Abstract
Physicochemical parameters (pH, colour and texture), proximate composition (moisture, protein, lipid and ash content), amino acid content, and taste profile of beef patties elaborated with soy (control), pulses (pea, lentil and bean) and microalgal (Chlorella and Spirulina) proteins were assessed. The pH, colour, ash content, total, essential and non-essential amino acids and amino acid content were significantly different among the beef patties studied. In this regard, beef patties prepared with pea protein presented the highest values for pH; whereas beef patties manufactured with pea also showed the highest values for lightness and patties elaborated with bean the highest values for redness. Similar textural parameters were observed among the six batches of beef patties manufactured. Regarding ash content, a significant higher content was observed in the beef patties prepared with soy compared to the other ones. On the other hand, the beef patties elaborated with bean and seaweeds showed the highest values for the total amino acids content. The inclusion of bean and seaweed proteins increased the concentrations of all amino acids in beef patties, being glutamic acids, lysine and aspartic acid the predominant amino acids. Regarding the taste analysis, a similar profile was found among the six batches of beef patties studied. Considering all studied parameters, beef patties elaborated with bean protein could be used as an alternative protein source respect to soy protein because of it showed a similar nutritional content and taste profile and higher amino acid content.
Keywords: Seaweeds, Pulses, Amino acid profile, Chlorella and Spirulina, Chemical composition, Color parameters, Textural traits
Introduction
For several decades, consumers´ interest in healthy and nutritious foods with balanced macro- and micronutrients from both animal and vegetal origins has triggered researchers and food industries to explore products from diverse sources. Meat and meat products are important sources of a great variety of nutrients, such as lipids, proteins, minerals, and vitamins (Jiménez-Colmenero and Delgado Pando 2013; Lorenzo and Pateiro 2013; Lorenzo et al. 2014a), which make them an important group of foods consumed by a wide range of people worldwide. Since the consumers demands are changing and the market is constantly growing, the improvement of the quality and image of the meat is needed, as well as the development of products with health beneficial properties (dos Santos et al. 2016; Lorenzo et al. 2016, Domínguez et al. 2017; Heck et al. 2017).
There has been a noticeable increase in the development of meat products partially replaced with plant food materials. Soy is a very important source of proteins and represents a highly nutritious food with a desirable amount of amino acids and fatty acids (Rinaldoni et al. 2014). Various processed food products incorporate soy proteins because of their health beneficial properties. However, due to the recognition of soy as an allergenic food, interest in using microalgae proteins in food has been growing in recent years (Madeira et al. 2017). In addition to soy proteins, the legume family proteins have gained importance among the food ingredients as meat analogs and in other food products. Beans, lentils, peas, and chickpeas, are among the most nutritious and accessible protein sources, representing about 20% of dry weight in pea and beans (Neacsu et al. 2017).
On the other hand, recently, there has been a growing interest in developing products with algal proteins (Parniakov et al. 2018). Depending on the species, strain and algae growing conditions, the nutrient composition of microalgae differs (Barba et al. 2014; Roohinejad et al. 2017). Microalgae are mainly composed of proteins, polysaccharides, polyunsaturated fatty acids (PUFAs), especially EPA and DHA, and antioxidants (phenolic acids, flavonoids and carotenoids) (Barba et al. 2014; Poojary et al. 2016; Agregán et al. 2017a, b, c; Lorenzo et al. 2017). Due to these nutritionally and bioactive valuable compounds, algae have been recognized for the manufacture of nutraceuticals, cosmeceuticals, and feed supplements (Barba 2017; Madeira et al. 2017; Roselló-Soto et al. 2015) where their incorporation in food and feed may improve nutritional value, texture, resistance to lipid oxidation and color loss (Kovač et al. 2013; Lorenzo et al. 2014b; Agregán et al. 2018a, b).
These matrices can be applied in animal nutrition in order to improve growth, meat and eggs quality (Madeira et al. 2017) as well as to reduce cholesterol levels (Kovač et al. 2013). Several researchers have focused their attention on microalgae as important sources of proteins and essential amino acids (Madeira et al. 2017; Parniakov et al. 2015a; b, c), thus constituting an alternative protein source (Kovač et al. 2013). Moreover, algal proteins have similar or higher quality compared to plant sources, such as wheat, beans or rice (Barba et al. 2014; Becker 2007; Zhu et al. 2017). For instance, Milovanović et al. (2012) observed that several cyanobacterial strains contain a high content of proteins (42.8–76.5%).
Spirulina biomass, the blue-green algae (filamentous cyanobacteria), has attracted attention of researchers because of its remarkable protein content and it is considered to be one of the richest protein sources of microalgal origin, with the protein levels comparable to meat (71–76% dry basis) and soy (~ 40% dry basis). The use of Spirulina in partial meat protein replacement can be beneficial to human health owing to its amino acid composition, absence of cholesterol, high amounts of vitamins, minerals, essential fatty acids, polyphenols and pigments (Lupatini et al. 2017).
Similarly, Chlorella is a photosynthetic microorganism, which also plays an important role in this scenario. It has a fast growth rate and is industrially produced not only for its lipids, but also because of its high protein content (48–58% dry basis) (Postma et al. 2014; Ursu et al. 2014). Because of its emulsifying properties, the use of Chlorella biomass as an ingredient in the food industry is highlighted (Lupatini et al. 2017). Moreover, both Spirulina and Chlorella have the ability to accumulate high amounts of bioactive compounds with technological, functional, antibacterial, antifungal and antiviral properties, which can be used at an industrial level (e.g. incorporation in several formulations of bread, snacks, and pasta) (Roohinejad et al. 2017).
When food products are manufactured with Spirulina and Chlorella biomasses, their taste should be assessed by potential consumers. It is known that alanine, glycine, proline, serine, and threonine provide a sweet taste, whereas histidine, allo-isoleucine, isoleucine, leucine, methionine, phenylalanine, tryptophan and valine are related to a bitter taste. On the other hand, aspartic acid and glutamic acid are correlated to the umami taste, which is considered as one of the primary tastes (Barba et al. 2017) and can be found in different types of foods, such as meat, soy sauce, seafood and some processed foods (Suess et al. 2015; Zhang and Peterson 2018).
Patties are very popular food worldwide. Regardless of a quality, color, texture and nutritive value, the taste profile is still often a key driver for a consumption of a product (Zamuz et al. 2018). A current focus of food industry is the enrichment of various products to improve a healthy lifestyle. Therefore, the addition of soy to the meat products has been found to positively influence the nutritional profile. Following a technological trend, the objective of this work was to determine the improvement of the physicochemical and nutritional quality profile of the meat in beef patties by using different protein sources of plant (pea, lentil and bean) and algal origin (Chlorella and Spirulina).
Materials and methods
Experimental design and manufacture of beef patties
Beef meat was provided by Novafrigsa S.A.—Grupo Coren (Lugo, Spain). All the used spices and additives were of food grade. Different vegetable protein (soy, pea, lentil and bean) were supplied by Vitessence™ Pulse Proteins, with 90, 55, 55 and 60% of purity, respectively. Seaweed proteins (Spirulina, Chlorella) sources were supplied by Algaenergy (Madrid, Spain) with 70 and 60% of purity, respectively. All the chemicals used for the analysis were of analytical grade.
Six batches of beef patties with different protein sources were manufactured in the pilot plant of the Meat Technology Center of Galicia (Fig. 1). A total of 30 beef patties were elaborated: 5 control (1% soy protein), 15 pulse protein (5 with 1% pea, 5 with 1% lentil and 5 with 1% bean) and, 10 seaweed protein (5 with 1% Chlorella and 5 with 1% Spirulina). Patties of 100 g were manufactured using beef lean meat with a fat content between 3 and 6% and the meat was ground through a 6 mm diameter mincing plate in a refrigerated mincer machine (La Minerva, Bologna, Italy).
Fig. 1.
Beef patties formulated with different protein sources (control = soy; pea; lentil; bean; Chlorella and Spirulina) manufactured in the pilot plant of the Meat Technology Center of Galicia
This meat was vacuum minced in a vacuum mincer machine (Fuerpla, Valencia, Spain) for 3 min with water (10%) and all additives except protein extract. Meat mass was separated in six batches and to each one the necessary quantity of extract protein was added. These six mixed masses were kept at 4 ± 2 °C during 4 h and then beef patties were elaborated in a burger-maker (Gaser, A-2000, Girona, Spain) and their physicochemical properties, amino acid content, and taste profile were analysed.
Physicochemical parameters
Physicochemical parameters were analysed according to method previously reported by Pateiro et al. (2013). Colour was measured using a portable colorimeter (CM-600d-Konica Minolta, Japan) with pulsed xenon arc lam, 0° viewing angle geometry and 8 mm aperture size, to estimate patties colour in the CIELAB space: lightness, (L*); redness, (a*); yellowness, (b*). The colour was measured in three different points of each sample in homogeneous and representative areas, free of fat. Water-holding capacity (WHC) was measured as cooking loos (%), and textural profile analysis (TPA) test was conducted using a texture analyser (TA-XT2, Stable Micro Systems, Godalming, UK). Likewise, pH of patties was measured using a digital portable pH-meter (HI 99163, Hanna Instruments, Eibar, Spain) equipped with a penetration probe.
Proximate composition
Moisture, protein and ash were determined following the ISO recommendations (ISO 1442:1997, ISO 937:1978 and ISO 936:1998, respectively). Moisture content was determined by measuring sample (3 g) weight loss at 105 °C in an oven (Memmert UFP 600, Schwabach, Germany), until constant weight. Kjeldahl total nitrogen method was used to determine protein percentage (total nitrogen content was multiplied × 6.25). Samples were subjected to reaction with sulfuric acid in a digester (Gerhardt Kjeldatherm KB, Bonn, Germany) and cupric sulfate was employed as a catalyst, then the organic nitrogen was transformed into ammonium sulfate, and distilled in alkali condition (Gerhardt Vapodest 50 carroused, Bonn, Germany). Ash content was assessed by weight loss by maintaining the sample in a muffle furnace (Carbolite RWF 1200, Hope Valley, England) at 600 °C. Fat content was extracted according to the AOCS Official Procedure Am 5-04 (AOCS 2005). Samples were subjected to a liquid–solid extraction using petroleum ether in an extractor apparatus (AnkomHCI Hydrolysis System, Macedon NY, USA) at 90 °C during 60 min and, the fat content was obtained based on gravimetric difference.
Amino acid content and taste profile
Taste profile was determined from amino acid profile. Hydrolyzed amino acid composition (g/100 g of meat) of manufactured meat products was estimated using the procedure previously described by Lorenzo et al. (2011). Amino acids derivatization was performed using the 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (Waters AccQ-Fluor reagent kit) and determined by RP-HPLC (Waters 2695 Separations Module + Waters 2475 Multi Fluorescence Detector + Waters AccQ-Tag amino acids analysis column).
Statistical analysis
The differences in physicochemical parameters, amino acid profile, and taste profile among elaborated beef patties were examined using one-way analysis of variances (ANOVA) and the means were compared using the Duncan’s test. A linear discriminate function containing an optimal subset of variables was done to determine the coefficients that maximize the differences between samples. Statistical tests were performed for a significance level P < 0.05 and using IBM SPSS Statistics® 23.0 program (IBM Corp). Data were presented as means of 5 replicates and standard error of the mean (SEM) was calculated.
Results and discussion
Color, pH, and textural parameters of beef patties
The pH and color and textural parameters of the different formulations of patties are presented in Table 1. Concerning the pH values, changes among the different formulations of protein addition of patties samples were normally considered significant (P < 0.001). Pea patties presented a slight increase in pH values compared to the other samples with values ranging between 6.56 and 6.28. Although other authors (Cofrades et al. 2008, 2017; López-López et al. 2009b) reported a pH reduction with the addition of Himanthalia elongata to a meat product, Chlorella and Spirulina showed similar values or slightly higher than control group.
Table 1.
Physical properties of beef patties manufactured with different protein sources
| Soy | Pea | Lentil | Bean | Spirulina | Chlorella | SEM | Sig. | |
|---|---|---|---|---|---|---|---|---|
| pH | 6.32c | 6.56a | 6.35c | 6.28c | 6.30c | 6.47b | 0.22 | *** |
| Colour parameters | ||||||||
| L* | 39.83b | 41.84a | 40.38ab | 38.90b | 30.34c | 28.40d | 0.99 | *** |
| a* | 22.37ab | 22.22b | 22.92ab | 23.25a | 3.24c | − 2.67d | 1.99 | *** |
| b* | 18.37a | 19.17a | 19.09a | 18.41a | 11.33b | 5.97c | 0.94 | *** |
| Textural parameters | ||||||||
| Cooking loss (%) | 8.28 | 9.73 | 8.78 | 9.79 | 9.65 | 8.19 | 0.23 | ns |
| Hardness (N) | 31.88 | 28.05 | 27.96 | 30.60 | 29.04 | 27.17 | 0.07 | ns |
| Adhesiveness (g s) | − 0.63 | − 0.77 | − 0.98 | − 1.20 | − 0.69 | − 0.72 | 0.11 | ns |
| Elasticity (mm) | 0.84 | 0.80 | 0.79 | 0.81 | 0.87 | 0.81 | 0.02 | ns |
| Cohesiveness | 0.50 | 0.45 | 0.47 | 0.46 | 0.49 | 0.44 | 0.01 | ns |
| Gumminess (N) | 16.09 | 12.75 | 13.14 | 14.03 | 12.95 | 11.97 | 0.04 | ns |
| Chewiness (N mm) | 13.53 | 10.20 | 10.40 | 11.88 | 12.36 | 9.71 | 0.06 | ns |
SEM standard error of the mean. Means with the different superscript letters in a row are differ significantly
Sig significance, ns not significant; ***P < 0.001
Colorimetric parameters (L*, a*, b* coordinates) were also significantly (P < 0.001) affected by incorporating protein from plant and algal origin. Lightness (L* coordinate) was lower in patties with added Chlorella (28.40), followed by Spirulina (30.34) than in other samples. This decrease in L* value is mainly due to the different color (green and blue-green pigments) of the protein extracts from microalgae species compared to pulses. Moreover, it could be also promoted by Maillard reactions. The highest L* values were observed in pea (42.84) and lentil (40.38) patties. In a similar way, a significant decrease in values of redness (a*) and yellowness (b*) after replacement by Chlorella (− 2.67 and 5.97, respectively) and Spirulina 3.24 and 11.33, respectively), was observed. This fact can be attributed to chlorophyll pigments.
Concerning to the textural profile analysis (TPA), cooking loss, hardness, adhesivity, elasticity, cohesiveness, gumminess and chewiness of the patties, it was not affected (P > 0.05) by the type of formulation. However, López-López et al. (2009b) showed that the addition of H. elongata extracts led to a twofold increase in hardness and chewiness values, while it reduced the springiness and cohesiveness of the frankfurters. Similar results were reported by Cofrades et al. (2008) in meat gel/emulsion model systems when they evaluated different edible seaweeds, including H. elongata.
Proximate chemical composition of beef patties
Regarding chemical composition (Table 2), no significant changes (P > 0.05) were found in moisture, protein and fat content of patties manufactured using different types of protein. However, significant differences (P < 0.001) were observed for ash contents, especially for patties prepared with lentil protein added, which had a reduction of 6% in ash content compared to control samples. This fact could be related with the high amount of ash in soy (2.2%) compared to the other ones (below 1.5%) (Taghdir et al. 2017). Our results contrast to those reported by López-López et al. (2009b), who reported a lower moisture and protein contents as well as higher ash and fat contents of seaweed-enriched meat products compared to the control sample with soy.
Table 2.
Chemical of beef patties manufactured with different protein sources
| Soy | Pea | Lentil | Bean | Spirulina | Chlorella | SEM | Sig. | |
|---|---|---|---|---|---|---|---|---|
| Moisture | 70.65 | 71.34 | 71.13 | 71.17 | 70.76 | 70.76 | 0.11 | ns |
| Lipids | 6.77 | 6.67 | 6.85 | 6.69 | 6.94 | 6.34 | 0.13 | ns |
| Proteins | 18.25 | 17.84 | 17.93 | 17.92 | 18.06 | 18.20 | 0.07 | ns |
| Ash | 2.15a | 2.08b | 2.02c | 2.11b | 2.10b | 2.11b | 0.01 | *** |
SEM standard error of the mean. Means with the different superscript letters in a row are differ significantly
Sig significance, ns not significant; ***P < 0.001
Impact of protein replacement on amino acid profile of beef patties
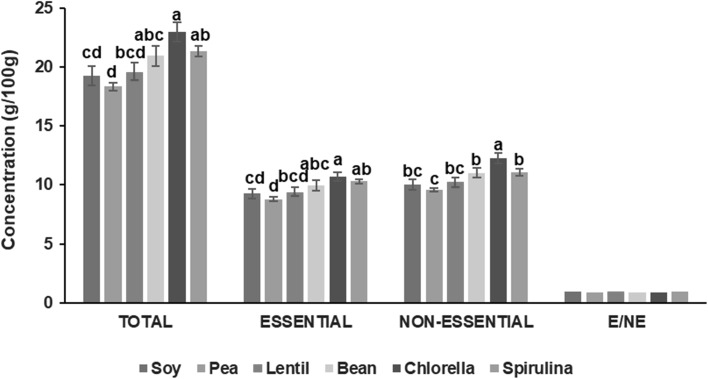
Figure 2 shows the total, essential (E) and non-essential (EN) amino acid contents and E/EN ratio of elaborated beef patties. As can be seen in the figure, significant differences were observed for total (P < 0.01), essential (P < 0.01) and non-essential (P < 0.001) amino acid content. Chlorella patties showed the highest content in the total hydrolyzed amino acids (22.98 g/100 g) followed by Spirulina patties (21.73 g/100 g) and bean patties (20.97 g/100 g), while pea patties exhibited the lowest (18.36 g/100 g) values. The results were concordant with the results found by Parniakov et al. (2018) in chicken rotti elaborated with legumes and algae protein. The same pattern was found to essential and non-essential amino acids. Chlorella patties had a 10.71 g/100 g of essential amino acids followed to Spirulina patties with a 10.31 g/100 g and bean patties with a 9.95 g/100 g and not significant differences was found among the three batches studied. The content of non-essential amino acids for these three patties was 12.25, 11.07 and 11.02 g/100 g, for Chlorella, Spirulina and bean samples, respectively. The content of non-essential amino acids on Chlorella beef patties showed significant differences (P < 0.05) respect to the values found on Spirulina and bean batches.
Fig. 2.
Total, essential (E) and non-essential (NE) amino acid content and E/EN relation of beef patties. Different letters for the formulations represent statistically different results (P < 0.01; P < 0.001)
The amino acid score confirmed that the quality of the protein in algal-meat systems is good from the nutritional standpoint (López-López et al. 2009a). Essential amino acids are those that cannot be synthesized de novo by the organism, and thus must be supplied in human diet. Statistical analysis did not show significant differences on the ratio of essential and non-essential amino acid, presenting values close to 1 for all batches studied. This result indicated that, proportionally essential amino acids content was higher than in other samples, and that the nonessential amino acids content was lower.
The hydrolyzed amino acid profile (Table 3) in the formulated patties included 17 amino acids out of the 20 amino acids constituting food proteins, including the nine essential amino acids. Arginine was included in the essential amino acid group (Domínguez et al. 2015). All amino acids showed significant differences (P < 0.05), except glycine, cysteine and tyrosine. With respect to the control sample (soy protein), the addition of Spirulina followed by Chlorella and bean produced an increase in the levels of all the amino acids, except for histidine and threonine, which did not present any significant differences (Table 3), and showed values higher to those found at the available literature (Cofrades et al. 2017). These results are generally consistent with the study of Dawczynski et al. (2007) and Fleurence (1999) in which they showed that the addition of seaweeds caused some changes in the proportion of several amino acids.
Table 3.
Amino acid content (expressed as g/100 g) of beef patties manufactured with different protein sources
| Soy | Pea | Lentil | Bean | Spirulina | Chlorella | SEM | Sig. | |
|---|---|---|---|---|---|---|---|---|
| Asp | 1.88bc | 1.72c | 1.81bc | 1.98abc | 2.19a | 2.00ab | 0.04 | * |
| Ser | 0.91b | 0.88b | 0.87b | 1.13a | 1.10a | 0.95ab | 0.03 | * |
| Glu | 3.30bc | 3.14c | 3.30bc | 3.51abc | 3.85a | 3.61ab | 0.07 | * |
| Gli | 1.24 | 1.15 | 1.22 | 1.24 | 1.50 | 1.22 | 0.04 | ns |
| Hys | 0.65ab | 0.60b | 0.63ab | 0.69a | 0.70a | 0.68a | 0.01 | * |
| Arg | 1.58bc | 1.51c | 1.60bc | 1.64abc | 1.78a | 1.73ab | 0.03 | * |
| Thr | 0.94abc | 0.86c | 0.91bc | 0.97ab | 1.02a | 1.02a | 0.02 | ** |
| Ala | 1.33bc | 1.23c | 1.32bc | 1.40bc | 1.64a | 1.47ab | 0.03 | ** |
| Pro | 0.87b | 0.83b | 0.94b | 0.96b | 1.16a | 0.95b | 0.03 | ** |
| Cis | 0.18 | 0.16 | 0.18 | 0.18 | 0.20 | 0.22 | 0.01 | ns |
| Tyr | 0.50 | 0.48 | 0.59 | 0.62 | 0.62 | 0.65 | 0.02 | ns |
| Val | 0.92bc | 0.87c | 0.93bc | 1.00ab | 1.10a | 1.03ab | 0.02 | ** |
| Met | 0.08b | 0.06b | 0.08b | 0.12a | 0.12a | 0.12a | 0.01 | ** |
| Lys | 1.83b | 1.76b | 1.86b | 2.00ab | 2.21a | 2.12a | 0.04 | ** |
| Isoleu | 0.89 cd | 0.85d | 0.90bcd | 0.97abc | 1.04a | 0.99ab | 0.02 | ** |
| Leu | 1.58 cd | 1.51d | 1.62bcd | 1.72abc | 1.87a | 1.77ab | 0.03 | ** |
| Phe | 0.80bc | 0.76c | 0.81bc | 0.85ab | 0.88a | 0.86ab | 0.01 | ** |
SEM standard error of the mean. Means with the different superscript letters in a row are differ significantly
Sig significance; *P < 0.05; **P < 0.01
The highest non-essential amino acid in all samples was glutamic acid with values ranging from 3.85 g/100 g in the patties prepared with Spirulina protein to 3.14 g/100 g in the patties formulated with pea protein. Aspartic acid followed in amount importance to glutamic acid, with values of 2.19 g/100 g in samples elaborated with Spirulina protein and 1.75 g/100 g in those with pea protein.
In the case of essential amino acids, lysine was the predominant amino acid followed by leucine and arginine. Lysine presented values ranging from 2.21 g/100 g in patties manufactured with Spirulina protein to 1.76 g/100 g in patties formulated with pea protein. As it is well known, glutamic and aspartic acids are perceived to have a savory, umami taste (Dermiki et al. 2013), which is perceived as tasteful by consumers. Leucine showed values ranging from 1.87 g/100 g in batches manufactured with Spirulina protein to 1.51 g/100 g in those formulated with pea proteins. Moreover, arginine showed values between 1.78 and 1.51 g/100 g in patties prepared with Spirulina and pea proteins, respectively.
Thus, the content of all amino acid was increased when microalgal protein extracts were incorporated into beef patties. The increased content of hydrolyzed amino acids is in agreement with the amount of the hydrolyzed amino acids found in different algae samples. For instance, according to the literature, most of the algal samples contain all essential amino acids and some also present high levels of acidic amino acids (8.0–44.0 g/100 g on a dry basis) and low levels of histidine, threonine, tryptophan, lysine, methionine, and cysteine (Fleurence 2004). However, in our study, the levels of histidine, threonine, and lysine presented were higher to those found in the available literature. As it is well known, and it was confirmed in the present study, Chlorella and Spirulina proteins are a good source of sulfur containing amino acids. For instance, the methionine content of beef patties manufactured with Chlorella and Spirulina proteins was 0.12 g/100 g, equal to the values obtained for beef patties prepared with bean proteins, while patties formulated with lentil and pea protein presented methionine values of 0.08 and 0.06 g/100 g, respectively. From the results obtained, it can be suggested that the soy proteins can be replaced with the bean and algal proteins taking into account only amino acids profile.
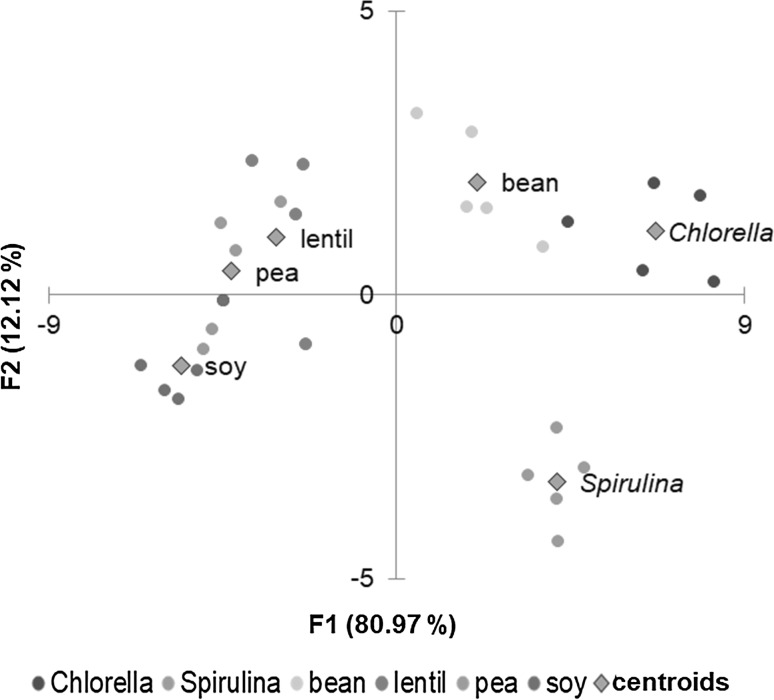
Discriminate analysis defines an optimal combination of varieties in a way that the first function furnishes the most general discrimination between groups, the second provides the second most, and so on (Benitez, et al. 2006). In order to determine which amino acids discriminate between the six beef patties batches a canonical discriminate analysis (CDA) was developed using a new matrix of data integrated by the standardized reduce original variables (Fig. 3). All amino acids that showed significant differences were reconsidered to find which one will contribute most to the discrimination between groups. CDA was able to separate the beef patties according to the amino acid profile and the first two discriminate functions of classification (F1 and F2) accounted 93.09% of the observed variance, so only they were considered in the CDA. F1 explaining 80.97% of total variability and F2 explaining the 12.12% of the variability.
Fig. 3.
Scattered plot of the beef patties projected in the plane defined by two canonical discriminant functions according to amino acid profile
Impact of protein replacement on taste profile of beef patties
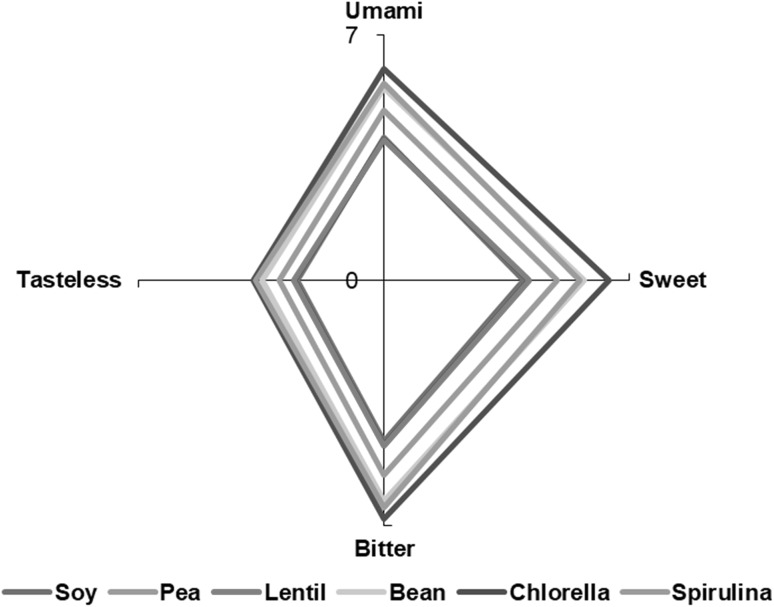
Taste is an important attribute of meat and meat products’ quality and acceptability (Yang et al. 2018). Metabolites responsible for meat taste are those having low molecular weight and non-volatile properties. Free amino acids have an important influence on the assessment of sensory quality and they are also contributing to the taste (Pérez-Santaescolastica et al. 2018a). In such a manner, alanine, glycine, proline, serine, and threonine provide a sweet taste, whereas histidine, allo-isoleucine, isoleucine, leucine, methionine, phenylalanine, tryptophan and valine are related to a bitter taste (Pérez-Santaescolastica et al. 2018b). On the other hand, aspartic acid and glutamic acid are correlated to the umami taste, which is considered as one of the primary tastes (FIB 2017). The taste profiles (Fig. 4) of beef patties formulated with different protein sources did not present any significant changes (P > 0.05). Despite of the fact that the content of most amino acid had significant differences in all patties studied, those differences were mitigated when the individual amino acid were combined to obtain the taste profile. This result is indicative that the replacement of soy by pulses and algal proteins did not affect taste profile of beef patties, being bitter taste the predominant one in all the studied samples.
Fig. 4.
Taste profile of beef patties elaborated with different protein sources
Conclusion
Among the six batches of beef patties studied, there were differences in pH values, colour parameters, ash content, total amino acid content and essential and non-essential amino acids and amino acids profile. The decrease showed in lightness values in beef patties prepared with algal proteins was also normally due to the green and blue-green pigments of the algal extracts. On the other hand, chemical composition was not modified in modified patties and the nutritional values were the same for the six different patties studied. The addition of bean and seaweed proteins increased the concentrations of all amino acids in beef patties. Umami taste amino acids (aspartic and glutamic acids) and lysine were the predominant amino acids found. However, the taste profile was similar in the different protein modified beef patties. The results obtained in this work indicate that Chlorella and Spirulina protein could be useful candidates for the manufacture of new meat products. Considering all the studied parameters, from a nutritional point of view, beef patties formulated with bean protein could be used as an alternative protein source compared to soy protein because it showed a similar nutritional content and taste profile and higher amino acid content.
Acknowledgements
This work was supported by FEDER INTERCONECTA (Grant Number ITC-20151395). The authors would like to thank Algaenergy S.A. (Madrid, Spain) for the Chlorella and Spirulina seaweeds samples supplied for this research. Jose M. Lorenzo is member of the MARCARNE network, funded by CYTED (Ref. 116RT0503). T.Z. holds an award from ERASMUS + TRAINEESHIPS program 2017/18. R.A. would like to acknowledge the République Tunisienne, Ministére de I’Enseignement Supérieur et de Ia Recherche Scientifique, Université de Gabés for the fellowship to do a stay in the Universitat de València. F.J.B. and A.R.J. would like to acknowledge the Croatian Science Foundation for their financing of the project titled “High voltage discharges for green solvent extraction of bioactive compounds from Mediterranean herbs (IP-2016-06-1913)”.
Contributor Information
Francisco J. Barba, Phone: +34 963544972, Email: francisco.barba@uv.es
José M. Lorenzo, Phone: +34 988548277, Email: jmlorenzo@ceteca.net
References
- Agregán R, Munekata PES, Franco D, Dominguez D, Carballo J, Lorenzo JM. Phenolic compounds from three brown seaweed species using LC-DAD–ESI-MS/MS. Food Res Int. 2017;99:979–985. doi: 10.1016/j.foodres.2017.03.043. [DOI] [PubMed] [Google Scholar]
- Agregán R, Munekata PES, Franco D, Dominguez D, Carballo J, Lorenzo JM. Assessment of the antioxidant activity of Bifurcaria bifurcata aqueous extract on canola oil. Effect of extract concentration on the oxidation stability and volatile compound generation during oil storage. Food Res Int. 2017;99:1095–1102. doi: 10.1016/j.foodres.2016.10.029. [DOI] [PubMed] [Google Scholar]
- Agregán R, Munekata PES, Franco D, Dominguez D, Carballo J, Lorenzo JM. Proximate composition, phenolic content and in vitro antioxidant activity of aqueous extracts of the seaweeds Ascophyllum nodosum, Bifurcaria bifurcata and Fucus vesiculosus. Effect of addition of the extracts on the oxidative stability of canola oil under accelerated storage conditions. Food Res Int. 2017;99:986–994. doi: 10.1016/j.foodres.2016.11.009. [DOI] [PubMed] [Google Scholar]
- Agregán R, Munekata PES, Franco D, Carballo J, Barba FJ, Lorenzo JM. Antioxidant potential of extracts obtained from macro- (Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata) and micro-algae (Chlorella vulgaris and Spirulina platensis) assisted by ultrasound. Medicines. 2018;5:33. doi: 10.3390/medicines5020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agregán R, Franco D, Carballo J, Tomasevic I, Barba FJ, Gómez B, Munchenje V, Lorenzo JM. Shelf life study of healthy pork liver pâté with added seaweed extracts from Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Food Res Int. 2018;112:400–411. doi: 10.1016/j.foodres.2018.06.063. [DOI] [PubMed] [Google Scholar]
- AOCS . Official Procedure Am 5-04. Rapid determination of oil/fat utilizing high temperature solvent extraction. Urbana: American Oil Chemists Society; 2005. [Google Scholar]
- Barba FJ. Microalgae and seaweeds for food applications: challenges and perspectives. Food Res Int. 2017;99:969–970. doi: 10.1016/j.foodres.2016.12.022. [DOI] [PubMed] [Google Scholar]
- Barba FJ, Grimi N, Vorobiev E. New approaches for the use of non-conventional cell disruption technologies to extract potential food additives and nutraceuticals from microalgae. Food Eng Rev. 2014;7(1):45–62. doi: 10.1007/s12393-014-9095-6. [DOI] [Google Scholar]
- Barba FJ, Poojary MM, Wang J, Olsen K, Orlien V. Effect of high pressure processing and storage on the free amino acids in seedlings of Brussels sprouts. Innov Food Sci Emerg Technol. 2017;41:188–192. doi: 10.1016/j.ifset.2017.03.004. [DOI] [Google Scholar]
- Becker EW. Microalgae as a source of protein. Biotechnol Adv. 2007;25:207–210. doi: 10.1016/j.biotechadv.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Benitez E, Nogales R, Campos M, Ruano F. Biochemical variability of olive–orchard soils under different management systems. Appl Soil Ecol. 2006;32:221–231. doi: 10.1016/j.apsoil.2005.06.002. [DOI] [Google Scholar]
- Cofrades S, López-López I, Solas MT, Bravo L, Jiménez-Colmenero F. Influence of different types and proportions of added edible seaweeds on characteristics of low-salt gel/emulsion meat systems. Meat Sci. 2008;79(4):767–776. doi: 10.1016/j.meatsci.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Cofrades S, Benedí J, Garcimartin A, Sánchez-Muniz FJ, Jimenez-Colmenero F. A comprehensive approach to formulation of seaweed-enriched meat products: from technological development to assessment of healthy properties. Food Res Int. 2017;99:1084–1094. doi: 10.1016/j.foodres.2016.06.029. [DOI] [PubMed] [Google Scholar]
- Dawczynski C, Schubert R, Jahreis G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007;103:891–899. doi: 10.1016/j.foodchem.2006.09.041. [DOI] [Google Scholar]
- Dermiki M, Phanphensophon N, Mottram DS, Methven L. Contributions of non-volatile and volatile compounds to the umami taste and overall flavour of shiitake mushroom extracts and their application as flavour enhancers in cooked minced meat. Food Chem. 2013;141(1):77–83. doi: 10.1016/j.foodchem.2013.03.018. [DOI] [PubMed] [Google Scholar]
- Domínguez R, Crecente S, Borrajo P, Agregán R, Lorenzo JM. Effect of slaughter age on foal carcass traits and meat quality. Animal. 2015;9:1713–1720. doi: 10.1017/S1751731115000671. [DOI] [PubMed] [Google Scholar]
- Domínguez R, Pateiro M, Agregán R, Lorenzo JM. Effect of the partial replacement of pork backfat by microencapsulated fish oil or mixed fish and olive oil on the quality of frankfurter type sausage. J Food Sci Technol. 2017;54:26–37. doi: 10.1007/s13197-016-2405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos LAA, Lorenzo JM, Gonçalves CAA, dos Santos BA, Heck RT, Cichoski AJ, Campagnol PCB. Production of healthier bologna type sausages using pork skin and green banana flour as a fat replacers. Meat Sci. 2016;121:73–78. doi: 10.1016/j.meatsci.2016.06.001. [DOI] [PubMed] [Google Scholar]
- FIB (Food Ingredients Brazil) (2017) Os aminoácidos e o sabor. Food Ingredients Braz, 31. www.revista-fi.com
- Fleurence J. Seaweed proteins: biochemical, nutritional aspects and potential uses. Trends Food Sci Technol. 1999;10(1):25–28. doi: 10.1016/S0924-2244(99)00015-1. [DOI] [Google Scholar]
- Fleurence J. Seaweed proteins. Proteins in food processing. Cambridge: Woodhead Publishing; 2004. pp. 197–213. [Google Scholar]
- Heck RT, Guidetti R, Etchepare MA, dos Santos LAA, Cichoski AJ, Ragagnin C, Smanioto J, Lorenzo JM, Wagner R, Campagnol PCB. Is it possible to produce a low-fat burger with a healthy n − 6/n − 3 PUFA ratio without affecting the technological and sensory properties? Meat Sci. 2017;130:16–25. doi: 10.1016/j.meatsci.2017.03.010. [DOI] [PubMed] [Google Scholar]
- ISO . Determination of nitrogen content, ISO 937:1978 standard. International standards meat and meat products. Genéve: International Organization for Standardization; 1978. [Google Scholar]
- ISO . Determination of moisture content, ISO 1442:1997 standard. International standards meat and meat products. Genéve: International Organization for Standardization; 1997. [Google Scholar]
- ISO . Determination of ash content, ISO 936:1998 standard. International standards meat and meat products. Genéve: International Organization for Standardization; 1998. [Google Scholar]
- Jiménez-Colmenero F, Delgado Pando G. Fibre-enriched meat products. Fibre-rich and wholegrain foods: improving quality. Amsterdam: Elsevier; 2013. [Google Scholar]
- Kovač D, Simeunović J, Babić O, Mišan A, Milovanović I. Algae in food and feed. Food Feed Res. 2013;40(1):21–31. [Google Scholar]
- López-López I, Bastida S, Ruiz-Capillas C, Bravo L, Larrea MT, Sánchez-Muniz F, et al. Composition and antioxidant capacity of low-salt meat emulsion model systems containing edible seaweeds. Meat Sci. 2009;83(3):492–498. doi: 10.1016/j.meatsci.2009.06.031. [DOI] [PubMed] [Google Scholar]
- López-López I, Cofrades S, Jiménez-Colmenero F. Low-fat frankfurters enriched with n-3 PUFA and edible seaweed: effects of olive oil and chilled storage on physicochemical, sensory and microbial characteristics. Meat Sci. 2009;83(1):148–154. doi: 10.1016/j.meatsci.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Lorenzo JM, Pateiro M. Influence of type of muscles on nutritional value of foal meat. Meat Sci. 2013;93:630–638. doi: 10.1016/j.meatsci.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Lorenzo JM, Purriños L, Temperán S, Bermúdez R, Tallón S, Franco D. Physicochemical and nutritional composition of dry-cured duck breast. Poult Sci. 2011;90(4):931–940. doi: 10.3382/ps.2010-01001. [DOI] [PubMed] [Google Scholar]
- Lorenzo JM, Sarriés MV, Tateo A, Polidori P, Franco D, Lanza M. Carcass characteristics, meat quality and nutritional value of horsemeat: a review. Meat Sci. 2014;96:1478–1488. doi: 10.1016/j.meatsci.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Lorenzo JM, Sineiro J, Amado IR, Franco D. Influence of natural extracts on the shelf life of modified atmosphere-packaged pork patties. Meat Sci. 2014;96:526–534. doi: 10.1016/j.meatsci.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Lorenzo JM, Munekata PES, Pateiro M, Campagnol PCB, Dominguez D. Healthy Spanish salchichón enriched with encapsulated n − 3 long chain fatty acids in konjac glucomannan matrix. Food Res Int. 2016;86:289–295. doi: 10.1016/j.foodres.2016.08.012. [DOI] [PubMed] [Google Scholar]
- Lorenzo JM, Agregán R, Munekata PE, Franco D, Carballo J, Şahin S, Barba FJ. Proximate composition and nutritional value of three macroalgae: Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Mar Drugs. 2017;15(11):360. doi: 10.3390/md15110360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupatini AL, Colla LM, Canan C, Colla E. Potential application of microalga Spirulina platensis as a protein source. J Sci Food Agric. 2017;97(3):724–732. doi: 10.1002/jsfa.7987. [DOI] [PubMed] [Google Scholar]
- Madeira MS, Cardoso C, Lopes P, Coelho D, Afonso C, Bandarra NM, Prates JAM. Microalgae as feed ingredients for livestock production and meat quality: a review. Livest Sci. 2017;205:111–121. doi: 10.1016/j.livsci.2017.09.020. [DOI] [Google Scholar]
- Milovanović I, Mišan AT, Šarić B, Kos J, Mandić AI, Simeunović J, Kovač D (2012) Evaluation of protein and lipid content and determination of fatty acid profile in selected species of cyanobacteria. In: Proceedings of 6th Central European Congress on Food, CEFood 2012, 13–17
- Neacsu M, McBey D, Johnstone AM. Chapter 22: Meat reduction and plant-based food: replacement of meat: nutritional, health, and social aspects. In: Nadathur SR, Wanasundara JPD, Scanlin L, editors. Sustainable protein sources. San Diego: Academic Press; 2017. pp. 359–375. [Google Scholar]
- Parniakov O, Apicella E, Koubaa M, Barba FJ, Grimi N, Lebovka N, et al. Ultrasound-assisted green solvent extraction of high-added value compounds from microalgae Nannochloropsis spp. Bioresour Technol. 2015;198:262–267. doi: 10.1016/j.biortech.2015.09.020. [DOI] [PubMed] [Google Scholar]
- Parniakov O, Barba FJ, Grimi N, Marchal L, Jubeau S, Lebovka N, Vorobiev E. Pulsed electric field and pH assisted selective extraction of intracellular components from microalgae Nannochloropsis. Algal Res. 2015;8:128–134. doi: 10.1016/j.algal.2015.01.014. [DOI] [Google Scholar]
- Parniakov O, Barba FJ, Grimi N, Marchal L, Jubeau S, Lebovka N, Vorobiev E. Pulsed electric field assisted extraction of nutritionally valuable compounds from microalgae Nannochloropsis spp. using the binary mixture of organic solvents and water. Innov Food Sci Emerg Technol. 2015;27:79–85. doi: 10.1016/j.ifset.2014.11.002. [DOI] [Google Scholar]
- Parniakov O, Teopfl S, Barba FJ, Granato D, Zamuz S, Galvez F, Lorenso JM. Impact of the soy protein replacement by legumes and algae based proteins on the quality on chicken rotti. J Food Sci Technol. 2018;55:2552–2559. doi: 10.1007/s13197-018-3175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pateiro M, Lorenzo JM, Diaz S, Gende JA, Fernandez M, Gonzalez J, Garcia L, Rial FJ, Franco D. Meat quality of veal: discriminatory ability of weaning status. Span J Agric Res. 2013;11:1044–1056. doi: 10.5424/sjar/2013114-4363. [DOI] [Google Scholar]
- Pérez-Santaescolastica C, Carballo C, Fulladosa E, Garcia-Perez JV, Benedito J, Lorenzo JM. Application of temperature and ultrasound as corrective measures to decrease the adhesiveness in dry-cured ham. Influence on free amino acid and volatile compound profile. Food Res Int. 2018 doi: 10.1016/j.foodres.2018.08.006. [DOI] [PubMed] [Google Scholar]
- Pérez-Santaescolastica C, Carballo C, Fulladosa E, Garcia-Perez JV, Benedito J, Lorenzo JM. Effect of proteolysis index level on instrumental adhesiveness, free amino acids content and volatile compounds profile of dry-cured ham. Food Res Int. 2018;107:559–566. doi: 10.1016/j.foodres.2018.03.001. [DOI] [PubMed] [Google Scholar]
- Poojary M, Barba F, Aliakbarian B, Donsì F, Pataro G, Dias D, Juliano P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar Drugs. 2016;14(11):214. doi: 10.3390/md14110214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma PR, Miron T, Olivieri G, Barbosa MJ, Wijffels R, Eppink M. Mild disintegration of the green microalgae Chlorella vulgaris using bead milling. Bioresour Technol. 2014;184:297–304. doi: 10.1016/j.biortech.2014.09.033. [DOI] [PubMed] [Google Scholar]
- Rinaldoni AN, Palatnik DR, Zaritzky N, Campderrós ME. Soft cheese-like product development enriched with soy protein concentrates. LWT Food Sci Technol. 2014;55(1):139–147. doi: 10.1016/j.lwt.2013.09.003. [DOI] [Google Scholar]
- Roohinejad S, Koubaa M, Barba FJ, Saljoughian S, Amid M, Greiner R. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Res Int. 2017;99:1066–1083. doi: 10.1016/j.foodres.2016.08.016. [DOI] [PubMed] [Google Scholar]
- Roselló-Soto E, Galanakis CM, Brnčić M, Orlien V, Trujillo FJ, Mawson R, et al. Clean recovery of antioxidant compounds from plant foods, by-products and algae assisted by ultrasounds processing. Modeling approaches to optimize processing conditions. Trends Food Sci Technol. 2015;42(2):134–149. doi: 10.1016/j.tifs.2015.01.002. [DOI] [Google Scholar]
- Suess B, Festring D, Hofmann T. Umami compounds and taste enhancers. In: Parker JK, Elmore JS, Methven L, editors. Flavour development, analysis and perception in food and beverages. Cambridge: Woodhead Publishing; 2015. pp. 331–351. [Google Scholar]
- Taghdir M, Mazloomi SM, Honar N, Sepandi M, Ashourpour M, Salehi M. Effect of soy flour on nutritional, physicochemical, and sensory characteristics of gluten-free bread. Food Sci Nutr. 2017;5(3):439–445. doi: 10.1002/fsn3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursu A-V, Marcati A, Sayd T, Sante-Lhoutellier V, Djelveh G, Michaud P. Extraction, fractionation and functional properties of proteins from the microalgae Chlorella vulgaris. Bioresour Technol. 2014;157(Supplement C):134–139. doi: 10.1016/j.biortech.2014.01.071. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ye Y, Wang Y, Sun Y, Pan D, Cao J. Effect of high pressure treatment on metabolite profile of marinated meat in soy sauce. Food Chem. 2018;240:662–669. doi: 10.1016/j.foodchem.2017.08.006. [DOI] [PubMed] [Google Scholar]
- Zamuz S, López-Pedrouso M, Barba FJ, Lorenzo JM, Domínguez H, Franco D. Application of hull, bur and leaf chestnut extracts on the shelf-life of beef patties stored under MAP: evaluation of their impact on physicochemical properties, lipid oxidation, antioxidant, and antimicrobial potential. Food Res Int. 2018;112:263–273. doi: 10.1016/j.foodres.2018.06.053. [DOI] [PubMed] [Google Scholar]
- Zhang L, Peterson DG. Identification of a novel umami compound in potatoes and potato chips. Food Chem. 2018;240:1219–1226. doi: 10.1016/j.foodchem.2017.08.043. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Wu Q, Di X, Li S, Barba FJ, Koubaa M, et al. Multistage recovery process of seaweed pigments: investigation of ultrasound assisted extraction and ultra-filtration performances. Food Bioprod Process. 2017;104:40–47. doi: 10.1016/j.fbp.2017.04.008. [DOI] [Google Scholar]