Abstract
Several studies have found that smoking increases the risk of abdominal aortic aneurysm, however, the strength of the association has differed between studies and data from cohort studies have not yet been summarized. A systematic review and meta-analysis was therefore conducted to clarify this association. We searched PubMed and Embase databases up to May 2nd 2018. A random effects model was used to estimate summary relative risks (RRs) and 95% confidence intervals (CIs). Twenty three prospective studies were included. Comparing current, former and ever smokers with never smokers the summary RRs were 4.87 (95% CI: 3.93–6.02, I2 = 92%, n = 20), 2.10 (95% CI: 1.76–2.50, I2 = 71%, n = 15) and 3.28 (95% CI: 2.60–4.15, I2 = 96%, n = 18), respectively. The summary RR was 1.87 (95% CI: 1.45–2.40, I2 = 97%) per 10 cigarettes per day, 1.78 (95% CI: 1.54–2.06, I2 = 83%) per 10 pack-years was and 0.45 (95% CI: 0.32–0.63, I2 = 92.3%) per 10 years of smoking cessation. There was evidence of nonlinearity for cigarettes per day and pack-years (pnonlinearity < 0.0001 and pnonlinearity = 0.02, respectively), but not for smoking cessation, pnonlinearity = 0.85. Among smokers who quit, the RR was similar to that of never smokers by 25 years of smoking cessation. These findings confirm a strong association between smoking and the risk of developing abdominal aortic aneurysms.
Introduction
Aortic aneurysms are localized dilatations of the aorta that exceeds the normal diameter by 50% or >3 cm1. Rupture of an aortic aneurysm can cause massive internal bleeding and is usually fatal; 80% of those reaching hospital and 50% of those undergoing surgery for ruptured aortic aneurysms die as a consequence2,3. Globally aortic aneurysms accounted for 168200 deaths and 2.9 million disability-adjusted life years in 20154,5. Most aortic aneurysms are abdominal aortic aneurysms, and these are more common in men than in women6, although the reason for these sex differences are unclear. The incidence of new abdominal aortic aneurysms is 0.4–0.67% in Western populations7–9, and ten-fold lower in Asian populations10. Secular trend studies of abdominal aortic aneurysms have shown conflicting results. An early American study found increased incidence of abdominal aortic aneurysms between 1951 and 198011 and a Swedish study found an approximate doubling in the incidence rate of ruptured abdominal aortic aneurysms between 1971–1986 and 2000–2004 in spite of increased use of surgical treatment12. However, another study found an increased incidence of non-ruptured aneurysms between 1990 and 2005, but no change in the incidence of interventions for ruptured aneurysms13, while a third study found a lower prevalence of abdominal aortic aneurysm detected through ultrasound screening over time which the authors attributed to a lower prevalence of smoking in more recent decades14. A study from Finland found a reduced incidence and mortality from ruptured abdominal aortic aneurysms between 2003 and 201315. Some of the established risk factors include age, ethnicity, height, hypertension, coronary heart disease, peripheral artery disease, while diabetes appears to be associated with a reduced risk16.
Although a large number of studies have found a positive association between smoking and risk of abdominal aortic aneurysm7,16–36, there has been variation observed with regard to the strength of the association between studies and by sex, with stronger associations observed in women than in men in some30,33, although not all studies22,32,34. Several studies found a dose-response relationship between the intensity of smoking and risk of abdominal aortic aneurysms7,18,28,29, however, in a few studies there was a flattening of the curve with a larger number of cigarettes smoked per day20,22,25,27. More recently, studies of smoking cessation have quite consistently reported reduced risk with longer duration of smoking cessation7,26,30,34, however, while some studies suggested a risk similar as in never smokers with very long durations of smoking cessation7,30, other studies found some elevation in risk even after long durations of smoking cessation26,30,34. Although a previous meta-analysis found an increased risk of abdominal aortic aneurysms among smokers, only screening studies (cross-sectional studies) were included in that analysis37. As cross-sectional studies cannot be used to draw causal inferences we therefore conducted a systematic review and meta-analysis of prospective studies on the association between tobacco smoking and the risk of abdominal aortic aneurysms to provide better evidence from studies with a stronger study design. We aimed to clarify the strength and shape of the dose-response relationship between tobacco smoking and risk of abdominal aortic aneurysm, potential differences of the association by sex, and the effects of smoking cessation.
Material and Methods
Search strategy
We searched the databases Pubmed, and Embase up to May 2nd 2018 for eligible studies (DA, SS). The search terms used for the searches are found in the Supplementary Text. We also screened the reference lists of the identified publications for additional potentially relevant studies. We followed the MOOSE criteria with regard to reporting of meta-analyses38.
Study selection
Published retrospective and prospective cohort studies and nested case-control studies within cohort studies on tobacco smoking and the risk of abdominal aortic aneurysms were eligible for inclusion. For studies to be included adjusted relative risk (RR) estimates had to be published with the 95% confidence intervals (CIs). A list of the excluded studies and the reasons for exclusion are found in Supplementary Table 1.
Data extraction
We extracted the following data from each study: The first author’s last name, publication year, country where the study was conducted, study period, sample size, number of cases and participants, subgroup, relative risks and 95% CIs for smoking exposure and the confounders adjusted for in the analysis. The data extraction was done by DA and checked for accuracy by SS.
Statistical methods
We calculated summary RRs and 95% CIs of abdominal aortic aneurysm by smoking status, cigarettes per day, pack-years, and time since quitting smoking using random-effects models39 which take into account heterogeneity between studies. A weighted average of the natural logarithm of the RRs was estimated using random effects weights39. The main analysis included studies that analyzed current, former, or ever smokers vs. never smokers (which was used as the reference category). As there is evidence that even former smokers are at increased risk, we conducted a separate analysis of studies that analyzed current vs. non-current smokers (former + never smokers). Linear dose-response analyses were conducted using the method of Greenland and Longnecker40. Study-specific linear trends and 95% CIs were computed from the natural log of the RRs and CIs across categories of cigarettes per day, pack-years and years since smoking cessation. A potential nonlinear association was investigated using restricted cubic splines with three knots at 10%, 50%, and 90% percentiles of the distribution, which was combined using multivariate meta-analysis41,42. For one study23 we used a previously described method to estimate approximate confidence intervals based on the size of the hazard ratios and the distribution of the participants for each smoking category43.
Q and I2 statistics were used to assess heterogeneity between studies44. I2 is a measure of the amount of heterogeneity that is due to between study variation rather than chance. Subgroup analyses stratified by duration of follow-up, outcome type, sex, geographic location, number of cases, study quality and adjustment for confounding factors were conducted to investigate potential sources of heterogeneity. The Newcastle Ottawa scale was used to assess study quality and it rates studies according to selection, comparability and outcome assessment with a score range from 0 to 945. Egger’s test46, Begg-Mazumdar’s test47 and inspection of funnel plots were used to assess publication bias. Stata 13.0 software (StataCorp, Texas, US) was used for the statistical analyses.
Results
Twenty three prospective studies (22 publications)7,16–36 were included in the meta-analysis of smoking and risk of abdominal aortic aneurysms (Fig. 1, Supplementary Table 2). Thirteen studies were from Europe, eight studies were from the USA, and two studies were from Asia (Supplementary Table 2). The study characteristics of the studies included in the meta-analysis are provided in Supplementary Table 2 including study period, number of cases and participants, age, comparisons and risk estimates and confounders adjusted for.
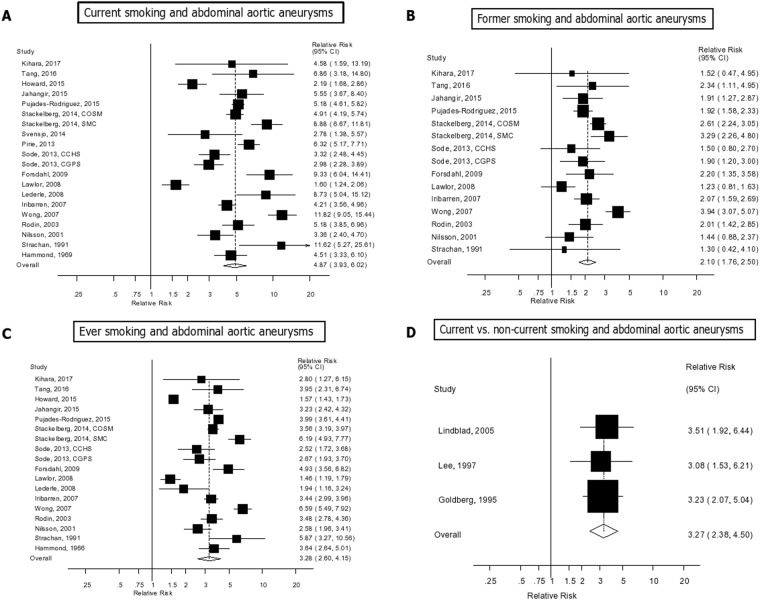
Figure 1.

Flow-chart of study selection.
Smoking status
Twenty studies (18 publications)7,16,18,19,22,23,25–36 of current smokers (8901 cases, 4716185 participants), fifteen studies (thirteen publications)7,19,22,23,25–27,29–31,33,35,36 of former smokers (7824 cases, 3060503 participants), and eighteen studies (16 publications)7,16,17,19,22,23,25–27,29,30,32–36 of ever smokers (8448 cases, 3934635 participants) and abdominal aortic aneurysms were included in the analyses. The summary RR for current smokers vs. never smokers was 4.87 (95% CIs: 3.93–6.02, I2 = 91.5%, pheterogeneity < 0.0001) (Fig. 2A), for former smokers it was 2.10 (95% CI: 1.76–2.50, I2 = 71.3%, pheterogeneity < 0.0001) (Fig. 2B), and for ever smokers it was 3.28 (95% CI: 2.60–4.15, I2 = 95.8%, pheterogeneity < 0.0001) (Fig. 2C). There was no evidence of publication bias with Egger’s test, p = 0.86 for current smokers, 0.18 for former smokers, and 0.48 for ever smokers, respectively (Supplementary Figs 1–3). In three studies20,21,24 of current smokers vs. never/former smokers combined the summary RR was 3.27 (95% CI: 2.38–4.50, I2 = 0%, pheterogeneity = 0.96) (Supplementary Fig. 2d).
Figure 2.
Smoking status and abdominal aortic aneurysm.
Cigarettes per day, pack-years and years since quitting smoking
Seven studies18,20,22,25,26,28,29 analysed the association between number of cigarettes per day and abdominal aortic aneurysms (2219 cases, 1658103 participants) and the summary RR per 10 cigarettes per day was 1.87 (95% CI: 1.45–2.40, I2 = 96.7%, pheterogeneity < 0.0001) (Fig. 3A). There was evidence of a nonlinear association between number of cigarettes per day and abdominal aortic aneurysms, pnonlinearity < 0.0001, with a strong increase in risk up to 15–20 cigarettes per day, but no further increase beyond 20 cigarettes per day (Fig. 3B). The summary RRs for 5, 10, 15, 20 and 25 cigarettes per day compared to zero 1.99 (1.67–2.37), 3.52 (2.58–4.80), 4.99 (3.41–7.29), 5.81 (3.92–8.60), 5.91 (4.04–8.64), and 5.61 (3.88–8.11), respectively (Fig. 3B, Supplementary Table 3).
Figure 3.

Cigarettes per day and abdominal aortic aneurysm, linear and nonlinear dose-response analysis.
Three studies (two publications)30,35 on the association between number of pack-years of smoking and abdominal aortic aneurysms (1747 cases, 93938 participants) were included and the summary RR was 1.78 (95% CI: 1.54–2.06, I2 = 82.5%, pheterogeneity < 0.0001) per 10 pack-years (Fig. 4A). There was also evidence of nonlinearity in the analysis of pack-years of smoking and abdominal aortic aneurysms, pnonlinearity = 0.02, with a strong increase in risk up to 20–25 pack-years, but little further increase in risk above 25 pack-years (Fig. 4B), however, there were few data points at higher levels of pack-years. The summary RRs for 5, 10, 15, 20, 25 and 30 pack-years compared to zero pack-years were 2.01 (1.38–2.92), 3.60 (1.87–6.94), 5.34 (2.46–11.63), 6.61 (3.11–14.11), 7.17 (3.81–13.45), and 7.08 (4.55–11.01), respectively (Fig. 4B, Supplementary Table 4).
Figure 4.
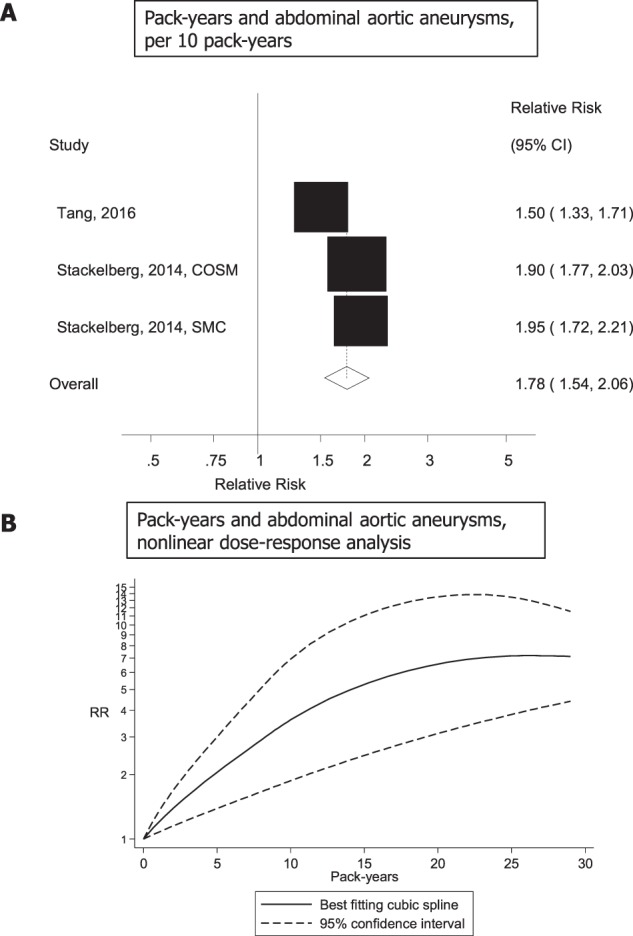
Pack-years and abdominal aortic aneurysm, linear and nonlinear dose-response analysis.
Five studies (4 publications)7,26,30,34 investigating the number of years since quitting smoking and risk of abdominal aneurysms (4787 cases, 2059203 participants) were included and the summary RR per 10 years of smoking cessation was 0.45 (95% CI: 0.32–0.63, I2 = 92.3%, pheterogeneity < 0.0001) (Fig. 5A). There was no evidence of nonlinearity, pnonlinearity = 0.85, and the relative risk at 25 years since quitting, summary RR = 0.13 (95% CI: 0.06–0.30), was similar to that of never smokers (summary RR = 0.14, 95% CI: 0.10–0.19) (Fig. 5B, Supplementary Table 5).
Figure 5.
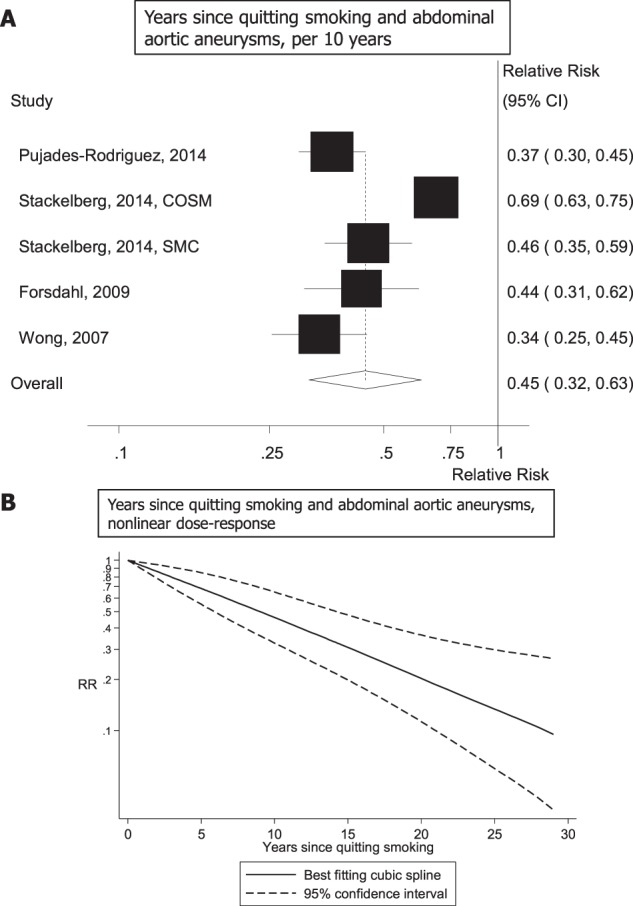
Years since quitting smoking and abdominal aortic aneurysm, linear and nonlinear dose-response analysis.
Subgroup and sensitivity analyses
Positive associations were observed between current, former and ever smoking and risk of abdominal aortic aneurysms across nearly all subgroups defined by duration of follow-up, outcome type, sex, geographic location, number of cases, study quality and adjustment for confounding factors (including age, education, alcohol, height, BMI, and physical activity, cardiovascular disease, hypertension, hypercholesterolemia, serum cholesterol, and diabetes) (Table 1). There was no evidence that the results differed between these subgroups in meta-regression analyses, except for two subgroup analyses stratified by adjustment for hypertension and hypercholesterolemia among former smokers, where there was a stronger association among studies with such adjustment compared to studies without such adjustment (pheterogeneity = 0.04 for both). The association between current and ever smoking versus never smoking and abdominal aortic aneurysms appeared to be slightly stronger among women than among men, although the test for heterogeneity between subgroups was not significant, pheterogeneity = 0.44 and pheterogeneity = 0.75, respectively.
Table 1.
Subgroup analyses of smoking status and abdominal aortic aneurysms.
| Current Smoking | Former smoking | Ever smoking | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | RR (95% CI) | I2 (%) | P h 1 | P h 2 | n | RR (95% CI) | I2 (%) | P h 1 | P h 2 | n | RR (95% CI) | I2 (%) | P h 1 | P h 2 | ||
| All studies | 20 | 4.87 (3.93–6.02) | 91.5 | <0.0001 | 15 | 2.10 (1.76–2.50) | 71.3 | <0.0001 | 18 | 3.28 (2.60–4.15) | 95.8 | <0.0001 | ||||
| Follow-up | ||||||||||||||||
| <10 years | 7 | 5.06 (3.84–6.65) | 80.0 | <0.0001 | 0.82 | 4 | 1.94 (1.66–2.27) | 0 | 0.96 | 0.69 | 6 | 3.43 (2.81–4.19) | 68.2 | 0.008 | 0.94 | |
| ≥10 years | 13 | 4.79 (3.52–6.51) | 93.7 | <0.0001 | 11 | 2.12 (1.67–2.70) | 76.8 | <0.0001 | 12 | 3.28 (2.37–4.53) | 96.9 | <0.0001 | ||||
| Outcome type | ||||||||||||||||
| Incidence | 16 | 4.94 (3.84–6.36) | 92.9 | <0.0001 | 0.82 | 13 | 2.30 (2.12–2.50) | 73.3 | <0.0001 | 0.26 | 16 | 3.02 (2.89–3.16) | 96.3 | <0.0001 | 0.99 | |
| Mortality | 4 | 4.67 (3.33–6.53) | 72.8 | 0.01 | 2 | 1.45 (0.92–2.29) | 0 | 0.93 | 2 | 3.51 (2.61–4.72) | 0 | 0.55 | ||||
| Sex | ||||||||||||||||
| Men | 9 | 4.55 (4.19–4.94) | 94.8 | <0.0001 | 0.63/0.443 | 6 | 2.43 (2.17–2.73) | 88.2 | <0.0001 | 0.94/0.443 | 8 | 2.57 (2.42–2.73) | 97.2 | <0.0001 | 0.47/0.753 | |
| Women | 7 | 5.79 (5.14–6.53) | 83.1 | <0.0001 | 3 | 3.14 (2.29–4.32) | 52.1 | 0.12 | 6 | 3.41 (2.95–3.95) | 93.5 | <0.0001 | ||||
| Men and women | 8 | 4.24 (3.82–4.70) | 72.5 | 0.001 | 7 | 2.00 (1.70–2.36) | 0 | 0.96 | 8 | 3.69 (3.44–3.96) | 52.7 | 0.04 | ||||
| Geographic location | ||||||||||||||||
| Europe | 11 | 4.68 (3.67–5.97) | 89.9 | <0.0001 | 0.60 | 8 | 2.12 (1.74–2.58) | 57.0 | 0.02 | 0.49 | 9 | 3.40 (2.41–4.80) | 97.1 | <0.0001 | 0.32 | |
| America | 7 | 6.17 (4.39–8.67) | 87.1 | <0.0001 | 5 | 2.40 (1.72–3.35) | 77.5 | 0.001 | 7 | 3.66 (2.80–4.77) | 86.5 | <0.0001 | ||||
| Asia | 2 | 2.38 (0.88–6.45) | 72.1 | 0.06 | 2 | 1.25 (0.89–1.75) | 0 | 0.74 | 2 | 1.80 (0.99–3.27) | 59.3 | 0.12 | ||||
| Number of cases | ||||||||||||||||
| <250 | 9 | 5.01 (3.21–7.81) | 90.6 | <0.0001 | 0.99 | 6 | 2.06 (1.51–2.80) | 43.0 | 0.12 | 0.73 | 8 | 3.17 (1.96–5.14) | 95.8 | <0.0001 | 0.60 | |
| 250–<500 | 7 | 4.68 (2.72–8.03) | 95.4 | <0.0001 | 5 | 1.98 (1.24–3.18) | 88.0 | <0.0001 | 6 | 2.92 (1.65–5.16) | 95.8 | <0.0001 | ||||
| ≥500 | 4 | 4.84 (4.32–5.42) | 37.7 | 0.19 | 4 | 2.22 (1.85–2.66) | 53.7 | 0.09 | 4 | 3.70 (3.43–3.99) | 19.8 | 0.29 | ||||
| Study quality | ||||||||||||||||
| 0–3 stars | 1 | 11.62 (5.27–25.61) | 0.70 | 1 | 1.30 (0.42–4.10) | 0.25 | 1 | 5.87 (3.27–10.56) | 0.83 | |||||||
| 4–6 stars | 6 | 4.71 (2.89–7.66) | 95.4 | <0.0001 | 3 | 1.88 (1.60–2.21) | 0 | 0.52 | 6 | 3.16 (1.93–5.19) | 95.8 | <0.0001 | ||||
| 7–9 stars | 14 | 4.92 (3.63–6.66) | 93.1 | <0.0001 | 11 | 2.21 (1.77–2.76) | 74.5 | <0.0001 | 11 | 3.50 (2.67–4.58) | 93.4 | <0.0001 | ||||
| Adjustment for confounding factors3 | ||||||||||||||||
| Age | Yes | 19 | 4.72 (3.81–5.86) | 91.7 | <0.0001 | 0.19 | 14 | 2.12 (1.77–2.53) | 72.8 | <0.0001 | 0.47 | 17 | 3.20 (2.52–4.06) | 96.0 | <0.0001 | 0.26 |
| No | 1 | 11.62 (5.27–25.62) | 1 | 1.30 (0.42–4.10) | 1 | 5.87 (3.27–10.56) | ||||||||||
| Education | Yes | 5 | 5.48 (4.16–7.22) | 80.0 | <0.0001 | 0.60 | 5 | 2.41 (2.00–2.90) | 39.7 | 0.16 | 0.35 | 5 | 3.85 (3.09–4.81) | 82.2 | <0.0001 | 0.41 |
| No | 15 | 4.71 (3.54–6.26) | 93.0 | <0.0001 | 10 | 1.94 (1.48–2.53) | 76.6 | <0.0001 | 13 | 3.12 (2.27–4.30) | 96.5 | <0.0001 | ||||
| Alcohol | Yes | 10 | 4.55 (3.32–6.22) | 92.4 | <0.0001 | 0.59 | 8 | 2.05 (1.61–2.62) | 68.1 | 0.003 | 0.83 | 9 | 2.96 (2.22–3.95) | 92.1 | <0.0001 | 0.36 |
| No | 10 | 5.22 (3.80–7.17) | 90.8 | <0.0001 | 7 | 2.12 (1.57–2.85) | 77.6 | <0.0001 | 9 | 3.64 (2.47–5.37) | 97.3 | <0.0001 | ||||
| Height | Yes | 6 | 4.69 (2.95–7.44) | 93.9 | <0.0001 | 0.82 | 4 | 1.80 (1.36–2.38) | 53.8 | 0.09 | 0.32 | 5 | 2.65 (1.75–4.03) | 92.7 | <0.0001 | 0.22 |
| No | 14 | 4.97 (3.88–6.37) | 90.4 | <0.0001 | 11 | 2.22 (1.81–2.74) | 70.8 | <0.0001 | 13 | 3.56 (2.66–4.78) | 96.5 | <0.0001 | ||||
| BMI, weight, adiposity | Yes | 9 | 5.53 (3.81–8.03) | 94.5 | <0.0001 | 0.37 | 7 | 2.32 (1.74–3.09) | 82.9 | <0.0001 | 0.20 | 8 | 3.34 (2.38–4.68) | 95.2 | <0.0001 | 0.88 |
| No | 11 | 4.33 (3.37–5.56) | 86.6 | <0.0001 | 8 | 1.89 (1.65–2.17) | 0 | 0.89 | 10 | 3.25 (2.33–4.54) | 95.8 | <0.0001 | ||||
| Physical activity | Yes | 4 | 4.85 (1.89–12.48) | 97.6 | <0.0001 | 0.99 | 3 | 2.02 (0.79–5.20) | 93.1 | <0.0001 | 0.80 | 3 | 3.01 (0.93–9.77) | 98.3 | <0.0001 | 0.77 |
| No | 16 | 4.79 (3.99–5.75) | 84.9 | <0.0001 | 12 | 2.12 (1.86–2.42) | 37.1 | 0.09 | 15 | 3.33 (2.64–4.20) | 95.1 | <0.0001 | ||||
| Cardiovascular disease | Yes | 6 | 5.50 (4.19–7.24) | 81.2 | <0.0001 | 0.56 | 4 | 2.43 (2.00–2.96) | 49.8 | 0.11 | 0.28 | 5 | 3.63 (2.86–4.60) | 85.7 | <0.0001 | 0.68 |
| No | 14 | 4.66 (3.47–6.25) | 93.3 | <0.0001 | 11 | 1.92 (1.49–2.49) | 74.2 | <0.0001 | 13 | 3.21 (2.32–4.43) | 96.5 | <0.0001 | ||||
| Hypertension | Yes | 10 | 5.79 (4.32–7.77) | 90.3 | <0.0001 | 0.16 | 9 | 2.41 (1.97–2.95) | 65.7 | 0.003 | 0.05 | 10 | 3.69 (2.96–4.59) | 88.0 | <0.0001 | 0.29 |
| No | 10 | 4.09 (2.95–5.68) | 92.5 | <0.0001 | 6 | 1.71 (1.41–2.07) | 26.7 | 0.23 | 8 | 2.94 (2.00–4.33) | 96.9 | <0.0001 | ||||
| Hyperchole-sterolemia | Yes | 7 | 5.83 (3.97–8.57) | 92.4 | <0.0001 | 0.28 | 6 | 2.53 (1.96–3.28) | 73.1 | 0.002 | 0.04 | 7 | 3.61 (2.66–4.88) | 91.2 | <0.0001 | 0.51 |
| No | 13 | 4.37 (3.36–5.69) | 90.9 | <0.0001 | 9 | 1.83 (1.61–2.09) | 7.1 | 0.38 | 11 | 3.12 (2.29–4.24) | 96.1 | <0.0001 | ||||
| Serum cholesterol | Yes | 7 | 4.01 (2.74–5.87) | 91.6 | <0.0001 | 0.24 | 7 | 1.83 (1.54–2.18) | 19.8 | 0.28 | 0.16 | 7 | 2.98 (2.16–4.11) | 90.6 | <0.0001 | 0.48 |
| No | 13 | 5.44 (4.28–6.92) | 89.3 | <0.0001 | 8 | 2.34 (1.82–3.01) | 77.0 | <0.0001 | 11 | 3.49 (2.51–4.85) | 97.0 | <0.0001 | ||||
| Diabetes | Yes | 6 | 5.51 (3.49–8.69) | 95.7 | <0.0001 | 0.48 | 6 | 2.37 (1.76–3.18) | 85.5 | <0.0001 | 0.16 | 7 | 3.39 (2.38–4.83) | 95.9 | <0.0001 | 0.80 |
| No | 14 | 4.49 (3.58–5.63) | 85.2 | <0.0001 | 9 | 1.88 (1.64–2.16) | 0 | 0.93 | 11 | 3.22 (2.34–4.44) | 95.3 | <0.0001 | ||||
n denotes the number of studies.
1P for heterogeneity within each subgroup.
2P for heterogeneity between subgroups with meta-regression analysis.
3P for heterogeneity between men and women (excluding studies of men and women combined) with meta-regression analysis.
In sensitivity analyses where each study was excluded from the analysis at a time the association between current, former and ever versus never smoking and abdominal aortic aneurysms was robust (Supplementary Tables 4–6).
The mean (median) study quality scores were 6.6 (7.0) for the studies of current smokers, 6.8 (7.0) for former smokers, and 6.6 (7.0).
Discussion
This meta-analysis of twenty two cohort studies confirm that smoking is a strong risk factor for abdominal aortic aneurysms, with 5-fold, 2-fold and 3.3-fold increases in the risk among current, former and ever smokers compared to never smokers, respectively. In addition, there was a strong dose-response relation between increasing number of cigarettes per day and pack-years and increasing risk of abdominal aortic aneurysms. The nonlinearity observed in the latter analyses may have been due to a modest number of studies included in the analyses and few data points at higher levels of cigarettes per day and pack-years of smoking. In addition, we found a linear inverse association between increasing duration of smoking cessation and risk of abdominal aortic aneurysms, with risk approaching that of never smokers at 25 years of smoking cessation.
Our study has some limitations which could have affected the results. Although publication bias can affect meta-analyses of published literature we found no evidence of publication bias in the current meta-analysis. Heterogeneity between studies was high in most of the analyses, however, because all the studies found risk estimates in the direction of increased risk the heterogeneity appeared to be driven more by differences in the sizes of the risk estimates rather than differences in the direction or presence or absence of an association. Smokers oftentimes have a less healthy lifestyle than non-smokers with lower physical activity, more abdominal adiposity, and unhealthier diets, however, we found that the associations persisted across subgroup analyses of adjustment for physical activity, adiposity and other established risk factors. Although the studies did not adjust for dietary factors, few dietary risk factors have been established and associations observed with dietary intake have generally been much weaker than those found in the current meta-analysis48. Although residual confounding by other risk factors or unidentified risk factors cannot entirely be excluded, the strong dose-response relation by smoking status (stronger association in current and ever smokers than former smokers), cigarettes per day and pack-years as well as the reduced risk with increasing duration of smoking cessation provides strong epidemiological support for a causal interpretation of the observed association between smoking and increased risk of abdominal aortic aneurysms.
In addition, a growing body of mechanistic studies support an association between smoking and the development of abdominal aortic aneurysms. Abdominal aortic aneurysms are characterized by loss of normal medial arterial structure and the near complete absence of normal lamellar elastin matrix49. The breakdown of the elastin and collagen of the arterial media is mediated by matrix metalloproteinases released by macrophages, and is also related to chronic inflammatory infiltration and loss or dysfunction of parenchymal cells central to matrix deposition and repair49. Smoking may activate tissue plasminogen activator which induces production of matrix metalloproteinases by macrophages and has also shown to disrupt collagen synthesis50–52. A rat study showed that nicotine administration weakened the vascular wall, increased gelatinolytic activity and promoted the destruction of elastin and collagen in the abdominal aorta and led to upregulation of matrix metalloproteinase-12 expression53, although another study found that smoking-induced aortic dilatation was not related to expression of matrix metalloproteinase-9 and -1254. Other experimental studies have found that both tobacco smoke and benzopyrene, a compound found in cigarette smoke, increased aortic muscular cell apoptosis and aortic macrophage infiltration and expression of matrix metalloproteinases-2, 9 and 12 and nuclear factor-κB and increased formation of abdominal aortic aneurysms in angiotensin-2 induced hypertension55,56.
Our study also has several strengths including the prospective design of the included studies (which avoids recall bias and reduces the potential for selection bias), the consistency of the findings across a range of subgroup and sensitivity analyses, and the moderately high study quality of the included studies. The large sample size (with >7800–8900 cases and 3–4.7 million participants) provided robust risk estimates of the relationship between tobacco smoking and abdominal aortic aneurysms. Given the ageing population, the strong associations observed between smoking and abdominal aortic aneurysms and the poor survival of patients with the disease the current findings provide strong support for interventions and policies to curb the tobacco epidemic.
Conclusion
There was a 5-fold and 2-fold increase in the risk of abdominal aortic aneurysms among current and former smokers compared to never smokers, respectively. A positive dose-response relationship was observed between increasing number of cigarettes smoked per day and pack-years smoked and the risk of abdominal aortic aneurysm, while there was a reduced risk with increasing duration of smoking cessation with a risk similar to that of never smokers by 25 years of smoking cessation. This together with supportive experimental data provides strong evidence for a causal relationship between smoking and abdominal aortic aneurysms. The findings provide further support for interventions and policies to curb the global tobacco epidemic.
Electronic supplementary material
Acknowledgements
This work has been supported by funding from the Imperial College National Institute of Health Research (NIHR) Biomedical Research Centre (BRC), the School of Public Health, Imperial College London and the South-East Regional Health Authorities of Norway.
Author Contributions
D.A. designed the research, conducted the literature search and analyses and wrote the first draft of the paper. D.A. and S.S. conducted the screening of the literature search. All authors interpreted the data, revised the subsequent drafts for important intellectual content, read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-32100-2.
References
- 1.Johnston KW, et al. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J. Vasc. Surg. 1991;13:452–458. doi: 10.1067/mva.1991.26737. [DOI] [PubMed] [Google Scholar]
- 2.Verhoeven EL, et al. Mortality of ruptured abdominal aortic aneurysm treated with open or endovascular repair. J. Vasc. Surg. 2008;48:1396–1400. doi: 10.1016/j.jvs.2008.07.054. [DOI] [PubMed] [Google Scholar]
- 3.Basnyat PS, Biffin AH, Moseley LG, Hedges AR, Lewis MH. Mortality from ruptured abdominal aortic aneurysm in Wales. Br. J. Surg. 1999;86:765–770. doi: 10.1046/j.1365-2168.1999.01170.x. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GBD 2015 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1603–1658. doi: 10.1016/S0140-6736(16)31460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat. Rev. Cardiol. 2011;8:92–102. doi: 10.1038/nrcardio.2010.180. [DOI] [PubMed] [Google Scholar]
- 7.Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromso Study, 1994-2001. Circulation. 2009;119:2202–2208. doi: 10.1161/CIRCULATIONAHA.108.817619. [DOI] [PubMed] [Google Scholar]
- 8.Vardulaki KA, et al. Incidence among men of asymptomatic abdominal aortic aneurysms: estimates from 500 screen detected cases. J. Med. Screen. 1999;6:50–54. doi: 10.1136/jms.6.1.50. [DOI] [PubMed] [Google Scholar]
- 9.Lederle FA, et al. Yield of repeated screening for abdominal aortic aneurysm after a 4-year interval. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch. Intern. Med. 2000;160:1117–1121. doi: 10.1001/archinte.160.8.1117. [DOI] [PubMed] [Google Scholar]
- 10.Salem MK, et al. Should Asian men be included in abdominal aortic aneurysm screening programmes? Eur. J. Vasc. Endovasc. Surg. 2009;38:748–749. doi: 10.1016/j.ejvs.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Melton LJ, III, et al. Changing incidence of abdominal aortic aneurysms: a population-based study. Am. J. Epidemiol. 1984;120:379–386. doi: 10.1093/oxfordjournals.aje.a113902. [DOI] [PubMed] [Google Scholar]
- 12.Acosta S, et al. Increasing incidence of ruptured abdominal aortic aneurysm: a population-based study. J. Vasc. Surg. 2006;44:237–243. doi: 10.1016/j.jvs.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 13.Larsson E, Granath F, Swedenborg J, Hultgren R. More patients are treated for nonruptured abdominal aortic aneurysms, but the proportion of women remains unchanged. J. Vasc. Surg. 2008;48:802–807. doi: 10.1016/j.jvs.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Svensjo S, et al. Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation. 2011;124:1118–1123. doi: 10.1161/CIRCULATIONAHA.111.030379. [DOI] [PubMed] [Google Scholar]
- 15.Laine MT, Laukontaus SJ, Kantonen I, Venermo M. Population-based study of ruptured abdominal aortic aneurysm. Br. J. Surg. 2016;103:1634–1639. doi: 10.1002/bjs.10200. [DOI] [PubMed] [Google Scholar]
- 16.Lederle FA, et al. Abdominal aortic aneurysm events in the women’s health initiative: cohort study. BMJ. 2008;337:a1724. doi: 10.1136/bmj.a1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammond EC. Smoking in relation to the death rates of one million men and women. Natl. Cancer Inst. Monogr. 1966;19:127–204. [PubMed] [Google Scholar]
- 18.Hammond EC, Garfinkel L. Coronary heart disease, stroke, and aortic aneurysm. Factors in the etiology. Arch. Environ. Health. 1969;19:167–182. doi: 10.1080/00039896.1969.10666825. [DOI] [PubMed] [Google Scholar]
- 19.Strachan DP. Predictors of death from aortic aneurysm among middle-aged men: the Whitehall study. Br. J. Surg. 1991;78:401–404. doi: 10.1002/bjs.1800780407. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg RJ, et al. Lifestyle and biologic factors associated with atherosclerotic disease in middle-aged men. 20-year findings from the Honolulu Heart Program. Arch. Intern. Med. 1995;155:686–694. doi: 10.1001/archinte.1995.00430070036004. [DOI] [PubMed] [Google Scholar]
- 21.Lee AJ, Fowkes FG, Carson MN, Leng GC, Allan PL. Smoking, atherosclerosis and risk of abdominal aortic aneurysm. Eur. Heart J. 1997;18:671–676. doi: 10.1093/oxfordjournals.eurheartj.a015314. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson S, Carstensen JM, Pershagen G. Mortality among male and female smokers in Sweden: a 33 year follow up. J. Epidemiol. Community Health. 2001;55:825–830. doi: 10.1136/jech.55.11.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodin MB, et al. Middle age cardiovascular risk factors and abdominal aortic aneurysm in older age. Hypertension. 2003;42:61–68. doi: 10.1161/01.HYP.0000078829.02288.98. [DOI] [PubMed] [Google Scholar]
- 24.Lindblad B, Borner G, Gottsater A. Factors associated with development of large abdominal aortic aneurysm in middle-aged men. Eur. J. Vasc. Endovasc. Surg. 2005;30:346–352. doi: 10.1016/j.ejvs.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 25.Iribarren C, et al. Traditional and novel risk factors for clinically diagnosed abdominal aortic aneurysm: the Kaiser multiphasic health checkup cohort study. Ann. Epidemiol. 2007;17:669–678. doi: 10.1016/j.annepidem.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Wong DR, Willett WC, Rimm EB. Smoking, hypertension, alcohol consumption, and risk of abdominal aortic aneurysm in men. Am. J. Epidemiol. 2007;165:838–845. doi: 10.1093/aje/kwk063. [DOI] [PubMed] [Google Scholar]
- 27.Lawlor DA, Song YM, Sung J, Ebrahim S, Smith GD. The association of smoking and cardiovascular disease in a population with low cholesterol levels: a study of 648,346 men from the Korean national health system prospective cohort study. Stroke. 2008;39:760–767. doi: 10.1161/STROKEAHA.107.494823. [DOI] [PubMed] [Google Scholar]
- 28.Pirie K, Peto R, Reeves GK, Green J, Beral V. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133–141. doi: 10.1016/S0140-6736(12)61720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sode BF, Nordestgaard BG, Gronbaek M, Dahl M. Tobacco smoking and aortic aneurysm: two population-based studies. Int. J. Cardiol. 2013;167:2271–2277. doi: 10.1016/j.ijcard.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Stackelberg O, Bjorck M, Larsson SC, Orsini N, Wolk A. Sex differences in the association between smoking and abdominal aortic aneurysm. Br. J Surg. 2014;101:1230–1237. doi: 10.1002/bjs.9526. [DOI] [PubMed] [Google Scholar]
- 31.Svensjo S, Bjorck M, Wanhainen A. Eur. J. Vasc. Endovasc. Surg. 2014. Editor’schoice: five-year outcomes in men screened for abdominal aortic aneurysm at 65 years of age: a population-based cohort study; pp. 37–44. [DOI] [PubMed] [Google Scholar]
- 32.Howard DP, et al. Age-specific incidence, risk factors and outcome of acute abdominal aortic aneurysms in a defined population. Br. J. Surg. 2015;102:907–915. doi: 10.1002/bjs.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jahangir E, et al. Smoking, sex, risk factors and abdominal aortic aneurysms: a prospective study of 18 782 persons aged above 65 years in the Southern Community Cohort Study. J. Epidemiol. Community Health. 2015;69:481–488. doi: 10.1136/jech-2014-204920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pujades-Rodriguez M, et al. Heterogeneous associations between smoking and a wide range of initial presentations of cardiovascular disease in 1937360 people in England: lifetime risks and implications for risk prediction. Int. J. Epidemiol. 2015;44:129–141. doi: 10.1093/ije/dyu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang W, et al. Lifetime Risk and Risk Factors for Abdominal Aortic Aneurysm in a 24-Year Prospective Study: The ARIC Study (Atherosclerosis Risk in Communities) Arterioscler. Thromb. Vasc. Biol. 2016;36:2468–2477. doi: 10.1161/ATVBAHA.116.308147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kihara T, Yamagishi K, Iso H, Tamakoshi A. Passive smoking and mortality from aortic dissection or aneurysm. Atherosclerosis. 2017;263:145–150. doi: 10.1016/j.atherosclerosis.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 37.Cornuz J, Sidoti PC, Tevaearai H, Egger M. Risk factors for asymptomatic abdominal aortic aneurysm: systematic review and meta-analysis of population-based screening studies. Eur. J. Public Health. 2004;14:343–349. doi: 10.1093/eurpub/14.4.343. [DOI] [PubMed] [Google Scholar]
- 38.Stroup DF, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 39.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 40.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am. J. Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 41.Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat. Med. 2010;29:1282–1297. doi: 10.1002/sim.3602. [DOI] [PubMed] [Google Scholar]
- 42.Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aune D, et al. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann. Oncol. 2012;23:843–852. doi: 10.1093/annonc/mdr398. [DOI] [PubMed] [Google Scholar]
- 44.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 45.Wells, G. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp, Accessed 08.11.2017.
- 46.Egger M, Davey SG, Schneider M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 48.Stackelberg O, Bjorck M, Larsson SC, Orsini N, Wolk A. Fruit and vegetable consumption with risk of abdominal aortic aneurysm. Circulation. 2013;128:795–802. doi: 10.1161/CIRCULATIONAHA.112.000728. [DOI] [PubMed] [Google Scholar]
- 49.Norman PE, Curci JA. Understanding the effects of tobacco smoke on the pathogenesis of aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 2013;33:1473–1477. doi: 10.1161/ATVBAHA.112.300158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindholt JS, Jorgensen B, Shi GP, Henneberg EW. Relationships between activators and inhibitors of plasminogen, and the progression of small abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2003;25:546–551. doi: 10.1053/ejvs.2002.1872. [DOI] [PubMed] [Google Scholar]
- 51.Raveendran M, et al. Cigarette suppresses the expression of P4Halpha and vascular collagen production. Biochem. Biophys. Res. Commun. 2004;323:592–598. doi: 10.1016/j.bbrc.2004.08.129. [DOI] [PubMed] [Google Scholar]
- 52.Wilson KA, et al. The relationship between abdominal aortic aneurysm distensibility and serum markers of elastin and collagen metabolism. Eur. J Vasc. Endovasc. Surg. 2001;21:175–178. doi: 10.1053/ejvs.2001.1303. [DOI] [PubMed] [Google Scholar]
- 53.Kugo H, et al. The effects of nicotine administration on the pathophysiology of rat aortic wall. Biotech. Histochem. 2017;92:141–148. doi: 10.1080/10520295.2017.1287428. [DOI] [PubMed] [Google Scholar]
- 54.Bergoeing MP, et al. Cigarette smoking increases aortic dilatation without affecting matrix metalloproteinase-9 and -12 expression in a modified mouse model of aneurysm formation. J. Vasc. Surg. 2007;45:1217–1227. doi: 10.1016/j.jvs.2007.01.058. [DOI] [PubMed] [Google Scholar]
- 55.Ji K, et al. Exploration of the mechanisms by which 3,4-benzopyrene promotes angiotensin II-induced abdominal aortic aneurysm formation in mice. J Vasc. Surg. 2014;59:492–499. doi: 10.1016/j.jvs.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 56.Stolle K, Berges A, Lietz M, Lebrun S, Wallerath T. Cigarette smoke enhances abdominal aortic aneurysm formation in angiotensin II-treated apolipoprotein E-deficient mice. Toxicol. Lett. 2010;199:403–409. doi: 10.1016/j.toxlet.2010.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.