Abstract
Significant amounts of citrus by-products remain after juice processing, which is then used to obtain pectin. The pectin industry then generates a new waste. No study has characterized this residue or suggested applications for it. The main goal of this study was to compare citrus industrial by-products that remain after juice (CJB) and pectin (CPB) extraction, aiming to obtain bioactive compounds. The residues were evaluated for their chemical composition, antioxidant capacity, and polyphenols content. CJB had 2-fold higher total phenols than CPB. Moreover, CJB exhibited higher antioxidant capacity than CPB. Nine polyphenols were detected; hesperidin was the main compound on both residues. CPB had higher content of polyphenols than CJB, which can be attributed to the industry procedure of pectin extraction. Thus, this study provides support for the reuse of CPB to obtain nutraceutical compounds, converting waste into added-value products.
Keywords: Agro-industrial residues, Polyphenols, Flavanones, Phenolic acids, Methoxylated flavones
Introduction
Oranges are the world’s largest fruit crop, reaching a production of ~ 71 million tons in 2014, and Brazil is the main producer (~ 17 million tons) (FAOSTAT, 2017). About 40% of this world production is for the juice industry (Delgado and Fleuri, 2015). As a consequence, millions of tons of residues are also generated, yielding approximately 45% of the original whole fruit mass, and ~ 12.8 million tons of by-products. Citrus peel is the primary waste product from juice processing, which is usually dried and either sold as raw material for pectin extraction or pelletized for animal food. Citrus peel contains several functional components, such as carotenoids, polyphenols, and limonoids; therefore, it has become an important raw material for chemical, food, and pharmaceutical industries (Bocco et al., 1998; Mamma and Christakopoulos, 2014).
Among all the bio-compounds obtained from citrus by-products, pectin is widely used as a functional ingredient for food, pharmaceutical, and cosmetic industries. Its utilization is mainly based on its gelling and stabiling properties, e.g. for production of jams, jellies, fruit juice, yogurts, milk drinks, and gels/pastes in cosmetic industry. Due to the innumerable products that use pectin as an ingredient, the worldwide consumption of this compound is estimated to be around 415 million kg per year, with a global market value of ~ 400 million Euros (Mamma and Christakopoulos, 2014). Currently, the major sources of pectin are citrus and apple juice residues, in which the yield is about 25% of the dry matter of the citrus peel (Braddock, 2004). Thus, the main problem faced by pectin industry is the generation of a considerable volume of a new waste. Pectin industries are commercially interested in achieving low environmental impact applications for their residues, by converting waste into added-value products. However, this residue composition needs to be characterize, with particular focus on its bioactive molecules.
It is well known that citrus by-products represent an important inexpensive source of polyphenols, such as flavonoids and phenolic acids. According to Bocco et al. (1998), orange peels contain about 13.5 g of flavonoids per kg of dry matter. The main flavonoids found in citrus are the glycosilated flavanones, such as hesperidin, narirutin, and naringin, which are only found in citrus. Among the aglycone forms, naringenin and hesperetin are the most important ones (Escobedo-Avellaneda et al., 2014; Tripoli et al., 2007). Other important compounds found in citrus are hydroxybenzoic acids and methoxylated flavones, e.g. ellagic acid, tangeritin, and diosmetin (Madeira Jr. and Macedo, 2015). In general, citrus phenolic compounds are associated with many benefic health effects, such as anti-inflammatory, antimicrobial, cancer prevention, inhibition of human platelet aggregation, and hypocholesterolemic effects (Khan et al., 2010; Nakajima et al., 2014). In this context, there is a growing interest in obtaining these substances, supplied as natural food components (replacing synthetic antioxidants and antimicrobials) or as specific preventive pharmaceuticals and nutraceuticals. Thus, the sustainable exploration of citrus pectin waste to recover these valuable compounds will reduce the amount of residue disposal and may be even a more economically interesting source than citrus juice by-products.
Until now, studies on citrus bioactive compounds have focused in citrus juice and its by-products (CJB). However, the waste remaining after the citrus pectin is obtained (CPB) has not been previously characterized. For the reasons presented above, the aim of this study was to investigate the potential use of this new CPB residue to obtainment valuable compounds. Thus, the chemical composition, antioxidant effectiveness, and polyphenol content of CJB and CPB residues were investigated. In addition, the residues were compared to each other to better understand the effect of industry processes used to obtain pectin on citrus bioactive compounds.
Materials and methods
Materials
Gallic acid, narirutin, hesperidin, hesperetin, naringin, naringenin, ellagic acid, tangeritin, diosmetin, 2,2′-azobis(2-methylpropionamidine) (AAPH), 2,2-diphenyl-1-picrylhydrazyl (DPPH), fluorescein, and trolox were purchased from Sigma–Aldrich (St. Louis, MO, USA). Folin Ciocalteu’s reagent, monobasic and dibasic sodium phosphate, sodium carbonate, and formic acid were purchased from Dinâmica Química Contemporânea (Diadema, SP, Brazil). LC grade methanol was purchased from JT Baker (Center valley, PA, USA). All other chemicals were used in analytical grade.
Citrus by-products
Two citrus residues were used in this study. They were supplied by CP Kelco Industry Headquarters (Limeira, SP, Brazil), which specializes in pectin production. The citrus juice by-products (CJB) were derived from orange juice production (flavedo, albedo, and seeds from Citrus latifolia and four cultivars from Citrus sinensis: Hamlin, Valencia, Pera riu, and Pera Natal). The citrus pectin by-products (CPB) are the residues that remained after citrus pectin extraction from CJB. Both residues were dried at 70 °C in a convection oven (Tecnal, Brazil), crushed in a blender (OXY, Brazil), and sieved at 0.80 mm (Bertel Metallurgical Industries Ltda, Brazil).
Chemical composition of residues
All chemical analyses of citrus residues were performed according to the Association of Official Analytical Chemists (1995). Moisture and fat content were determined gravimetrically. Fat in the samples were extracted following the method of Bligh and Dyer (1959). The total protein was determined by Kjeldahl nitrogen determination, using the factor 6.25 to protein conversion. Ash content was determined by mineralization of the samples at 450 °C. The content of carbohydrate was calculated based on the difference (100—moisture—fat—protein—ash). The experiments were carried out in triplicate, and the results of each component were expressed as percentage (%) based on dry matter.
Polyphenols extraction
The phenolic compounds of the citrus by-products were extracted with aqueous-ethanol solution (1:1) according to Nakajima et al. (2016). One gram of the dried citrus residues was added to 25 mL of water–ethanol mixture. Then, the samples were treated for 15 min in ultrasonic bath at 30 °C and in shaker for 15 min at 200 rpm. After that, the samples were filtered on Whatman paper (number 1), and the products obtained were concentrated on a rotary evaporator at 40 °C for 15 min to remove the ethanol. The aqueous solutions were freeze-dried and frozen until the analyses.
Total polyphenols content
The total polyphenols content (TPC) of the lyophilized citrus extracts was measured by Folin–Ciocalteu method, according to Singleton et al. (1999). Gallic acid standard or test samples were mixed with Folin–Ciocalteu phenol reagent in the dark. After 3 min, Na2CO3 was added and mixed. Then, the mixtures were kept in the dark at room temperature for 2 h, and the assay was carried out on a NovoStar Microplate reader (BMG LABTECH, Germany) with absorbance filters of 725 nm wavelength. Gallic acid was used as a standard to calibration curve construction; its concentration ranged from 15 to 300 µg mL−1. Results were expressed as mg of gallic acid equivalents in 100 g of dry matter (GAE 100 g−1 DM).
Antioxidant capacity
DPPH radical scavenging activities of lyophilized citrus extracts were determined following the method of Macedo et al. (2011). The reaction mixtures containing the standard trolox or test samples were added to DPPH solution. The decolorizing process was recorded for 90 min of reaction, and the assay was carried out on a microplate reader with absorbance filters of 520 nm wavelength. The DPPH radical-scavenging activity was evaluated by trolox calibration curve. The results were expressed as µmol of trolox equivalent per g of dry matter (µmol TE g−1 DM).
ORAC assay was performed using the fluorescent probe fluorescein (FL), as described by Ferreira et al. (2013). Trolox standard or test samples were distributed in black-walled 96-well plate, followed by the addition of fluorescein. The reaction was initiated by adding the AAPH solution. The fluorescence was monitored every 56 s for 75 min in a microplate reader at 37 °C with excitation filter of 485 nm and emission filter of 520 nm. All preparation of reagents and samples was done in sodium phosphate buffer 75 mM (pH 7.4). Results were calculated by the difference between the area under the FL decay curve of the samples and the blank (net AUC). Regression equations between net AUC and samples concentration were made. Trolox was used as standard to construct calibration curve, and the results were expressed as µmol of Trolox equivalent per g of DM (µmol TE g−1 DM).
Identification and quantification of main citrus polyphenols by HPLC–DAD
Citrus extracts and standards were combined with 70% methanol solution. All phenolic compounds were quantified using a Dionex UltiMate 3000 (Dreieich, Germany) liquid chromatography system, equipped with a C-18 Acclaim 120 column (Dionex, 3 μm, 4.6 × 150 mm) maintained at 30 °C. The method was adapted by Madeira Jr. and Macedo (2015). The mobile phases were A, H2O (0.1% of formic acid); and B, Methanol (0.1% of formic acid). The gradient elution was 90% A (0–5 min), 20% A (5–80 min), 90% A (80–85 min), and 90% A, with a flow rate of 0.6 mL min−1. The detection was carried out at 280 nm using a diode array detector (DAD-3000). Individual flavonoids were identified by comparison with retention time and UV–Vis spectra of the standard compounds naringin, naringenin, hesperidin, hesperitin, tangeritin, gallic acid, ellagic acid, and diosmetin. The contents of these compounds in the citrus by-products extracts were calculated using the standard calibration curves.
Statistical analysis
All measurements were performed in triplicate, and the results are presented as mean ± standard deviation (SD). The statistical difference between the samples was analyzed using t test (p ≤ 0.05). All statistical analyses were performed using Minitab 16.1.1.
Results and discussion
Chemical composition
The citrus industrial by-products remained after obtaining juice (CJB) and after pectin extraction (CPB) were studied and compared to each other for their chemical composition, antioxidant activity, and phenolic content. Table 1 provides the chemical composition of CJB and CPB. The results demonstrate that the residues had similar composition of fat and protein, slightly differed in moisture and carbohydrate concentration. The main difference was the mineral content, indicated by ash values. The CJB residue had mineral composition > 4-fold higher than the CPB. The hypothesis for this observation could be the capacity of pectin to absorb and maintain other substances e.g. minerals (Xu et al., 2008). Thus, the procedure used to obtain pectin also extracts minerals, so the residue left after pectin extraction has lower ash content. According to citrus pectin yields (~ 18%, data provided by the company of the residues), Fig. 1 demonstrates the material balance of the chemical composition of CJB and CPB, during the process of pectin obtainment.
Table 1.
Chemical composition of different citrus by-products
| Components (g/100 g) | Citrus by-products | p value | |
|---|---|---|---|
| CJB | CPB | ||
| Moisture | 7.92 ± 0.09a | 7.23 ± 0.10b | 0.003 |
| Literature* | 9.17–10.55 | – | |
| Ash | 2.81 ± 0.01a | 0.56 ± 0.02b | 0.0001 |
| Literature* | 3.68–3.75 | – | |
| Protein | 6.67 ± 0.08a | 6.76 ± 0.40a | 0.741 |
| Literature* | 9.80 | – | |
| Fat | 3.81 ± 0.11a | 4.15 ± 0.45a | 0.331 |
| Literature* | 1.60–1.88 | – | |
| Carbohydrate | 78.79 ± 0.52b | 81.30 ± 0.06a | 0.015 |
| Literature* | 75.68–77.78 | – | |
Fig. 1.
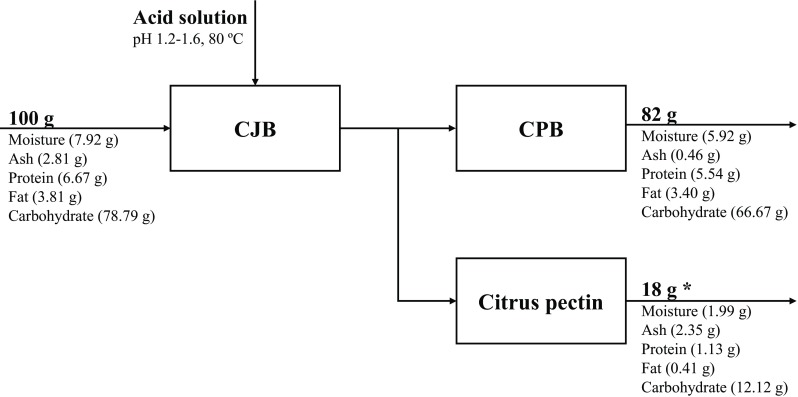
Material balance of the chemical composition of CJB and CPB (*Data provided by the company of the citrus residues)
Many studies have already evaluated CJB residue, reporting its composition, reuse, and new methods to obtain pectin and its bioactive compounds. O’Shea et al. (2015) and Senevirathne et al. (2009) studied the chemical composition of citrus juice by-products. The composition found in our CJB was similar, or slightly differed, from the results reported by the authors.
The study of Aravantinos-Zafiris et al. (1994) is the only literature that reports the use of CPB. According to them, the chemical composition of CPB in dry basis was 87% carbohydrate, 6.9% protein, 3.3% ash, and 0.4% fat. They also determined that the carbohydrate fraction mainly corresponded to fibers (83.9% of dry matter), and Ca, Mg, P, and K were the predominant minerals. Their results mainly differed from ours in fat and ash content. The reason can be attributed to differences in citrus growing conditions, such as the soil characteristics and maturity of the fruits, or because of the different process of pectin extraction used by the authors, which may influenced the final composition of the residue.
Aravantinos-Zafiris et al. (1994) proved CPB to be a useful source of fiber for food or feed ingredient. However, its bioactive potential was not taken into account and is still unknown. Thus, studying the phenolic composition of CPB and its antioxidant potential could provide greater added value to this residue.
Total polyphenols content and antioxidant capacity
The total phenols content and the antioxidant properties of the citrus by-products were assessed using the assays of TPC by Folin–Ciocalteu method, DPPH radical scavenging capacity, and oxygen radical absorption capacity (ORAC). Table 2 shows the results for extract yields, TPC, DPPH, and ORAC assays compared with literature. The results indicated that CJB is approximately 2-fold higher in extraction yield and TPC than CPB (Table 2). Our TPC results also exceeded the values in orange peels obtained by Anagnostopoulou et al. (2006) (3.63–254 mg GAE/100 g DM) and in fresh orange peels by Casquete et al. (2015) (284 mg GAE/100 g). However, when compared to flavedo extracts, our results presented lower content than those found by Escobedo-Avellaneda et al. (2014) (588.6–679.9 mg GAE/100 g DM). According to Abeysinghe et al. (2007), the content and composition of phenolic compounds vary in different citrus tissues. In this context, the CJB residue used in this study contains peel (flavedo and albedo), pulp (juice sac residue), rag (membranes and cores), seeds, and pectin. Thus, the composition of different residues may make it difficult to compare our results with other data.
Table 2.
Yield, total phenolic content, and antioxidant capacity of citrus by-product extracts, CJB and CPB
| Citrus by-product | p value | ||
|---|---|---|---|
| CJB | CPB | ||
| Yield (%) | 7.37 | 3.31 | – |
| TPC (mg GAE/100 g DM) | 386 ± 23a | 170 ± 10b | 0.0001 |
| DPPH (µmol TE/g DM) | 11,035 ± 549a | 2571 ± 146b | 0.0001 |
| Literature* | 9188 ± 187 | – | – |
| ORAC (µmol TE/g DM) | 91,570 ± 12,153a | 37,588 ± 6207b | 0.002 |
| Literature* | 90,075 ± 9132 | – | – |
CJB citrus juice byproducts, CPB citrus pectin by-products, DM dry matter, GAE gallic acid equivalent, TE trolox equivalent
a,bValues (mean ± SD) within different letters in the line significantly differ by t test (p ≤ 0.05)
*Madeira Jr and Macedo (2015)
Usually, phenolic compounds are extracted with organic solvent, such as methanol and acetone. However, in industry, the methanol application is unfeasible due to its toxicity. According to Dahmoune et al. (2013), the type of solvent significantly influences the TPC results, and aqueous-ethanol is the best extractive solution for polyphenols. Results obtained by Nakajima et al. (2016) confirmed the potential of aqueous-ethanol (1:1) solution for phenolics extractions. In this study, we chose to use the same solution, aiming to obtain higher polyphenols, lower solvent use, and less harmful to health solvent.
Previous studies reported that the high presence of phenolic compounds contributes to stronger antioxidant activity in citrus fruits (Sun et al. 2013; Xu et al., 2008). This observation is consistent with our results, in which CJB exhibited higher antioxidant activity and higher TPC than CPB (Table 2). In addition, the ORAC and DPPH values in CJB were similar to those reported by Madeira Jr. and Macedo (2015) for citrus juice residues (Table 2).
In citrus peel, Xi et al. (2014) obtained values from 9.10 to 19.75 µmol TE g−1 of DW in DPPH and 126.6–331.29 µmol TE g−1 of DW in ORAC. Regarding DPPH, Casquete et al. (2015) found 102.39 mg TE 100 g−1 of fresh orange peels. In this context, CJB and CPB appear to have higher and stronger antioxidant capacity.
According to Ferreira et al. (2013), orange juice is recognized as a rich source of antioxidants. However, it appears to have lower TPC and antioxidant activity than its by-products (Escobedo-Avellaneda et al., 2014). Ferreira et al. (2013) found in orange juice TPC values of 6.7 mg GAE 100 mL−1 and antioxidant activity equivalent to 5319 µmol TE L−1 (DPPH assay) and 18,750 µmol TE L−1 (ORAC assay). According to the USDA (2017) database, orange juice presents TPC of 67 mg GAE 100 g−1 and antioxidant activity of 726 μmol TE 100 g−1 (ORAC assay). Thus, the data found in this study indicates that citrus and pectin by-products can be considered important and higher sources of polyphenols and antioxidant compounds than orange juice.
Polyphenolic composition
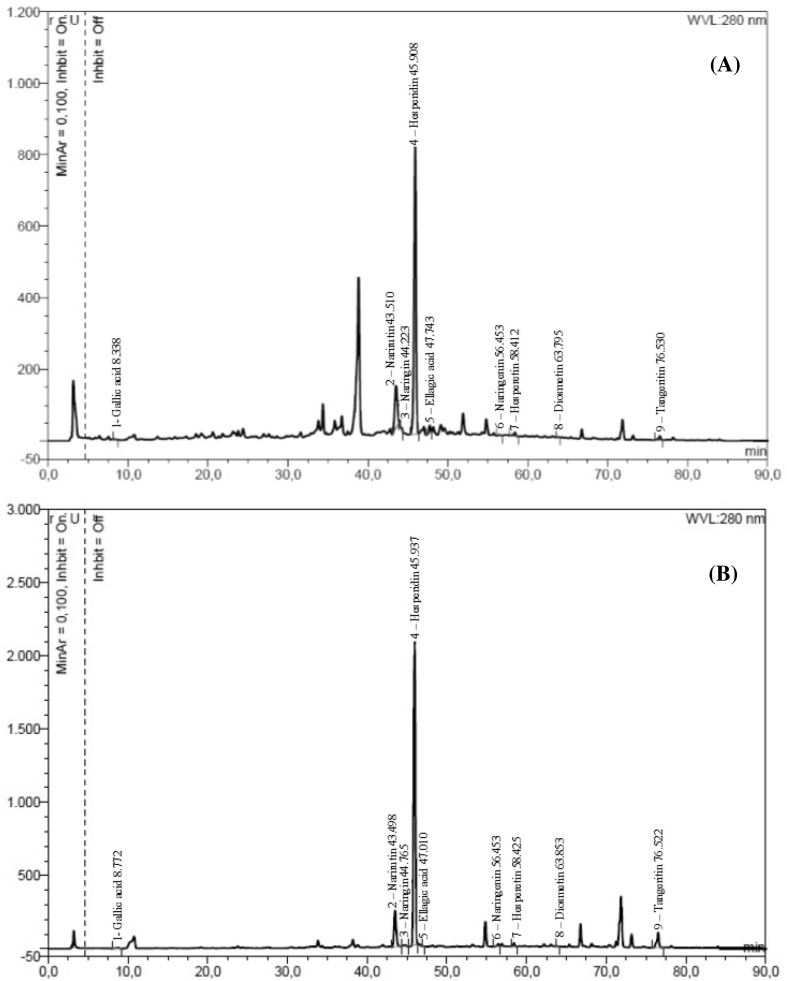
Some of the most important citrus polyphenols compounds were detected and quantified by HPLC–DAD in CJB and CPB, and the results are summarized in Table 3. The major polyphenols present in both citrus by-products were the glycoside flavanone hesperidin, followed by narirutin (Fig. 2). Besides flavanones, CJB presented high ellagic acid content, and tangeretin was predominantly found in CPB. Gallic acid, naringin, naringenin, hesperetin, and diosmetin were found in both samples, but in low amounts.
Table 3.
Content of polyphenols (mg/100 g DM) in citrus by-products, quantified by HPLC–DAD
| Peak | Polyphenol | Citrus by-product | p value | |
|---|---|---|---|---|
| CJB | CPB | |||
| Hydroxybenzoic acids | ||||
| 1 | Gallic acid | 0.57 ± 0.02b | 1.07 ± 0.01a | 0.02 |
| – | – | |||
| 5 | Elagic acid | 10.97 ± 0.08a | 0.27 ± 0.05b | 0.004 |
| Literature* | 17.25* | – | ||
| Glycoside flavanones | ||||
| 2 | Narirutin | 29.34 ± 0.43a | 17.50 ± 0.41b | 0.022 |
| Literature* | 85.54* | – | ||
| 3 | Naringin | 1.02 ± 0.05b | 3.11 ± 0.08a | 0.021 |
| Literature* | 5.1 - 70.30* | – | ||
| 4 | Hesperidin | 232.65 ± 10.47b | 314.44 ± 18.82a | 0.033 |
| Literature* | 205.20–258.38* | – | ||
| Aglycone flavanones | ||||
| 6 | Naringenin | 0.47 ± 0.01b | 1.00 ± 0.09a | 0.015 |
| Literature* | 0.13* | – | ||
| 7 | Hesperetin | 1.05 ± 0.04a | 0.91 ± 0.05a | 0.108 |
| Literature* | 0.13* | – | ||
| Methoxyflavones | ||||
| 8 | Diosmetin | tr | 0.32 ± 0.13 | – |
| – | – | |||
| 9 | Tangeretin | 1.41 ± 0.04b | 6.07 ± 0.36a | 0.003 |
| Literature* | 6.52* | – | ||
| Total | 277.46 ± 9.90 | 344.68 ± 19.12 | ||
Fig. 2.
Chromatogram of the citrus residues CJB (A) and CPB (B) polyphenols obtained using HPLC–DAD
The obtained results are consistent with previous findings, showing that hesperidin, narirutin, naringin, and eriocitrin are the main flavonoids found in citrus; the first one being the most abundant (Khan et al., 2010). Khan et al. (2010) used similar techniques to extract phenolics from orange peel. The content of hesperidin obtained from our citrus by-products was similar and higher than those obtained by Khan et al. (2010) (205.2 mg/100 g FW). On the other hand, the naringin content obtained in CJB and CPB was lower than expected. The absence and low content of naringin in the samples can be explained by a study by Coll et al. (1998), who observed that peels and other solid residues of lemon predominantly had the flavonoids hesperidin and eriocitrin, while naringin was mainly found in liquid waste. A previous study of our group observed low content of naringin in Brazilian citrus residues (Nakajima et al., 2016). In addition, Chinapongtitiwat et al. (2013) showed that the composition and amount of flavonoids in citrus is highly linked to species, and factors such as growth conditions, harvest time, and type of processing. The other phenolic compounds, the aglycones, and phenolic acids were found in low concentrations (Table 3), in agreement with other studies (Madeira Jr. and Macedo 2015; Nakajima et al., 2014; Tripoli et al., 2007).
All the quantified phenolic compounds were significantly higher (p ≤ 0.05) in CPB than CJB, except narirutin, ellagic acid, and hesperetin. The sum of the individual levels of the polyphenols was calculated, and CPB presented the highest amount of polyphenols. In industry, the raw materials used for pectin obtainment are usually CJB and apple pomace. The extraction of pectin is accomplished by pH decrease (around 1.2–1.6), using mineral acids (hydrochloric or nitric acid) and high temperatures (around 80 °C) (Oreopoulou, 2007). Previous studies indicated that phenolic compounds could be released by simple heat treatment from citrus peel (Xu et al., 2007, 2008). Acidified extraction of orange peel polyphenols yields high content of these compounds (Hedge et al., 2015). Based on these, the industry procedure of pectin extraction may release the polyphenols compounds from the matrix, justifying the better results obtained in CPB.
In general, polyphenols compounds are considered potent antioxidant molecules. In agreement with this, the sample with higher TPC values (CJB) also presented higher antioxidant capacity (Table 2). However, the comparison between the quantified polyphenols content (Table 3) and the antioxidant capacity of the samples did not show any clear relationship. According to Granato et al. (2016), this might be because the Folin–Ciocalteu assay is not specific just to TPC, as it can also react with reducing sugars, ascorbic acid, transition metals, and reducing amino acids in the sample, which may have improved the TPC of CJB. In addition, the results of antioxidant capacity (Table 2) and polyphenols content of the extracts (Table 3) demonstrate that not only the detected polyphenols, but other compounds with reducing power must be responsible for the major bioactivity of CJB, such as vitamin C and pectic polysaccharides, which may contribute to this extract hydroxyl radical-scavenging activity (Dalonso and Petkowicz, 2012; Papoutsis et al., 2016). Thus, the hypothesis is that these bioactive compounds, which contribute to CJB antioxidant capacity, might have been lost in the pectin-extraction process, which reduced CPB antioxidant power.
According to Russo et al. (2015), the primary product of orange industry, the juice, has the lowest amount of bioactive compounds and polyphenols. Nakajima et al. (2014) observed that the main flavonoids of C. sinensis juice are hesperidin (28.6 mg 100 mL−1) and narirutin (5.2 mg 100 mL−1). However, they were found in minor concentration when compared to the CJB and CPB residues. This data reinforces the health benefits of CJB and CPB.
Because the studied residues were obtained from industrial waste and commonly employed for animal feed, their use to extract citrus bioactive compounds is a possibility to increase the commercial added-value of these residues through profitable applications. Especially polyphenols are related to many functional and nutraceutical activities, e.g. antioxidant, inhibition of juice browning, antimicrobial action, and protection against inflammatory process (González-Gómez et al., 2014; Nakajima et al., 2014). Moreover, other compounds can be investigated and exploited in these by-products, including enzymes (lipases, proteases, and peroxidases), dietary fiber, and vitamins (Delgado and Fleuri, 2015; Mamma and Christakopoulos, 2014). Thus, this study may provide significant information for a future profitable use of both residues as natural antioxidants, and to obtain polyphenols and other bioactive compounds.
In conclusion, as already known, a significant amount of citrus by-products remain after juice processing. Previous studies have shown the high presence of phenolic compounds in this residue. However, until now, studies had not been conducted on the residues that remain after citrus pectin extraction or industry application for it. Our results may prove that the treatment applied to the extraction of pectin does not promote polyphenol degradation, and the by-product obtained after this industrial procedure contains even higher content of the quantified phenolic compounds. These results provide support for the reuse of both studied residues, which can be in the future applied as ingredients to functional foods development, natural antioxidants in food to prevent oxidative alterations (replacing synthetic antioxidants), and to extract molecules with therapeutic action.
Acknowledgements
Acknowledgements for the financial support of FAPESP (grant number 2015/04555-2). The authors also appreciate the CNPq scholarships.
References
- Abeysinghe DC, Xian L, Chongde S, Wangshu Z, Chunhua Z, Kunsong C. Bioactive compounds and antioxidant capacities in different edible tissues of citrus fruit of four species. Food Chem. 2007;104:1338–1344. doi: 10.1016/j.foodchem.2007.01.047. [DOI] [Google Scholar]
- Anagnostopoulou MA, Kefalas P, Papageorgiou VP, Assimopoulou NA, Boskou D. Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis) Food Chem. 2006;94:19–25. doi: 10.1016/j.foodchem.2004.09.047. [DOI] [Google Scholar]
- Aravantinos-Zafiris G, Oreopoulou V, Tzia C, Thomopoulos CD. Fibre fraction from orange peel residues after pectin extraction. LWT - Food Sci. Technol. 1994;27:468–471. doi: 10.1006/fstl.1994.1094. [DOI] [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official methods of analysis of the AOAC International, 16th ed. Texas: Arlington, (1995).
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- Bocco A, Cuvelier M-E, Richard H, Berset C. Antioxidant activity and phenolic composition of citrus peel and seed extracts. J. Agric. Food Chem. 1998;46:2123–2129. doi: 10.1021/jf9709562. [DOI] [Google Scholar]
- Braddock RJ. Importance of byproducts to citrus juice processing. Fruit Processing. 2004;5:310–313. [Google Scholar]
- Casquete R, Sonia MC, Martín A, RuizMoyano S, Saraiva JA, María GC, Paula T. Evaluation of the effect of high pressure on total phenolic content, antioxidant and antimicrobial activity of citrus peels. Innov. Food Sci. Emerg. Technol. 2015;31:37–44. doi: 10.1016/j.ifset.2015.07.005. [DOI] [Google Scholar]
- Chinapongtitiwat V, Jongaroontaprangsee S, Chiewchan N, Devahastin S. Important flavonoids and limonin in selected Thai citrus residues. J. Funct. Foods. 2013;5:1151–1158. doi: 10.1016/j.jff.2013.03.012. [DOI] [Google Scholar]
- Coll MD, Coll L, Laencina J, Tomas-Barberan FA. Recovery of flavonons from wastes of industrially processed lemons. Eur. Food Res. Technol. 1998;206:404–407. [Google Scholar]
- Dahmoune F, Boulekbache L, Moussi K, Aoun O, Spigno G, Madani K. Valorization of citrus limon residues for the recovery of antioxidants: evaluation and optimization of microwave and ultrasound application to solvent extraction. Ind. Crops Prod. 2013;50:77–87. doi: 10.1016/j.indcrop.2013.07.013. [DOI] [Google Scholar]
- Dalonso N, Petkowicz CLO. Guarana powder polysaccharides: characterisation and evaluation of the antioxidant activity of a pectic fraction. Food Chem. 2012;134:1804–1812. doi: 10.1016/j.foodchem.2012.03.088. [DOI] [PubMed] [Google Scholar]
- Delgado CHO, Fleuri LF. Orange and mango by-products: agro-industrial waste as source of bioactive compounds and botanical versus commercial description—A review. Food Rev. Int. 2015;32(1):1–14. doi: 10.1080/87559129.2015.1041183. [DOI] [Google Scholar]
- Escobedo-Avellaneda Z, Gutiérrez-Uribe J, Valdez-Fragoso A, Torres JA, Welti-Chanes J. Phytochemicals and antioxidant activity of juice, flavedo, albedo and comminuted orange. J. Funct. Foods. 2014;6:470–481. doi: 10.1016/j.jff.2013.11.013. [DOI] [Google Scholar]
- FAOSTAT. Statistics division, food and agriculture organization of the United Nations. Available from: http://www.fao.org/faostat/en/#home. Accessed Apr. 13, 2017.
- Ferreira LR, Macedo JA, Ribeiro ML, Macedo GA. Improving the chemopreventive potential of orange juice by enzymatic biotransformation. Food Res. Int. 2013;51:526–535. doi: 10.1016/j.foodres.2013.01.018. [DOI] [Google Scholar]
- González-Gómez D, Cardoso V, Bohoyo D, Ayuso MC, Delgado-Adamez J. Application of experimental design and response surface methodology to optimize the procedure to obtain a bactericide and highly antioxidant aqueous extract from orange peels. Food Control. 2014;5:252–259. doi: 10.1016/j.foodcont.2013.07.013. [DOI] [Google Scholar]
- Granato D, Santos JS, Maciel LG, Nunes DS. Chemical perspective and criticism on selected analytical methods used to estimate the total content of phenolic compounds in food matrices. Trends Anal. Chem. 2016;80:266–279. doi: 10.1016/j.trac.2016.03.010. [DOI] [Google Scholar]
- Hedge P, Agrawal P, Gupta PK. Isolation and optimization of polyphenols from the peels of orange fruit. J. Chem. Pharm. Sci. 2015;8:463–468. [Google Scholar]
- Khan MK, Abert-Vian M, Fabiano-Tixier A-S, Dangles O, Chemat F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010;119:851–858. doi: 10.1016/j.foodchem.2009.08.046. [DOI] [Google Scholar]
- Macedo JA, Battestin V, Ribeiro ML, Macedo GA. Increasing the antioxidant power of tea extracts by biotransformation of polyphenols. Food Chem. 2011;126:491–497. doi: 10.1016/j.foodchem.2010.11.026. [DOI] [Google Scholar]
- Madeira JV, Jr, Macedo GA. Simultaneous extraction and biotransformation process to obtain high bioactivity phenolic compounds from Brazilian citrus residues. Biotechnol. Prog. 2015;31:1273–1279. doi: 10.1002/btpr.2126. [DOI] [PubMed] [Google Scholar]
- Mamma D, Christakopoulos P. Biotransformation of citrus by-products into value added products. Waste Biomass Valori. 2014;5:529–549. doi: 10.1007/s12649-013-9250-y. [DOI] [Google Scholar]
- Nakajima VA, Macedo GA, Macedo JA. Citrus bioactive phenolics: role in the obesity treatment. LWT - Food Sci. Technol. 2014;59:1205–1212. doi: 10.1016/j.lwt.2014.02.060. [DOI] [Google Scholar]
- Nakajima VA, Madeira JV, Jr, Macedo GA, Macedo JA. Biotransformation effects on anti lipogenic activity of citrus extracts. Food Chem. 2016;197:1046–1053. doi: 10.1016/j.foodchem.2015.11.109. [DOI] [PubMed] [Google Scholar]
- O’Shea N, Ktenioudaki A, Smyth TP, McLoughlin P, Doran L, Auty MAE, Arendt E, Gallagher E. Physicochemical assessment of two fruit by-products as functional ingredients: Apple and orange pomace. J. Food Eng. 2015;153:89–95. doi: 10.1016/j.jfoodeng.2014.12.014. [DOI] [Google Scholar]
- Oreopoulou V, Tzia C. Utilization of plant by-products for the recovery of proteins, dietary fibers, antioxidants, and colorants. In: Oreopoulou V, Russ W, editors. Utilization of by-products and treatment of waste in the food industry. Boston: Springer; 2007. pp. 209–232. [Google Scholar]
- Papoutsis K, Pristijono P, Golding JB, Stathopoulos CE, Scarlett CJ, Bowyer MC, Vuong QV. Impact of different solvents on the recovery of bioactive compounds and antioxidant properties from lemon (Citrus limon L.) pomace waste. Food Sci. Biotechnol. 2016;25:971–977. doi: 10.1007/s10068-016-0158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo M, Bonaccorsi I, Inferrera V, Dugo P, Mondello L. Phytochemicals and antioxidant activity of juice, flavedo, albedo and comminuted orange. J. Funct. Foods. 2015;12:150–157. doi: 10.1016/j.jff.2014.11.008. [DOI] [Google Scholar]
- Senevirathne M, Jeon Y-J, Ha J-H, Kim S-H. Effective drying of citrus by-product by high speed drying: A novel drying technique and their antioxidant activity. J. Food Eng. 2009;92:157–163. doi: 10.1016/j.jfoodeng.2008.10.033. [DOI] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteau reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Sun Y, Qiao L, Shen Y, Jiang P, Chen J, Ye X. Phytochemical profile and antioxidant activity of physiological drop of citrus fruits. J. Food Sci. 2013;78:37–42. doi: 10.1111/j.1750-3841.2012.03002.x. [DOI] [PubMed] [Google Scholar]
- Tripoli E, La Guardia M, Giammanco S, Di Majo D, Giammanco M. Citrus flavonoids: molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007;104:466–479. doi: 10.1016/j.foodchem.2006.11.054. [DOI] [Google Scholar]
- United States Department of Agriculture (USDA). Agricultural research service, USDA oxygen radical absorbance capacity (ORAC) of selected foods, release 2. Available from: http://www.ars.usda.gov/nutrientdata/orac. Accessed Apr. 15, 2017.
- Xi W, Zhang Y, Sun Y, Shen Y, Ye X, Zhou Z. Phenolic composition of Chinese wild mandarin (Citrus reticulate Balnco) pulps and their antioxidant properties. Ind. Crops Prod. 2014;52:466–474. doi: 10.1016/j.indcrop.2013.11.016. [DOI] [Google Scholar]
- Xu G, Ye X, Chen J, Liu D. Effect of heat treatment on the phenolic compounds and antioxidant capacity of citrus peel extract. J. Agric. Food Chem. 2007;55:330–335. doi: 10.1021/jf062517l. [DOI] [PubMed] [Google Scholar]
- Xu GH, Chen JC, Liu DH, Zhang YH, Jiang P, Ye XQ. Minerals, phenolic compounds, and antioxidant capacity of citrus peel extract by hot water. J. Food Sci. 2008;73:11–18. doi: 10.1111/j.1750-3841.2007.00546.x. [DOI] [PubMed] [Google Scholar]