Abstract
The development of edible films and coatings from food-grade biopolymers has advanced significantly during the past decade. The current state-of-the-art lies in the formulation of composite edible films and coatings from such biomolecules. Composite films and coatings refer to systems where multiple biopolymers have been combined to achieve beneficial properties. Carbohydrate, protein, and lipids have been preferred for developing such systems. Binary films and coatings have been prepared from multiple combinations including protein–protein, carbohydrate–carbohydrate and protein-carbohydrate. Similarly, ternary films and coatings have been prepared from protein–protein–carbohydrate combinations. In addition, several active ingredients including antimicrobial compounds have been loaded to these systems for the preparation of functional films and coatings. Therefore, the goal of this manuscript is to review the multitude of composite systems that are currently available for food packaging purposes. In addition, we discuss the application of composite coatings to fruits and vegetables, dairy, meat and seafood as model food systems.
Keywords: Composite, Binary, Ternary, Edible films and coatings, Food packaging
Introduction
The usages of minimally-processed foods such as fresh fruits and vegetables have found great applications in the modern world. There is a persistent demand for nutritious and healthy foods across all sections of the society. Different techniques have been utilized for enhancing the shelf-life and quality of foods. One such technique is by the use of edible films and coatings. The preparation of films and coating materials from food-grade biopolymers is an emerging area of research. In general, edible film and coating system perform multiple functions when applied to food systems. For example, they bolster the natural structure of the food and provide resistance against moisture loss. In addition, they allow controlled exchange of gases required for respiration. These systems can also be used as delivery vehicles for the prolonged protection and controlled release of different active ingredients such as vitamins, antimicrobial compounds and antioxidants (Gutiérrez 2017). Some model scenario in food processing where edible films and coatings have found great applications include enhancing the shelf-life of fresh produce, reducing oil absorption during frying process and reducing the rate of aroma and color loss during prolonged storage of food systems (Khazaei et al. 2016).
Even though the terms edible films and coatings are used together as a phrase, there is a fundamental difference between them. Coatings are systems where the food product is dipped into a liquid which contains the biopolymers (Kang et al. 2013). On the other hand, films are systems that are prepared as sheets and then used as a wrapper on food materials (Falguera et al. 2011). Based on the hydrophobic-hydrophilic characteristics of biomolecules, the properties of edible films and coatings can be manipulated. For example, hydrophobic molecules demonstrate strong moisture barrier properties whereas hydrophilic molecules demonstrate efficient mechanical properties. In addition, such hydrophilic biopolymers are also good gas barriers such as oxygen and carbon dioxide. Therefore, in general, these molecules with varying characteristics can be combined to yield films and coatings with added advantages. In addition to beneficial mechanical or barrier properties, these biopolymers also confer a certain amount of nutritional value as well. For example, a number of health advantages have been reported including cardiovascular health effects, immune development, obesity control and gastrointestinal issues (Gutiérrez 2017).
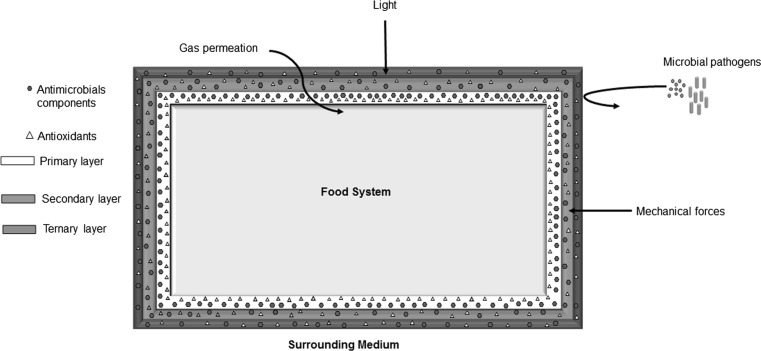
In recent years, a major thrust of food packaging research has been in the area of composite films and coatings. Composite films can also be termed as multicomponent systems. These films are characterized by the complexation of a hydrophilic layer with a hydrophobic layer (including lipids) which provides excellent barrier properties (Tavassoli-Kafrani et al. 2016). In addition, emulsion-based films are also being formulated where the lipid layer is uniformly distributed within the biopolymer matrix. Based on the number of biopolymers, composite edible films have been classified as binary or ternary as shown in Fig. 1. A classical example of a binary edible film is a combination of κ-carrageenan and locust bean gum (LBG) (Martins et al. 2012). In such systems, several combinations of carbohydrate–carbohydrate, carbohydrate–protein and protein–protein are possible (Podshivalov et al. 2017; Suppakul et al. 2016; Thakur et al. 2016). Extensive literature exists for composite films and coatings prepared from the combination of two hydrocolloids, but the combination of three hydrocolloids for the formulation of edible films or coating is rare.
Fig. 1.
Schematic representation of barrier properties of composite edible films and coatings
A multitude of polysaccharide-based materials have been used recently in the preparation of edible films including starches from tamarind (Chandra mohan et al. 2016), chitosan and its nanoparticles (Hosseini et al. 2016), cellulose and its derivatives including methyl cellulose and hydroxypropyl methyl cellulose (Choi et al. 2016), pullulan (Pan et al. 2014) and gums. Gums have been extracted from various sources such as gum ghatti (Zhang et al. 2016a), locust bean gum (Martins et al. 2012), and sage seed gum (Razavi et al. 2015). In addition to polysaccharides, proteins have also found enormous applications in the formulation of edible films and coatings. Some of the model proteins include whey protein (Zhang et al. 2016b), sodium caseinate (Eghbal et al. 2016), soy protein isolate (Jia et al. 2009), and collagen (Wang et al. 2017). Therefore, the focus of this review is to compile the latest information regarding formulation and development of composite edible films and coatings. In addition, the application of composite coatings to different food systems including fresh produce, dairy, meat and seafoods have been discussed.
Methods for the preparation of edible films and coatings
Edible films
There are two popular techniques that are used for the preparation of edible films: solvent casting (wet technique) and compression molding or extrusion (dry technique). In the former method, dispersion of edible biopolymeric materials is spread over appropriate base material followed by drying and holding for a suitable time for the development of the film. As a consequence of solvent evaporation during drying, the solubility of the polymer decreases (Tharanathan 2003).
The major drawbacks encountered during peeling operation of the film are wrinkling and tearing. Several investigations report that deleterious effects on film formation can be mitigated with proper selection of the base material. Thus, the optimum moisture content for easy peeling of the film is observed to be between 5 and 8% (Tharanathan 2003).
Edible coatings
Edible coatings can be prepared by three methods: spraying, dipping and spreading. Spraying can be done using classical as well as electro-spraying method. In the classical method, the droplet size of the solution is around 20 μm whereas in electro-spraying the droplet size was around 100 nm (González-Forte et al. 2014). The dipping method of edible coating is applied where thicker coatings are required. The dipping method is generally used to improve the physicochemical quality attributes of minimally processed fruits and vegetables as well as meat products. The coating thickness can be controlled by various parameters such as density, viscosity and surface tension. A special variation in dipping method can be achieved by using a layer-by-layer electrostatic technique where two or more layers can be connected using physical or chemical interactions. Mostly, the electrostatic method has been used to coat confectionary type products. In the third technique, the coating dispersion is brushed on the food material surface. In addition to the above three methods, edible coatings have also been prepared by the combination of brushing and spraying techniques. Firstly, sodium alginate solution was spread by brushing on the surface of biscuits and then calcium chloride solution was applied by spraying method (González-Forte et al. 2014).
Even though edible coatings have demonstrated potential benefits, there are certain challenges associated with such systems. For example, the thickness of edible coatings should be carefully controlled which might lead to potential issues within the food systems. Specifically, a thick coating layer reduces the internal oxygen concentration and increases the carbon dioxide concentration within the food material. This type of situation is detrimental from food quality standpoint since anaerobic fermentation occurs (Dehghani et al. 2018). Therefore, in order to control such issues, several strategies can be adopted. For example, the wettability of edible coatings can be controlled to minimize such a situation. In addition, information about the gas permeation, diffusion characteristics of the food surface and knowledge about the internal gas composition of foods can help to decide the suitability of edible coatings (Dehghani et al. 2018).
Composite edible films and coatings
Binary films and coatings
Some studies involving protein–protein, carbohydrate–carbohydrate, protein–carbohydrate and lipid-based edible films are discussed in the following section.
Protein–protein combination films
Soy protein isolate and corn zein were complexed to form a bi-layer edible film for the coating of olive oil condiments. The laminates were tested for their mechanical, physical and oxidative stability properties of olive oil. The bilayer was compared with monolayer controls. The study reported that addition of corn zein enhanced tensile strength and moisture barrier. Meanwhile, the combination process reduced elongation at break and oxygen barrier properties (Suppakul et al. 2016).
In another study, zein was combined with gliadin which improved film flexibility and decreased film brittleness. With an added amount of gliadins, elongation at break improved significantly. However, gliadin demonstrated minor impact on film moisture content and solubility. Both Fourier-transform infrared spectroscopy (FTIR) and scanning electron microscope (SEM) data suggested that changes in secondary structures were responsible for improved film brittleness. Glass transition temperature study demonstrated that film moisture content affected thermal characteristics of the films (Gu and Wang 2013).
The composite film was formulated from barley bran protein and gelatin. Mechanical properties such as tensile strength and elongation at break were measured (Song et al. 2012). The study concluded that barley bran protein could be used as a suitable candidate because of excellent film-forming capability. Optimum conditions of the film were obtained with 3% barley bran protein, 3% gelatin and 1% plasticizer (sorbitol). In addition, this composite film was combined with grapefruit seed extract for studying antibacterial and antioxidant properties using salmon as the model food. It was reported that Escherichia coli O157:H7 and Listeria monocytogenes count reduced significantly when compared with control preparation (without grapefruit seed extract). In addition, peroxide and Thiobarbituric acid reactive substances (TBARS) value reduced by 23 and 23.4%, respectively (Song et al. 2012).
Salicylic acid and acetyl salicylic acid was used as a filler material for the preparation of the zein films, and their structure and mechanical properties were studied. The results revealed that glycerol level affected the α-helix and β-sheet confirmations within zein microstructure. It was observed that the presence of salicylic acid enhanced β-sheet levels where as acetyl salicylic acid increased α-helix levels. In terms of film mechanical properties, tensile strength, strain at failure and modulus of zein films were studied (Singh et al. 2010). It was observed that zein films without salicylic acid or acetyl salicylic acid incorporation demonstrated the tensile strength of 23.9 MPa. However, with the addition of filler materials, the tensile strength decreased to 12 and 16 MPa. However, strain at failure initially decreased but then increased with enhanced levels of acetyl salicylic acid. Lastly, release kinetics of salicylic acid, and acetyl salicylic acid was studied for 75 min at 37 °C. It was found that glycerol containing zein films demonstrated the lowest level of acetyl salicylic acid. In addition, in glycerol devoid films a gradual increase in acetyl salicylic acid level up to 1% was observed till 75 min, where as in glycerol treated films, acetyl salicylic acid release started after 40 min (Singh et al. 2010).
In a study, focused on the impact of iodine on zein films and zein precipitates, zein films were prepared by solution casting method using glycerol as plasticiser and iodine (2–8%). The secondary structure of the zein films was characterized by FTIR in addition to its dielectric and mechanical properties. It was found that iodine increased the intensity of the α-helix structure. In addition, the relative intensity of the α-helix was higher with glycerol when compared with non-glycerol films. It was observed that the α-helix was found around the wavenumber of 1648–1659 cm−1, while β-sheet was found around 1627–1629 cm−1, 1681–1682 cm−1, and 1694–1697 cm−1. Similarly, random coil and β-turn structures were observed at 1638–1640 cm−1 and 1681–1682 cm−1, respectively. Thus, the study revealed the ability of zein films usage as controlled release matrix and carrier for bioactive components such as iodine (Singh et al. 2009).
Carbohydrate–carbohydrate combination films
In this study, rice starch was combined with ι-carrageenan to form a bi-layered edible film. Stearic acid and glycerol were used as plasticizers with varied concentrations of the biopolymers. A multitude of response parameters was studied including water vapor permeability, solubility, moisture content and color properties. In addition, advanced characterizations were performed using Fourier-transform infrared spectroscopy (FTIR) and X-ray diffraction (XRD) to study biopolymer interactions. The study concluded that the combination process improved tensile strength and elongation at break properties. Meanwhile, FTIR analysis demonstrated that band shifting between 1200 and 1300 cm−1 could be possibly due to interactions between polysaccharides (Thakur et al. 2016).
Chitosan or chitosan nanoparticles were complexed with tara gum and characterized for a host of parameters including thermomechanical, antimicrobial and barrier properties. In addition, thermogravimetric analysis (TGA), FTIR and XRD were also performed to study the thermal stability and molecular interactions, respectively. The microstructure of the nanocomposites was studied using atomic force microscopy and scanning electron microscopy (Antoniou et al. 2015). Tensile strength increased significantly whereas elongation at break reduced by 7.21%. Meanwhile, water solubility and water vapor permeability demonstrated a decreasing trend. The incorporation of chitosan nanoparticles demonstrated a rough surface with uniform distribution. On the other hand, micro-sized chitosan demonstrated stronger antimicrobial activity when compared with nano-sized chitosan (Antoniou et al. 2015).
Water chestnut starch and chitosan were combined for the formulation of bi-layered films containing fruit extract. In addition, the formulations also contained glycerol monolaurate, nisin and pine needle essential oil. The study concluded that addition of fruit extract affected the pH and moisture content of the system. In addition, nisin incorporated films demonstrated better mechanical characteristics. In general, most formulations demonstrated elevated water vapor permeability. All the antimicrobial agents could inhibit bacterial growth significantly when tested against E. coli O157:H7, Staphylococcus aureus and L. monocytogenes (Mei et al. 2013).
Locust bean gum was combined with κ-carrageenan to form edible films. Different analytical techniques such as thermogravimetric analysis, XRD, dynamic mechanical analysis (DMA) and FTIR were used for characterization of the molecular interactions. The study concluded that there was a strong synergy between the two biopolymers. FTIR and TGA data suggested a higher level of miscibility within the biopolymers. In addition, the principal component analysis demonstrated that a combination of 40:60 κ-carrageenan: locust bean gum was the optimum choice for food applications (Martins et al. 2012).
Protein-carbohydrate combination films
A study investigated the microstructure and operational properties for the formation of edible biocomposite films (gelatin/potato starch/glycerol). During the formulation of the biocomposite films, the starch content was varied from 0 to 50%, and further, it was subjected to a drying operation for the duration of 15 h at 36 °C. The impact of the phase separation during the drying operation on the size of starch granules and its influence on various other properties such as mechanical, water-barrier properties and optical properties was investigated. The resultant edible films demonstrated phase separated heterogeneous morphology with gelatin matrix and microgranules of starch as a continuous phase and minor phase, respectively (Podshivalov et al. 2017). The outcome of the study revealed that phase separation is dependent on the starch content. When the starch content was less than or equal to 30 wt% the phase separation mechanism was attributed to nucleation and growth, whereas with starch content was greater than 30 wt%, the dominant mechanism was spinodal decomposition (Podshivalov et al. 2017).
In order to enhance the physicochemical properties of gelatin-based edible films, formulations were prepared with increasing concentration of chitosan. Characterization of the formulated composite films for physical and functional properties was observed. On the one hand, the substitution of chitosan exhibited significant (p < 0.05) increase in tensile strength and elastic modulus. On the other hand, elongation at break was reported to reduce significantly. Furthermore, FTIR and differential scanning calorimetry (DSC) analysis of the structural properties demonstrated that a reasonable interaction between fish gelatin and chitosan with superior mechanical properties (Hosseini et al. 2013).
In another similar study, edible films of chitosan-fish gelatin were subjected to electron beam irradiation. This study was conducted with the goal to determine the effect of irradiation dose of 40 and 60 kGy on the release kinetics of quercetin incorporated in the composite films. Irradiation led to reduced release profile for quercetin leading to entrapment of the antioxidant in the composite film. Quercetin complexation in the films was attributed to the possible occurrence of cross-linking between the biopolymers and quercetin post-irradiation. The non-irradiated film showed 23.4 ± 5.7% retention of quercetin whereas the edible composite film subjected to 60 kGy irradiation dose retained 33.6 ± 2.1% of quercetin (Benbettaïeb et al. 2016).
Recently, interest was drawn on the formulation of composite edible films (gelatin/chitosan) incorporated with antimicrobial extracts for attaining enhanced shelf life (Bonilla and Sobral 2016). Various ethanolic extracts of cinnamon, rosemary, guarana and boldo-do-chile possessing antimicrobial and antioxidant properties were utilized for blending along with the film forming solutions. On one hand, microstructural, optical, mechanical, barrier properties were studied. On the other hand, examination of antimicrobial and antioxidant properties was also carried out. As a consequence of increased chitosan addition, films containing higher elasticity and reduced water vapor permeability were formed. The bioactive-loaded composite films witnessed inhibition of E. coli and S. aureus showcasing higher antimicrobial properties, and Trolox-Equivalent-Antioxidant-Capacity test (TEAC) exhibited good antioxidant properties as well (Bonilla and Sobral 2016).
Several studies have been carried out on the physical properties of emulsion-based films. In a recent study, the microstructure of the emulsion based films was investigated which was formulated by stabilization with hydroxypropyl methyl cellulose (HPMC)/whey protein isolate (WPI) with and without sodium dodecyl sulfate (SDS). These films were compared to HPMC/WPI emulsion-based films. On the evaluation of the film physical properties, it was revealed that the addition of oil and SDS led to an increase in parameters such as whiteness index and ΔE and a decrease in elongation at break and tensile strength (Rubilar et al. 2015).
Bacterial cellulose (BC) films (BC1: produced in the laboratory from a static culture of G. xylinus ATCC 53582; BC2: procured from a commercial source) obtained from two different sources were modified by adsorption to bovine lactoferrin (bLF). These functional films (BC + bLF) possessing antimicrobial properties were applied on meat products, specifically fresh sausages and were tested for various quality parameters. Mechanical and barrier properties along with bactericidal efficacy were evaluated against food-borne pathogens especially E. coli and S. aureus. Composite functional films proved to be antibacterial, non-toxic and could be used for bio-based meat product casings owing to advanced technological characteristics (Padrão et al. 2016).
Cationic chitosan and anionic quinoa protein extract were blended by solution casting technique. Composite films formed from the blend were characterized for film microstructure and mechanical strength. In addition, the film was evaluated for sorption studies. The interaction between chitosan and quinoa protein in the blended films attained improved mechanical properties which were determined by analytical techniques such as XRD, TGA and FTIR. The findings of water barrier properties revealed that the blended films (chitosan/quinoa protein extract) possessed greater hydrophilic character compared to chitosan films (Abugoch et al. 2011).
Chitosan and zein were utilized as base materials for the production of composite films. Furthermore, phenolic compounds (ferulic acid and gallic acid) and dicarboxylic acids were used as substrates to provide functional attributes to the biopolymer composite films. In addition, dicarboxylic acids such as adipic acid or succinic acid was utilized for supplementation of the films. Regarding recovery from the films, it was observed that phenolics had accounted for 71–84%, while the dicarboxylic acids recovered to 48–68%. Antimicrobial efficacy of the films was tested against target microorganisms especially S. aureus and E. coli. The functional films proved to be effective against these food-borne pathogens (Cheng et al. 2015).
Edible films prepared using whey protein isolate were characterized for getting the optimized ratio of WPI and glycerol. The WPI: glycerol ratios selected for the study were 3.6:1; 3:1; and 2:1 with three different WPI concentrations 5, 7 and 9% (w/v). The results revealed that 5% WPI concentration with WPI: glycerol ratio of 3.6:1 exhibited the best combination of WVP and thickness. Similarly, a sample with 9% WPI concentration having WPI: glycerol ratio of 3.6:1 demonstrated the best result for oxygen permeability. Furthermore, pullulan was added at various ratios. Experiments were conducted by the addition of pullulan (Benítez et al. 2015) with WPI at different ratios for the film formation. The various WPI:PUL ratios selected for the study were 1:0; 1:1; 2:1; 3:1; 4:1; 5:1; 6:1; 8:1; 10:1, among which WPI:PUL combination with 1:1 ratio was observed to have improved results for oxygen permeability, water vapor permeability (WVP), moisture content and film solubility (Gounga et al. 2008).
Coacervation between protein and polysaccharide at different ratio was evaluated. The protein and polysaccharide selected for the study at pH 3.0 were sodium caseinate (CAS) and low methoxyl pectin (LMP), respectively. The best combination of CAS: LMP for highest complex coacervates formation was observed to be at the ratio of 2.0, resulting in ζ-potential value as zero and having higher turbidity values. Furthermore, the films with complex coacervates were characterized to understand its effect on various properties of film formation. The findings suggested that with higher protein content, the films resulted in reduced water content and water sorption values. The study on film properties such as tensile strength and Young’s modulus exhibited higher values of 15.64 ± 1.74 MPa and 182.97 ± 6.48 MPa, respectively. These findings indicated that novel packaging systems with active molecules could be produced due to interactions between LMP and CAS (Eghbal et al. 2016).
Lipid-based binary films
Several studies have focused on the formulation of edible films from lipid molecules. In one work, the effect of lipid addition was studied on the physical, mechanical and barrier properties of edible films from wheat gluten. The results indicated the lipid addition reduced water vapor permeability and water affinity. A lower surface hydrophilicity was observed for films with lipid addition. However, lipid incorporation reduced bond formation within the film matrix that led to the development of weak structure. DSC data suggested that films with lipid addition showed a physical change from a glass state to rubber-like state (Rocca-Smith et al. 2016).
The effect of β-glucan-fatty acid esters on the film physical, barrier and microstructural properties was investigated. β-glucan was conjugated with various hydrophobic saturated and unsaturated fatty acids such as palmitic, lauric, stearic, myristic and oleic acids producing corresponding β-glucan-fatty acid esters with an approximately constant degree of substitution (Podshivalov et al. 2017). Consequently, these fatty acid esters were incorporated into arabinoxylan based films for evaluation of different properties. Overall, the results revealed that film properties were significantly affected by the incorporation of β-glucan-fatty acid esters. The β-glucan-fatty acid ester films demonstrated improved thermal stability compared to the controls (arabinoxylan-alone and arabinoxylan-β-glucan films). Saturated fatty acids generated a laminar morphology due to self-association within the film matrices. This type of structure improved moisture barrier property. The laminar structure of films also contributed towards decreased tensile strength. The study concluded that stearic acid based films produced the best conditions required for edible film applications (Ali et al. 2017).
Edible films were formulated from tara gum, and oleic acid was added to improve its hydrophobic characteristics. Oleic acid concentration ranged from 0 to 20% w/w of gum basis. FTIR data suggested that there was no bond formation between tara gum and oleic acid molecules. It also revealed that oleic acid weakened the hydrogen bond strength between individual gum molecules. The study confirmed that oleic acid incorporation produced non-homogeneous systems which were responsible for reduced transmittance and tensile strength of edible films. Oleic acid was found to improve the moisture barrier properties of films. It also produced a film with improved contact angle which had significant effects on film thermal properties (Ma et al. 2016).
Ternary edible films and coatings
A triple layered film was formulated from Konjac glucomannan, chitosan and soy protein isolate using glycerol as the plasticizer. Different physicochemical properties were measured including water vapor permeability, tensile strength, and percentage elongation at break (Jia et al. 2009). The study reported a range of values for all the response parameters: for example, water vapor permeability ranged between 3.29 to 9.63 × 10−11 gm−1 s−1 Pa−1. In addition, tensile strength ranged between 16.77 to 51.07 MPa, and the addition of plasticizer reduced the water vapor permeability and tensile strength but elevated the % elongation value (Jia et al. 2009).
Whey protein isolate, gelatin, and sodium alginate were combined in different ratios and various mechanical properties (tensile strength, puncture strength, elongation at break and tear strength) and barrier properties such as water vapor permeability and oxygen permeability were quantified (Wang et al. 2010). In addition, scanning electron microscopy demonstrated the microstructural aspects of the composite film. A simplex centroid design was used for optimization purpose. The study recorded the lowest oxygen permeability and water vapor permeability as 8.00 cm3 μm/m2 d kPa and 48.00 g mm/kPa d m2, respectively. Scanning electron microscopy images indicated that formulations containing higher concentrations of whey protein and gelatin had less porous structures compared to the remaining formulations. The optimized condition was obtained as follows—whey protein isolate: gelatin: sodium alginate as 8:12:15 (Wang et al. 2010).
Konjac glucomannan, chitosan and nisin based ternary edible film was developed and the physical, mechanical, barrier, optical, structural and antimicrobial properties were studied. Agar diffusion method was used to test the antimicrobial efficacy of E. coli, S. aureus, L. monocytogenes, and Bacillus cereus. It was observed that the blend film demonstrated high tensile strength and optimum transparency, solubility in water and water vapor permeability. FTIR analysis showed that hydrogen bond was formed between the macromolecules. Nisin (42,000 IU/g) demonstrated strong antimicrobial efficacy against S. aureus, L. monocytogenes, and B. cereus (Li et al. 2006).
A complex edible film from pullulan, alginate and other types of polysaccharides such as propyleneglycol alginate, pectin, carrageenan, and aloe polysaccharide was prepared by co-drying method. Physical, mechanical, optical, and barrier properties were studied. The research concluded that ternary-co-blended films can be used for the preservation of different types of food systems (Pan et al. 2014).
Ternary blended films were formulated from agar, alginate and collagen. In addition, the blended films were functionalized with silver nanoparticles and grape fruit seed extract as antimicrobial agents. The study demonstrated that such films could significantly reduce both Listeria and Escherichia counts on fresh potatoes as model food system (Wang and Rhim 2015).
Solution casting method was used to prepare chitosan, polyvinyl alcohol (PVA) and pectin based ternary edible films. Structural, morphological, thermal, and antimicrobial properties of the film was studied. The research revealed that the film was crystalline, rough, heterogeneous and possessed higher antimicrobial efficacy against E. coli, S. aureus, B. subtilis, Pseudomonas spp., and Candida albicans (Tripathi et al. 2010).
Quadruple edible films and coatings
Quadruple edible films were formulated from polycaprolactone, methylcellulose, polycaprolactone in combination with different antimicrobial compounds such as organic acids, rosmarinic acid, Asian essential oil and Italian essential oil mixture. These films were inserted into containers hosting broccoli and stored at 4 °C for 12 days. The antimicrobial films could significantly inhibit the growth of both E. coli, and S. aureus (Takala et al. 2013).
Applications of composite edible coatings to food systems
Fruit and vegetable products
A number of studies have been conducted on the application of multiple biopolymers for the preparation of edible coatings on model fruits and vegetables. Chitosan and gelatin were mixed and coated to peppers for extension of shelf life. It was observed that chitosan coating reduced spoilage. In addition, gelatin coating improved firmness. On the other hand, the composite film helped to reduce microbial spoilage by 2 log counts (Poverenov et al. 2014).
Combinations of biopolymers hydroxypropyl methylcellulose (HPMC) and beeswax were used to coat cherry tomatoes as the model food system. In addition, three classes of food preservatives were complexed with the film: sodium methyl paraben, sodium ethyl paraben and sodium benzoate. Alternaria alternata was used as the target microorganism in the study. It was reported that sodium benzoate complexed films were the most successful in reducing the black spot formation due to A. alternata. On the other hand, sodium benzoate complexed films also demonstrated strong potential to reduce the loss of weight and respiration rate (Fagundes et al. 2015).
In a layer-by-layer approach, carboxymethyl cellulose was combined with chitosan, and edible coatings were developed. The performance of these composite films was compared with individual layers comprising of methylcellulose (MC), hydroxypropyl methylcellulose (HPMC), carboxymethyl cellulose (CMC) and chitosan (CH). The model food system used in this study was mandarin fruits. The study reported that there was a positive correlation between the concentration of chitosan and coating firmness and coating gloss. The composite film also demonstrated better protection against weight loss (Arnon et al. 2015).
Fruit commodities such as strawberry are easily perishable having shorter life span. In addition, they are also susceptible to the infectious attacks caused by bacterial pathogens leading to deterioration in fruit quality. Few researchers worked with edible composite films based on cellulose derivatives carboxymethyl cellulose (CMC) and hydroxypropylmethyl cellulose (HPMC) and their application on strawberry. Attempts were made to ensure food safety and to extend shelf life. Formulations of edible coatings with cellulose derivatives were made individually and in composites with chitosan (CH) and were tested for various quality parameters. These research findings suggest that the application of polysaccharide-based edible films had resulted in higher retention of total phenolics and total anthocyanin content, which gradually decreased in control fruit samples exhibiting biological processes such as over-ripening and senescence. Furthermore, the composites of CMC + CH and HPMC + CH were found to be effective against cell wall degradation inhibiting enzyme activity (Gol et al. 2013).
Japanese plums (also known as stone fruits) are quite susceptible to postharvest fungal diseases, especially brown rot caused by Monilinia spp. In a study, fifteen generally recognized as safe (GRAS) antimicrobial food agents have been tested at various concentrations in vitro as coating ingredients against the fungal pathogen Monilinia fructicola. Overall, it was observed that thirteen out of fifteen antifungal edible coatings that were used exhibited significant antimicrobial activity against fungal pathogens. Coatings with antimicrobial agents such as sodium methylparaben and ammonium carbonate were found to be effective when compared with the rest of the antifungal edible coating formulations (Karaca et al. 2014).
Composite films of Indian gooseberry puree (IGP) and methylcellulose (MC) were formulated using response surface methodology (Suppakul et al. 2016). Indian gooseberry extract (IGE) was blended with resultant composite films and simultaneously tested for antioxidant assays such as the ferric reducing ability of plasma assay and (1,1-Diphenyl-2-picrylhydrazyl) assay. IGE-composite films were applied on roasted cashew nut, which was selected as the model food system and its efficacy was investigated over a prolonged storage period (90 days at 27 °C and 27 days at 7 °C). The findings suggested that composite film of IGP-MC would serve as reservoirs for IGE. These films would release IGE and are responsible for inhibitory effect over the surface of roasted cashew nut. In addition, it would assist in extended shelf life and maintenance of nut quality (Suppakul et al. 2016).
A comparative study was conducted on various edible film formulations formed from Aloe vera, sodium alginate and chitosan modified with acetic acid (AC) or citric acid (C). These edible film coatings were applied to minimally processed kiwi fruit and were evaluated for quality and shelf life for storage duration of 12 days. The selected quality parameters for the study were pH, soluble solids content, color, texture, titratable acidity, ascorbic acid, microbial, textural and sensory properties. It was reported that chitosan-AC and alginate-based films had good gas barrier properties. Aloe vera was reported to maintain the firmness of the fruit, and it was observed to inhibit color loss. Overall, it was suggested that coating-based on aloe vera had a maximum shelf life and excellent sensory properties during the entire storage period (Benítez et al. 2015).
Dairy products
In another study related to the application of edible films on dairy products, ricotta cheese samples were dipped into film forming solution prepared from 2.4% w/v lyophilized milk whey and 0.8% w/v chitosan for a duration of 30 s. During storage, these samples were evaluated for various quality parameters at regular intervals of 7, 14, 21 and 30 days. After 30 days of storage under modified atmosphere conditions, it was observed that the texture of coated cheese were found to be superior to uncoated cheese samples. The pH and titratable acidity values of uncoated samples were found to be around 6.48 and 0.34 meq/100 g, respectively. Similarly, for coated ricotta cheese samples, the pH and titratable acidity values were observed as 6.51 and 0.33 meq/100 g, respectively. In addition, coated samples were observed to possess significantly lower viable plate count of mesophilic, psychrotrophic, and lactic acid bacteria. In case of control samples, the viable count was greater than 7 log CFU/g during the storage period. Conversely, the viable count was evaluated to be lower than the permissible limit in coated samples (Di Pierro et al. 2011).
Meat products
Tartaric acid (0.75% w/v) was incorporated into a solution of apple peel powder and carboxymethylcellulose to form an active edible coating. Raw and cooked beef patties were used as model foods. Effect of antioxidant on the lipid oxidation of beef product and efficacy of antimicrobial were tested for mold, yeast, mesophilic aerobic bacteria (MAB) and S. enterica.
The results revealed that coating treatment prevents the lipid oxidation activity and retards the growth of microorganisms on the beef patties. TBARS value of control and coated beef sample were 3-malondialdehyde (Hamedi et al. 2017) mg/kg meat and 1-MDA mg/kg meat respectively, after 7 days of storage. This is possibly because apple peel powder has polyphenol compounds which trap the hydroxyl radicals or oxygen and prevents lipid oxidation. The total log count of yeast and mold, MAB and S. enterica were found to be 5.2 log CFU/g, 5.7 log CFU/g and 3.7 log CFU/g respectively, for the control sample. On the other hand, the coated sample demonstrated counts of 4.9 log CFU/g, 5.7 log CFU/g and 4.5 log CFU/g respectively, after 7 days of storage. The active coating did not affect the sensory quality of fresh and cooked beef patties (Shin et al. 2017).
In a study, sodium alginate was combined with a novel gum for the production of edible coatings on chicken fillets. Sodium alginate and galbanum oleo-resin gum based composite edible coatings were developed and were evaluated for antimicrobial and antioxidant properties on the shelf life and quality of chicken fillets. Besides galbanum oleo-resin gum and sodium alginate, different concentrations of Ziziphora persica essential oil such as 0, 0.5 and 1% w/v was added to the film-forming solution. During coating, the chicken fillets were first dipped into the active coating solution followed by second immersion into CaCl2 solution. Overall, the findings indicate that the coated samples had improved inhibitory effect on the growth of L. monocytogenes when compared to control samples. The findings revealed that in case of control samples the inoculated microbial count of L. monocytogenes was 6.8 log CFU g−1, on the other hand, it was under 2.7 log CFU g−1 in coated chicken fillet samples after 12 days of cold storage (Hamedi et al. 2017).
Seafood products
Seafoods constitute one among the highly perishable food commodities and possess shorter shelf life. Despite improved processing facilities and process control procedures, contamination with pathogenic microorganisms and occurrence of food borne illness has substantially increased. In addition, during storage, various endogenous chemical and enzymatic reactions lead to deteriorative changes in the quality of the product. Various researchers have reported the application of protein-based composite edible coatings for the improved shelf life of seafood products. In one such study, the shelf life of smoked rainbow trout (Oncorhynchus mykiss), a fish species with great economic importance was investigated by application of the protein-based edible coating. The coatings were prepared from different protein sources such as soy protein isolates, whey protein isolate, egg white powder protein, wheat gluten, corn protein, gelatin, and collagen. Based on the microbiological and sensory analysis, it was revealed that shelf life of the seafood commodity was extended by 2–3 weeks (Dehghani et al. 2018).
In another similar study carried out on rainbow trout fillets, the effect of chitosan–gelatin based films and coatings on rancidity development was investigated. The quality attributes were monitored over a period of 16 days under refrigerated conditions (4 ± 1 °C). The results indicated that the films were superior to coatings to retain the quality characteristics and in reducing lipid oxidation of fish fillets (Nowzari et al. 2013).
Conclusion
The field of edible films and coatings has seen great advancements in recent times. There is a major focus on the development of composite edible films and coatings where multiple biopolymers have been combined. Composite edible films and coatings have been utilized extensively for improving the shelf life and quality of different food systems including fresh produce, meat, dairy and seafood products. The effectiveness of a particular composite film or coating depends on the selection of the individual biopolymer and the compatibility between them. Greater research is needed in the area of sensory evaluation to improve the applicability of these systems on real food materials. In addition, these composite films prepared from various sources of polysaccharides, proteins, and lipids can also be used as delivery vehicles for the protection and controlled release of a multitude of bioactive compounds including antimicrobials, antioxidants, probiotics and vitamins within different food matrices (Table 1).
Table 1.
A summary of binary and ternary composite edible films and coatings showing various biopolymers and their properties
| S. no. |
Film category | First bizopolymer |
Second biopolymer | Third biopolymer |
Properties of the composites | Inference | References |
|---|---|---|---|---|---|---|---|
| 1 | Binary | Chitosan | Gelatin | – | Antimicrobial treated samples demonstrated 2–3 log CFU/g of L. monocytogenes reduced count compared to controls. Chitosan films containing Ziziphora clinopodioides essential oil (1%), pomegranate peel extract (1%) and cellulose nanoparticle (1%) demonstrated the best antimicrobial properties | Chitosan and Gelatin based films were enriched with various active ingredients such as Ziziphora clinopodioides essential oil (ZEO), pomegranate peel extract (PPE) and cellulose nanoparticle, separately and in combinations. It was observed that composite films incorporated with ZEO and PPE had exhibited strong antimicrobial activity against L. monocytogenes, while the effect of chitosan nanoparticles in the study was found to be insignificant | Mohebi and Shahbazi (2017) |
| 2 | Binary | FucoPol (Microbial polymer produced from Enterobacter A47) | Chitosan | – |
Mechanical properties: Tensile strength at break (MPa) 11.9 ± 6.2 Elongation at break (%) 38.4 ± 11.3 Elastic modulus (MPa) 137.0 ± 36.8 Barrier properties: Water vapor permeability (10−11 mol/m s Pa) 1.65 ± 0.40 O2 Permeability (10−16 mol m/m2 s Pa) 0.47 ± 0.19 CO2 Permeability (10−16 mol m/m2 s Pa) 5.8 ± 0.7 Selectivity (α) 12.6 |
Bilayer films produced from FucoPol and chitosan were evaluated for mechanical and barrier properties along with morphological and hygroscopic properties. These tests revealed that the bilayer films possess dense and homogenous layers with enhanced barrier properties when compared with monolayer FucoPol films | Ferreira et al. (2016) |
| 3 | Binary | Konjac glucomannan | Gellan gum | – |
Mechanical properties of composite films
Tensile strength (MPa) of blend (70:30:: Konjac glucomannan: gellan gum) 17.51 Elongation at break (%) 5.4 Antimicrobial properties of composite film against Staphylococcus aureus Zone of inhibition (mm): 20 mm for blend with 0% Konjac glucomannan Zone of inhibition (mm): 18 mm for blend with 50% Konjac glucomannan and 50% gellan gum Zone of inhibition (mm): 16 mm for blend with 100% Konjac glucomannan |
The blended films were characterized for Fourier transform infrared spectroscopy, differential scanning calorimetry, scanning electron microscopy and wide-angle X-ray diffraction. These findings confirmed the existence of hydrogen bonding in between the two polysaccharides. The properties of the film were found to vary with the change in the ratio of the film blend. It was noticed that blended films incorporated with nisin had exhibited antimicrobial activity against Staphylococcus aureus | Xu et al. (2007) |
| 4 | Binary | Whey protein isolate | Chitosan | – |
Mechanical properties: Tensile strength (MPa) 18.04 ± 1.30 Elongation at break (%) 177.57 ± 9.54 Barrier properties: Water vapor permeability (10−9 g m−1 s−1 Pa−1) 1.82 ± 0.05 Monolayer moisture content (g water/g dry solid) 0.207 |
Composite films produced from whey protein isolate and chitosan were incorporated with sodium laurate, modified with TiO2 nanoparticles and were characterized by physicochemical properties. It was observed that addition of nanoparticles to the system had enhanced the mechanical properties and compatibility between the biopolymers and reduced the WVP | Zhang et al. (2016b) |
| 5 | Binary | Hydroxypropyl methylcellulose (HPMC) | Beeswax (BW) | – | Disease severity for sodium benzoate coated samples after 14 days of storage: 3% Disease incidence for sodium benzoate coated samples after 14 days of storage: 25% Weight loss of sodium benzoate coated tomatoes after 15 days of storage at 5 °C and 5 days of storage at 20 °C: 3.3% Firmness of sodium benzoate coated tomatoes after 15 days of storage at 5 °C and 5 days of storage at 20 °C: 18% Respiration rate of sodium benzoate coated tomatoes after 15 days of storage at 5 °C and 5 days of storage at 20 °C (mg CO2/Kg h): 10 |
Hydroxypropyl methylcellulose (HPMC), beeswax (BW) and selected antifungal agents such as sodium methyl paraben, sodium ethyl paraben and sodium benzoate were used in the production of edible composite films. Cherry tomatoes were selected as model food system and were inoculated with Alternaria alternate followed by antifungal coating. Edible coatings with antifungal agent sodium benzoate were found to reduce the weight loss and respiration rate and maintained the firmness of the fruit | Fagundes et al. (2015) |
| 6 | Ternary | Konjac glucomannan |
Chitosan | Soy protein isolate |
Barrier properties
Range of water vapor permeability (× 10−11 gm−1 s−1 Pa−1) 3.29–9.63 Mechanical properties Range of tensile strength (MPa) 16.77 and 51.07 Percentage elongation at break (%) between 1.29 and 10.73 |
Film characterization studies were carried out and its barrier properties have been highlighted | Jia et al. (2009) |
| 7 | Ternary | Whey protein isolate | Gelatin | Sodium alginate | Composite films produced from WPI: G:SA 10.0:16.0:14.0 demonstrated the best oxygen barrier property (8.00 cm3 µm/m2 d kPa); Composite films produced from WPI: G:SA 20.0: 8.0:12.0 demonstrated the best water vapor barrier property (48.04 g mm/kPa d m2); Composite films produced from WPI:G:SA 10.0:17.5:22.5 demonstrated the best mechanical properties Tensile strength (MPa) 9.47 ± 1.54 Elongation at break (%) 29.42 ± 5.34 |
Composite films were characterized to investigate the microstructure and film barrier properties | Wang et al. (2010) |
| 8 | Ternary | Agar | Alginate | Collagen |
Agar/alginate/collagen
L* 92.72 ± 0.17 a* 0.63 ± 0.08 b* 5.68 ± 0.54 ∆E 3.21 ± 0.53 T660 (%) 87.1 ± 1.0 T280 (%) 39.1 ± 2.8 Tensile strength (MPa) 20.9 ± 2.4 Elongation (%) 26.5 ± 6.3 Elastic modulus (MPa) 500 ± 150 Water vapor permeability (10−9 g m/m2 Pa s) 59.3 ± 4.9 Contact angle (o) 46.8 ± 1.9 Swelling ratio (%) 2363.7 ± 50.9 Water solubility (%) 65.7 ± 0.9 |
Ternary blend films were demonstrated to prevent condensation of water on the surface of the film and prevented the greening of food systems such as potatoes during the storage period | Wang and Rhim (2015) |
| 9 | Ternary | Pullulan | Alginate | Polysaccharides (Propyleneglycol alginate, carrageenan, and aloe polysaccharide, pectin) |
Pullulan/Alginate
Water vapor permeability (g mm/m2 h kPa) 1.69 ± 0.08 Tensile strength (MPa) 69.34 ± 4.45 Elongation (%) 5.71 ± 0.16 Oxygen permeability (cm3 mm/m2 da kPa) 326.58 ± 12.07 Thickness (mm) 45.17 ± 0.47 L* 86.06 ± 0.19 a* 0.42 ± 0.04 b* 0.77 ± 0.04 Transparency 1.62 ± 0.04 Pullulan/Alginate/Propyleneglycol alginate Water vapor permeability (g mm/m2 h kPa) 1.61 ± 0.033 Tensile strength (MPa) 91.47 ± 8.12 Elongation (%) 7.89 ± 0.33 Oxygen permeability (cm3 mm/m2 da kPa) 311.38 ± 8.82 Thickness (mm) 48.30 ± 1.01 L* 86.00 ± 0.20 a* 0.54 ± 0.04 b* 1.03 ± 0.07 Transparency 0.67 ± 0.05 |
Ternary co-blended films were applied on intermediate moisture foods to help retain product characterises such as moisture, texture, and flavour | Pan et al. (2014) |
References
- Abugoch LE, Tapia C, Villamán MC, Yazdani-Pedram M, Díaz-Dosque M. Characterization of quinoa protein–chitosan blend edible films. Food Hydrocoll. 2011;25:879–886. doi: 10.1016/j.foodhyd.2010.08.008. [DOI] [Google Scholar]
- Ali U, Bijalwan V, Basu S, Kesarwani AK, Mazumder K. Effect of β-glucan-fatty acid esters on microstructure and physical properties of wheat straw arabinoxylan films. Carbohydr Polym. 2017;161:90–98. doi: 10.1016/j.carbpol.2016.12.036. [DOI] [PubMed] [Google Scholar]
- Antoniou J, Liu F, Majeed H, Zhong F. Characterization of tara gum edible films incorporated with bulk chitosan and chitosan nanoparticles: a comparative study. Food Hydrocoll. 2015;44:309–319. doi: 10.1016/j.foodhyd.2014.09.023. [DOI] [Google Scholar]
- Arnon H, Granit R, Porat R, Poverenov E. Development of polysaccharides-based edible coatings for citrus fruits: a layer-by-layer approach. Food Chem. 2015;166:465–472. doi: 10.1016/j.foodchem.2014.06.061. [DOI] [PubMed] [Google Scholar]
- Benbettaïeb N, Chambin O, Karbowiak T, Debeaufort F. Release behavior of quercetin from chitosan-fish gelatin edible films influenced by electron beam irradiation. Food Control. 2016;66:315–319. doi: 10.1016/j.foodcont.2016.02.027. [DOI] [Google Scholar]
- Benítez S, Achaerandio I, Pujolà M, Sepulcre F. Aloe vera as an alternative to traditional edible coatings used in fresh-cut fruits: a case of study with kiwifruit slices. LWT Food Sci Technol. 2015;61:184–193. doi: 10.1016/j.lwt.2014.11.036. [DOI] [Google Scholar]
- Bonilla J, Sobral PJ. Investigation of the physicochemical, antimicrobial and antioxidant properties of gelatin-chitosan edible film mixed with plant ethanolic extracts. Food Biosci. 2016;16:17–25. doi: 10.1016/j.fbio.2016.07.003. [DOI] [Google Scholar]
- Chandra Mohan C, Rakhavan K, Sudharsan K, Babuskin S, Sukumar M. Design and characterization of spice fused tamarind starch edible packaging films. LWT Food Sci Technol. 2016;68:642–652. doi: 10.1016/j.lwt.2016.01.004. [DOI] [Google Scholar]
- Cheng S-Y, Wang B-J, Weng Y-M. Antioxidant and antimicrobial edible zein/chitosan composite films fabricated by incorporation of phenolic compounds and dicarboxylic acids. LWT Food Sci Technol. 2015;63:115–121. doi: 10.1016/j.lwt.2015.03.030. [DOI] [Google Scholar]
- Choi WS, Singh S, Lee YS. Characterization of edible film containing essential oils in hydroxypropyl methylcellulose and its effect on quality attributes of ‘Formosa’ plum (Prunus salicina L.) LWT Food Sci Technol. 2016;70:213–222. doi: 10.1016/j.lwt.2016.02.036. [DOI] [Google Scholar]
- Dehghani S, Hosseini SV, Regenstein JM. Edible films and coatings in seafood preservation: a review. Food Chem. 2018;240:505–513. doi: 10.1016/j.foodchem.2017.07.034. [DOI] [PubMed] [Google Scholar]
- Di Pierro P, Sorrentino A, Mariniello L, Giosafatto CVL, Porta R. Chitosan/whey protein film as active coating to extend Ricotta cheese shelf-life. LWT Food Sci Technol. 2011;44:2324–2327. doi: 10.1016/j.lwt.2010.11.031. [DOI] [Google Scholar]
- Eghbal N, Yarmand MS, Mousavi M, Degraeve P, Oulahal N, Gharsallaoui A. Complex coacervation for the development of composite edible films based on LM pectin and sodium caseinate. Carbohydr Polym. 2016;151:947–956. doi: 10.1016/j.carbpol.2016.06.052. [DOI] [PubMed] [Google Scholar]
- Fagundes C, Palou L, Monteiro AR, Pérez-Gago MB. Hydroxypropyl methylcellulose-beeswax edible coatings formulated with antifungal food additives to reduce alternaria black spot and maintain postharvest quality of cold-stored cherry tomatoes. Sci Hortic Amst. 2015;193:249–257. doi: 10.1016/j.scienta.2015.07.027. [DOI] [Google Scholar]
- Falguera V, Quintero JP, Jiménez A, Muñoz JA, Ibarz A. Edible films and coatings: structures, active functions and trends in their use. Trends Food Sci Technol. 2011;22:292–303. doi: 10.1016/j.tifs.2011.02.004. [DOI] [Google Scholar]
- Ferreira AR, et al. Development and characterization of bilayer films of FucoPol and chitosan. Carbohydr Polym. 2016;147:8–15. doi: 10.1016/j.carbpol.2016.03.089. [DOI] [PubMed] [Google Scholar]
- Gol NB, Patel PR, Rao TR. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol Technol. 2013;85:185–195. doi: 10.1016/j.postharvbio.2013.06.008. [DOI] [Google Scholar]
- González-Forte L, Bruno E, Martino M. Application of coating on dog biscuits for extended survival of probiotic bacteria. Anim Feed Sci Technol. 2014;195:76–84. doi: 10.1016/j.anifeedsci.2014.05.015. [DOI] [Google Scholar]
- Gounga M, Xu SY, Wang Z, Yang W. Effect of whey protein Isolate–Pullulan edible coatings on the quality and shelf life of freshly roasted and freeze-dried Chinese chestnut. J Food Sci Technol. 2008;73:E155–E161. doi: 10.1111/j.1750-3841.2008.00694.x. [DOI] [PubMed] [Google Scholar]
- Gu L, Wang M. Effects of protein interactions on properties and microstructure of zein–gliadin composite films. J Food Eng. 2013;119:288–298. doi: 10.1016/j.jfoodeng.2013.05.022. [DOI] [Google Scholar]
- Gutiérrez TJ. Surface and nutraceutical properties of edible films made from starchy sources with and without added blackberry pulp. Carbohydr Polym. 2017;165:169–179. doi: 10.1016/j.carbpol.2017.02.016. [DOI] [PubMed] [Google Scholar]
- Hamedi H, Kargozari M, Shotorbani PM, Mogadam NB, Fahimdanesh M. A novel bioactive edible coating based on sodium alginate and galbanum gum incorporated with essential oil of Ziziphora persica: the antioxidant and antimicrobial activity, and application in food model. Food Hydrocoll. 2017;72:35–46. doi: 10.1016/j.foodhyd.2017.05.014. [DOI] [Google Scholar]
- Hosseini SF, Rezaei M, Zandi M, Ghavi FF. Preparation and functional properties of fish gelatin–chitosan blend edible films. Food Chem. 2013;136:1490–1495. doi: 10.1016/j.foodchem.2012.09.081. [DOI] [PubMed] [Google Scholar]
- Hosseini SF, Rezaei M, Zandi M, Farahmandghavi F. Development of bioactive fish gelatin/chitosan nanoparticles composite films with antimicrobial properties. Food Chem. 2016;194:1266–1274. doi: 10.1016/j.foodchem.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Jia D, Fang Y, Yao K. Water vapor barrier and mechanical properties of konjac glucomannan–chitosan–soy protein isolate edible films. Food Bioprod Process. 2009;87:7–10. doi: 10.1016/j.fbp.2008.06.002. [DOI] [Google Scholar]
- Kang H-J, Kim S-J, You Y-S, Lacroix M, Han J. Inhibitory effect of soy protein coating formulations on walnut (Juglans regia L.) kernels against lipid oxidation. LWT Food Sci Technol. 2013;51:393–396. doi: 10.1016/j.lwt.2012.10.019. [DOI] [Google Scholar]
- Karaca H, Pérez-Gago MB, Taberner V, Palou L. Evaluating food additives as antifungal agents against Monilinia fructicola in vitro and in hydroxypropyl methylcellulose–lipid composite edible coatings for plums. Int J Food Microbiol. 2014;179:72–79. doi: 10.1016/j.ijfoodmicro.2014.03.027. [DOI] [PubMed] [Google Scholar]
- Khazaei N, Esmaiili M, Emam-Djomeh Z. Effect of active edible coatings made by basil seed gum and thymol on oil uptake and oxidation in shrimp during deep-fat frying. Carbohydr Polym. 2016;137:249–254. doi: 10.1016/j.foodhyd.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Li B, Kennedy J, Peng J, Yie X, Xie B. Preparation and performance evaluation of glucomannan–chitosan–nisin ternary antimicrobial blend film. Carbohydr Polym. 2006;65:488–494. doi: 10.1016/j.carbpol.2006.02.006. [DOI] [Google Scholar]
- Ma Q, Hu D, Wang H, Wang L. Tara gum edible film incorporated with oleic acid. Food Hydrocoll. 2016;56:127–133. doi: 10.1016/j.foodhyd.2015.11.033. [DOI] [Google Scholar]
- Martins JT, Cerqueira MA, Bourbon AI, Pinheiro AC, Souza BW, Vicente AA. Synergistic effects between κ-carrageenan and locust bean gum on physicochemical properties of edible films made thereof. Food Hydrocoll. 2012;29:280–289. doi: 10.1016/j.foodhyd.2012.03.004. [DOI] [Google Scholar]
- Mei J, Yuan Y, Guo Q, Wu Y, Li Y, Yu H. Characterization and antimicrobial properties of water chestnut starch–chitosan edible films. Int J Biol Macromol. 2013;61:169–174. doi: 10.1016/j.ijbiomac.2013.06.026. [DOI] [PubMed] [Google Scholar]
- Mohebi E, Shahbazi Y. Application of chitosan and gelatin based active packaging films for peeled shrimp preservation: a novel functional wrapping design. LWT Food Sci Technol. 2017;76(Part 1):108–116. doi: 10.1016/j.lwt.2016.10.062. [DOI] [Google Scholar]
- Nowzari F, Shábanpour B, Ojagh SM. Comparison of chitosan–gelatin composite and bilayer coating and film effect on the quality of refrigerated rainbow trout. Food Chem. 2013;141:1667–1672. doi: 10.1016/j.foodchem.2013.03.022. [DOI] [PubMed] [Google Scholar]
- Padrão J, et al. Bacterial cellulose-lactoferrin as an antimicrobial edible packaging. Food Hydrocoll. 2016;58:126–140. doi: 10.1016/j.foodhyd.2016.02.019. [DOI] [Google Scholar]
- Pan H, Jiang B, Chen J, Jin Z. Assessment of the physical, mechanical, and moisture-retention properties of pullulan-based ternary co-blended films. Carbohydr Polym. 2014;112:94–101. doi: 10.1016/j.carbpol.2014.05.044. [DOI] [PubMed] [Google Scholar]
- Podshivalov A, Zakharova M, Glazacheva E, Uspenskaya M. Gelatin/potato starch edible biocomposite films: correlation between morphology and physical properties. Carbohydr Polym. 2017;157:1162–1172. doi: 10.1016/j.carbpol.2016.10.079. [DOI] [PubMed] [Google Scholar]
- Poverenov E, et al. Effects of a composite chitosan–gelatin edible coating on postharvest quality and storability of red bell peppers. Postharvest Biol Tec. 2014;96:106–109. doi: 10.1016/j.postharvbio.2014.05.015. [DOI] [Google Scholar]
- Razavi SMA, Mohammad Amini A, Zahedi Y. Characterisation of a new biodegradable edible film based on sage seed gum: influence of plasticiser type and concentration. Food Hydrocoll. 2015;43:290–298. doi: 10.1016/j.foodhyd.2014.05.028. [DOI] [Google Scholar]
- Rocca-Smith JR, et al. Effect of lipid incorporation on functional properties of wheat gluten based edible films. J Cereal Sci. 2016;69:275–282. doi: 10.1016/j.jcs.2016.04.001. [DOI] [Google Scholar]
- Rubilar JF, Zúñiga RN, Osorio F, Pedreschi F. Physical properties of emulsion-based hydroxypropyl methylcellulose/whey protein isolate (HPMC/WPI) edible films. Carbohydr Polym. 2015;123:27–38. doi: 10.1016/j.carbpol.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Shin S-H, Chang Y, Lacroix M, Han J. Control of microbial growth and lipid oxidation on beef product using an apple peel-based edible coating treatment. LWT Food Sci Technol. 2017;84:183–188. doi: 10.1016/j.lwt.2017.05.054. [DOI] [Google Scholar]
- Singh N, Georget DM, Belton PS, Barker SA. Zein–iodine complex studied by FTIR spectroscopy and dielectric and dynamic rheometry in films and precipitates. J Agric Food Chem. 2009;57:4334–4341. doi: 10.1021/jf900436q. [DOI] [PubMed] [Google Scholar]
- Singh N, Georget DM, Belton PS, Barker SA. Physical properties of zein films containing salicylic acid and acetyl salicylic acid. J Cereal Sci. 2010;52:282–287. doi: 10.1016/j.jcs.2010.06.008. [DOI] [Google Scholar]
- Song HY, Shin YJ, Song KB. Preparation of a barley bran protein–gelatin composite film containing grapefruit seed extract and its application in salmon packaging. J Food Eng. 2012;113:541–547. doi: 10.1016/j.jfoodeng.2012.07.010. [DOI] [Google Scholar]
- Suppakul P, Boonlert R, Buaphet W, Sonkaew P, Luckanatinvong V. Efficacy of superior antioxidant Indian gooseberry extract-incorporated edible Indian gooseberry puree/methylcellulose composite films on enhancing the shelf life of roasted cashew nut. Food Control. 2016;69:51–60. doi: 10.1016/j.foodcont.2016.04.033. [DOI] [Google Scholar]
- Takala PN, et al. Antimicrobial effect and physicochemical properties of bioactive trilayer polycaprolactone/methylcellulose-based films on the growth of foodborne pathogens and total microbiota in fresh broccoli. J Food Eng. 2013;116:648–655. doi: 10.1016/j.jfoodeng.2013.01.005. [DOI] [Google Scholar]
- Tavassoli-Kafrani E, Shekarchizadeh H, Masoudpour-Behabadi M. Development of edible films and coatings from alginates and carrageenans. Carbohydr Polym. 2016;137:360–374. doi: 10.1016/j.carbpol.2015.10.074. [DOI] [PubMed] [Google Scholar]
- Thakur R, et al. Characterization of rice starch-ι-carrageenan biodegradable edible film. Effect of stearic acid on the film properties. Int J Biol Macromol. 2016;93:952–960. doi: 10.1016/j.ijbiomac.2016.09.053. [DOI] [PubMed] [Google Scholar]
- Tharanathan R. Biodegradable films and composite coatings: past, present and future. Trends Food Sci Technol. 2003;14:71–78. doi: 10.1016/S0924-2244(02)00280-7. [DOI] [Google Scholar]
- Tripathi S, Mehrotra G, Dutta P. Preparation and physicochemical evaluation of chitosan/poly (vinyl alcohol)/pectin ternary film for food-packaging applications. Carbohydr Polym. 2010;79:711–716. doi: 10.1016/j.carbpol.2009.09.029. [DOI] [Google Scholar]
- Wang L-F, Rhim J-W. Preparation and application of agar/alginate/collagen ternary blend functional food packaging films. Int J Biol Macromol. 2015;80:460–468. doi: 10.1016/j.ijbiomac.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Wang L, Auty MA, Kerry JP. Physical assessment of composite biodegradable films manufactured using whey protein isolate, gelatin and sodium alginate. J Food Eng. 2010;96:199–207. doi: 10.1016/j.jfoodeng.2009.07.025. [DOI] [Google Scholar]
- Wang K, Wang W, Ye R, Liu A, Xiao J, Liu Y, Zhao Y. Mechanical properties and solubility in water of corn starch-collagen composite films: effect of starch type and concentrations. Food Chem. 2017;216:209–216. doi: 10.1016/j.foodchem.2016.08.048. [DOI] [PubMed] [Google Scholar]
- Xu X, Li B, Kennedy J, Xie B, Huang M. Characterization of konjac glucomannan–gellan gum blend films and their suitability for release of nisin incorporated therein. Carbohydr Polym. 2007;70:192–197. doi: 10.1016/j.carbpol.2007.03.017. [DOI] [Google Scholar]
- Zhang P, Zhao Y, Shi Q. Characterization of a novel edible film based on gum ghatti: effect of plasticizer type and concentration. Carbohydr Polym. 2016;153:345–355. doi: 10.1016/j.carbpol.2016.07.082. [DOI] [PubMed] [Google Scholar]
- Zhang W, Chen J, Chen Y, Xia W, Xiong YL, Wang H. Enhanced physicochemical properties of chitosan/whey protein isolate composite film by sodium laurate-modified TiO2 nanoparticles. Carbohydr Polym. 2016;138:59–65. doi: 10.1016/j.carbpol.2015.11.031. [DOI] [PubMed] [Google Scholar]