Abstract
Yogurt is a fermented dairy food produced by growth of lactic acid bacteria (LAB). Green tea is associated with beneficial health effects. Thus, the aim of this study was to evaluate the effects of green tea powder (GTP) on the fermentation and bioactive properties of yogurt. Yogurt was supplemented with 0–3% GTP (w/v), and effects on fermentation were determined. In addition, antioxidant and anti-inflammatory effects of GTP supplemented yogurts were determined in HT-29 colon cells. GTP (1–3%) supplementation significantly increased the acidification rate and growth of LAB during yogurt fermentation. Removal of free radicals and cellular H2O2, and an increase of antioxidant Nrf2 and HO-1 proteins were observed in the 1–3% GTP groups. Yogurt extracts with 0–3% GTP showed decreased expression of TNF-α and IL-1β in cells. In summary, addition of GTP can enhance the beneficial health effects of yogurt by increasing its antioxidant activity, LAB growth and anti-inflammatory effects.
Keywords: Green tea, Yogurt, Antioxidant activity, Lactic acid bacteria, Human colorectal cells
Introduction
Yogurt is one of the most widely consumed, fermented dairy products in the world, and produced by the coagulation of milk proteins during fermentation with lactic acid bacteria (LAB). Diverse LAB are utilized in yogurt production, including Lactobacillus acidophilus, Bifidobacterium longum, and Streptococcus thermophilus. LAB are known to exert beneficial effects on the human body by fostering a balanced intestinal microbiota, enhancing immunity, and reducing blood cholesterol levels [1]. Moreover, bioactive peptides are formed during yogurt fermentation [2], some of which have been found to have antioxidant properties. Although the total antioxidant activity of yogurt is relatively low, purified peptide fractions exert more notable antioxidant effects [3].
Tea (from Camellia sinensis) is the most consumed beverage worldwide. Consumption of tea provides many health benefits, including cholesterol reduction, protection against cardiovascular disease, and anticarcinogenic and anti-obesity effects [4]. These beneficial properties are primarily due to tea catechins, powerful phenolic antioxidants [5]. Epigallocatechin gallate (EGCG) is a particularly abundant green tea catechin, representing more than 50% of the total catechins in green tea leaves. Catechins from green tea have been reported to have anticancer, anti-obesity, anti-atherosclerotic, antidiabetic, antibacterial, and antiviral effects, among other favorable impacts on health [6].
Owing to such positive health effects, green tea has been widely used as a natural food additive. The addition of green tea extract to foods can improve flavor and shelf life and present a healthier image to consumers [7]. In addition, green tea has been reported to have an antimicrobial effect against pathogenic bacteria without killing LAB. This selective activity towards microorganisms can promote a balanced intestinal microbiota, similar to the effects of yogurt intake [8]. In fact, the influence of green tea on antioxidant and physicochemical properties during yogurt manufacturing has been reported previously. For example, addition of green tea extract was found to improve the antioxidant activity of yogurt, as measured by 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, ferric reducing antioxidant power, and ferrous ion chelating assays [9]. Moreover, green tea extract has been shown to decrease yogurt syneresis over 21 days of storage [10]. However, previous studies have not tested the effect of green tea yogurt on living cells. Since yogurt is known to be beneficial to the digestive system, including the colon, it would be particularly informative to establish whether the addition of green tea enhances the health-promoting effects of yogurt on the colon.
Therefore, the goals of our study were: (1) to characterize the properties of green tea yogurt, such as microbiological and physicochemical qualities, and (2) to evaluate its biological activity with respect to colon cells, including cellular antioxidant effects. In this work, green tea powder (GTP) was used instead of green tea extract, as all green tea leaf components are preserved in the former and its properties make it suitable for use as a food additive.
Materials and methods
Yogurt preparation
Yogurt was produced using 1, 2, or 3% GTP originated from Jeju Island (Teazen, Anyang, Korea) (w/v). Non-fat milk (Seoul Dairy Cooperative, Seoul, Korea) (12%) was mixed with GTP before being pasteurized at 85 °C for 30 min. Samples of milk were then cooled to 42 °C and inoculated with 2% yogurt starter culture (v/v). Starter culture was prepared by incubation of a mixture with 100 mL 12% non-fat milk and 0.34 g yogurt starter culture powder (Samik Dairy and Food Co. Ltd., Seoul, Korea) for 5 h. The starter culture powder is mixed strains of L. acidophilus (35%), B. longum (30%), and S. thermophilus (35%). All inoculated milk samples were placed in an incubator at 42 °C until they reached a pH of 4.5–4.6. Plain yogurt (as a control) was produced in the same manner but without addition of GTP. The yogurts were analyzed during fermentation (42 °C; at 0, 2, 4, and 6 h) and storage (4 °C; at 1, 7, 14, and 21 days).
Yogurt water extract preparation
The yogurt water extract was prepared as previously described [11]. The yogurts (10 g) were diluted with 2.5 mL sterile distilled water and homogenized at the highest speed possible (30,000 rpm) for 15 s (Benchmark Scientific, Edison, NJ, USA). Using a pH meter and 0.1 N HCl, the pH of the yogurts was then lowered to 4.0. The acidified yogurts were subsequently incubated in a water bath at 45 °C for 10 min before being centrifuged at 5000×g at 4 °C for 10 min. The resulting supernatant was adjusted to pH 7.0 by adding 0.1 N NaOH and centrifuged again at 5000×g at 4 °C for 10 min. The clear supernatant obtained was stored at − 20 °C and used for analysis within 2 weeks.
Kinetic parameters and acidity measurement
Acidification kinetics were estimated by a previously described method [12]. The maximum acidification rate (Vmax) was calculated based on the variation of pH over time (dpH/dt), expressed in pH units × 10−3/min. At the end of fermentation, the following kinetic parameters were calculated: tmax (h), the time taken to reach Vmax; tpH5.0 (h), the time taken to reach pH 5.0; and tf (h), the time taken to complete fermentation. The pH of all yogurt samples was determined using a SevenEasy pH meter (Mettler-Toledo, Columbus, OH, USA). After mixing 10 g yogurt with 10 mL distilled water and titrating it to pH 8.3 using 0.1 N NaOH, titratable acidity (TA) was calculated as follows:
Lactic acid bacteria counts
Total LAB counts in starter culture and fermented milk (0, 2, 4, and 6 h) were measured on bromocresol purple (BCP) plate count agar (MB cell, Seoul, Korea) after aerobic incubation at 37 °C for 48 h. Strains such as L. acidophilus and S. thermophilus were counted using MRS (Difco Laboratories, Detroit, MI, USA) with bile salt (Oxoid, Basingstoke, UK) and M17 agar (Oxoid, Basingstoke, UK) plates, respectively after aerobic incubation at 37 °C for 24 h. B. longum was enumerated on MRS with LiCl, sodium propionate, and l-cysteine (Sigma-Aldrich, St. Louis, MO, USA) after anaerobic incubation at 37 °C for 24 h in anaerobic gas packs (Mitsubishi Gas Chemical Company, Inc., Tokyo, Japan).
Measurement of syneresis
Yogurt syneresis was analyzed by the centrifugal acceleration test with minor modifications [13]. Yogurt (10 g) was centrifuged at 600×g for 6 min at 4 °C. The clear serum having separated from the yogurt was then poured off and weighed. Syneresis is expressed as grams of whey lost.
Radical scavenging assay
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) (Sigma-Aldrich, St. Louis, MO, USA) stock solution (14.8 mM) was mixed with 5 mM potassium persulfate (1:1, v/v) and allowed to react in the dark at room temperature for 16 h. The ABTS+ solution was then diluted with distilled water to an absorbance at 734 nm of 0.700 ± 0.05 before use. Samples of yogurt water extract (20 μL) were each mixed with 180 μL ABTS+ solution in a 96-well plate and incubated in the dark at room temperature for 15 min. A mixture of 20 μL distilled water and 180 μL ABTS+ solution served as a control. ABTS+ scavenging activity was then calculated as follows:
where Abs is absorbance at 734 nm.
In a separate assay, samples of yogurt water extract (20 μL) were mixed with 180 μL DPPH (Sigma-Aldrich, St. Louis, MO, USA) reagent (0.1 mM; from a stock solution of 39.43 mg DPPH powder in 1 L ethanol) in a 96-well plate and incubated in the dark at room temperature for 30 min. A mixture of 20 μL ethanol and 180 μL DPPH reagent was used as a control. DPPH scavenging activity was then calculated as follows:
where Abs is absorbance at 515 nm.
Cell culture and treatment
Human colorectal cell line, HT-29 was originally obtained from ATCC (Manassas, VA, USA). Cells were maintained at 37 °C in RPMI 1640 medium (Lonza, Walkersville, MD, USA) supplemented with 10% Fetal bovine serum (FBS) (Atlas Biologicals, Fort Collins, CO, USA) and penicillin/streptomycin (Gibco, Grand Island, NY, USA) in a humidified atmosphere containing 5% CO2. Cells were grown to approximately 90% confluency and synchronized overnight in medium containing 1% FBS before initiating treatment. Cells were pretreated with yogurt extracts for 15 h before exposure to 1 µg/mL Escherichia coli O111:B4 lipopolysaccharide (LPS) (Sigma-Aldrich, St. Louis, MO, USA) for 6 h to induce oxidative stress.
Detection of cellular oxidative stress
Cells were grown to confluency in 6-well plates, treated for 15 h with yogurt extract supplemented with 0, 1, 2, or 3% GTP (w/v), and exposed for 6 h to 1 µg/mL LPS. They were then incubated with Dimethyl sulfoxide, 2′,7′-dichlorofluorescin diacetate (DCFDA) (Sigma-Aldrich, St. Louis, MO, USA) at a final concentration of 20 μM for 30 min, before being washed 3 times with PBS. The plates were subsequently evaluated under an Olympus IX71 fluorescence microscope and digital images were captured using an Olympus DP71 camera and DP controller software (Olympus Optical Co. Ltd, Tokyo, Japan). DCFDA is oxidized in the presence of reactive oxygen species (ROS), especially H2O2, staining cells bright green.
Cell lysate preparation, SDS-PAGE, and Western blot analysis
Cells were lysed in RIPA buffer containing 50 mM Tris (pH 8.0), 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, and a protease inhibitor mixture (2 µg/mL aprotinin, 10 µg/mL leupeptin, 1 µg/mL pepstatin A, 1 mM PMSF, 5 mM EDTA, 1 mM EGTA, 10 mM sodium fluoride, and 1 mM sodium orthovanadate). Lysed cells were centrifuged at 21,000×g for 10 min at 4 °C and the resulting supernatants were collected. Protein concentrations were then determined using Bradford reagent (Sigma-Aldrich, St. Louis, MO, USA). Proteins (30 μg per sample) were separated by SDS-PAGE and transferred onto nitrocellulose membranes, which were subsequently blocked with 3% non-fat milk buffer and incubated with a primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 3 h. After washing, the membranes were incubated with HRP-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Bands were visualized using ECL detection reagents (Thermo Fisher scientific, Pittsburgh, PA, USA), and their intensities were quantified using ImageJ software and normalized to the housekeeping protein glyceraldehyde 3-phosphate (GAPDH).
Real-time PCR analysis
To examine gene expression of interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), cells were pretreated with yogurt extracts for 15 h, then stimulated with 1 µg/mL LPS for 12 h. Cells were cultured in 6-well plates and total RNA was extracted from the cells using TRIzol reagent (Ambion, Austin, TX, USA). The TOPscript RT DryMIX kit (Enzynomics, Daejeon, Korea) was used for reverse transcription. The levels of mRNA expression were estimated by real-time PCR using the Real-Time PCR System (Thermo Fisher Scientific, Pittsburgh, PA, USA) and 2 × Real-Time PCR mix (SolGent, Daejeon, Korea). The thermal conditions were as follows: 95 °C for 15 min, followed by 40 cycles of 95 °C for 20 s and 58 °C for 40 s, followed by 60 °C for 30 s and a hold at 4 °C. Relative quantification of mRNA expression was conducted by ΔΔCq method using the reference control gene (i.e., GAPDH). The primer sequences used were IL-1β, 5′-TAC CTG AGC TCG CCA GTG AAA T-3′ and 5′-CCT GGA AGG AGC ACT TCA TCT GTT-3′; TNF-α, 5′-AAG CCC TGG TAT GAG CCC ATC TAT-3′ and 5′-AGG GCA ATG ATC CCA AAG TAG ACC-3′; and GAPDH, 5′-GAC CCC TTC ATT GAC CTC AAC TAC-3′ and 5′-ATG ACA AGC TTC CCG TTC TCA G-3′.
Statistical analysis
Data are expressed as means ± standard errors of the mean (SEM). Statistical significance was determined using SPSS–PASW statistics software version 18.0 for Windows (SPSS, Chicago, IL, USA), by one-way ANOVA and the Turkey post hoc test. p values less than 0.05 were considered to indicate statistically significant differences.
Results and discussion
Acidity measurements and acidification kinetics during fermentation
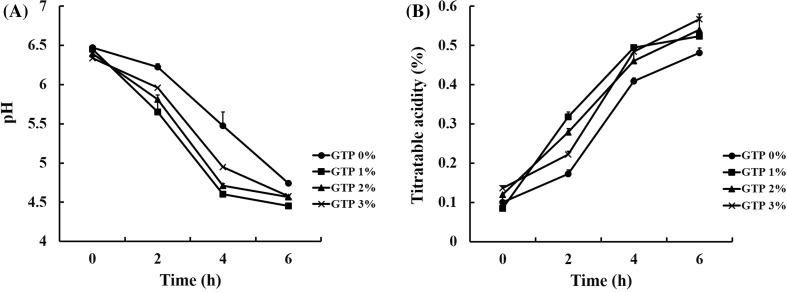
The pH and TA of GTP-supplemented yogurt were measured during fermentation. The pH of GTP-treated yogurt samples was markedly lower than that of control yogurt (0% GTP) [Fig. 1(A)]. Moreover, TA increased in both control yogurt and 1–3% GTP yogurt during fermentation [Fig. 1(B)], but was higher in the latter than in the former. These results are similar to those of previous studies in which yogurts supplemented with black tea or mulberry powder exhibited a faster acidification rate than control yogurts with no additives [14, 15].
Fig. 1.
Changes in (A) pH and (B) TA during the fermentation of yogurts supplemented with 0, 1, 2, or 3% green tea powder (GTP) (w/v). Values are mean ± SEM (n = 3)
To further study the effects of GTP on acidification during yogurt fermentation, acidification kinetics were examined. Addition of GTP significantly accelerated the yogurt fermentation rate (Table 1). Yogurts supplemented with 1% GTP showed a twofold increase in Vmax, and tmax was significantly reduced in the presence of 1–2% GTP. Moreover, tpH5.0 and tf were decreased by addition of GTP. These results indicate that GTP supplementation may significantly enhance the metabolic activity of LAB in yogurt, since acidity increased more rapidly in the GTP yogurts tested. In a previous report, the pH of milk fermented with herbs was found to decrease at a faster rate than that of the control owing to promotion of LAB metabolism [16]. However, the acidification rate and pH of 2–3% GTP yogurt were slightly lower than those of 1% GTP yogurt [Fig. 1(A) and Table 1]. This was probably due to the buffering capacity of the organic acids and phenolic compounds in GTP. Such effects have also been reported in previous research, in which the acidification rate was seen to decrease with increasing additions of passion fruit peel powder during yogurt fermentation [17]. Our results indicate that supplementation with an appropriate amount of GTP may be useful in yogurt production, particularly for shortening fermentation time.
Table 1.
Kinetic parameters and acidity measurement of green tea powder (GTP)
| Kinetic parameters | GTP concentrations | |||
|---|---|---|---|---|
| 0% | 1% | 2% | 3% | |
| Vmax (10−3 pH units/min) | 6.76 ± 0.90a | 12.66 ± 0.56b | 10.58 ± 0.42b | 8.70 ± 0.66a |
| tmax (h) | 3.90 ± 0.31a | 2.13 ± 0.13b | 2.39 ± 0.04b | 3.02 ± 0.42a |
| tpH5.0 (h) | 4.87 ± 0.27a | 2.80 ± 0.12c | 3.17 ± 0.11bc | 3.72 ± 0.21b |
| tf (h) | 6.37 ± 0.29a | 3.95 ± 0.09c | 4.63 ± 0.06b | 5.55 ± 0.16a |
Vmax, acidification rate; tmax, time at which Vmax was reached; tpH5.0, time to reach pH 5.0; and tf, time to complete the fermentation
a–cDifferent letters within columns denote significant differences (p < 0.05)
Growth of lactic acid bacteria
The viability of yogurt microbiota can be affected by many factors, including starter culture quantity and type, incubation time, and presence of available nutrients [18]. In the present study, the viability of LAB was measured during fermentation to observe the effect of GTP, as a potential prebiotic ingredient, on the yogurt microbiota (Fig. 2). LAB counts in 1–3% GTP-supplemented yogurts were higher than those in the control yogurt, averaging 7.2–7.4 and 6.9 log CFU/mL, respectively, at the end of fermentation (after 6 h) [Fig. 2(A)]. Then, the prebiotic effect of GTP was investigated on each starter strain. During fermentation, the number of L. acidophilus, B. longum, and S. thermophilus increased by 0.35–0.64, 0.03–0.27, and 0.11–0.19 log CFU/mL, respectively, in GTP supplemented yogurts, compared to control [Fig. 2(B–D)]. Generally, green tea was known to have antimicrobial effects due to its catechin contents [19]. Catechin can inhibit bacteria growth through damaging cell membrane, inhibition of enzyme activity and decreased fatty acid synthesis [19]. On the other hand, others reported that phenolic compounds promoted the growth of probiotic bacteria. For example, Lactobacillus spp. counts in green tea extract-treated yogurt are increased by approximately twofold, compared to those in plain control yogurt [20]. The number of S. thermophilus cells is also significantly higher in yogurt supplemented with green tea extract than in untreated control yogurt [20]. In addition, addition of green tea extract to yogurts maintains S. thermophilus and Bifidobacterium levels during 21 days of cold storage [21]. Thus, based on these past results and our own, it seems that LAB growth is accelerated during fermentation and can be sustained during refrigeration by green tea components. The decreased pH and increased TA observed in the yogurts in the current work [Fig. 1(A, B)] therefore likely resulted from a higher rate of LAB population growth due to the addition of GTP during fermentation. The mechanisms associated with the promoting effects of GTP on the growth of probiotic bacteria is not clear. A possible explanation is that polyphenols in green tea have stimulatory influence to probiotic bacteria as reported previously [22]. Additionally, polyphenol metabolites generated by probiotic bacteria could modulate the oxidative stress, and provide an beneficial environment for the growth of probiotic bacteria [23]. Collectively, our data indicate that GTP can serve as a prebiotic ingredient during yogurt fermentation by promoting the growth of LAB and shorten fermentation time.
Fig. 2.
Growth of LAB during fermentation in yogurts supplemented with 0, 1, 2, or 3% green tea powder (GTP) (w/v). (A) Total lactic acid bacteria, (B) L. acidophilus, (C) S. thermophilus, and (D) B. longum. Values are mean ± SEM (n = 9)
Syneresis
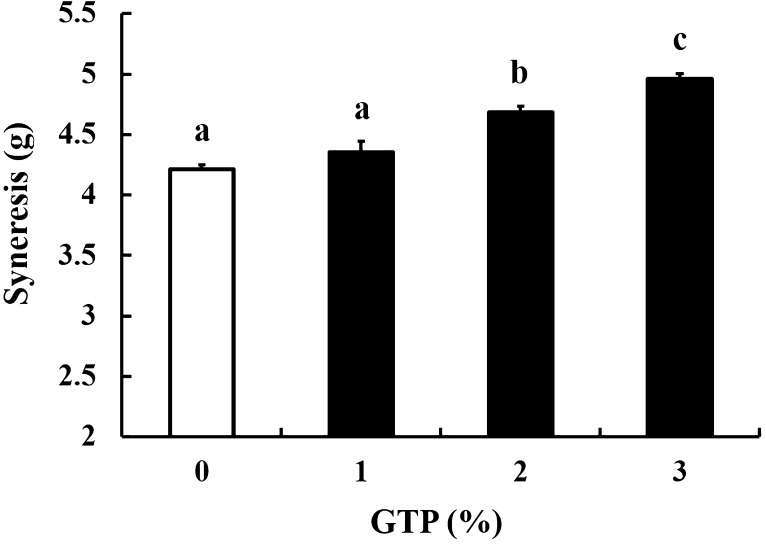
Syneresis, the emission of serum from the yogurt gel matrix, is an important parameter in the evaluation of yogurt quality. We therefore assessed the syneresis of GTP-supplemented yogurt by a centrifugal acceleration test. Syneresis increased in a GTP dose-dependent manner (Fig. 3), and 2 and 3% GTP-treated yogurts demonstrated significantly higher levels of syneresis than the control yogurt (Fig. 3). However, syneresis was not significantly affected by administration of 1% GTP (Fig. 3). The increased syneresis of 2–3% GTP-supplemented yogurt noted was probably due to excessive quantities of green tea components, such as polyphenols. It has been reported that an overabundance of green tea polyphenols can induce syneresis by reducing the gel matrix that confines the yogurt serum [10]. Therefore, based on our data, supplementation of yogurt with GTP at a concentration of 1% would be most suitable in order to maintain the gel matrix.
Fig. 3.
Syneresis of yogurts supplemented with 0, 1, 2, or 3% green tea powder (GTP) (w/v). Values are mean ± SEM (n = 4). Different letters denote significant differences (p < 0.05)
Radical scavenging activity
Green tea is rich in phenolic compounds such as EGCG. These compounds exert antioxidant effects through their ability to donate an electron or a hydrogen atom [24]. To evaluate the antioxidant activity of GTP yogurts, both ABTS and DPPH radical scavenging tests were conducted during a 21-day storage period (Fig. 4). Extracts of 1–3% GTP yogurts exhibited significantly higher radical scavenging potential than those of non-supplemented control yogurts over this period (Fig. 4). Both ABTS and DPPH assays indicated similarly strong radical scavenging effects. Previous studies have also reported that yogurt supplemented with additives containing phenolic compounds (e.g., cinnamon and pomegranate) demonstrates enhanced antioxidant activity [25, 26]. In our study, the ABTS scavenging activity of the control yogurt, although lower than that of the GTP yogurt, was nevertheless around 50% [Fig. 4(A)]. This was likely due to the antioxidant properties of metabolites (e.g., polypeptides, peptides, and amino acids) produced during fermentation by LAB. Indeed, this effect has been demonstrated in a prior study, in which the antioxidant activity of yogurt was found to increase due to production of peptides by LAB during fermentation [27]. Therefore, the antioxidant activity of green tea yogurts is considered to derive from both the phenolic compounds present in green tea and the metabolites generated by LAB [28]. In summary, supplementation with GTP enhances antioxidant properties by increasing both the phenol content and LAB population of yogurt.
Fig. 4.
(A) ABTS and (B) DPPH radical scavenging activity of yogurt extracts over 21 days of storage at 4 °C. Values are mean ± SEM (n = 3). Different letters denote significant differences (p < 0.05)
Cellular antioxidant activity
Oxidative stress is linked to diseases of the human colon, including inflammatory bowel diseases and colorectal cancers [29]. To assess the antioxidant effects of GTP yogurt on human cells, levels of intracellular ROS (e.g., H2O2) in HT-29 colorectal cells were measured using the dye DCFDA, which generates a fluorescent compound upon reaction with H2O2 inside cells [Fig. 5(A)]. It has been reported that green tea possesses direct ROS scavenging activity [30]; however, little is known of the antioxidant effect of green tea yogurt on human colon cells. To measure the ROS scavenging activity of GTP yogurt extracts, HT-29 cells were pretreated with such extracts for 15 h, before being stimulated with 1 µg/mL LPS for 6 h to intentionally trigger intracellular ROS production. LPS is a component of gram-negative bacteria and known to increase cellular oxidative stress and inflammatory responses [31]. In the present investigation, ROS (H2O2) production was increased in LPS-treated cells, as shown in Fig. 5(A). However, LPS-induced H2O2 production was significantly lower in cells pretreated with GTP yogurt extracts, with levels of fluorescence similar to those in non-LPS-stimulated control cells [Fig. 5(A)]. Extracts of yogurt not supplemented with GTP also markedly decreased LPS-induced H2O2 production [Fig. 5(A)]. These data are in agreement with the results of our ABTS assay, which demonstrated that even plain yogurt extract exerted strong radical scavenging effects [Fig. 4(A)].
Fig. 5.
(A) Antioxidant effects of extracts of green tea powder (GTP) yogurt on human colorectal cells. Cells were pretreated with extracts of 0, 1, 2, or 3% GTP (w/v) yogurts for 15 h, before being stimulated with 1 µg/mL lipopolysaccharide (LPS) for 6 h, and stained with the fluorogenic dye DCFDA to detect H2O2 production. The intensity of green fluorescence was assessed using a fluorescence microscope and analyzed with ImageJ. The images shown here are representative of three independent experiments. (B) Western blot analysis of the antioxidant proteins Nrf2 and HO-1 in HT-29 cells. Protein expression in cells treated for 15 h with yogurt extract supplemented with 0, 1, 2, or 3% GTP (w/v) was measured. GAPDH levels were measured as a loading control. The blot shown is representative of three independent experiments. Protein bands were quantified using ImageJ software. (C) mRNA expression of IL-1β and TNF-α in HT-29 cells. Cells were pretreated with extracts of 0, 1, 2, or 3% GTP (w/v) yogurts for 15 h, followed by stimulation with 1 µg/mL LPS for 12 h. The mRNA expression levels were measured using quantitative real-time PCR technique. GAPDH was used as a control gene. In all graphs, values are mean ± SEM (n = 3). *Significant differences versus control (p < 0.05). #Significant differences versus LPS-only group (p < 0.05)
To identify the intracellular mechanisms responsible for these findings, the expression of antioxidant proteins was measured. Nrf2, an antioxidant transcription factor, plays a major role in the reduction of cellular oxidative stress. Upon binding of this transcription factor to antioxidant response elements in its target genes in the nucleus, several antioxidant enzymes are transcriptionally activated, including HO-1 [32]. Therefore, in the present study, the expression of Nrf2 and HO-1 proteins in HT-29 cells treated for 15 h with yogurt extracts supplemented with 0, 1, 2, or 3% GTP was tested by Western blotting. GTP increased Nrf2 and HO-1 expression in a dose-dependent manner [Fig. 5(B)]. Moreover, our results indicated that the components of GTP were responsible for such altered expression, as extracts of control yogurt did not significantly increase levels of these proteins. According to previous reports, EGCG, the most abundant compound in green tea, is responsible for this increased antioxidant protein expression. For example, treatment of cells with EGCG results in increased nuclear translocation and expression of Nrf2 and HO-1 in human mammary epithelial cells [33]. Taken together, these results suggest that yogurt extract supplemented with GTP can suppress ROS production through increased expression of the antioxidant proteins Nrf2 and HO-1 in human colon cells.
Since antioxidant effect is normally linked to anti-inflammatory effect, the level of mRNA for pro-inflammatory cytokine was measured using quantitative real-time PCR technique. LPS was used to induce inflammatory responses in HT-29 cells. Treatment of cells to LPS increased mRNA levels of IL-1β and TNF-α in HT-29 cells [Fig. 5(C)]. However, pretreatment of cells to yogurt extracts significantly decreased LPS-induced expression of IL-1β and TNF-α, in GTP concentration-dependent manner [Fig. 5(C)]. In general, LPS binds to toll-like receptor4 which normally results in activation of inflammatory response through mitogen-activated protein kinases and NF-κB pathway. These cellular events lead to the production of pro-inflammatory cytokines such as TNF-α and IL-1β [34]. However, in an alternative pathway, LPS can induce cellular inflammation such as expression of TNF-α and IL-1β by producing ROS upon activation of NADPH oxidase [35]. In the same context, control yogurt was able to lower LPS-induced ROS production and upregulated antioxidant proteins (Nrf2 and HO-1). The supplementation of GTP during yogurt fermentation further provided these positive cellular effects as shown in the current study. As a result, the anti-inflammatory properties of GTP yogurt were due to the decreased ROS generation, the increase of antioxidant proteins (i.e., Nrf2 and HO-1) and the subsequent decrease of proinflammatory cytokines (i.e., TNF-α and IL-1β).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Masood MI, Qadir MI, Shirazi JH, Khan IU. Beneficial effects of lactic acid bacteria on human beings. Crit. Rev. Microbiol. 2011;37:91–98. doi: 10.3109/1040841X.2010.536522. [DOI] [PubMed] [Google Scholar]
- 2.Farvin KS, Baron CP, Nielsen NS, Jacobsen C. Antioxidant activity of yoghurt peptides: Part 1-in vitro assays and evaluation in ω-3 enriched milk. Food Chem. 2010;123:1081–1089. doi: 10.1016/j.foodchem.2010.05.067. [DOI] [Google Scholar]
- 3.Aloğlu HŞ, Öner Z. Determination of antioxidant activity of bioactive peptide fractions obtained from yogurt. J. Dairy Sci. 2011;94:5305–5314. doi: 10.3168/jds.2011-4285. [DOI] [PubMed] [Google Scholar]
- 4.da Silva Pinto M. Tea: A new perspective on health benefits. Food Res. Int. 2013;53:558–567. doi: 10.1016/j.foodres.2012.12.043. [DOI] [Google Scholar]
- 5.Yang J, Liu RH. The phenolic profiles and antioxidant activity in different types of tea. Int. J. Food Sci. Technol. 2013;48:163–171. doi: 10.1111/j.1365-2621.2012.03173.x. [DOI] [Google Scholar]
- 6.Suzuki Y, Miyoshi N, Isemura M. Health-promoting effects of green tea. Proc. Jpn. Acad. Ser. B-Phys. Biol. Sci. 2012;88:88–101. doi: 10.2183/pjab.88.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Provan G, Helliwell K, Ransom W. The functional benefits of flavonoids: the case of tea. Phytochem. Funct. Food. 1 (2003)
- 8.Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013;24:1415–1422. doi: 10.1016/j.jnutbio.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Muniandy P, Shori AB, Baba AS. Influence of green, white and black tea addition on the antioxidant activity of probiotic yogurt during refrigerated storage. Food Packag. Shelf Life. 2016;8:1–8. doi: 10.1016/j.fpsl.2016.02.002. [DOI] [Google Scholar]
- 10.Dönmez Ö, Mogol BA, Gökmen V. Syneresis and rheological behaviors of set yogurt containing green tea and green coffee powders. J. Dairy Sci. 2017;100:901–907. doi: 10.3168/jds.2016-11262. [DOI] [PubMed] [Google Scholar]
- 11.Shori A, Baba A. Antioxidant activity and inhibition of key enzymes linked to type-2 diabetes and hypertension by Azadirachta indica-yogurt. J. Saudi Chem. Soc. 2013;17:295–301. doi: 10.1016/j.jscs.2011.04.006. [DOI] [Google Scholar]
- 12.Oliveira RP, Florence AC, Silva RC, Perego P, Converti A, Gioielli LA, Oliveira MN. Effect of different prebiotics on the fermentation kinetics, probiotic survival and fatty acids profiles in nonfat symbiotic fermented milk. Int. J. Food Microbiol. 2009;128:467–472. doi: 10.1016/j.ijfoodmicro.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Keogh MK, O’Kennedy BT. Rheology of Stirred Yogurt as Affected by Added Milk Fat. Protein and Hydrocolloids. J. Food Sci. 1998;63:108–112. [Google Scholar]
- 14.Sung JM, Choi HY. Effect of mulberry powder on antioxidant activities and quality characteristics of yogurt. J. Korean Soc. Food Sci. Nutr. 2014;43:690–697. doi: 10.3746/jkfn.2014.43.5.690. [DOI] [Google Scholar]
- 15.Jaziri I, Slama MB, Mhadhbi H, Urdaci MC, Hamdi M. Effect of green and black teas (Camellia sinensis L.) on the characteristic microflora of yogurt during fermentation and refrigerated storage. Food Chem. 2009;112:614–620. doi: 10.1016/j.foodchem.2008.06.017. [DOI] [Google Scholar]
- 16.Amirdivani S, Baba AS. Changes in yogurt fermentation characteristics, and antioxidant potential and in vitro inhibition of angiotensin-1 converting enzyme upon the inclusion of peppermint, dill and basil. LWT-Food Sci. Technol. 2011;44:1458–1464. doi: 10.1016/j.lwt.2011.01.019. [DOI] [Google Scholar]
- 17.do Espírito Santo AP, Perego P, Converti A, Oliveira MN. Influence of milk type and addition of passion fruit peel powder on fermentation kinetics, texture profile and bacterial viability in probiotic yoghurts. LWT - Food Sci. Technol. 47: 393–399 (2012)
- 18.Cais-Sokolińska D, Pikul J. Proportion of the microflora of Lactobacillus and Streptococcus genera in yoghurts of different degrees of condensation. Bull. Vet. Inst. Pulawy. 2004;48:443–447. [Google Scholar]
- 19.Reygaert WC. The antimicrobial possibilities of green tea. Front. Microbiol. 5 (2014) [DOI] [PMC free article] [PubMed]
- 20.Amirdivani S, Baba AS. Green tea yogurt: major phenolic compounds and microbial growth. J. Food Sci. Technol. 2015;52:4652–4660. doi: 10.1007/s13197-014-1670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Najgebauer-Lejko D. Effect of green tea supplementation on the microbiological, antioxidant, and sensory properties of probiotic milks. Dairy Sci. Technol. 2014;94:327–339. doi: 10.1007/s13594-014-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Najgebauer-Lejko D, Sady M, Grega T, Walczycka M. The impact of tea supplementation on microflora, pH and antioxidant capacity of yoghurt. Int. Dairy J. 2011;21:568–574. doi: 10.1016/j.idairyj.2011.03.003. [DOI] [Google Scholar]
- 23.Molan AL, Flanagan J, Wei W, Moughan PJ. Selenium-containing green tea has higher antioxidant and prebiotic activities than regular green tea. Food Chem. 2009;114:829–835. doi: 10.1016/j.foodchem.2008.10.028. [DOI] [Google Scholar]
- 24.Rice-Evans CA, Sampson J, Bramley PM, Holloway DE. Why do we expect carotenoids to be antioxidants in vivo? Free Radic. Res. 1997;26:381–398. doi: 10.3109/10715769709097818. [DOI] [PubMed] [Google Scholar]
- 25.Shori A, Baba A. Cinnamomum verum improved the functional properties of bioyogurts made from camel and cow milks. J. Saudi Soc. Agric. Sci. 2011;10:101–107. [Google Scholar]
- 26.Trigueros L, Wojdyło A, Sendra E. Antioxidant activity and protein–polyphenol interactions in a pomegranate (Punica granatum L.) yogurt. J. Agric. Food Chem. 2014;62:6417–6425. doi: 10.1021/jf501503h. [DOI] [PubMed] [Google Scholar]
- 27.Virtanen T, Pihlanto A, Akkanen S, Korhonen H. Development of antioxidant activity in milk whey during fermentation with lactic acid bacteria. J. Appl. Microbiol. 2007;102:106–115. doi: 10.1111/j.1365-2672.2006.03072.x. [DOI] [PubMed] [Google Scholar]
- 28.Amirdivani S, Baba ASH. Green tea yogurt: major phenolic compounds and microbial growth. J. Food Sci. Technol. 2015;52:4652–4660. doi: 10.1007/s13197-014-1670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014;94:329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch. Biochem. Biophys. 2010;501:65–72. doi: 10.1016/j.abb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang S, Lee JS, Lee HC, Petriello MC, Kim BY, Do JT, Lim DS, Lee HG, Han SG. Phytoncide Extracted from Pinecone Decreases LPS-Induced Inflammatory Responses in Bovine Mammary Epithelial Cells. J. Microbiol. Biotechnol. 2016;26:579–587. doi: 10.4014/jmb.1510.10070. [DOI] [PubMed] [Google Scholar]
- 32.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes. Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Na H-K, Kim E-H, Jung J-H, Lee H-H, Hyun J-W, Surh Y-J. (−)-Epigallocatechin gallate induces Nrf2-mediated antioxidant enzyme expression via activation of PI3K and ERK in human mammary epithelial cells. Arch. Biochem. Biophys. 2008;476:171–177. doi: 10.1016/j.abb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Joh E-H, Gu W, Kim D-H. Echinocystic acid ameliorates lung inflammation in mice and alveolar macrophages by inhibiting the binding of LPS to TLR4 in NF-κB and MAPK pathways. Biochem. Pharmacol. 2012;84:331–340. doi: 10.1016/j.bcp.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 35.Meng Z, Yan C, Deng Q. Gao D-f, Niu X-l. Curcumin inhibits LPS-induced inflammation in rat vascular smooth muscle cells in vitro via ROS-relative TLR4-MAPK/NF-κB pathways. Acta Pharmacol. Sin. 2013;34:901. doi: 10.1038/aps.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]