Cyclic GMP-AMP synthase (cGAS) is activated by DNA through direct binding. Active cGAS catalyzes the production of cyclic GMP-AMP (cGAMP), and consequently activates the innate immune responses. However, the detailed biological processes are poorly understood. Du and Chen demonstrated in a recent paper published on Science that DNA binding to cGAS robustly induced the formation of liquid like droplets in which cGAS was activated.
The innate immune system utilizes cGAS to detect cytosolic foreign DNA, which is essential genetic information carriers for most living organisms including viral, bacterial, and eukaryotic pathogens.1 Upon recognition of foreign DNA, the cytosolic sensor-cGAS triggers cGAMP production. cGAMP in turn functions as an endogenous second messenger to activate STING, leading to the production of type I interferons, as well as proinflammatory cytokines.2
Research in the past few years has led to significant progress toward understanding the mechanism of cytosolic DNA sensing and signaling. Although the crystal structure of cGAS alone or in complex with cytosolic foreign DNA has been resolved, the detailed in vivo biological processes remain unresolved.3,4 Recently, a physicochemical model of phase separation of fluids based on principles of polymer chemistry and soft matter physics explains a series of biological events including RNA metabolism, ribosome biogenesis, transcription, DNA damage response and signal transduction.5 Phase-separated biomolecular condensates produced by liquid-liquid phase separation of protein-nucleic acid-macromolecules allow rapid biological reaction of components within the dense phase.6 Based on this model, Du and Chen hypothesized that cGAS and cytosolic DNA interactions could lead to the formation of biomolecular condensates through liquid phase separation. To this end, they incubated fluorescently labeled cGAS protein with double-stranded DNA oligonucleotides in vitro. As expected, cGAS and DNA formed micrometer-sized liquid droplets, in which cGAS and reactants were concentrated to greatly enhance the production of cGAMP.7 Under physiological conditions, the formation of these droplets may have two functions: one is to concentrate reactants to amplify the signaling, and the other is to collect the invading nucleic acid molecules. cGAS also formed liquid droplets with dsRNA, but RNA did not activate cGAS to produce cGAMP, which suggests that liquid phase separation is insufficient for cGAS activation. These results confirm the validity of the previous structural studies indicating that DNA but not RNA binding induces a conformational change that activates cGAS and the following signaling.
Within cells, the formation of cGAS-DNA foci was also detected. Furthermore, the cGAS-DNA foci could fuse with each other and exhibited near-complete fluorescence recovery after photobleaching, indicating that cGAS was in dynamic liquid like granules. This raises the question of whether the cGAS-DNA liquid phase separation is a dynamic and reversible process in vivo, which is important to timely terminate the activation of immune responses. Besides, the cGAS-DNA liquid like granules were distinct from cellular organelles and vesicles as evidenced by the fact that most cGAS activities were present in subcellular fractions containing the nuclear and heavy fractions. This corresponds to the physical properties of the phase separation condensates of protein-nucleic acid-macromolecules.
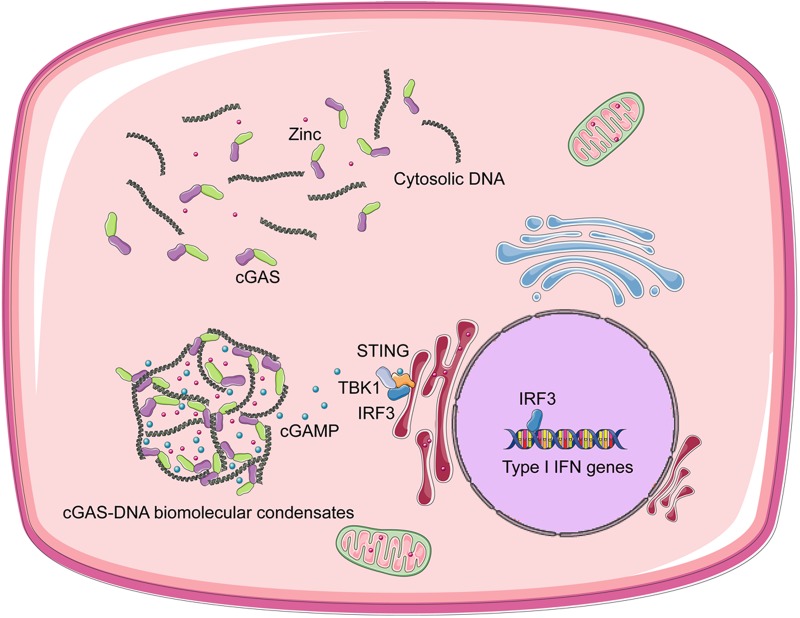
Based on the premise that liquid phase separation is driven by multivalent interactions, valency is important for this physicochemical process. Thus, Du and Chen tested the DNA length, full-length cGAS, cytoplasmic salt concentrations and free zinc ions as important factors to regulate the formation of cGAS-DNA biomolecular condensates: (1) Long DNA has more binding sites (valency) for cGAS than short DNA, and full-length cGAS has higher valency for DNA than core-cGAS, thus long DNA and full-length cGAS phase separation are more efficient; (2) such DNA-cGAS multivalent interactions are vulnerable to cytoplasmic salt concentrations, which may be a mechanism to prevent unwanted activation of cGAS by self-DNA below a certain threshold8; (3) free zinc ions facilitate cGAS activation by promoting DNA-cGAS phase transition. To a certain extent, this discovery unified our understanding of previous studies about cytosolic DNA sensing and signaling (Fig. 1).
Fig. 1.
Cytosolic DNA and cGAS form liquid droplets, which function as micro reactors in which the enzyme and reactants are concentrated to greatly enhance the production of cGAMP.7 cGAMP transduces signals to the ER-localized adaptor protein STING. STING recruits and activates the kinase TBK1, which then activates the transcription factor IRF3 to induce type I IFNs.1,2
A recent study suggested that STING could function as a direct sensor for DNA.9 The binding affinity of STING to dsDNA was several orders of magnitude lower than that of cGAS. However, it is still necessary to explore whether STING is involved in the formation of cGAS-DNA condensates, and it will also be interesting to study details of cGAS-STING anti-infection signal pathway activation in cGAS-DNA condensates. Besides, since this study was carried out in vitro at cellular level, it is necessary to further validate these conclusions in vivo in the future. This is of particular interest given that cytosolic DNA-cGAS induces type I IFNs and other cytokines can also cause autoimmune diseases in addition to resisting infection,10 further investigation on the dynamics and composition of the DNA-cGAS condensates in autoimmune diseases may provide more mechanisms and therapeutic approaches. In addition, there are other immune signaling activations and transductions that may also happen in phase separated droplets, such as inflammasomes.
Acknowledgements
This work was supported by the National Natural Science Committee of China (No. 91753141 to Hua-Bing Li), the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (Hua-Bing Li), the start-up fund from the Shanghai Jiao Tong University School of Medicine (Hua-Bing Li), and the Howard Hughes Medical Institute (Richard A. Flavell).
Competing interests
Yongbo Liu, Hua-Bing Li and Richard A. Flavell declare that they have no conflict of interest. This manuscript is a review article and does not require an approved research protocol by the relevant institutional review board of ethics committee.
Contributor Information
Hua-Bing Li, Email: huabing.li@shsmu.edu.cn.
Richard A. Flavell, Email: richard.flavell@yale.edu
References
- 1.Sun L, Wu J, Du F, Chen X, Chen ZJ. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu J, et al. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao P, et al. Cell. 2013;153:1094–1107. doi: 10.1016/j.cell.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Civril F, et al. Nature. 2013;498:332–337. doi: 10.1038/nature12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyman AA, Simons K. Science. 2012;337:1047–1049. doi: 10.1126/science.1223728. [DOI] [PubMed] [Google Scholar]
- 6.Banani SF, Lee HO, Hyman AA, Rosen MK. Nat. Rev. Mol. Cell Biol. 2017;18:285–298. doi: 10.1038/nrm.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du M, Chen ZJ. Science. 2018 doi: 10.1126/science.aat1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rongvaux A, et al. Cell. 2014;159:1563–1577. doi: 10.1016/j.cell.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abe T, et al. Mol. Cell. 2013;50:5–15. doi: 10.1016/j.molcel.2013.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krug A. Handb. Exp. Pharmacol. 2008;183:129–151. doi: 10.1007/978-3-540-72167-3_7. [DOI] [PubMed] [Google Scholar]