Abstract
Water-soluble polysaccharides were isolated from Colpomenia peregrina to determine their chemical characteristics and immunomodulatory properties. High extraction yields were obtained for CP1 (17.6%) and CP2 (5.2%) polysaccharides. Polysaccharides were mainly consisted of neutral sugars (67.01–73.79%), uronic acids (9.43–14.89%), proteins (3.44–14.89%) and small amounts of sulfates (4.87–4.91%). Polysaccharides were composed of fucose (20.62–24.56%), galactose (25.5–26.94%) and glucose (50.00–52.91%) residues. The average molecular weights of the CP1 and CP2 polysaccharides were 1890 × 103 g/mol and 639 × 103 g/mol, respectively. The polysaccharides exerted a relatively low cytotoxicity against HeLa cancer cells (< 40%). The CP1 and CP2 polysaccharides were nontoxic and induced RAW264.7 murine macrophage cells to release considerable amounts of nitric oxide (NO). Inflammatory cytokines including IL-1β, TNF-α, IL-6, IL-10 and IL-12 from were secreted from RAW264.7 cells induced with CP1 polysaccharides. As the most immunostimulating fraction, CP1 polysaccharides were homogeneous and formed of 1,3-linked galactose, 1,4-linked glucose and 1,3-linked fucose residues. Overall, these findings suggested that the polysaccharides isolated from C. peregrina can be utilized as potential natural immunostimulant in functional foods or pharmaceutical industries.
Keywords: Colpomenia peregrina, Polysaccharide, Molecular weight, Structure, Immunostimulating activity
Introduction
Fucoidan is a general name used for sulfated and fucose containing polysaccharides isolated from the cell wall of brown seaweeds. As it appears from the definition given above, these polysaccharides are consisted of different chemical and molecular structures depending on seaweed species (Yuguchi et al. 2016). However, growing conditions of the seaweeds and the method of choice employed to isolate the fucoidans add more complexity and diversity to their structures (Saravana et al. 2016).
Some of the well-established chemical structures of fucoidans which have been found in brown seaweeds include fucan sulfate with a (1→3)-α-Fucp backbone, fucogalactan with a (1→6)-β-Manp and (1→2)-β-Manp backbone, fucoglucuronomannan with a (1→2)-α-Manp, (1→4)-β-GlupA and α-Fucp backbone, and fucoglucuronan with a (1→3)-β-GlupA or (1→4)-β-GlupA and α-Fucp backbone (Duarte et al. 2001; Li et al. 2006; Bilan et al. 2010; Shevchenko et al. 2015).
Sulfates, uronic acids, proteins and monosaccharides as well as molecular weights and types of glycosidic linkages are the structural features of fucoidans varying among different species (Wu et al. 2016; Li et al. 2008). Any variations in the chemical structures of fucoidans have determinant effects on their bioactivities either through enhancing the activity or compromising the function (Li et al. 2008). This was evidently shown by an investigation conducted on the bioactivities of fucoidans from nine brown seaweeds including Laminaria, Cladosiphon, Fucus and Ascophyllum genera (Cumashi et al. 2007). The study reported various levels of fucose (23.0–58.7%), uronic acids (1.0–23.4%) and sulfates (15.1–36.3%) for the isolated fucoidans and then concluded that the discrepancy in the chemical structure is responsible for their different anti-inflammatory and anticoagulant properties (Cumashi et al. 2007). Although, the chemical complexity of fucoidans has made their structural elucidation rather a tedious and laborious task, it resulted in a wide spectrum of therapeutic activities such as anticancer, immunomodulatory, antithrombotic and anti-inflammatory (Cumashi et al. 2007; Shao et al. 2015). In spite of numerous researches conducted on the structural characterization and biological properties of fucoidans from brown seaweeds, there are many more species remaining to be explored for their bioactive polysaccharides with novel structural specifications and health promoting effects.
Colpomenia peregrina is one of the brown seaweeds widely distributed along the coastal lines of Caspian Sea in North and Persian Gulf and Oman Sea in South of Iran. Despite the abundance and easy access, seaweeds have not been used as food materials in Iran due to strong reliance on land vegetables. Hence, it seems exploring new bioactive compounds from available species and introducing seaweed-based functional foods and nutraceuticals could promote seaweed consumption. Therefore, in the present study, water-soluble polysaccharides were isolated from C. peregrina to evaluate their potential utilization as functional foods with immunostimulatory properties. To that end, chemical compositions, monosaccharide components, molecular weights and glycosidic linkages were determined for the isolated polysaccharides. In vitro anticancer activity in association with immunomodulatory properties of the polysaccharides were evaluated on both cellular and molecular levels.
Materials and methods
Materials
Colpomenia peregrina samples were randomly collected from the coast of Caspian Sea, Noor, Iran in January 2016. Sample species was identified in the College of Marine Sciences at Tarbiat Modares University through morphological studies. The raw material was washed with tap water and air dried at 60 °C. The dried seaweed was milled using a blender, sieved (< 0.5 mm) and kept in plastic bags at − 20 °C. All other chemicals and reagents were of analytical grade. RPMI-1640 medium and fetal bovine serum (FBS) used in cell culture were purchased from Lonza (Walkersville, MD, USA). Alcalase was purchased from Sigma-Aldrich (USA). The following antibodies (Abs) were used in this study: anti-phospho-NF-κB rabbit polyclonal, anti-phospho-JNK rabbit polyclonal, anti-phospho-pERK1/2 rabbit polyclonal, anti-phospho-p38 rabbit polyclonal (Cell Signaling Technology) and anti-β-actin mAb (Sigma).
Isolation and fractionation of the polysaccharides
Seaweed powder (20 g) was treated with ethanol (80% EtOH, 200 mL) under constant stirring overnight at room temperature to remove pigments, lipids and low molecular weight compounds. The supernatants were discarded after centrifugation at 10 °C and 6080×g for 10 min. The sediment was rewashed with EtOH (99%) under the same conditions, rinsed with acetone (99%), centrifuged at 10 °C and 6080×g for 10 min and dried at room temperature in a fume hood. Polysaccharide extraction was carried out from 20 g of depigmented material with alcalase, Sigma (5% W:W, pH 8.0, 50 °C, 24 h). Enzymes were inactivated by keeping the extraction mixture at 100 °C for 10 min. The supernatants were collected after centrifugation at 10 °C and 6080×g for 10 min. The extraction was carried out twice and the supernatants were combined and concentrated by evaporation under reduced pressure at 60 °C. Then, 1% CaCl2 was added into the supernatants and kept at 4 °C overnight. The precipitated alginates were discarded after centrifugation. The polysaccharide precipitation was conducted in two steps using EtOH (99%) to obtain a final EtOH concentration of 30% (CP1) and 70% (CP2). The mixture was kept at 4 °C overnight and the precipitate was obtained after centrifugation at 10 °C and 6080×g for 10 min. Polysaccharides were washed and dehydrated with EtOH (99%), acetone (99%), and then dried at room temperature. Polysaccharides were dissolved in water and dialyzed against distilled water using filter membrane (3500 Da MWCO) for 3 days after which solutions freeze dried to obtain polysaccharide powder (Borazjani et al. 2017). The yields of isolated polysaccharides were calculated in relation to the depigmented material obtained after 80% EtOH treatment.
Purification of polysaccharides
The CP1 polysaccharides (250 mg) were dissolved in distilled water (10 mL) and fractionated on DEAE Sepharose fast flow column (17-0709-01; GE Healthcare Bio-Science AB, Uppsala, Sweden) using water and different concentrations of NaCl (from 0.5 to 2 M) as eluents. Only a single fraction was obtained after ion exchange chromatography. Therefore, CP1 polysaccharides were eluted on a Sepharose CL-2B column (65099-79-8; Sigma) using water. To prepare the sample, 250 mg of CP1 polysaccharides were dissolved in distilled water (10 mL) at 65 °C for 15 min and filtered using a 3.0 µm filter. The elution of CP1 polysaccharides also resulted in one fraction.
Chemical compositions
Lowry method was employed to measure the amount of protein using a DC protein assay kit (Bio-Rad, CA, USA) (Lowry et al. 1951). The determination of neutral sugar amount of the isolated polysaccharides was carried out using the phenol–sulfuric acid method and d-glucose as a standard (Dubois et al. 1956). After hydrolysis of polysaccharides (3 mg) with 0.5 M HCl and addition of BaCl2 containing gelatin, the amount of sulfate was evaluated using K2SO4 as a standard (Dodgson and Price 1962). The amount of uronic acid was determined by sulfamate/m-hydroxydiphenyl method and glucuronic acid was used as a standard (Filisetti-Cozzi and Carpita 1991).
Monosaccharide composition of polysaccharides
The monosaccharide composition of polysaccharides (3 mg) extracted from C. peregrina were determined after hydrolysis with 4 M trifluoroacetic acid at 100 °C for 6 h. This was followed by reduction reaction using NaBD4 (5 mg) at room temperature. Then, acetylation process was carried out by acetic anhydride (0.5 mL) at 100 °C (Ciucanu and Kerek 1984). Finally, a gas chromatography-mass spectrometry (6890N/MSD5973, Agilent Technologies, Santa Clara, CA) equipped with HP-5MS capillary column (30 m × 0.25 mm × 0.25 µm) (Agilent Technologies, Santa Clara, CA) was used to analyze the derivatives.
Glycosidic linkage analysis
The methylation analysis of polysaccharides were carried out based on Ciucanu and Kerek method (1984). Briefly, 0.5 mL of DMSO (dimethyl sulfoxide) was added to lyophilized samples (3 mg) under nitrogen, followed by NaOH (20 mg) addition. Methyl iodide (CH3I, 0.5 mL) was added after 15 min and the reaction proceeded for 45 min. The methylated derivatives were hydrolyzed by 0.5 mL of 4 M TFA at 100 °C for 6 h. The hydrolyzed derivatives were reduced by NaBD4 (5 mg) and acetylated with 0.5 mL of acetic anhydride at 100 °C. A gas chromatography mass spectrometry (GC–MS) system (6890N/MSD 5973, Agilent Technologies, Santa Clara, CA) equipped with HP-5MS capillary column (30 m × 0.25 mm × 0.25 µm) (Agilent Technologies, Santa Clara, CA) was used to identify the partially methylated alditol acetate derivatives. The carrier gas was Helium which was used as at a constant flow rate of 1.2 mL/min. The oven temperature was set as follows: from 160 to 210 °C for 10 min and then to 240 °C for 10 min. The temperature gradient was 5 °C/min and the inlet temperature was kept constant at 250 °C. The mass range was set to measure between 35 and 450 m/z (Tabarsa et al. 2017).
Molecular properties
Polysaccharides were solubilized in distilled water (2 mg/mL) and heated for 30 s in a microwave bomb (#4872; Parr Instrument Co., Moline, IL, USA) for complete solubilization (Rahimi et al. 2016). Prior to injection, samples were filtered through a 3 µm cellulose acetate filter unit (ADVANTEC) to remove any large contaminants or dust particles. Twenty microliters of each sample were loaded onto the size exclusion chromatography system operating at a flow rate of 0.4 mL/min with 0.15 M NaNO3 and 0.02% NaN3. The high performance size exclusion chromatography (HPSEC) system was composed of a TSK G5000PW column (7.5 × 600 mm; Toso Biosep, Montgomeryville, PA, USA) connected to a multi-angle laser light scattering (HELEOS; Wyatt Technology Corp, Santa Barbara, CA, USA) and a refractive index detector (Waters, 2414) (HPSEC-MALLS-RI). The volume delays among the MALLS and RI detectors were determined using bovine serum albumin (BSA). The weight average molecular weight (Mw) and radius of gyration (Rg) was calculated by ASTRA 5.3 software (Wyatt Technology Corp.) (Anvari et al. 2016).
The specific volume of gyration (SVg) is another important property of the polysaccharide molecules that can be estimated using Mw and Rg values by the following equation (You and Lim 2000):
where the units for SVg, Mw and Rg are cm3/g, g/mol and nm respectively, and N is Avogadro’s number (6.02 × 1023/mol).
In vitro anticancer activity assay
The anticancer activity of the polysaccharides was evaluated using a human cervical cancer cell line (HeLa, ATCC). The HeLa cells were seeded in a 96 well microplate and incubated for 4 h at 37 °C in the presence of 5% CO2. The polysaccharide solutions (100 µL) were added into the wells at concentrations of 100, 200 and 400 µg/mL. 5-fluorouracil was used as positive control (10 µg/mL). After incubation for 72 h, the anticancer activity of the polysaccharides was determined using the WST-1 colorimetric assay kit (Roche Diagnostics, Madison, WI, USA).
RAW264.7 macrophage proliferation and nitric oxide production assays
Aliquots (1 × 104 cells/well, 100 µL volume) of a RAW264.7 macrophage cells grown in an RPMI-1640 medium containing 10% fetal bovine serum (FBS) were plated in a 96-well microplate. One hundred microliter of samples with different concentration (10, 25 and 50 µg/mL) was incubated with cells for 24 h at 37 °C in humidified atmosphere containing 5% CO2. Then, the WST-1 solution (20 µL) was added to each well and incubated for 4 h at 37 °C. The optical density of the mixture was determined at 450 nm using a microplate reader (EL-800; BioTek Instruments, Winooski, VT, USA) (Tabarsa et al. 2015). The level of macrophage proliferation was calculated using the following equation:
Macrophage proliferation (%) = At/Ac × 100, in which At the absorbance of the test group and Ac is the absorbance of the control group (cells without samples addition).
To indicate immunoenhancing activity of polysaccharides extracted with different methods, the nitric oxide (NO) secreted by RAW264.7 cells into culture supernatants was measured by Griess reagent (Green et al. 1982). In brief, RAW264.7 cells (1 × 105 cells/well) in a 96-well plate were treated with varying concentration of either polysaccharide samples (10, 25 and 50 µg/mL) or LPS (1.0 µg/mL) at 37 °C for 18 h. The level of NO production was quantified using the Griess reagent. A range of 1–200 µM NaNO2 in culture medium was used as reference to quantify the NO amount produced by macrophages (Tabarsa et al. 2015).
Cytokine gene expression
Different concentrations of either polysaccharide or LPS were added to RAW264.7 macrophage cells with density of 1 × 105 cells/well and incubated at 37 °C for 18 h. The total RNA was extracted from RAW264.7 cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and according to the manufacturer’s protocol. The isolated RNA concentration was determined using a spectrophotometer and construction of cDNA was carried out with an oligo-(dT)20 primer and Superscript III RT (Invitrogen, Carlsbad, CA, USA). The GoTaq Flexi DNA Polymerase (Promega, Madison, WI, USA) and specific primers were employed for PCR amplification. The primers used were as follows: iNOS, 5′-CCCTTCCGAAGTTTCTGGCAGCAGC-3′ (forward) and 5′-GGCTGTCAGAGCCTCGTGGCTTTGG-3′ (reverse); IL-1β, 5′ ATGGCAACTATTCCTGAACTCAACT-3′ (forward) and 5′-CAGGACAGGTATAGATTCTTTCCTTT-3′ (reverse); IL-6, 5′-TTCCTCTCTGCAAGAGACT-3′ (forward) and 5′-TGTATCTCTCTGAAGGACT-3′ (reverse); IL-10, 5′-TACCTGGTAGAAGTGATGCC-3′ (forward) and 5′-CATCATGTATGCTTCTATGC-3′ (reverse); TNF-α, 5′-ATGAGCACAGAAAGCATGATC-3′ (forward) and 5′-TACAGG CTTGTCACTCGAATT-3′ (reverse); IL-12, 5′- CCACAAAGGAGGCGAGACTC-3′ (forward) and 5′- CTCTACGAGGAACGCACCTT-3′ (reverse)and β-actin, 5′-TGGAATCCTGTGGCATCCATGAAAC-3′ (forward) and 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′ (reverse). To run the reverse transcriptase amplification, a sequential process (30 cycles) of denaturation (94 °C for 30 s), annealing (56 °C for 40 s) and extension (72 °C for 1 min) was carried out which was ended with a final extension step at 72 °C for 10 min. The PCR products were run on a 1% agarose gel electrophoresis and visualized by ethidium bromide staining (Tabarsa et al. 2015).
Statistical analyses
All experiments were conducted in triplicate (n = 3) and statistical analysis performed using SPSS software (Version 16; SPSS Inc., Chicago, IL, USA). Significant differences were identified by one-way analysis of variance (ANOVA) and Duncan’s multiple-range test. A probability value of p < 0.05 was considered to be statistically significant.
Results and discussions
Chemical composition of polysaccharides from C. peregrina
The water-soluble polysaccharides extracted from C. peregrina were partitioned through stepwise precipitations using 30% and 70% EtOH resulting polysaccharides namely CP1 and CP2, respectively. The yields, chemical compositions and sugar constituents of the partitioned polysaccharides are presented in Table 1. As calculated with respect to the dry weight of depigmented seaweed powder, the yield of CP1 polysaccharides was found to be notably high (17.6%); whereas that of CP2 polysaccharides was significantly lower (5.2%). So far, a wide range of extraction yields has been reported for the polysaccharides from brown seaweeds ranging from 1.5 to 14.7%. While seaweeds such as Sargassum muticum and Agarum cribrosum contained higher levels of water soluble polysaccharides, S. fusiforme and S. mcclurei showed lower extraction yields (Cho et al. 2014; Thinh et al. 2013; Flórez-Fernández et al. 2017). The chemical analysis revealed that CP1 polysaccharides were chiefly consisted of neutral sugars (73.79%) with small amounts of uronic acids (9.43%), sulfates (4.91%) and proteins (3.44%). The amount of neutral sugars was slightly lower in CP2 polysaccharides whereas uronic acids (14.89%) and proteins (14.78%) were measured to be at higher levels. Similar amount of sulfate esters (4.87%) was determined in CP2 polysaccharides. A similar chemical composition has been reported for fucoidans from A. cribrosum, S. glaucescens and S. binderi (Cho et al. 2014; Huang et al. 2016; Lim et al. 2016).
Table 1.
Yield and chemical composition of the polysaccharides from C. peregrina
| CP1 | CP2 | |
|---|---|---|
| Chemical compositions (%) | ||
| Yield (%) | 17.6 | 5.2 |
| Neutral sugars | 73.79 ± 0.29 | 67.01 ± 0.31 |
| Protein | 3.44 ± 0.76 | 14.78 ± 1.52 |
| Uronic acid | 9.43 ± 0.26 | 14.89 ± 0.50 |
| Sulfate | 4.91 ± 0.12 | 4.87 ± 0.19 |
| Monosaccharide (%) | ||
| Fucose | 24.56 ± 0.09 | 20.62 ± 0.07 |
| Glucose | 50.00 ± 0.15 | 52.91 ± 0.13 |
| Galactose | 25.5 ± 0.11 | 26.94 ± 0.13 |
| Molecular characteristics | ||
| MW × 103 (g/mol)a | 1890.95 ± 1.34 | 639.05 ± 1.62 |
| Rg (nm)b | 56.60 ± 0.00 | 57.55 ± 0.63 |
| SVg (cm3/g)c | 0.24 ± 0.00 | 0.75 ± 0.02 |
aWeight average molecular weight (Mw)
bRadius of gyration (Rg)
cSpecific volume of gyration (SVg)
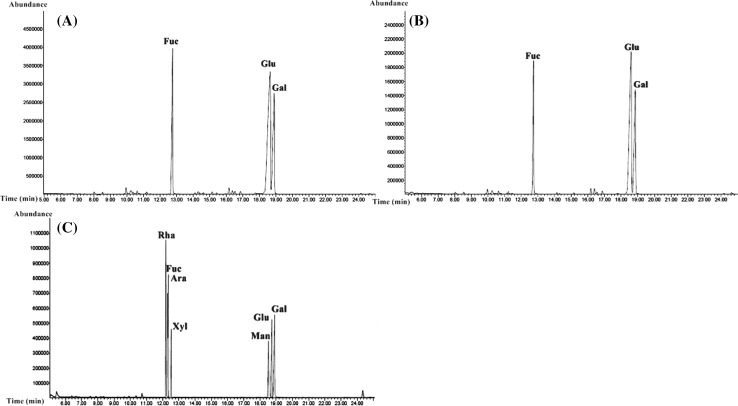
The sugar composition of the polysaccharide chains was determined and the results are presented in Table 1 and Fig. 1. As shown in the GC–MS chromatogram, there were three peaks recorded for the monosaccharide composition of CP1 polysaccharides where the predominantly occurring sugar was glucose (50.00%). In addition, as the characteristic monosaccharide of brown seaweeds, the fucose content (24.56%) was found to be nearly as high as that of galactose (25.50%) in the polysaccharide chain. Likewise, the CP2 polysaccharides were mainly formed of glucose (52.91%) with lower levels of fucose (20.62%) and galactose (26.94%).
Fig. 1.
GC chromatograms of monosaccharides of CP1 (a) and CP2 (b) polysaccharides from C. peregrina and standard (c)
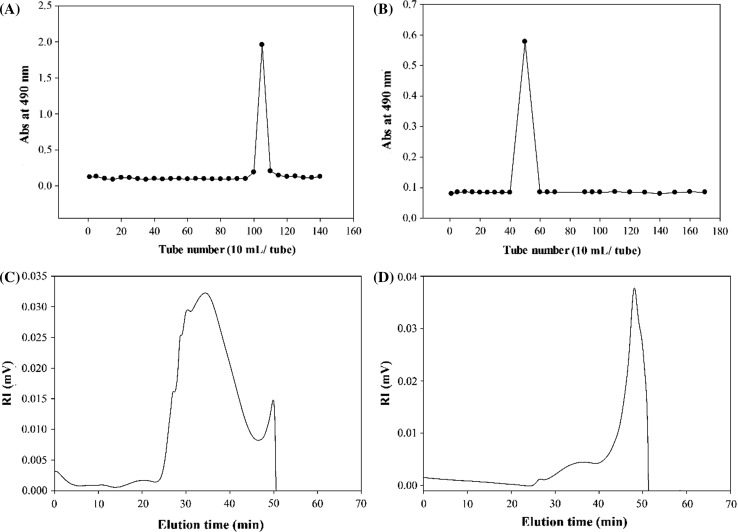
The fraction with the highest yield, CP1 polysaccharide, was chosen for further purification. As shown in Fig. 2a, the employment of DEAE Sepharose fast flow column resulted only a single fraction. Similarly, the CP1 polysaccharides were eluted from Cellulose CL-2B column as one fraction (Fig. 2b). These results implied that CP1 polysaccharides were formed of molecules with homogeneous charge intensity and size distribution. Therefore, the rest of experiments were carried out on CP1 and CP2 polysaccharides.
Fig. 2.
The DEAE Sepharose FF (a) and Sepharose CL-2B (b) elution profiles of CP1 polysaccharides. The HPSEC chromatograms of CP1 (c) and CP2 (d) polysaccharides determined by a TSK G5000 PW column at a flow rate of 0.4 mL/min
Molecular properties of the polysaccharides
The RI (refractive index) chromatograms of CP1 and CP2 polysaccharides eluted from the SEC column are presented in Fig. 2c, d. The molecules of CP1 polysaccharides were eluted from the SEC column as one peak between elution times of 24 and 46 min indicating the presence of one type of polymers with similar molecular weights. In contrast, the polysaccharide polymers of CP2 were eluted from SEC column as two peaks between elution times of 25 and 51 min which implied the existence of heterogeneous polysaccharides with different molecular weights. The Mw of CP1 and CP2 polysaccharides were 1890.95 × 103 and 639.05 × 103 g/mol, respectively (Table 1). Similar molecular sizes were also estimated by the calculated Rg for CP1 (56.60 nm) and CP2 (57.55 nm) polysaccharides. The molecular weights of fucoidans reported from brown seaweeds seem to be very diverse and vary greatly from the very high molecular weight polysaccharides of 2420.0 × 103 g/mol from A. cribrosum to the very low molecular weight polysaccharides of 27.9 × 103 g/mol from S. binderi (Dubois et al. 1956; Lim et al. 2016). These large discrepancies in the molecular weights of the fucoidans on one hand has been attributed to species differences of tested seaweeds and on the other hand were related to the variables involved in the extraction protocols and seaweeds environmental conditions (Saravana et al. 2016; Huang et al. 2016; Fletcher et al. 2017).
Using Mw and Rg, the SVg values were calculated for CP1 and CP2 polysaccharides. Basically, the SVg value is the theoretical gyration volume per unit of molar mass with an inverse relationship to the degree of molecular compactness. The SVg value of CP1 polysaccharide was 0.24 cm3/g; whereas the SVg value of CP2 polysaccharide was considerably high (0.75 cm3/g) (Table 1). These findings indicated that the molecules of CP1 polysaccharides had compact structures and CP2 polysaccharides had more loosed and expanded conformation.
Bioactivities of partitioned polysaccharides
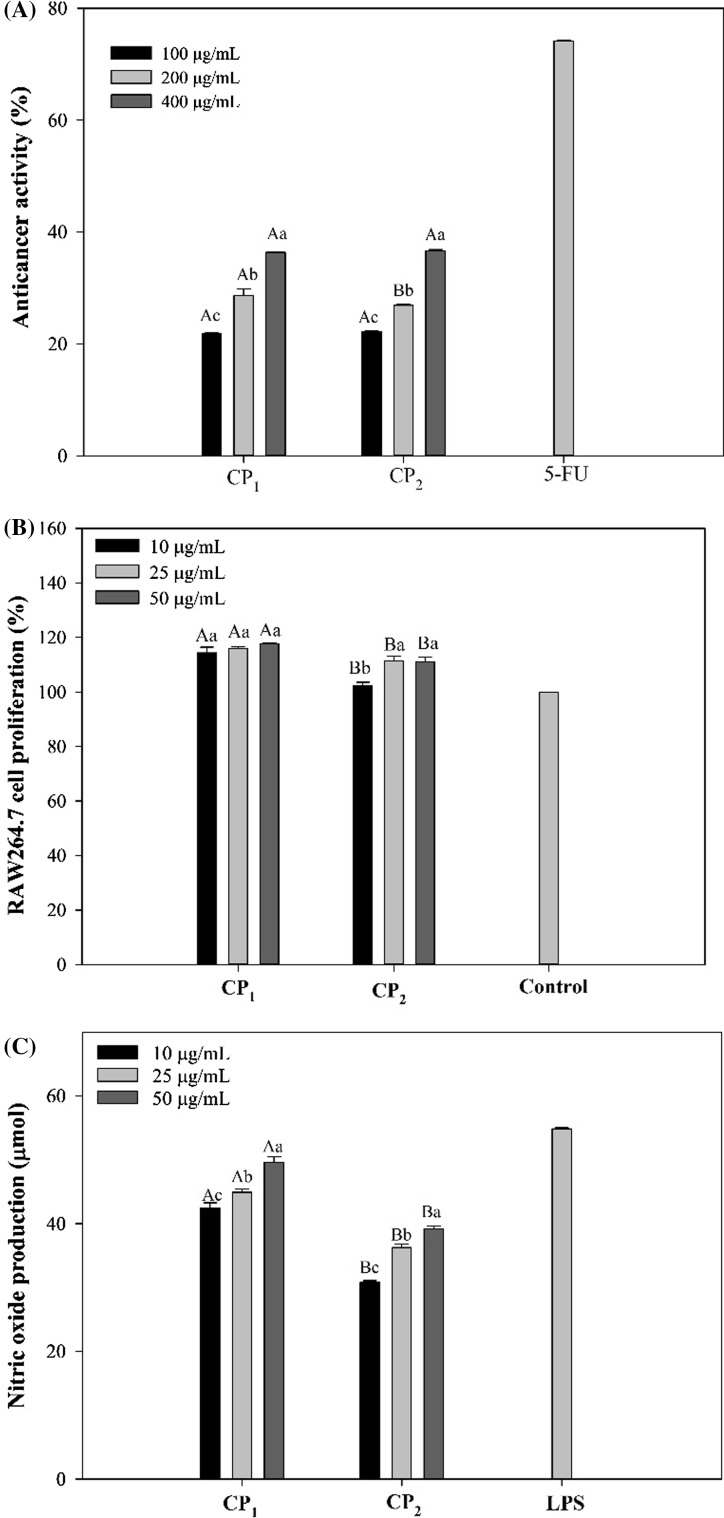
The effect of extracted polysaccharides on the growth of HeLa cancer cells was studied over the concentration range of 100–400 µg/mL. Both CP1 and CP2 polysaccharides exhibited similar inhibitions on the growth rate of HeLa cells (Fig. 3a). The tested polysaccharides exerted relatively lower cytotoxicity against cancer cells which reached nearly 40% at the highest concentrations. An overview of the previous studies shows that there are many reports on the effectiveness of fucoidans on the growth inhibition of various cancers through different pathways (Wu et al. 2016). However, it has to be mentioned that the magnitude of fucoidans direct anticancer activities vary significantly depending on polysaccharide structure and cancer type (Wu et al. 2016; Cho et al. 2014). The polysaccharides with little anticancer activities were found to be potent agents in treating cancers through the stimulation of immune system (Tabarsa et al. 2015). The immune system is involved in defense against tumors during which macrophage cells play a pivotal role because of their ability to selectively destroy a broad range of tumor types after specific activation (Weiming et al. 2002). The effect of CP1 and CP2 polysaccharides on the proliferation of RAW264.7 macrophage cells was examined over the concentration range of 10–50 µg/mL. As shown in Fig. 3b, CP1 polysaccharides not only were safe and nontoxic to RAW264.7 cells at all concentrations, but also stimulated the macrophage cells to proliferate up to 118% (p < 0.05). Although it was less effective than CP1 polysaccharides, CP2 polysaccharides showed significant proliferating effect when incubated with macrophage cells.
Fig. 3.
Anticancer activity against HeLa cells (a), RAW264.7 cells proliferation activity (b) and nitric oxide production (c) of the RAW264.7 macrophage cells after treatment with CP1 and CP2 polysaccharides. Superscripts a, b, c, d show significant differences (p < 0.05) among different treatments, and A, B, C, D show significant differences (p < 0.05) among different concentrations
The immunostimulatory effect of extracted polysaccharides was evaluated by measuring the amount of NO released from RAW264.7 cells after incubation with CP1 and CP2 polysaccharides. The level of NO secreted from macrophage cells after treating with CP1 and CP2 polysaccharides at concentrations of 10, 25 and 50 µg/mL is presented in Fig. 3c. The CP1 polysaccharides induced a considerable NO release from the cells in a dose dependent manner up to 49.6 µmol (p < 0.05). This level of stimulation was comparable with that of lipopolysaccharide (LPS) used at 1 µg/mL. The CP2 polysaccharides showed weaker effect on macrophage cells releasing only 39 µmol at the highest concentration (p < 0.05). In basic, macrophage NO is a free radical gasotransmitter that its regulation is under the transcriptional control of cytokines, LPS and other mediators. Activated macrophages generate large concentrations of NO after which tumor cells can be killed through mechanisms such as inhibition of DNA synthesis and direct damage of DNA (MacMicking et al. 1997).
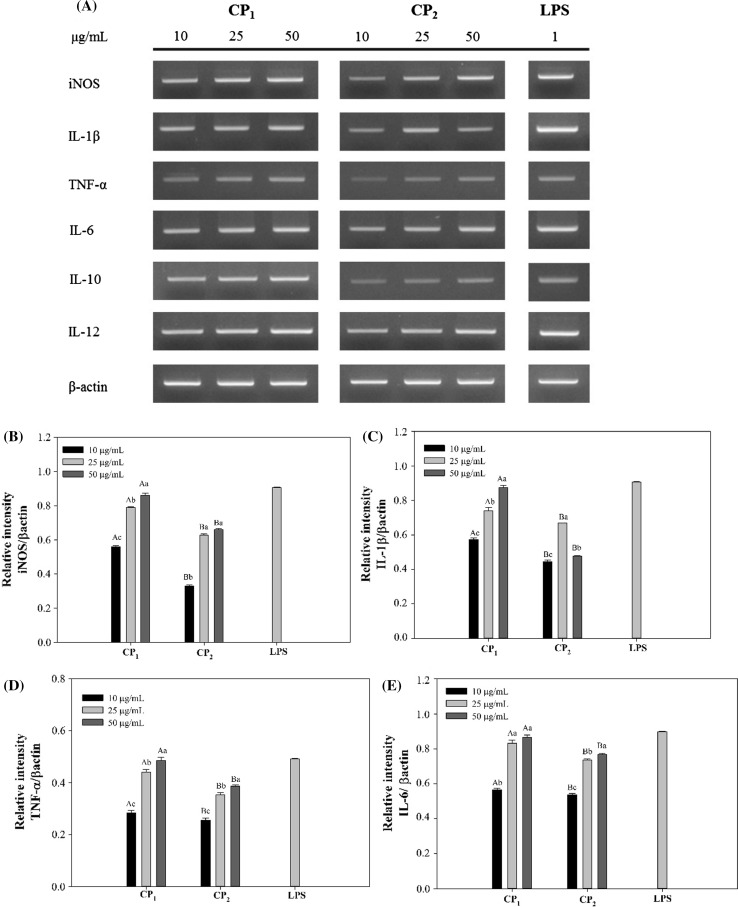
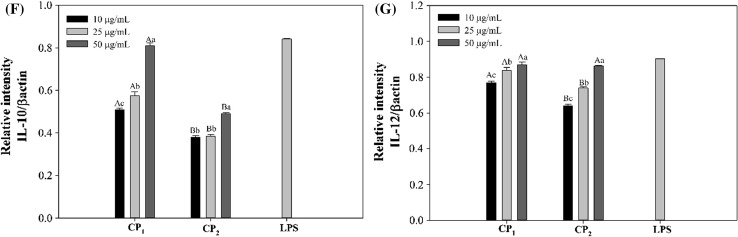
To further confirm the results obtained above, the RAW264.7 macrophage cells stimulated with polysaccharides and LPS were collected and the whole mRNA contents of the cells were extracted afterwards. Using specific primers, the possibility of mRNA expression of inducible nitric oxide synthase (iNOS) which catalyzes the production of NO from l-arginine, was investigated on the molecular level (Förstermann and Sessa 2011). As shown in Fig. 4a, strong and distinctive bands were observed for the PCR products indicating the expression of iNOS mRNA and thus synthesis of NO after polysaccharide stimulations. The level of iNOS mRNA expression induced by polysaccharides and LPS was consistent with that of NO production, as calculated by graphic analysis of the PCR products on the agarose gel (Fig. 4b). Cytokines are signaling proteins that are released from macrophages and their primary function is to regulate inflammation (Arango Duque and Descoteaux 2014). In the present study, the mRNA expression of cytokines including IL-1β, TNF-α, IL-6, IL-10 and IL-12 was also studied in the macrophage cells (Fig. 4a, c–g). In accordance with NO production, CP1 polysaccharides were able to significantly increase the mRNA expressions of these cytokines dose dependently. It is well known that the inflammatory response is triggered by pro-inflammatory cytokines such as IL-1β, IL-6 and TNFα. Consequently, these cytokines affect other cell types leading to the secretion of other inflammatory mediators and creation of multiple inflammatory cascades (Hernandez-Rodriguez et al. 2003). The overexpression of proinflammatory cytokines leads to sever inflammation symptoms and inflammatory diseases to prohibit which anti-inflammatory cytokines are produced to down regulate their expressions (Sultani et al. 2012). In current study, the mRNA expression of IL-10 was studied and the results showed the potential anti-inflammatory effect of CP1 and CP2 polysaccharides (Fig. 4f).
Fig. 4.
Effects of CP1 and CP2 polysaccharides on the mRNA expression of cytokines in RAW264.7 cells. Superscripts a–d show significant differences (p < 0.05) among different treatments, and A–D show significant differences (p < 0.05) among different concentrations. β-actin was used as a control
Glycosidic linkage analysis of CP1 polysaccharides
The CP1 polysaccharides were chosen as the most potent macrophage stimulating fraction for further structural analysis. The types and ratios of glycosidic linkages of CP1 polysaccharides were found from the partially methylated alditol acetate products after GC–MS injection. As presented in Table 2, the most abundant derivatives were 2,3,6-tri-O-methyl-Glu and 2,4,6-tri-O-methyl-Gal which indicated the presence of 1,4-linked glucose and 1,3-linked galactose residues in the backbone of CP1 polysaccharides. Lower amounts of 1,3-linked glucose residues were also included in the polysaccharide chain. The existence of 2,6-di-O-methyl-Glu derivative showed the inclusion of branch points as 1,3,4-linked glucose in the CP1 polysaccharide. The inclusion of fucose monosaccharides in the polysaccharide structure was in form of 1,3-linked fucose residues. Besides, some levels of fucose and galactose residues were found to be presented as non-reducing terminals. The ratios of glycosidic linkages of (1→)-Fuc, (1→3)-Fuc, (1→)-Gal, (1→3)-Gal, (1→4)-Glu, (1→3)-Glu and (1→3,4)-Glu were calculated to 0.26:0.35:0.21:0.69:1.0:0.31:0.58.
Table 2.
Glycosidic linkage analysis of the constituent sugars of the fraction F1 from C. peregrina
| Glycosidic linkage | Methylation | Peak ratio (%) |
|---|---|---|
| Fuc-(1→ | 1,5-di-O-acetyl-2,3,4-tri-O-methyl-Fuc | 7.62 |
| →3)-Fuc-(1→ | 1,3,5-tri-O-acetyl-2,4-di-O-methyl-Fuc | 10.25 |
| Gal-(1→ | 1,5-di-O-acetyl-2,3,4,6-tetra-O-methyl-Gal | 6.23 |
| →3)-Gal-(1→ | 1,3,5-tri-O-acetyl-2,4,6-tri-O-methyl-Gal | 20.30 |
| →4)-Glu-(1→ | 1,4,5-tri-O-acetyl-2,3,6-tri-O-methyl-Glu | 29.31 |
| →3)-Glu-(1→ | 1,3,5-tri-O-acetyl-2,4,6-tri-O-methyl-Glu | 9.12 |
| →3,4)-Glu-(1→ | 1,3,4,5-tetra-O-acetyl-2,6-di-O-methyl-Glu | 17.17 |
In conclusion, alcalase extraction of polysaccharides from C. peregrina resulted in high extraction yield. Stepwise ethanol precipitations produced two fractions with different chemical compositions and molecular weights. The CP1 polysaccharides contained higher acidic sugars and notably lower molecular weight. The CP1 polysaccharides were the most potent immunostimulating polysaccharides with the highest potential to release cytokines from macrophage cells via NF-κB and MAPKs signaling pathways. CP1 polysaccharide was consisted of 1,3-linked galactose, 1,4-linked glucose and 1,3-linked fucose residues. The novel findings of the present study suggested the potential application of fucogalactoglucan isolated from C. peregrina as immunostimulatory agent to improve innate immunity function. However, further researches need to be conducted on structure–activity relationships of these polysaccharides before any applications.
References
- Anvari M, Tabarsa M, Cao R, You S, Joyner HS, Behnam S, Rezaei M. Compositional characterization and rheological properties of an anionic gum from Alyssum homolocarpum seeds. Food Hydrocol. 2016;52:766–773. doi: 10.1016/j.foodhyd.2015.07.030. [DOI] [Google Scholar]
- Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilan MI, Grachev AA, Shashkov AS, Kelly M, Sanderson CJ, Nifantiev NE, Usov AI. Further studies on the composition and structure of a fucoidan preparation from the brown alga Saccharina latissima. Carbohydr Res. 2010;345:2038–2047. doi: 10.1016/j.carres.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Borazjani NJ, Tabarsa M, You S, Rezaei M. Purification, molecular properties, structural characterization, and immunomodulatory activities of water soluble polysaccharides from Sargassum angustifolium. Int J Biol Macromol. 2017;109:793–802. doi: 10.1016/j.ijbiomac.2017.11.059. [DOI] [PubMed] [Google Scholar]
- Cho M, Lee DJ, Kim JK, You S. Molecular characterization and immunomodulatory activity of sulfated fucans from Agarum cribrosum. Carbohydr Polym. 2014;113:507–514. doi: 10.1016/j.carbpol.2014.07.055. [DOI] [PubMed] [Google Scholar]
- Ciucanu I, Kerek F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr Res. 1984;131:209–217. doi: 10.1016/0008-6215(84)85242-8. [DOI] [Google Scholar]
- Cumashi A, Ushakova NA, Preobrazhenskaya ME, D’incecco A, Piccoli A, Totani L, Tinari N, Morozevich GE, Berman AE, Bilan MI, Usov AI. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology. 2007;17:541–552. doi: 10.1093/glycob/cwm014. [DOI] [PubMed] [Google Scholar]
- Dodgson KS, Price RG. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem J. 1962;84:106. doi: 10.1042/bj0840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte ME, Cardoso MA, Noseda MD, Cerezo AS. Structural studies on fucoidans from the brown seaweed Sargassum stenophyllum. Carbohydr Res. 2001;333:281–293. doi: 10.1016/S0008-6215(01)00149-5. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Filisetti-Cozzi TM, Carpita NC. Measurement of uronic acids without interference from neutral sugars. Anal Biochem. 1991;197(1):157–162. doi: 10.1016/0003-2697(91)90372-Z. [DOI] [PubMed] [Google Scholar]
- Fletcher HR, Biller P, Ross AB, Adams JM. The seasonal variation of fucoidan within three species of brown macroalgae. Algal Res. 2017;22:79–86. doi: 10.1016/j.algal.2016.10.015. [DOI] [Google Scholar]
- Flórez-Fernández N, López-García M, González-Muñoz MJ, Vilariño JM, Domínguez H. Ultrasound-assisted extraction of fucoidan from Sargassum muticum. J App Phycol. 2017;29:1553–1561. doi: 10.1007/s10811-016-1043-9. [DOI] [Google Scholar]
- Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2011;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15 N] nitrate in biological fluids. Anal Biochem. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- Hernandez-Rodriguez J, Segarra M, Vilardell C, Sanchez M, Garcia-Martinez A, Esteban MJ, Queralt C, Grau JM, Urbano-Marquez A, Palacin A, Colomer D. Tissue production of pro-inflammatory cytokines (IL-1β, TNFα and IL-6) correlates with the intensity of the systemic inflammatory response and with corticosteroid requirements in giant-cell arteritis. Rheumatology. 2003;43:294–301. doi: 10.1093/rheumatology/keh058. [DOI] [PubMed] [Google Scholar]
- Huang CY, Wu SJ, Yang WN, Kuan AW, Chen CY. Antioxidant activities of crude extracts of fucoidan extracted from Sargassum glaucescens by a compressional-puffing-hydrothermal extraction process. Food Chem. 2016;197:1121–1129. doi: 10.1016/j.foodchem.2015.11.100. [DOI] [PubMed] [Google Scholar]
- Li B, Wei XJ, Sun JL, Xu SY. Structural investigation of a fucoidan containing a fucose-free core from the brown seaweed, Hizikia fusiforme. Carbohydr Res. 2006;341:1135–1146. doi: 10.1016/j.carres.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Li B, Lu F, Wei X, Zhao R. Fucoidan: structure and bioactivity. Molecules. 2008;13(8):1671–1695. doi: 10.3390/molecules13081671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SJ, Aida WM, Maskat MY, Latip J, Badri KH, Hassan O, Yamin BM. Characterisation of fucoidan extracted from Malaysian Sargassum binderi. Food Chem. 2016;209:267–273. doi: 10.1016/j.foodchem.2016.04.058. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Ann Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- Rahimi F, Tabarsa M, Rezaei M. Ulvan from green algae Ulva intestinalis: optimization of ultrasound-assisted extraction and antioxidant activity. J Appl Phycol. 2016;28:2979–2990. doi: 10.1007/s10811-016-0824-5. [DOI] [Google Scholar]
- Saravana PS, Cho YJ, Park YB, Woo HC, Chun BS. Structural, antioxidant, and emulsifying activities of fucoidan from Saccharina japonica using pressurized liquid extraction. Carbohydr Polym. 2016;153:518–525. doi: 10.1016/j.carbpol.2016.08.014. [DOI] [PubMed] [Google Scholar]
- Shao P, Liu J, Chen X, Fang Z, Sun P. Structural features and antitumor activity of a purified polysaccharide extracted from Sargassum horneri. Int J Biol Macromol. 2015;73:124–130. doi: 10.1016/j.ijbiomac.2014.10.056. [DOI] [PubMed] [Google Scholar]
- Shevchenko NM, Anastyuk SD, Menshova RV, Vishchuk OS, Isakov VI, Zadorozhny PA, Sikorskaya TV, Zvyagintseva TN. Further studies on structure of fucoidan from brown alga Saccharina gurjanovae. Carbohydr Polym. 2015;121:207–216. doi: 10.1016/j.carbpol.2014.12.042. [DOI] [PubMed] [Google Scholar]
- Sultani M, Stringer AM, Bowen JM, Gibson RJ. Anti-inflammatory cytokines: important immunoregulatory factors contributing to chemotherapy-induced gastrointestinal mucositis. Chemotherapy Res Pract. 2012;2:1–11. doi: 10.1155/2012/490804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarsa M, Shin IS, Lee JH, Surayot U, Park W, You S. An immune-enhancing water-soluble α-glucan from Chlorella vulgaris and structural characteristics. Food Sci Biotechnol. 2015;24:1933–1941. doi: 10.1007/s10068-015-0255-0. [DOI] [Google Scholar]
- Tabarsa M, Anvari M, Joyner HS, Behnam S, Tabarsa A. Rheological behavior and antioxidant activity of a highly acidic gum from Althaea officinalis flower. Food Hydrocol. 2017;69:432–439. doi: 10.1016/j.foodhyd.2017.02.009. [DOI] [Google Scholar]
- Thinh PD, Menshova RV, Ermakova SP, Anastyuk SD, Ly BM, Zvyagintseva TN. Structural characteristics and anticancer activity of fucoidan from the brown alga Sargassum mcclurei. Marine Drugs. 2013;11:1456–1476. doi: 10.3390/md11051456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiming XU, Liu LZ, Loizidou M, Ahmed M, Charles IG. The role of nitric oxide in cancer. Cell Res. 2002;12:311–320. doi: 10.1038/sj.cr.7290133. [DOI] [PubMed] [Google Scholar]
- Wu L, Sun J, Su X, Yu Q, Yu Q, Zhang P. A review about the development of fucoidan in antitumor activity: progress and challenges. Carbohydr Polym. 2016;154:96–111. doi: 10.1016/j.carbpol.2016.08.005. [DOI] [PubMed] [Google Scholar]
- You S, Lim ST. Molecular characterization of corn starch using an aqueous HPSEC-MALLS-RI system under various dissolution and analytical conditions. Cereal Chem. 2000;77:303–308. doi: 10.1094/CCHEM.2000.77.3.303. [DOI] [Google Scholar]
- Yuguchi Y, Bui LM, Takebe S, Suzuki S, Nakajima N, Kitamura S, Thanh TT. Primary structure, conformation in aqueous solution, and intestinal immunomodulating activity of fucoidan from two brown seaweed species Sargassum crassifolium and Padina australis. Carbohydr Polym. 2016;147:69–78. doi: 10.1016/j.carbpol.2016.03.101. [DOI] [PubMed] [Google Scholar]