Abstract
Excessive production and restricted elimination of free radicals like superoxide, hydroxyl radical (·OH), anion radical (O·−2), and non-radical hydrogen peroxide (H2O2) are related to the development of cancer, arteriosclerosis, arthritis and neurodegenerative diseases. According to a report of World Health Organisation, about 80% of the population living in the developing countries predominantly depends on the traditional medicine for their primary healthcare. Plants possess innate ability to synthesize a wide variety of enzymatic and non-enzymatic antioxidants capable of attenuating ROS-induced oxidative damage. The ethanolic leaf extracts of Syzygium jambos L. and Terminalia citrina Roxb. exhibited a significant in vitro antioxidant activity when compared with natural antioxidant, ascorbic acid. The extracts also provided strong cellular protection against the damaging effects of H2O2 induced oxidative stress in the mutant strains (tsa1Δ and sod1Δ) of Saccharomyces cerevisiae. The GC–MS analysis of the leaf extracts revealed the presence of phytoconstituents majorly constituting of terpenes, vitamin and fatty acids contributing to the antioxidant property. The plant extracts may serve as a potential source of exogenous antioxidants to combat the undesirable effects of oxidative stress.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3355-z) contains supplementary material, which is available to authorized users.
Keywords: Antioxidant, Saccharomyces cerevisiae, sod1Δ, Syzygium jambos, T. citrina, tsa1Δ
Introduction
In living organisms, reactive oxygen species (ROS) are continuously formed as a by-product of different metabolic process. The balance between the formation and removal of ROS is required to regulate numerous physiological functions such as mitogenic response, signal transduction, and immunity. Antioxidant enzymes involved in oxidative stress responses such as superoxide dismutases (SOD), thioredoxin peroxidase (TSA1), catalase (CAT), and glutathione peroxidase (G-Px) naturally neutralize the excess free radicals (Li et al. 2013). However, the overproduction of ROS during injury, inflammation, aging, and chronic diseases result in oxidative stress. The oxidative stress damages the cell membrane, proteins, nucleic acid and lipids, consequently contributing to the development of ulcer, diabetes, neurodegenerative diseases, cancer, and rheumatoid arthritis (Oliveira et al. 2016).
Medicinal plants have attracted enormous attention as a source of natural antioxidants to combat the development and progression of life-threatening diseases, including neurodegenerative and cardiovascular disease (Kasote et al. 2015). Syzygium jambos L. and Terminalia citrina Roxb. have been traditionally used in the treatment of numerous health disorders and infectious diseases. Syzygium jambos (L.) Alston, commonly known as rose apple is native to Southeast Asia and widely distributed in the north-east region of India. In Indian traditional system of medicine, the decoction of the fruit and leaves are traditionally administered as a diuretic, an expectorant, in the treatment of tooth ailments, sore eyes and in rheumatism. In Indo-China, the flowers are used to reduce fever, and the seeds in the treatment of diabetes, diarrhea and dysentery. The decoction of the bark is believed to relieve asthma and bronchitis. Recent studies have shown that the plant posses significant antibacterial and anti-inflammatory properties (Sharma et al. 2013). Terminalia citrina (Gaertn.) Roxb. ex Fleming is a plant of extensive ethnomedicinal significance and widely distributed in North East India. The fruit of the plant is used in the treatment of asthma, migraine, dental disorders, hemorrhoids, anemia, chronic fever, diarrhea and other digestive disorders. It is also known to possess anti-inflammatory, analgesic, antipyretic and diuretic properties (Akhtar et al. 2016). Considering their broad spectrum pharmacological properties, the current study was executed to determine the antioxidant efficacy of S. jambos and T. citrina. The in vivo antioxidant property of the ethanolic leaf extracts in response to H2O2 induced oxidative stress was also investigated using wild-type and isogenic mutant strains (tsa1Δ and sod1Δ) of Saccharomyces cerevisiae.
Materials and methods
Collection of plant materials and preparation of extracts
Fresh leaves of S. jambos and T. citrina were collected from Palace Compound, Imphal East, Manipur, India. The leaves were washed, shade dried and grounded into a fine dry powder. The plant extracts were prepared by mixing 50 g of the powdered leaves with 250 mL of ethanol in an Erlenmeyer flask. The flasks were kept under agitation at 28 °C for 48 h. The extracts were filtered and concentrated to complete dryness. The crude residues obtained was reconstituted in dimethyl sulfoxide (DMSO) and stored at − 20 °C until further use.
Yeast strains and culture conditions
Saccharomyces cerevisiae wild-type BY4741 and its isogenic mutants, tsa1Δ and sod1Δ were kindly provided by Department of Biochemistry and Molecular biology, Pondicherry University, Puducherry, India. The yeast cells were grown at 28 °C in YPD broth composed of 1% yeast extract, 2% glucose and 2% peptone.
Antioxidant activity
The plant extracts were diluted to concentrations ranging from 100 to 500 μg/mL. The free radical scavenging activity was determined using 2,2′-diphenyl-1-picrylhydrazyl (DPPH) assay as described earlier by Husein et al. (2014) with few modifications. Briefly, 2 mL of DPPH solution (0.2 mM) was supplemented with 100 μL of the plant extract (100–500 μg/mL). The reaction mixture was vortexed and incubated for 20 min, in dark. Ascorbic acid which is involved in the non-enzymatic system of radical scavenging was used as positive control. The optical density was recorded at 517 nm (Husein et al. 2014).
The ability of the test extracts in scavenging hydroxyl radicals generated by Fenton reaction (Fe3+-ascorbate-EDTA-H2O2) was evaluated according to the method described by Saeed et al. (2012).
Ferric reducing antioxidant power (FRAP) assay was evaluated as described by Udayaprakash et al. (2015).
The total antioxidant capacity of the extracts was measured using phosphomolybdate method with ascorbic acid as a standard. An aliquot containing 0.1 mL of the plant extract (100–500 μg/mL) was added to a reagent solution (1 mL) constituting of sulphuric acid (0.6 M), sodium phosphate (28 mM) and ammonium molybdate (4 mM). The reaction mixture was incubated at 95 °C for 90 min and the absorbance was recorded at 695 nm. The total antioxidant capacity was interpreted in terms of ascorbic acid equivalent (μg/mL) (Hossain and Shah 2015).
Antioxidant activity
Owing to the homology between the stress response genes between S. cerevisiae and humans, the wild-type and antioxidant mutant strains (tsa1Δ and sod1Δ) of S. cerevisiae were used to investigate the antioxidant activity of the test extracts. The yeast cells were grown up to the first exponential phase (OD600 = 0.6) in YPD broth at 28 °C (Golla and Bhimathati 2014).
The hydrogen peroxide (H2O2) sensitivity assay was performed to evaluate the sensitivity of S. cerevisiae against the oxidative agent. Exponential phase culture of S. cerevisiae, wild-type and mutant strains (20 μL) were serially diluted in a 96 well microtitre plate and spot inoculated on YPD agar plates supplemented with 1, 1.5, 2, 2.5, 3 and 3.5 mM of H2O2. The plates were incubated for 3 days at 30 °C and sensitivity of the mutant strains towards increasing concentration of H2O2 was determined. YPDA plates devoid of H2O2 served as control (Subhaswaraj et al. 2017).
Wild-type and antioxidant mutants (sod1Δ and tsa1Δ) of S. cerevisiae were exposed to the plant extracts (500 μg/mL) and incubated for 2 h. Subsequently, serial dilutions (10−1, 10−2, 10−3, 10−4and 10−5) of the yeast cells were made and 4 μL of the culture was spot inoculated on YPD agar plates supplemented with 2.5 mM H2O2. The plates were incubated for 72 h at 28 °C and the growth was observed. The cells treated only with H2O2 serve as a negative control (Golla and Bhimathati 2014).
For the cell viability assay, S. cerevisiae (wild-type and mutants) were treated with plant extracts (500 μg/mL) and incubated for 1 h. The broth cultures were further supplemented with H2O2 (2.5 mM) and incubated for 1 h at 28 °C. The extract treated and untreated cells were further incubated at 30 °C for 18 h and the optical density was recorded at 600 nm (Toussaint and Conconi 2006).
The free radical scavenging efficacy of the test extracts was determined using fluorescent dye, 2′, 7′-dichlorofluorescein diacetate (DCF-DA). The plant extracts treated and untreated cells were mixed with 20 μL of H2O2 (1 mM) and incubated for 1 h at 28 °C under agitation (160×g). The cells were harvested by centrifugation at 6000×g. The harvested cells were washed thrice with 1X phosphate-buffered saline (PBS) and resuspended in PBS containing 0.8 μL of DCF-DA (20 μM). Further, the cells were incubated in dark for 30 min, recollected by centrifugation and washed thrice with PBS. The cell pellet was diluted with 10 µL of PBS, mounted onto a slide and examined using a fluorescence microscope. The relative fluorescent intensity of the samples was recorded at 488 and 520 nm (Wong et al. 2002).
Phytochemical analysis
The plant extracts were subjected to GC–MS analysis to identify the phytoconstituents. The phytocompounds were identified based on the retention indices and the spectrum obtained in comparison to the National Institute Standard and Technology (NIST-2008) library database (Musthafa et al. 2013).
Results and discussion
Antioxidant activity
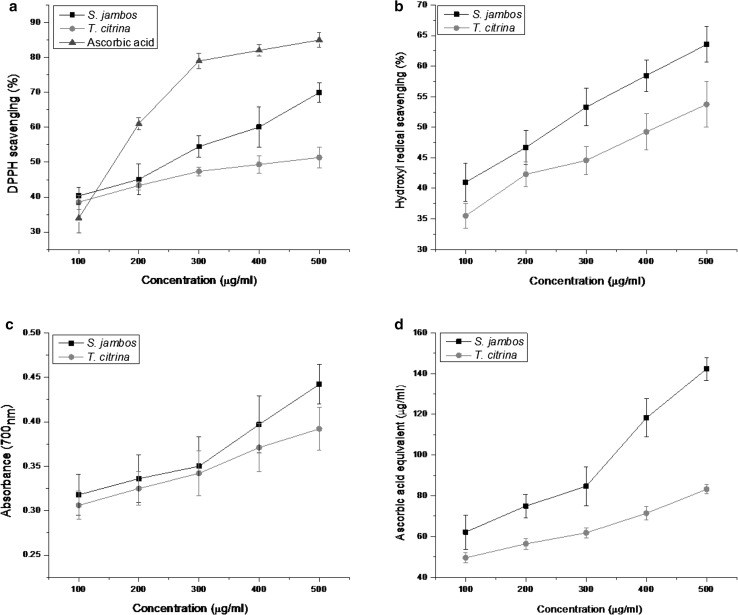
In the living system, excessive production of ROS exerts oxidative damages to the biological molecules like protein, lipid, amino acids and DNA. These damages subsequently result in various chronic and degenerative diseases, such as cancer, diabetes, hypertension, Alzheimers, Parkinsons and coronary heart diseases. Various parts of plants used in the herbal medicine have been documented as a good source of natural antioxidants with wide therapeutic applications (Sahoo et al. 2013). The ethanolic extracts of S. jambos and T. citrina showed potent antioxidant activity in a dose-dependent manner. The significant reduction in the intensity of DPPH solution indicated the free radical scavenging effect of the phytocompounds present in the plant extract. The extracts exhibited an increase in DPPH scavenging activity with a concomitant increase in concentration from 100 to 500 µg/mL (Fig. 1a). A maximum free radical scavenging activity of 69.91 and 51.34% was observed at 500 μg/mL for S. jambos and T. citrina, respectively. The IC50 value of S. jambos was 38.73 μg/mL while for T. citrina it was found to be 111.86 μg/mL. The positive control, ascorbic acid exhibited a higher DPPH scavenging activity of 85% at 500 μg/mL with an IC50 value of 2.83 μg/mL. Hydroxyl radicals (OH·) are considered as one of the most reactive species and known to exert damaging effects to DNA, protein, and lipid. The OH· induced oxidative damage to DNA have been recognized to exert mutagenic effects and aging (Nimse and Pal 2015). Therefore, the elimination of OH· radical may be considered as one of the best indicators to detect the antioxidant potential of a compound. The OH· radical scavenging activity was quantified by measuring the ability of the extracts to compete with 2-deoxyribose for the free radicals generated in the Fenton reaction. The OH· radical scavenging efficacy of S. jambos and T. citrina extracts at a concentration of 500 µg/mL were found to be 63.54 and 53.75% respectively (Fig. 1b). Ascorbic acid exhibited OH· radical scavenging efficacy of 68.55%.
Fig. 1.
In vitro antioxidant activities of the ethanolic extract of S. jambos and T. citrina at various concentrations (100–500 μg/mL): a DPPH radical scavenging activity; b Hydroxyl radical scavenging activity; c Reducing power; d Total antioxidant capacity. Value are represented as mean ± SD (n = 3)
The reducing power of a compound is generally corresponds to the presence of reductants, which imparts antioxidant property by giving a hydrogen atom and thereby breaking the free radical chain (Jan et al. 2013). The reducing power of a compound is measured by its ability to reduce Fe[(CN)6]3+, subsequently resulting in an increase in absorbance due to the formation of the Fe[(CN)6]2+ complex. The dose–response curves of plant extracts (100–500 µg/mL) exhibited a gradual increase in reducing power, indicated by the increase in optical density (Fig. 1c). The extracts of S. jambos and T. citrina showed a significant reducing power of 0.44 and 0.39 respectively, at a concentration of 500 μg/mL. However, the reducing power of ascorbic acid was recorded to be 0.48. Hence, the antioxidant potential could be attributed to the presence of the reductants which resulted in the reduction of Fe3+ to Fe2+ (ferrous) form.
The total antioxidant capacity of the extracts was determined using phosphomolybdenum method in terms of ascorbic acid equivalent. A significantly high total antioxidant activity was displayed by the ethanolic leaf extracts of S. jambos and T. citrina. S. jambos exhibited a total antioxidant activity with an ascorbic acid equivalent of 142.32 μg/mL while T. citrina exhibited 83.17 μg/mL, at a concentration of 500 μg/mL (Fig. 1d). In this study, both the plant extracts registered prominent antioxidant activity in a concentration-dependent manner. However, S. jambos showed higher antioxidant potential in all the in vitro assays. The difference observed in the antioxidant efficacy of the plant extracts may be attributed to the unequal distribution of the antioxidant compounds in the plant extracts.
Determination of in vivo antioxidant activity
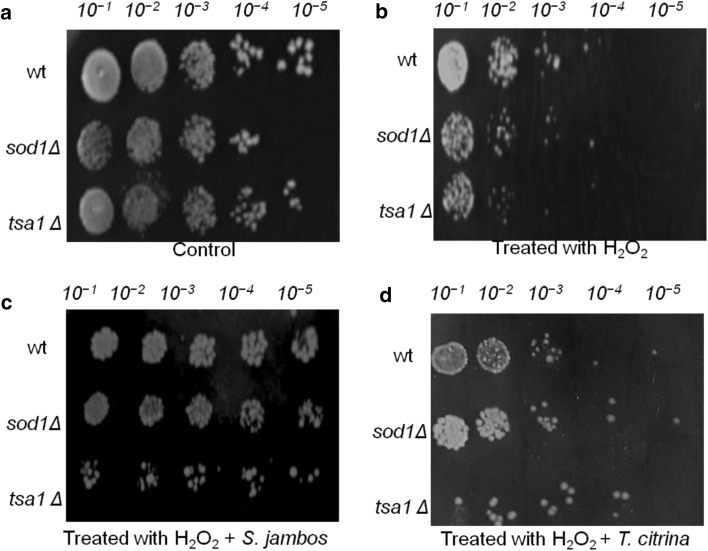
Understanding the regulation of cellular redox balance and identifying novel natural antioxidants are of great importance for human health. S. cerevisiae serves as a reliable in vivo model system to identify bioactive antioxidants (Meng et al. 2017). In S. cerevisiae, the enzymatic antioxidant defenses viz. superoxide dismutase (SOD), glutathione peroxidase (GPx), thioredoxin reductase (TR) and catalase (CAT) maintains the ROS balance and controls the over-accumulation of ROS in the cells. The endogenous antioxidant enzymes, SOD encoded by genes sod1 converts O·−2 to O2 and H2O2. Thioredoxin reductase (TR) encoded by tsa1 controls the levels of H2O2 by converting it into H2O and O2 (Li et al. 2013). Hence, S. cerevisiae mutants deficient in antioxidases (sod1∆ and tsa1∆) were exploited for evaluating the possible antioxidant mechanism of S. jambos and T.citrina. The hydrogen peroxide sensitivity assay was performed to determine the sensitivity of the wild-type and its isogenic mutants (tsa1Δ and sod1Δ) of S. cerevisiae against the H2O2 induced oxidative stress and to optimize the concentration of H2O2 for the subsequent assay. It was determined that 2.5 mM concentration of H2O2 induces high cell death in the mutants while no toxicity was observed in the wild-type strains. The H2O2 produces highly reactive hydroxyl radical and proves to be more toxic against the mutant strains, devoid of the ability to produce defense enzymes. It was observed that 500 µg/mL of the plant extract showed no toxicity towards the growth of both the wild-type and rescued the mutant strains against the oxidative damage caused by H2O2 (Fig. 2). Hence, the determined doses of H2O2 (2.5 mM) and plant extract (500 µg/mL) were used for further assays.
Fig. 2.
In vivo antioxidant activity of ethanolic extract of S. jambos and T. citrina. a Wild-type and antioxidant mutant (sod1Δ, tsa1Δ) of S. cerevisiae grown on YPDA plates; b wild-type and antioxidant mutant (sod1Δ, tsa1Δ) of S. cerevisiae grown on YPDA plates supplemented with stressing agent (H2O2); b S. jambos treated wild-type and antioxidant mutant (sod1Δ, tsa1Δ) of S. cerevisiae grown on YPDA plates supplemented with stressing agent (H2O2); c T. citrina treated wild-type and antioxidant mutant (sod1Δ, tsa1Δ) of S. cerevisiae grown on YPDA plates supplemented with stressing agent (H2O2)
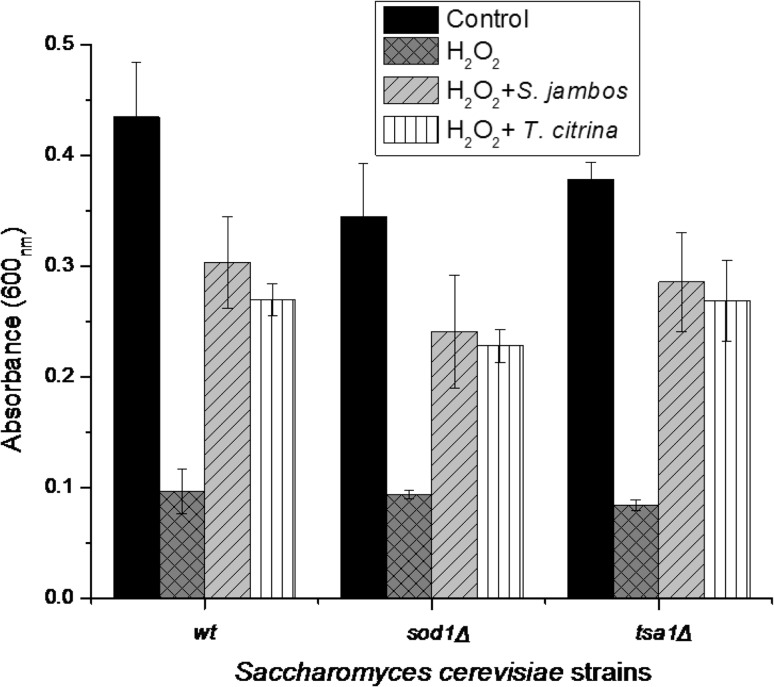
The plant extracts exhibited a significant radical scavenging activity in both sod1Δ and tsa1Δ mutants as evident from the spot assay (Fig. 3). The plant extracts significantly improved the viability of yeast cells under oxidative stress imposed by hydrogen peroxide. The extract treated cells showed a decrease in the susceptibility to oxidative stress and grew normally in presence of the stressing agent (H2O2). On the contrary, the viability of the cells was found to decrease in the untreated cells exposed to H2O2. In an earlier report, a significant increase the survival rates of sod1Δ mutant strains exposed to H2O2 was observed in presence of Lisosan G, powder of the grain of Triticum sativum (Frassinetti et al. 2012).
Fig. 3.
Effect of S. jambos and T. citrina extracts on the cell viability of S. cerevisiae wild-type strain BY4741 and antioxidant mutants (tsa1Δ and sod1Δ), in presence of stressing agent (H2O2, 2.5 mM). Values are represented as mean ± SD (n = 3)
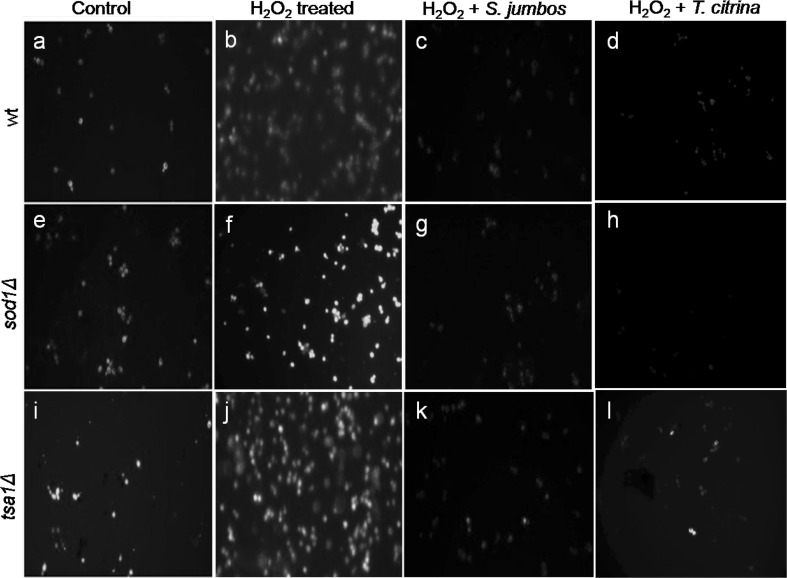
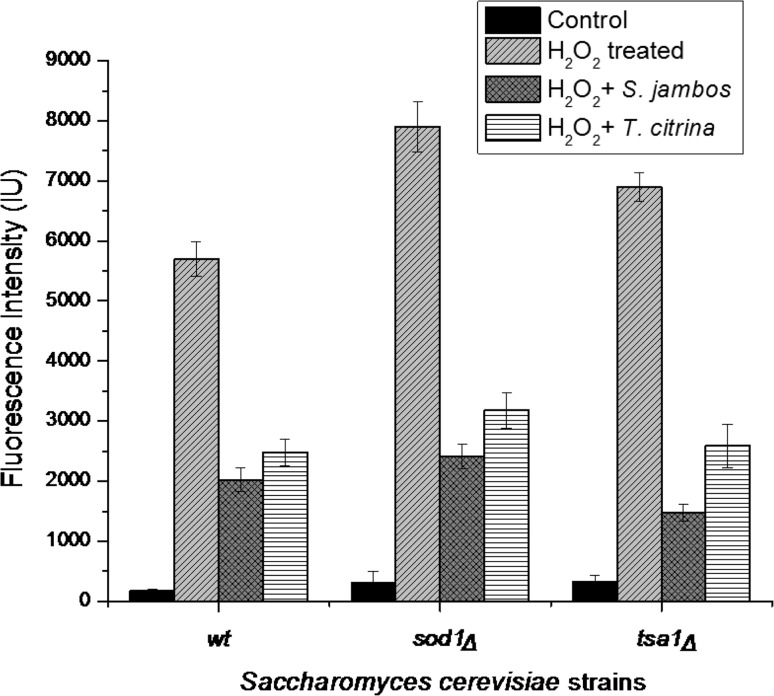
To measure the intracellular ROS generation in the yeast cells, a fluorescence based assay was performed using fluorescent dye, DCF-DA. DCF-DA specifically reacts with H2O2 to produce high fluorescent intensity which corresponds to the intracellular redox level. An increase in fluorescence intensity was observed in cells treated with H2O2 indicating high oxidative stress while a significantly lower fluorescent intensity was observed in both sod1∆ and tsa1∆ mutants on treatment with plant extract (Fig. 4). Frassinetti et al. (2012) also employed DCF-DA to evaluate intracellular ROS in S. cerevisiae. A notable decreased the intracellular ROS level induced by H2O2 in S. cerevisiae D7 strain was observed on treatment with Lisosan G (Frassinetti et al. 2012).
Fig. 4.
Effect of S. jambos and T. citrina extracts on the intracellular ROS generation in S. cerevisiae (wild-type and antioxidant mutants, tsa1Δ and sod1Δ) as observed under fluorescent microscope using DCF-DA. (1st row) Untreated wild-type and antioxidant mutants, tsa1Δ and sod1Δ; (2nd row) wild-type and antioxidant mutants, tsa1Δ and sod1Δ treated with H2O2; (3rd row) wild-type and antioxidant mutants, tsa1Δ and sod1Δ treated with H2O2 and S. jambos; (4th row) wild-type and antioxidant mutants, tsa1Δ and sod1Δ treated with H2O2 and T. citrina
The decrease in fluorescence intensity in wild-type, as well as mutant yeast strains stressed with H2O2 on treatment with the plant extracts, indicated that the plant extracts substantially neutralize the ROS level generated by H2O2 in the mutant strains (Fig. 5). The remarkable H2O2 scavenging potential of the plant extract may be attributed to the antioxidant property of the phytoconstituent present in the plant extract. Similar observations were reported by Dani et al. (2008) using S. cerevisiae antioxidant mutants against oxidative stress caused by H2O2. The antioxidant property of the polyphenols, catechin and resveratrol contributed to the increase in H2O2 tolerance and survival rates of the mutant strains (Dani et al. 2008).
Fig. 5.
Effect of S. jambos and T. citrina extract on the intracellular ROS generation of S. cerevisiae, wild-type strain and antioxidant mutants (tsa1Δ and sod1Δ) showing relatively lesser fluorescence intensity on treatment with the plant extracts, indicating the decrease in oxidative stress. Values are represented as mean ± SD (n = 3)
Hence, the decrease of intracellular ROS and increase in cell viability induced by the plant extracts indicated the to the H2O2 scavenging potential of S. jambos and T.citrina.
Phytochemical analysis
Medicinal plants and their derivatives have played a vital role in the discovery of novel compounds with a wide range of therapeutic properties. Many extracts prepared from plants traditionally used for medicinal applications are known to contain a variety of phytochemicals with antioxidant property. Supplementation of exogenous antioxidants or boosting endogenous antioxidant defenses of the body is a promising way of combating the undesirable effects of oxidative stress (Kasote et al. 2015). The phytoconstituents present in the ethanolic extract of S. jambos and T. citrina were identified using GC–MS analysis (supplementry Fig. 1 and Fig. 2). The GC–MS spectra of the ethanolic leaf extract of S. jambos and T. citrina revealed the presence of terpenes and vitamin E in considerably high amount. Vitamin E (α-tocopherol) is a well known lipid soluble antioxidant with the ability to protect cell membranes from oxidation by reacting with lipid radicals generated during lipid peroxidation chain reaction (Nimse and Pal 2015). The major phytoconstituents present in the plant extracts previously reported for their anti-oxidant property along with their retention time (RT), and concentration (%) are presented in Table 1. As evident in supplementry Fig. 1 and Fig. 2, there was relatively more antioxidant compounds present in S. jambos leaf extracts as compared to that of T. citrina which might be responsible for the differential antioxidant activity observed in this study.
Table 1.
List of phytochemicals present in the ethanolic leaf extract of S. jambos and T. citrina identified from the GC–MS analysis and previously reported for antioxidant activity
| Phyto-compound | Class | Retention time | Peak area (%) | References | |
|---|---|---|---|---|---|
| Phytoconstituents identified from the leaf extract of S. jambos | |||||
| 2-furancarboxaldehyde,5-(hydroxymethyl)- | 5-hydroxymethylfurfural, heterocyclic aldehyde | 9.626 | 0.878 | Zhao et al. (2013) | |
| Tetradecanoic acid, ethyl ester | Myristic acid, fatty acid | 16.619 | 0.758 | Henry et al. (2002) | |
| Phytol | Diterpene alcohol | 19.775 | 1.465 | Costa et al. (2016) | |
| Ethyl 9,12,15-octadecatrienoate | Linolenic acid, fatty acid | 20.306 | 6.680 | Palacios et al. (2003) | |
| Squalene | Triterpene | 25.463 | 0.770 | Gunes (2013) | |
| Alpha-tocopherol | Vitamin E | 27.964 | 0.586 | Yamauchi (1997) | |
| Betulin | Triterpenoid | 31.450 | 2.38 | Peng et al. (2015) | |
| Phytoconstituents identified from the leaf extract of T. citrina | |||||
| 3,7,11,15-tetramethyl-2-hexadecen-1-ol | Phytol, diterpene alcohol | 19.51 | 18.62 | Costa et al. (2016) | |
| Alpha-tocopherol | Vitamin | 27.40 | 10.74 | Yamauchi (1997) | |
The presence of natural antioxidants like phytol, tocopherol and 5-hydroxymethylfurfural in the plant extracts indicated their possible role in providing antioxidant property (Costa et al. 2016; Yamauchi 1997; Zhao et al. 2013). In addition, the presence of fatty acids i.e. myristic acid and linolenic acid and other terpenes like Squalene and Betulin might have also contributed significantly to the antioxidant potential of S. jambos thereby, protecting the antioxidant mutants of S. cerevisiae against oxidative stress (Gunes 2013; Henry et al. 2002; Palacios et al. 2003; Peng et al. 2015). Frassinetti et al. (2012) evaluated the antioxidant activity of Lisosan G in S. cerevisiae D7 and SOD-deficient yeast strains. It was found that the presence of polyphenols, vitamins, and polyunsaturated fatty acids contributed to the antioxidant and antigenotoxic activity of Lisosan G (Frassinetti et al. 2012). The present findings also corroborate well with the reports of Udayprakash et al. (2015) where the methanolic extract of Cinnamomum iners containing fatty acids as the major component along with phenols and polyphenols exhibited significant antioxidant ability (Udayaprakash et al. 2015). Hence, leaf extract of S. jambos and T. citrina might serve as a potential source of exogenous antioxidants to combat the toxicity generated by the free radicals and to maintain a balance between ROS generation and cellular defense mechanism.
Conclusion
The present work revealed significant antioxidant activity of S. jambos and T. citrina against the H2O2 induced oxidative stress in S. cerevisiae mutant strains, deficient in antioxidant defense system. The findings suggested that the plant extracts may be exploited for the development of effective antioxidant to resist oxidative stress and maintain the cellular redox balance. Hence, the plant extract serve as promising alternative to their synthetic antioxidants.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to the Sophisticated Instrumentation Facility, VIT University, Vellore, India for the GCMS studies. The authors extend their appreciation to the Lady Tata Memorial Trust for scholarship.
Compliance with ethical standards
Conflict of interests
The authors declare no conflict of interest in this work.
References
- Akhtar MF, et al. Genotoxic and cytotoxic action potential of Terminalia citrina, a medicinal plant of ethnopharmacological significance. EXCLI J. 2016;15:589–598. doi: 10.17179/excli2016-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa JP, et al. Evaluation of antioxidant activity of phytol using non- and pre-clinical models. Curr Pharm Biotechnol. 2016;17:1278–1284. doi: 10.2174/1389201017666161019155715. [DOI] [PubMed] [Google Scholar]
- Dani C, Bonatto D, Salvador M, Pereira MD, Henriques JA, Eleutherio E. Antioxidant protection of resveratrol and catechin in Saccharomyces cerevisiae. J Agric Food Chem. 2008;56:4268–4272. doi: 10.1021/jf800752s. [DOI] [PubMed] [Google Scholar]
- Frassinetti S, Della Croce CM, Caltavuturo L, Longo V. Antimutagenic and antioxidant activity of Lisosan G in Saccharomyces cerevisiae. Food Chem. 2012;135:2029–2034. doi: 10.1016/j.foodchem.2012.06.090. [DOI] [PubMed] [Google Scholar]
- Golla U, Bhimathati SSR. Evaluation of antioxidant and DNA damage protection activity of the hydroalcoholic extract of Desmostachya bipinnata L. Stapf Sci World J. 2014 doi: 10.1155/2014/215084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunes FE. Medical use of squalene as a natural antioxidant. MÜSBED. 2013;3:220–228. doi: 10.5455/musbed.20131213100404. [DOI] [Google Scholar]
- Henry GE, Momin RA, Nair MG, Dewitt DL. Antioxidant and cyclooxygenase activities of fatty acids found in food. J Agric Food Chem. 2002;50:2231–2234. doi: 10.1021/jf0114381. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Shah MD. A study on the total phenols content and antioxidant activity of essential oil and different solvent extracts of endemic plant Merremia borneensis. Arab J Chem. 2015;8:66–71. doi: 10.1016/j.arabjc.2011.01.007. [DOI] [Google Scholar]
- Husein AI, Ali-Shtayeh MS, Jondi WJ, Zatar NA, Abu-Reidah IM, Jamous RM. In vitro antioxidant and antitumor activities of six selected plants used in the Traditional Arabic Palestinian herbal medicine. Pharm Biol. 2014;52:1249–1255. doi: 10.3109/13880209.2014.886274. [DOI] [PubMed] [Google Scholar]
- Jan S, Khan MR, Rashid U, Bokhari J. Assessment of antioxidant potential, total phenolics and flavonoids of different solvent fractions of Monotheca buxifolia fruit. Osong Public Health Res Perspect. 2013;4:246–254. doi: 10.1016/j.phrp.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasote DM, Katyare SS, Hegde MV, Bae H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int J Biol Sci. 2015;11:982–991. doi: 10.7150/ijbs.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Li W, Jiang ZG, Ghanbari HA. Oxidative stress and neurodegenerative disorders. Int J Mol Sci. 2013;14:24438–24475. doi: 10.3390/ijms141224438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng D, Zhang P, Li SM, Ho CT, Zhao H. Antioxidant activity evaluation of dietary phytochemicals using Saccharomyces cerevisiae as a model. J Funct Foods. 2017;38:36–44. doi: 10.1016/j.jff.2017.08.041. [DOI] [Google Scholar]
- Musthafa KS, Sahu SK, Ravi AV, Kathiresan K. Anti-quorum sensing potential of the mangrove Rhizophora annamalayana. World J Microb Biot. 2013;29:1851–1858. doi: 10.1007/s11274-013-1347-8. [DOI] [PubMed] [Google Scholar]
- Nimse SB, Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. Rsc Adv. 2015;5:27986–28006. doi: 10.1039/c4ra13315c. [DOI] [Google Scholar]
- Oliveira BD, et al. Antioxidant, antimicrobial and anti-quorum sensing activities of Rubus rosaefolius phenolic extract. Ind Crop Prod. 2016;84:59–66. doi: 10.1016/j.indcrop.2016.01.037. [DOI] [Google Scholar]
- Palacios A, Piergiacomi V, Catala A. Antioxidant effect of conjugated linoleic acid and vitamin A during non enzymatic lipid peroxidation of rat liver microsomes and mitochondria. Mol Cell Biochem. 2003;250:107–113. doi: 10.1023/A:1024977613141. [DOI] [PubMed] [Google Scholar]
- Peng J, et al. Betulinic acid downregulates expression of oxidative stress-induced lipoprotein lipase via the PKC/ERK/c-Fos pathway in RAW264.7 macrophages. Biochimie. 2015;119:192–203. doi: 10.1016/j.biochi.2015.10.020. [DOI] [PubMed] [Google Scholar]
- Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complem Altern Med. 2012;12:221. doi: 10.1186/1472-6882-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo S, Ghosh G, Das D, Nayak S. Phytochemical investigation and in vitro antioxidant activity of an indigenous medicinal plant Alpinia nigra B. L. Burtt Asian Pac J Trop Biomed. 2013;3:871–876. doi: 10.1016/S2221-1691(13)60171-9. [DOI] [Google Scholar]
- Sharma R, Kishore N, Hussein A, Lall N. Antibacterial and anti-inflammatory effects of Syzygium jambos L. (Alston) and isolated compounds on acne vulgaris. BMC Complement Altern Med. 2013;13:292. doi: 10.1186/1472-6882-13-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subhaswaraj P, Sowmya M, Jobina R, Sudharshan SJ, Dyavaiah M, Siddhardha B. Determination of antioxidant potential of Acacia nilotica leaf extract in oxidative stress response system of Saccharomyces cerevisiae. J Sci Food Agric. 2017;97:5247–5253. doi: 10.1002/jsfa.8409. [DOI] [PubMed] [Google Scholar]
- Toussaint M, Conconi A. High-throughput and sensitive assay to measure yeast cell growth: a bench protocol for testing genotoxic agents. Nat Protoc. 2006;1:1922–1928. doi: 10.1038/nprot.2006.304. [DOI] [PubMed] [Google Scholar]
- Udayaprakash NK, Ranjithkumar M, Deepa S, Sripriya N, Al-Arfaj AA, Bhuvaneswari S. Antioxidant, free radical scavenging and GC–MS composition of Cinnamomum iners Reinw. ex Blume. Ind Crop Prod. 2015;69:175–179. doi: 10.1016/j.indcrop.2015.02.018. [DOI] [Google Scholar]
- Wong CM, Zhou Y, Ng RWM, Kung HF, Jin DY. Cooperation of yeast peroxiredoxins Tsa1p and Tsa2p in the cellular defense against oxidative and nitrosative stress. J Biol Chem. 2002;277:5385–5394. doi: 10.1074/jbc.M106846200. [DOI] [PubMed] [Google Scholar]
- Yamauchi R. Vitamin E: mechanism of its antioxidant activity. Food Sci Technol Int Tokyo. 1997;3:301–309. doi: 10.3136/fsti9596t9798.3.301. [DOI] [Google Scholar]
- Zhao L, et al. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J Agric Food Chem. 2013;61:10604–10611. doi: 10.1021/jf403098y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.