Abstract
Background
The incidence of lymphoplasmacytic lymphoma (LPL) is lower in Asian than in Western populations. Few studies have described the clinical features and treatment outcomes of patients with LPL, including non-IgM LPL, in East Asia.
Methods
We retrospectively analyzed patients diagnosed with LPL at Asan Medical Center between January 2001 and March 2016. We evaluated the clinical features and survival outcomes of patients with LPL and non-IgM LPL and compared these data with those of patients with LPL/Waldenström's macroglobulinemia (WM).
Results
The median age at diagnosis of patients with LPL was 61.5 years (range, 34–77 yr); most patients were male (91%). Approximately three-quarters of the 22 patients with LPL were in the low or intermediate risk groups according to the International Prognostic Scoring System for Waldenström's Macroglobulinemia classification. The median follow-up duration was 75 months [95% confidence interval (CI), 48–102 mo], and the median overall survival (OS) was 81 months (95% CI, 0–167 mo). The number of patients in the non-IgM LPL group who exhibited extramedullary involvement was higher than in the LPL/WM group. OS of the LPL/WM group was improved compared with that of the non-IgM LPL group [median not reached vs. 10.0 mo (95% CI, 0–36.7); P=0.05].
Conclusion
We present a single-center experience of 22 patients with LPL, including a non-IgM cohort, in Korea. The treatment of non-IgM LPL was heterogeneous, and patients with non-IgM LPL showed a higher 5-year mortality rate and more adverse prognostic factors than those with LPL/WM.
Keywords: Lymphoplasmacytic lymphoma, Non-IgM, Waldenström's macroglobulinemia
INTRODUCTION
According to the latest World Health Organization (WHO) classification revised in 2016, lymphoplasmacytic lymphoma (LPL) is a monoclonal expansion of B-lymphocytes with varying degrees of B-cell differentiation from small lymphocytes to plasma cells [1,2]. Waldenström's macroglobulinemia (WM) is a subset of LPL with bone marrow involvement and the presence of circulating immunoglobulin M (IgM) paraprotein [2]. LPL presenting with serum monoclonal IgM, referred to as LPL/WM in clinical practice, is frequent, whereas non-IgM LPL presenting with IgG or IgA is rare and accounts for <5% of all LPL cases [3].
The incidence of LPL is approximately 0.3–0.4 cases per million persons per year in Asia, which is 10-fold lower than in Western countries [4]. Possible reasons for this difference in incidence likely include race-dependent genetic predisposition to LPL, lifestyle differences, and environmental factors [5].
Recently, a mutation in MYD88 (MYD88 L265P) has been recognized in most patients with IgM LPL/WM, accounting for >90% of tumor samples from patients with LPL [6]. This mutation plays a crucial role in the pathogenesis of LPL/WM and can help to differentiate B-cell lymphomas and plasma cell myeloma, both of which warrant further research [6]. A previous study reported that fewer patients with non-IgM LPL harbor this mutation than those with classic LPL/WM; consequently, these 2 diseases may have different disease entities [7].
Limited information is available regarding the clinical features of patients with non-IgM LPL and LPL/WM in East Asia. Therefore, in this study, we present our experiences of evaluating the clinical features and survival outcomes of 22 patients with LPL. Moreover, we present the clinical characteristics and treatment outcomes of patients with non-IgM LPL at our institution in Korea.
MATERIALS AND METHODS
Study design and patients
This retrospective cohort study used prospectively collected data from the lymphoma registry of Asan Medical Center, a university-affiliated, tertiary referral center located in Seoul, Korea. The registry contains demographic, clinical, and laboratory data, as well as outcomes of patients with LPL, treated at Asan Medical Center. Twenty-two patients who were enrolled in the registry and who were diagnosed with LPL between January 2001 and March 2016 were included. Among these patients, we identified 8 patients diagnosed with non-IgM LPL. The institutional review board of Asan Medical Center approved this study and waived the requirement for informed consent owing to its retrospective nature.
The diagnosis of LPL was based on clinical, laboratory, molecular, and morphological findings, according to the WHO criteria. Patients' clinical presentations and laboratory data were obtained from medical records and reviewed. Laboratory findings such as platelet and white blood cell counts; hemoglobin, beta2-microglobulin (β2-MG), serum albumin, and serum lactate dehydrogenase (LDH) levels; serum protein electrophoresis results; and immunofixation results were reviewed. Additionally, the cytogenetic and morphologic results of bone marrow biopsies were reviewed.
Treatment and definition of response
Considering the small study cohort and the heterogeneous nature of the associated treatment regimens, data and outcomes are described for each patient. Response to treatment was evaluated using consensus-based uniform response criteria that were established at the 6th International Workshop on LPL/WM [8]. Complete response (CR) was defined as immunofixation negativity in serum and no histologic evidence of bone marrow involvement with any resolution of adenopathy/organomegaly and other signs of symptoms. Very good partial response was defined as a ≥90% reduction in M protein; partial response (PR) was defined as at least a 50% reduction in serum M protein and reduction in extramedullary disease without any evidence of new symptoms or signs of active disease. Stable disease (SD) was defined as a <25% reduction and <25% increase in serum IgM by electrophoresis without progression of adenopathy/organomegaly, cytopenias, or clinically significant symptoms due to disease and/or signs of WM. Minor response (MR) was defined as at least a 25% reduction in M protein; progressive disease (PD) was defined as a 25% increase in serum IgM by protein electrophoresis, confirmed by a second measurement.
Statistical analysis
Patient characteristics and treatment outcomes were summarized with the use of descriptive statistics. Overall survival (OS) was measured from the date of diagnosis to the date of death from any cause; survivors were tracked to the time of the last follow-up. Progression-free survival (PFS) was measured from the date of diagnosis to the date of progression or death from any cause, whichever occurred first. OS and PFS curves were estimated using the Kaplan-Meier method and compared using the log-rank test. Analyses were performed using IBM SPSS Statistics, version 23.0 (IBM, Armonk, NY, USA).
RESULTS
Clinical characteristics of patients
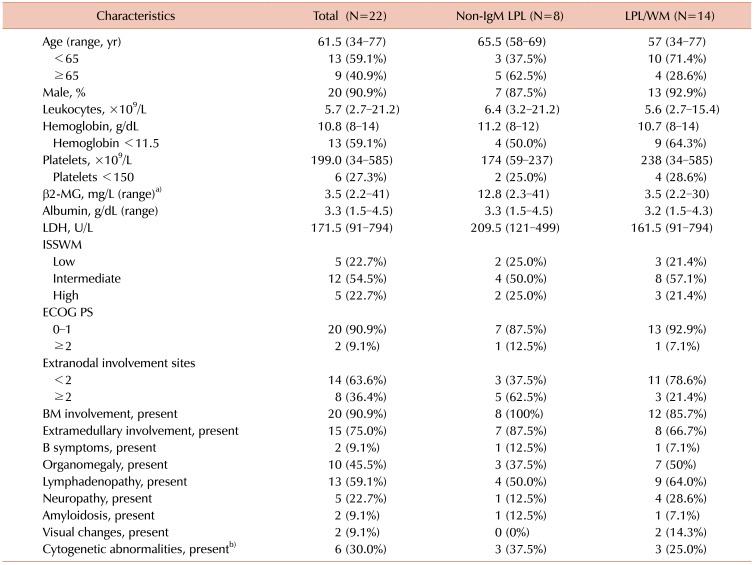
A total of 22 patients with LPL were identified, including 8 patients with non-IgM LPL and 14 patients with LPL/WM [identified from within our non-Hodgkin lymphoma (NHL) cohort of 4,469 patients], at the time of analysis. The baseline characteristics of patients with LPL, consisting of patients with non-IgM LPL and LPL/WM, are summarized in Table 1. LPL was more common among men (N=20, 90.9%) with a median age at diagnosis of 61.5 years (range, 34–77 yr). Regarding the International Prognostic Scoring System for Waldenström's Macroglobulinemia classification, approximately three-quarters of patients (77.2%) were in the low or intermediate risk group. There was bone marrow involvement in all except 2 (90.9%) patients. Approximately 75% (N=20) of patients had extramedullary involvement.
Table 1. Baseline patient characteristics.
a)One patient with LPL/WM was excluded because of missing data for β2-MG. b)Two patients with LPL/WM were excluded because of missing data for cytogenetics.
Abbreviations: β2-MG, beta2-microglobulin; BM, bone marrow; ECOG PS, Eastern Cooperative Oncology Group Performance Status; ISSWM, International Prognostic Scoring System for Waldenström's Macroglobulinemia; LDH, lactate dehydrogenase; LPL, lymphoplasmacytic lymphoma; WM, Waldenström's macroglobulinemia.
The most common symptoms at initial presentation were dyspnea on exertion (N=4, 18.2%) due to anemia (N=2), pleural effusion (N=1), or heart failure (N=1) secondary to cardiac amyloidosis, and edema with newly developed or progressive azotemia (N=4, 18.2%). Four (18%) patients were asymptomatic. All of the asymptomatic patients initially visited a clinic for reversed albumin/globulin ratios on a regular screening test. Median LDH and β2-MG levels were 171.5 U/L (range, 91–794 U/L) and 3.5 µg/L (range, 2.2–41 µg/L), although serum β2-MG data were available for only 21 patients.
Comparison between non-IgM LPL and LPL/WM
We compared non-IgM LPL patients with LPL/WM patients using descriptive statistics, given the relatively small sample sizes of these 2 groups. In the non-IgM LPL cohort, 5 (62.5%) patients exhibited IgG paraproteins, 1 (12.5%) patient exhibited IgA paraproteins, and 2 (25.0%) patients were non-secretory. The median age of the non-IgM LPL group was older than that of the LPL/WM group, with similar gender distributions. Regarding symptoms at initial presentation in the 2 groups, edema with newly developed or progressive azotemia (N=3, 37.5%) was the most common symptom in the non-IgM LPL group, while dyspnea on exertion (N=3, 21.4%) and dizziness due to anemia (N=3, 21.4%) were the most common symptoms in patients with LPL/WM. The laboratory results were similar across the 2 groups, except for β2-MG and LDH levels (non-IgM LPL group vs. LPL/WM group; 12.8 µg/L vs. 3.5 µg/L, 210 µg/L vs. 162 U/L, respectively).
Treatment and outcome
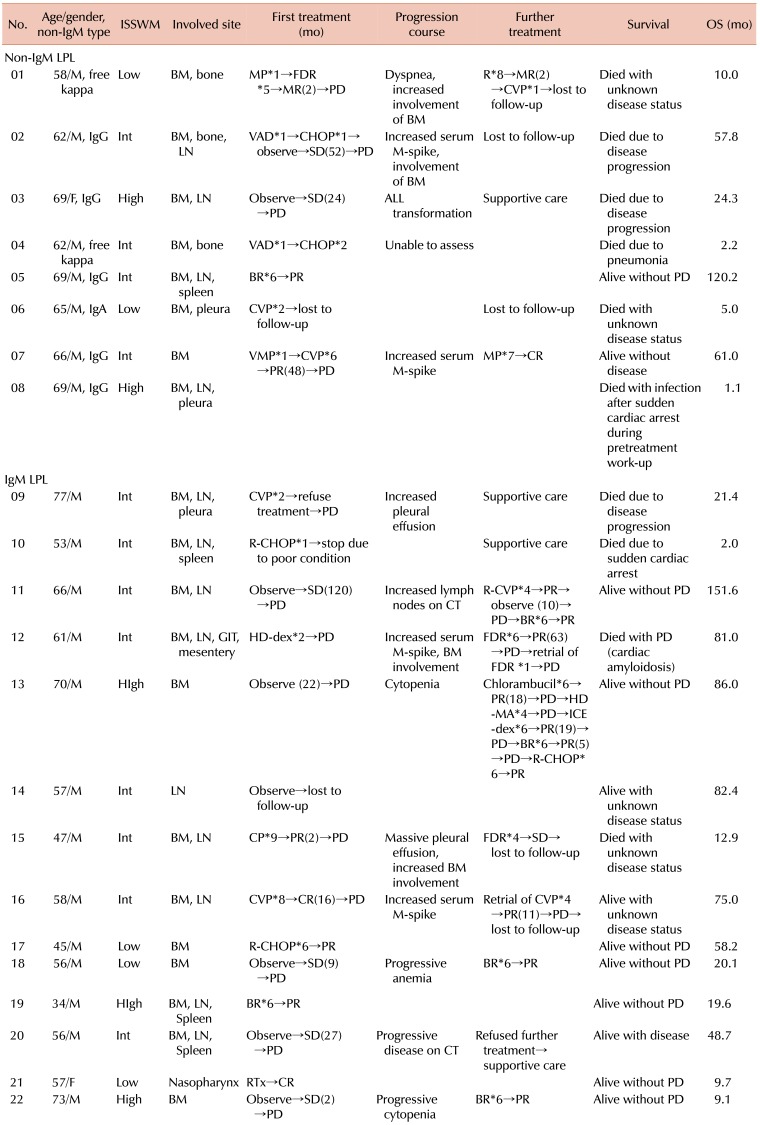
Disease courses and treatment outcomes of the 22 patients with LPL are summarized in Table 2. Fourteen patients with systemic symptoms or organ impairment were treated immediately after diagnosis, while 7 patients without systemic symptoms were only observed. One patient died of infection after sudden cardiac arrest unrelated to the disease during pretreatment work-up. Four patients received rituximab (R)-based treatment including R combined with cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) and R combined with bendamustine (BR). CHOP or CHOP-like regimens, including CVP or CP regimens that consisted of cyclophosphamide and prednisolone, with or without vincristine, were administered to 7 patients. Fludarabine or high-dose dexamethasone was used as a first-line treatment for 2 patients showing durable responses. Among the 7 patients who were observed at the time of diagnosis, 6 showed SD durations from 2 months (patient No. 22) to 120 months (patient No. 11). One patient (patient No. 21) received radiation therapy to the primary lesion (nasopharynx) without systemic therapy and showed CR at the time of analysis.
Table 2. Outcomes and treatments of patients.
Abbreviations: BM, bone marrow; GIT, gastrointestinal tract; LN, lymph node; LPL, lymphoplasmacytic lymphoma; OS, overall survival; BR, bendamustine+rituximab; R, rituximab; CHOP, cyclophosphamide+doxorubicin+vincristine+prednisolone; C(V)P, cyclophosphamide+(vincristine)+prednisolone; FDR, fludarabine; HD-dex, high-dose dexamethasone; HD-MA, high-dose methotrexate+ara-C; ICE-dex, ifosfamide+carboplatin+etoposide+dexamethasone; MP, melphalan+prednisolone; VAD, vincristine+doxorubicin+dexamethasone; VMP, bortezomib+melphalan+prednisolone; ISSWM, International Prognostic Scoring System for Waldenstrom Macroglobulinemia; Int, Intermediate; CR, complete response; PR, partial response; MR, minimal response; SD, stable disease; PD, progressive disease.
Among the 11 patients who experienced disease progression or recurrence after the initial treatment, 2 patients refused further treatment, while 9 patients received salvage treatment regimens. Salvage treatments included R-based regimens, fludarabine, CVP, and chlorambucil and MP (melphalan+prednisolone). The R-based regimens were most commonly used for salvage treatment and included R alone (patient No. 1), R-CVP (patient No. 11), and BR (patients No. 18 and 22). Fludarabine was administered to 2 patients. Intriguingly, 1 patient who received the MP regimen as salvage treatment achieved CR at the time of analysis. This patient initially received 6 cycles of CVP regimen as first-line treatment.
Overall survival and progression-free survival
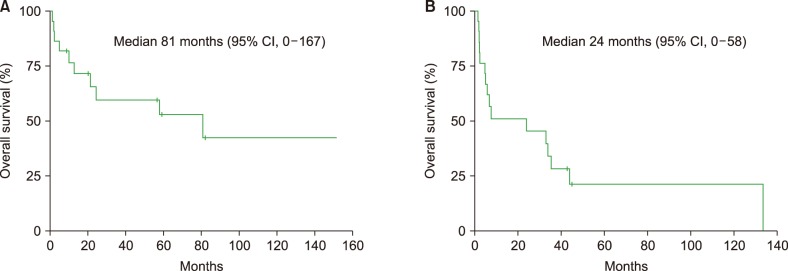
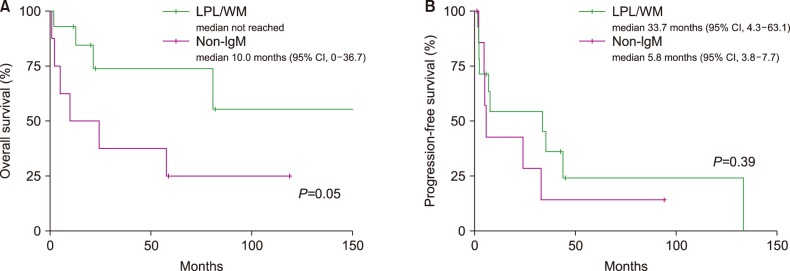
Of the 22 patients in the LPL cohort, 10 patients had died by the time of analysis. Four patients died of disease progression, 2 died of unknown causes, 2 died of infection, and 1 died of sudden cardiac arrest unrelated to the disease. The median follow-up duration for the 22 patients was 75 months [95% confidence interval (CI), 48–102 mo] in surviving patients. The median OS and PFS were 81 months (95% CI, 0–167 mo) and 24 months (95% CI, 0–58 mo; Fig. 1A, B), respectively. OS was better in the LPL/WM group than in the non-IgM LPL group [median not reached vs. 10.0 mo (95% CI, 0–36.7); P=0.05], while the median PFS rates were not significantly different between the 2 groups [33.7 mo (95% CI, 4.3–63.1) vs. 5.8 mo (95% CI, 3.8–7.7); P=0.39] (Fig. 2A, B, Table 3). Compared to patients who received conventional chemotherapy as first-line therapy, patients who received the R-based regimen showed numerically better OS [R-based regimen vs. conventional regimen; median not reached vs. 57.8 mo (95% CI, 0–130.1); P=0.49] and PFS [median not reached vs. 7.6 mo (95% CI, 0–30.5); P=0.16] (Supplementary Fig. S1A,B), although these differences were not statistically significant.
Fig. 1. Overall survival (A) and progression-free survival (B) curves in overall lymphoplasmacytic lymphoma patients.
Fig. 2. Overall survival (A) and progression-free survival (B) curves in patients with lymphoplasmacytic lymphoma (LPL)/Waldenström's macroglobulinemia (WM) and non-IgM LPL.
Table 3. OS and PFS of patients in the 2 groups (LPL/WM vs. non-IgM).
Abbreviations: CI, confidence interval; LPL, lymphoplasmacytic lymphoma; OS, overall survival; PFS, progression-free survival; WM, Waldenström's macroglobulinemia.
DISCUSSION
LPL accounts for <1% of NHL [3], and the incidence of LPL is approximately 8.3 cases per million per year in Western countries [9]. The incidence of LPL in Asia is 10-fold lower than that in Western countries [4]. In Korea, LPL accounts for 0.8%–1.7% of all cases of NHL [10,11]. We evaluated the clinical characteristics and treatment outcomes of 22 patients with LPL from among the 4,469 patients within our NHL registry, which has been maintained at our institution for 15 years. Here, patients with LPL were 0.5% of those with NHL. Although previous studies reported the incidence of non-IgM LPL as <5% of LPL cases [12], our incidence (36.3%) was markedly higher. However, since there are few studies on the incidence of non-IgM LPL in Asia, this finding warrants further population-based investigations.
In the current study, LPL was common in elderly patients with a median age of 61.5 years, and patients in the LPL cohort were predominantly male (20/22, 90.9%), consistent with previous reports [11,13]. Patients with LPL/WM in Korea were more commonly male (77.0%–90.9%) than those in Western countries (54.5%–66.0%) despite the similar median age of diagnosis between Korea (57–66 yr) and Western countries (62–69 yr) [14,15]. However, unlike a previous report [3], patients in the non-IgM LPL cohort were elderly with a median age of 65.5 years with a male preponderance (N=7, 87.5%), although further studies based on larger patient samples are required to verify this result. Notably, extramedullary involvement was more common in the non-IgM LPL group than in patients with LPL/WM. This was because a number of non-IgM LPL patients had lymph node, lytic bone lesion, or pleural involvement. This finding was also consistent with the finding of the previous report that indicated that the rate of extramedullary involvement was significantly higher in patients with non-IgM LPL [16].
Interestingly, half (N=4) of patients (patients No. 1, 2, 4, and 7) in the non-IgM group were initially diagnosed with multiple myeloma and received 1 cycle of VAD, VMP, or MP treatment. In contrast, all patients in the LPL/WM group were appropriately diagnosed initially. Of the 4 patients misdiagnosed with multiple myeloma at initial presentation, 3 had osteolytic bone lesions, whereas the other patient had no bone lesions. This patient without bone lesions was referred from another institution with a diagnosis of multiple myeloma and had already undergone 1 cycle of VMP treatment. All 4 patients were reclassified to non-IgM LPL group after confirmation of results via bone marrow biopsy. This suggests that the diagnosis of non-IgM LPL can be challenging, given that some plasma cell myelomas can exhibit lymphoplasmacytoid features at the histomorphological level and resemble low-grade lymphomas, particularly in cases of osteolytic lesions [17,18]. Moreover, plasma cell neoplasms producing IgM are relatively rare, while those producing IgG or IgA are common. This may be one of the reasons why we observed patients misdiagnosed as having multiple myeloma only in the non-IgM LPL group.
Although there are no prospective studies regarding standard treatment regimens for patients with LPL, current National Comprehensive Cancer Network guidelines recommend treatment including alkylating agents, nucleoside analogs, bortezomib, and R for symptomatic patients with LPL/WM [12]. Treatment of patients with non-IgM LPL also follows these guidelines. In this study, a total of 9 patients with LPL were available for response evaluation, and 6 of 9 patients received an R-based regimen, including R-CHOP and BR, as well as a CHOP or CHOP-like regimen, including CVP and CP, and all patients achieved CR or PR. Currently, R-CHOP and BR are commonly-used combination regimens based on their active treatment efficacy of at least 90% overall response rates in previous randomized trials, supporting response in our cases [19,20]. However, the lack of significant changes in OS [R-based regimen vs. conventional regimen; median not reached vs. 57.8 mo (95% CI, 0–130.1); P=0.49] and PFS [median not reached vs. 7.6 mo (95% CI, 0–30.5); P=0.16] in patients who received the R-based regimen as first-line therapy should be verified in future studies.
In the current study, the median OS of patients with LPL was 81 months (95% CI, 0–167 mo), which is comparable to a previous Korean report (70.8 mo, 95% CI, 31–109 mo). In contrast to PFS in the LPL/WM and non-IgM groups, OS was better in the LPL/WM group than in the non-IgM group in the present study, although this finding should be cautiously interpreted given the small sample size. Furthermore, there appears to be a higher mortality rate in the non-IgM LPL group than in the LPL/WM group in terms of 1- and 5-year mortality in our case series. Four (50%) and 7 (75%) patients in the non-IgM LPL group died within 1 and 5 years, respectively, while 1 (8.3%) patient and 3 (25%) patients in the LPL/WM group died within 1 and 5 years, respectively. Although 2 patients died of causes unrelated to disease progression within 1 year, the mortality rate within 5 years in the non-IgM LPL group suggests that patients with non-IgM LPL might have a worse prognosis than those with LPL/WM. This hypothesis is supported by a previous study that showed a shorter survival in patients with non-IgM within 1 year [3]. Furthermore, the previous study reported that a worse prognosis in patients with non-IgM LPL was associated with a higher frequency of extramedullary involvement, which is consistent with our results. In addition, age and β2-MG are known prognostic factors in patients with LPL/WM [21,22,23,24]. In the present study, the non-IgM LPL group was older and had a higher median level of β2-MG than the LPL/WM group. This may partly explain the poorer survival outcomes in the non-IgM LPL group compared with the LPL/WM group.
Our study is limited by its retrospective design and the small number of patients. In addition, a number of patients were lost to follow-up or refused further treatments, which rendered response evaluation available for only 9 patients, and data on MYD88 mutations in our registry were not available for most patients. Considering the small sample size, we were unable to perform formal statistical comparisons regarding clinical characteristics between the groups or evaluate prognostic factors. Moreover, the higher incidence of patients with non-IgM LPL may reflect a referral bias inherent to the study hospital. Furthermore, each patient received heterogeneous treatments, which likely confounded our study outcomes.
In this study, we described our 15 years of experience in evaluating the clinical characteristics and treatment outcomes of patients with LPL at a single institution. Moreover, we reviewed the clinical characteristics and treatment outcomes of patients with non-IgM LPL within our cohort and compared their clinical features with those of patients with LPL/WM. The treatment of non-IgM LPL was heterogeneous, and patients with non-IgM LPL showed a higher 5-year mortality rate and more adverse prognostic factors than those with LPL/WM.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
SUPPLEMENTARY MATERIAL
Overall survival (A) and progression-free survival (B) of patients treated with rituximab-based regimen and conventional chemotherapy.
References
- 1.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Harris NL, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC Press; 2008. [Google Scholar]
- 3.Cao X, Medeiros LJ, Xia Y, et al. Clinicopathologic features and outcomes of lymphoplasmacytic lymphoma patients with monoclonal IgG or IgA paraprotein expression. Leuk Lymphoma. 2016;57:1104–1113. doi: 10.3109/10428194.2015.1096357. [DOI] [PubMed] [Google Scholar]
- 4.Iwanaga M, Chiang CJ, Soda M, et al. Incidence of lymphoplasmacytic lymphoma/Waldenström's macroglobulinaemia in Japan and Taiwan population-based cancer registries, 1996–2003. Int J Cancer. 2014;134:174–180. doi: 10.1002/ijc.28343. [DOI] [PubMed] [Google Scholar]
- 5.Kristinsson SY, Björkholm M, Goldin LR, McMaster ML, Turesson I, Landgren O. Risk of lymphoproliferative disorders among first-degree relatives of lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia patients: a population-based study in Sweden. Blood. 2008;112:3052–3056. doi: 10.1182/blood-2008-06-162768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström's macroglobulinemia. N Engl J Med. 2012;367:826–833. doi: 10.1056/NEJMoa1200710. [DOI] [PubMed] [Google Scholar]
- 7.King RL, Gonsalves WI, Ansell SM, et al. Lymphoplasmacytic lymphoma with a non-IgM paraprotein shows clinical and pathologic heterogeneity and may harbor MYD88 L265P mutations. Am J Clin Pathol. 2016;145:843–851. doi: 10.1093/ajcp/aqw072. [DOI] [PubMed] [Google Scholar]
- 8.Owen RG, Kyle RA, Stone MJ, et al. Response assessment in Waldenström macroglobulinaemia: update from the VIth International Workshop. Br J Haematol. 2013;160:171–176. doi: 10.1111/bjh.12102. [DOI] [PubMed] [Google Scholar]
- 9.Sant M, Allemani C, Tereanu C, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116:3724–3734. doi: 10.1182/blood-2010-05-282632. [DOI] [PubMed] [Google Scholar]
- 10.Ko YH, Kim CW, Park CS, et al. REAL classification of malignant lymphomas in the Republic of Korea: incidence of recently recognized entities and changes in clinicopathologic features. Hematolymphoreticular Study Group of the Korean Society of Pathologists. Revised European-American lymphoma. Cancer. 1998;83:806–812. [PubMed] [Google Scholar]
- 11.Won YW, Kim SJ, Kim K, Ko YH, Kim WS. Clinical features and treatment outcomes of lymphoplasmacytic lymphoma: a single center experience in Korea. Ann Hematol. 2010;89:1011–1018. doi: 10.1007/s00277-010-0978-1. [DOI] [PubMed] [Google Scholar]
- 12.The National Comprehensive Cancer Network. NCCN Guidelines for patients. Waldenström's macroglobulinemia: lymphoplasmacytic lymphoma. Version 1. Fort Washington, PA: NCCN; 2017. [Accessed November 25, 2017]. at https://www.nccn.org/patients/guidelines/waldenstroms/files/assets/common/downloads/files/waldenstroms.pdf. [Google Scholar]
- 13.Vitolo U, Ferreri AJ, Montoto S. Lymphoplasmacytic lymphoma-Waldenstrom's macroglobulinemia. Crit Rev Oncol Hematol. 2008;67:172–185. doi: 10.1016/j.critrevonc.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Bang SM, Seo JW, Park KU, et al. Molecular cytogenetic analysis of Korean patients with Waldenström macroglobulinemia. Cancer Genet Cytogenet. 2010;197:117–121. doi: 10.1016/j.cancergencyto.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Lee HS, Kim K, Yoon DH, et al. Clinical factors associated with response or survival after chemotherapy in patients with Waldenström macroglobulinemia in Korea. Biomed Res Int. 2014;2014:253243. doi: 10.1155/2014/253243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tursz T, Brouet JC, Flandrin G, Danon F, Clauvel JP, Seligmann M. Clinical and pathologic features of Waldenström's macroglobulinemia in seven patients with serum monoclonal IgG or IgA. Am J Med. 1977;63:499–502. doi: 10.1016/0002-9343(77)90193-0. [DOI] [PubMed] [Google Scholar]
- 17.Braggio E, Fonseca R. Genomic abnormalities of Waldenström macroglobulinemia and related low-grade B-cell lymphomas. Clin Lymphoma Myeloma Leuk. 2013;13:198–201. doi: 10.1016/j.clml.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Heerema-McKenney A, Waldron J, Hughes S, et al. Clinical, immunophenotypic, and genetic characterization of small lymphocyte-like plasma cell myeloma: a potential mimic of mature B-cell lymphoma. Am J Clin Pathol. 2010;133:265–270. doi: 10.1309/AJCPUS3PRRT5ZXVS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimopoulos MA, García-Sanz R, Gavriatopoulou M, et al. Primary therapy of Waldenstrom macroglobulinemia (WM) with weekly bortezomib, low-dose dexamethasone, and rituximab (BDR): long-term results of a phase 2 study of the European Myeloma Network (EMN) Blood. 2013;122:3276–3282. doi: 10.1182/blood-2013-05-503862. [DOI] [PubMed] [Google Scholar]
- 20.Treon SP, Ioakimidis L, Soumerai JD, et al. Primary therapy of Waldenström macroglobulinemia with bortezomib, dexamethasone, and rituximab: WMCTG clinical trial 05-180. J Clin Oncol. 2009;27:3830–3835. doi: 10.1200/JCO.2008.20.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owen RG, Barrans SL, Richards SJ, et al. Waldenström macroglobulinemia. Development of diagnostic criteria and identification of prognostic factors. Am J Clin Pathol. 2001;116:420–428. doi: 10.1309/4LCN-JMPG-5U71-UWQB. [DOI] [PubMed] [Google Scholar]
- 22.Morel P, Monconduit M, Jacomy D, et al. Prognostic factors in Waldenström macroglobulinemia: a report on 232 patients with the description of a new scoring system and its validation on 253 other patients. Blood. 2000;96:852–858. [PubMed] [Google Scholar]
- 23.Morel P, Duhamel A, Gobbi P, et al. International prognostic scoring system for Waldenstrom macroglobulinemia. Blood. 2009;113:4163–4170. doi: 10.1182/blood-2008-08-174961. [DOI] [PubMed] [Google Scholar]
- 24.Merlini G, Baldini L, Broglia C, et al. Prognostic factors in symptomatic Waldenstrom's macroglobulinemia. Semin Oncol. 2003;30:211–215. doi: 10.1053/sonc.2003.50064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overall survival (A) and progression-free survival (B) of patients treated with rituximab-based regimen and conventional chemotherapy.