospermone, 1,2,3-cyclohexanetrione-1,3-dioxime and 2,2,4,4,6,6-hexamethyl-1,3,5-cyclohexanetrione was less than that of tetracycline against five food-borne bacteria. Although leptospermone and its derivatives presented higher MIC values than tetracycline, it was possible that leptospermone and its derivatives (1,2,3-cyclohexanetrione-1,3-dioxime and 2,2,4,4,6,6-hexamethyl-1,3,5-cyclohexanetrione) had potential as substitutes for more problematic, commercial antibiotics. Especially, earlier studies (van Klink et al., 2005) showed that leptospermone possessed antimicrobial activities against methicillin-resistant Staphylococcus aureus and methicillin-resistant S. pseudintermedius).
Table 3.
Antimicrobial activities of leptospermone and its derivatives against foodborne bacteria
| Compound | Conc. (mg/disc) | Bacterial species | |||||
|---|---|---|---|---|---|---|---|
| Gram positive bacteria | Gram negative bacteria | ||||||
| L. monocytogenes | S. aureus | S. intermedius | S. typhimurium | S. flexneri | S. sonnei | ||
| Leptospermone | 2.0 | +++a | ++++ | ++++ | ++++ | +++ | +++ |
| 1.0 | +++ | +++ | ++++ | ++++ | +++ | +++ | |
| 0.5 | ++ | ++ | ++ | +++ | ++ | ++ | |
| 0.25 | ++ | + | + | ++ | + | + | |
| 0.125 | + | + | + | + | − | − | |
| 1,3-Cyclohexanedione | 2.0 | − | − | − | − | − | − |
| 5,5-Dimethyl-1,3-cyclohexanedione | 2.0 | − | − | − | − | − | − |
| 2,2,4,4,6,6-Hexamethyl-1,3,5-cyclohexanetrione | 2.0 | ++ | +++ | ++ | ++ | ++ | ++ |
| 1.0 | + | ++ | ++ | + | + | + | |
| 0.5 | − | + | + | − | + | + | |
| 0.25 | − | + | − | − | − | − | |
| 1,2,3-Cyclohexanetrione-1,3-dioxime | 2.0 | +++ | ++ | ++ | +++ | ++ | ++ |
| 1.0 | ++ | + | + | +++ | + | + | |
| 0.5 | + | + | − | ++ | + | − | |
| 0.25 | − | − | − | + | − | − | |
Cultured on Mueller–Hinton agar at 37 °C for 24 h in an incubator
aDiameter of inhibition zone > 30 mm, ++++ ; 21–30 mm, +++; 16–20 mm, ++; 10–15 mm, +; and < 10 mm, −
Table 4.
Minimum inhibition concentration (MIC) values of leptospermone and its derivatives against foodborne bacteria
| Compound | MIC (μg/mL)a | |||||
|---|---|---|---|---|---|---|
| Gram positive bacteria | Gram negative bacteria | |||||
| L. monocytogenes | S. aureus | S. intermedius | S. typhimurium | S. flexneri | S. sonnei | |
| Leptospermone | 41.4 | 53.5 | 58.1 | 23.6 | 65.3 | 69.7 |
| 1,3-Cyclohexanedione | 100 < | 100 < | 100 < | 100 < | 100 < | 100 < |
| 5,5-Dimethyl-1,3-cyclohexanedione | 100 < | 100 < | 100 < | 100 < | 100 < | 100 < |
| 2,2,4,4,6,6-Hexamethyl-1,3,5-cyclohexanetrione | 71.4 | 48.1 | 66.4 | 68.3 | 69.9 | 75.8 |
| 1,2,3-Cyclohexanetrione-1,3-dioxime | 65.3 | 81.7 | 79.8 | 43.9 | 73.4 | 88.5 |
| Tetracyclineb | 20.2 | 5.85 | 19.8 | 25.4 | 26.1 | 25.2 |
Cultured on Mueller–Hinton broth at 37 °C for 24 h in an incubator
aMIC values < 100 μg/mL
bTetracycline was served as positive control
Considering the chemical structure of leptospermum and its derivatives, the difference was the number of ketone groups. Leptospermone, which has a cyclic triketone conjugated with carbons in an acyl side chain had the most antimicrobial activity against six foodborne bacteria. In addition, 1,2,3-cyclohexanetrione-1,3-dioxime and 2,2,4,4,6,6-hexamethyl-1,3,5-cyclohexanetrione, containing hydroxylamine or methyl functional group on cyclic triketone, also had strong antimicrobial activities against six foodborne bacteria. However, 1,3-cyclohexanedione and 5,5-dimethyl-1,3-cyclohexanedione, containing cyclic diketone, observed no antimicrobial activities against six foodborne bacteria. Therefore, the higher antimicrobial activity of triketone group (leptospermone, 1,2,3-cyclohexanetrione-1,3-dioxime and 2,2,4,4,6,6-hexamethyl-1,3,5-cyclohexanetrione) than diketone group (1,3-cyclohexanedione and 5,5-dimethyl-1,3-cyclohexanedione) could be related to this property. These results indicated that the good antimicrobial activity of L. scoparium oil could also be associated with the presence of cyclic triketones, as described by Porter and Wilkins (1999) and Christoph et al. (2000). Kashman et al. (1974) also suggested that the core part of the cyclic triketone, which contains a syncarpic acid residue exhibited antimicrobial activity against Gram-positive bacteria. Moreover, cyclic triketones may disrupt the bacteria cytoplasmic membrane because of the function of the hydrophobic properties (van Klink et al., 2005). Earlier L. scoparium studies reported that the triketone group (leptospermone and 2,2,4,4,6,6-Hexamethyl-1,3,5-cyclohexanetrione) possessed potent acaricidal activities against Dermatophagoides pteronyssinus, D. farinae, and Tyrophagus putrescentiae, but the diketone group (1,3-cyclohexanedione and 5,5-Dimethyl-1,3-cyclohexanedione) had no acaricidal activity (van Klink et al., 1999). In addition, the essential oil of Eucalyptus nitens, rich in triketones, showed repellent and larvicidal activities against Aedes aegypti and A. albopictus (Alvarez Costa et al., 2017). The highly antimicrobial compound of L. scoparium, which is grown widely in most areas of New Zealand, gives us a chance to develop natural food preservatives rather than hazardous synthetic preservatives. Furthermore, the findings of the correlation between triketones and antimicrobial activity would also serve as a guide for using triketones as natural antimicrobial agents. Our studies are just a first step in releasing the complex mechanisms of six foodborne bacteria by leptospermone isolated from L. scoparium seeds and its derivatives. The pharmacological safety and efficacy of leptospermone isolated from L. scoparium seeds and its derivatives makes it a potential compound for treatment and prevention foodborne diseases. Nonetheless, leptospermone and its derivatives has not yet been studied the relative bioavailability. Further study is required to evaluate the bioavailability of leptospermone isolated from L. scoparium seeds and its derivatives.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (2016R1A2A2A05918651).
References
- Alvarez Costa A, Naspi CV, Lucia A, Masuh HM. Repellent and larvicidal activity of the essential oil from Eucalyptus nitens against Aedes aegypti and Aedes albopictus (Diptera: Culicidae) J. Med. Entomol. 2017;54:670–676. doi: 10.1093/jme/tjw222. [DOI] [PubMed] [Google Scholar]
- Bhargava K, Conti DS, da Rocha SR, Zhang Y. Application of an oregano oil nanoemulsion to the control of foodborne bacteria on fresh lettuce. Food Microbiol. 2015;47:69–73. doi: 10.1016/j.fm.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Cho M, Ko SB, Kim JM, Lee OH, Lee DW, Kim JY. Influence of extraction conditions on antioxidant activities and catechin content from bark of Ulmus pumila L. Appl. Biol. Chem. 2016;59:329–336. doi: 10.1007/s13765-016-0165-8. [DOI] [Google Scholar]
- Christoph F, Kaulfers PM, Stahl-Biskup E. A comparative study of the in vitro antimicrobial activity of tea tree oils with special reference to the activity of β-triketones. Planta Med. 2000;66:556–560. doi: 10.1055/s-2000-8604. [DOI] [PubMed] [Google Scholar]
- Douglas MH, van Klink JW, Smallfield BM, Perry NB, Anderson RE, Johnstone P, Weavers RT. Essential oils from New Zealand Manuka: triketone and other chemotypes of Leptospermum scoparium. Phytochemistry. 2004;65:1255–1264. doi: 10.1016/j.phytochem.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Gyawali R, Ibrahim SA. Natural products as antimicrobial agents. Food control. 2014;46:412–429. doi: 10.1016/j.foodcont.2014.05.047. [DOI] [Google Scholar]
- Hellyer RO. The occurrence of β-triketones in the steam-volatile oils of some myrtaceous Australian plants. Aust. J. Chem. 1968;21:2825–2828. doi: 10.1071/CH9682825. [DOI] [Google Scholar]
- Henriques AF, Jenkins RE, Burton NF, Cooper RA. The intracellular effects of Manuka honey on Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2010;29:45–50. doi: 10.1007/s10096-009-0817-2. [DOI] [PubMed] [Google Scholar]
- Kashman Y, Rotstein A, Lifshitz A. The structure determination of two new acylphloroglucinols from Myrtus communis L. Tetrahedron. 1974;30:991–997. doi: 10.1016/S0040-4020(01)97486-1. [DOI] [Google Scholar]
- Kim HJ, Koo M, Hwang D, Choi JH, Kim SM, Oh SW. Contamination patterns and molecular typing of Bacillus cereus in fresh-cut vegetable salad processing. Appl. Biol. Chem. 2016;59:573–577. doi: 10.1007/s13765-016-0198-z. [DOI] [Google Scholar]
- Kim MG, Lee HS. Insecticidal toxicities of naphthoquinone and its structural derivatives. Appl. Biol. Chem. 2016;59:3–8. doi: 10.1007/s13765-015-0115-x. [DOI] [Google Scholar]
- Law JWF, Ab Mutalib MS, Chan KG, Lee LH. Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front. Microbiol. 2015;5:770. doi: 10.3389/fmicb.2014.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis-Balchin M, Hart SL, Deans SG. Pharmacological and antimicrobial studies on different tea-tree oils (Melaleuca alternifolia, Leptospermum scoparium or Manuka and Kunzea ericoides or Kanuka), originating in Australia and New Zealand. Phytother. Res. 2000;14:623–629. doi: 10.1002/1099-1573(200012)14:8<623::AID-PTR763>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Perry NB, Brennan NJ, Van Klink JW, Harris W, Douglas MH, McGimpsey JA, Smallfield BM, Anderson RE. Essential oils from New Zealand Manuka and Kanuka: chemotaxonomy of Leptospermum. Phytochemistry. 1997;44:1485–1494. doi: 10.1016/S0031-9422(96)00743-1. [DOI] [Google Scholar]
- Porter NG, Wilkins AL. Chemical, physical and antimicrobial properties of essential oils of Leptospermum scoparium and Kunzea ericoides. Phytochemistry. 1999;50:407–415. doi: 10.1016/S0031-9422(98)00548-2. [DOI] [PubMed] [Google Scholar]
- Reichling J, Koch C, Stahl-Biskup E, Sojka C, Schnitzler P. Virucidal activity of a β-triketone-rich essential oil of Leptospermum scoparium (Manuka oil) against HSV-1 and HSV-2 in cell culture. Planta Med. 2005;71:1123–1127. doi: 10.1055/s-2005-873175. [DOI] [PubMed] [Google Scholar]
- Sanyacharernkul S, Nantapap S, Sangrueng K, Nuntasaen N, Pompimon W, Meepowpan P. Antifungal of modified neolignans from Mitrephora wangii Hu. Appl. Biol. Chem. 2016;59:385–389. doi: 10.1007/s13765-016-0178-3. [DOI] [Google Scholar]
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Griffin PM. Foodborne illness acquired in the United States-major pathogens. Emerg. Infect. Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan B, Cai YZ, Brooks JD, Corke H. Antibacterial properties and major bioactive components of cinnamon stick (Cinnamomum burmannii): activity against foodborne pathogenic bacteria. J. Agric. Food Chem. 2007;55:5484–5490. doi: 10.1021/jf070424d. [DOI] [PubMed] [Google Scholar]
- Smith-Palmer A, Stewart J, Fyfe L. Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett. Appl. Microbiol. 1998;26:118–122. doi: 10.1046/j.1472-765X.1998.00303.x. [DOI] [PubMed] [Google Scholar]
- Song CY, Nam EH, Park SH, Hwang CY. In vitro efficacy of the essential oil from Leptospermum scoparium (Manuka) on antimicrobial susceptibility and biofilm formation in Staphylococcus pseudintermedius isolates from dogs. Vet. Dermatol. 2013;24:404-e87. doi: 10.1111/vde.12045. [DOI] [PubMed] [Google Scholar]
- van Klink JW, Brophy JJ, Perry NB, Weavers RT. β-Triketones from Myrtaceae: Isoleptospermone from Leptospermum scoparium and papuanone from Corymbia dallachiana. J. Nat. Prod. 1999;62:487–489. doi: 10.1021/np980350n. [DOI] [PubMed] [Google Scholar]
- van Klink JW, Larsen L, Perry NB, Weavers RT, Cook GM, Bremer PJ, Kirikae T. Triketones active against antibiotic-resistant bacteria: synthesis, structure-activity relationships, and mode of action. Bioorg. Med. Chem. 2005;13:6651–6662. doi: 10.1016/j.bmc.2005.07.045. [DOI] [PubMed] [Google Scholar]
- Wilcock A, Pun M, Khanona J, Aung M. Consumer attitudes, knowledge and behavior: a review of food safety issues. Trends Food Sci. Technol. 2004;15:56–66. doi: 10.1016/j.tifs.2003.08.004. [DOI] [Google Scholar]