Abstract
Aim:
The primary aim of this study was to evaluate the association between chest compression rates and 1) arterial blood pressure and 2) survival outcomes during pediatric in-hospital cardiopulmonary resuscitation (CPR).
Methods:
Prospective observational study of children ≥37 weeks gestation and <19 years old who received CPR in an intensive care unit (ICU) as part of the Pediatric Intensive Care Unit Quality of CPR Study (PICqCPR) of the Collaborative Pediatric Critical Care Research Network (CPCCRN). Arterial blood pressure and compression rate were determined from manually extracted arterial line waveform data during the first 10 minutes of CPR. The primary outcome was survival to hospital discharge. Modified Poisson regression models assessed the association between rate categories (80-<100, 100–120 [Guidelines], >120–140, >140) and outcomes.
Results:
Compression rate data were available for 164 patients. More than half (98/164; 60%) were < 1 year old. Return of circulation was achieved in 148/164 (90%); survival to hospital discharge in 77/164 (47%). Percentage of events with average rate within Guidelines was 32.9%. Compared to Guidelines, higher rate categories were associated with lower systolic blood pressures (>120–140, p=0.010; >140, p=0.077), but not survival. A rate between 80-<100 per minute was associated with a higher rate of survival to hospital discharge (aRR 1.92, CI95 1.13, 3.29, p=0.017) and survival with favorable neurological outcome (aRR 2.12, CI95 1.09, 4.13, p=0.027) compared to Guidelines.
Conclusion:
Non-compliance with compression rate Guidelines was common in this multicenter cohort. Among ICU patients, slightly lower rates were associated with improved outcomes compared to Guidelines.
Keywords: cardiac arrest, cardiopulmonary resuscitation, intensive care, unit, pediatric
Introduction
Thousands of hospitalized children are treated with cardiopulmonary resuscitation (CPR) for a cardiac arrest each year in the United States (1). Although survival rates have been improving over the last 20 years, still more than half of these children do not live to hospital discharge (2). Of those who survive, neurological morbidity is common (1).
CPR quality has been implicated as a modifiable risk factor to improve survival from cardiac arrest (3–8). Several large adult studies have demonstrated that achieving evidence-based targets for chest compression rate (4) and depth (8), release velocity (i.e., recoil between compressions)(9), and chest compression fraction (5, 6) (i.e., percentage of time that compressions are provided during arrest) improves survival. Unfortunately, most of the corresponding pediatric data has been collected from out-of-hospital resuscitations (10) or from single center studies (3, 11–14), and may not be generalizable. Thus, larger prospective pediatric studies that can evaluate the association between CPR quality metrics and outcomes are necessary.
To that end, the Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN)(15) Pediatric Intensive Care Unit Quality of CPR (PICqCPR)(16) study provides a unique opportunity to evaluate chest compression rates across several pediatric institutions. This study prospectively collected data on pediatric cardiac arrests that occurred in the network ICUs over a three-year period. Using this dataset, the objectives of this investigation were to 1) quantitatively describe compression rates during pediatric cardiac arrest in a multi-institutional collaborative, 2) describe variability in compression rates across the institutions, and 3) associate compression rate with both arterial blood pressure and survival outcomes.
Methods
Setting and Design
CPCCRN is a network of pediatric institutions that conducts investigations related to pediatric critical care practice in their pediatric and pediatric cardiac ICUs (15). The clinical sites and the data coordinating center (DCC) supporting the Network have been funded by the National Institute of Child Health and Human Development since 2004. Further details on the Network can be found at https://www.cpccrn.org.
Between July 2013 and June 2016, CPCCRN conducted the PICqCPR Study to evaluate the association between invasively monitored arterial blood pressures during pediatric CPR and cardiac arrest survival outcomes (16). This study represents a secondary observational analysis of this multi-center cohort study.
PICqCPR was approved with waiver of informed consent by the Institutional Review Board at each clinical site and the DCC. Data collected on subjects included Utstein-style standardized cardiac arrest and CPR data (17), with assessments of neurological outcomes (pediatric cerebral performance category (PCPC)(18) and functional status scale (FSS)(19, 20)) for pre-admission status and at hospital discharge. Because the primary objective of PICqCPR was to associate arterial blood pressure with outcomes, all patients had an arterial line in place at the time of the arrest. Blood pressures were extracted from arterial waveform printouts by manual digitization (PlotDigitizer; Version 2.0; Department of Physics, University of South Alabama). Please see previous publication for more details regarding the methods of the CPCCRN PICqCPR study (16).
Patient Population
All children ≥37 weeks gestation and <19 years old who received external chest compressions for at least 1 minute and who had invasive arterial blood pressure monitoring prior to and during CPR in a CPCCRN ICU were eligible for inclusion. Subjects were excluded if the first compression was not captured on the waveform data or compression rate was unable to be determined (e.g., lack of arterial waveform due to line interruption for blood draw, flushing, pressure zeroing).
Measurements
The first 10 minutes of CPR data were collected for each event. For each one minute epoch, the following data points were extracted from the waveform data: 1) the number of compressions given; 2) the time (sec) that compressions were not being performed (pause time); 3) total time (sec) that rate could not be determined (e.g., missing data due to arterial line interruption); and 4) mean SBP and DBP (mmHg). Chest compression rate was defined as the “instantaneous” rate between adjacent compressions during periods of uninterrupted compression delivery by the following equation: 60 / time between compressions (sec). Chest compression fraction (CCF; proportion of time compressions are performed during arrest) was defined as: 1 – (pause time / (60 – missing data time)). For each minute of CPR, an average of compression rate, CCF, systolic blood pressure, and diastolic blood pressure was calculated (minute-level average), and then for each event, the average of all the available epochs was calculated (event-level average). Interruptions in compression delivery were defined as any interruption in compression delivery > 1.5 seconds (i.e., a compression rate < 40). Compliance with American Heart Association (AHA) Guidelines was defined as a compression rate of 100–120 per minute (21). Of note, CPR recording defibrillators were not commonly used in the Network at the time of this study. As such, other important CPR quality variables such as compression depth (11) and release velocity (9) were not able to be considered in the analysis.
Outcomes
The prospectively selected primary outcome was survival to hospital discharge of index events. Secondary outcomes included: 1) return of spontaneous circulation (ROSC) of all events; 2) diastolic blood pressure (mmHg); 3) systolic blood pressure (mmHg); and 4) survival with favorable neurological outcome (PCPC 1–3 or no worsening)(17, 18) of index events.
Statistical Analysis
Patient and event characteristics were summarized using frequencies and percentages or median and interquartile ranges (IQR). Differences in these characteristics between those who did and did not survive to discharge were examined using Fisher’s exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables (Table 1). Minute-level averages were used to determine the association between arterial blood pressure and compression rate, while event-level averages were used for the survival outcome models. The reference group in all models was a rate of 100–120 per minute (Guideline recommendations)(21). Guideline recommendation for CCF of 0.80 was also utilized (21, 22). In all models, compression rate was categorized into 4 groups: < 100; 100–120; >120–140; and >140 per minute. Modified Poisson regression models were used to calculate relative risks (RRs) of achieving the patient outcome of interest across rate categories (Table 4). Survival models were adjusted for age (23), initial cardiac rhythm (24), illness category (25), and time of CPR (26), which were specified a priori based on previously established associations with inhospital cardiac arrest outcomes. Backward selection with an exit criterion of p>0.1 was then used for inclusion of other candidate variables. For arterial blood pressures, mixed effects linear regression models were used with an AR-1 correlation structure to account for the correlation between minutes of an event (Table 3). Blood pressure models were adjusted for fluid bolus and vasopressor administration during arrest, and backward selection with an exit criterion of p>0.1 was used to consider additional covariates. Site was considered as a potential confounder in all models due to known differences in survival rates across the clinical sites in the network (27). A mixed effects linear regression model with site as the fixed effect and an AR-1 correlation structure for minutes within an event was used to determine if there was significant variability in the average minute-level compression rate between sites. Similarly, a modified Poisson regression model with an AR-1 correlation structure and site as a fixed effect was used to determine if site was associated with higher likelihood of a minute of CPR having an average rate within Guidelines. Analyses of survival used only index events, while blood pressure and ROSC analyses included all events. Relative risks are presented with their 95% confidence intervals (CIs). P-values are reported based on a 2-sided alternative and considered statistically significant when less than 0.05.
Table 1:
Patient Characteristics by Survival to Hospital Discharge
| Survival to hospital discharge | ||||
|---|---|---|---|---|
| No (N = 87) |
Yes (N = 77) | Overall (N = 164) | P-value | |
| Age | 0.0381 | |||
| < 1 year | 45 (51.7%) | 53 (68.8%) | 98 (59.8%) | |
| >= 1 year | 42 (48.3%) | 24 (31.2%) | 66 (40.2%) | |
| Sex | 0.1581 | |||
| Male | 43 (49.4%) | 47 (61.0%) | 90 (54.9%) | |
| Female | 44 (50.6%) | 30 (39.0%) | 74 (45.1%) | |
| Race | 0.2481 | |||
| Unknown or Not Reported | 14 (16.1%) | 23 (29.9%) | 37 (22.6%) | |
| White | 45 (51.7%) | 37 (48.1%) | 82 (50.0%) | |
| Black or African American | 25 (28.7%) | 12 (15.6%) | 37 (22.6%) | |
| Other | 3 (3.4%) | 5 (6.5%) | 8 (4.9%) | |
| Preexisting conditions | ||||
| Respiratory insufficiency | 73 (83.9%) | 59 (76.6%) | 132 (80.5%) | 0.3241 |
| Hypotension | 77 (88.5%) | 51 (66.2%) | 128 (78.0%) | <.0011 |
| Congestive heart failure | 12 (13.8%) | 7 (9.1%) | 19 (11.6%) | 0.4651 |
| Pneumonia | 5 (5.7%) | 8 (10.4%) | 13 (7.9%) | 0.3861 |
| Sepsis | 24 (27.6%) | 20 (26.0%) | 44 (26.8%) | 0.8611 |
| Renal insufficiency | 16 (18.4%) | 8 (10.4%) | 24 (14.6%) | 0.1861 |
| Malignancy | 4 (4.6%) | 1 (1.3%) | 5 (3.0%) | 0.3721 |
| Congenital heart disease | 44 (50.6%) | 55 (71.4%) | 99 (60.4%) | 0.0071 |
| Illness Category | 0.0901 | |||
| Surgical cardiac | 39 (44.8%) | 49 (63.6%) | 88 (53.7%) | |
| Medical cardiac | 17 (19.5%) | 8 (10.4%) | 25 (15.2%) | |
| Surgical non-cardiac | 8 (9.2%) | 5 (6.5%) | 13 (7.9%) | |
| Medical non-cardiac | 23 (26.4%) | 14 (18.2%) | 37 (22.6%) | |
| Unknown | 0 (0.0%) | 1 (1.3%) | 1 (0.6%) | |
| Initial cardiac rhythm | 0.0681 | |||
| Asystole/PEA | 29 (33.3%) | 19 (24.7%) | 48 (29.3%) | |
| VF/VT | 14 (16.1%) | 5 (6.5%) | 19 (11.6%) | |
| Bradycardia with pulses | 43 (49.4%) | 48 (62.3%) | 91 (55.5%) | |
| Unknown | 1 (1.1%) | 5 (6.5%) | 6 (3.7%) | |
| Baseline PCPC score | 0.4522 | |||
| 1 - Normal | 45 (51.7%) | 32 (41.6%) | 77 (47.0%) | |
| 2 - Mild disability | 21 (24.1%) | 26 (33.8%) | 47 (28.7%) | |
| 3 - Moderate disability | 10 (11.5%) | 13 (16.9%) | 23 (14.0%) | |
| 4 - Severe disability | 7 (8.0%) | 6 (7.8%) | 13 (7.9%) | |
| 5 - Coma/vegetative state | 4 (4.6%) | 0 (0.0%) | 4 (2.4%) | |
| Baseline total FSS | 7.0 [6.0,11.0] | 8.0 [6.0,11.0] | 8.0 [6.0,11.0] | 0.1202 |
| Interventions in place | ||||
| Vascular access | 83 (95.4%) | 72 (93.5%) | 155 (94.5%) | 0.736 |
| Arterial catheter | 87 (100.0%) | 76 (98.7%) | 163 (99.4%) | 0.4701 |
| Central venous catheter | 76 (87.4%) | 66 (85.7%) | 142 (86.6%) | 0.8211 |
| Vasoactive infusion | 75 (86.2%) | 53 (68.8%) | 128 (78.0%) | 0.0081 |
| Invasive mechanical ventilation | 77 (88.5%) | 57 (74.0%) | 134 (81.7%) | 0.0251 |
| Immediate Cause | ||||
| Hypotension | 61 (70.1%) | 49 (63.6%) | 110 (67.1%) | 0.4081 |
| Arrhythmia | 17 (19.5%) | 14 (18.2%) | 31 (18.9%) | 0.8451 |
| Respiratory decompensation | 38 (43.7%) | 34 (44.2%) | 72 (43.9%) | 1.0001 |
| CPR Time Category | 0.3371 | |||
| Weekday | 51 (58.6%) | 51 (66.2%) | 102 (62.2%) | |
| Weeknight/Weekend | 36 (41.4%) | 26 (33.8%) | 62 (37.8%) | |
| Duration of CPR (minutes) | 17.5 [4.0,38.0] | 5.0 [2.0,13.0] | 8.0 [3.0,27.0] | <.001 |
| Epinephrine | 78 (89.7%) | 65 (84.4%) | 143 (87.2%) | 0.3551 |
| Total number of epinephrine doses | 3.0 [2.0,7.0] | 2.0 [1.0,3.0] | 3.0 [1.0,5.0] | <.0012 |
| Calcium | 50 (57.5%) | 28 (36.4%) | 78 (47.6%) | 0.0081 |
| Sodium bicarbonate | 57 (65.5%) | 36 (46.8%) | 93 (56.7%) | 0.0181 |
Fisher’s Exact Test is used for 2×2 tables. Categorical variables are summarized using n (%).
The Wilcoxon rank-sum test is used for ordinal variables. Continuous variables are summarized using median [Q1,Q3].
Table 4:
Multivariable Association of Recommended Chest Compression Rates with Outcomes
| Adjusted Relative Risk of Outcome (95% CI) |
P-value | |
|---|---|---|
| ROSCa | ||
| Rate in Guidelines | ||
| Yes | Reference | |
| No | 1.02 (0.83, 1.25) | 0.876 |
| Rate Category | ||
| <100 | 1.34 (0.98,1.82) | 0.069 |
| 100–120 (Guidelines) | Reference | |
| >120–140 | 0.92 (0.72, 1.18) | 0.511 |
| >140 | 1.13 (0.89,1.43) | 0.322 |
| Survival to Hospital Dischargeb | ||
| Rate in Guidelines | ||
| Yes | Reference | |
| No | 1.27 (0.84, 1.92) | 0.257 |
| Rate Category | ||
| <100 | 1.92 (1.13, 3.29) | 0.017 |
| 100–120 (Guidelines) | Reference | |
| >120–140 | 1.15 (0.74, 1.80) | 0.527 |
| >140 | 1.31 (0.79, 2.18) | 0.293 |
| Survival with Favorable Neuroc | ||
| Rate in Guidelines | Reference | |
| Yes | ||
| No | 1.54 (0.96, 2.49) | 0.246 |
| Rate Category | ||
| <100 | 2.12 (1.09, 4.13) | 0.027 |
| 100–120 (Guidelines) | Reference | |
| >120–140 | 1.42 (0.85, 2.37) | 0.177 |
| >140 | 1.62 (0.93, 2.82) | 0.085 |
Results are based on a modified Poisson model adjusting for age (<1 year, >= 1 year), initial cardiac rhythm, illness category, time of CPR (night/weekend), baseline PCPC score, and vascular access. All CPR events are considered for this analysis.
Results are based on a modified Poisson model adjusting for age (<1 year, >= 1 year), initial cardiac rhythm, illness category, time of CPR (night/weekend), vasoactive infusion, and invasive mechanical ventilation. All index CPR events are considered for this analysis.
Results are based on a modified Poisson model adjusting for age (<1 year, >= 1 year), initial cardiac rhythm, illness category, time of CPR (night/weekend), and vasoactive infusion. All index CPR events are considered for this analysis.
Table 3:
Multivariable Association Between Minute-Level Compression Rate and Blood Pressures
| Diastolic blood pressurec | Systolic blood pressured | |||
|---|---|---|---|---|
| Effect estimate (95% CI) | P-value | Effect estimate (95% CI) | P-value | |
| Chest compression rate (per minute)a | −0.02 (−0.06, 0.03) | 0.427 | −0.17 (−0.27, −0.07) | < 0.001 |
| Chest compression rate (per minute)b | 0.148 | 0.083 | ||
| 80-<100 (n=57) | −1.33 (−3.53, 0.87) | 0.237 | 2.55 (−4.57, 9.68) | 0.483 |
| 100–120 (n=323) | Reference | Reference | ||
| >120–140 (n=350) | 0.15 (−1.08, 1.38) | 0.813 | −4.07 (−7.17, −0.97) | 0.010 |
| >140 (n=204) | 1.62 (−0.31, 3.56) | 0.099 | −4.57 (−9.63, 0.50) | 0.077 |
Chest compression rate is analyzed as a continuous predictor and an AR-1 correlation structure is assumed to control for minute-level correlations within events. In the continuous models, minutes of CPR with compression rates less than 100 are excluded because the linearity assumption was only satisfied for rates greater than or equal to 100.
Chest compression rate is analyzed as a categorical predictor and an AR-1 correlation structure is assumed to control for minute-level correlations within events. Every minute is included in the categorical models.
The DBP models adjust for vasopressor administration, fluid bolus, sex, CPR location (PICU/CICU), arrhythmia as an immediate cause, respiratory decompensation as an immediate cause, and age (< 1 year, >= 1 year).
The SBP models adjust for vasopressor administration, fluid bolus, initial cardiac rhythm, vascular access, non-invasive ventilation, respiratory decompensation as an immediate cause, and age (< 1 year, >- 1 year).
Results
Between July 2013 and June 2016, there were 244 index CPR events in patients who received at least 1 minute of chest compressions and who had an arterial line in place at the time of the arrest making them eligible for inclusion in PICqCPR. Of these, 80 subjects were excluded due to inability to determine arterial diastolic blood pressure (n=36) or inability to determine starts and stops in chest compressions (n=44), leaving 164 (67%) patients in the analytical cohort. The range of events reported per site was 4 to 70. The subjects for this secondary analysis are the same 164 patients who were included in the main study. Please see Figure 1 for more details.
Figure 1:
Utstein-style flow diagram of patients included in this secondary analysis of the Pediatric Intensive Care Quality of Cardiopulmonary Resuscitation (PICqCPR) study. ROSC indicates Return of Spontaneous Circulation; ROC, return of circulation with extracorporeal support; neuro, neurological; favorable neuro outcome, Pediatric Cerebral Performance Category of 1–3 or no worse.
Patient and index event characteristics are contained in Table 1. More than half of the patients (98/164; 60%) were < 1 year old and more than half were also classified as cardiac surgical patients (88/164; 54%). Respiratory insufficiency (132/164; 80%), hypotension (128/164; 78%), and congenital heart disease (99/164; 60%) were the most common preexisting conditions. Hypotension (110/164; 67%) and respiratory decompensation (72/164; 44%) were common immediate causes of arrest. Among the pre-arrest patient characteristics, pre-existing hypotension was associated with a significantly lower rate of survival to hospital discharge and congenital heart disease with a higher rate. Among the index event characteristics, lower survival rates were associated with vasoactive infusions and invasive mechanical ventilation in place at the time of event, longer CPR duration, number of epinephrine doses, and the administration of either calcium or sodium bicarbonate during CPR. The characteristics and outcomes of the 164 patients included in the main study, and in this secondary analysis, were similar to those excluded (16).
Outcomes are summarized in Figure 1. Ninety percent of patients survived the event (68% with ROSC; 22% by extracorporeal life support during CPR (E-CPR)). Forty seven percent survived to hospital discharge and 43% survived to discharge with favorable neurologic outcome. Among the 77 patients surviving to discharge, 70 (91%) had favorable neurologic outcome. Outcomes of subcohort requiring E-CPR are in Supplementary eTable1.
The summaries of compression rate and CCF are contained in Table 2. The median eventlevel average compression rate was 125.6 [IQR 114.3, 139.4]. Percentage of events with average compression rate within Guideline recommendations was 32.9%. Percentage of minutes with average compression rate within Guideline recommendations was 32.6%. The median CCF was 0.92 [IQR 0.84, 0.97]. Neither average rate nor CCF was different between those who did and did not survive to hospital discharge.
Table 2:
Compression Rate & Chest Compression Fraction by Survival to Hospital Discharge
| Survival to Hospital Discharge | ||||
|---|---|---|---|---|
| No | Yes | Overall | P-value | |
| Event Average Compression Rate | 124.4 [114.9,138.1] |
127.1 [110.8,140.3] |
125.6 [114.3,139.4] |
0.7742 |
| Percent of Events: Rate 100–120 | 39.1% | 26.0% | 32.9% | 0.0961 |
| Percent of Minutes: Rate 100–120 | 33.5% | 31.2% | 32.6% | 0.3963 |
| Event Average CCF | 0.92 [0.84,0.97] | 0.91 [0.84,0.96] | 0.92 [0.84,0.97] | 0.4372 |
Fisher’s exact test is used for comparison of categorical variables.
The Wilcoxon rank-sum test is used for comparison of continuous variables. Continuous variables are summarized using median [Q1,Q3].
The association between percent of minutes in guidelines and survival to hospital discharge was assessed using a modified Poisson model assuming an AR (1) correlation structure for minutes within an event.
Across sites, there was significant variability in average delivered compression rates (p=0.001). Additionally, there was a trend of association between site and likelihood of an event achieving Guideline recommendations for rate (p=0.058). Please see Supplementary eFigure 1 for a graphical representation of the variability in compression rates across this cohort.
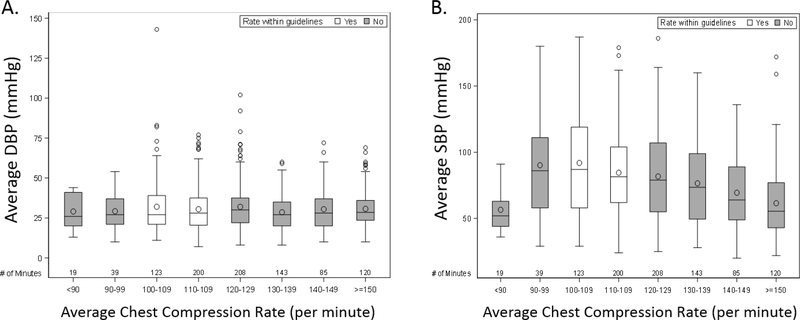
The association between compression rates and arterial blood pressures is summarized in Table 3. In the top part of the table, compression rate was modeled as a continuous variable. Among rates ≥ 100 per minute, there is a statistically significant decline in systolic blood pressure (point estimate 1.7 mmHg; CI95 2.7, 0.7; p<0.001) for every 10 compression per minute increase in rate. In the bottom part of the table, chest compression rate was modeled as a categorical predictor with Guideline recommendations (100–120 per minute) as the reference category. In this model, compared to Guidelines, systolic blood pressure was lower when compressions were performed between >120–140/min (point estimate −4.07; CI95 −7.17, −0.97; p=0.010) and tended to be lower when >140/min (point estimate −4.57; CI95 −9.63, 0.50; p=0.077). In neither model was there a significant relationship between diastolic blood pressure and compression rate. Please see Figure 2 for a graphical representation of the relationship between minute-level average compression rates and average diastolic (A) and systolic (B) blood pressures.
Figure 2:
Box plot minute-level average diastolic blood pressures (A) and systolic blood pressures (B) across compression rate categories. DBP indicates diastolic blood pressure, SBP, systolic blood pressure. Circles inside of each box represent the mean BP. The horizontal line inside of each box represents the median BP. The bottom and top edges of the box indicate the interquartile range (IQR). The whiskers that extend from each box indicate the range of values that are outside of the IQR (up to a distance of 1.5*IQR from the box). Any points that are more than 1.5*IQR from the box are considered outliers and are represented by circles beyond the edge of the whiskers.
The association between compression rates and outcomes is summarized in Table 4. Compared to Guidelines, a rate between 80–100 per minute was associated with a higher rate of survival to hospital discharge (aRR 1.92, CI95 1.13, 3.29, p=0.017) and survival with favorable neurological outcome (aRR 2.12, CI95 1.09, 4.13, p=0.027). Of note, among the 8 patients in this category, 6 (75%) had average rates ≥90 per minute (median of category: 94.6 per minute).
Discussion
In this study, event-level average compression rates were frequently outside Guideline recommendations, with nearly 70% of events not achieving the target. Across study sites, there was significant variability in the rate at which compressions were delivered (p=0.001) and a trend towards some sites being more likely to achieve Guidelines compared to others (0.058). These data establish that rates exceeding Guideline recommendations are associated with mildly decreased systolic blood pressure, but not differences in diastolic blood pressure or survival outcomes. Compared to Guidelines, a rate between 80-<100 per minute was associated with increased rates of survival, but not differences in blood pressures during CPR.
There has been a growing emphasis on the delivery of high quality CPR to improve outcomes from cardiac arrest (22). Unfortunately, there is little data collected from children during cardiac arrest to support pediatric evidence-based guidelines. As many current CPR recording defibrillators are either not approved for infants or use pads that are too large for most infants, even the limited data that exists regarding pediatric CPR quality has been collected during the resuscitation of older children and adolescents (13). By using monitor waveforms, this study overcomes these technological limitations.
Animal and adult out-of-hospital cardiac arrest studies have demonstrated that faster compression rates (>120/min) may be associated with decreased coronary perfusion pressures (28) and lower survival rates (4, 29). Although of questionable clinical significance (~4mmHg change in systolic blood pressure), our results similarly demonstrate a potentially detrimental physiologic consequence at faster rates (Figure 2b). We propose two possible mechanisms. First, during compression, the generated arterial pressure is related to the amount of blood available for ejection during the compression phase. If compressions are delivered too quickly, diastolic filling time is reduced and the amount of blood available for the next compression phase may be limited. In addition, other studies have demonstrated an inverse relationship between compression depth and rate (i.e., higher rates lead to shallower compressions). Given the relationship between compression depth/force and arterial pressure (30, 31), it is not surprising that faster rates would lead to lower arterial pressures, particularly systolic pressure, if depth was compromised. In the end, irrespective of the underlying mechanism, these data provide some evidence that rescuers should be cautious when providing faster compressions. As mean arterial pressure, mathematically defined as [(2 × diastolic pressure) + systolic pressure] /3, is a primary determinant of cerebral perfusion pressure and cerebral blood flow, it is reasonable to speculate that lower systolic blood pressures during CPR could lead to lower cerebral perfusion and have clinical implications.
While a compression rate between 80-<100 per minute was associated with improved survival, this statistical finding must be interpreted with caution given the following factors. First, there were only 8 patients in this category and as such, despite statistical significance, it is difficult to make definitive conclusions in regards to an optimal compression rate applicable to all pediatric patients. Second, the median rate in this category was ~95 per minute (within 5 per minute of Guidelines). A reasonable interpretation of our data therefore could be that rates of “approximately 100 per minute” as per 2005 Guidelines (32) should be recommended or that no change be made. Finally, in the main PICqCPR paper, threshold diastolic blood pressure targets (25mmHg in infants, 30mmHg in older children) were associated with improved outcomes (16). Therefore, we speculate that providers in this cohort may have been focusing on blood pressures rather than rescuer-centric targets such as compression rate. Although diastolic pressures were similar across rate categories (Figure 2), a slightly higher percentage of patients achieved the diastolic thresholds associated with survival when rates were between 80–100 (63%) compared to Guidelines (54%; difference not statistically significant; data not shown). This may explain part of the observed survival difference and is consistent with a growing body of translational literature supporting a hemodynamic-directed approach to resuscitation (33, 34). Coupled with the strong association of diastolic blood pressure and outcomes in the main PICqCPR manuscript, we believe that these data should be used to highlight the importance of prioritizing physiologic targets over rescuer-centric targets (35).
This investigation has strengths worth noting. Prior reports of pediatric CPR quality have utilized CPR recording defibrillators (3, 11–14), and as such, have two main limitations. First, many infants are too small to accommodate the chest pads / sensors that are used to record quality data (36). In a series of younger children at The Children’s Hospital of Philadelphia (n=8), all patients were at least 1 year of age (12). In contrast, more than half of the patients in this report are < 1 year of age. In addition, placement of the sensors themselves can take up to 5 minutes after the start of compressions. Therefore, previous reports have likely either missed data from the initial minutes of the resuscitation or, worse, failed to include entire events. The magnitude of the number of potential missed events in these prior reports is highlighted by the fact that 37 (18.6%) events were less than 3 minutes in this investigation.
This study also has limitations. First, there may be concern that our findings are not generalizable because all patients had an arterial line in place at the time of arrest; however, more than 95% of pediatric in-hospital cardiac arrests now occur in ICUs, and over half will have an arterial line in place at the time of the arrest (37). Second, other important CPR quality variables (compression depth (11), release velocity (9), ventilation rate (38)) and quality of postarrest care (39, 40) are not evaluated. While inclusion of CCF did not substantially affect our results (data not shown), the contribution of depth to this association remains unknown highlighting the need for further research in this area. Finally, this study was conducted in a network comprised of large academic pediatric ICUs and may represent a “best-case” scenario.
Conclusions
Compliance with Guideline recommendations for rate was not common in this multi-center cohort with substantial variability in compression rates across the sites. Rates exceeding Guidelines were associated with mildly decreased systolic blood pressure, but not diastolic pressure or survival. Slightly lower rates were associated with improved outcomes. These data do not support a pediatric compression rate of 100–120 per minute during in-hospital resuscitations.
Supplementary Material
Acknowledgements
This study was supported, in part, by the following cooperative agreements from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, Department of Health and Human Services: UG1HD050096, UG1HD049981, UG1HD049983, UG1HD063108, UG1HD083171, UG1HD083166, UG1HD083170, U10HD050012, U10HD063106, U10HD063114 and U01HD049934.
The CPCCRN Investigators include: Athena F. Zuppa, Katherine Graham, Carolann Twelves, Mary Ann Diliberto, Elyse Tomanio, Jeni Kwok, Michael J. Bell, Alan Abraham, Anil Sapru, Mustafa F. Alkhouli, Sabrina Heidemann, Ann Pawluszka, Mark W. Hall, Lisa Steele, Thomas P. Shanley, Monica Weber, Heidi J. Dalton, Aimee La Bell, Peter M. Mourani, Kathryn Malone, Russell Telford, Christopher Locandro, Whitney Coleman, Alecia Peterson, Julie Thelen, and Allan Doctor.
Footnotes
Conflicts of Interest
The authors report no conflicts of interest related specifically to this manuscript. Unrelated disclosures include the following: Robert M. Sutton reports grant funding from the National Institutes of Health (NIH); Murray M. Pollack reports grant funding from the NIH and the Department of Defense, collaborative projects with Cerner Corporation, and philanthropy from Mallinckrodt Pharmaceuticals; Frank W. Moler reports NIH funding paid to his institution; Daniel A. Notterman reports grant funding from the NIH; and Christopher J. Newth reports consulting services for both Philips Research of North America and Medtronics.
CPCCRN Investigators
Athena F. Zuppa M.D. M.S.C.E.1, Katherine Graham B.S.1, Carolann Twelves R.N.1, Mary Ann Diliberto R.N.1, Elyse Tomanio R.N.5, Jeni Kwok J.D.6, Michael J. Bell M.D.5,7, Alan Abraham M.B.A.7, Anil Sapru M.D.6,8, Mustafa F. Alkhouli B.A.8, Sabrina Heidemann M.D.3, Ann Pawluszka R.N.3, Mark W. Hall M.D.4, Lisa Steele R.N.4, Thomas P. Shanley M.D.10,14, Monica Weber R.N.10, Heidi J. Dalton M.D.11, Aimee La Bell R.N.11, Peter M. Mourani M.D.12, Kathryn Malone R.N.12, Russell Telford MAS2, Christopher Locandro MSPH2, Whitney Coleman2, Alecia Peterson MS2, Julie Thelen2, Allan Doctor M.D.15
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Berg RA, Nadkarni VM, Clark AE, Moler F, Meert K, Harrison RE, et al. Incidence and Outcomes of Cardiopulmonary Resuscitation in PICUs. Crit Care Med. 2016;44(4):798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girotra S, Nallamothu BK, Spertus JA, Li Y, Krumholz HM, Chan PS, et al. Trends in survival after in-hospital cardiac arrest. N Engl J Med. 2012;367(20):1912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfe H, Zebuhr C, Topjian AA, Nishisaki A, Niles DE, Meaney PA, et al. Interdisciplinary ICU cardiac arrest debriefing improves survival outcomes*. Crit Care Med. 2014;42(7):1688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Idris AH, Guffey D, Pepe PE, Brown SP, Brooks SC, Callaway CW, et al. Chest compression rates and survival following out-of-hospital cardiac arrest. Crit Care Med. 2015;43(4):840–8. [DOI] [PubMed] [Google Scholar]
- 5.Vaillancourt C, Everson-Stewart S, Christenson J, Andrusiek D, Powell J, Nichol G, et al. The impact of increased chest compression fraction on return of spontaneous circulation for out-of-hospital cardiac arrest patients not in ventricular fibrillation. Resuscitation. 2011;82(12):1501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christenson J, Andrusiek D, Everson-Stewart S, Kudenchuk P, Hostler D, Powell J, et al. Chest compression fraction determines survival in patients with out-of-hospital ventricular fibrillation. Circulation. 2009;120(13):1241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilgannon JH, Kirchhoff M, Pierce L, Aunchman N, Trzeciak S, Roberts BW. Association between chest compression rates and clinical outcomes following in-hospital cardiac arrest at an academic tertiary hospital. Resuscitation. 2017;110:154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stiell IG, Brown SP, Nichol G, Cheskes S, Vaillancourt C, Callaway CW, et al. What is the optimal chest compression depth during out-of-hospital cardiac arrest resuscitation of adult patients? Circulation. 2014;130(22):1962–70. [DOI] [PubMed] [Google Scholar]
- 9.Cheskes S, Common MR, Byers AP, Zhan C, Silver A, Morrison LJ. The association between chest compression release velocity and outcomes from out-of-hospital cardiac arrest. Resuscitation. 2015;86:38–43. [DOI] [PubMed] [Google Scholar]
- 10.Sutton RM, Case E, Brown SP, Atkins DL, Nadkarni VM, Kaltman J, et al. A quantitative analysis of out-of-hospital pediatric and adolescent resuscitation quality--A report from the ROC epistry-cardiac arrest. Resuscitation. 2015;93:150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutton RM, French B, Niles DE, Donoghue A, Topjian AA, Nishisaki A, et al. 2010 American Heart Association recommended compression depths during pediatric in-hospital resuscitations are associated with survival. Resuscitation. 2014;85(9):1179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutton RM, Niles D, French B, Maltese MR, Leffelman J, Eilevstjonn J, et al. First quantitative analysis of cardiopulmonary resuscitation quality during in-hospital cardiac arrests of young children. Resuscitation. 2014;85(1):70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutton RM, Niles D, Nysaether J, Abella BS, Arbogast KB, Nishisaki A, et al. Quantitative analysis of CPR quality during in-hospital resuscitation of older children and adolescents. Pediatrics. 2009;124(2):494–9. [DOI] [PubMed] [Google Scholar]
- 14.Sutton RM, Wolfe H, Nishisaki A, Leffelman J, Niles D, Meaney PA, et al. Pushing harder, pushing faster, minimizing interruptions... but falling short of 2010 cardiopulmonary resuscitation targets during in-hospital pediatric and adolescent resuscitation. Resuscitation. 2013;84(12):1680–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willson DF, Dean JM, Newth C, Pollack M, Anand KJ, Meert K, et al. Collaborative Pediatric Critical Care Research Network (CPCCRN). Pediatr Crit Care Med. 2006;7(4):301–7. [DOI] [PubMed] [Google Scholar]
- 16.Berg RA, Sutton RM, Reeder RW, Berger JT, Newth CJ, Carcillo JA, et al. Association Between Diastolic Blood Pressure During Pediatric In-Hospital Cardiopulmonary Resuscitation and Survival. Circulation. 2018;137(17):1784–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs I, Nadkarni V, Bahr J, Berg RA, Billi JE, Bossaert L, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries. A statement for healthcare professionals from a task force of the international liaison committee on resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa). Resuscitation. 2004;63(3):233–49. [DOI] [PubMed] [Google Scholar]
- 18.Becker LB, Aufderheide TP, Geocadin RG, Callaway CW, Lazar RM, Donnino MW, et al. Primary outcomes for resuscitation science studies: a consensus statement from the American Heart Association. Circulation. 2011;124(19):2158–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollack MM, Holubkov R, Funai T, Clark A, Moler F, Shanley T, et al. Relationship between the functional status scale and the pediatric overall performance category and pediatric cerebral performance category scales. JAMA Pediatr. 2014;168(7):671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollack MM, Holubkov R, Glass P, Dean JM, Meert KL, Zimmerman J, et al. Functional Status Scale: new pediatric outcome measure. Pediatrics. 2009;124(1):e18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Caen AR, Berg MD, Chameides L, Gooden CK, Hickey RW, Scott HF, et al. Part 12: Pediatric Advanced Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2): S526–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meaney PA, Bobrow BJ, Mancini ME, Christenson J, de Caen AR, Bhanji F, et al. Cardiopulmonary resuscitation quality: [corrected] improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American Heart Association. Circulation. 2013;128(4):417–35. [DOI] [PubMed] [Google Scholar]
- 23.Meaney PA, Nadkarni VM, Cook EF, Testa M, Helfaer M, Kaye W, et al. Higher survival rates among younger patients after pediatric intensive care unit cardiac arrests. Pediatrics. 2006;118(6):2424–33. [DOI] [PubMed] [Google Scholar]
- 24.Donoghue A, Berg RA, Hazinski MF, Praestgaard AH, Roberts K, Nadkarni VM, et al. Cardiopulmonary resuscitation for bradycardia with poor perfusion versus pulseless cardiac arrest. Pediatrics. 2009;124(6):1541–8. [DOI] [PubMed] [Google Scholar]
- 25.Matos RI, Watson RS, Nadkarni VM, Huang HH, Berg RA, Meaney PA, et al. Duration of cardiopulmonary resuscitation and illness category impact survival and neurologic outcomes for in-hospital pediatric cardiac arrests. Circulation. 2013;127(4):442–51. [DOI] [PubMed] [Google Scholar]
- 26.Bhanji F, Topjian AA, Nadkarni VM, Praestgaard AH, Hunt EA, Cheng A, et al. Survival Rates Following Pediatric In-Hospital Cardiac Arrests During Nights and Weekends. JAMA Pediatr. 2017;171(1):39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollack MM, Holubkov R, Funai T, Berger JT, Clark AE, Meert K, et al. Simultaneous Prediction of New Morbidity, Mortality, and Survival Without New Morbidity From Pediatric Intensive Care: A New Paradigm for Outcomes Assessment. Crit Care Med. 2015;43(8):1699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolfe JA, Maier GW, Newton JR Jr., Glower DD, Tyson GS Jr., Spratt JA, et al. Physiologic determinants of coronary blood flow during external cardiac massage. The Journal of thoracic and cardiovascular surgery. 1988;95(3):523–32. [PubMed] [Google Scholar]
- 29.Idris AH, Guffey D, Aufderheide TP, Brown S, Morrison LJ, Nichols P, et al. Relationship between chest compression rates and outcomes from cardiac arrest. Circulation. 2012;125(24):3004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ornato JP, Levine RL, Young DS, Racht EM, Garnett AR, Gonzalez ER. The effect of applied chest compression force on systemic arterial pressure and end-tidal carbon dioxide concentration during CPR in human beings. Annals of Emergency Medicine. 1989;18(7):732–7. [DOI] [PubMed] [Google Scholar]
- 31.Bellamy RF, DeGuzman LR, Pedersen DC. Coronary blood flow during cardiopulmonary resuscitation in swine. Circulation. 1984;69(1):174–80. [DOI] [PubMed] [Google Scholar]
- 32.American Heart A 2005 American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) of pediatric and neonatal patients: pediatric basic life support. Pediatrics. 2006;117(5):e989–1004. [DOI] [PubMed] [Google Scholar]
- 33.Morgan RW, Sutton RM, Karlsson M, Lautz AJ, Mavroudis CD, Landis WP, et al. Pulmonary Vasodilator Therapy in Shock-associated Cardiac Arrest. Am J Respir Crit Care Med. 2018;197(7):905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutton RM, Friess SH, Naim MY, Lampe JW, Bratinov G, Weiland TR, 3rd, et al. Patient-centric blood pressure-targeted cardiopulmonary resuscitation improves survival from cardiac arrest. Am J Respir Crit Care Med. 2014;190(11):1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutton RM, French B, Meaney PA, Topjian AA, Parshuram CS, Edelson DP, et al. Physiologic monitoring of CPR quality during adult cardiac arrest: A propensity-matched cohort study. Resuscitation. 2016;106:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutton RM, Niles D, Nysaether J, Arbogast KB, Nishisaki A, Maltese MR, et al. Pediatric CPR quality monitoring: analysis of thoracic anthropometric data. Resuscitation. 2009;80(10):1137–41. [DOI] [PubMed] [Google Scholar]
- 37.Berg RA, Sutton RM, Holubkov R, Nicholson CE, Dean JM, Harrison R, et al. Ratio of PICU versus ward cardiopulmonary resuscitation events is increasing. Crit Care Med. 2013;41(10):2292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aufderheide TP, Sigurdsson G, Pirrallo RG, Yannopoulos D, McKnite S, von Briesen C, et al. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation. 2004;109(16):1960–5. [DOI] [PubMed] [Google Scholar]
- 39.Bembea MM, Nadkarni VM, Diener-West M, Venugopal V, Carey SM, Berg RA, et al. Temperature patterns in the early postresuscitation period after pediatric inhospital cardiac arrest. Pediatr Crit Care Med. 2010;11(6):723–30. [DOI] [PubMed] [Google Scholar]
- 40.Topjian AA, Telford R, Holubkov R, Nadkarni VM, Berg RA, Dean JM, et al. Association of Early Postresuscitation Hypotension With Survival to Discharge After Targeted Temperature Management for Pediatric Out-of-Hospital Cardiac Arrest: Secondary Analysis of a Randomized Clinical Trial. JAMA Pediatr. 2018;172(2):143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.