Abstract
The survival of Sacharomyces cerevisiae in Trypticase Soy Broth and natural orange juice processed by combined use of thermo-ultrasound and cinnamon leaf essential oil has been evaluated and modelled. Minimal inhibitory concentration of cinnamon leaf essential oil against S. cerevisiae was determined using absorbance measurements based on the microtiter plate assay. The resistance of S. cerevisiae cells to the combined action of thermal treatment with ultrasound was analyzed in Trypticase Soy Broth with different concentrations of cinnamon leaf essential oil at 30, 40 and 50 °C. The best conditions of inactivation in TSB to study the inactivation of S. cerevisiae in natural orange juice. Experimental data were fitted by using the “shoulder + log-linear” and “Weibull” models (GInaFiT). The combined use of thermo-ultrasound and cinnamon leaf essential oil enhanced the inactivation of S. cerevisiae in TSB and natural orange juice.
Keywords: Sacharomyces cerevisiae, Orange juice, Cinnamon leaf essential oil, Thermo-ultrasound, Inactivation, Modelling
Introduction
Yeasts are responsible of food spoilage and the specie S. cerevisiae is one of the most common spoilage agents in orange juice. This is a serious problem for the food industry because it renders products unacceptable for human consumption and produce severe economical losses (Patrignani et al. 2009).
Nowadays, the application of high-temperature short-time pasteurization (95–98 °C, 20–30 s) is the most common method for processing foods in order to inactivate microorganisms and enzymes. However heat treatments may alter the organoleptic and nutritional properties of food products (Soria and Villamiel 2010).
Actually, the growing demand for fresh foods of higher quality has generated an increased interest in non-thermal processing technologies for the inactivation of the microorganisms with negligible changes in the organoleptic and nutritional value of the raw materials. Therefore, the food industry is constantly searching for emergent mild processing technologies such as UV light, pulsed electric and magnetic fields, high hydrostatic pressure, ultrasound (US), etc., not only to obtain high-quality food with “fresh-like” characteristics, but also food with improved or even novel functionalities (Patrignani et al. 2009; Ravanfar et al. 2015; Soria and Villamiel 2010; Sun et al. 2015).
Among these emergent technologies, special attention has been paid during the past few years to US as a possible emerging preservation technology in combination with other hurdles to reach the desired inactivation effect. US refers to pressure waves with a frequency of 20 kHz or more (Soria and Villamiel 2010). US at lower frequencies (20–100 kHz) has the ability to cause cavitation, phenomenon which is described as a rapid creation, growth, and abrupt breakdown of bubbles, yielding localized extremely high temperatures (5500 °C) and pressures (5 MPa), disrupting cell structures and causing microbial death. The energy released by cavitation depends on surface tension; thus, foods with high surface tension such as fruit juices are candidates of being processed by ultrasound technology (Soria and Villamiel 2010). It has been suggested that US could be more effective for inactivation of microorganisms when it is used in combination with other stress factors in a multifactorial approach such as heating, extremes of pH, chlorination or addition of preservatives (Guerrero et al. 2001; López-Malo et al. 1999). Accordingly, the combined effects of US and heat treatment (thermo-ultrasound), static pressure (mano-ultasound) and heat treatment under pressure (mano-thermo-ultrasound) have been also investigated (Alzamora et al. 2011; Guerrero et al. 2001; Soria and Villamiel 2010). Specifically, sensitivity of microbial cells to the action of US combined with moderate temperatures, addition of preservatives (synthetic and/or naturally occurring ones) and control of pH has been examined (Gastélum et al. 2012; López-Malo et al. 2005b). The studies conducted to date have shown that resistance of different microbial species to US differs widely. In fact sporulated microorganisms are much more resistant than fungi and these are more resistant in general than vegetative bacteria (Guerrero et al. 2001; Soria and Villamiel 2010).
After several decades using chemically synthesized preservatives, essential oils (EOs), aromatic oil liquids of plant origin, are once again being proposed as good candidates for food preservation because of the growing demand of consumers for natural food additives (Tserennadmid et al. 2011). They have wide antimicrobial spectra against bacteria, yeasts and moulds. The most EOs is regarded as GRAS substances (Prakash et al. 2012) and could be accepted by consumers in the foods at certain concentrations. Cinnamon (Cinnamomum sp.) belongs to Lauraceae, an economically important family consisting mostly of trees. Cinnamomum sp. EO is recognized as a safe food additive, widely used as flavouring agent and by its inhibitory effects against pathogenic bacteria, fungi, viruses and spoilage organisms (Burt 2004). So, it is possible to use cinnamon EOs or theirs components as preservatives to extend the shelf life of selected foods considering their GRAS status and antimicrobial activities. The main constituents of cinnamon EO (Cinnamomum zeylanicum) are cinnamaldehyde (main component of cinnamon bark EO) and eugenol [main component of cinnamon leaf EO (CLEO)] (Ranasinghe et al. 2002).
The objective of this research was to study the lethality of the combined effect of US, CLEO and mild heat treatment on S. cerevisiae viable cells suspended in Trypticase Soy Broth (TSB) and in natural orange juice.
Materials and methods
Microorganism and inoculum preparation
S. cerevisiae was chosen as the target microorganism in the present study. This strain was isolated and identified by Valverde et al. (2010) and kept at − 80 °C in Microbank™ vials (Pro-labo Diagnostics, Neston, Wirrall, UK). Every 2 months, one of the vials was opened and the stock culture grown in Trypticase Soy Broth (TSB) (Cultimed, Barcelona, Spain) for 24 h at 25 °C and streaked onto Standars Methods agar (SMA) plates (Cultimed, Panreac Barcelona, Spain) with chloramphenicol (Ch) at 0.1 g l−1 (Tournas et al. 2001). The S. cerevisiae inocula were prepared by transferring a colony obtained in SMA-Ch plates to TSB, which was incubated for 24 h at 25 °C before being stored at − 20 °C in a solution of 40% TSB and 60% glycerol until use. The fresh cultures for the experiments were made by incubating one loopful of pure culture in TSB for 24 h at 25 °C. The inocula were standardized by dilution in TSB until an optical density (OD) of 0.1 at 600 nm (Nicolet Evolution 300, Thermo Electron Corporation) was reached to obtain a yeast concentration of 107 cfu ml−1. The yeast populations were estimated by spreading suitable diluted aliquots onto SMA-Ch plates, followed by incubation at 25 °C for 48 h.
Cinnamon leaf EO (CLEO)
CLEO (ρ = 1.0524 g ml−1; GC-FID: 74.32% eugenol, 2.98% benzyl benzoate) was donated by Destilerías Muñóz Gálvez, SA (Murcia, Spain). TSB (pH 7.1 ± 0.2) stock solutions were prepared following the methodology of Souza et al. (2005), with Tween 20 (0.8%). The tested concentrations in TSB microtiter plate assay were 5200, 2600, 1300, 650, 320, 160, 80, 40, 20, 10 and 0 mg l−1 (ppm). Depending on these growth inhibition results on the microtiter plate assay, the concentrations that are going to be assayed in orange juice will be lower or equal to the minimum inhibitory concentration.
Antimicrobial activity, minimal inhibitory concentration (MIC) and growth inhibition
The changes in the absorbance based on the microtiter plate assay (MPA) were used to determine the MIC of CLEO in TSB. Ninety-six well microtiter plates (Sarstedt Ltd.) were used to perform the MPA. This assay was based on previous works (Gutierrez et al. 2009) but with some modifications. The wells were filled with 150 μl of TSB. Aliquots (150 µl) of EO stock solutions were added into the first column of a microtiter plate. The EOs were then diluted twofold along each row. Finally, 150 μl of broth containing ~ 105 cfu ml−1 of inoculum were added to all wells. Positive controls contained 150 μl of growth medium were inoculated with 150 μl of broth containing ~ 105 cfu ml−1 of inoculum. Negative controls contained EOs concentrations and sterile growth broth only. The plates were then placed in the Multiskan Ascent® microplate spectrophotometer (Thermo Electron Corporation) set at 25 °C. The absorbance was recorded at 595 nm every 1 h over a 24 h incubation period. The area under the OD/time curve of the control was compared with the areas of the tests and expressed as percent of growth inhibition (GI%) to determine the antimicrobial activity of each concentration tested. Minimal inhibitory concentration (MIC) was considered as the lowest concentration of CLEO that caused 100 GI%. Partial inhibitory concentrations (PICs) were any concentration of CLEO lower than the MIC that produced an inhibition of between > 0 and < 100% (Cava-Roda et al. 2012b). Each experiment was repeated three times and results were expressed in ppm.
Thermal and thermo-US treatments with CLEO in TSB. Enumeration of S. cerevisiae
Thermal and thermo-US treatments were carried out in a double-wall cylindrical vessel (4-cm internal diameter, 6.5 cm height) which water was circulated with a refrigerated bath (model Digit-Cool J.P. Selecta®) to attain and fix 30, 40 or 50 °C in the TSB (pH 7.1 ± 0.2). The refrigerated bath temperature was set as needed for each thermal, thermo-US and US treatments and monitored with a sterile thermometer to maintain the desired temperature in the TSB. US (24 kHz; 105 μm; 33.31 W ml−1; 30 min) was continuously applied with a UP200H ultrasonic processor (Hielscher Ultrasound Technology) using a S3 probe (Hielscher) (Gastélum et al. 2012). The effect of treatments was also tested with concentrations of CLEO of 650, 320,160, 80, 40 and 0 ppm. For each treatment, yeast was inoculated (~ 105 cfu ml−1) into sterile TSB that was previously heated to the desired temperature. At fixed intervals during treatments, samples were taken and serially diluted in peptone water. Three trials were performed for every treatment. The surviving viable cells counts were determined immediately after treatment using the pouring method on SMA-Ch. Two plates were used for each dilution and were incubated at 25 °C for 48 h.
Thermal and thermo-US treatments with CLEO in natural orange juice: enumeration of S. cerevisiae
After treatments in TSB, the best factors conditions (50 °C, US, 650 ppm of CLEO and their combination) obtained in TSB were selected to study the inactivation of S. cerevisiae in natural orange juice (pH: 3.97 ± 0.01; °Brix: 11.6 ± 0.15). The experimental process was the same as described above. The oranges were harvested in a local orchard (Beniaján, Murcia, Spain). The fruits were kept at 5 °C for 1 day before juice extraction. Damaged fruits were discarded. Fruits were washed in cold tap water before drained. Orange juice was obtained by usual squeezing (Citro New 100 W, Solac) in aseptic conditions. The natural orange juice was pasteurized and stored at − 20 °C in the dark until thermo-US treatments (Gastélum et al. 2012). The CLEO suspension (650 and 0 ppm) in natural orange juice (pH: 3.97 ± 0.01; °Brix: 11.6 ± 0.15) without Tween 20 was prepared using a vigorous shaking method by stirrer (TQTECH, multipoint magnetic stirrer, Spain) (Ait-Ouazzou et al. 2013). For each treatment, yeast was inoculated (~ 105 cfu ml−1) into sterile natural orange juice, previously heated to the desired temperature. At fixed intervals, samples were taken and serially diluted in peptone water. Three trials were performed for every treatment. The surviving viable cells counts were determined immediately after treatment using the pouring method on SMA-Ch. Two plates were used for each dilution and were incubated at 25 °C for 48 h.
Curve fitting
Survival curves were obtained by plotting the logarithm of survivors against the treatment time. Data that did not fit the usual linear model of thermal destruction were adjusted by the “log-linear + shoulder” and “Weibull” models, because they more closely fit such kinds of data. GInaFiT, a freeware add-in for Microsoft Excel, was utilized to model inactivation kinetics (Geeraerd et al. 2005). The “shoulder + log-linear” model took into account a shoulder before a decrease in the population size and the data were fitted using the equation (Eq. 1) of Geeraerd et al. (2000):
| 1 |
where N, cell concentration (cfu ml−1) after a treatment time t (min); N0, initial cell concentration (cfu ml−1); kmax, maximum inactivation rate (min−1); and Sl, shoulder length (min) (i.e., the length of the lag phase).
The other survival model tested was the cumulative form of the Weibull distribution (Eq. 2). The survival data were fitted using the equation described by Mafart et al. (2002):
| 2 |
where t (min) is time, N(t) (cfu ml−1) is the cell concentration as a function of time, N0 (cfu ml−1) is the initial cell concentration, δ or scale parameter is the time that leads to the first tenfold reduction of the surviving population and ρ is the shape parameter. When ρ > 1, the survival curve shows a convex shape, ρ < 1 means a concave shape and ρ = 1 corresponds to the log-linear shape.
Statistical analysis
An analysis of variance of treatment effect on S. cerevisiae log reduction and D50 values were performed. These analyses were conducted with the Statgraphic Plus for Windows 3.0®, version 5.0 (StatPoint, Inc., Herndon, Va.). Results were considered significant at p < 0.05.
Results and discussion
Antimicrobial activity of CLEO: determination of MIC, PICs and percent of growth inhibition (%GI)
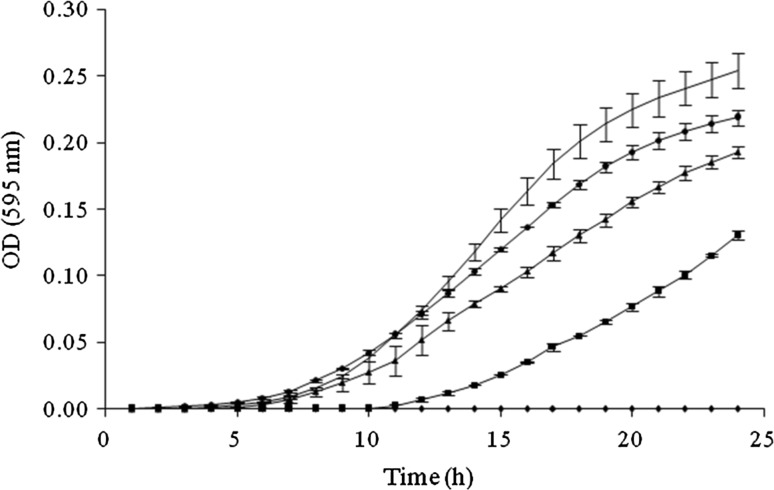
The growth curves of S. cerevisiae in TSB supplemented with different concentrations of CLEO and incubated for 24 h at 25 °C in microtiter are shown in Fig. 1. The concentrations of 5200, 2600, 1300 and 650 ppm produced 100% GI, so the MIC obtained was 650 ppm (100% GI) and the PICs values were 320 ppm (69.36% GI), 160 ppm (30.59% GI) and 80 ppm (11.97% GI) in TSB at 25 °C/24 h. Concentrations of 40, 20 and 10 ppm did not produce growth inhibition.
Fig. 1.
Growth of Saccharomyces cerevisiae in Trypticase Soy Broth with different concentrations of cinnamon leaf essential oil (
 : control,
: control,
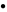 : 80 ppm,
: 80 ppm,
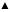 : 160 ppm,
: 160 ppm,
 : 320 ppm,
: 320 ppm,
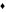 : 650 ppm). Experimental (points) and predicted (lines) values
: 650 ppm). Experimental (points) and predicted (lines) values
The major constituents of the CLEO used in this study were eugenol (74.32%) and benzyl benzoate (2.98%), These were close to those found by others authors (Ranasinghe et al. 2002). Darvishi et al. (2013) determined the MIC100 of eugenol (Sigma-Aldrich; Oakville, ON, Canada) on S. cerevisiae S288C by the broth microdilution assay and obtained that the MIC100 for eugenol was in the range of 270–320 ppm. These authors determined that eugenol interferes with transporters responsible for uptake of aromatic and branched-chain amino acids across the S. cerevisiae cytoplasmic membrane (Darvishi et al. 2013). The mechanism of action of phenylpropanes, as eugenol, methyl-eugenol, and methyl-chavicol, was similar to terpenes and phenolic compounds and was involved in disruption of the cytoplasmic membrane and coagulation of cell content (Sukatta et al. 2008). Specifically, the antimicrobial activity of eugenol is attributed to its aromatic nucleus and to the phenolic –OH group that are known to be reactive and can form hydrogen bonds with –SH groups in the active sites of target enzymes, resulting in the deactivation of some essential enzymes in fungi (Sukatta et al. 2008). Bennis et al. (2004) studied the alteration induced by thymol and eugenol on S. cerevisiae, and concluded that antifungal activity of eugenol and thymol involves alterations of both membrane and cell wall of yeast.
Thermal, thermo-US and US treatments with CLEO
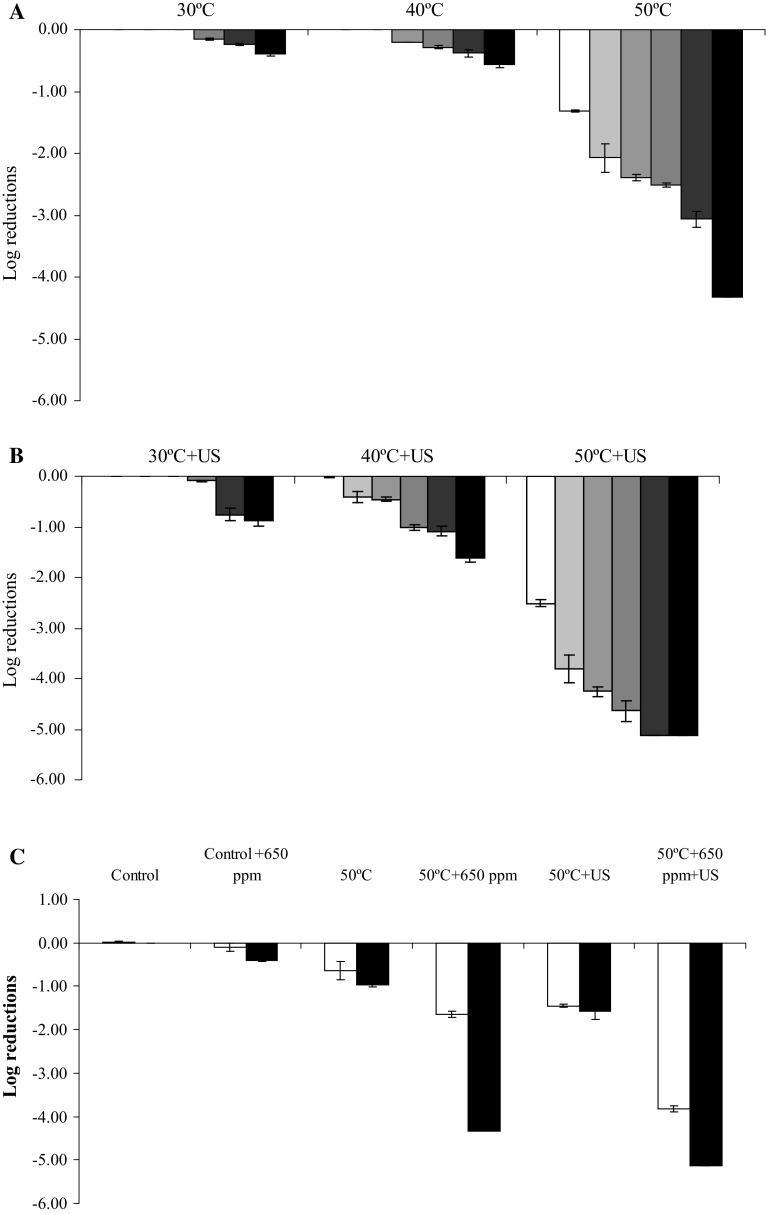
Figure 2a shows S. cerevisiae log reductions obtained in TSB by application of temperature (30–40–50 °C) and CLEO (650, 320, 160, 80, 40 and 0 ppm) but not US. All the treatments lasted 30 min. No logarithmic reductions were obtained at temperatures of 30 and 40 °C in TSB without CLEO after 30 min. But ~ 1.2 significant log reductions (p < 0.05) were obtained when temperature of 50 °C was applied in TSB without CLEO after 30 min. However, significant differences in log reduction (p < 0.05) were found in TSB when the same temperatures were combined with different concentrations of CLEO, obtaining ~ 4 significant log reductions at 50 °C in TSB with 650 ppm of CLEO after 30 min.
Fig. 2.
a
Saccharomyces cerevisiae log reductions obtained by application of temperature (30 °C, 40 °C and 50 °C, 30 min) in Trypticase Soy Broth with cinnamon leaf essential oil (
 0 ppm;
0 ppm;
 40 ppm;
40 ppm;
 80 ppm;
80 ppm;
 160 ppm;
160 ppm;
 320 ppm;
320 ppm;
 650 ppm). b
Saccharomyces cerevisiae log reductions obtained by application of ultrasound treatments (24 kHz; 105 μm; 33.31 W ml−1; 30°, 40° and 50 °C, 30 min) in Trypticase Soy Broth with cinnamon leaf essential oil (
650 ppm). b
Saccharomyces cerevisiae log reductions obtained by application of ultrasound treatments (24 kHz; 105 μm; 33.31 W ml−1; 30°, 40° and 50 °C, 30 min) in Trypticase Soy Broth with cinnamon leaf essential oil (
 0 ppm;
0 ppm;
 40 ppm;
40 ppm;
 80 ppm;
80 ppm;
 160 ppm;
160 ppm;
 320 ppm;
320 ppm;
 650 ppm). c
Saccharomyces cerevisiae log reductions obtained by application of ultrasound (24 kHz; 105 μm; 33.31 W ml−1), temperature (50 °C) and cinnamon leaf essential oil (0 ppm; 650 ppm) in Trypticase Soy Broth (
650 ppm). c
Saccharomyces cerevisiae log reductions obtained by application of ultrasound (24 kHz; 105 μm; 33.31 W ml−1), temperature (50 °C) and cinnamon leaf essential oil (0 ppm; 650 ppm) in Trypticase Soy Broth (
 ) and natural orange juice (
) and natural orange juice (
 ). (All the treatments lasted 30 min)
). (All the treatments lasted 30 min)
Figure 2b shows S. cerevisiae log reductions obtained in TSB by application of temperature (30–40–50 °C), US (24 kHz; 105 μm; 33.31 W ml−1) and CLEO (650, 320, 160, 80, 40 and 0 ppm). All the treatments lasted 30 min. The application of thermo-US combined with CLEO improved the lethal effect of treatments and significant differences were found for the tested combinations. The significant highest log reduction (5.13 log cycles) was obtained by applying thermo-US at 50 °C and concentrations of 650 ppm during 24 min (Fig. 3b) or 320 ppm during 30 min (Fig. 3b).
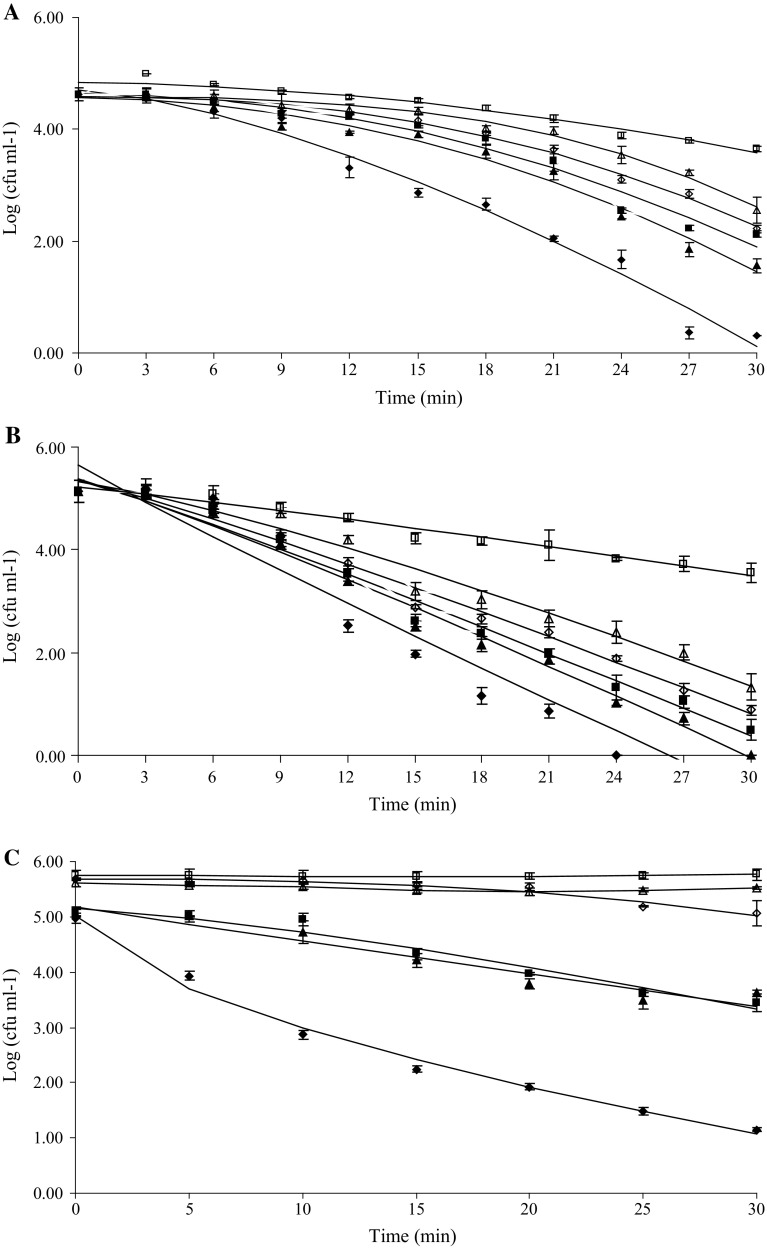
Fig. 3.
a Fit of Weibull model to survival data sets of Saccharomyces cerevisiae suspended in Trypticase Soy Broth treated (50 °C, 30 min) and supplemented or not with cinnamon leaf essential oil (
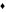 : 650 ppm,
: 650 ppm,
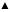 : 320 ppm,
: 320 ppm,
 : 160 ppm,
: 160 ppm,
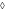 : 80 ppm,
: 80 ppm,
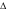 : 40 ppm,
: 40 ppm,
 : control). b Fit of Weibull model to survival data sets of Saccharomyces cerevisiae suspended in Trypticase Soy Broth treated (ultrasound + 50 °C 30 min) and supplemented or not with cinnamon leaf essential oil (
: control). b Fit of Weibull model to survival data sets of Saccharomyces cerevisiae suspended in Trypticase Soy Broth treated (ultrasound + 50 °C 30 min) and supplemented or not with cinnamon leaf essential oil (
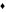 : 650 ppm,
: 650 ppm,
 : 320 ppm,
: 320 ppm,
 : 160 ppm, '
: 160 ppm, '
 : 80 ppm,
: 80 ppm,
 : 40 ppm,
: 40 ppm,
 : control). c Fit of Weibull model to survival data sets of Saccharomyces cerevisiae suspended in natural orange juice (
: control). c Fit of Weibull model to survival data sets of Saccharomyces cerevisiae suspended in natural orange juice (
 : control,
: control,
 : control 650 ppm,
: control 650 ppm,
 : 50 °C,
: 50 °C,
 : 50 °C + 650 ppm,
: 50 °C + 650 ppm,
 : 50 °C + ultrasound,
: 50 °C + ultrasound,
 : 50 °C + 650 ppm + ultrasound). Experimental (points) and predicted (lines) values
: 50 °C + 650 ppm + ultrasound). Experimental (points) and predicted (lines) values
Figures 2c shows the significant S. cerevisiae log reductions obtained in natural orange juice treated by application of temperature (50 °C), US (24 kHz; 105 μm; 33.31 W ml−1) and CLEO (650 or 0 ppm). All the treatments lasted 30 min. The highest inactivation was obtained in TSB (5.13 log reductions) with 650 ppm of CLEO and treated by thermo-US at 50 °C for 30 min compared with natural orange juice (~ 3.8 log reductions) with 650 ppm of CLEO and treated by thermo-US at 50 °C for 30 min.
The effectiveness of US in the inactivation of various strains of S. cerevisiae at low (23–35 °C) and mild (35–55 °C) temperatures, wave amplitudes (71–124 μm), W ml−1 (0.252–822.24) and time (5–200 min) has been studied by several authors. They obtained log reductions ranged between ~ 0.5 and ~ 4 (Ciccolini et al. 1997; Guerrero et al. 2001; López-Malo et al. 1999; López-Malo et al., 2005a, b). Our results are in according with previously data, so we obtained a significant 1.32 log reductions at 50 °C and a significant 2.52 log reductions at 50 °C combined with US. It can be concluded that combination of mild temperatures and US improve the effectiveness of US.
It has been studied the combined effect of mild heat (40–60 °C), US (60–105 μm; 33.31–167.72 W ml−1) and antimicrobials (EDTA, Na-benzoate, potassium sorbate, citrus extract, vanillin, chitosan, Chinese and Ceylan cinnamon EOs) in different strains of bacteria, fungi and yeast (Listeria monocytonenes, L. innocua, Aspergillus flavus spore, Penicillium digitatum spore, spoiling yeasts, S. cerevisiae) (Alzamora et al. 2011; Ferrante et al. 2007; Gastélum et al. 2012; Guerrero et al. 2005; López-Malo et al. 2005a, b). All the mentioned authors observed that the combination of US, antimicrobials and mild temperatures increased inactivation of bacteria, fungi and yeasts studied. They obtained log reductions ranged between ~ 1.15 and ~ 5. It has been found that the combined action of US (95.2 μm; 167.72 W ml−1), mild heat (45 °C) and 50 ppm of chinese cinnamon EO resulted in 2.74 log cycles reductions of the initial population of S. cerevisiae in 20 min, whereas when US was used alone at 45 °C only 0.70 log reduction were obtained in 20 min of treatment (Alzamora et al. 2011). Our results are in agreement with all these previous studies, as we found that the combination of CLEO and thermo-US caused a greater yeast log reduction, especially at 50 °C (5.13 log reductions) (Fig. 2a, b). These results suggest that probably there is a synergistic effect between mild heat, US and CLEO in the inactivation of S. cerevisiae.
Comparing the inactivation obtained in TSB and natural orange juice (Fig. 2c), similar results were obtained by Char et al. (2010), who studied the inactivation of Escherichia coli in peptone water (0.1% w/w) and orange juice (pH 3.4, °Brix 10) treated by combination of temperature (40 °C ± 1 °C) and US (20 kHz, 95.2 μm, 20 min) and they obtained the highest log reduction in peptone water. It has been determinate by Salleh-Mack and Roberts (2007) that the higher soluble solids samples required longer sonication time to achieve 5-log reduction and that the pH has a significant effect on the US inactivation in solutions having soluble solids 12 g/100 ml. Guerrouj et al. (2016) studied the effect of sonication (24 kHz) on microbial quality of natural orange juice and they obtained the highest log reduction when US was applied for 30 min and the temperature rose until 45.6 °C.
The effect of pH on ultrasound effectiveness has been studied for different authors. Some authors found no influence of pH on resistance, while a higher ultrasound sensitivity at acidic pH values was reported by others authors. For example, Guerreo et al. (2001) only found a significant difference when WA = 71.4 µm and T = 45 °C were used. However, this could be attributed to the enhanced heat inactivation of the yeast at pH 3.0 and the less pronounced effect of sonication at low amplitude. Agad and Collins (1992) studied the effect of pH on thermal inactivation at moderate temperatures and they found that pH reduction effect on thermal inactivation of the yeast was noticed at temperatures < 55 °C probably because at the highest temperature the death cell mechanism mainly involved cell disruption by the lethal effect of heat.
It is known that microbial strain, culture broth, aw, pH, wave amplitude, intensity, power, temperature, antimicrobial agent and its concentration, time of preincubation and treatment time influence the lethality of US treatments (Alzamora et al. 2011; Ferrante et al. 2007; Gastélum et al. 2011; Guerrero et al. 2001, 2005; López-Malo et al. 2005a, b), specifically when low temperatures are used (López-Malo et al. 2005b; Luo et al. 2012). It is known that cavitation efficiency is affected by the fluid rheology, in particular by the viscosity, the vapour pressure and surface tension of the culture broth (Luo et al. 2012). The lethality of US treatments is also highly influenced by the amplitude of US waves (López-Malo et al. 1999); with larger amplitudes, the bubbles may grow so larges on rarefaction and the time available for collapse is insufficient. Therefore, depending on liquid properties (vapour pressure, surface tension, and viscosity) the cavitation effect can be diminished with larger amplitudes (Ciccolini et al. 1997; López-Malo et al. 1999). The efficiency of US treatment for killing bacteria by cavitational effects could be reduced with an increase in temperature. López-Malo et al. (1999) found that, at temperatures above 50 °C, the benefits of US application are reduced, probably as a result of an increased thermal effect and reduced intensity (power) of cavitation. This trend could be the result of an increased thermal effect that either hinders the effect of US or decreases the violence of the bubble implosion due to the increased vapour pressure at higher temperatures (Guerrero et al. 2001).
Curve fitting
D-values (decimal reduction times at 50 °C combined with US) were calculated from the linear survival curve slopes in TSB with 0, 40, 80, 160, 320 and 650 ppm of CLEO (Fig. 3b). D-values for S. cerevisiae varied from 4.74 to 17.26 min. D-values were significantly lower when the CLEO concentrations increased (Table 1). Reduction percentage of D-values was 73% with the combination of the three factors (50 °C, US, CLEO) (Table 1). The bibliography shows that the combination of US with mild heat (35–60 °C) at different wave amplitudes (71–107.1 μm), W ml−1 values (0.33–167.72) and time (10–200 min) improves the inactivation of S. cerevisiae and fungi spore (Aspergillus flavus and Penicillium digitatum) getting smaller D values (78.7–2.0 min). The inactivation of spore fungi is even greater in the presence of natural antimicrobial (vanillin 500 ppm) during thermo-US, resulting in lower D values (78.7 to < 0.5), (Ciccolini et al. 1997; Guerrero et al. 2001; López-Malo et al. 1999, 2005a). Furthermore, it has been determinate that S. cerevisiae inactivation in laboratory medium by thermo-US at high temperatures generally followed first-order kinetics during the most of the process (Guerrero et al. 2001, 2005; López-Malo et al. 1999, 2005b), and the combination of antimicrobials (cinnamon EOs, vanillin, chitosan) and thermo-US at low temperatures resulted in non-linear survival curves (Guerrero et al. 2005; López-Malo et al. 2005b). López-Malo et al. (2005a) studied the inactivation of Aspergillus flavus strain ATCC 16872 spores and Penicillium digitatum strain LMUDLA-2 spores by the combined effects of thermo-US (20 kH; 90 µm; 167.72 W ml−1; 45 °C; 9 min) and antimicrobials (vanillin, potassium sorbate), as well as for thermal inactivation and they also found a first order reaction kinetics in all the cases.
Table 1.
Decimal reduction times (D) at 50 °C for S. cerevisiae inactivation during thermo-US (D50/US) treatments in TSB with and without CLEO
| CLEO (ppm) | D50/US (min) |
|---|---|
| 650 | 4.74 ± 0.12a |
| 320 | 5.50 ± 0.10ab |
| 160 | 5.96 ± 0.21ab |
| 80 | 6.53 ± 0.23bc |
| 40 | 7.44 ± 0.36c |
| 0 | 17.26 ± 1.39d |
Only are represented the data that have a good fit to log-linear survival curves. Data are expressed as mean of three replications ± SD. Different letters denote significant differences between data (p < 0.05)
For the data obtained in TSB, the fitting of the models was not good for some conditions studied, with a correlation coefficient (R^2), which is a measure of how well inactivation data fit the prediction, lower than 0.97 (Table 2). It is probably due to the low inactivation degree (less than 1 log cycle) (Figs. 2a, b), so the errors in experimental data are big and the fitting of the data becomes worse (Table 2). However, both models, “Shoulder + log-linear” and Weibull, fitted accurately the survivor curves in the most intense treatments, with the correlation coefficient, ranging from 0.97 to 0.99 (Table 2).
Table 2.
Parameters for survival curves of S. cerevisiae in TSB, at different temperatures, supplemented with sub-inhibitory concentrations of CLEO and subjected to US treatment
| Treatments | “Log-linear + shoulder” model | Weibull model | ||||
|---|---|---|---|---|---|---|
| CLEO (ppm) | kmax (min−1) | Sl (min) | R^2 | δ | ρ | R^2 |
| 30 °C | ||||||
| 650 | 0.09 ± 0.02 | 22.05 ± 5.66 | 0.93 | 55.52 ± 7.41 | 1.37 ± 0.29 | 0.93 |
| 320 | 0.07 ± 0.04 | 28.84 ± 4.86 | 0.90 | 64.17 ± 12.29 | 1.90 ± 0.57 | 0.92 |
| 40 °C | ||||||
| 650 | 0.21 ± 0.02 | 23.84 ± 0.97 | 0.97 | 35.37 ± 1.16 | 3.34 ± 0.41 | 0.97 |
| 320 | 0.19 ± 0.04 | 27.40 ± 0.82 | 0.93 | 37.35 ± 2.00 | 4.06 ± 0.79 | 0.93 |
| 50 °C | ||||||
| 650 | 0.43 ± 0.03 | 6.43 ± 1.79 | 0.97 | 10.76 ± 1.52 | 1.48 ± 0.19 | 0.97 |
| 320 | 0.38 ± 0.04 | 11.93 ± 1.72 | 0.96 | 17.13 ± 1.33 | 2.04 ± 0.27 | 0.97 |
| 160 | 0.35 ± 0.04 | 12.65 ± 1.67 | 0.96 | 18.22 ± 1.57 | 2.03 ± 0.33 | 0.96 |
| 80 | 0.34 ± 0.03 | 13.94 ± 1.21 | 0.98 | 20.86 ± 0.74 | 2.32 ± 0.21 | 0.99 |
| 40 | 0.30 ± 0.04 | 16.78 ± 1.38 | 0.96 | 23.86 ± 0.73 | 2.94 ± 0.36 | 0.97 |
| 0 | 0.17 ± 0.03 | 13.44 ± 2.93 | 0.93 | 26.60 ± 1.39 | 1.85 ± 0.39 | 0.93 |
| 30 °C/US | ||||||
| 650 | 0.21 ± 0.03 | 9.56 ± 5.20 | 0.92 | 30.10 ± 1.95 | 1.41 ± 0.31 | 0.93 |
| 320 | 0.11 ± 0.03 | 22.92 ± 1.14 | 0.95 | 33.27 ± 0.99 | 3.42 ± 0.55 | 0.95 |
| 40 °C/US | ||||||
| 650 | 0.21 ± 0.02 | 11.68 ± 1.25 | 0.99 | 22.04 ± 0.85 | 1.80 ± 0.18 | 0.98 |
| 320 | 0.15 ± 0.02 | 11.71 ± 2.05 | 0.98 | 27.86 ± 0.92 | 1.08 ± 0.14 | 0.98 |
| 160 | 0.13 ± 0.01 | 13.01 ± 1.70 | 0.98 | 28.69 ± 0.75 | 1.74 ± 0.18 | 0.98 |
| 80 | 0.09 ± 0.03 | 17.46 ± 6.98 | 0.91 | 52.46 ± 8.13 | 1.11 ± 0.29 | 0.90 |
| 40 | 0.07 ± 0.02 | 20.87 ± 3.73 | 0.94 | 47.26 ± 4.51 | 1.79 ± 0.32 | 0.95 |
| 50 °C/US | ||||||
| 650 | 0.51 ± 0.06 | 1.81 ± 3.60 | 0.93 | 4.21 ± 2.01 | 0.94 ± 0.21 | 0.92 |
| 320 | 0.45 ± 0.02 | 2.75 ± 1.41 | 0.99 | 6.65 ± 1.10 | 1.12 ± 0.11 | 0.98 |
| 160 | 0.41 ± 0.02 | 2.47 ± 1.76 | 0.98 | 6.79 ± 1.32 | 1.08 ± 0.13 | 0.98 |
| 80 | 0.38 ± 0.02 | 2.80 ± 1.68 | 0.98 | 7.81 ± 1.30 | 1.13 ± 0.13 | 0.98 |
| 40 | 0.35 ± 0.03 | 4.47 ± 2.23 | 0.97 | 9.86 ± 1.85 | 1.24 ± 0.19 | 0.96 |
| 0 | 0.14 ± 0.03 | 2.23 ± 6.01 | 0.91 | 18.21 ± 3.08 | 1.09 ± 0.26 | 0.91 |
The values for the different constants were calculated by GInaFiT (Geeraerd et al. 2005). All values are expressed as the mean of three replications ± SD
Parameters obtained by the “Shoulder + log-linear” model may allow conclude that the presence of CLEO increased mortality rate (kmax) and reduced the S1 of S. cerevisiae at the most treatments tested. This effect considerably depended on the amount of CLEO present in the thermo-US treated medium. The kmax-values were lower for TSB control than those for TSB with CLEO at all the temperature tested, but the effect was most pronounced at the highest temperature (50 °C). The presence of CLEO also reduced the shoulder length of S. cerevisiae in TSB, but this effect was more variable. Cava-Roda et al. (2012a) studied the destruction kinetics of Listeria monocytogenes Scott A in semi-skim milk with vanillin (0, 900, 1400, 1800 ppm) and heated (55, 58, 60, 62 °C) and found that the presence of vanillin increased mortality rate (kmax) and reduced the shoulder length of L. monocytogenes Scott A at any temperature tested, specially at the lowest temperatures (55, 58 °C) and the biggest amounts of vanillin (1400, 1900 ppm).
The effect of combined treatments was also analyzed from a different approach by means of the Weibull distribution of resistance model. This model considers that the entire microbial population is not equally resistant to the proposed treatment and so each cell is not destroyed at the same time during processing (Peleg and Cole 1998). As a result, the survival curve is the cumulative form of a temporal distribution of lethal events where each individual organism is inactivated at a specific time, thus generating a spectrum of heat resistances (Peleg and Cole 1998). Table 2 displays δ and ρ parameters obtained from fitting the Weibullian distribution model to the survival curves along with the adjusted correlation coefficient. Weibull distribution parameters δ and ρ varied according to the severity of stress conditions and R^2 values obtained were ranged between 0.90 and 0.99. Our results showed that treatments involving mild heat (30, 40, 50 °C) and US applied to S. cerevisiae in TSB with or without CLEO yielded ρ values greater than 1, correlating with a downward concavity of the survival curve that in general was lower with higher temperatures, greater concentrations of CLEO and US application. It has been argued (Peleg 2000) that a possible explanation of these curve shapes could be that the more sensitive members were weakened leaving a large fraction of more resistant members which were affected in much lesser extent. Some of the predicted S. cerevisiae survival curves corresponding to treatments involving US at 50 °C in TSB with CLEO exhibited ρ values close to 1, indicating first order inactivation kinetics (ρ ~ 1 corresponds to the log-linear shape). Our results are similar to those obtained by other researchers that fitted the survival data of S. cerevisiae to the Weibull model. López-Malo et al. (2005a) found that survival curves of S. cerevisiae KE162 US treated (20 kHz; 95.2 μm; 822.24 W ml−1; 35 °C; 25 min) in buffer solution were characterized by n value greater than 1. When Guerrero et al. (2005) treated S. cerevisiae KE162 in Sabouraud broth with US (20 kHz; 95.2 μm; 167.72 W ml−1; 45 °C; 35 min) get survival curves that exhibited n (the parameter n is equivalent to parameter ρ) value close to 1, and when they studied the combined effect of US (same conditions) in Sabouraud broth with 1000 ppm of chitosan get n value greater than 1. The parameter δ, or scale parameter, in general decreased as CLEO concentrations and heating temperature increased or when US is applied. The greater inactivation effect was observed at heating temperatures of 50 °C applied in combination with US and CLEO. This effect depended mainly on the concentration of CLEO: to higher concentration, bigger inactivation is obtained. In this case the survival curves followed a first order reaction kinetic because in the Weibull fit ρ is very close to 1 (See Table 2). For example, in TSB with 650 ppm of CLEO and thermo-US at 50 °C we obtained a S. cerevisiae inactivation of 5.13 log cycles at 24 min and the same result was obtained with 320 ppm of CLEO in 30 min of treatment (Fig. 3a, b).
In natural orange juice, both models, “log-linear + Shoulder” and Weibull, fitted accurately the survivor curves of some treatments, with the correlation coefficient ranging from 0.97 to 0.99 (Table 3). However, when the data obtained in the sample treated by 50 °C + 650 ppm + US were modelled, it was obtained a negative value for the shoulder length Sl, which is physically not possible. So, model with shoulder is unlikely for this data. Figure 3c shows the fit of Weibull model to survival data sets of S. cerevisiae suspended in natural orange juice and the highest inactivation was obtained with the combination of US and 650 ppm CLEO at 50 °C. López-Malo et al. (2005a) evaluated and modelled (Weibull model) the survival of S. cerevisiae and L. monocytogenes in apple juice (pH: 3.5; 12 °B) and in a model system (buffer phosphate; pH: 3.5) processed by short wave ultraviolet radiation (UV-C) and high intensity ultrasound (US: 20 kHz, 95.2 µm, 35 ± 1 °C, 25 mm probe). The inactivation of both microorganisms was highly dependent of the medium (apple juice or buffer system). In general, treatments in buffer solution were more effective as compared to those applied in apple juice, which could be due to the presence of organic compounds which reduced the efficiency of treatments.
Table 3.
Parameters for survival curves of S. cerevisiae in natural orange juice (pH: 3.97; °Brix: 11.6) at 50 °C, supplemented with CLEO and subjected to US treatment
| Treatments | “Log-linear + shoulder” model | Weibull model | ||||
|---|---|---|---|---|---|---|
| kmax (min−1) | Sl (min) | R^2 | δ | ρ | R^2 | |
| 50 °C | 0.17 ± 0.04 | 22.25 ± 2.30 | 0.95 | 35.15 ± 2.27 | 2.69 ± 0.78 | 0.95 |
| 50 °C + 650 ppm | 0.18 ± 0.03 | 6.41 ± 4.18 | 0.97 | 18.86 ± 2.69 | 1.29 ± 0.30 | 0.96 |
| 50 °C + US | 0.14 ± 0.04 | 0.54 ± 8.43 | 0.93 | 16.67 ± 4.27 | 0.99 ± 0.32 | 0.93 |
| #50 °C + 650 ppm + US | – | – | – | 3.12 ± 0.90 | 0.61 ± 0.07 | 0.99 |
The values for the different constants were calculated by GInaFiT (Geeraerd et al. 2005). All values are expressed as the mean of three replications ± SD. #50 °C + 650 ppm + US: it was obtained a negative value for the shoulder length Sl. So, model with shoulder is unlikely for this data
The combination of US, mild temperature and antimicrobials would have a synergistic effect on the inactivation of bacteria, fungi and yeast. The effectiveness of treatment would depend of the US wave amplitude, W ml−1, temperature, treatment time, concentration and type of antimicrobial (López-Malo et al. 1999; Guerrero et al. 2005) and also of the microbial cell wall, which differs significantly between species (Luo et al. 2012). During US treatment, microbial cell alterations involved disruption of subcellular particles with fine membrane fragmentation, internal cavitation as well as internal micro-streaming, with modification of the cellular structure, as well as fragmented cells and marked irregularity in density (López-Malo et al. 2005a). It has been demonstrated that extra and intracellular cavitation erodes the cell wall and disrupts structural and functional components up to cell lysis (Ferrante et al. 2007; Guerrero et al. 2005). In S. cerevisiae cells, thermo-US provoked puncturing of cell walls and breakage of plasmalemma with leakage of content, wall rupture or fragmentation, and cytological disruption of organelles (Guerrero et al. 2001, 2005). Thermo-US and phenolic compounds damage the structure and functionality of the membrane proteins, enhancing the simultaneous effect of these active agents against membrane (Burt 2004; Guerrero et al. 2001). Phenolic compounds have a lipophilic nature and could accumulate in the lipid bilayer of the cell (Burt 2004), disturbing and sensitizing the membrane to US action. In addition, US waves improve the antimicrobial action by weakening the cell wall (Ferrante et al. 2007). Sonication applied to orange juice is not only able to control of spoilage microorganisms, but also improves the sensory characteristics and produces an general enhancement in bioactive compounds: total phenolics, flavonoids, carotenoids, anthocyanins and ascorbic acid (Guerrouj et al. 2016).
Conclusion
The combined use of temperature, US and CLEO was highly effective in the inactivation of S. cerevisiae in TSB and natural orange juice, especially in TSB, results that was enhanced with high temperatures. Thermo-US combined with CLEO could act synergistically in inactivation of S. cerevisiae in both, TSB and natural orange juice. Lowest D-values were obtained when samples were treated with US in presence of CLEO at 50 °C. Both models used, “shoulder + log-linear” and “Weibull”, fitted the inactivation curves accurately for some conditions. The later model was a useful tool to select CLEO concentration combined with US-50 °C necessary to achieve the inactivation of most part of the yeast population.
The combination of US, mild thermal treatments (30–50 °C) and natural antimicrobials as EOs show considerable promise for the future in order to control S. cerevisiae as important spoiling microorganism in orange juice.
References
- Agad MA, Collins M. Effect of treatments environment (temperature, pH, water activity) on the heat resistence of yeast. J Food Sci Technol. 1992;29:5–9. [Google Scholar]
- Ait-Ouazzou A, Espina L, García-Gonzalo D, Pagán R. Synergistic combination of physical treatments and carvacrol for Escherichia coli O157:H7 inactivation in apple, mango, orange, and tomato juices. Food Control. 2013;32:159–167. doi: 10.1016/j.foodcont.2012.11.036. [DOI] [Google Scholar]
- Alzamora SM, Guerrero SN, Schenk M, Raffellini S, López-Malo A. Inactivation of microorganisms. In: Feng H, Barbosa-Cánovas GV, Weiss J, editors. Ultrasound technologies for food and bioprocessing. Berlin: Springer; 2011. pp. 321–345. [Google Scholar]
- Bennis S, Chami F, Chami N, Bouchikhi T, Remmal A. Surface alteration of Saccharomyces cerevisiae induced by thymol and eugenol. Lett Appl Microbiol. 2004;38:454–458. doi: 10.1111/j.1472-765X.2004.01511.x. [DOI] [PubMed] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods: a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Cava-Roda RM, Taboada A, Palop A, López-Gómez A, Marin-Iniesta F. Heat resistance of Listeria monocytogenes in semi-skim milk supplemented with vanillin. Int J Food Microbiol. 2012;157:314–318. doi: 10.1016/j.ijfoodmicro.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Cava-Roda RM, Taboada-Rodríguez A, Valverde-Franco MT, Marín-Iniesta F. Antimicrobial activity of vanillin and mixtures with cinnamon and clove essential oils in controlling Listeria monocytogenes and Escherichia coli O157:H7 in milk. Food Bioprocess Tech. 2012;5(6):2120–2131. doi: 10.1007/s11947-010-0484-4. [DOI] [Google Scholar]
- Char CD, Mitilinaki E, Guerrero SN, Alzamora SM. Use of high-intensity ultrasound and UV-C light to inactivate some microorganisms in fruit juices. Food Bioprocess Technol. 2010;3:797–803. doi: 10.1007/s11947-009-0307-7. [DOI] [Google Scholar]
- Ciccolini L, Taillandier P, Wilhem AM, Delmas H, Strehaiano P. Low frequency thermo-ultrasonication of Saccharomyces cerevisiae suspensions: effect of temperature and of ultrasonic power. Chem Eng J. 1997;65:145–149. doi: 10.1016/S1385-8947(96)03172-5. [DOI] [Google Scholar]
- Darvishi E, Omidi M, Bushehri AAS, Golshani A, Smith ML. The antifungal eugenol perturbs dual aromatic and branched-chain amino acid permeases in the cytoplasmic membrane of yeast. PLoS ONE. 2013;8(10):e76028. doi: 10.1371/journal.pone.0076028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante S, Guerrero S, Alzamora SM. Combined use of ultrasound and natural antimicrobials to inactivate Listeria monocytogenes in orange juice. J Food Protect. 2007;70(8):1850–1856. doi: 10.4315/0362-028X-70.8.1850. [DOI] [PubMed] [Google Scholar]
- Gastélum GG, Avila-Sosa R, López-Malo A, Palou E. Listeria innocua multi-target inactivation by thermo-sonication and vanillin. Food Bioprocess Tech. 2012;5(2):665–671. doi: 10.1007/s11947-010-0334-4. [DOI] [Google Scholar]
- Geeraerd AH, Herremans CH, Van Impe JF. Structural model requirements to describe microbial inactivation during a mild heat treatment. Int J Food Microbiol. 2000;59(3):185–209. doi: 10.1016/S0168-1605(00)00362-7. [DOI] [PubMed] [Google Scholar]
- Geeraerd AH, Valdramidis VP, Van Impe JF. GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. Int J Food Microbiol. 2005;102(1):95–105. doi: 10.1016/j.ijfoodmicro.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Guerrero S, López-Malo A, Alzamora SM. Effect of ultrasound on the survival of Saccharomyces cerevisiae: influence of temperature, pH and amplitude. Innov Food Sci Emerg Technol. 2001;2:31–39. doi: 10.1016/S1466-8564(01)00020-0. [DOI] [Google Scholar]
- Guerrero S, Tognon M, Alzamora SM. Modeling the response of Saccharomyces cerevisiae to the combined action of ultrasound and low weight chitosan. Food Control. 2005;16:131–139. doi: 10.1016/j.foodcont.2004.01.003. [DOI] [Google Scholar]
- Guerrouj K, Sánchez-Rubio M, Taboada-Rodríguez A, Cava-Roda RM, Marín-Iniesta F. Sonication at mild temperatures enhances bioactive compounds and microbiological quality of orange juice. Food Bioprod Process. 2016 doi: 10.1016/j.fbp.2016.03.007. [DOI] [Google Scholar]
- Gutierrez J, Barry-Ryan C, Bourke P. Antimicrobial activity of plant essential oils using food model media: efficacy, synergistic potential and interactions with food components. Food Microbiol. 2009;26:142–150. doi: 10.1016/j.fm.2008.10.008. [DOI] [PubMed] [Google Scholar]
- López-Malo A, Guerrero S, Alzamora SM. Saccharomyces cerevisiae thermal inactivation kinetics combined with ultrasound. J Food Protect. 1999;62(10):1215–1217. doi: 10.4315/0362-028X-62.10.1215. [DOI] [PubMed] [Google Scholar]
- López-Malo A, Guerrero SN, Santiesteban A, Alzamora SM (2005a) Inactivation kinetics of Saccharomyces cerevisiae and Listeria monocytogenes in apple juice processed by novel technologies. In: Proceedings of the 2nd Mercosur congress on chemical engineering. http://www.e-papers.com.br
- López-Malo A, Palou E, Jiménez-Fernández M, Alzamora SM, Guerrero S. Multifactorial fungal inactivation combining thermosonication and antimicrobials. J Food Eng. 2005;67:87–93. doi: 10.1016/j.jfoodeng.2004.05.072. [DOI] [Google Scholar]
- Luo H, Schmid F, Grbin PR, Jiranek V. Viability of common wine spoilage organisms after exposure to high power ultrasonics. Ultrason Sonochem. 2012;19:415–420. doi: 10.1016/j.ultsonch.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Mafart P, Couvert O, Gaillard S, Leguerinel I. On calculating sterility in thermal preservation methods: application of the Weibull frequency distribution model. Int J Food Microbiol. 2002;72:107–113. doi: 10.1016/S0168-1605(01)00624-9. [DOI] [PubMed] [Google Scholar]
- Patrignani F, Vannini L, Kamdem SLS, Lanciotti R, Guerzoni ME. Effect of high pressure homogenization on Saccharomyces cerevisiae inactivation and physico-chemical features in apricot and carrot juices. Int J Food Microbiol. 2009;136:26–31. doi: 10.1016/j.ijfoodmicro.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Peleg M. Microbial survival curves. The reality of flat shoulders and absolute thermal death times. Food Res Int. 2000;33(7):531–538. doi: 10.1016/S0963-9969(00)00088-0. [DOI] [Google Scholar]
- Peleg M, Cole MB. Reinterpretation of microbial survival curves. Crit Rev Food Sci. 1998;38:353–380. doi: 10.1080/10408699891274246. [DOI] [PubMed] [Google Scholar]
- Prakash B, Singh P, Mishra PK, Dubey NK. Safety assessment of Zanthoxylum alatum Roxb. essential oil, its antifungal, antiaflatoxin, antioxidant activity and efficacy as antimicrobial in preservation of Piper nigrum L. fruits. Int J Food Microbiol. 2012;153:183–191. doi: 10.1016/j.ijfoodmicro.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Ranasinghe L, Jayawardena B, Abeywickrama K. Fungicidal activity of essential oils of Cinnamomum zeylanicum (L.) and Syzygium aromaticum (L.) Merr et L. M. Perry against crown rot and anthracnose pathogens isolated from banana. Lett Appl Microbiol. 2002;35:208–211. doi: 10.1046/j.1472-765X.2002.01165.x. [DOI] [PubMed] [Google Scholar]
- Ravanfar R, Tamadon AM, Niakousari M. Optimization of ultrasound assisted extraction of anthocyanins from red cabbage using Taguchi design method. J Food Sci Technol. 2015;52(12):8140–8147. doi: 10.1007/s13197-015-1880-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salleh-Mack SZ, Roberts JS. Ultrasound pasteurization: the effects of temperature, soluble solids, organic acids and pH on the inactivation of Escherichia coli ATCC 25922. Ultrason Sonochem. 2007;14:323–329. doi: 10.1016/j.ultsonch.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Soria AC, Villamiel M. Effect of ultrasound on the technological properties and bioactivity of food: a review. Trends Food Sci Tech. 2010;21:323–331. doi: 10.1016/j.tifs.2010.04.003. [DOI] [Google Scholar]
- Souza EL, Lima EO, Luna Freire KR, Sousa CP. Inhibitory action of some essential oils and phytochemicals on the growth of various moulds isolated from foods. Braz Arch Biol Technol. 2005;48(2):245–250. doi: 10.1590/S1516-89132005000200011. [DOI] [Google Scholar]
- Sukatta U, Haruthaithanasan V, Chantarapanont W, Dilokkunanant U, Suppakul P. Antifungal activity of clove and cinnamon oil and their synergistic against postharvest decay fungi of grape in vitro. Kasetsart J Nat Sci. 2008;42:169–174. [Google Scholar]
- Sun Y, Zhong L, Cao L, Lin W, Ye X. Sonication inhibited browning but decreased polyphenols contents and antioxidant activity of fresh apple (malus pumilamill, cv. Red Fuji) juice. J Food Sci Technol. 2015;52(12):8336–8342. doi: 10.1007/s13197-015-1896-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournas V, Stack ME, Mislivec PB, Koch HA, Bandler R (2001) Bacteriological analytical manual. Chapter 18. Yeasts, Molds and Mycotoxins. https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm071435.htm. Accessed 15 Jan 2018
- Tserennadmid R, Takó M, Galgóczy L, Papp T, Pesti M, Vágvölgyi C, Almássy K, Krisch J. Anti yeast activities of some essential oils in growth medium, fruit juices and milk. Int J Food Microbiol. 2011;144:480–486. doi: 10.1016/j.ijfoodmicro.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Valverde MT, Marín-Iniesta F, Calvo L. Inactivation of Saccharomyces cerevisiae in conference pear with high pressure carbon dioxide and effects on pear quality. J Food Eng. 2010;98:421–428. doi: 10.1016/j.jfoodeng.2010.01.022. [DOI] [Google Scholar]