Abstract
Ensuring the safety of baby bottle teats and kitchen tools made from rubber is critical. Therefore, the migration of N-nitrosamines and N-nitrosatable substances from 30 teats and 45 kitchen tools to artificial saliva was analyzed by liquid chromatography–tandem mass spectrometry. The method was validated by assessing the limits of detection (0.46–3.87 μg/kg), limits of quantification (1.38–11.73 μg/kg), and recoveries (86.3–108.6%) of seven compounds. Nitrosodimethylamine (NDMA), N-nitrosopiperidine, and N-nitrosomorpholine (NMOR) migrated from baby bottle teats at concentrations of not detected (ND) to 3.67 μg/kg. NDMA and NMOR concentrations ranged from ND to 1.72 μg/kg after migration from 45 rubber kitchen tools. N-nitrosatable substances ranged from ND to 42.16 μg/kg after migration from baby bottle teats but did not migrate from rubber kitchen tools. All tested products were considered safe for use, as N-nitrosamine and N-nitrosatable substance levels did not exceed the permitted management specifications.
Keywords: N-nitrosamines, N-nitrosatable substances, Baby bottle teat, Rubber kitchen tool, LC–MS/MS
Introduction
N-nitrosamines, characterized by the N-nitroso functional group (> N–N=O), are usually formed from the reaction of an amine (primarily secondary amines) with a nitrosating agent (NOX) derived from either nitrite salts or nitrogen oxides [1]. Some N-nitrosamines are classified as carcinogenic by the International Agency for Research on Cancer (IARC) [2]: N-nitrosodimethylamine (NDMA) and N-nitrosodiethylamine (NDEA) are classified as Group 2A compounds (probably carcinogenic to humans), while N-nitrosodipropylamine (NDPA), N-nitrosodibutylamine (NDBA), N-nitrosopiperidine (NPIP), N-nitrosopyrrolidine (NPYR), and N-nitrosomorpholine (NMOR) are Group 2B compounds (possibly carcinogenic to humans). N-nitrosatable substances are nitrosamine precursors; when reacted with nitrite and released into solution, they undergo nitrosation to form N-nitrosamines under specific conditions [3]. N-nitrosamines and N-nitrosatable substances are present, usually in trace quantities, in various environmental media, as well as certain food products, cosmetics, and rubber products. In particular, those found in rubber products are thought to originate from certain additives used for the vulcanization of rubber [4].
European Union (EU) Directive 93/11/EEC limits the total migration of N-nitrosamines to 10 μg/kg or less and the total migration of N-nitrosatable substances to 100 μg/kg or less for rubber teats and soothers [5]. The same limits have been established in Korea [6]. The American Society for Testing and Materials (ASTM) F1313-90, the U.S. standard, allows N-nitrosamine levels of 10 μg/kg or less per chemical compound, with total amounts not exceeding 20 μg/kg [7]. There are no distinct standards for N-nitrosamines and N-nitrosatable substances in rubber kitchen tools, either in Korea or worldwide. N-nitrosamines and N-nitrosatable substances in migrated solutions are generally measured using either EN 12868, which is in line with EU regulations and is used worldwide [8], or ASTM F1313-90 as the chosen test solution preparation methods; together, these are the most widely used testing methods for N-nitrosamines in rubber teats and soothers.
Several researchers have analyzed the concentrations of N-nitrosamines in teats and soothers using techniques involving nitrogen chemiluminescence detectors (NCDs), thermal energy analyzers (TEAs), gas chromatography–mass spectrometry (GC–MS), or liquid chromatography–tandem mass spectrometry (LC–MS/MS) [9–14]. Furthermore, Motoh et al. [15] developed an improved method for detecting N-nitrosamines and N-nitrosatable substances in rubber teats and soothers. Baby bottle teats, which come into contact daily with babies, are mostly made of rubber materials, which are also widely used in current kitchen tool products. Therefore, it is necessary to monitor the safety of various baby bottle teats and kitchen tool products that are currently on the market. However, earlier studies on N-nitrosamines present in baby bottles have mainly focused on the development of analytical methods [16–18], and studies reporting the monitoring of baby bottle teats or rubber kitchen tool products are still lacking. In particular, the monitoring of N-nitrosamine migration from baby bottle teats and rubber kitchen tools has not been performed using LC–MS/MS. Therefore, the aim of this study was to assess the migration of N-nitrosamines and N-nitrosatable substances from baby bottle teats and rubber kitchen tools to artificial saliva in order to inform safety management practices in Korea.
Materials and methods
Chemicals and working standard solutions
Standard solutions (100 mg/kg in methanol) of NDMA (99.5%), NDEA (99.5%), NDPA (99.5%), NDBA (99.5%), NPIP (99.5%), NPYR (99.5%), and NMOR (99.5%) were purchased from Chem Service, Inc. (West Chester, PA, USA). A solution of N-nitrosodi-n-propylamine-d14 (NDPA-d14, 99.5%; 1000 mg/kg in methanol), used as an internal standard (IS), was also purchased from Chem Service, Inc. A mixed stock solution was prepared by dissolving the seven standard solutions in methanol to give a final concentration of 1 mg/kg for each N-nitrosamine. Working standard solutions diluted with methanol ranged from 20 to 500 μg/kg for each of the seven N-nitrosamines. All standard solutions were stored at − 5 to 5 °C in the dark until use.
Preparation of migration solution (artificial saliva)
Artificial saliva was prepared according to the method of the Ministry of Food and Drug Safety (MFDS) [18] and EN 12868 [8]. Sodium hydrogen carbonate (4.2 g), sodium chloride (0.5 g), potassium carbonate (0.2 g), and sodium nitrite (30 mg) were dissolved in deionized water (900 mL). The solution was adjusted to pH 9.0 using 0.1 M sodium hydroxide solution (NaOH) or 0.1 M hydrochloric acid solution (HCl), then transferred to a 1-L volumetric flask and made up to 1 L with deionized water.
Method validation
Linearity was confirmed with a calibration curve and the correlation coefficient (R2) of the standard solutions at 20–500 μg/kg. The σ and S values from this evaluation were determined by the slope of the calibration curve and the standard deviation of the peak area of the standard solutions, respectively. The limit of detection (LOD) and limit of quantitation (LOQ) were calculated as 3.3 σ/S and 10 σ/S, respectively [19]. Recovery was determined by adding standard mixture solutions at three concentrations to migrated solutions containing artificial saliva. Intra-day and inter-day precision was assessed using the relative standard deviations (RSD%) of recovery obtained by performing three replicates per day and three replicates on three separate days, respectively.
Preparation of samples
Thirty teat samples made from silicone rubber and natural rubber and 45 kitchen tool product samples made from silicone rubber were purchased from markets in Korea. Rubber kitchen tool products were mostly supplies designed for babies (bottles, cups, and spoons), bakeware items (baking sheets and molds), and high-temperature cooking utensils (steamers, spatulas, and ladles). The migration of N-nitrosamines and N-nitrosatable substances was assessed using the MFDS method [6]. Samples were pretreated by boiling in water for 10 min before testing; after drying, each sample was cut vertically into two pieces, and samples (10 ± 0.2 g) were exposed to artificial saliva (40 mL) at 40 °C for 24 h in the dark [20, 21]. Migration solutions were then transferred to 50-mL measuring cylinders. Each sample was then washed with 5 mL of artificial saliva, and this washing solution was added to the rest of the migration solution, along with distilled water up to 50 mL. N-nitrosamine test solutions were prepared as follows: migration solution (40 mL) was transferred to a separating funnel, and IS solution (0.5 mL), NaOH solution (1 mL, 0.1 mol/L), and dichloromethane (20 mL) were added. After vigorous shaking, the dichloromethane layer was separated from the upper layer, which was further extracted with dichloromethane (20 mL). Acetonitrile (1 mL) was added to the combined dichloromethane extracts before concentrating the solution to 1 mL via nitrogen evaporation. The N-nitrosatable substance test solutions were prepared as follows: migration solution (10 mL) was added to HCl solution (1 mL, 0.1 mol/L), then left in the dark for 30 min. Next, the solution was transferred to a separating funnel, and IS solution (0.5 mL), NaOH solution (2 mL, 0.1 mol/L), and dichloromethane (20 mL) were added. After vigorous shaking, the dichloromethane layer was separated from the upper layer, which was further extracted with dichloromethane (20 mL). Acetonitrile (1 mL) was added to the combined dichloromethane extracts before concentrating the solution to 1 mL via nitrogen evaporation. N-nitrosamine and N-nitrosatable substance test solutions concentrated to 1 mL were analyzed by LC–MS/MS after filtering with a polytetrafluoroethylene (PTFE) syringe filter.
LC–MS/MS conditions
N-nitrosamine analysis was performed using LC–MS/MS. The LC system was a Shiseido (Tokyo, Japan) separation module consisting of an SP3133 injector, SP3004 oven, and SP3201 pump. Separation was carried out on an Imtak Cadenza CD-C18 (250 mm × 2 mm, 3 μm) column at 40 °C. The LC mobile phase solvents were 0.1% formic acid in distilled water (A) and acetonitrile (B). The gradient was initiated using 20% B for 3 min and continued with a linear increase to 100% B over 7 min before being maintained at 100% B for 5 min, followed by a linear decrease to 20% B for 5 min. The flow rate was 200 μL/min, and the injection volume was 5 μL for a run time of 20 min. Detection was performed using a Thermo TSQ Quantum ULTRA (Thermo-Fisher, Waltham, MA, USA). MS/MS conditions were optimized with a spray voltage of 3800 V, capillary temperature of 330 °C, and SRM mode and collision energies of 10–40 V.
Results and discussion
Method validation
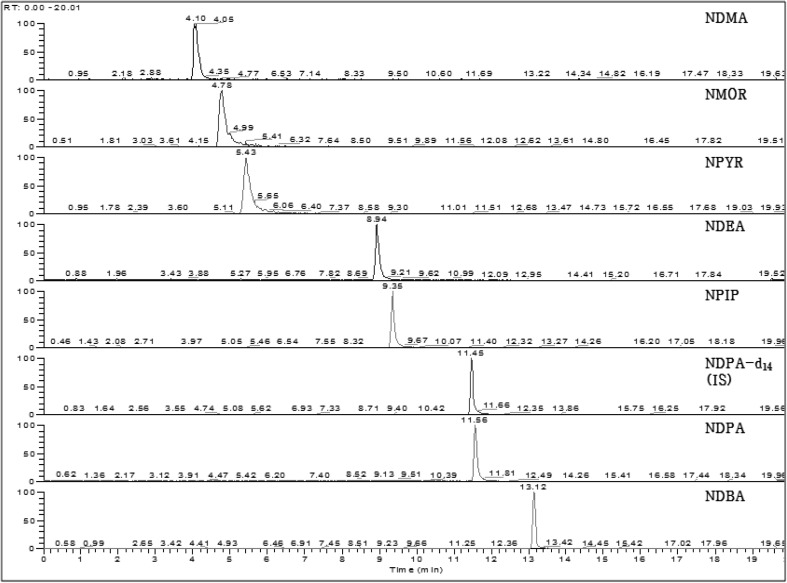
Chromatograms of NDMA, NDEA, NDPA, NDBA, NPIP, NPYR, and NMOR in a standard solution (500 μg/kg, respectively) are shown in Fig. 1 and the specific ions are shown in Table 1 analyzed by LC–MS/MS. According to the Association of Official Analytical Chemists (AOAC) guidelines, recovery assessments should be performed as a function of concentration; for a concentration of 1 mg/kg, there is an acceptable recovery range of 75–120% and repeatability (RSDr) of 8% [19]. The method was validated with good analytical results, including good linearities (r2 > 0.99), low LODs (0.46–3.87 μg/kg), and low LOQs (1.38–11.73 μg/kg) compared to the reported values of LODs (1–20 μg/kg) of N-nitrosamines [11]. Recovery and precision of intra-day and inter-day of N-nitrosamines migrated from baby bottle teats were determined with the spiked concentrations of 20, 100, and 500 μg/kg, respectively (Table 2). The recoveries were ranged from 86.3 to 108.6% and the standard deviations of recovery were less than ± 0.86 for six kinds of N-nitrosoamines except NDMA (up to ± 2.26). The precision of intra-day and inter-day of N-nitrosamines were from 0.18 to 2.08% and from 0.40 to 6.88%, respectively.
Fig. 1.
Chromatograms of N-nitrosamine standards (500 μg/kg) by LC–MS/MS
Table 1.
LOD, LOQ, and specific ions of N-nitrosamines detected by LC–MS/MS
| N-nitrosamine | LOD (μg/kg) | LOQ (μg/kg) | Precursor ion (m/z) | Fragment ion (m/z) |
|---|---|---|---|---|
| NDMA | 2.55 | 7.71 | 75.0 | 43.2 |
| NDEA | 3.87 | 11.73 | 103.0 | 43.3/75.3 |
| NDPA | 1.11 | 3.38 | 131.0 | 43.2/89.2 |
| NDBA | 2.15 | 6.51 | 159.1 | 57.2/103.2 |
| NPIP | 0.66 | 2.01 | 115.0 | 41.2/69.0 |
| NPYR | 0.46 | 1.38 | 101.1 | 55.2 |
| NMOR | 2.25 | 6.82 | 117.1 | 86.2 |
| NDPA-d14 | 145.0 | 50.8/97.0 |
Table 2.
Recovery, standard deviation (SD), and precision (relative standard deviation, RSD) of N-nitrosamines migrated from baby bottle teats
| N-Nitrosamine | Spiked level (μg/kg) | Recovery ± SD (%) | Precision (RSD %) | |
|---|---|---|---|---|
| Intra-day | Inter-day | |||
| NDMA | 20 | 108.6 ± 2.26 | 2.08 | 4.22 |
| 100 | 101.2 ± 0.79 | 0.79 | 3.00 | |
| 500 | 86.31 ± 1.57 | 1.82 | 6.88 | |
| NDEA | 20 | 101.3 ± 0.43 | 0.42 | 2.81 |
| 100 | 101.7 ± 0.40 | 0.39 | 2.50 | |
| 500 | 100.1 ± 0.18 | 0.18 | 0.40 | |
| NDPA | 20 | 101.0 ± 0.86 | 0.86 | 2.49 |
| 100 | 101.2 ± 0.50 | 0.50 | 2.32 | |
| 500 | 101.6 ± 0.18 | 0.18 | 4.86 | |
| NDBA | 20 | 104.2 ± 0.59 | 0.59 | 5.51 |
| 100 | 102.8 ± 0.50 | 0.50 | 1.69 | |
| 500 | 100.7 ± 0.41 | 0.41 | 0.84 | |
| NPIP | 20 | 104.3 ± 0.55 | 0.53 | 2.29 |
| 100 | 100.2 ± 0.19 | 0.19 | 1.81 | |
| 500 | 93.70 ± 0.56 | 0.50 | 4.42 | |
| NPYR | 20 | 101.8 ± 0.21 | 0.20 | 3.72 |
| 100 | 100.2 ± 0.64 | 0.64 | 3.08 | |
| 500 | 93.95 ± 0.57 | 0.61 | 3.41 | |
| NMOR | 20 | 102.7 ± 0.25 | 0.24 | 2.64 |
| 100 | 100.7 ± 0.86 | 0.86 | 1.50 | |
| 500 | 100.5 ± 0.37 | 0.36 | 0.56 | |
All data were calculated from measurements carried out in triplicate
Migration of N-nitrosamines
The migration of N-nitrosamines was measured from 30 teats made from silicone rubber and natural rubber and from 45 kitchen tool products made from silicon rubber (Table 3). Among baby bottle teats, the migration of three N-nitrosamines (NDMA, NPIP, and NMOR) was detected at concentrations ranging from 0.38 to 3.67 μg/kg with 17 positive samples of total N-nitrosamine migration. Migrated NDMA concentrations ranged from 1.02 to 3.67 μg/kg in 11 samples, while NPIP and NMOR concentrations ranged from 0.38 to 0.55 and 0.89 to 1.96 μg/kg, respectively, in three samples. Among the rubber kitchen tools, concentrations of NDMA ranged from 1.07 to 1.72 μg/kg in five migrated samples from baby products and ranged from 1.38 to 1.67 μg/kg in three migrated samples from bakeware, while NMOR was found at 0.94 μg/kg from only one cooking utensil sample. Overall, NDMA was the most commonly found among the seven N-nitrosamines. There are no N-nitrosamines exceeding the concentration permitted by specific legislation, such as that enacted by the MFDS and EU, which limits the concentration to 10 μg/kg in baby bottle teats [5, 18]. In comparison, Motoh et al. [14] and Bouma et al. [4] reported detectable levels of five N-nitrosamines in rubber sheets (from 2.1 to 15 μg/kg), all of which were present at higher levels than those in this study.
Table 3.
Mean concentrations of N-nitrosamines migrated from baby bottle teats and rubber kitchen tools
| Sample | Number of sample | NDMA (μg/kg) | NPIP (μg/kg) | NMOR (μg/kg) | |
|---|---|---|---|---|---|
| Baby bottle teat | 30 | 2.17 ± 0.73 (11)a (1.02–3.67)b |
0.38 (1) 0.52 (1) 0.55 (1) |
0.89 (1) 0.98 (1) 1.96 (1) |
|
| Kitchen tool | Baby product | 18 | 1.40 ± 0.24 (5)a (1.07–1.72)b |
NDc | ND |
| Bakeware | 11 | 1.38 (1) 1.53 (1) 1.67 (1) |
ND | ND | |
| Cooking utensil | 16 | ND | ND | 0.94 (1) | |
aMean ± SD for the positive samples (number of positive samples in parenthesis)
bRange from minimum to maximum
cND not detected or below LOQ
Migration of N-nitrosatable substances
Levels of N-nitrosatable substances were measured in the same 30 teats and 45 kitchen tool products as above (Table 4). N-nitrosatable substances are categorized by the N-nitrosamines that they form. NDMA-forming N-nitrosatable substances were found in concentrations of 4.70 μg/kg from one baby bottle teat and 42.16 μg/kg from the other one baby bottle teats. NPIP-, and NMOR-forming N-nitrosatable substances were found in concentrations of 1.39 and 5.77 μg/kg from each of one baby bottle teat, respectively. The total migration of N-nitrosatable substances from the four positive samples ranged from 1.39 to 42.16 μg/kg, which does not exceed the concentration permitted by specific legislation, such as that enacted by the MFDS and EU, which limits N-nitrosatable substances to 100 μg/kg in baby bottle teats (5,18). N-nitrosatable substances were not found in any rubber kitchen tools. The large variation of positive concentrations occurred because of different origins of samples. Motoh et al. [14] and Bouma et al. [4] reported N-nitrosatable substances at levels of 30 to 17,000 μg/kg in rubber sheets and at levels of 8.2–55 μg/kg in rubber teats, all of which are higher than the levels observed in this study.
Table 4.
Concentrations of N-nitrosatable substances migrated from baby bottle teats and rubber kitchen tools
| Sample | Number of sample | NDMA (μg/kg) | NPIP (μg/kg) | NMOR (μg/kg) |
|---|---|---|---|---|
| Baby bottle teat | 30 | 4.70 (1)a 42.16 (1) |
1.39 (1) | 5.77 (1) |
| Kitchen tool | 45 | NDb | ND | ND |
aNumber of positive samples in parenthesis
bND not detected or below LOQ
In conclusion, the migration of N-nitrosamines and N-nitrosatable substances from baby bottle teats and rubber kitchen tools was investigated. Samples from 30 baby bottle teats and 45 kitchen tool products were monitored and determined to be safe in terms of N-nitrosamines and N-nitrosatable substances, as levels did not exceed the concentrations permitted by management specifications. Three N-nitrosamines (NDMA, NPIP, and NMOR) were found among migrated samples from baby bottle teats, while two (NDMA and NMOR) were found among rubber kitchen tools. The migration results for N-nitrosamines and N-nitrosatable substances in this study could be used in determining a scientific basis for the safe management of food contact materials.
Acknowledgements
This research was supported by a Grant from the Korea Ministry of Food and Drug Safety (14161MFDS015).
Compliance with ethical standards
Conflict of interest
No potential conflicts of interest are reported by the authors.
Contributor Information
Se-Jong Park, Email: sjpark517@korea.kr.
Mi-Jin Jeong, Email: alwls812@hanmail.net.
So-Ra Park, Email: psr6658@hanmail.net.
Jae Chun Choi, Email: chjatu@korea.kr.
Heeju Choi, Email: iamheeju@korea.kr.
MeeKyung Kim, Phone: +82-43-719-4351, Email: mkim@korea.kr.
References
- 1.Challis BC. Nutrition and nitrosamine formation. J. Proc. Nutr. Soc. 1985;44:95–100. doi: 10.1079/PNS19850015. [DOI] [PubMed] [Google Scholar]
- 2.IARC. List of Classifications. International Agency Research on Cancer, Lyon, France (2015). Available from: http://monographs.iarc.fr/ENG/Classification/latest_classif.php.
- 3.CEN. EN 12868: Child use and care articles–methods for determining the release of N-nitrosamines and N-nitrosatable Substances from elastomer or rubber teats and soothers. European Committee for Standardization (1999).
- 4.Bouma K, Nab FM, Schothorst RC. Migration of N-nitrosamines, N-nitrosatable substances and 2-mercaptobenzthiazol from baby bottle teats and soothers: a Dutch retail survey. Food Addit. Contam. 2003;20:853–858. doi: 10.1080/0265203031000156105. [DOI] [PubMed] [Google Scholar]
- 5.EU. Commission Directive 93/11/EEC. Concerning the release of the N-nitrosamines and N-nitrosatable substances from elastomer or rubber teats and soothers. Official Journal of European Union L93/37 (1993). Available from: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31993L0011&from=en.
- 6.ASTM. Standard specification for volatile N-nitrosamine levels in rubber nipples on pacifiers. F1313-90. American Society for Testing and Materials (2011). Available from: http://filesinstrument.com.cn/bbs/upfile/2007113010288.pdf; 10.1520/f1313-90r99.
- 7.European Committee for Standardization. Child use and care articles. Methods for determining the release of N-nitrosamines and N-nitrosatable substances from elastomer or rubber teats and soothers. EN 12868 (1999).
- 8.Masahiro T, Kazuko M, Hideo Y, Yohya W. Determination of volatile N-nitrosamines in rubber nipples by gas chromatography using thermal energy analyzer. J. Anal. Sci. 1986;2:577–580. doi: 10.2116/analsci.2.577. [DOI] [Google Scholar]
- 9.Nrishinha PS, Santosh C, Kushwaha Stephen WS, Steven GC. Nitrosamines in baby bottle nipples and pacifiers: occurrence, migration, and effect of infant formulas and fruit juices on in vitro formation of nitrosamines under simulated gastric conditions. J. Agric. Food Chem. 1985;33:428–433. doi: 10.1021/jf00063a026. [DOI] [Google Scholar]
- 10.Heavy DC, Fazio T. Estimation of volatile N-nitrosamines in rubber nipples for babies’ bottles. J. Food Chem. Toxicol. 1982;20:939–944. doi: 10.1016/S0015-6264(82)80232-0. [DOI] [PubMed] [Google Scholar]
- 11.Sung JH, Kwak IS, Park SK, Kim HI, Lim HS, Park HJ, Kim SH. Liquid chromatography–tandem mass spectrometry determination of N-nitrosamines released from rubber or elastomer teats and soothers. Food Addit. Contam. 2010;27:1745–1754. doi: 10.1080/19440049.2010.508184. [DOI] [PubMed] [Google Scholar]
- 12.Feng D, Wang H, Cheng X, Wang J, Ning L, Zhou Q, Zhou Y, Yang Q. Detection and toxicity assessment of nitrosamines migration from latex gloves in the Chinese market. Int. J. Hyg. Environ. Health. 2009;212:533–540. doi: 10.1016/j.ijheh.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Feng D, Yang H, Qi D, Li Z. Extraction, confirmation, and screening of non-target compounds in silicone rubber teats by purge-and-trap and SPME combined with GC-MS. Polym. Test. 2016;56:91–98. doi: 10.1016/j.polymertesting.2016.09.021. [DOI] [Google Scholar]
- 14.Motoh M, Yamaguchi M, Kawamura Y. Analysis of N-nitrosamine migration from rubber teats and soothers. J. Anal. Chem. 2013;4:277–285. doi: 10.4236/ajac.2013.46035. [DOI] [Google Scholar]
- 15.Sen NP, Kushwaha SC, Seaman SW, Clarkson SG. Nitrosamines in baby bottle nipples and pacifiers: Occurrence, migration, and effect of infant formulas and fruit juices on in vitro formation of nitrosamines under simulated gastric conditions. J Agric. Food Chem. 1985;33:428–433. doi: 10.1021/jf00063a026. [DOI] [Google Scholar]
- 16.Niessner G, Klampfl CW. Direct comparison of solid-phase extraction and solid-phase microextraction for the gas chromatographicdetermination of dibenzylamine in artificial saliva leachates from baby bottle teats. Anal. Chimi. Acta. 2000;414:133–140. doi: 10.1016/S0003-2670(00)00833-3. [DOI] [Google Scholar]
- 17.Reche F, Garrigos MC, Marin ML, Canto A, Jimenez A. Optimization of parameters for the supercritical fluid extraction in the determination of N-nitrosamines in rubbers. J Chromatog A. 2002;963:419–426. doi: 10.1016/S0021-9673(02)00549-6. [DOI] [PubMed] [Google Scholar]
- 18.MFDS. Standards and specifications for food utensil, containers and packages. 206-210. Ministry of Food and Drug Safety (2015). Available from: https://www.mfds.go.kr/eng/eng/download.do;jsessionid=mp8xvroGqonS7pLm4YMS2aPFE3a4135esS9AVWaRCcsDaaRjglpV6IphR6ABjjIu?boardCode=17837&boardSeq=70089&fileSeq=1.
- 19.AOAC. Appendix K, Part I: Guidelines for single laboratory validation of chemical methods for dietary supplements and botanicals. Association of Official Analytical Chemists (2013). Available from: http://www.eoma.aoac.org/app_k.pdf.
- 20.Bouma K, Nab FM, Schothorst RC. Migration of N-nitrosamines, N-nitrosatable substances and 2-mercaptobenzthiazol from baby bottle teats and soothers: a Dutch retail survey. Food Add. Contam. 2003;20:853–858. doi: 10.1080/0265203031000156105. [DOI] [PubMed] [Google Scholar]
- 21.Feng D, Liu L, Zhao L, Zhou Q, Tan T. Evaluation of simulant migration of volatile nitrosamines from Latex Gloves and Balloons by HS-SPME-GC-MS. J Chromatog Sci. 2012;50:733–738. doi: 10.1093/chromsci/bms057. [DOI] [PubMed] [Google Scholar]