Abstract
The leaves of seventeen cultivars of olive growing in the north of Iran were investigated for total phenol content and antioxidant activity. The identification and quantification of main phenolic compounds were performed by reverse phase high performance liquid chromatography with diode array detector. The cultivars Kalamon, Gordal, and Coratina contained the highest concentration of phenolic compounds (190.65 ± 0.03, 184.72 ± 0.001, and 155.91 ± 0.06 mg GAE/g extract, respectively). The maximum radical scavenging activities were found in Gordal, Coratina, and Kalamon extracts (IC50 20.66, 22.95, and 26.74 µg ml−1, respectively). The extracts of Mishen, Fishomi, and Arbequina (1971.37 ± 0.007, 1794.57 ± 0.001, and 1760.57 ± 0.005 µmol Fe II/g dried extract, respectively) showed highest antioxidant activity in FRAP assay. The identification analysis demonstrated the present of vanillin, rutin, luteolin 7-O-glucoside, oleuropein, and quercetin. The highest oleuropein concentrations were detected in cultivars Mishen, Beleidi, Kalamon, and Roghani while it was not detected in cultivars Conservolea, Amigdalolia, Leccino, and Fishomi.
Keywords: Olea europaea L, Olive, Antioxidant activity, Total phenol, Oleuropein, HPLC
Introduction
Oxidative stress has been associated with various diseases like atherosclerosis, cancer, and tissue damage in rheumatoid arthritis (McDonald et al. 2001). Overproduction of free radicals in the body can lead to oxidative injuries to biomolecules including lipids, proteins, and DNA, finally causing many chronic diseases (Cai et al. 2004). In addition, the process of lipid peroxidation is an important reason for deterioration of food products during processing and storage (Singh et al. 2002). In this case, the use of antioxidants in food products, particularly in lipids and lipid-containing foods, can increase the shelf life by retarding the process of lipid peroxidation (Singh et al. 2002). As synthetic antioxidants, including butylated hydroxyanisole (BHA), and butylated hydroxytolune (BHT) are doubted to be carcinogenic, their use has been restricted in food industries. Consequently, the search for natural antioxidants, particularly of plant origin has considerably increased in recent years (Singh et al. 2002). Free radical scavenging molecules, such as phenolic compounds (e.g. phenolic acids, flavonoids, quinones, coumarins, lignans, stilbenes, and tannins), nitrogen compounds (alkaloids, amines, and betalains), vitamins, and terpenoids (including carotenoids), are widely found in plant species and acted as potential antioxidant agents (Cai et al. 2004). Plant materials that are rich in antioxidant compounds, especially phenolics, are recommended because of their protective effects against cardiovascular diseases, certain cancers, and many chronic diseases (Benavente-Garcıa et al. 2000).
The olive tree (Olea europaea L., Oleaceae) is widely distributed in the European Mediterranean islands and countries like Spain, Italy, France, Greece, Morrocco, Tunisia, and Turkey (Somova et al. 2003). It is also found in northern Iran, western Asia, and northern Africa (Hashmi et al. 2015). Over the centuries, the extracts of olive leaves have been used for preservation and promotion of health because of medical and nutritional values (Ghanbari et al. 2012). The leaves of this plant have a long history of usage in folk medicine for medical conditions like fevers, and malaria (Benavente-Garcıa et al. 2000). The herbal tea and infusion or extract of olive leaves have been commonly used as a traditional remedy in the Mediterranean region (Romani et al. 2016). Previous studies indicated that the olive leaves extract had hypotensive and hypoglycemic properties (Silva et al. 2006). It has been shown that this extract can lower blood pressure in animal models, alleviate arrhythmia, and exert spasmolytic activity on intestinal muscle (Khemakhem et al. 2017). Also, hypouricaemic, antimicrobial, and antioxidant activities have been reported (Silva et al. 2006). Olive leaves extract has revealed significant antimicrobial and anti-HIV-1 virus activities (Agalias et al. 2005). There are also numerous nutritional supplements and cosmetics containing olive leaves extract (Agalias et al. 2005).
Generally, the fruits, leaves, and oil of olive are considered as a potential natural antioxidant source because of their phenolic contents (Khemakhem et al. 2017). Phenolic compounds, flavonoids, and secoiridoids such as oleuropein, hydroxytyrosol, tyrosol, and elenolic acid are present in leaves and fruits of O. europaea tree (Hashmi et al. 2015).
Among these compounds, the bitter secoiridoid oleuropein is the major constituent of the leaf (Benavente-Garcıa et al. 2000) as well as seed, pulp, and the peel of unripe olives (Barbaro et al. 2014). Studies suggested that oleuropein and its derivatives had significant biological activities including antioxidant, antihypertensive, antiatherogenic, anti-inflammatory, hypoglycemic, hypocholesterolemic, antiproliferative, and antifungal activities (Romani et al. 2016). Oleuropein and hydroxytyrosol are effective in the treatment of numerous diseases, including coronary artery disease, by preventing LDL oxidation, cancer, and osteoporosis. It was demonstrated that Oleuropein is effective against viruses, retroviruses, bacteria, yeasts, fungi, molds, and other parasites (Benavente-Garcıa et al. 2000).
There are about 2500 identified varieties of olives and of which 250 are considered by the International Olive Oil Council as commercial cultivars (Ghanbari et al. 2012). Among these, around 17 different cultivars have been growing in Olive Research Center, Roudbar, Guilan province, north of Iran. These cultivars include: Manzanilla, Conservolea, Arbequina, Mishen, Coratina, Roghani, Kalamon, Amphissis, Yellow, Amigdalifolia, Mary, Leccino, Shenge, Gordal, Sevillenca, Fishomi, and Beleidi.
Until now, there is not any study about the chemical compositions and antioxidant differences between the leaves of these cultivars and, therefore, their possible therapeutic value.
The aim of this study was to investigate the main phenolic compounds present in olive tree leaves obtained from 17 different cultivars of Olea europaea L. growing in the north of Iran, using reverse phase high performance liquid chromatography (HPLC) with diode array detector (PDA). The total phenolic contents were measured by the Folin–Ciocalteu method. Finally, the antioxidant activities of these samples were assessed by DPPH and FRAP methods.
Materials and methods
Reagents and chemicals
All used solvents were HPLC grade and they were obtained from Merck (Germany). Oleuropein, apigenin-7-glucoside, luteolin-7-glucoside, rutin, quercetin, gallic acid, vanillin, catechin, 2,2-Diphenyl-1-picrylhydrazyl (DPPH∙), butylated hydroxyanisole (BHA), and ferric tripyridyl triazine (Fe [III]-TPTZ) were purchased from Sigma-Aldrich chemical Company (United States of America). Folin–Ciocalteu reagent and α-tocopherol were purchased from Merck (Germany).
Plant material and extraction
The leaves of 17 cultivars (Manzanilla, Conservolea, Arbequina, Mishen, Coratina, Roghani, Kalamon, Amphissis, Yellow, Amigdalifolia, Mary, Leccino, Shenge, Gordal, Sevillenca, Fishomi, and Beleidi) were collected from Olive Research Center, Guilan Agricultural and Natural Resources Research and Education Center, Roudbar, Guilan province, Iran, in March 2015. A voucher specimen of each cultivar is deposited in the herbarium of school of pharmacy, Guilan university of medical sciences, Rasht, Iran.
The samples (600 g of each) were dried at room temperature (22–25 °C) and powdered. Then, the leaves powder (200 g) of each cultivar was extracted separately by the percolation method with MeOH/H2O (50/50) three times (Golfakhrabadi et al. 2016). Briefly, the solvent was added to each cultivar and stand for 24 h. Then the lower orifice of percolator was opened and the solvent was collected. This process was repeated for 3 times. The extracts were evaporated by rotary evaporator and stored in refrigerator.
Total phenolic contents
Total phenolic contents were measured by the Folin-Ciocalteu method. Briefly, 1 ml of each extract (in the concentration of 1 mg ml−1) was mixed with 5 ml of Folin-Ciocalteu reagent (previously diluted tenfold with distilled water) and stand at room temperature for 10 min. Then 4 ml sodium bicarbonate solution (75 g l−1) was added. The mixture was allowed to stand for a further 30 min in the dark at room temperature, and absorbance was measured at 765 nm using a UV/VIS spectrophotometer (Lambda 25 PerkinElmer). Total phenolic contents were quantified by calibration curve obtained from measuring the absorbance of four known concentrations of gallic acid (GA) standard (25–50–70–100–200 µg l−1). The concentrations are expressed as milligrams of gallic acid equivalents (GAE) per gram of dry extract (Yousefbeyk et al. 2014).
DPPH radical scavenging activity
The DPPH radical scavenging activity was measured using the method described by Yokozawa et al. (1998). 1 ml of different concentrations of each extract was added to 2 ml DPPH methanol solution and the absorbance was measured at λmax 517 nm after 30 min. The experiment was repeated three times. Vitamin E and butylated hydroxyanisole (BHA) were used as positive controls.
Percentage of radical scavenging activity of the extracts was calculated by using the equation: inhibition% = [(A0 − As)/A0] × 100, that A0 is the absorbance of the control and As is the absorbance of the sample. IC50 values (indicate the concentration of the extract (µg ml−1) providing 50% radical scavenging) were calculated from the graph-plotted scavenging percentage against extract concentration (Golfakhrabadi et al. 2016).
Ferric reducing antioxidant potential (FRAP scavenging) assay
The FRAP assay was measured using the method described by Benzie and Strain (1996). The FRAP reagent contained 5 ml of TPTZ (2,4,6-tripyridyl-s-triazine) solution (10 mmol l−1) in HCl (40 mmol l−1) plus 5 ml of FeCl3 solution (20 mmol l−1) and 50 ml of a 0.3 mol.l−1 acetate buffer solution (pH 3.6) that was prepared freshly and warmed at 37 °C. Aliquots of each extract (50 μl) were mixed with the FRAP reagent (1.5 ml) and incubated at 37 °C for 10 min. The absorbance was measured at 593 nm. The calibration curve was plotted by using five concentrations of FeSO4 .7H2O (125, 250, 500, 750, and 1000 mmol l−1) and the absorbance values were measured by the same method. Vitamin E and butyl hydroxyanisole (BHA) were used as the positive control. The antioxidant activity of each extract was expressed as the concentration of antioxidants with a ferric reducing ability equivalent to that of 1 mmol l−1 FeSO4 (Golfakhrabadi et al. 2015; Yousefbeyk et al. 2014).
HPLC analysis for identification of phenolic compounds
All the extracts were dissolved in HPLC grade methanol and distilled water (1:1) (10 mg ml−1). The solutions were filtered through a 0.45 mm nylon membrane. The phenolic compounds analysis was performed using a Waters Aliance e2695 HPLC apparatus equipped with the Waters 2998 photodiode array (PDA) detector, C18 reversed-phase column (Waters spherisorb, S5 ODS2, 4 × 250 mm, 5 µm), and a security guard (Waters spherisorb, S5 ODS2 4.6 × 10 mm). The absorbance changes were monitored at 210 nm to 400 nm. Data acquisition and quantitation were performed with Empower 3 software (Waters, USA).
The mobile phases for chromatographic analysis were: (A): acetic acid/water (2.5:97.5) and (B): acetonitrile. A linear gradient was used, starting from 95% (A) and 5% (B) to 75% (A) and 25% (B) during 20 min; in 20 min it altered to 50%(A) and (B) (40 min, total time); then it changed to 20% (A) and 80% (B) in 10 min (50 min, total time), and finally it changed to 100% B in 20 min (70 min, total time) (Benavente-Garcıa et al. 2000). The flow rate was 1 ml min−1 and equilibration time was 15 min. The oven temperature was 30 °C. The injection volume of all samples was 20 µl. UV/Vis spectra were recorded in the range 210–400 nm.
Chromatographic peaks were identified by comparing the retention times (Rt) of samples with the corresponding standards. Moreover, their UV spectra obtained from the PDA detector (from 210 to 400) were compared with UV spectra of authentic standards. For quantification, four different concentration levels (0.25, 0.5, 1, and 2 mg ml−1) of external standards, including luteolin 7-O-glucoside, quercetin, oleuropein, vanillin, gallic acid, rutin, and catechin were used for plotting calibration curves. Each concentration was measured in triplicate. The peak areas of samples were correlated with the concentrations according to the calibration curve.
Statistics
Data were expressed as means ± standard errors of three replicate determinations. The results were statistically analyzed using SPSS 22.0 for Windows. The Pearson correlation analysis was done between total phenolic contents and antioxidant activities.
Results and discussion
Total phenol content and antioxidant activity
The total phenol contents in the leaves of all cultivars were measured by the Folin- Ciocalteu method. The results are presented in Table 1. The assay indicated wide variation from 42.35 ± 0.002 mg GAE/g extract (for Arbequina extract) to 190.65 ± 0.03 mg GAE/g extract (for Kalamon extract). The cultivars Kalamon, Gordal, and Coratina contain the highest concentration of total phenolic compounds (190.65 ± 0.03, 184.72 ± 0.001, and 155.91 ± 0.06 mg GAE/g extract, respectively).
Table 1.
Total phenol contents and antioxidant activities of different cultivars of Olive leaves extracts
| Cultivars | Total phenol (mg GAE/g dry extract) | FRAP (µmol Fe II/g dried extract) | DPPH (IC50: µg/ml) | |
|---|---|---|---|---|
| 1 | Manzanilla | 134.5 ± 0.013 | 1107.71 ± 0.007 | 33.93 |
| 2 | Conservolea | 92.35 ± 0.001 | 1277.33 ± 0.009 | 62.94 |
| 3 | Arbequina | 42.35 ± 0.002 | 1760.57 ± 0.005 | 62.56 |
| 4 | Mishen | 71.93 ± 0.01 | 1971.37 ± 0.007 | 63.48 |
| 5 | Coratina | 155.91 ± 0.06 | 358.66 ± 0.004 | 22.95 |
| 6 | Roghani | 121.755 ± 0.017 | 1400.76 ± 0.003 | 29.58 |
| 7 | Kalamon | 190.65 ± 0.03 | 532.76 ± 0.004 | 26.74 |
| 8 | Amphissis | 50.7 ± 0.001 | 1110.38 ± 0.003 | 95.39 |
| 9 | Yellow | 73.85 ± 0.014 | 1400.95 ± 0.004 | 53.8 |
| 10 | Amigdalolia | 42.73 ± 0.002 | 1341.05 ± 0.006 | 74.3 |
| 11 | Mary | 62.24 ± 0.012 | 1203.81 ± 0.001 | 60.26 |
| 12 | Leccino | 59.23 ± 0.005 | 568.280 ± 0.002 | 69.3 |
| 13 | Shenge | 61.97 ± 0.006 | 614.19 ± 0.004 | 60.18 |
| 14 | Gordal | 184.72 ± 0.001 | 450.86 ± 0.002 | 20.66 |
| 15 | Sevillenca | 83.63 ± 0.002 | 432.19 ± 0.003 | 34.92 |
| 16 | Fishomi | 109.98 ± 0.063 | 1794.57 ± 0.001 | 32.82 |
| 17 | Beleidi | 113.18 ± 0.025 | 525.43 ± 0.003 | 33.6 |
| 18 | Vitamin E | – | 313 ± 0.01 | 14.12 |
| 19 | BHA | – | 880 ± 0.06 | 7.8 |
Results are expressed as mean ± standard deviation of 3 determinations
The antioxidant activities of all cultivars were tested by the DPPH and FRAP methods (Table 1). The results of DPPH assay varied from IC50 20.66 µg m−1 for Gordal extract to 95.39 µg m−1 for Amphissis extract. Also, the highest radical scavenging activities were found in Gordal, Coratina, and Kalamon extracts (IC50 20.66, 22.95, and 26.74 µg m−1, respectively).
The FRAP ranged from 358.66 ± 0.004 (µmol Fe II/g dried extract) in the Coratona extract to 1971.37 ± 0.007 (µmol Fe II/g dried extract) in Mishen extract. Moreover, the extracts of Mishen, Fishomi, and Arbequina (1971.37 ± 0.007, 1794.57 ± 0.001, and 1760.57 ± 0.005 µmol Fe II/g dried extract, respectively) showed highest antioxidant activities.
The Pearson’s correlation coefficients between phenolic contents and IC50 values in the DPPH assay were performed. The results showed a strong negative significant correlation between DPPH radical scavenging activities (IC50 values) and contents of phenolic (-0.843, p < 0.05). Moreover, this correlation coefficient between phenolic contents and FRAP assay was low (-0.509, p < 0.05). It showed that the DPPH radical scavenging activities of olive leaves from different cultivars were in accordance with their amount of total phenolic contents.
So far, antioxidant activity of O. europaea leaves extract growing in different countries and its constituents have been assessed in several studies by using various methods (Hashmi et al. 2015). The investigation of antioxidant activity of olive leaf extract from plants growing in Ireland showed the IC50 of 34.58 µg m−1 in the DPPH radical scavenging assay (Hayes et al. 2011). This result is in the range of IC50 reported in the present study and is comparable with cultivar Sevillana extract (IC50 34.92 µg m−1). It has been demonstrated that olive tree (oil, fruits, and leaves) are one of the plant species with potent antioxidant activity (Jemai et al. 2009). Many phenolic antioxidant molecules have been recognized in the olive tree. For example, the nitrite-scavenging abilities of oleuropein, rutin, vanillin, caffeic acid, and their mixture at 500 μM were reported as 72.7, 47.8, 6.3, 92.2, and 66.6%, respectively (Lee and Lee 2010). It was suggested that the phenolic compounds of olive leaves can defend against injury from nitrite and nitrosamine related cancer (Lee and Lee 2010). Also, caffeic acid had the highest DPPH radical scavenging activity (85.7%), followed by rutin (57.0%) and oleuropein (55.0%) (Lee and Lee 2010).
Furthermore, total phenolic content of olive leaf extract was estimated as 1.6 GAE/g dry weight of olive growing in Ireland. Also the major polyphenolic compounds present in olive leaf extract were reported as: oleuropein, verbascoside, luteolin-7-O-glucoside, and apigenin-7-O-glucoside (Hayes et al. 2011).
It has been reported that phenolic compounds can exert antioxidant activity by a number of potential pathways. The principal is likely through free radical scavenging in which the phenolic molecules can break the free radical chain reaction (Lee and Lee 2010).
Identification and quantitative analysis of phenolic compounds present in olive leaves
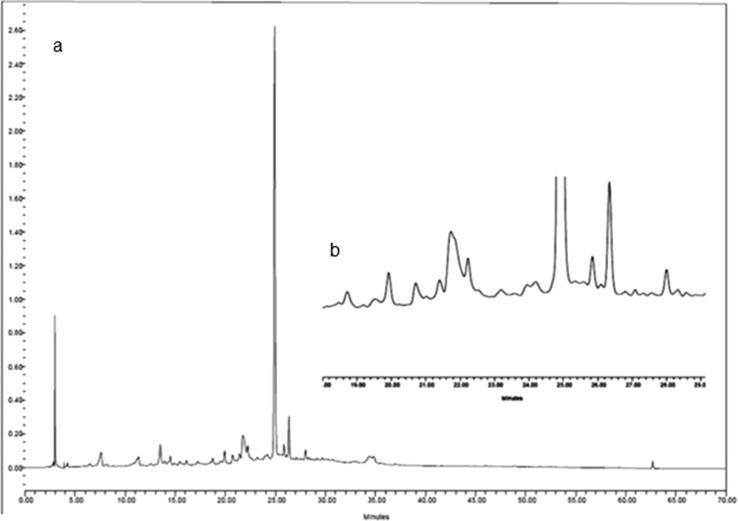
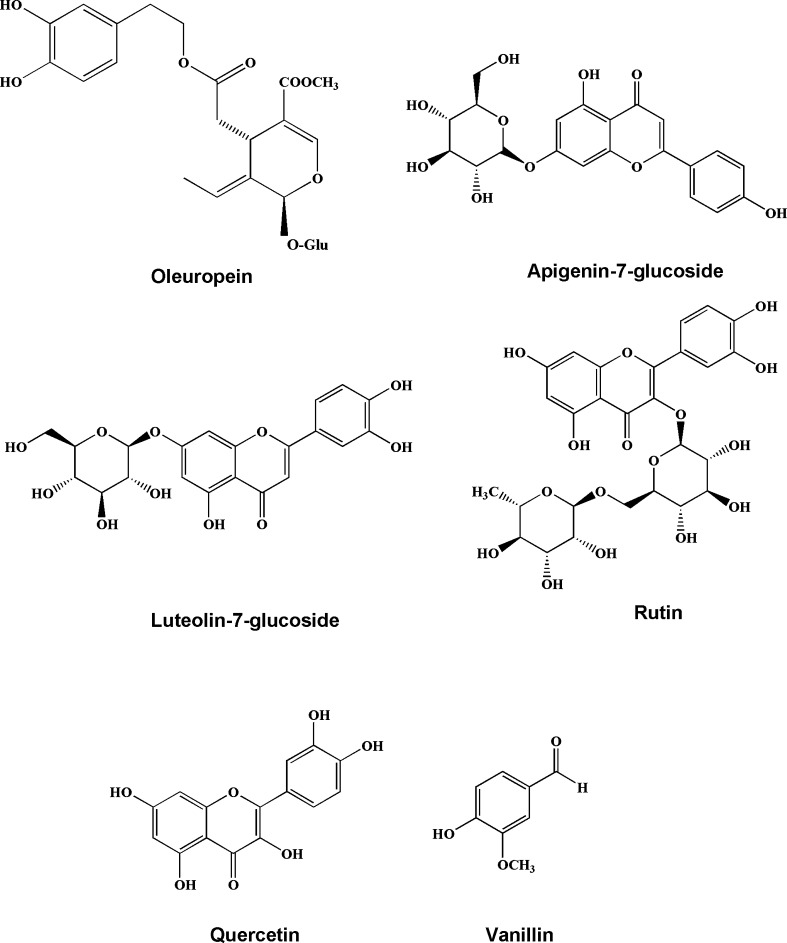
All the extracts were further studied by the HPLC to investigate the presence of phenolic compounds. The chromatographic profile of phenolic compounds in the extract of Beleidi is shown as the example in Fig. 1. The chromatogram of extracts was compared to authentic standards and 5 phenolic compounds, including vanillin, rutin, luteolin 7-O-glucoside, oleuropein, and quercetin were identified. Gallic acid and catechin were not detected in the leaves extracts of all studied cultivars. The structures of these compounds are shown in Fig. 2.
Fig. 1.
HPLC chromatogram of the extract from leaves of O. europea ‘beleidi’ cultivar (a) and detail of 18–30 min retention time (b): 1: vanillin (Rt = 18.8 min), 2: rutin (Rt = 21.05 min), 3: luteolin 7-O-glucoside (Rt = 22.14 min), 4: oleuropein (Rt = 24.9 min) and 5: quercetin (Rt = 29.9 min). Detection was at 254 nm
Fig. 2.
Chemical structures of identified compounds in O. europaea leaves extracts
The quantity of phenolic compounds varied from 0.006 to 0.85% (percent (w/w) of dry extract) for vanillin, 0.05 to 0.19% for rutin, 0.62 to 2.97% for luteolin 7-O-glucoside, 0 to 11.12% for oleuropein, and 1.47% to 4.07 for quercetin (Table 2). The Regression equation for each standard is shown in Table 3. The most abundant compound in the most cultivars was oleuropein while it was not detected in Conservolea, Amigdalolia, Leccino, and Fishomi. The highest oleuropein concentrations were observed in Mishen and Beleidi (11.12%), Kalamon (10.7%) and Roghani (10.07%). Moreover, the cultivars Sevillenca and Kalamon had the highest amount of quercetin (4.07% and 2.47%, respectively). Shenge, kalamon, and Leccino showed the highest content of luteolin 7-O-glucoside (2.97, 2.43, and 2.21%, respectively).
Table 2.
Retention time and abundance of the main phenolic compounds present in olive leaves extracts of 17 different cultivars (expressed in percent (w/w) of dry extract)
| No | Cultivars | gallic acid | vanillin | rutin | luteolin 7-O-glucoside | catechin | oleuropein | quercetin |
|---|---|---|---|---|---|---|---|---|
| 1 | Manzanilla | –* | – | 0.18 | 0.92 | – | 6.15 | 1.84 |
| 2 | Conservolea | – | 0.03 | 0.12 | 0.65 | – | – | 1.47 |
| 3 | Arbequina | – | – | 0.12 | 2.08 | – | 0.58 | 1.47 |
| 4 | Mishen | – | 0.006 | 0.11 | 0.62 | – | 11.12 | 1.85 |
| 5 | Coratina | – | 0.05 | 0.12 | 1.12 | – | 0.51 | 1.84 |
| 6 | Roghani | – | 0.32 | 0.13 | 0.715 | – | 10.07 | 1.45 |
| 7 | Kalamon | – | – | 0.08 | 2.43 | – | 10.7 | 2.47 |
| 8 | Amphissis | – | 0.02 | 0.08 | 0.68 | – | 2.44 | 1.51 |
| 9 | Yellow | – | 0.65 | 0.19 | 2 | – | 7.13 | 1.48 |
| 10 | Amigdalolia | – | – | 0.097 | 0.57 | – | – | 1.47 |
| 11 | Mary | – | – | 0.13 | 2.04 | – | 0.94 | 1.48 |
| 12 | Leccino | – | 0.02 | 0.13 | 2.21 | – | – | 1.47 |
| 13 | Shenge | – | 0.18 | 0.19 | 2.97 | – | 9.92 | 1.48 |
| 14 | Gordal | – | 0.85 | 0.17 | 0.86 | – | 7.20 | 1.47 |
| 15 | Sevillenca | – | 0.13 | 0.07 | 0.898 | – | 8.7 | 4.07 |
| 16 | Fishomi | – | 0.03 | 0.17 | 0.87 | – | – | 1.48 |
| 17 | Beleidi | – | 0.58 | 0.05 | 1.84 | – | 11.12 | 1.48 |
*– not detected
Table 3.
The Regression equations, LODs and LOQs of standard compounds
| Compound | Regression equation | Correlation coefficient (R2) | LOD (mg ml−1) | LOQ (mg ml−1) | |
|---|---|---|---|---|---|
| 1 | gallic acid | Y = 1.65 × 103X + 1.12 × 106 | 0.9999 | 0.106 | 0.323 |
| 2 | catechin | Y = 1.34 × 105X + 3.05 × 104 | 0.9980 | 0.096 | 0.293 |
| 3 | vanillin | Y = 7.31 × 105X + 1.34 × 105 | 0.999 | 0.064 | 0.195 |
| 4 | rutin | Y = 1.42 × 106X-2.65 × 105 | 0.9997 | 0.062 | 0.188 |
| 5 | luteolin7-O-glucoside | Y = 7.98 × 106X-2.55 × 105 | 0.9998 | 0.047 | 0.143 |
| 6 | oleuropein | Y = 4.86 × 105X + 1.26 × 106 | 0.9966 | 0.049 | 0.151 |
| 7 | quercetin | Y = 3.18 × 107X-1.87 × 106 | 0.9997 | 0.056 | 0.169 |
LOD limit of detection, LOQ limit of quantification
In a study, the main phenolic compounds present in O. europaea leaves of five cultivars, Villalonga, Alfafarenca, Picual, Cornicabra, and Blanqueta, from the regions in the south of Spain were investigated. The most abundant compounds were oleuropein (24%), hydroxytyrosol (1.46%), luteolin-7-glucoside (1.38%), and apigenin-7-glucoside (1.37%). In addition, verbascoside, diosmetin-7-glucoside, luteolin, diosmetin, rutin, and catechin were detected (Benavente-Garcıa et al. 2000).
In another study, the decoctions of O. europaea leaves from ten Greek cultivated varieties were investigated. According to the results, the decoction of variety Gaidouroelia had the highest amount of oleuropein (121.934 mg/50 g leaves) (Agalias et al. 2005).
Moreover, 11 French varieties (Aglandau, Cailletier, Cayet Rouge, Cayon, Grossanne, Lucques, Picholine, Picholine Noire, Tanche, Verdale de l’He´rault, and Verdale-Picholine hybrid) and 3 commonly cultivated varieties in Tunisia were evaluated in another investigation (Savournin et al. 2001). The three Tunisian varieties had concentrations of 12 ± 2% (percent (w/w) of powdered leaves) of oleuropein. Almost all of the French varieties had concentrations less than 12% of this compound with the exception of Cailletier, Lucques, Tanche, and Verdale-Picholine hybrid. Aglandau had the lowest content (9.04–9.62%) and Lucques the highest content (12.56–14.16% percent (w/w) of powdered leaves) of oleuropein (Savournin et al. 2001). The concentration of oleuropein was 2.44 g/100 g dry weight in the leaves extract of trees growing in Tunisia (Somova et al. 2003). It is apparent that there is an important variation for all the compounds among the 17 studied cultivars from Iran and other cultivars in other parts of the world.
Conclusion
The chemical composition and antioxidant activity of the leaves extracts from 17 olive cultivars growing in the north of Iran was evaluated. The highest contents of phenolics and DPPH radical scavenging activities were detected in Kalamon, Gordal, and Coratina. Consequently, the leaves of these cultivars can be considered as the subject of further studies to use as sources of natural antioxidants in food products. Also, the highest amount of secoiridoid oleuropein was determined in Mishen, Beleidi, Kalamon, and Roghani. In contrast, this compound was absent in Conservolea, Amigdalolia, Leccino, and Fishomi.
Acknowledgements
This work was supported by Guilan University of Medical Sciences.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
References
- Agalias A, Melliou E, Magiatis P, Mitaku S, Gikas E, Tsarbopoulos A. Quantitation of oleuropein and related metabolites in decoctions of Olea europaea leaves from ten Greek cultivated varieties by HPLC with diode array detection (HPLC-DAD) J Liq Chromatogr Relat Technol. 2005;28(10):1557–1571. doi: 10.1081/JLC-200058355. [DOI] [Google Scholar]
- Barbaro B, Toietta G, Maggio R, Arciello M, Tarocchi M, Galli A, et al. Effects of the olive-derived polyphenol oleuropein on human health. Int J Mol Sci. 2014;15(10):18508–18524. doi: 10.3390/ijms151018508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavente-Garcıa O, Castillo J, Lorente J, Ortuno A, Del Rio J. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000;68(4):457–462. doi: 10.1016/S0308-8146(99)00221-6. [DOI] [Google Scholar]
- Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74(17):2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanbari R, Anwar F, Alkharfy KM, Gilani A-H, Saari N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)—a review. Int J Mol Sci. 2012;13(3):3291–3340. doi: 10.3390/ijms13033291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golfakhrabadi F, Yousefbeyk F, Mirnezami T, Laghaei P, Hajimahmoodi M, Khanavi M. Antioxidant and antiacetylcholinesterase activity of Teucrium hyrcanicum. Pharmacogn Res. 2015;7(Suppl 1):S15. doi: 10.4103/0974-8490.157993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golfakhrabadi F, Ardekani MRS, Saeidnia S, Yousefbeyk F, Jamalifar H, Ramezani N, et al. Phytochemical analysis, antimicrobial, antioxidant activities and total phenols of Ferulago carduchorum in two vegetative stages (flower and fruit) Pak J Pharm Sci. 2016;29(2):623–628. [PubMed] [Google Scholar]
- Hashmi MA, Khan A, Hanif M, Farooq U, Perveen S. Traditional uses, phytochemistry, and pharmacology of Olea europaea (olive) Evid Based Complement Alternat Med. 2015 doi: 10.1155/2015/541591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J, Allen P, Brunton N, O’grady M, Kerry J. Phenolic composition and in vitro antioxidant capacity of four commercial phytochemical products: olive leaf extract (Olea europaea L.), lutein, sesamol and ellagic acid. Food Chem. 2011;126(3):948–955. doi: 10.1016/j.foodchem.2010.11.092. [DOI] [Google Scholar]
- Jemai H, El Feki A, Sayadi S. Antidiabetic and antioxidant effects of hydroxytyrosol and oleuropein from olive leaves in alloxan-diabetic rats. J Agric Food Chem. 2009;57(19):8798–8804. doi: 10.1021/jf901280r. [DOI] [PubMed] [Google Scholar]
- Khemakhem I, Gargouri OD, Dhouib A, Ayadi MA, Bouaziz M. Oleuropein rich extract from olive leaves by combining microfiltration, ultrafiltration and nanofiltration. Sep Purif Technol. 2017;172:310–317. doi: 10.1016/j.seppur.2016.08.003. [DOI] [Google Scholar]
- Lee O-H, Lee B-Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour Technol. 2010;101(10):3751–3754. doi: 10.1016/j.biortech.2009.12.052. [DOI] [PubMed] [Google Scholar]
- McDonald S, Prenzler PD, Antolovich M, Robards K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001;73(1):73–84. doi: 10.1016/S0308-8146(00)00288-0. [DOI] [Google Scholar]
- Romani A, Mulas S, Heimler D. Polyphenols and secoiridoids in raw material (Olea europaea L. leaves) and commercial food supplements. Eur Food Res Technol. 2016 doi: 10.1007/s00217-016-2756-3. [DOI] [Google Scholar]
- Savournin C, Baghdikian B, Elias R, Dargouth-Kesraoui F, Boukef K, Balansard G. Rapid high-performance liquid chromatography analysis for the quantitative determination of oleuropein in Olea europaea leaves. J Agric Food Chem. 2001;49(2):618–621. doi: 10.1021/jf000596+. [DOI] [PubMed] [Google Scholar]
- Silva S, Gomes L, Leitao F, Coelho A, Boas LV. Phenolic compounds and antioxidant activity of Olea europaea L. fruits and leaves. Food Sci Technol Int. 2006;12(5):385–395. doi: 10.1177/1082013206070166. [DOI] [Google Scholar]
- Singh R, Chidambara Murthy K, Jayaprakasha G. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J Agric Food Chem. 2002;50(1):81–86. doi: 10.1021/jf010865b. [DOI] [PubMed] [Google Scholar]
- Somova L, Shode F, Ramnanan P, Nadar A. Antihypertensive, antiatherosclerotic and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies africana leaves. J Ethnopharmacol. 2003;84(2):299–305. doi: 10.1016/S0378-8741(02)00332-X. [DOI] [PubMed] [Google Scholar]
- Yokozawa T, Chen CP, Dong E, Tanaka T, Nonaka G-I, Nishioka I. Study on the inhibitory effect of tannins and flavonoids against the 1, 1-diphenyl-2-picrylhydrazyl radical. Biochem Pharmacol. 1998;56(2):213–222. doi: 10.1016/S0006-2952(98)00128-2. [DOI] [PubMed] [Google Scholar]
- Yousefbeyk F, Gohari AR, Hashemighahderijani Z, Ostad SN, Sourmaghi MHS, Amini M, et al. Bioactive terpenoids and flavonoids from Daucus littoralis Smith subsp. hyrcanicus Rech. f, an endemic species of Iran. DARU. J Pharm Sci. 2014;22(1):1. doi: 10.1186/2008-2231-22-12. [DOI] [PMC free article] [PubMed] [Google Scholar]