Abstract
Chronic alcohol consumption induces damage to the brain that can cause various forms of dementia. An abundance of acetaldehyde is produced by excessive alcohol consumption and accumulates in the body to induce oxidative stress, apoptosis, and inflammation in neuronal cells, which results in learning and cognitive decline. In the present study, C57BL/N mice were orally administered alcohol (16%) and Carthamus tinctorius L. seed (CTS) (100 and 200 mg/kg/day). Behavioral experiments showed that memory and cognitive abilities were significantly higher in the CTS groups than the alcohol-treated control group in the T-maze test, novel object recognition test, and Morris water maze test. In addition, CTS inhibited alcohol-induced lipid peroxidation and nitric oxide production in the brain, kidney, and liver. Moreover, alcohol increased acetylcholinesterase activity in the brain, but this was significantly decreased by the administration of CTS. Therefore, CTS may play role in the prevention of alcohol-related dementia.
Keywords: Carthamus tinctorius L. seed, Alcohol, Dementia, Cognition, Oxidative stress
Introduction
Alcohol is known to cause various medical symptoms and has been attributed to the occurrence of alcoholic disease (Room et al., 2005). Alcoholism is one of the most prevalent causes of mental disorders worldwide, and chronic consumption may induce the deterioration of cognitive function (Grant et al., 2004). Neurological dysfunctions, including difficulties with abstract problem-solving abilities, visual and language learning and memory, perceptual motor skills, and even motor functions are commonly found in alcoholism (Harper and Matsumoto, 2005). In particular, chronic alcohol consumption is the main cause of alcohol-related dementia (ARD) (Thomas and Rockwood, 2001). Alcohol is metabolized to acetaldehyde, which causes brain damage and contributes to the progression of ARD (Holownia et al., 1996; Holownia et al., 1999; Tong et al., 2011).
Antioxidants may be beneficial for the reduction of oxidative stress, which is one of the causes of ARD. Vitamins, flavonoids, and carotenoids from various natural sources can be used to prevent or reduce the progression of ARD. Polyphenols are reported to exert antioxidant activity and to attenuate oxidative stress caused by reactive oxygen species (ROS). In particular, serotonin and kaempferol have been shown to improve cognitive performance in Alzheimer’s disease (Seyedabadi et al., 2014; Zhang et al., 2016).
Carthamus tinctorius L., which is distributed widely throughout the world, including China, India, Southern Europe, and North America, has been reported to have anti-inflammatory (Jun et al., 2011), anti-oxidative, and neuro-protective effects (Wang et al., 2014). It contains several bioactive flavonoids and polyunsaturated fatty acids such as linoleic acid. In particular, safflower yellow and carthamin, a red pigment, from C. tinctorius have been used widely as natural dyes (Hiramatsu et al., 2014). C. tinctorius L. seed (CTS) has been reported to exert anti-cancer (Hou et al., 2010), anti-oxidant (Herrera et al., 2003), anti-inflammatory effects (Bae et al., 2002), and to improve lipid metabolism (Kang et al., 1999; Roh et al., 1999; Takii et al., 1999). Lignans and flavonoids isolated from CTS, such as serotonin, kaempferol, and acacetin, showed health-beneficial activities (Cho et al., 2004). Moreover, CTS oil comprises more than 70% of linoleic acid, which is known to play a protective role in brain function (Kalmijn, 2000; Zhang et al., 2016). However, a study of the protective effects of CTS on cognitive function impaired by alcohol consumption has not yet been conducted. In this study, we investigated the neuro-protective effect of CTS on cognitive dysfunction induced by chronic alcohol intake in an in vivo mouse model.
Materials and methods
Sample preparation
CTS was obtained from Geunyang Oriental Medicine Co. (Chungcheongbuk-do, Korea). A voucher herbarium specimen has been deposited at the Department of Medicinal Crop Research, National Institute of Horticultural and Herbal Science, Rural Development Administration, and was identified by a plant systematist from the Herbal Crop Research Division. Dried CTS was pulverized and extracted twice in 70% ethanol (EtOH) by ultrasound (60 Hz, 19 °C±2 °C, 3 h). The solvent was evaporated in vacuo to give an extract with a yield of 4.65% by weight of the original CTS. The EtOH extracts from CTS were dissolved in 1% carboxymethyl cellulose sodium salt (CMC) solution.
Animals and experimental protocols
Male C57BL/6 N mice (Orient Inc., Gyeonggi-do, Korea) were given ad libitum access to food and distilled water, and maintained in a 12 h light–dark cycle at a controlled temperature (20 °C±2 °C) and humidity (50%±10%). The experimental protocols complied with the animal care and use guidelines approved by the Pusan National University-Institutional Animal Care and Use Committee (PNU-IACUC, Approval No. PNU-2017-1510). The animals were divided into four groups each consisting of nine individuals. The normal group was administered distilled water + 1% CMC solution. The alcohol-treated control group was administered 16% EtOH (Duksan Co., Gyeonggi-do, Korea) (5.0 g/kg/day) + 1% CMC solution. The CTS 100 group was administered 16% EtOH (5.0 g/kg/day) + CTS (100 mg/kg/day in 1% CMC solution). The CTS 200 group was administered 16% EtOH (5.0 g/kg/day) + CTS (200 mg/kg/day in 1% CMC solution). All reagents were administered orally by using a feeding needle. The schedule of treatment and behavioral experiments is shown in Fig. 1.
Fig. 1.
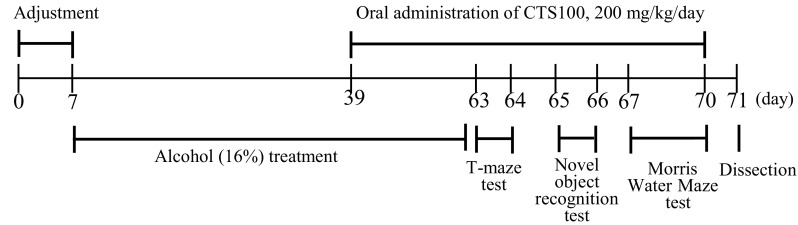
Experimental schedule of behavioral tests for mice treated with alcohol (16%). CTS Carthamus tinctorius L. seed
T-maze test
The T-maze test was performed according to the method of Montgomery (1952). The T-maze box consisted of black acrylic with no visible outside, starting arm, left arm, and right arm (width 12 cm, height 20 cm, starting arm 76 cm, right arm and left arm, 31.5 cm each). A blocking door was installed and used. On the first day, the mice were placed at the beginning of the T-maze box, the right arm was blocked, and the mice were allowed to freely navigate for 10 min. The number of times that the left arm was entered was recorded. After 24 h, the blocking door was removed, the mice were placed in the same position, and the number of entries to the old route and the new route were measured over 10 min. The spatial perception ability (%) was calculated by recording the number of entries to the whole passage and the number of entries to the left and right arm, multiplied by 100.
Novel object recognition test
For the object recognition test, a black acrylic box (40 × 40 × 40 cm3) was made to be invisible from the inside according to the method of Bevins and Besheer (2006). Two objects of the same shape and size (A, A’) were fixed in the box. On the first day, the mice were started from the center of the box and allowed to touch each object freely for 10 min, and the number of touches was recorded. After 24 h, one of the two objects was changed to a new object (A, B), and the mice were started at the same position. The number of touches of the old object (A) and the new object (B) was observed. The object cognitive ability (%) was calculated from the comparison of the number of touches of both objects with the number of touches of the old object and the new object.
Morris water maze test
The Morris water maze test was conducted according to the experimental method of Morris (1984). The round water pool (150 cm in diameter, 30 cm in height) used in the experiment was divided into four quadrants and marked with different marks at each position. The temperature of the water was maintained at 22 °C±1 °C and non-toxic white paint was mixed with water to make it opaque, so that the mice could not go directly to the escape platform. An invisible escape platform (8 cm in diameter) was placed 1 cm below the surface of the water in the center of one quadrant. During the training period, the position of the escape platform was not changed, and visual cues were provided to locate the escape platform by attaching four spatial cues to the walls of each and quadrant of the water pool. The training period was three intervals of 4 h for 3 days. In the training trials, the mouse was placed in the water facing the pool wall at an arbitrary starting point and allowed 60 s to find the hidden platform; the time taken was recorded. When the mouse found the hidden platform, a rest of 15 s was allowed on the platform. If the hidden platform was not found within 60 s, the mice were guided to the escape platform and trained for 15 s to recognize the clues on the escape platform. Three tests were performed on the fourth day after the training was completed. The first test was performed in the same way as before to record the time to find the hidden platform. The second test was to remove the escape platform and allow the mouse to freely navigate in the water pool for 60 s. Then, the percentage of time spent in the target quadrant was calculated. The final test measured the time to reach the exposed platform.
Measurement of lipid peroxidation
The malondialdehyde (MDA) content of the tissues was measured by using the method of Ohkawa et al. (1979). The brain, kidney, and liver tissue were homogenized with physiological saline (0.9% NaCl) using a homogenizer (Next Advance Inc., Averill Park, NY, USA) and centrifuged at 1150×g for 10 min (UNION 32R, Hanil Science Industrial Co., Ltd., Incheon, Korea). The tissue supernatant was mixed with 1% phosphoric acid (Samchun Pure Chemical Co., Gyeonggi-do, Korea) and 0.67% thiobarbituric acid solution (Acros Organics, Morris Plains, NJ, USA) and incubated at 100 °C for 20 min. After cooling on ice, 7.5 mL of n-butanol was added, centrifuged at 1150×g for 10 min, and the absorbance of supernatant was measured using microplate reader at 540 nm (BMG LAB-TECH, Ortenberg, Germany). The degree of lipid peroxidation was calculated from the standard curve.
Measurement of nitric oxide (NO)
The NO content of tissues was measured by using the method of Schmidt et al. (1992). Briefly, 150 μL of brain, kidney, and liver tissue homogenate was mixed with 130 μL of distilled water. Subsequently, 5% phosphoric acid containing 1% sulfanilic acid and 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride solution were mixed with 1:1 (v/v). After incubation at room temperature for 15 min, the absorbance at 540 nm was measured. The inhibition of NO production was calculated from a standard curve of NaNO2 (Junsei Chemical Co., Tokyo, Japan) concentration.
Acetylcholinesterase (AChE) activity assay
The AChE activity was determined in brain tissue by using an AChE assay kit (Sigma Aldrich Co., St Louis, MO, USA) in accordance with the manufacturer’s instructions. After centrifugation at 18,000×g for 5 min, the working reagent was added to the supernatant for 2 min (initial) or 10 min (final). The absorbance at 412 nm was measured and calculated relative to the control group (100%).
Statistical analysis
All results were expressed as the mean ± SD. The statistical significances of results were analyzed by one-way ANOVA and Duncan’s multiple test (p < 0.05) using IBM SPSS statistics programs 23 (IBM Corporation, Armonk, NY, USA). In the T-maze test and novel recognition test experiments, significance differences between the training session and the test session were compared by two-tailed Student’s t-test (p < 0.05).
Results and discussion
Chronic alcohol consumption is a typical risk factor for liver disease (Wang et al., 2018), neuropsychiatric disorders (Gerridzen et al., 2018), brain damage (Guo and Li, 2017), and various forms of dementia, including ARD (Huang et al., 2016). Chronic alcohol consumption causes morphological changes in the brain and it accelerates brain shrinkage. These changes in the brain are associated with the loss of neuronal cells, leading to critical neurodegenerative function and cognitive decline (Jensen and Pakkenberg, 1993; Kril and Halliday, 1999). In addition, chronic intake of alcohol impairs cholinergic neurons or decreases receptors of cholinergic system, which plays important role in memory function (Arendt, 1994). More than 90% of alcohol is metabolized to acetaldehyde by alcohol dehydrogenase (ADH) in the liver. Acetaldehyde is converted into acetic acid and released from the body (Smith et al., 1997). However, when excessive consumption of alcohol occurs, acetaldehyde is not converted to acetic acid and accumulates in the body. The accumulation of acetaldehyde and fatty acid ethyl ester, which are toxic metabolites of EtOH, inhibits functions of mitochondria and causes damage to cells or tissues (Nakamura et al., 2003; Quertemont et al., 2005). Brain is easily damaged by free radical attacks as compared to other organs because of high density of polyunsaturated fatty acids, its high metabolic activity, and relatively low antioxidant defense system (Friedman, 2011; Halliwell and Gutteridge, 1985). EtOH can cross the blood-brain-barrier very easily and is metabolized in the brain by ADH, catalase, or cytochrome P450 (CYP2E1). Exposure to a high concentration of EtOH or its metabolic products (acetaldehyde) enhances excessive ROS generation via this process, leading to damage to neuronal cell (Haorah et al., 2008; Koop, 2006). Furthermore, increased ROS are associated with neuroinflammation and neuronal apoptosis, contributing to cognitive impairment (Tiwari and Chopra, 2012). Climent et al. (2002) reported that exposure to EtOH downregulates brain-derived neurotrophic factor and interferes with intracellular signaling systems involved in cell survival, growth, and differentiation of brain. In addition, chronic intake of EtOH also leads to elevate pro-inflammatory mediators (such as inducible nitric oxide synthase, cyclooxygenase-2, and interleukin-1β) in the brain and to activate neuroinflammation—related signaling pathway. They are responsible for the neuropathological responses associated with behavioral deficits (Qin et al., 2008; Vallés et al., 2004). Many previous studies have suggested that natural antioxidants are attractive candidates for scavenging ROS and alcohol neurotoxicity due to its safety and tolerance by oral administration (Asari et al., 2013; Chen and Luo, 2016; Tiwari et al., 2010).
CTS is an important source of linoleic acid, proteins, and dietary fiber. In addition, it contains a variety of polyphenols, such as serotonin, kaempferol, and acacetin, which are now widely consumed as antioxidants (Sakamura et al., 1980). Recent studies have reported its physiological effects such as fracture healing (Seo et al., 2000), anti-oxidative activity (Kang et al., 1999; Kim et al., 2007; Roh et al 1999; Zhang et al., 1997), LDL oxidation inhibition (Cho et al., 2006), and improvement of lipid metabolism (Cho et al., 2004; Moon et al., 2001). In particular, serotonin and kaempferol in CTS have been reported to exert protection against memory impairment (Buhot et al., 2000; Yu et al., 2013). However, the ability of CTS to ameliorate the cognitive impairment induced by alcohol consumption was not previously studied. Therefore, we investigated the protective effect of CTS on cognitive impairment in a chronic alcohol-induced mice model.
The intake of alcohol (from 12 to 35%) showed learning and memory impairment and apoptotic neurodegenerative responses (Olney et al., 2002; Tiwari et al., 2009; Wagner et al., 2014). On the basis of these evidences, in the present study, the EtOH exposure of 5 g/kg/day (16%) was determined. Our preliminary study showed that intake of EtOH increased in serum GPT, whereas the activity of GPT was significantly reduced in CTS-administered group. No significant differences were found in the serum GOT activity by administration of EtOH or CTS (data not shown). According to previous study, non-toxic effect was observed in serum or urine after feeding the CTS diet at oral doses of 1 to 350 mg/kg/day (Lee et al., 2009; Song et al., 2002). Supplementation of 350 mg/kg/day of CTS extract for 8 weeks enhanced bone formation in rat model without any toxicity. In addition, Park et al. (2018) demonstrated that administration of CTS extracts (100 and 200 mg/kg/day) inhibited cisplatin-induced renal damage in mice model, thus we decided to assess the effect of CTS at a concentration 100 and 200 mg/kg/day.
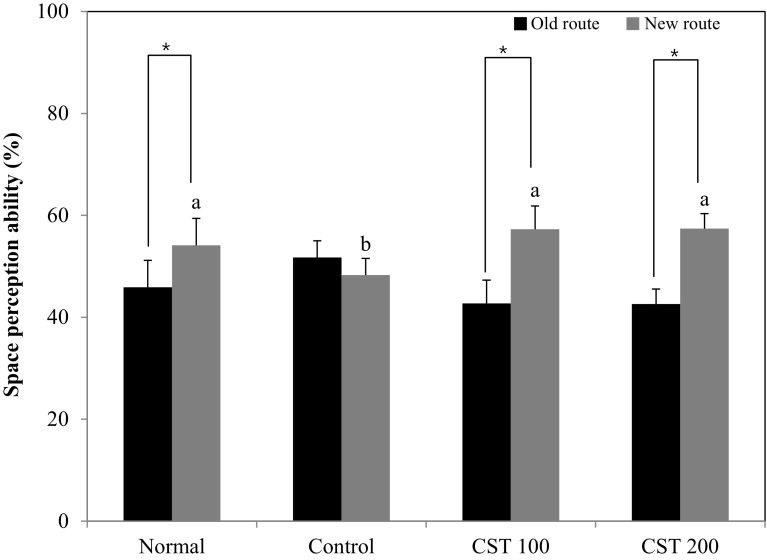
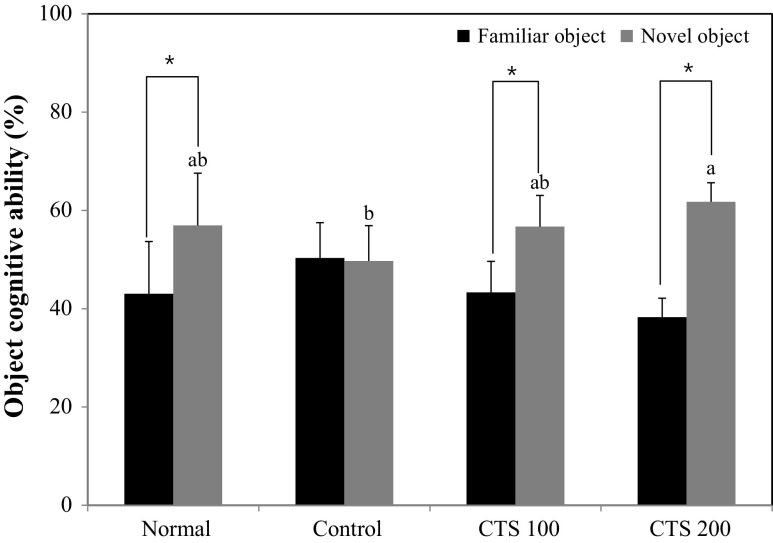
The T-maze test and novel object recognition test are based on the spontaneous and natural exploratory behavior of mice on new environment and serve to examine short-term memory and learning function (Lalonde, 2002). In the T-maze test, the normal group showed higher perception of the new route than the old route, whereas the alcohol-treated control group did not show any significant preference for the old route or the new route (Fig. 2). However, the CTS 100 and CTS 200 groups had an increased number of entries to the new route, suggesting that CTS ameliorated alcohol-induced cognitive impairment and improved spatial learning memory. The novel object recognition test showed that normal group had a higher number of touches on the novel object than the familiar object, whereas the alcohol-treated control group was not able to recognize the novel object, with no significant variation in the exploration rate of the novel and familiar objects (Fig. 3). In contrast, the CTS 100 and 200-treated groups were more interested in the novel object than the familiar object, as shown by the significantly higher the number of touches on the novel object. These results suggested that the administration of CTS could protect against the alcohol-induced impairment of object recognition. The Morris water maze test was performed to investigate the effect of CTS administration on long-term learning and memory ability. During the training period of 3 days, the time to reach the platform decreased, except for the alcohol-treated control group [Fig. 4(A)]. The CTS 100 and CTS 200 groups showed a considerable decrease in the time to reach the escape platform compared with the alcohol-treated control group [Fig. 4(B)]. In the final test, the normal group showed higher occupancy of the target quadrant (63.0%) than the alcohol-treated control group (25.0%). Likewise, The CTS 100 and 200 groups spent significantly more time in the target quadrant than the alcohol-treated control group, showing 40.5% and 49.7%, respectively [Fig. 4(C)]. In addition, The CTS-treated group required a shorter time to reach the hidden platform than the alcohol-treated control group, whereas the time to reach the exposed platform was not significantly different among the experimental groups [Fig. 4(D)]. These results indicated that the protective effect of CTS was related to the improvement of cognitive function regardless of visual perception or physical activity.
Fig. 2.
Effect of the Carthamus tinctorius L. seed on the T-maze test in alcohol-treated mice. The values are the mean ± SD (n = 7). *The space perception of the old and new routes was significantly different as determined by Student’s t-test (p < 0.05). a,bMeans with different letters among groups are significantly different (p < 0.05) by Duncan’s multiple range test. Normal = distilled water + 1% CMC solution; Control = 16% EtOH (5.0 g/kg/day) + 1% CMC solution; CTS 100 = 16% EtOH (5.0 g/kg/day) + Carthamus tinctorius L. seed (100 mg/kg/day in 1% CMC solution); CTS 200 = 16% EtOH (5.0 g/kg/day) + Carthamus tinctorius L. seed (200 mg/kg/day in 1% CMC solution)
Fig. 3.
Effect of the Carthamus tinctorius L. seed on novel object recognition test in alcohol-treated mice. The values are the mean ± SD (n = 7). *The object cognitive abilities for familiar and novel objects were significantly different as determined (p < 0.05) by Student t-test. a~bMeans with different letters among groups are significantly different (p < 0.05) by Duncan’s multiple range test Normal = distilled water + 1% CMC solution; Control = 16% EtOH (5.0 g/kg/day) + 1% CMC solution; CTS 100 = 16% EtOH (5.0 g/kg/day) + Carthamus tinctorius L. seed (100 mg/kg/day in 1% CMC solution); CTS 200 = 16% EtOH (5.0 g/kg/day) + Carthamus tinctorius L. seed (200 mg/kg/day in 1% CMC solution)
Fig. 4.
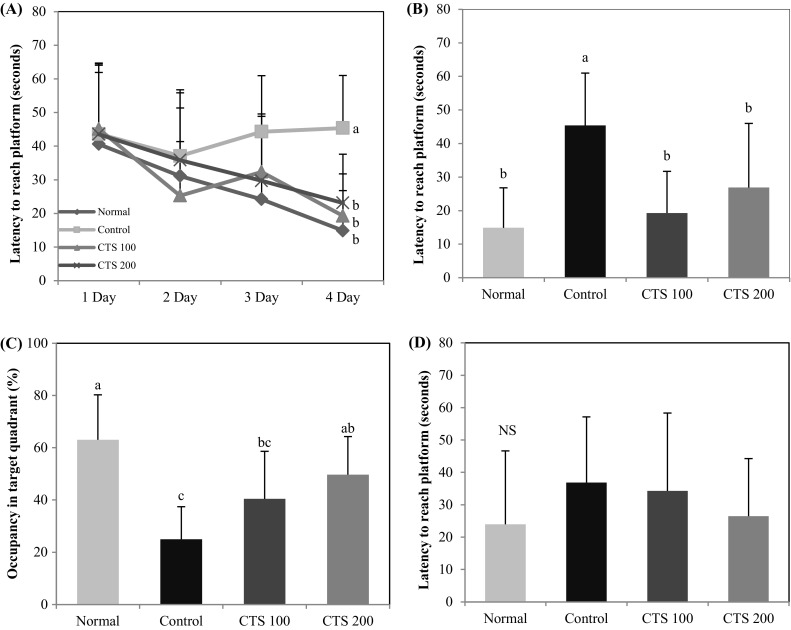
Effect of Carthamus tinctorius L. seed on the Morris water maze test in alcohol-treated mice. (A) Effect of Carthamus tinctorius L. seed on escape latency to the platform in alcohol-treated mice. (B) Effect of Carthamus tinctorius L. seed on the time required to reach the hidden platform on the final test in alcohol-treated mice. (C) Effect of Carthamus tinctorius L. seed on occupancy time of the target quadrant in alcohol-treated mice. (D) Effect of Carthamus tinctorius L. seed on the time required to reach the exposed platform in alcohol-treated mice. NS No significance differences. The values are mean ± SD (n = 7). a,b,cMeans with different letters are significantly different (p < 0.05) by Duncan’s multiple range test. Normal = distilled water + 1% CMC solution; Control = 16% EtOH (5.0 g/kg/day) + 1% CMC solution; CTS 100 = 16% EtOH (5.0 g/kg/day) + Carthamus tinctorius L. seed (100 mg/kg/day in 1% CMC solution); CTS 200 = 16% EtOH (5.0 g/kg/day) + Carthamus tinctorius L. seed (200 mg/kg/day in 1% CMC solution)
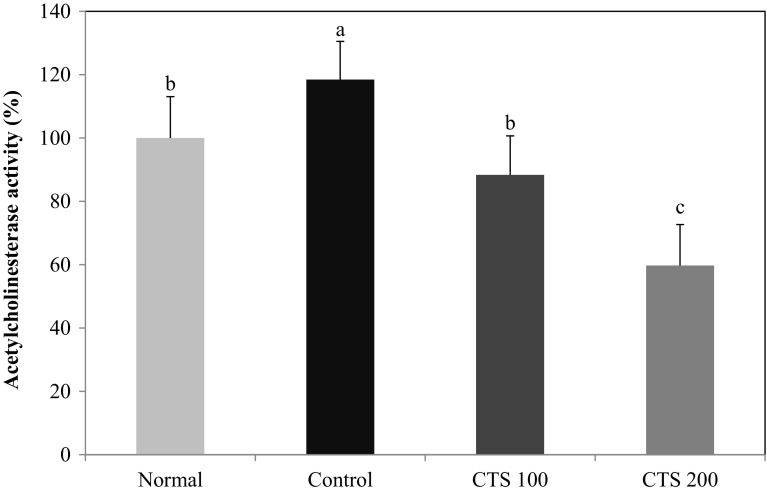
AChE degrades acetylcholine (ACh) in the cholinergic system into acetate and choline (Woolf, 1997). ACh activates the muscarinic receptors bound to phospholipid metabolism to induce the proliferation of neurons and can act as a nutrient factor to prevent apoptotic cell death and to grow neurons (Croxson et al., 2012). Alcohol was shown to inhibit the effects of ACh in vitro (Balduini and Costa, 1989) and to induce nerve dysfunction and loss of neurons (Guerri, 1998). A previous report demonstrated that AChE activity in animal brains was elevated by chronic alcohol consumption with cognitive impairment (Tiwari and Chopra, 2013). In this study, the effect of CTS administration on AChE activity was measured in the brain (Fig. 5). The administration of alcohol resulted in a high AChE activity of 118.47% relative to the normal group (100%). In contrast, the CTS 100 and CTS 200 groups had reduced enzymatic activity of 88.36% and 59.72%, respectively. Many studies indicated that dysfunction of learning and memory was caused by the degeneration of cholinergic nervous system, and increased AChE activity in the brain could elevate the risk of cognitive disorders (Cohen et al., 2007; Jamal et al., 2010; Srikumar et al., 2004). Tiwari et al. (2009) demonstrated that enhanced AChE activity in cerebral cortex and hippocampus of EtOH-treated rats is involved in cognitive deficit. Our results also showed that AChE activity was increased in the brain tissue of chronic alcohol-administered mice, while supplementation of CTS significantly decreased in elevated AChE activity. Therefore, we suggested that suppression of AChE activity by administration of CTS may be one of the mechanisms involved in the improvement of cognitive deficits in alcohol-treated mice.
Fig. 5.
Effect of Carthamus tinctorius L. seed on AChE activity in mice brain. The values are the mean ± SD (n = 7). a,b,cMeans with the different letters are significantly different (p < 0.05) by Duncan’s multiple range test. Normal = distilled water + 1% CMC solution; Control = 16% EtOH (5.0 g/kg/day) + 1% CMC solution; CTS 100 = 16% EtOH (5.0 g/kg/day) + Carthamus tinctorius L. seed (100 mg/kg/day in 1% CMC solution); CTS 200 = 16% EtOH (5.0 g/kg/day) + Carthamus tinctorius L. seed (200 mg/kg/day in 1% CMC solution)
Alcohol metabolism is directly or indirectly involved in the production of ROS and reactive nitrogen species (RNS). These cause the modification of biological structures and consequently result in cellular or tissue dysfunction, which can be found in most organs. In particular, brain, liver and kidneys are major organs affected by the administration of alcohol consumption (Montoliu et al., 1994; Shaw and Jayatilleke, 1990). Alcohol is mainly broken down in the liver and stimulates ROS production, leading to cell and tissue injury (Wu and Cederbaum, 2003). Kidney, like brain, is highly vulnerable to oxidative damage due to abundance of long-chain polyunsaturated fatty acids in lipid composition (Ozbek, 2012). Tissue damage induced by alcohol consumption can release MDA and NO, in turn, further stimulate oxidative stress (Epstein, 1997). Consistent with previous study, oral administration of plant extract significantly restored the antioxidant defense system in kidney and liver of rat against alcohol-induced oxidative stress (Esmaeili et al., 2009). Therefore, we investigated whether CTS prevent oxidative damage induced by alcohol consumption in the brain, kidney, and liver. The accumulation of MDA not only affects membrane function, but also promotes protein degeneration and DNA damage (Niess et al., 1999), ultimately resulting in nerve oxidative stress and cell death. Previous studies have shown that chronic alcohol intake increased MDA levels (Draper and Hadley, 1990). Our results showed that the administration of alcohol significantly increased the concentration of MDA in the brain, kidney, and liver (Table 1A). In the alcohol-administered control group, the levels of MDA were significantly increased compared to normal group (from 24.22 to 33.22 nmol/mg protein). However, CTS 100 and 200 groups decreased the MDA concentration in the mice brain, showing 30.37 and 26.49 nmol/mg protein, respectively. The CTS 100 (5.26 nmol/mg protein) and CTS 200 (5.48 nmol/mg protein) groups exhibited lower MDA concentration in the kidneys than the alcohol-treated control group (6.22 nmol/mg protein). In addition, MDA concentration in the liver of the CTS 100 (1.69 nmol/mg protein) and CTS 200 (1.44 nmol/mg protein) groups was significantly lower than the alcohol-treated control group (2.24 nmol/mg protein). Therefore, we confirmed that CTS administration inhibited alcohol-induced lipid peroxidation in the brain, kidney and the liver.
Table 1.
Effect of Carthamus tinctorius L. seed on lipid peroxidation (A) and NO production (B) in the brain, kidney, and liver of alcohol-treated mice
| (A) | |||
|---|---|---|---|
| Group | MDA (nmol/mg protein) | ||
| Brain | Kidney | Liver | |
| Normal | 24.22 ± 2.98b | 5.77 ± 0.53ab | 1.59 ± 0.29b |
| Control | 33.22 ± 7.69a | 6.22 ± 0.53a | 2.24 ± 0.72a |
| CTS 100 | 30.37 ± 6.92ab | 5.26 ± 0.25b | 1.69 ± 0.22b |
| CTS 200 | 26.49 ± 6.72ab | 5.48 ± 0.32b | 1.44 ± 0.27b |
| (B) | |||
|---|---|---|---|
| Group | NaNO2 (μmol/L/mg protein) | ||
| Brain | Kidney | Liver | |
| Normal | 8.96 ± 0.54b | 29.42 ± 1.95b | 35.72 ± 7.73b |
| Control | 10.14 ± 0.84a | 31.98 ± 2.35a | 45.97 ± 8.50a |
| CTS 100 | 10.14 ± 0.58a | 27.58 ± 1.14bc | 31.70 ± 8.90b |
| CTS 200 | 9.10 ± 0.64ab | 26.26 ± 0.49c | 31.51 ± 9.75b |
The values are the mean ± SD (n = 6)
a,b,cMeans with different letters are significantly different (p < 0.05) by Duncan’s multiple range test. Normal = distilled water + 1% CMC solution; Control = 16% EtOH (5.0 g/kg/day) + 1% CMC solution; CTS 100 = 16% EtOH (5.0 g/kg/day) + Carthamus tinctorius L. seed (100 mg/kg/day in 1% CMC solution); CTS 200 = 16% EtOH (5.0 g/kg/day) + Carthamus tinctorius L. seed (200 mg/kg/day in 1% CMC solution)
NO plays an important role in the regulation of a variety of physiological activities through mediation of the signaling pathways of neuronal regulation, neurotransmission, and synaptic plasticity (Lowenstein et al., 1994). Several studies demonstrated that excessive alcohol consumption increased the plasma concentration of NO precursors and metabolites (Kavitha et al., 2008). Excessive alcohol in the brain increases O-2 and NO production; these molecules are associated with ADH and CYP2E1, which play an important role in neuronal cell death (Zimatkin et al., 2006). Therefore, measurement of NO concentration provides an indication of nerve cell damage. In this study, the effect of CTS on NO production in the brain, kidney, and liver of alcohol-treated mice is shown in Table 1B. The NO concentration in the brain of the normal group was 8.96 μmol/L/mg protein, whereas the concentration in the alcohol-treated control group was 10.14 μmol/L/mg protein. However, the elevated NO concentration was attenuated to 9.10 μmol/L/mg protein in the CTS 200 group. The NO concentration in the kidney in the alcohol-treated control group was 31.98 μmol/L/mg protein, whereas the NO concentration was decreased in the CTS 100 and CTS 200 groups to 27.58 and 26.26 μmol/L/mg protein, respectively. The NO concentration in the liver of the alcohol-treated control group was increased from 35.72 μmol/L/mg protein to 45.97 μmol/L/mg protein. However, the CTS 100 and CTS 200 groups had significantly lower NO concentrations of 31.70 and 31.51 μmol/L/mg protein, respectively. These findings suggested that the oral administration of CTS inhibited the alcohol-induced formation of NO in the brain, kidney, and liver.
In conclusion, the administration of alcohol resulted in impairment of learning ability and cognitive function, and caused oxidative stress in the brain, kidney, and liver. Our results showed that the oral administration of 100 and 200 mg/kg/day CTS exerted protective effects against ARD through the improvement of cognitive and memory function. The alleviation of short-term memory decline was confirmed in the T-maze test and novel object recognition test through a comparison of alcohol-administered mice treated with or without CTS. The Morris water maze test demonstrated that CTS improved long-term memory and spatial cognitive ability. In addition, CTS attenuated oxidative stress through the inhibition of lipid peroxidation and NO production in the brain, kidney and liver. Furthermore, alcohol-induced AChE activity was suppressed by administration of CTS in the brain. These results indicated that CTS was a good resource for the inhibition of cognitive impairment and the increase of learning ability. CTS may therefore be a promising agent for the protection and delay of memory impairment observed in ARD.
Acknowledgements
This work was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01312301)” Rural Development Administration, Republic of Korea.
Compliance with ethical standards
Conflict of interest
The authors declare no potential conflicts of interests.
Contributor Information
Seung Hak Choi, Email: merrymison@pusan.ac.kr.
Ah Young Lee, Email: aylee@pusan.ac.kr.
Chan Hum Park, Email: ptman123@korea.kr.
Yu Su Shin, Email: totoro69@korea.kr.
Eun Ju Cho, Phone: 82-51-510-2837, Email: ejcho@pusan.ac.kr.
References
- Arendt T. Cell and Animal Models in Aging and Dementia Research. Vienna: Springer Vienna; 1994. Impairment in memory function and neurodegenerative changes in the cholinergic basal forebrain system induced by chronic intake of ethanol; pp. 173–187. [DOI] [PubMed] [Google Scholar]
- Asari MA, Mohd Ismail ZI, Mohd Yusof NA. Evaluation of spirulina supplementation on intermittent binge ethanol-induced neurotoxicity in dentate gyrus of rats. Int. J. Appl. Res. Nat. Prod. 2013;6:8–14. [Google Scholar]
- Bae SJ, Shim SM, Park YJ, Lee JY, Chang EJ, Choi SW. Cytotoxicity of phenolic compounds isolated from seeds of safflower (Carthamus tinctorius L.) on cancer cell lines. Food Sci. Biotechnol. 2002;11:140–146. [Google Scholar]
- Balduini W, Costa LG. Effects of ethanol on muscarinic receptor-stimulated phosphoinositide metabolism during brain development. J. Pharmacol. Exp. Ther. 1989;250:541–547. [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching to-sample learning task to study ‘recognition memory’. Nat. Protoc. 2006;1:1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Buhot MC, Martin S, Segu L. Role of serotonin in memory impairment. Ann. Med. 2000;32:210–221. doi: 10.3109/07853890008998828. [DOI] [PubMed] [Google Scholar]
- Chen G, Luo J. Anthocyanins: Are they beneficial in treating ethanol neurotoxicity? Neurotox. Res. 2016;17:91–101. doi: 10.1007/s12640-009-9083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SH, Choi SW, Choi YS, Lee WJ. Effects of defatted safflower seed extract and phenolic compounds in diet on plasma and liver lipid in ovariectomized rats fed high-cholesterol diets. J. Nutr. Sci. Vitaminol. 2004;50:32–37. doi: 10.3177/jnsv.50.32. [DOI] [PubMed] [Google Scholar]
- Cho SH, Park YY, Yoon JY, Choi SW, Ha TY. The effect of polyphenols from safflower seed on HMG-CoA reductase (HMGR) activity, LDL oxidation and Apo A1 secretion. Korean J. food Sci. Technol. 2006;38:279–283. [Google Scholar]
- Climent E, Pascual M, Renau-Piqueras J, Guerri C. Ethanol exposure enhances cell death in the developing cerebral cortex: role of brain-derived neurotrophic factor and its signaling pathways. J. Neurosci. Res. 2002;68:213–225. doi: 10.1002/jnr.10208. [DOI] [PubMed] [Google Scholar]
- Cohen AC, Tong M, Wands JR, de la Monte SM. Insulin and insulin-like growth factor resistance with neurodegeneration in an adult chronic ethanol exposure model. Alcohol Clin. Exp. Res. 2007;31:1558–1573. doi: 10.1111/j.1530-0277.2007.00450.x. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Browning PG, Gaffan D, Baxter MG. Acetylcholine facilitates recovery of episodic memory after brain damage. J. Neurosci. 2012;32:13787–13795. doi: 10.1523/JNEUROSCI.2947-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Meth. Enzymol. 1990;186:421. doi: 10.1016/0076-6879(90)86135-I. [DOI] [PubMed] [Google Scholar]
- Epstein M. Alcohol’s impact on kidney function. Alcohol Health and Res. World. 1997;21:84–92. [PMC free article] [PubMed] [Google Scholar]
- Esmaeili MA, Sonboli A, Kanani MR, Sadeghi H. Salvia sahendica prevents tissue damages induced by alcohol in oxidative stress conditions: Effect on liver and kidney oxidative parameters. J. Med. Plants Res. 2009;3:276–283. [Google Scholar]
- Friedman Joseph. Oxidative Stress and Free Radical Damage in Neurology. Totowa, NJ: Humana Press; 2010. Why Is the Nervous System Vulnerable to Oxidative Stress? pp. 19–27. [Google Scholar]
- Gerridzen IJ, Hertogh CMPM, Depla MF, Veenhuizen RB, Verschuur EML, Joling KJ. Neuropsychiatric symptoms in people with Korsakoff syndrome and other alcohol-related cognitive disorders living in specialized long-term care facilities: prevalence, severity, and associated caregiver distress. J. Am. Med. Dir. Assoc. 2018;19:240–247. doi: 10.1016/j.jamda.2017.09.013. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug Alcohol Depen. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Guerri C. Neuroanatomical and neurophysiological mechanisms involved in central nervous system dysfunctions induced by prenatal alcohol exposure. Alcohol Clin. Exp. Res. 1998;22:304–312. doi: 10.1111/j.1530-0277.1998.tb03653.x. [DOI] [PubMed] [Google Scholar]
- Guo Z, Li J. Chlorogenic acid prevents alcohol-induced brain damage in neonatal rat. Transl. Neurosci. 2017;8:176–181. doi: 10.1515/tnsci-2017-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Oxygen radicals in the nervous system. Trends Neurosci. 1985;8:22–26. doi: 10.1016/0166-2236(85)90010-4. [DOI] [Google Scholar]
- Haorah J, Ramirez SH, Floreani N, Gorantla S, Morsey B, Persidsky Y. Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic. Biol. Med. 2008;45:1542–1550. doi: 10.1016/j.freeradbiomed.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Matsumoto I. Ethanol and brain damage. Curr. Opin. Pharmacol. 2005;5:73–78. doi: 10.1016/j.coph.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, Romero FJ, Garcia-Verdugo JM. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc. Natl. Acad. Sci. U.S.A. 2003;100:7919–7924. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu M, Takahashi T, Komatsu M, Kido T, Kasahara Y. Antioxidant and neuroprotective activities of Mogami-benibana (safflower, Carthamus tinctorius Linne) Neurochem. Res. 2014;34:795–805. doi: 10.1007/s11064-008-9884-5. [DOI] [PubMed] [Google Scholar]
- Holownia A, Ledig M, Brszko JJ, Ménez JF. Acetaldehyde cytotoxicity in cultured rat astrocytes. Brain Res. 1999;833:202–208. doi: 10.1016/S0006-8993(99)01529-2. [DOI] [PubMed] [Google Scholar]
- Holownia A, Ledig M, Mapoles J, Ménez JF. Acetaldehyde-induced growth inhibition in cultured rat astroglial cells. Alcohol. 1996;13:93–97. doi: 10.1016/0741-8329(95)02019-5. [DOI] [PubMed] [Google Scholar]
- Hou Y, Aboukhatwa MA, Lei DL, Manaye K, Khan I, Luo Y. Anti-depressant natural flavonols modulate BDNF and beta amyloid in neurons and hippocampus of double TgAD mice. Neuropharmacology. 2010;58:911–920. doi: 10.1016/j.neuropharm.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WJ, Zhang X, Chen WW. Association between alcohol and Alzheimer’s disease. Exp. Ther. Med. 2016;12:1247–1250. doi: 10.3892/etm.2016.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal M, Ameno K, Miki T, Tanaka N, Ohkubo E, Kinoshita H. Effects of systemic nicotine, alcohol or their combination on cholinergic markers in the frontal cortex and hippocampus of rat. Neurochem. Res. 2010;35:1064–1070. doi: 10.1007/s11064-010-0155-x. [DOI] [PubMed] [Google Scholar]
- Jensen GB, Pakkenberg B. Do alcoholics drink their neurons away? Lancet. 1993;342:1201–1204. doi: 10.1016/0140-6736(93)92185-V. [DOI] [PubMed] [Google Scholar]
- Jun MS, Ha YM, Kim HS, Jang HJ, Kim YM, Lee YS, Kim HJ, Seo HG, Lee JH, Lee SH, Chang KC. Anti-inflammatory action of methanol extract of Carthamus tinctorius involves in heme oxygenase-1 induction. J. Ethnopharmacol. 2011;133:524–530. doi: 10.1016/j.jep.2010.10.029. [DOI] [PubMed] [Google Scholar]
- Kalmijn S. Fatty acid intake and the risk of dementia and cognitive decline: a review of clinical and epidemiological studies. J. Nutr. Health Aging. 2000;4:202–207. [PubMed] [Google Scholar]
- Kang GH, Chang EJ, Park SW. Antioxidative activity of phenolic compounds in roasted safflower (Carthamus tinctorius L.) seeds. Prev. Nutr. Food Sci. 1999;4:221–225. [Google Scholar]
- Kavitha G, Damodara Reddy V, Paramahamsa M, Akhtar PM, Varadacharyulu NC. Role of nitric oxide in alcohol-induced changes in lipid profile of moderate and heavy alcoholics. Alcohol. 2008;42:47–53. doi: 10.1016/j.alcohol.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Kim EO, Oh JH, Lee SK, Lee JY, Choi SW. Antioxidant properties and quantification of phenolic compounds from safflower (Carthamus tinctorius L.) seeds. Food Sci. Biotechnol. 2007;16:71–77. [Google Scholar]
- Koop DR. Alcohol metabolism’s damaging effects on the cell: a focus on reactive oxygen generation by the enzyme cytochromeP450 2E1. Alcohol Res. Health. 2006;29:274–280. [PMC free article] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM. Brain shrinkage in alcoholics: a decade on and what have we learned? Prog. Neurobiol. 1999;58:381–387. doi: 10.1016/S0301-0082(98)00091-4. [DOI] [PubMed] [Google Scholar]
- Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav. Rev. 2002;26:91–104. doi: 10.1016/S0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- Lee YS, Choi CW, Kim JJ, Ganapathi A, Udayakumar R, Kim SC. Determination of mineral content in methanolic safflower (Carthamus tinctorius L.) seed extract and its effect on osteoblast markers. Int. J. Mol. Sci. 2009;10:292–305. doi: 10.3390/ijms10010292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein CJ, Dinerman JL, Synder SH. Nitric oxide: a physiologic messenger. Ann. Intern. Med. 1994;120:227–237. doi: 10.7326/0003-4819-120-3-199402010-00009. [DOI] [PubMed] [Google Scholar]
- Montgomery KC. A test of two explanations of spontaneous alternation. J. Comp. Physiol. Psych. 1952;45:287–293. doi: 10.1037/h0058118. [DOI] [PubMed] [Google Scholar]
- Montoliu C, Valles S, Renau-Piqueras J, Guerii C. Ethanol-induced oxygen radical formation and lipid peroxidation in rat brain: Effect of chronic ethanol consumption. J. Neurochem. 1994;63:1855–1862. doi: 10.1046/j.1471-4159.1994.63051855.x. [DOI] [PubMed] [Google Scholar]
- Moon KD, Back SS, Kim JH, Jeon SM, Lee MK, Choi MS. Safflower seed extract lowers plasma and hepatic lipids in rats fed high-cholesterol diet. Nutr. Res. 2001;21:895–904. doi: 10.1016/S0271-5317(01)00293-7. [DOI] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying a spatial learning in the rat. J. Neurosci. Meth. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Iwahashi K, Furukawa A, Ameno K, Kinoshita H, Ijiri I, Sekine Y, Suzuki K, Iwata Y, Minabe Y, Mori N. Acetaldehyde adducts in the brain of alcoholics. Arch. Toxicol. 2003;77:591–593. doi: 10.1007/s00204-003-0465-8. [DOI] [PubMed] [Google Scholar]
- Niess AM, DickHuth HH, Northoff H, Fehrenbach E. Free radicals and oxidative stress in exercise-immunological aspects. Exerc. Immunol. Rev. 1999;5:22–56. [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Olney JW, Tenkova T, Dikranian K, Qun YQ, Labruyere J, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing C57BL/6 mouse brain. Dev. Brain Res. 2002;133:115–126. doi: 10.1016/S0165-3806(02)00279-1. [DOI] [PubMed] [Google Scholar]
- Ozbek E. Induction of oxidative stress in kidney. Int. J. Nephrol. 2012;2012:465897. doi: 10.1155/2012/465897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Lee AY, Kim JH, Seong SH, Jang GY, Cho EJ, Choi JS, Kwon J, Kim YO, Lee SW, Yokozawa T, Shin YS. Protective effect of safflower seed on cisplatin-induced renal damage in mice via oxidative stress and apoptosis-mediated pathways. Am. J. Chin. Med. 2018;46:1–18. doi: 10.1142/S0192415X18500015. [DOI] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J. Neuroinflammation. 2008;18:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quertemont E, Grant KA, Correa M, Arizzi MN, Salamone JD, Tambour S, Aragon CM, McBride WJ, Rodd ZA, Goldstein A, Zaffaroni A, Li TK, Pisano M, Diana M. The role of acetaldehyde in the central effects of ethanol. Alcohol Clin. Exp. Res. 2005;29:221–234. doi: 10.1097/01.ALC.0000156185.39073.D2. [DOI] [PubMed] [Google Scholar]
- Roh JS, Sun WS, Oh SU, Lee JI, Oh WT, Kim JH. In vitro antioxidant activity of safflower (Carthamus tinctorius L.) seeds. Food Sci. Biotechnol. 1999;8:88–92. [Google Scholar]
- Room R, Babor T, Rehm J. Alcohol and public health. Lancet. 2005;365:519–530. doi: 10.1016/S0140-6736(05)70276-2. [DOI] [PubMed] [Google Scholar]
- Sakamura A, Terayama Y, Kawakatsu S, Ichihara A, Saito H. Conjugated serotonins and phenolic constituents in safflower seed (Carthamus tinctorius L.). Argric. Biol. Chem. 1980;44:2951–2954. [Google Scholar]
- Schmidt HH, Warner TD, Nakane M, Forstermann U, Murad F. Regulation and sub cellular location of nitrogen oxide synthases in RAW264.7 macrophages. Mol. Pharmacol. 1992;41:615–624. [PubMed] [Google Scholar]
- Seo HJ, Kim JH, Kwak DY, Jeon SM, Ku SK, Lee JH, Moon KD, Choi MS. The effects of safflower seed powder and its fraction on bone tissue in rib-fractured rats during the recovery. Korean J. Nutr. 2000;33:411–420. [Google Scholar]
- Seyedabadi M, Fakhfouri G, Ramezani V, Mehr SE, Rahimian R. The role of serotonin in memory: interactions with neurotransmitters and downstream signaling. Exp. Brain Res. 2014;232:723–738. doi: 10.1007/s00221-013-3818-4. [DOI] [PubMed] [Google Scholar]
- Shaw S, Jayatilleke E. The role of aldehyde oxidase in ethanol-induced hepatic lipid peroxidation in the rat. Biochem. J. 1990;268:579–583. doi: 10.1042/bj2680579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BR, Aragon CM, Amit Z. Catalase and the production of brain acetaldehyde: a possible mediator for the psychopharmacological effects of ethanol. Addict. Biol. 1997;2:277–289. doi: 10.1080/13556219772570. [DOI] [PubMed] [Google Scholar]
- Song HR, Ra DK, Kim JS, Jung TS, Kim YH, Kang HJ, Kang CB, Yeon SC, Kim EH, Lee HJ, Shin GW, Park MR, Kim GS. Effects of safflower seed on new bone formation. J. Vet. Clinic. 2002;19:66–72. [Google Scholar]
- Srikumar BN, Ramkumar K, Raju TR, Shankaranarayana Rao BS, Assay of acetylcholinesterase activity in the brain. In: Brain and Behavior. Raju TR, Kutty BM, Sathyaprabha TN, Shanakranarayana Rao BS (eds). National Institute of Mental Health and Neurosciences, Bangalore, India, pp. 142–144 (2004)
- Takii T, Hayashi M, Hiroma H, Chiba T, Kawashima S, Zhang HL, Nagatsu A, Sakakibara J, Onozaki K. Serotonin derivative, N-(p-coumaroyl)serotonin, isolated from safflower (Carthamus tinctorious L.) oil cake augments the proliferation of normal human and mouse fibroblasts in synergy with basic fibroblast growth factor (FGF) of epidermal growth factor (EGF) J. Biochem. 1999;125:910–915. doi: 10.1093/oxfordjournals.jbchem.a022368. [DOI] [PubMed] [Google Scholar]
- Thomas VS, Rockwood KJ. Alcohol abuse, cognitive impairment, and mortality among older people. J. Am. Geriatr. Soc. 2001;49:415–420. doi: 10.1046/j.1532-5415.2001.49085.x. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Chopra K. Attenuation of oxidative stress, neuroinflammation and apoptosis by prevents cognitive deficits in rats postnatally exposed ethanol. Psychopharmacology. 2012;224:519–535. doi: 10.1007/s00213-012-2779-9. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Chopra K. Protective effect of curcumin against chronic alcohol-induced cognitive deficits and neuroinflammation in the adult rat brain. Neuroscience. 2013;244:147–158. doi: 10.1016/j.neuroscience.2013.03.042. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Kuhad A, Chopra K. Suppression of neuro-inflammatory signaling cascade by tocotrienol can prevent chronic alcohol-induced cognitive dysfunction in rats. Behav. Brain Res. 2009;203:296–303. doi: 10.1016/j.bbr.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Kuhad A, Chopra K. Epigallocatechin-3-gallate ameliorates alcohol-induced cognitive dysfunctions and apoptotic neurodegeneration in the developing rat brain. Int. J. Neuropsychopharmacol. 2010;13:1053–1066. doi: 10.1017/S146114571000060X. [DOI] [PubMed] [Google Scholar]
- Tong M, Longato L, Nguyen QGL, Chen WC, Spaisman A, Monte SM. Acetaldehyde-mediated neurotoxicity: relative to fetal alcohol spectrum disorders. Oxid. Med. Cell Longev. 2011;2011:213–286. doi: 10.1155/2011/213286. [DOI] [Google Scholar]
- Vallés SL, Blanco AM, Pascual M, Guerri C. Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain Pathol. 2004;14:365–371. doi: 10.1111/j.1750-3639.2004.tb00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JL, Zhou FC, Goodlett CR. Effects of one-and three-day binge alcohol exposure in neonatal C57BL/6 mice on spatial learning and memory in adolescence and adulthood. Alcohol. 2014;38:99–111. doi: 10.1016/j.alcohol.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chen P, Tang C, Wang Y, Li Y, Zhang H. Antinociceptive and anti-inflammatory activities of extract and two isolated flavonoids of Carthamus tinctorius L. J. Ethnopharmacol. 2014;151:944–950. doi: 10.1016/j.jep.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Wang X, Liu M, Zhang C, Li S, Yang Q, Zhang J, Gong Z, Han J, Jia L. Antioxidant activity and protective effects of enzyme-extracted Oudemansiella radiata polysaccharides on alcohol-induced liver injury. Molecules. 2018;23:481. doi: 10.3390/molecules23020481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf NJ. A possible role for cholinergic neurons of the basal forebrain and pontomesencephalon in consciousness. Conscious. Cogn. 1997;6:574–596. doi: 10.1006/ccog.1997.0319. [DOI] [PubMed] [Google Scholar]
- Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res. Health. 2003;27:277–284. [PMC free article] [PubMed] [Google Scholar]
- Yu L, Chen C, Wang LF, Kuang X, Liu K, Zhang H, Du JR. Neuroprotective effect of kaempferol glycosides against brain injury and neuroinflammation by inhibiting the activation of NF-κB and STAT3 in transient focal stroke. Plos One. 2013;8:e55839. doi: 10.1371/journal.pone.0055839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HL, Nagatsu A, Watanabe T, Sakakibara J, Okuyama H. Antioxidative compounds isolated from safflower (Carthamus tinctorius L.) oil cake. Chem. Pharm. Bull. 1997;45:1910–1914. doi: 10.1248/cpb.45.1910. [DOI] [PubMed] [Google Scholar]
- Zhang LL, Tian K, Tang ZH, Chen XJ, Bian ZX, Wang YT, Lu JJ. Phytochemistry and Pharmacology of Carthamus tinctorius L. Am. J. Chin. Med. 2016;44:197–226. doi: 10.1142/S0192415X16500130. [DOI] [PubMed] [Google Scholar]
- Zimatkin SM, Pronko SP, Vasiliou V, Gonzalez FJ, Deitrich RA. Enzymatic mechanisms of ethanol oxidation in the brain. Alcohol Clin. Exp. Res. 2006;30:1500–1505. doi: 10.1111/j.1530-0277.2006.00181.x. [DOI] [PubMed] [Google Scholar]