Abstract
This study was conducted to determine the effects of hazelnut drying machine (DM1 and DM2; at 45 °C and 50 °C, respectively) and sun-drying (concrete ground and grass ground) methods on the chemical properties of Tombul, Palaz, and Ordu Levant hazelnuts. For this purpose, protein, lipid and moisture content, water activity, free fatty acid (FFA), peroxide value (PV), rancimat value (RV) and fatty acid composition were analyzed. As expected, it was observed that monounsaturated fatty acid (MUFA) was the main fatty acid group (81.58–84.80%) followed by polyunsaturated (PUFA; 9.53–11.42%) and saturated fatty acids (SFA; 5.87–6.92%), and the major group constituted ~ 99.00% of the total fatty acids, whereas the minor group constituted ~ 0.5% of these acids. However, caproic (C6: 0), caprylic (C8: 0), capric (C10: 0), lauric (C12: 0), eicosadienoic (20: 2), erucic (22: 1), docosadienoic (22: 2), and lignoceric (C24: 0) fatty acids were below limit of detection (< 0.001%). Samples dried in DM1 and DM2 had more MUFA (84.49%, 84.80, respectively), and lower SFA and PUFA than those using sun-drying methods. Following the drying process, the lowest FFA and PV (0.04–0.17%, 0.00–0.27 meq O2 kg−1, respectively) and the highest RV (5.46–6.05 h) were recorded in the DM1 method. Furthermore, it was also observed that as the heat increased (DM1 and DM2; 45–50 °C, respectively), oleic/linoleic acidity ratio, FFA, and PV increased and iodine value and RV decreased. Therefore, DM1 was thought to be a promising method for hazelnut drying.
Keywords: Drying, Fatty acids, Nut quality, Oil oxidation, Sun-dried
Introduction
In Turkey, areas where hazelnut is produced are majorly located between 40°–41° latitudes and 37–42° longitudes. The most suitable areas within these borders in terms of ecological conditions are Black Sea coasts. Hazelnut cultivation extends 60 km inland from the Black Sea coast and up to 750 m high. These areas are categorized in three sections depending on their distance from the coast and their altitude. These section are the coastal section (0–250 m; up to 10 km inland), mid-section (251–500 m; 10–20 km inland), and high section (510–750 m; ≥ 20 km inland; Turan 2017).
In the Black Sea region, modern hazelnut cultivation is not common, although this region has the highest quality hazelnut cultivars and ecology. The failure to apply modern techniques to cultural applications results in decreased nut quality and an increase in the post–harvest losses. Faulty practices particularly during the drying stage lead to significant losses of hazelnut, and pave the way for great issues in nut preservation and marketing stages (Turan and İslam 2016). Therefore, it is of great importance to separate the husks following the picking process, and to dry hazelnuts in a short time as postponing the drying process poses the risk of damaging the hazelnuts owing to mold formation and pests. The conventional drying methods [on concrete ground (CG) and grass grounds (GG)] require rainless and sunny days. However, consecutive sunny days are rare during the harvest season because of the ecology of the area, and thus it is difficult to find windows for continuous drying in certain time periods.
The drying process is one of the oldest methods of agricultural products preservation (Kaveh et al. 2018). Therefore, drying is crucial while processing postharvest hazelnut for ensuring food safety and quality during storage. The recommend safe moisture content for in–shell hazelnuts is 6 s/100 g (Wang et al. 2018). Dehydration is one of the best preservation methods, which could extend the shelf life of the food and has been used for drying and preserving the fruit for several centuries (Zhang et al. 2018). The method is based on the removal of moisture contained in the product by means a complex process involving simultaneous heat and mass transfer.
The traditional drying method is based on solar energy, but the products can be easily spoiled due to varying environmental conditions that can cause a major loss in nut quality (Kaveh et al. 2018). Moreover, the heterogeneity of the internal structure of fruit within single variety, or even single fruit is causing the plant tissue to be a material susceptible to various types of thermal, mechanical or enzymatic processes. Technological treatment changes the structure of the raw fruit by modifying not only enzymatic reactions occurring in the tissue, but most of all, by affecting the conditions of the heat and mass exchange that occur in the plant material (Janowicz and Lenart 2018). In addition, undesirable range of moisture content and water activity of kernels as a result of drying can increase hydrolytic or oxidative enzyme activities, including lipase, peroxidase, and polyphenol oxidase. Thus, drying process should be applied carefully to postharvest hazelnut in-shells not only suppressing microbial growth, but also delaying deterioration quality deterioration associated with lipid oxidation in kernels (Wang et al. 2018). Hazelnut has a high fat content, so it is much utilized as raw oilseeds for food and industrial purposes. Studies of the hazelnut oil showed that the average oil content was 60% of the dry weight of the kernel. Oxidative stabilities of the hazelnut oils were higher than that of soybean oil, while the cloud points of hazelnut oils were lower than that of soybean oil. These findings indicate that hazelnut oil is a potential feedstock for oleochemicals (Guine et al. 2015).
In response to the changes in light and heat, lipid molecules are released to form free fatty acids, which can affect the stability of nut oil (Fu et al. 2016). Light is a major factor to control photooxidation, although its importance reduces if the temperature increases. The effect of oxygen concentration on the oxidation of oil was more significant with increasing temperatures and with exposure to light (Rabadan et al. 2018). Therefore, it is important to maintain oil stability during the hazelnut drying process. Moreover, the rapid postharvest processing of hazelnut, particularly drying, is an important parameter in terms of the quality of the final product during the storage phase. In sum, to ensure their long shelf-life and to protect them from rancidification processes, hazelnuts must be dried, immediately after harvest (Ghirardello et al. 2013). Unfortunately, in comparison with other food products, studies on the drying of hazelnut are limited (Turan and İslam 2018). Therefore, it is necessary to design and accurately simulate drying system for hazelnuts to sustain better quality.
It is known that, among sun-drying methods, concrete ground is more suitable for drying hazelnuts in comparison with the grass ground method. However, comprehensive studies on this topic have not been conducted so far. In addition, there is no comprehensive information on the effects of artificial and natural drying methods on Turkish nut quality. The purpose of this study is to determine the effects of sun drying methods (CG and GG) and artificial drying methods (DM1 and DM2) on the chemical properties of Tombul, Palaz, and Ordu Levant hazelnuts. Data that will be obtained in the study will make important contributions to the hazelnut industry and, in particular, to the literature.
Materials and methods
Materials
Experiments were carried out on Tombul, Palaz, and Ordu Levant hazelnuts (Mostly these cultivars are grown in Turkey) that were harvested in a single orchard, located in the Cumhuriyet neighborhood (40°58″21.72 N, 37°58″48.14E, altitude: 43 m) in the Altınordu district, Ordu, Turkey. Ordu Levant hazelnuts comprised of 45.09% Tombul, 37.05% Palaz and, 17.86% Kalinkara cultivars [According to hazelnut quality specifications, Giresun and Levant quality are divided into two. Tombul hazelnuts produced in this region, including Vakfıkebir district of Trabzon province, from Piraziz district east of Ordu province are named as Giresun quality. Hazelnuts produced in regions outside Giresun region are called Levant quality hazelnuts (Turan 2017). It is named for the region where it is produced in trade (Akçakoca, Ordu and Trabzon) and is of lower quality than Giresun quality hazelnuts (Alaşalvar et al. 2010)].
Drying methods
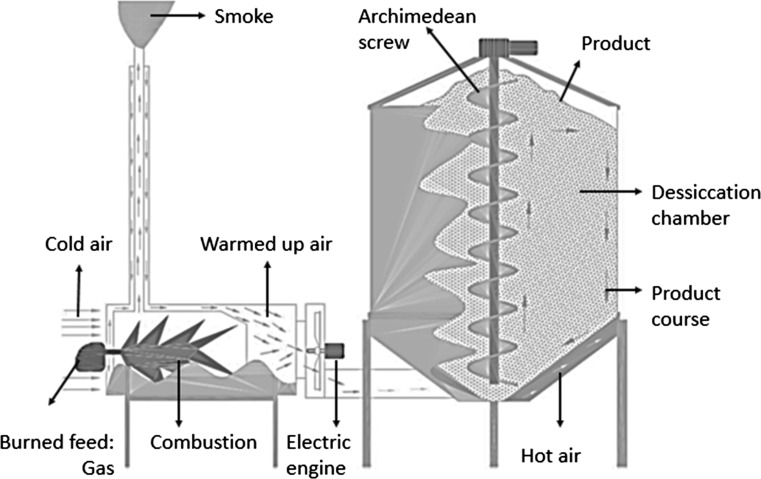
Nuts in husks were harvested by hand from August 5 to August 15, 2015. Average kernel moisture content was ~ 28% at harvest (Refsan RK 55, Kütahya, Turkey; Turan and İslam 2016). Clusters were laid on a grass ground and dehydrated for 4 days (August 15 to August 19, 2015) to lose moisture (Tombul, Palaz and Ordu Levant; 22, 21%.01% and 19%, respectively. Nuts were separated from husks using machines (Dinçler Makine, FPHM 2500, Samsun, Turkey) and divided into three groups. The first group was dried in the sun on grass ground (Dimensions of the ground: 4 × 3 m, 30 kg cv. used in each drying, total 90 kg; GG). Grass was cut with string trimmer (Oleo–Mac 440 T, Italy), a canvas was laid on the ground, and samples were placed on the canvas (TS 4739, TS 1534–2; EN ISO 2286-2, Kale Tente, İstanbul, Turkey) to be dried in the sun with occasional turning. The second group was dried on concrete ground (Dimensions of the ground: 5 × 5 m, 30 kg cv. used in each drying, total 90 kg; CG). Nuts were placed on CG and dried in the sun with occasional mixing. It is mention that CG and GG methods were performed in similar sunshine and environmental conditions (average of wind velocity, ambient air temperature and relative humidity and sunshine duration; 1.2 h km−1, 25.7 °C, 69.3% and 5.45 h, respectively). The hazelnut on CG and GG methods were dried every day from 8:00 a.m. to 8:00 p.m. continuously. After 8 p.m., plastic cover (Metroplast, İstanbul, Turkey) was used to prevent the samples from getting wet. The last group was dried in a drying machine. Nuts were placed in a drying machine (FACMA s.r.l ES 3000) and dried with hot air at 45–50 °C [3000 kg cv. used each drying (in–shell nut samples of ~ 30 kg were randomly selected for analysis), total 18,000 kg; DM1 and DM2, respectively; Turan 2017]. Namely, the desiccation was obtained by the forced ventilation of hot air, which the heat-exchanger sends to the ventilator, which at the same time pushes it inside the body of the dryer. The sample, continuously ventilated, was mixed by a central Archimedean screw and it can be ventilated also with non heated air. The dryer adjusted in temperature was conditioned about 3 h each operation and 1.5 h cease. Meanwhile, the Archimedean screw has continued circulation for 1.5 h in every cycle (Fig. 1). Shell and kernel moisture contents were measured before and after dehydration (Table 1). Drying time (in hours) has been represented in Table 1. Drying process were carried out 20 and 25th day of August 2015 in the Karapınar neighborhood (l 40°58′17.53′′N, 37°56′00.41′′ E, altitude 10 m) in the Altınordu district, Ordu, Turkey (Ordu OSB, Gürsoy Tarımsal Ürünler Gıda Sanayi ve Ticaret A.Ş. Entegre Tesisi). At the end of the drying process, samples were stored (Faculty of Agriculture, Ordu University, Ordu, Turkey, l 40°58′30.35′′N, 37°58′09.06′′E, altitude 4 m) at an ambient temperature (~ 25 °C and 70–80% relative humidity) in jute bags (three 3 replicates, ~ 800 g in–shell hazelnut in each replication) until further analyses. A total of ~ 30 kg of nuts was utilized in this research.
Fig. 1.
Schematic diagram of the dryer used for hazelnut drying
Table 1.
Moisture content of hazelnuts before and after dehydration, and after drying and drying time
| Cultivar | M | MC (%) | Drying time (h) | |||||
|---|---|---|---|---|---|---|---|---|
| Initially | After dehydration | After drying | ||||||
| Shell | Kernel | Shell | Kernel | Shell | Kernel | |||
| Tombul | CG | 28.02 | 26.13 | 25.33 | 22.00 | 7.50 | 5.45 | 85 |
| GG | 8.10 | 6.00 | 96 | |||||
| DM1 | 8.00 | 6.02 | 25 | |||||
| DM2 | 8.50 | 5.89 | 23 | |||||
| Palaz | CG | 27.96 | 25.00 | 24.96 | 21.01 | 7.75 | 6.24 | 76 |
| GG | 7.70 | 6.27 | 85 | |||||
| DM1 | 7.69 | 6.32 | 26 | |||||
| DM2 | 7.48 | 6.35 | 24 | |||||
| Ordu Levant | CG | 29.00 | 26.59 | 21.50 | 19.00 | 9.00 | 5.68 | 80 |
| GG | 9.23 | 6.75 | 88 | |||||
| DM1 | 9.01 | 5.86 | 30 | |||||
| DM2 | 8.86 | 5.03 | 27 | |||||
M Drying, CG concrete ground, GG grass ground, DM1 and DM2 drying machine (45 °C and 50 °C, respectively), MC moisture content
Extraction of hazelnut oil
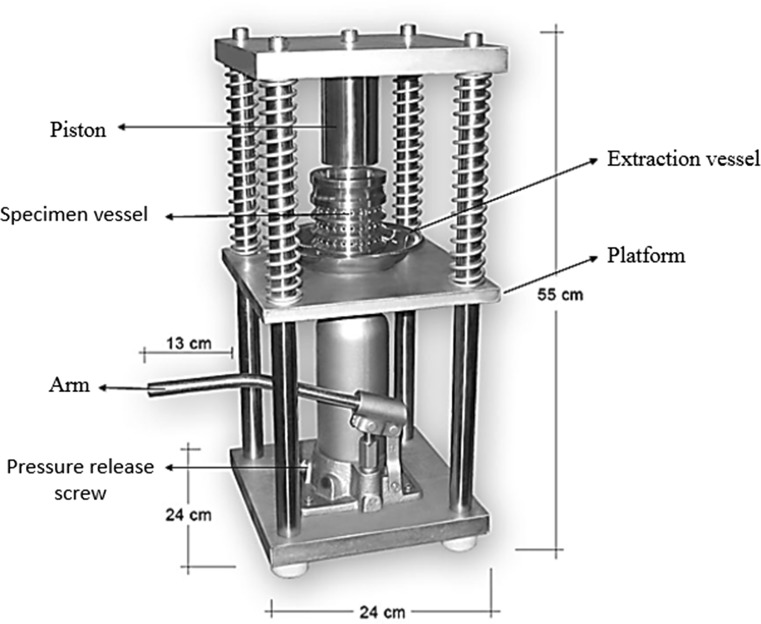
Hazelnut oil was extracted by a cold-pressing (Pressure force: 10,000 kgf, pressure: 34.7 MPa, temperature: – 5 ~ + 45 °C and capacity; 250 g kernel) method using Ceselsan’s nut oil extraction system (Fig. 2; AISI3004, Ceselsan, Giresun, Turkey). Kernel samples of ~ 800 g were randomly selected (Turan 2017) and pressed under up to 30 MPa and 40 °C for 10 min. The recovered oil was separated by centrifugation at 4800 rpm for 5 min, and the oil was stored at − 18 °C in a freezer until further analysis.
Fig. 2.
Schematic diagram of cold–pressed hazelnut oil extraction
Protein and fat content
Protein content (PC) was determined using AOAC Standard Methods. PC (N × 6.25) was estimated from 0.5 g samples by the macro Kjehldahl method (Velp UDK 149, Europe). Lipid content (LC) was determined according to AOAC Official Methods (AOAC 2000). LC was determined by extracting a known sample-weight (5 g) with petroleum ether, using a soxhlet apparatus (Velp Ser 148, Milano, Italy).
Moisture content and water activity
Moisture content (MC) was determined according to Turkish Standards Institution (TSE)–TS 3075/T1 standard (TSE 2001). MC was evaluated on ground hazelnut (Fakir Motto 800 w, Germany) samples in an oven (Refsan RK 55, Kütahya, Turkey) at 105 °C until a constant weight was reached. Water activity (aw) was determined using the Novasina aw Sprint TH 500 (Switzerland) water activity analyzer (WAA 2004).
Fatty acid composition
The fatty acid composition of hazelnut kernel oils was determined by gas chromatography (GC). Methyl esters of fatty acids (FAMEs) were prepared according to Ficarra et al. (2010) with slight modifications. Oil samples (0.5 g of oil) were weighed into erlenmayer flask and mixed with 4 mL of iso–octane and 2 mL of methanolic KOH solution followed by shaking for 30 s. Then, erlenmayer was closed and left in the dark for 6 min at the end of period, 2 drops of methyl orange indicator were dropped and the solution was titrated with 1 M HCL until pink color appeared. After the content was rested for 15 min, the colorless upper layer was transferred into glass vials and analyzed in GC. Fatty acids profiles were determined using Shimadzu brand (Model GC–2010, Japan) gas chromatography with a flame ionization detector (FID) and TR-CN100 column (60 m × 0.25 mm I.D., 0.20 µm; Teknokroma, Spain). The enjector temperature was set at 250 °C and the detector temperature was set at 250 °C. The amount of sample injected was 1.0 µL and helium at pressure of 200 kPa was used as carrier gas. Injection was performed at a ratio of 1:100. The column temperature was maintained at 90 °C for 7 min, and then the temperature increased to 240 °C increasing by 5 °C min−1. Eventually, it was held at 240 °C for 15 min. Fatty acids were identified by comparison with the time of arrival of the FAME mixture (Supelco 37 Component FAME Mixture, Cat. No. 18919–1 AMP, Bellefonte PA, USA) consisting of 37 standard components (Sarıcaoğlu and Turhan 2013).
The obtained fatty-acid composition was used to calculate the sum of saturated (∑SFA), monounsaturated (∑MUFA), and polyunsaturated (∑PUFA) fatty acids as well as the ratio of fatty acids (∑MUFA + PUFA/∑SFA).
Oxidation parameters
To determine free fatty acids (FFA), peroxide value (PV; expressed as meq O2 kg−1 oil), rancimat value (RV), ratio of oleic-to-linoleic acid (O/L), and iodine value (IV) were evaluated.
FFA was determined using the AOAC Standard Method (AOAC 1990a, b). ~ 2.5–5 g (M) of oil was transferred into a 250 mL erlenmayer flask with a known tare weight. 25–50 mL of a mixture of ether: ethanol (1/1, v/v) was added and shaken, 2–3 drops of phenolphthalein indicator were dropped and was titrated with 0.1 N NaOH solution until the pink color persisting for at least 10 s. The amount of FFA was calculated by the following formula. FFA (% oleic acid) = [(S × F × 0.0282)/M)] × 100, S: Amount of NaOH spent in the titration, F: factor of 0.1 NaOH solutions, M: Amount of oil (g).
To determine PV, ~ 2.5–5 g was weighted into a 250 mL erlenmayer, which has been tarred and wrapped with aluminum foil. 30 mL acetic acid: chloform (3/2, v/v) mixture was add and mixed. 0.5 mL of saturated potassium iodide solution was added. It was shaken for a minute and left in the dark. At the end of shaking period, 30 mL of distilled water and 0.5 mL of 1% starch solution was added. The solution was titrated with 0.01 N sodium thiosulfate solution until a clear cream color was formed and the PV was calculated according to following formula; PV (meq O2 kg−1 fat) = [(S × N × F)/M] × 1000; S: amount of sodium thiosulfate spent in the titration, F: factor of solution, N: normality of the sodium thiosulfate solution, M: amount of fat (g; Metrohm, Dosimat 799, Switzerland; 1990a, b).
RV was evaluated by the Rancimat method (Velasco et al. 2004). RV was expressed as the oxidation induction period measured with the Ransimat 743 device (Metrohm co., Basel, Switzerland). An oil sample of 3.5 g was used, warmed to 130 °C under an air flow of 20 L h−1. IV was determined according to the percentages of fatty acids using the following formula: (palmitoleic acid × 1.901) + (oleic acid × 0.899) + (linoleic acid × 1.814) + (linolenic acid × 2.737; Belviso et al. 2017).
Statistical analysis
Experiments were performed in triplicate with a randomized–block design. Descriptive statistics were obtained with SPSS v. 22.0 (Armok, New York: IBM Corp.). Statistical tests were performed using the SAS–JAMP v. 10.0 (SAS Institute Inc., Cary, North Carolina). A one-way ANOVA was conducted to assess significant differences among levels and the least significance difference (LSD) test was used to compare multiple means. Results were considered to be significantly different at p < 0.05, p < 0.01 and p < 0.001.
Results and discussion
Protein and fat content
The effect of drying methods on protein content (PC) was found to be significant (p < 0.001; Table 2). Consistent with our study, Delgado et al. (2017) and Turan and İslam (2016) reported that drying methods affected PC, whereas other studies reported that drying methods did not have any effect on PC (Gölükçü 2015; Thakur et al. 2014; Kermani et al. 2017). This contradiction may have arisen because of different cultivars or species as well as different drying methods. In our study, there were differences in PC values with regard to the effect of drying methods between cultivars. That is, there was no difference in PC value when different drying methods were applied to Tombul, and the variability was between 14.66 and 14.94%. For Palaz cultivar, the highest value was 17.43%, which was observed for CG, and the lowest value was 14.40%, which was observed for GG. PC value was between 14.38 and 13.52% (CG and GG, respectively) for Ordu Levant cultivar. Although drying methods did not affect LC values (p > 0.05), the difference between cultivars was statistically significant (p < 0.001; Table 2). Similarly, Kermani et al. (2017) reported that drying methods did not affect LC values, whereas Delgado et al. (2017) reported that drying methods affected LC values in chestnuts (2.14–3.07%). In our study, there were differences in the effect of drying methods between cultivars. For example, the highest LC values were observed for CG (59.18%), DM1 (60.05%), and DM1 (57.20%) methods for Tombul, Palaz, and Ordu Levant cultivars, respectively. In light of these results, it can be concluded that LC values vary in hazelnut cultivars depending on the selection of drying methods.
Table 2.
Effect of drying on protein content, lipid content, moisture content and water activity
| Cv | M | PC (%) | LC (%) | MC (%) | aw |
|---|---|---|---|---|---|
| Parameter | |||||
| Tombul | CG | 14.92 ± 0.03b | 59.18 ± 1.11ab | 4.38 ± 0.00f | 0.65 ± 0.00c |
| GG | 14.66 ± 0.32bcd | 57.47 ± 0.61a-e | 4.15 ± 0.01h | 0.63 ± 0.00d | |
| DM1 | 14.94 ± 0.26b | 57.13 ± 1.55cde | 4.30 ± 0.00g | 0.60 ± 0.00g | |
| DM2 | 14.81 ± 0.27bc | 57.35 ± 0.58b-e | 4.27 ± 0.03g | 0.60 ± 0.00g | |
| Palaz | CG | 17.43 ± 0.84a | 58.80 ± 0.76abc | 5.15 ± 0.00b | 0.61 ± 0.01f |
| GG | 14.40 ± 1.27bcd | 59.42 ± 1.46a | 4.88 ± 0.00d | 0.62 ± 0.00e | |
| DM1 | 14.97 ± 0.39b | 60.05 ± 0.92a | 4.75 ± 0.01e | 0.60 ± 0.00g | |
| DM2 | 14.88 ± 0.22b | 58.61 ± 1.14a-d | 4.74 ± 0.01e | 0.60 ± 0.00g | |
| Ordu Levant | CG | 14.38 ± 0.81bcd | 54.53 ± 0.47f | 5.00 ± 0.00c | 0.60 ± 0.00g |
| GG | 13.52 ± 1.29d | 56.89 ± 1.40de | 5.11 ± 0.01b | 0.63 ± 0.00d | |
| DM1 | 14.12 ± 0.73bcd | 57.20 ± 1.47cde | 5.35 ± 0.01a | 0.68 ± 0.00b | |
| DM2 | 13.63 ± 0.55cd | 56.52 ± 0.95de | 5.33 ± 0.08a | 0.70 ± 0.00a | |
| Sign. | Cv | *** | *** | *** | ns |
| M | *** | ns | ns | ns | |
| CvxM | *** | * | ** | * | |
Cv cultivar, M drying, CG concrete ground, GG grass ground and DM1 and DM2 drying machine (45 °C and 50 °C, respectively). PC protein content, LC lipid content, MC moisture content and aw Water activity. Values are expressed as mean ± standard deviation. Different letters in columns for each different drying method. Significant level; *, **, *** and “ns” mean significance at p < 0.05, 0.01, 0.001 and “not significant”, respectively, among drying methods
Moisture content and water activity
The effect of drying methods on MC values was not significant (p < 0.05; Table 2). MC value was 4.15–4.38%, 4.74–5.15% and 5.00–5.35% for Tombul, Palaz, and Ordu Levant hazelnuts, respectively, and the highest percentage change in MC value was for Palaz cultivar. Contrary to our results, Turan and İslam (2016) reported that drying methods affected MC values, and that the values obtained using drying machines were higher (4.47% and 4.33%, respectively) compared with those obtained using sun–drying methods. In our study, the highest MC was recorded for Ordu Levant hazelnuts. Such difference may have been due to the thicker nutshells of Ordu Levant in comparison with other cultivars. The aw value is an important property that affects fat oxidation and the desired aw value is > 0.70. Our study found that the effect of drying methods on aw value was not significant, and aw values ranged from 0.60 to 0.70 throughout the study duration.
Fatty acid composition
In this study, the effect of drying methods on 12 fatty acids [except for (C17:1)] was significant, and the results have been presented in Table 3 in detail. 13 fatty acids were identified in Tombul, Palaz, and Ordu Levant hazelnuts in total, and the major fatty acid was oleic acid (C18:1) followed by linoleic (C18:2), palmitic (C16:0), and stearic (C18:0) acids. Major fatty acids constituted ~ 99.00% of the total fatty acids while the minor fatty acids constituted only ~ 0.5%. In addition, caproic (C6: 0), caprylic (C8: 0), capric (C10: 0), lauric (C12: 0), eicosadienoic (20: 2), erucic (22: 1), docosadienoic (22: 2), and lignoceric (C24: 0) fatty acids were below limit of detection (< 0.001%). Fatty acid content varies depending on several factors such as cultivar, geographical origin, cultural applications, maturity, harvest time, season, soil type, climate, and altitude (Amaral et al. 2006; Turan 2018). For instance, Tüfekçi and Karataş (2018) reported that Central Black Sea hazelnuts contained high levels of saturated fatty acids (8.45%) and monounsaturated fatty acids (83.45%) and low levels of polyunsaturated fatty acids (7.85%), whereas Eastern Black Sea hazelnuts contained high levels of linoleic (9.10%) and linolenic (0.09%) acids. Besides, Alaşalvar et al. (2010) reported that Tombul cultivar contained 5.61% palmitic acid (C16:0), 82.16% oleic acid (C18:1), and 8.26% linoleic acid (C18:2); and Palaz cultivar contained 6.64% palmitic (C16:0), 81.97% oleic acid (C18:1), and 8.32% linoleic (C18:2) acids.
Table 3.
Effect of drying methods on fatty acids composition of hazelnut
| Cv | M | C14:O (%) | C16:O (%) | C16:1 (%) | C17:O (%) | C17:1 (%) | C18:O (%) | C18:1 (%) |
|---|---|---|---|---|---|---|---|---|
| Parameter | ||||||||
| Tombul | CG | 0.02 ± 0.00d | 4.25 ± 0.01f | 0.09 ± 0.01bc | 0.02 ± 0.00f | 0.05 ± 0.01 | 1.94 ± 0.01c | 84.25 ± 0.01d |
| GG | 0.02 ± 0.00d | 3.85 ± 0.01k | 0.08 ± 0.00c | 0.03 ± 0.01de | 0.05 ± 0.00 | 1.85 ± 0.00e | 83.90 ± 0.00f | |
| DM1 | 0.03 ± 0.00c | 3.95 ± 0.01ı | 0.07 ± 0.00d | 0.04 ± 0.00bc | 0.05 ± 0.00 | 2.08 ± 0.00a | 84.26 ± 0.01d | |
| DM2 | 0.03 ± 0.01c | 3.93 ± 0.01j | 0.07 ± 0.01d | 0.04 ± 0.01cd | 0.05 ± 0.01 | 1.98 ± 0.01b | 84.58 ± 0.01a | |
| Palaz | CG | 0.04 ± 0.00b | 4.80 ± 0.00a | 0.11 ± 0.00a | 0.04 ± 0.00bc | 0.05 ± 0.00 | 1.90 ± 0.00d | 81.35 ± 0.00j |
| GG | 0.03 ± 0.00c | 3.95 ± 0.01ı | 0.09 ± 0.00b | 0.03 ± 0.00e | 0.04 ± 0.00 | 1.95 ± 0.00c | 82.78 ± 0.00ı | |
| DM1 | 0.05 ± 0.01a | 4.62 ± 0.00b | 0.09 ± 0.00b | 0.04 ± 0.00bc | 0.05 ± 0.00 | 1.70 ± 0.01j | 82.80 ± 0.01h | |
| DM2 | 0.05 ± 0.00a | 4.57 ± 0.01c | 0.083 ± 0.01bc | 0.036 ± 0.01cd | 0.05 ± 0.01 | 1.68 ± 0.01k | 82.85 ± 0.01g | |
| Ordu Levant | CG | 0.03 ± 0.00c | 4.33 ± 0.01d | 0.10 ± 0.01a | 0.04 ± 0.00bc | 0.05 ± 0.00 | 1.83 ± 0.01f | 84.12 ± 0.03e |
| GG | 0.02 ± 0.00d | 4.26 ± 0.00e | 0.11 ± 0.01a | 0.04 ± 0.00bc | 0.05 ± 0.01 | 1.81 ± 0.01g | 84.11 ± 0.01e | |
| DM1 | 0.03 ± 0.00d | 4.21 ± 0.01g | 0.10 ± 0.01a | 0.04 ± 0.00bc | 0.04 ± 0.01 | 1.80 ± 0.00h | 84.45 ± 0.01c | |
| DM2 | 0.03 ± 0.01d | 4.18 ± 0.01h | 0.11 ± 0.01a | 0.043 ± 0.01b | 0.04 ± 0.01 | 1.78 ± 0.01ı | 84.50 ± 0.01b | |
| Sign. | Cv | *** | *** | *** | *** | ns | *** | *** |
| M | *** | *** | *** | ** | ns | *** | *** | |
| CvxM | * | *** | *** | *** | ns | *** | *** | |
| Cv | M | C18:2 (%) | C18:3 (%) | C20:0 (%) | C20:1 (%) | C22:0 (%) | C24:1 (%) |
|---|---|---|---|---|---|---|---|
| Parameter | |||||||
| Tombul | CG | 9.43 ± 0.01ı | 0.10 ± 0.00ef | 0.06 ± 0.00d | 0.06 ± 0.01abc | 0.03 ± 0.00d | 0.04 ± 0.00a |
| GG | 9.95 ± 0.00h | 0.12 ± 0.00ab | 0.08 ± 0.00a | 0.06 ± 0.00bc | 0.04 ± 0.00c | 0.03 ± 0.00c | |
| DM1 | 9.23 ± 0.03j | 0.12 ± 0.00ab | 0.07 ± 0.00b | 0.07 ± 0.00a | 0.05 ± 0.00a | 0.04 ± 0.00a | |
| DM2 | 9.21 ± 0.01j | 0.12 ± 0.01a | 0.07 ± 0.00b | 0.06 ± 0.01abc | 0.05 ± 0.01ab | 0.04 ± 0.01b | |
| Palaz | CG | 11.32 ± 0.01a | 0.10 ± 0.01e | 0.08 ± 0.00a | 0.05 ± 0.01d | 0.05 ± 0.00a | 0.02 ± 0.00d |
| GG | 10.82 ± 0.00b | 0.11 ± 0.00d | 0.08 ± 0.00a | 0.06 ± 0.00bc | 0.05 ± 0.00a | 0.04 ± 0.00a | |
| DM1 | 10.35 ± 0.01c | 0.10 ± 0.00ef | 0.07 ± 0.00b | 0.06 ± 0.00bc | 0.04 ± 0.00c | 0.02 ± 0.00d | |
| DM2 | 10.30 ± 0.01d | 0.09 ± 0.01f | 0.06 ± 0.01bc | 0.056 ± 0.01c | 0.04 ± 0.01bc | 0.03 ± 0.00c | |
| Ordu Levant | CG | 10.34 ± 0.01c | 0.12 ± 0.00ab | 0.08 ± 0.00a | 0.06 ± 0.01c | 0.04 ± 0.00c | 0.03 ± 0.00c |
| GG | 10.25 ± 0.01e | 0.12 ± 0.01bc | 0.07 ± 0.00b | 0.06 ± 0.00bc | 0.04 ± 0.00c | 0.03 ± 0.00c | |
| DM1 | 10.12 ± 0.01f | 0.12 ± 0.00ab | 0.06 ± 0.01bc | 0.07 ± 0.00a | 0.04 ± 0.00c | 0.03 ± 0.00c | |
| DM2 | 10.04 ± 0.03g | 0.11 ± 0.01cd | 0.06 ± 0.01cd | 0.07 ± 0.01ab | 0.04 ± 0.01bc | 0.03 ± 0.00c | |
| Sign. | Cv | *** | *** | ** | *** | ** | *** |
| M | *** | ** | *** | *** | * | ** | |
| CvxM | *** | *** | *** | * | *** | *** | |
Cv cultivar, M drying, CG concrete ground, GG grass ground and DM1 and DM2 drying machine (45 °C and 50 °C, respectively). Values are expressed as mean ± standard deviation. Different letters in columns for each different drying method. Significant level; *, **, *** and “ns” mean significance at p < 0.05, 0.01, 0.001 and “not significant”, respectively, among drying methods
Table 4 represents the ratio of saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), unsaturated/saturated fatty acids (MUFA + PUFA/SFA), oleic/linoleic ratio (O/L), iodine value (IV), free fatty acid (FFA), rancimat value (RV), and peroxide value (PV). As expected, MUFA (81.58–84.80%) constituted the main fatty-acid group and was followed by PUFA (9.53–11.42%), and SFA (5.87–6.92%). Alaşalvar et al. (2010) obtained similar results and reported that hazelnuts contained low levels of SFA (7.46–9.59%), moderate levels of PUFA (3.92–13.86%), and high levels of MUFA (78.10–87.26%). However, Amaral et al. (2006) reported that MUFA constituted the main fatty acids group while SFA and PUFA fatty acids had equal levels. It was observed that drying methods had a statistically significant effect on total fatty acids and fat oxidation (p < 0.001; Table 4). Highest SFA value was in Palaz (6.92%) cultivar while the lowest value was in Tombul (5.87%) cultivar. Among the four drying methods, the highest values were recorded for CG (Palaz, Ordu Levant, and Tombul; 6.92%, 6.35%, and 6.32%, respectively) method while the lowest values differed according to cultivars. For example, the lowest values for Tombul and Palaz cultivars were detected in GG method while the lowest value for Ordu Levant cultivar was obtained using DM2 method. Furthermore, SFA value decreased with increase in drying temperature and changed from 6.22 to 6.09% for Tombul. Özdemir et al. (2002) reported similar results in their study, and palmitic acid (C16:0) value decreased from 7.86 to 5.98% as the drying temperature increased (35–50 °C). In another study, it was observed that SFA value increased as the drying time extended (18.80–19.70%; Delgado et al. 2016), and that SFA values in chestnuts altered depending on drying methods (17.80–20.00%; Delgado et al. 2017).
Table 4.
Effect of drying methods on sum of fatty acids and oil oxidation of hazelnut
| Parameter | M | Cv | Sign. | ||||
|---|---|---|---|---|---|---|---|
| Tombul | Palaz | Ordu Levant | Cv | M | Cv × M | ||
| ∑SFA (%) | CG | 6.32 ± 0.01e | 6.92 ± 0.00a | 6.35 ± 0.01d | |||
| GG | 5.87 ± 0.01k | 6.09 ± 0.01j | 6.24 ± 0.01f | *** | *** | *** | |
| DM1 | 6.22 ± 0.01g | 6.52 ± 0.00b | 6.19 ± 0.01h | ||||
| DM2 | 6.09 ± 0.01j | 6.45 ± 0.01c | 6.14 ± 0.01ı | ||||
| ∑MUFA (%) | CG | 84.48 ± 0.01d | 81.58 ± 0.01ı | 84.36 ± 0.03e | |||
| GG | 84.12 ± 0.001f | 83.01 ± 0.00h | 84.35 ± 0.01e | *** | *** | *** | |
| DM1 | 84.49 ± 0.01d | 83.02 ± 0.01h | 84.70 ± 0.00c | ||||
| DM2 | 84.80 ± 0.02a | 83.07 ± 0.02g | 84.75 ± 0.02b | ||||
| ∑PUFA (%) | CG | 9.53 ± 0.01ı | 11.42 ± 0.02a | 10.46 ± 0.01c | |||
| GG | 10.07 ± 0.00h | 10.93 ± 0.00b | 10.37 ± 0.00e | *** | *** | *** | |
| DM1 | 9.35 ± 0.03j | 10.45 ± 0.01c | 10.24 ± 0.01f | ||||
| DM2 | 9.34 ± 0.02j | 10.40 ± 0.01d | 10.15 ± 0.03g | ||||
| ∑(MUFA + PUFA)/SFA | CG | 14.87 ± 0.02h | 13.44 ± 0.02k | 14.94 ± 0.02g | |||
| GG | 16.05 ± 0.03a | 15.42 ± 0.02c | 15.18 ± 0.02e | *** | *** | *** | |
| DM1 | 15.09 ± 0.02f | 14.34 ± 0.00j | 15.35 ± 0.03d | ||||
| DM2 | 15.45 ± 0.03b | 14.50 ± 0.01ı | 15.46 ± 0.02b | ||||
| O/L | CG | 8.93 ± 0.01c | 7.19 ± 0.01k | 8.13 ± 0.01g | |||
| GG | 8.43 ± 0.00d | 7.65 ± 0.00j | 8.20 ± 0.01f | *** | *** | *** | |
| DM1 | 9.13 ± 0.03b | 8.00 ± 0.00ı | 8.35 ± 0.01e | ||||
| DM2 | 9.18 ± 0.01a | 8.04 ± 0.00h | 8.42 ± 0.03d | ||||
| IV | CG | 93.29 ± 0.01j | 94.15 ± 0.03f | 94.91 ± 0.03a | |||
| GG | 93.96 ± 0.00g | 94.52 ± 0.00e | 94.74 ± 0.01c | *** | *** | *** | |
| DM1 | 92.96 ± 0.05l | 93.65 ± 0.01h | 94.80 ± 0.00b | ||||
| DM2 | 93.22 ± 0.04k | 93.60 ± 0.03ı | 94.69 ± 0.01d | ||||
| FFA | |||||||
| (%, oleic acid) | CG | 0.26 ± 0.00b | 0.10 ± 0.00g | 0.05 ± 0.00h | |||
| GG | 0.29 ± 0.01a | 0.13 ± 0.00e | 0.05 ± 0.01h | *** | *** | *** | |
| DM1 | 0.17 ± 0.00d | 0.10 ± 0.01g | 0.04 ± 0.00ı | ||||
| DM2 | 0.25 ± 0.00c | 0.12 ± 0.01f | 0.04 ± 0.00ı | ||||
| RV (h) | CG | 5.75 ± 0.01e | 5.92 ± 0.01c | 5.06 ± 0.02h | |||
| GG | 5.30 ± 0.00g | 5.90 ± 0.00c | 4.46 ± 0.01ı | *** | *** | *** | |
| DM1 | 5.90 ± 0.01c | 6.05 ± 0.00a | 5.45 ± 0.03f | ||||
| DM2 | 5.78 ± 0.01d | 5.99 ± 0.02b | 5.28 ± 0.04g | ||||
| PV ( meq O2 kg−1) | CG | 0.06 ± 0.00f | 0.23 ± 0.00d | 0.31 ± 0.01b | |||
| GG | 0.00 ± 0.00g | 0.08 ± 0.00e | 0.34 ± 0.01a | *** | *** | *** | |
| DM1 | 0.00 ± 0.00g | 0.07 ± 0.00ef | 0.27 ± 0.02c | ||||
| DM2 | 0.00 ± 0.00g | 0.08 ± 0.00e | 0.27 ± 0.02c | ||||
Cv cultivar, M drying, CG concrete ground, GG grass ground and DM1 and DM2 drying machine (45 °C and 50 °C, respectively). Values are expressed as mean ± standard deviation. Different letters in columns for each different drying. Significant level; *, **, *** and “ns” mean significance at p < 0.05, 0.01, 0.001 and “not significant”, respectively, among drying methods
MUFA primarily consisted of oleic acid (C18:1) followed by palmitoleic (C16:1), eicosenoic (C20:1), heptadecenoic (C17:1), and nervonic (C24:01) fatty acids, and the effect of drying methods and cultivars was statistically significant (p < 0.001; Table 4). For instance, MUFA values were comparable in Tombul and Ordu Levant cultivars (84.12–84.80% and 84.35–84.75%, respectively) while lower values were recorded for Palaz (81.58–83.07%; Table 4). The highest MUFA values were recorded in DM2 method for all three cultivars (Tombul, Palaz, and Ordu Levant; 84.80%, 83.07%, and 84.75%, respectively). Özdemir et al. (2002) reported similar results and determined that oleic (C18:1) acid value increased (79.50–83.50%) as the temperature increased. Furthermore, Delgado et al. (2016) reported that MUFA values decreased (36.20–32.30%) in chestnuts as the drying time increased, and this varied depending on cultivars and drying methods.
Linoleic (C18:2) and linolenic (C18:3) fatty acids are generally defined as the main fatty acids that constitute PUFA values (Delgado et al. 2017; Juhaimi et al. 2018; Turan 2018). The highest PUFA value was in GG for Tombul (10.07%) and in CG methods for Palaz and Ordu Levant (11.42% and 10.46%, respectively). According to the results of our study, hazelnuts dried under sun generally had higher PUFA values. Similar results were also reported in the studies carried out by Qu et al. (2016) and Fu et al. (2016), and linoleic (C18:2) acid level in walnuts were reported to be higher when dried under sun compared to drying in oven (70.41% and 61.05%, respectively).
Contrary to the results obtained by Juhaimi et al. (2018), the results of our study indicate that the effect of drying methods on the ratio of unsaturated/saturated fatty acids (MUFA + PUFA/SFA) was significant (p < 0.001; Table 4). MUFA + PUFA/SFA values (16.05%) in Tombul cultivar were generally higher than the other two cultivars. Similar results were also reported by Belviso et al. (2017), and demonstrated that MUFA + PUFA/SFA value in Ordu hazelnuts was higher compared with that of Tonda Gentile Trilobata (TGT) cultivar (12.97–9.16%, respectively). In addition, the highest values for Tombul and Palaz cultivars were detected in GG (16.05% and 15.42%, respectively) method while such values were obtained in DM2 for Ordu Levant hazelnuts (15.46%).
Oxidation of oil
Oleic (C18:1) and linoleic (C18:2) unsaturated fatty acids are present in high levels in hazelnuts and thus, hazelnut oils are sensitive against oxidation (Alasalvar et al. 2010). Furthermore, oleic/linoleic acid ratio (O/L) is an important factor that is used in the evaluation of nut quality. Linoleic acid is more sensitive against oxidation compared to oleic acid (Qu et al. 2016). Therefore, the higher ratio of O/L means that the hazelnut is more resistant to oxidation.
In our study, the effect of drying methods and cultivars on O/L ratio was statistically significant (p < 0.001; Table 4). The highest O/L values were identified for Tombul cultivar (8.43–9.18) and this was followed by Ordu Levant (8.13–8.42). The lowest values were identified for Palaz cultivar (7.19–8.04). Belviso et al. (2017) also demonstrated similar results and reported that the O/L value of TGT hazelnuts was higher compared to Ordu hazelnuts (12.91–12.13, respectively). In our study, the highest O/L values were recorded in DM1 and DM2 methods (9.13–9.18, respectively), and the O/L values increased as the temperature increased. Qu et al. (2016) demonstrated similar results and reported a considerable increase in oleic acid (C18:1; 12.52–21.11%, respectively) and a decrease in linoleic acid (C18:2; 70.41–61.05%) in walnuts dried in oven.
Iodine value (IV) is a measure of unsaturation level of fats. High IV values indicate that fats are sensitive to oxidation and rancidity (Belviso et al. 2017; Turan 2018). In our study, the highest IV values were found for Ordu Levant hazelnuts (94.69–94.91) while the lowest values were recorded for Tombul cultivar (92.96–93.96), and also, in generally, it is known that IV value varies according to cultivars. Our study demonstrated that the effect of the four drying methods on IV value was statistically significant (p < 0.001; Table 4). The lowest IV values were recorded in DM1 and DM2 methods (Tombul, Palaz and Ordu Levant; 92.96, 93.60 and 94.69, respectively), and IV value decreased with temperature increase (Palaz and Ordu Levant; 93.65–93.60, 94.80–94.69, respectively) consistent the study conducted by Özdemir et al. (2002) (88.30–87.50).
Free fatty acid (FFA) value is the first indicator of loss of quality, and it is also accepted that spoilage occurs if such value increases above FFA ≥ 1% (Turan 2017). In our study, FFA value varied depending on different cultivars and drying methods (p < 0.001; Table 4). The highest FFA values was noted for Tombul cultivar (0.17–0.29%, oleic acid) and the lowest values were recorded for Ordu Levant hazelnuts (0.04–0.05%, oleic acid); lower FFA values were recorded in DM1 and DM2 methods compared to CG and GG methods. Qu et al. (2016) and Fu et al. (2016) reported similar results, and that fat molecules of walnuts dried under sun for a long period secreted FFA; thus the value increased. In this study, FFA values increased with temperature increase in DM1 and DM2 methods. Similar results were also reported by Özdemir et al. (2002), Tavakolipour et al. (2010) and Venkitasamy et al. (2017), the rise in drying temperature was observed to lead to an increase in FFA values. However, Kashaninejad et al. (2003) reported that drying methods did not affect FFA values in pistachios. Such differences may have resulted due to several factors such as species, cultivars, and drying methods.
Rancimat value (RV) is a result of the peroxidation of unsaturated fatty acids in fats and is determined by absorption of degradation products into water and measuring the conductivity of water (Turan 2017). The highest RV was recorded in DM1 method (Tombul, Palaz and Ordu Levant; 5.90 h, 6.05 h and 5.45 h, respectively), however, the RV decreased with the increase of temperature. Similarly, Özdemir et al. (2002) reported that the increase in temperature caused RV to decrease (6.97–4.43 h) for Tombul cultivar. In addition, Turan (2017) reported that they obtained lower RV in sun–drying methods and that, for this reason, artificial means of drying should be preferred since they provide shorter drying times and higher RV. In our study, the highest values were recorded for Palaz cultivar (5.90–6.05 h) while the lowest values were noted for Ordu Levant hazelnuts (4.46–5.45 h).
Peroxide value (PV) is an important parameter used in hazelnut industry for the products to be stored (Ghirardello et al. 2013; Koç Güler et al. 2017; Turan 2017) and it is also one of the most important indicators of fat oxidation (Fu et al. 2016). The effect of drying methods and cultivars on PV was statistically significant (p < 0.001; Table 4). Among drying methods, the lowest values were recorded in DM1 and DM2 methods (0.00–0.27 meq O2 kg−1), and the PV of Palaz cultivar increased with an increase in drying temperature (0.07–0.08 meq O2 kg−1). Fu et al. (2016) and Qu et al. (2016) also reported that PV increased due to the extended time of sun-drying. Furthermore, Venkitasamy et al. (2017) reported that the PV in Kerman pistachio increased in parallel to the increase in drying temperature (0.80–1.87 meq O2 kg−1). In general, decreases RV and increases of FFA, PV and IV values and corresponding decreases of O/L values were recorded. Moreover, PV and IV indexes highlight as the primary oxidation besides the number of degree of unsaturation of the oils change proportionally due to the presence of much higher contents of oleic acid (Belviso et al. 2017). The latter is affected at high drying temperatures (from 45 to 50 °C), ultimately, increasing SFA and PUFA percentages, and so, the degradation rate of oleic acid led to an increase O/L value. Consequently, it is of great importance to dry hazelnuts in a short time and not to exceed 45 °C drying temperature in order to avoid peroxidation.
Conclusion
This is the first detailed study in literature on the effect of CG, GG, DM1, and DM2 methods on chemical properties of Tombul, Palaz, and Ordu Levant cultivars. The effect of drying methods on fatty acid composition and fat oxidation was statistically significant and varied depending on the cultivars. A total of 13 fatty acids were identified for Tombul, Palaz, and Ordu Levant hazelnuts, and major fatty acids were oleic acid (C18:1) followed by linoleic (C18:2), palmitic (C16:0), and stearic (C18:0) acid; the major fatty acids constituted ~ 99.00% of the total fatty acids whereas the minor fatty acids constituted only ~ 0.5%. Furthermore, caproic (C6:0), caprylic (C8:0), capric (C10: 0), lauric (C12:0), eicosadienoic (20:2), erucic (22:1), docosadienoic (22:2), and lignoceric (C24:0) fatty acids were below the level of detection (< 0.001%). Among drying methods, hazelnut samples dried by DM1 and DM2 methods yielded lower IV, FFA and PV values, and higher O/L and RV compared to CG and GG methods. In terms of artificial drying methods; MUFA, MUFA + PUFA/SFA, O/L fatty acids, and FFA and PV values increased with increase in temperature (DM1 and DM2, 45–50 °C, respectively) while SFA, PUFA fatty acids, and IV and RV decreased. In light of these findings, it can be concluded that the DM1 method is a promising method for hazelnut drying.
Acknowledgements
This study was supported by Altaş Oil Industry Inc and Gürsoy Tarımsal Urünler Gıda Sanayi ve Ticaret AŞ (Ordu, Turkey). The author wishes to thank Assist Prof Fatih ÖNER for statistical analysis.
References
- Alasalvar C, Pelvan E, Topal B. Effect of roasting oil and fatty acid composition of Turkish hazelnut varieties (Corylus avellana L.) Int J Food Sci Nutr. 2010;61:630–642. doi: 10.1021/f101039f. [DOI] [PubMed] [Google Scholar]
- Amaral JS, Casal S, Citová I, Santos A, Seabra RM, Oliveira BPP. Characterization of several hazelnut (Corylus avellana L.) cultivars based in chemical, fatty acid and sterol composition. Eur Food Res Technol. 2006;222:274–280. doi: 10.1007/s00217-005-0068. [DOI] [Google Scholar]
- AOAC (1990a) Oils and fats, 15th edn. Official Methods of analysis of the association of official analytical chemists. Washington, pp 485–518
- AOCS (1990b) Official methods and recommended practices of the american oil. Chemist’s Society, 5th edn. American Oil Chemist Society, Washington
- AOAC (2000) Official methods of analysis of AOAC international, 17th edn, vol 40, pp 1–3
- Belviso S, Bell BD, Giacosa S, Bertolino M, Ghirardello D, Giordano M, Rolle L, Gerbi V, Zeppa G. Chemical, mechanical and sensory monitoring of hot air and infrared roasted hazelnuts (Corylus avellana L.) during nine months of storage. Food Chem. 2017;217:398–408. doi: 10.1016/.2016.08.103. [DOI] [PubMed] [Google Scholar]
- Delgado T, Pereira JA, Ramalhosa E, Casal S. Effect of hot air convective drying on the fatty acid and vitamin E composition of chestnut (Castanea sativa Mill.) slices. Eur Food Res Technol. 2016;242:1299–1306. doi: 10.1007/s00217-015-2633-5. [DOI] [Google Scholar]
- Delgado T, Pereira JA, Ramalhosa E, Casal S. Comparison of different drying methods on the chemical and sensory properties of chestnut (Castanea sativa M.) slices. Eur Food Res Technol. 2017;243:1957–1971. doi: 10.1007/s00217-017-2902-6. [DOI] [Google Scholar]
- Ficarra A, Lo Fiego DP, Minelli G, Antonelli A. Ultra fast analysis of subcutaneous pork fat. Food Chem. 2010;121:809–814. doi: 10.1016/.2010.01.003. [DOI] [Google Scholar]
- Fu M, Qu Q, Yang X, Zhang X. Effect of intermittent oven drying on lipid oxidation, fatty acids composition and antioxidant activities of walnut. LWT Food Sci Technol. 2016;65:1126–1132. doi: 10.1016/.2015.10.002. [DOI] [Google Scholar]
- Ghirardello D, Contessa C, Valentini N, Zeppa G, Rolle R, Gerbi V, Botta R. Effect of storage condition on chemical and physical characteristics of hazelnut (Corylus avellana L.) Postharvest Biol Technol. 2013;81:37–43. doi: 10.1016/.2013.02.014. [DOI] [Google Scholar]
- Gölükcü M. The effects of drying methods, packaging atmosphere and storage time on dried pomegranate aril quality. J Agric Sci. 2015;21:207–219. doi: 10.15832/tbd.06219. [DOI] [Google Scholar]
- Guine RPF, Almeida CFF, Correia PMR. Influence of packaging and storage on some properties of hazelnuts. Food Meas. 2015;9:11–19. doi: 10.1007/s11694-014-9206-3. [DOI] [Google Scholar]
- Janowicz M, Lenart A. The impact of high pressure and drying processing on internal scructure and quality of fruit. Eur Food Res Technol. 2018;244:1329–1340. doi: 10.1007/s00217-018-3047-y. [DOI] [Google Scholar]
- Juhaimi FA, Ozcan MM, Uslu N, Ghafoor K. The effect of drying temperatures on antioxidant activity, phenolic compounds, fatty acid composition and tocopherol contents in citrus and oils. Eur Food Res Technol. 2018;55:190–197. doi: 10.1007/s13197-017-2895-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashaninejad M, Tabil LG, Mortazavi A, Safeordi A. Effect of drying methods on quality of pistachio nuts. Dry Technol. 2003;21:821–838. doi: 10.1081/DRT-120021688. [DOI] [Google Scholar]
- Kaveh M, Gilandeh YA, Chayjan RA, Taghinezhad E, Mohammadigol R. Mass transfer, physical, and mechanical characterictics of terebinth fruit (Pistacia atlantica L.) under convective infrared microwave drying. Heat Mass Transf. 2018;54:1879–1899. doi: 10.1007/s00231-018-2287-5. [DOI] [Google Scholar]
- Kermani AM, Khashehchi M, Kouravand S, Sadeghi A. Effect of intermittent microwave drying on quality characteristics of pistachio nuts. Dry Technol. 2017;35:1108–1116. doi: 10.1016/.2008.01.003. [DOI] [Google Scholar]
- Koc Güler S, Bostan SZ, Con AZ. Effects of gamma irradiation on chemical and sensory characteristics of natural hazelnut kernels. Postharvest Biol Technol. 2017;123:12–21. doi: 10.1016/.2016.08.007. [DOI] [Google Scholar]
- Özdemir M, Yıldız M, Gürcan TŞ. Effect of artificial trying air temperature on stability of the major Turkish hazelnut variety Tombul. GIDA. 2002;27:35–39. [Google Scholar]
- Qu Q, Yang X, Fu M, Chen Q, Zhang X, He Z, Qiao X. Effects of three conventional drying methods on the lipid oxidation, fatty acids composition, and antioxidant activities of walnut (Juglans regia L.) Dry Technol. 2016;34:822–829. doi: 10.1080/07373937.2015.1081931. [DOI] [Google Scholar]
- Rabadan A, Alvarez-Orti M, Pardo JE, Alvarruiz A. Storage stability and composition changes of three cold–pressed nut oils under refrigeration and room temperature conditions. Food Chem. 2018;259:31–35. doi: 10.1016/j.foodchem.2018.03.098. [DOI] [PubMed] [Google Scholar]
- Sarıcaoğlu FT, Turhan S. Chemical composition, colour and textural properties of Akçaabat meatball: a traditional Turkish meat product. GIDA. 2013;38(4):191–198. doi: 10.5505/gida.2013.58066. [DOI] [Google Scholar]
- Tavakolipour H, Armin M, Kalbasi AA. Storage stability of Kerman pistachio nuts (Pistacia vera L.) Int J Food Eng. 2010;6:1–11. doi: 10.2202/1556-3758.1740. [DOI] [Google Scholar]
- Thakur NS, Sharma S, Gupta R, Gupta A. Studies on drying and storage of chilgoza (Pinus gerardiana) nuts. J Food Sci Technol. 2014;51:2092–2098. doi: 10.1007/s13197-012-0692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSE (2001) Turkish Standardization Institution. Hazelnut Kernels Standard, TS 3075
- Tüfekci F, Karataş Ş. Determination of geographical origin Turkish hazelnuts according to fatty acid composition. Food Sci Nutr. 2018;00:1–6. doi: 10.1002/fsn3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan A (2017) Effect of drying methods on nut quality and storage of hazelnut. Ph. D., thesis, Ordu
- Turan A. Effect of drying methods on fatty acid profile and oil oxidation of hazelnut oil during storage. Eur Food Res Technol. 2018 doi: 10.1007/s00217-018-3128-y. [DOI] [Google Scholar]
- Turan A, İslam A. Çakıldak fındık çeşidinde kurutma ortamları ve muhafaza süresine bağlı olarak meydana gelen değişimler. Ordu Univ J Sci Tech. 2016;6:272–285. [Google Scholar]
- Turan A, İslam A. Effect of drying methods on some chemical characteristics of hazelnuts (Corylus avellana L.) during storage. Iğdır Univ J Ins Sci Technol. 2018;8:3. [Google Scholar]
- Velasco J, Anderson ML, Skibsted LH. Evaluation of oxidative stability of vegetable oils by monitoring the tendency to radical formation. A comparison of electron spins resonance spectroscopy with the rancimat method and differential scanning calorimetry. Food Chem. 2004;85:623–632. doi: 10.1016/.2003.07.020. [DOI] [Google Scholar]
- Venkitasamy C, Brandl MT, Wang B, MvHugh TH, Zhang R, Pan Z. Drying and decontamination of raw pistachios with sequential infrared drying, tempering and hot air drying. Int J Food Microbiol. 2017;246:85–91. doi: 10.1016/.2017.02.005. [DOI] [PubMed] [Google Scholar]
- WAA (2004) Operating Manual Novasina. AW Sprint TH 500 water activity analyzers
- Wang W, Jung J, McGorrin RJ, Traber MG, Leonard GC, Zhao Y. Investigation of drying conditions on bioactive compounds, lipid oxidation, and enzyme activity of Oregon hazelnuts (Corylus avellana L.) LWT Food Sci Technol. 2018;90:526–534. doi: 10.1016/j.lwt.2018.01.002. [DOI] [Google Scholar]
- Zhang L, Wang Z, Shi G, Yang H, Wang X, Zhao H, Zhao S. Effects of drying methods on the nutritional aspects, flavor, and processing properties of Chinese chestnuts. J Food Sci Technol. 2018 doi: 10.1007/s13197-018-3227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]