TO THE EDITOR: We read with great interest the recent paper “A case of synchronous multiple myeloma and chronic myeloid leukemia” published by Lee et al. [1] and report here a similar case that further highlights the management challenges in such complex cases. A 39-year-old female presented with left hip pain 5 years ago and a plain film showed a large lytic lesion in the neck of the femur. Her complete blood count revealed a hemoglobin level of 12 g/dL, platelet count of 233×109/L and total white blood cell (WBC) count of 10.8×109/L with normal serum calcium and creatinine levels. A bone marrow biopsy showed 40% bone marrow plasma cells (Fig. 1), lambda chain levels were elevated at 2,760 mg/L with a free light chain ratio of 0.0004, resulting in a diagnosis of multiple myeloma (MM). She completed 6 cycles of cyclophosphamide, bortezomib and dexamethasone chemotherapy in conjunction with radiation therapy to the fracture site and received monthly bisphosphonate infusions. This was consolidated with a melphalan-conditioned autologous stem cell transplant. She did not receive maintenance lenalidomide therapy given a previous history of pulmonary embolism. Two and a half years following her initial diagnosis, despite a normal serum free light chain ratio, it was noted that she had a rising WBC count with a peak level of 40.2×109/L, and a platelet count of 204×109/L, neutrophil count of 32.2×109/L, monocyte count of 2.7×109/L and basophil count of 0.59×109/L. Molecular analysis of the peripheral blood did not identify a JAK-2 mutation, however the BCR-ABL ratio, measured by real-time quantitative polymerase chain reaction, was elevated at 140.6%. Bone marrow (BM) sampling showed marked granulocytic hyperplasia without excess blasts consistent with chronic-phase chronic myeloid leukemia (CML) (Fig. 2). There was no increase in plasma cells and BM karyotype confirmed the presence of an abnormal clone that contained a translocation between the long arms of chromosomes 9 and 22 with breakpoints at 9q34 and 22q11.2. This BCR-ABL rearrangement was analyzed in 192 out of 200 cells and the results were consistent with CML. The patient initially commenced imatinib therapy at 400 mg daily, but at 6 months, her disease control was suboptimal with a BCR-ABL ratio of 23%. She subsequently started nilotinib 400 mg twice daily resulting in an improved molecular response with a most recent transcript ratio of 1.4%. Her most recent BM aspirate showed CML in morphological remission and her free light chain ratio remained normal in line with ongoing MM remission.
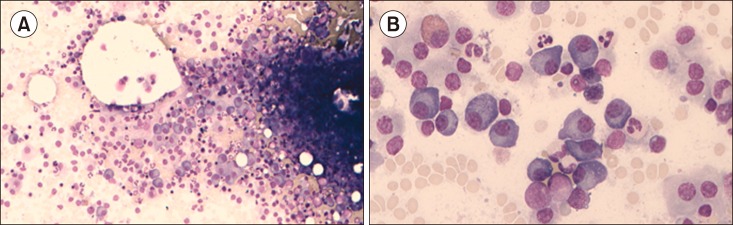
Fig. 1. Bone marrow at the time of multiple myeloma (MM) diagnosis. A hypercellular particle with atypical plasma cells under low power (A). Numerous atypical plasma cells under high power (B).
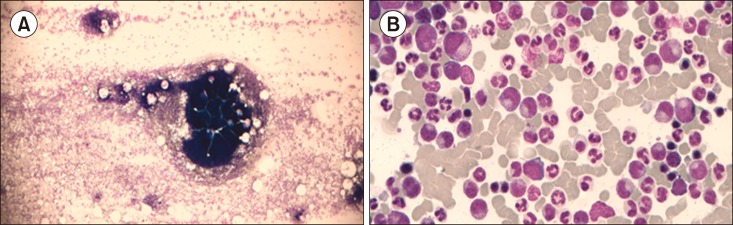
Fig. 2. Bone marrow at the time of chronic myeloid leukemia (CML) diagnosis. A hypercellular particle under low power (A). A high-power view of granulocytic hyperplasia (B).
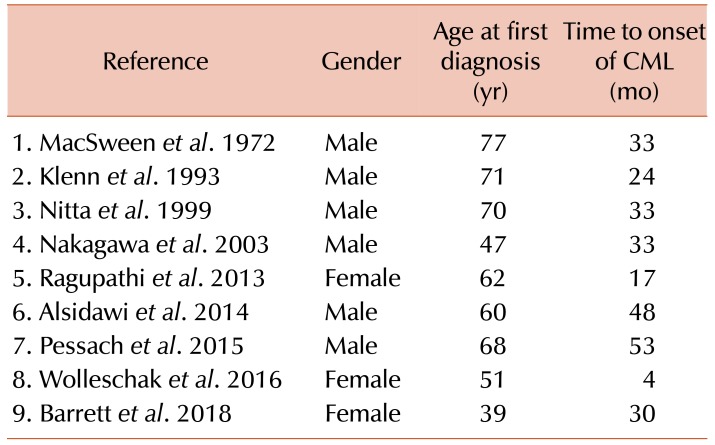
The co-diagnosis of MM and CML is a vanishing rare phenomenon, with literature on this topic confined to case reports and small series. Clonal expansion in both the lymphoid and the myeloid cell lineages is implied as pathology of the disease. Possible hypotheses for this include the development of a common malignant pluripotent hematopoietic stem cell, treatment-related toxicity of myeloid cells and environmental factors [2]. To date, there have been 27 published reports of cases of co-diagnosis of MM and CML. Eight of these included patients who initially presented with MM and were subsequently diagnosed with CML, as in our case (Table 1) [3,4,5,6,7,8,9,10]. The mean time to CML diagnosis in this cohort was 30.6 months (4–53 mo), comparable to the 30 months till onset of CML in our case. Six of 8 patients were male, and the mean age of the cohort at first diagnosis was 63.3 years (47–77 yr). Therefore, our patient is the youngest recorded patient to have developed this disease. Additionally, of note, and to the best of our knowledge, there has been no other case described to date that developed CML following autologous stem cell transplant.
Table 1. Reported cases of CML following MM diagnosis.
Abbreviations: CML, chronic myeloid leukemia; MM, multiple myeloma.
There are no evidence based-guidelines for the management of these patients. In 2 similar cases compared to our case, the use of imatinib led to stable remission of CML [3,5], and in 1 other case treatment with the more recent tyrosine kinase inhibitor (TKI) dasatinib also led to remission [10]. Another report described an elderly male patient with relapsed MM who was diagnosed with CML at the time of relapse. His disease was controlled after 11 months of treatment with bortezomib, dexamethasone, and dasatinib before he died of pulmonary hypertension [4]. There are no reports thus far of a similar patient who underwent allogeneic stem cell transplantation.
In conclusion, we here reported the youngest patient diagnosed with CML following successful treatment of MM and the first patient who has developed CML following autologous stem cell transplantation. We also described the initial poor response to first-line TKI therapy with subsequent improved disease control after use of second-line therapy.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Lee JY, Lee SM, Yoon HK, Kim KH, Choi MY, Lee WS. A case of synchronous multiple myeloma and chronic myeloid leukemia. Blood Res. 2017;52:219–222. doi: 10.5045/br.2017.52.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas A, Mailankody S, Korde N, Kristinsson SY, Turesson I, Landgren O. Second malignancies after multiple myeloma: from 1960s to 2010s. Blood. 2012;119:2731–2737. doi: 10.1182/blood-2011-12-381426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolleschak D, Heidel FH. Chronic myelogenous leukemia evolving after treatment of multiple myeloma. Blood. 2016;128:146. doi: 10.1182/blood-2016-03-706945. [DOI] [PubMed] [Google Scholar]
- 4.Alsidawi S, Ghose A, Qualtieri J, Radhakrishnan N. A case of multiple myeloma with metachronous chronic myeloid leukemia treated successfully with bortezomib, dexamethasone, and dasatinib. Case Rep Oncol Med. 2014;2014:962526. doi: 10.1155/2014/962526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pessach I, Bartzis V, Tzenou T, et al. Multiple myeloma and chronic myelogenous leukemia; an uncommon coexistence in 2 patients, with literature review. Ann Hematol Oncol. 2015;2:1030. [Google Scholar]
- 6.Nakagawa M, Noto S, Kobayashi H, Hayashi M. A case of a 47 year old man who developed chronic myelogenous leukemia after therapy for multiple myeloma. J Obihiro Kosei Gen Hosp. 2003;6:101–106. [Google Scholar]
- 7.Nitta M, Tsuboi K, Yamashita S, et al. Multiple myeloma preceding the development of chronic myelogenous leukemia. Int J Hematol. 1999;69:170–173. [PubMed] [Google Scholar]
- 8.Klenn PJ, Hyun BH, Lee YH, Zheng WY. Multiple myeloma and chronic myelogenous leukemia-a case report with literature review. Yonsei Med J. 1993;34:293–300. doi: 10.3349/ymj.1993.34.3.293. [DOI] [PubMed] [Google Scholar]
- 9.MacSween JM, Langley GR. Light-chain disease (hypogammaglobulinemia and Bence Jones proteinuria) and sideroblastic anemia-preleukemic chronic granulocytic leukemia. Can Med Assoc J. 1972;106:995–998. [PMC free article] [PubMed] [Google Scholar]
- 10.Ragupathi L, Najfeld V, Chari A, Petersen B, Jagannath S, Mascarenhas J. A case report of chronic myelogenous leukemia in a patient with multiple myeloma and a review of the literature. Clin Lymphoma Myeloma Leuk. 2013;13:175–179. doi: 10.1016/j.clml.2012.09.010. [DOI] [PubMed] [Google Scholar]