Abstract
To systematically study multi-stage countercurrent process for Antarctic krill protein extracting and to optimize the multi-stage countercurrent technology, the solubility of Antarctic krill proteins after multi-step dissolution was explored firstly; multi-step extraction was investigated; and then multi-stage countercurrent system for protein extraction was carried out. In single step extraction, krill-to-water ratio and pH were chosen as 1:10 and 12.5 respectively, in order to extract more protein. In the multi-step dissolution process, the protein solubility of aqueous solution at pH 12.5 was 33.0 ± 0.8 mg/mL. Multi-step cross-flow processing testified the feasibility of multi-stage countercurrent assumption. Three-stage countercurrent method using krill-to-water ratio 1:10 extracted, 95.1 ± 0.6% protein from krill, where almost the same water as previous works. The total recovery yield of 67.9 ± 1.6% was achieved after precipitation at pH 4.5.
Keywords: Euphausia superba, Protein solubility, Multi-stage countercurrent, Antarctic krill
Introduction
As a huge biomass, Antarctic krill (Euphausia superb) attracts increasing attention of researchers from various countries. Protein in Antarctic krill has a quite high quality (Suzuki and Shibata 1990; Tou et al. 2007; Gigliotti et al. 2008) and a quite high content, about 70% on a dry weight basis (Gigliotti et al. 2008). However, high fluoride level in Antarctic krill protein is a major constraint on its application.
So far, isoelectric solubilization/precipitation (ISP) process has been widely applied to extract protein (Chen and Jaczynski 2007; Taskaya et al. 2009; Vareltzis and Undeland 2012; Barac et al. 2015), and also used as a suitable method to extract the krill protein (Chen et al. 2009; Wang et al. 2011; Qi et al. 2016). Chen et al. (2009) paid more attention to application properties and chemical composition of extracted protein, where operation conditions were set referring to ISP extraction condition of other aquatic protein. Wang et al. (2011) tried to optimize extraction conditions, and found that two extraction steps could improve the yield of extracted protein obviously, and applied two-step washing after isoelectric precipitation to remove fluoride. As to remove fluoride, Qi et al. (2016) chosed multi-stage countercurrent system instead at acid condition and saved water consumption.
Multi-stage countercurrent process may be a good choice of protein extracting also. This technology was covered by a US patent in 1974 (Takahata et al. 1974) and widely used in extracting specific compounds, for example, extracting glycyrrhizic acid from licorice (Wang et al. 2004) and flavone glycosides from Ginkgo biloba leaves (Yu et al. 2012) In those works, higher recoveries were obtained and water consumptions were cut down, so less wastewater was released. Lestari et al. (2010) had ever applied the technology to extract plant protein, which saved water and increased protein recovery as well.
The purpose of this study was trying to improve the recovery of krill protein with low fluoride to a large extend by using multi-stage countercurrent system in protein alkaline extraction process of ISP technology. Before this task, protein dissolving principle in multi-stage countercurrent system should be systematically and gradually explored. To achieve these objectives, a series of experiments were conducted: (1) to explore the solubility of Antarctic krill proteins after multi-step dissolution; (2) to clarify the influence of the number of steps on the yield using a multi-step extraction method; and (3) to optimize Antarctic krill protein extraction using a multi-stage countercurrent system.
Materials and methods
Material
Antarctic krill (Euphausia superba) were obtained as plate-frozen blocks from Dalian Ocean Fishery Group Ltd. (Dalian, China) and transported frozen (at − 20 °C) to the laboratory. Each Antarctic krill was about 2 cm long. The average proximate compositions of whole Antarctic krill, which were determined according to AOAC methods (AOAC 2012), were 79.46% moisture, 8.73% total lipid (dry basis), 69.27% crude protein (dry basis), and 11.93% ash (dry basis). The krill blocks were stored at − 20 °C for no more than three months after krill was captured.
The frozen blocks were reduced in size with a band saw and then crushed using hammer mill (PSC-150, Longshi Machine Building Co. Ltd., Shanghai, China) at about 0 °C. The crushed krill were used that day.
Multi-step dissolution
Influence of krill-to-water ratio on Antarctic krill protein solubility in single step
To identify the best conditions for extraction, a series of krill-water ratios were studied. Twenty grams of frozen crushed Antarctic krill were put into 150, 110, 90 and 50 mL distilled water, stirred magnetically (200 rpm, RH basic, IKA group, Guangdong, China) at room temperature (25 °C) until the samples were almost thawed (about − 4 °C). Then the mixture was homogenized (1000 rpm, 30 s, ETS-2, Yi Tong Electronics Ltd., Hangzhou, China) at 4 °C. The homogenate’s pH was adjusted to 12.5 according to the procedures reported by Qi et al. (2016) (PHS-3C pH meter, INESA Scientific Instrument Co. Ltd., Shanghai, China) using 10 mol/L NaOH of 4 °C. Finally, additional water of 4 °C was added to final volumes of 200, 160, 120 and 80 mL, respectively (i.e. final ratios of 1:10, 1:8, 1:6 and 1:4 w/v). The following extracting procedures were all conducted at 4 °C.
After that, the homogenates at pH 12.5 were centrifuged (DL-7 M, Ping Fan Scientific Instruments Ltd., Changsha, China) at 7000 × g at 4 °C for 10 min. The crude protein in the raw material and dissolved in the supernatant were determined using Kjeldahl method (Kjeltec 8400, Foss, Hillerød, Denmark) (AOAC 2012), wherein non-protein N was measured (using trichloroacetic acid precipitation method) and deducted. 6.25 was used as Kjeldahl nitrogen-to-protein conversion factor (NPCF). The protein extraction percentage was calculated using the following equation:
| 1 |
Solubility of Antarctic krill protein after multi-step dissolution
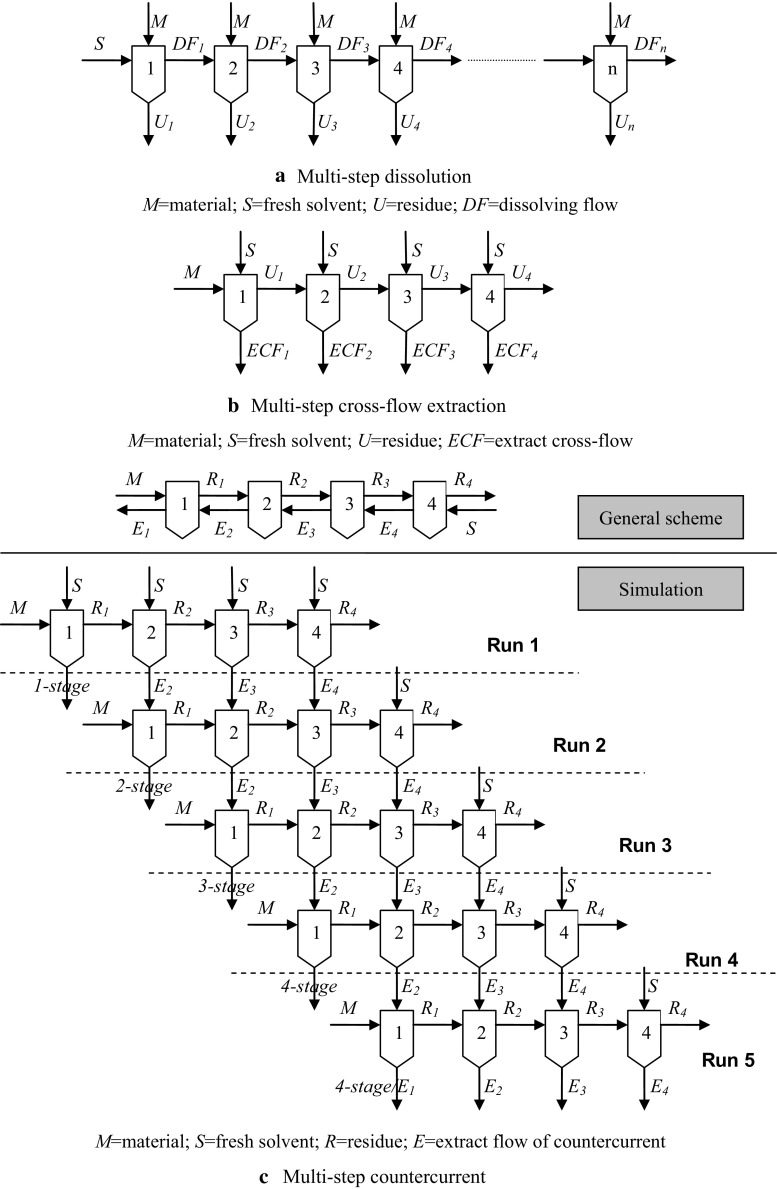
Since extract supernatant was used as solvent of next step, solubility of Antarctic krill protein after multi-step dissolution was determined following frame in Fig. 1a. Krill samples of first step were prepared as above, mixed with 10 mol/L NaOH to pH 12.5, and fresh distilled water was added at a set 1:10 ratio. Then the mixture was centrifuged, and the protein concentration of the new supernatant was determined. In the second steps, new krill samples were added into supernatants of first steps at 1:10 ratio (krill/supernatant, g/mL). The pH was adjusted to 12.5 again. And so on, these steps were repeated until the protein concentration in the supernatant became fairly constant, which happened after about 7–9 times dissolutions.
Fig. 1.
Flow diagram of multi-step dissolution, cross-flow and countercurrent extraction. a Multi-step dissolution. b Multi-step cross-flow extraction. c Multi-step countercurrent. M material; S fresh solvent; U residue; DF dissolving flow; ECF extract cross-flow; R residue; E extract flow of countercurrent
Protein solubility analysis using SDS-PAGE
The method reported by Walker (2002) was used. The samples which had been obtained in multi-step dissolution were mixed with an equal volume of sample buffer containing 1% β-mercaptoethanol (v/v), 1% SDS (sodium dodecyl sulfate, w/v), 0.02% bromphenol blue(w/v), 40% sugar(w/v), and 0.01 mol/L pH 8.0 Tris–HCl. After 10 min in a boiling water bath, 10 μL of each sample was placed in the sample well. The gel consists of a stacking gel (5% totalacrylamidein 0.1 mol/L pH6.7 Tris–HCl buffer with a ratio of acrylamide to methylene-bisacrylamide of 20:1) and a separating gel (10% totalacrylamide in 0.4 mol/L pH8.9 Tris–HCl buffer with a ratio of acrylamide to methylene-bisacrylamide of 29:1) with both gels containing 10% SDS, 1% TEMED (N, N, N’, N’-tetramethylethylenediamine) and 10% ammonium persulfate. Electrophoresis was initially run at a voltage of 80 V (DYCP-37B, Liu-Yi Instrument Factory, Beijing, China) through the stacking gel. Then the voltage was increased to 120 V until the electrophoresis ended. The electrophoresis buffer was pH 8.3 Tris–glycine containing 0.1% SDS. The gel was soaked in a fixation solution (45% methanol, 10% acetic acid) for 1 h and was stained with 0.05% Coomassie brilliant blue R-250 (Sangon Biotech Co., Ltd., Shanghai, China) for 20 min at 60 °C. After distaining at 70 °C with a 10% methanol, 10% acetic acid solution (until the background was clear, the distaining solution was changed 2-4 times), the gels were scanned using a gel imaging and analysis system (ChemiDoc™ MP System, Bio-Rad Laboratories, Inc., Hercules, CA, USA) and the data used directly from the results generated by the software Image Lab 3.0 (Bio-Rad, 2010). The quantitative procedure was based on the method reported by Nayak et al. (1996). However, accurate quantitation needs good separation of the bands. So the results were considered to be a semi-quantitative method to analyze the solubility of proteins.
Multi-step cross-flow extraction
Extraction percentage of protein
Multi-step extraction, or to be more specific, multi-step cross-flow extraction was often used to increase extraction efficiency (Wang et al. 2011). However more water was needed in the process. In order to compare with multi-stage countercurrent, multi-step cross-flow extraction was carried out following frame in Fig. 1b.
In each step, the procedures were similar to multi-step dissolution. The ratio was 1:10. Then the crude protein in the raw materials, residues and the supernatants were determined using Kjeldahl method as well. The precipitate was then extracted twice more with 200 mL distilled water at pH 12.5. The extraction percentage of each step was calculated using Eq. (1).
Protein yield of acid precipitation
In ISP technique, acid precipitation was always applied after alkali extraction to obtain protein product. The supernatants from the above experiment or similar samples were obtained following the procedures described in the above section. They were adjusted to pH 4.5 using 5 mol/L HCl and centrifuged immediately at 7000×g at 4 °C, and then the precipitated protein was washed by 3-stage countercurrent method reported by Qi et al. (2016) so as to assure that fluoride level was lower than 2 mg/kg. Then protein content was determined using Kjeldahl method, and recovery yield was calculated.
Influence of krill-to-water ratio
Experiments of 4 ratios were performed (1:10, 1:8, 1:6 and 1:4 w/v) and the insoluble protein was extracted twice more at pH 12.5.
Multi-stage countercurrent system of protein extraction
Extracting protein
To improve Antarctic krill protein yield and to save water in ISP process, a multi-stage countercurrent protein extraction system was designed based on the work of Lestari et al. (2010). A scheme of 4-stage countercurrent extraction was shown in Fig. 1c.
This system simulated a series of consecutive protein extractions by using a series of beakers with the protein and water moving in a counter-current fashion as previously reported (Adu-peasah et al. 1993; Moure et al. 2003; Lestari et al. 2010). Fresh material and fresh solvent were added and residues and extract flow were moved as schemed in Fig. 1c. The countercurrent system reached equilibrium after 4 runs. In the first run, each batch consisted of 20 g krill at pH 12.5 and a krill-to-water ratio of 1:10. Each beaker was homogenized (1000 rpm, 30 s) at 4 °C. Protein content of supernatant from each stage (1-stage, 2-stage, 3-stage and 4-stage/E1 as shown in Fig. 1c) was analyzed using Kjeldahl method. The extraction percentage was calculated by Eq. (1).
Effect of different factors on the multi-stage countercurrent system
Experiments of 2, 3 and 4-stage were carried out as described in above section and Fig. 1c so as to observe the influence of factors on extraction efficiency. By comparing the final protein concentrations of E1 in different stages, the optimal stages were determined. Then the influence of krill-to-water ratio was studied using the 3-stage system. All the 4 ratios (1:10, 1:8, 1:6 and 1:4 w/v) were then investigated.
Statistical analysis
Experiments were conducted in triplicates (excluding the semi-quantitative analysis of SDS-PAGE, which was done only once). Values are expressed as mean ± standard deviation of three measurements. Line and column figures were drawn using Origin Pro 8.6. One-way ANOVA was performed using SPSS v. 19.0 (IBM Corp., Armonk, NY, USA) to test difference. Significance was set at p < 0.05.
Results and discussion
Solubility of Antarctic krill protein
Solubility in solution of different krill-to-water ratios
To identify the proper condition, the protein concentrations of the supernatants at different ratios (1:10, 1:8, 1:6 and 1:4) were determined to be 12.2 ± 1.0 mg/mL, 14.5 ± 0.7 mg/mL, 16.6 ± 1.5 mg/mL and 18.5 ± 1.3 mg/mL respectively. Then the extraction percentage of the krill-to-water ratio 1:10 1:8, 1:6 and 1:4 were calculated to be 88.1%, 83.4%, 71.9% and 53.2%, respectively.
From the results, it can be seen that less krill protein had been extracted when krill-to-water ratio changed from 1:10 to 1:4. In previous studies on Antarctic krill protein extraction, the krill-to-water ratio was often set as 1:3 (Chen and Jaczynski 2007, Chen et al. 2009) or 1:6 (Gigliotti et al. 2008; Wang et al. 2011). Results obtained in similar condition of this work were close to the results reported by Chen et al. (2009) and Wang et al. (2011). The suggested that water content limited dissolution of krill protein.
Solubility of krill protein into aqueous solution after multi-step dissolution
What limited dissolution of krill protein into aqueous solution? To find dissolving behavior of krill protein into water and ensure the feasibility and necessity of using a countercurrent system, the solubility of multi-step dissolution was observed firstly (Fig. 2).
Fig. 2.
Protein concentration after multi-step dissolution
It was seen that as the dissolving times increased the dissolved krill protein in the unchanged solvent rose gradually. The maximum was 33.0 ± 0.8 mg/mL after 9 extractions by one share of solvent. Comparing to supernatant of single-step extraction, the concentration increased by almost 2 times. It seemed that in single step extractions of all 4 supernatants might have the ability to dissolve proteins continuously.
Lestari et al. (2010) considered that better diffusion or high saturation concentration would help to improve extraction rate. In these experiments, homogenization has been proved to be sufficient, and the equilibrium concentrations 4 supernatants were obviously below saturation concentration. According to protein alkali-solution theory (Vojdani 1996; Lestari et al. 2010), NaOH solutions provided sufficient amount of alkaline, which increased protein net charge and electrostatic repulsion, and then promoted protein solubilization into the extracting solvent protein-solvent interaction. So it might be explained that there was lack of impetus to dissolving proteins in the residues.
Analysis of the soluble protein using SDS-PAGE
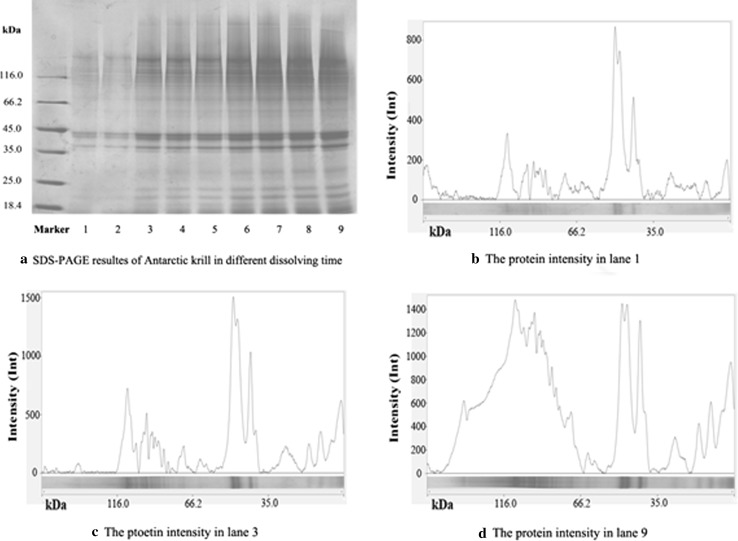
In order to interpret the above mentioned phenomena further, SDS-PAGE was used to further explore the content of the 9 solutions (Fig. 3). As the dissolving time increased, the band intensities of molecular weight between 66.2 and 35.0 kD reached a visual constant gradually. Meanwhile, the bands in the molecular weight range > 66.2 or < 35.0 kD both became more intensive continuously. The semi-quantitative results (Fig. 3b–d) agreed with the visual observations.
Fig. 3.
SDS-PAGE of Antarctic krill protein and its semi-quantitative analysis
As has been reported, molecular weights of sarcoplasmic proteins are mainly about 50 kDa (Jafarpour and Gorczyca 2009; Li et al. 2014). Meanwhile, myofibrillar proteins always divided into two main parts: myosin heavy chains (MHC), whose molecular weights are often more than 100 kDa, and myosin light chains (MLC), whose molecular weights are 16–28 KDa, varying depending on species) (Shahidi 1994; Ochiai and Chau-Jen 2000). It was assumed that, in this study, the bands in the molecular weight range > 66.2 kD were of MHC of myofibrillar proteins, bands in the molecular weight range < 35.0 kD were of MLC of myofibrillar proteins, and bands in the molecular weight range from 66.2 to 35.0 kD were of sarcoplasmic proteins. Consequently, it was implied that, in subsequent steps, this countercurrent system might dissolve more myofibrillar proteins when easily-soluble sarcoplasmic proteins became saturated in solution (Fig. 3). In other words, different proteins in the solution might have different saturation concentrations and different diffusion rates.
Analysis of multi-step cross-flow processing
Extraction percentage and recovery yield
The multi-step extractions with a krill-to-water ratio of 1:10 were conducted (Table 1). Most of protein was extracted in the first extraction step. In the following two extraction steps, 6.0 and 1.3% more protein of total were dissolved from the residue. Meanwhile, after precipitation and water-washing, only 62.3% recovery was obtained in the first step, suggesting a large amount of low molecular weight acid-soluble materials in that fraction. The second and third precipitation steps were almost completely acid-insoluble, which implied that protein recovered from these two steps might be myofibrillar protein. The overall recovery reached 69.3% of the total protein, higher than previously reported (Chen et al. 2009; Wang et al. 2011).
Table 1.
Extraction percentage and recovery yield of multi-step extraction
| Extraction step | Extraction percentage (%) | Recovery yield (%) |
|---|---|---|
| 1st | 88.1 ± 0.6 | 62.3 ± 0.4 |
| 2nd | 6.0 ± 0.4 | 5.7 ± 0.2 |
| 3rd | 1.3 ± 0.2 | 1.3 ± 0.1 |
| Sum up | 95.4 | 69.3 |
Values are mean ± standard deviation (n = 3)
Krill-to-water ratio
It was interesting if the water ratio could be lowered in the multi-step extraction. The ratios from 1:10 to 1:4 were tested (Table 2). Results showed that extraction percentage of the first step decreased significantly (p < 0.05) while the ratios changed from 1:10 to 1:4, which was in agreement with studies reported by Lestari et al. (2010). As krill-to-water ratios were 1:10 and 1:8, total extraction percentage of more than 90% could be obtained. At lower water ratios, for example 1:4, the total protein was lower, even though 20.9% protein was extracted in the second step.
Table 2.
Multi-step cross-flow extractions with different krill to water ratios
| Extraction step | 1st | 2nd | 3rd | Total E.P. | |||
|---|---|---|---|---|---|---|---|
| Conc. | E.P. | Conc. | E.P. | Conc. | E.P. | ||
| Ratio 1:10 | 12.2 ± 1.0 | 88.1 ± 0.5a | 0.8 ± 0.1 | 6.0 ± 0.4d | 0.2 ± 0.0 | 1.3 ± 0.2d | 95.4 |
| Ratio 1:8 | 14.5 ± 0.7 | 83.4 ± 1.7b | 1.3 ± 0.1 | 7.5 ± 0.2c | 0.3 ± 0.0 | 1.4 ± 0.0c | 92.3 |
| Ratio 1:6 | 16.6 ± 1.5 | 71.9 ± 1.4c | 3.2 ± 0.3 | 13.7 ± 1.1b | 0.5 ± 0.1 | 1.9 ± 0.3b | 87.5 |
| Ratio 1:4 | 18.5 ± 1.3 | 53.2 ± 2.1d | 7.3 ± 0.5 | 20.9 ± 0.8a | 1.8 ± 0.1 | 5.1 ± 0.1a | 79.2 |
Conc. concentration (mg/mL); E.P. extraction percentage (%); values are mean ± standard deviation (n = 3); means within the same column with different letters are significantly (p < 0.05) different
Multi-step countercurrent system for protein extraction
Effect of number of stage on extraction percentage
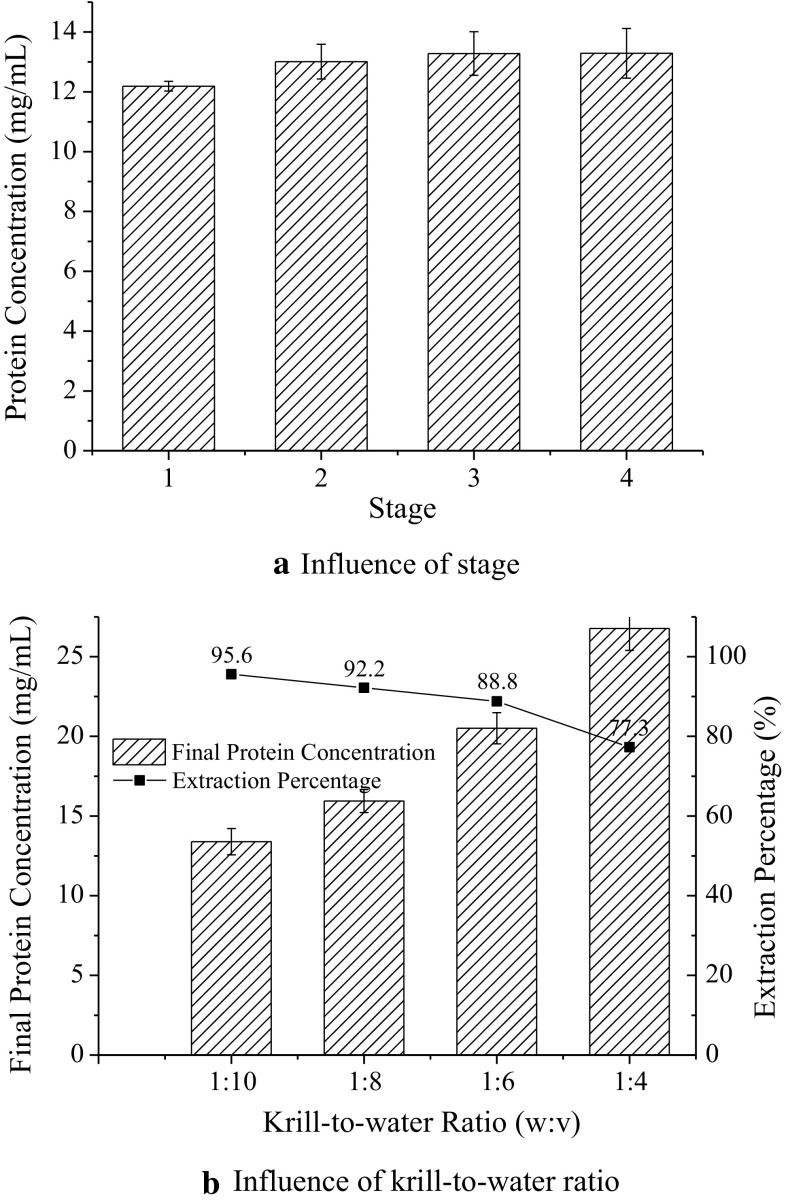
The main advantage of multi-stage countercurrent technology is that extraction percentage would increase with stage number rising. So countercurrent extractions with different numbers of stages were studied and the final protein concentrations were tested (Fig. 4a). The final protein concentration of 2-stage became higher while that of 3 and 4-stage were almost the same. So, 3 stages in the countercurrent system were enough.
Fig. 4.
Protein extraction in multi-stage countercurrent system. a Influence of stage. b Influence of krill-to-water ratio
Krill-to-water ratio
Using the 3-stage countercurrent system, the extraction percentages and final protein concentrations were examined using different krill-to-water ratios (Fig. 4b). As the amount of water increased, the final protein concentration decreased as well. Above all, the extraction percentages, which reflect extraction efficiency, were almost the same as results of multi-step cross-flow processing, while the water consumption were equal to single-step extraction.
Consequently, 3-stage countercurrent system of krill-to-water ratio 1:10 was applied to extract protein from krill, and extraction percentage 95.1 ± 0.6% was obtained. After acid precipitation and 3-stage countercurrent water-washing, the total recovery yield was up to 67.9 ± 1.6% with fluoride level less than 2 mg/kg.
Conclusion
The present study describes the possibility of extracting krill protein by multi-stage countercurrent system. First of all, in the multi-step dissolution process, it was found that protein concentration of supernatant rose up to 33.0 ± 0.8 mg/mL. As a comparison, the highest protein concentration in single step dissolution was only 18.5 ± 1.3 mg/mL. It indicated that multi-stage countercurrent system was feasible and reasonable for extracting protein from krill. Multi-step cross-flow processing extracted more protein than single-step extraction (at krill-to-water ratio 1:10, extraction percentage was raised from 88.1 to 95.4%, and recovery yield was raised from 62.3 to 69.3%), which testified the assumption further. When multi-stage countercurrent system was applied, the optimized result was obtained at krill-to-water ratio 1:10, in 3-stage countercurrent extracting system, where the extraction percentage 95.1 ± 0.6% and total recovery yield 67.9 ± 1.6% were achieved. Comparing to previous works, almost the same water was consumed when the better extraction efficiency was reached. All the results demonstrated a great potential for application of this technology in Antarctic krill processing industry.
Acknowledgements
This study was supported by the Key Research and Development Project of Shandong Province (No. 2015GSF115005), Huimin Special Fund of Qingdao Municipal achievement transformation plan (No.15-9-2-120-NSH), and the Natural Science Foundation of China project (No. 31101380).
Contributor Information
Xiangming Qi, Email: qixm@ouc.edu.cn.
Xiangzhao Mao, Phone: +86-532-82031360, Email: xzhmao@ouc.edu.cn.
References
- Adu-Peasah SP, Diosady LL, Rubin LJ. A multistage hydrocyclone/stirred-tank system for countercurrent extraction of canola oil. J Am Oil Chem Soc. 1993;70:755–762. doi: 10.1007/BF02542596. [DOI] [Google Scholar]
- AOAC (Association of Official Analytical Chemists) Official methods of analysis of AOAC international. 19. Washington DC: AOAC; 2012. [Google Scholar]
- Barac MB, Pesic MB, Stanojevic SP, Kostic AZ, Bivolarevic V. Comparative study of the functional properties of three legume seed isolates: adzuki, pea and soy bean. J Food Sci Tech. 2015;52:2779–2787. doi: 10.1007/s13197-014-1298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Jaczynski J. Gelation of protein recovered from whole Antarctic krill (Euphausia superba) by isoelectric solubilization/precipitation as affected by functional additives. J Agric Food Chem. 2007;55:1814–1822. doi: 10.1021/jf0629944. [DOI] [PubMed] [Google Scholar]
- Chen YC, Tou JC, Jaczynski J. Amino acid and mineral composition of protein and other components and their recovery yields from whole Antarctic krill (Euphausia superba) using isoelectric solubilization/precipitation. J Food Sci. 2009;74:H31–H39. doi: 10.1111/j.1750-3841.2008.01026.x. [DOI] [PubMed] [Google Scholar]
- Gigliotti JC, Jaczynski J, Tou JC. Determination of the nutritional value, protein quality and safety of krill protein concentrate isolated using an isoelectric solubilization/precipitation technique. Food Chem. 2008;111:209–214. doi: 10.1016/j.foodchem.2008.03.030. [DOI] [Google Scholar]
- Jafarpour A, Gorczyca EM. Characteristics of sarcoplasmic proteins and their interaction with surimi and kamaboko gel. J Food Sci. 2009;74:N16–N22. doi: 10.1111/j.1750-3841.2008.01009.x. [DOI] [PubMed] [Google Scholar]
- Lestari D, Mulder W, Sanders J. Improving Jatropha curcasseed protein recovery by using counter current multistage extraction. Biochem Eng J. 2010;50:16–23. doi: 10.1016/j.bej.2010.02.011. [DOI] [Google Scholar]
- Li K, Shen H, Li B, Wang H, Luo Y. Changes in physiochemical properties of water-soluble proteins from crucian carp (Carassius auratus) during heat treatment. J Food Sci Tech. 2014;51:1396–1400. doi: 10.1007/s13197-012-0651-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moure A, Franco D, Sineiro J, Domínguez H, Núñez MJ. Simulation of multistage extraction of antioxidants from Chilean hazelnut (Gevuina avellana) hulls. J Am Oil Chem Soc. 2003;80:389–396. doi: 10.1007/s11746-003-0709-x. [DOI] [Google Scholar]
- Nayak R, Kenney PB, Slider S. Protein extractability of turkey breast and thigh muscle with varying sodium chloride solutions as affected by calcium, magnesium and zinc chloride. J Food Sci. 1996;61:1149–1154. doi: 10.1111/j.1365-2621.1996.tb10950.x. [DOI] [Google Scholar]
- Ochiai Y, Chau-Jen C. Myosin ATPase. In: Haard NF, Simpson BK, editors. Seafood enzymes: utilization and influence on postharvest seafood quality. Basel: Marcel Dekker; 2000. pp. 69–90. [Google Scholar]
- Qi XM, Liao E, Wang L, Lin H, Xue CH. Extracting protein from antarctic krill (euphausia superba) J Aquat Food Prod T. 2016;25:597–606. doi: 10.1080/10498850.2014.904461. [DOI] [Google Scholar]
- Shahidi F. Seafood proteins and preparation of protein concentrates. In: Shahidi F, Botta JR, editors. Seafoods: chemistry, processing technology and quality. Berlin: Springerlink; 1994. pp. 3–9. [Google Scholar]
- Suzuki T, Shibata N. The utilization of Antarctic krill for human food. Food Rev Int. 1990;6:119–147. doi: 10.1080/87559129009540863. [DOI] [Google Scholar]
- Takahata S, Suemura M, Noguchi M, Ohdan K (1974) US Patent No. 3844723. US Patent and Trademark Office, Washington DC
- Taskaya L, Chen YC, Jaczynski J. Functional properties of proteins recovered from silver carp (Hypophthalmichthys molitrix) by isoelectric solubilization/precipitation. LWT-Food Sci Technol. 2009;42:1082–1089. doi: 10.1016/j.lwt.2009.02.007. [DOI] [Google Scholar]
- Tou JC, Jaczynski J, Chen YC. Krill for human consumption: nutritional value and potential health benefits. Nutr Rev. 2007;65:63–77. doi: 10.1111/j.1753-4887.2007.tb00283.x. [DOI] [PubMed] [Google Scholar]
- Vareltzis PK, Undeland I. Protein isolation from blue mussels (Mytilus edulis) using an acid and alkaline solubilisation technique—process characteristics and functionality of the isolates. J Sci Food Agric. 2012;92:3055–3064. doi: 10.1002/jsfa.5723. [DOI] [PubMed] [Google Scholar]
- Vojdani F. Solubility. In: Hall GM, editor. Methods of testing protein functionality. London: Blackie Academic & Professional an imprint of Chapman & Hall; 1996. pp. 9–60. [Google Scholar]
- Walker JM. SDS polyacrylamide gel electrophoresis of proteins. In: Walker JM, editor. The protein protocols handbook. 2. New York: Humana Press; 2002. pp. 61–68. [Google Scholar]
- Wang QE, Ma S, Fu B, Lee FS, Wang X. Development of multi-stage countercurrent extraction technology for the extraction of glycyrrhizic acid (GA) from licorice (Glycyrrhiza uralensis Fisch) Biochem Eng J. 2004;21:285–292. doi: 10.1016/j.bej.2004.06.002. [DOI] [Google Scholar]
- Wang LZ, Xue CH, Wang YM, Yang B. Extraction of proteins with low fluoride level from Antarctic krill (Euphausia superba) and their composition analysis. J Agric Food Chem. 2011;59:6108–6112. doi: 10.1021/jf201009t. [DOI] [PubMed] [Google Scholar]
- Yu CH, Chen J, Xiong YK, Li XX, Dai XY, Shi CC. Optimization of multi-stage countercurrent extraction of antioxidants from Ginkgo biloba L. leaves. Food Bioprod Process. 2012;90:95–101. doi: 10.1016/j.fbp.2011.05.003. [DOI] [Google Scholar]