Abstract
The E. coli gyrA promoter (PgyrA) is a DNA supercoiling sensitive promoter, stimulated by relaxation of DNA templates, and inhibited by (−) DNA supercoiling in bacteria. However, whether PgyrA can be inhibited by transient and localized transcription-coupled DNA supercoiling (TCDS) has not been fully examined. In this paper, using different DNA templates including the E. coli chromosome, we show that transient and localized TCDS strongly inhibits PgyrA in E. coli. This result can be explained by a twin-supercoiled domain model of transcription in which (+) and (−) supercoiled domains are generated around the transcribing RNA polymerase. We also find that fluoroquinolones, such as ciprofloxacin, can substantially increase the expression of the firefly luciferase under the control of the PgyrA coupled to a divergent IPTG-inducible promoter in the presence of IPTG. This stimulation of PgyrA by fluoroquinolones can be also explained by the twin-supercoiled domain model of transcription. This unique property of TCDS may be configured into a high throughput-screening (HTS) assay to identify antimicrobial compounds targeting bacterial DNA gyrase.
Introduction
DNA supercoiling plays a critical role in several crucial DNA transactions including DNA replication, recombination, transcription, and DNA repair1,2. In bacteria, DNA molecules are usually (−) supercoiled3. DNA supercoiling in vivo is determined by counteractions of DNA topoisomerase I & IV (relaxation) and DNA gyrase ((−) supercoiling)4,5. Inhibition of DNA gyrase activities by gyrase inhibitors causes the relaxation of the DNA templates or accumulation of (+) supercoiled plasmids6 and also induces the expression of DNA gyrase in bacteria7. Deletion of topA from the chromosome results in the production of hypernegatively supercoiled DNA molecules at the exponential phase of bacteria8. Recent genomic studies also showed that DNA supercoiling is critical for transcription regulation of many genes during bacterial cell growth9–13.
Transcription can also disrupt localized DNA supercoiling in vitro14–18 and in vivo6,8,19–23. Liu and Wang formulated a twin supercoiled domain model of transcription to explain how transcription affects localized DNA supercoiling24. As the length of the RNA transcript increases, it becomes more and more difficult for the RNA-RNA polymerase complex to rotate around the DNA molecule. At a turning point, energetically, it is more practical to rotate the DNA about its own helical axis. Further translocation of the RNA-RNA polymerase along the DNA template generates a positively supercoiled domain in front of the transcribing RNA polymerase and a negatively supercoiled domain behind it24. Many in vitro and in vivo results support this twin supercoiled domain model. For instance, in defined protein systems, transcription is able to drive close circular DNA templates to hypernegatively supercoiled status in the presence of DNA gyrase because DNA gyrase converts a fraction of the transient (+) supercoils into permanent (−) supercoils14–18. Likewise, in E. coli topA strains, transcription at the exponential phase is able to drive close circular DNA templates to hypernegatively supercoiled because DNA gyrase converts (+) supercoiled domain into (−) supercoils6,8,19–23.
Transcription-coupled DNA supercoiling (TCDS) is also able to activate supercoiling-sensitive promoters in bacteria25–29. The best-studied case is the activation of bacterial Leu-500 promoter (Pleu-500) by TCDS, a promoter containing a single A-to-G mutation in the promoter region of the leu operon30,31. Previous studies demonstrated that transcription-driven localized supercoiling rather than global supercoiling density was responsible for the activation of Pleu-50025–27,32–37. The orientation of TCDS had opposite effects where (−) supercoiling domain activated Pleu-500 and (+) supercoiling domain suppressed the promoter27. In our previously published studies38, using uniquely designed linear plasmids, we demonstrated that transient and localized (−) DNA supercoiling can strongly activate Pleu-500. The activation of Pleu-500 is dependent of the promoter strength and the length of RNA transcripts, unique properties of TCDS as predicted by the twin-supercoiled domain mechanism. We also demonstrated that TCDS could be generated on topologically open DNA molecules in E. coli cells. These results suggest that topological boundaries or barriers are not necessary for the generation of TCDS in vivo.
The E. coli gyrA promoter (PgyrA) is another supercoiling sensitive promoter and stimulated by relaxation of DNA templates7,39,40. Early mutation studies showed that the stimulation of PgyrA stems from a 20 bp DNA sequence around the −10 region of PgyrA39,40. Since this 20 bp DNA sequence is intrinsically bent or curved41, it is possible that the DNA bend or curvature functions as a supercoiling sensor for the activation by DNA relaxation41. Nevertheless, whether PgyrA can be inhibited by TCDS has not been examined. Here, using different DNA templates including the E. coli chromosome, we show that transient and localized (−) TCDS is able to strongly inhibit PgyrA in E. coli. We also found that fluoroquinolones, such as ciprofloxacin, were able to substantially increase the expression of the firefly luciferase controlled by PgyrA coupled to a divergent IPTG-inducible promoter in the presence of IPTG. This unique property of TCDS may be used to screen and identify antimicrobial compounds targeting bacterial DNA gyrase.
Results and Discussion
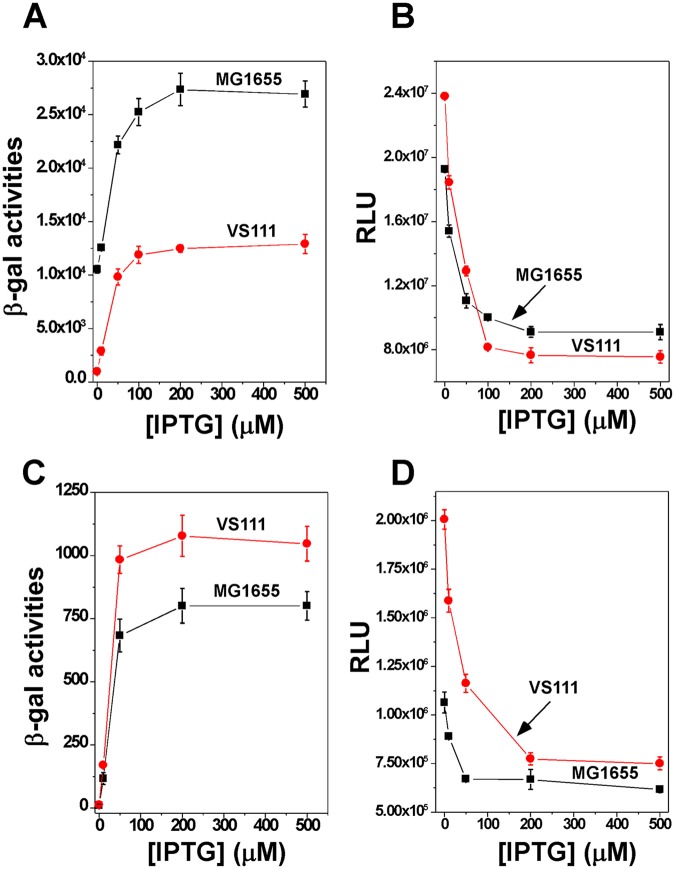
In our previous studies38, using an in vivo system that contains E. coli topA strain VS111(DE3)ΔlacZ or wild-type strain MG1655(DE3)ΔlacZ and a circular or linear plasmid DNA template, we demonstrated that transient and localized TCDS from a divergently-coupled transcription unit potently activated the supercoiling-sensitive promoter Pleu-500. In this study, we decided to utilize this system to examine whether and how TCDS inhibits a different supercoiling-sensitive promoter PgyrA. For this purpose, we substituted Pleu-500 with PgyrA divergently coupled to the strong IPTG-inducible promoter PT7A1/O4 (Fig. 1). The distance between these two promoters is 92 bp (Fig. 1A). As shown in Fig. 1C,D, we used 2 sets of 4 Rho-independent, rrnB T1 transcription terminators to block transcription from PT7A1/O4 and PgyrA, respectively. In this case, transcription is restricted to a selected region of the plasmids22. Circular plasmid pZXD144 and linear plasmid pZXD150 were used to transform VS111(DE3)ΔlacZ or MG1655(DE3)ΔlacZ. After IPTG was added to E. coli cells in the early log phase, luciferase activities were used to monitor the inhibition of PgyrA. Results in Fig. 2 show that TCDS strongly inhibits the supercoiling-sensitive PgyrA for both circular and linear plasmids. For example, TCDS from E. coli RNA polymerase on pZXD144 inhibited 53% and 68% of PgyrA in VS111(DE3)ΔlacZ and MG1655(DE3)ΔlacZ, respectively, comparing with the activities of PgyrA in the absence of IPTG (Fig. 2B). TCDS on pZXD150 inhibited 42% and 63% of PgyrA in VS111(DE3)ΔlacZ and MG1655(DE3)ΔlacZ, respectively (Fig. 2D). Due to the fact that linear DNA templates cannot be permanently supercoiled42, these results unambiguously demonstrated that transient and localized TCDS, rather than global supercoiling, inhibits the divergently coupled PgyrA. Interestingly, for circular plasmid pZXD144, the expression level of β-galactosidase is always higher in MG1655(DE3)ΔlacZ than that in VS111(DE3)ΔlacZ in the absence or presence of IPTG (Fig. 2A), which is consistent with our previously published results43. In contrast, for linear plasmid pZXD150, the expression level of β-galactosidase is lower in MG1655(DE3)ΔlacZ comparing with that in VS111(DE3)ΔlacZ (Fig. 2C). These results suggest that DNA supercoiling plays some roles in regulating the activities of PT7A1/O438. Please note that each E. coli cell carries approximate 1 copy of a linear plasmid, the overall expression levels of firefly luciferase are much lower for linear plasmids38. Since the topA strain VS111 is a DNA topoisomerase I deletion strain, it should have greater supercoiling fluctuations when disturbed by TCDS. As a result, PgyrA should be more sensitive to TCDS. Indeed, our results showed that PgyrA is more sensitive to the IPTG concentration, indicating that it is more sensitive to TCDS (Fig. 2B and D).
Figure 1.
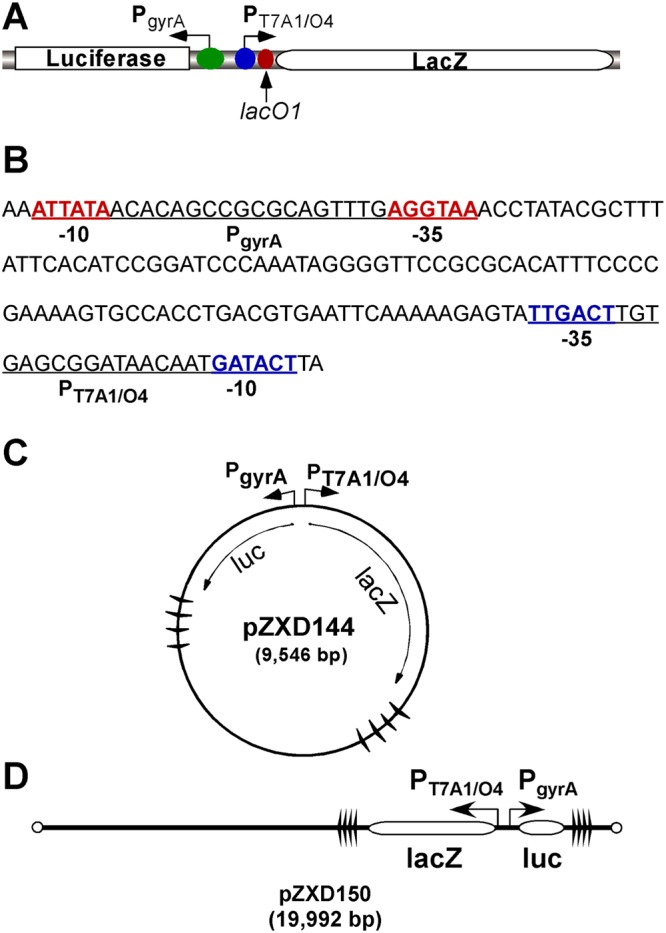
Experimental design of a pair of divergently coupled transcription units to examine transcription inhibition of PgyrA by TCDS in vivo. (A) Divergently coupled promoters PT7A1/O4 and PgyrA, respectively, control the expression of β-galactosidase (lacZ) and firefly luciferase (luc). (B) The DNA sequence of the pair of divergently coupled promoters, PT7A1/O4 and PgyrA. Underlined are PgyrA and PT7A1/O4 with −10 and −35 regions. (C,D) Maps of circular plasmid pZXD144 and linear plasmid pZXD150. Winged triangles represent Rho-independent rrnB T1 transcription terminators.
Figure 2.
Inhibition of PgyrA by TCDS for circular plasmid pZXD144 (A,B) and linear plasmid pZXD150 (C, D). The activities of β-galactosidase (Miller’s units) and firefly luciferase (RLU, relative light units) were determined as described under Methods and plotted versus the IPTG concentration. (A,B) E. coli strains MG1655(DE3)ΔlacZ (black squares and lines) and VS111(DE3)ΔlacZ (red circles and lines) carrying pZXD144 were used. (C,D) E. coli strains MG1655(DE3)ΔlacZ (black squares and lines) and VS111(DE3)ΔlacZ (red circles and lines) carrying pZXD150 were used. The standard deviation (SD) was determined according to results from three independent experiments.
Next, we examined how TCDS inhibits PgyrA on the E. coli chromosome. First, we placed a ~5 kb DNA fragment carrying the divergently coupled PgyrA and PT7A1/O4 promoters (Fig. 1A) into the attTn7 site of the E. coli chromosome (Fig. S1; the 84.2 min of the E. coli chromosome44) using a procedure of transposon Tn745 to yield a wild type strain FL1181 (MG1655(DE3)ΔlacZ attTn7::PT7A1/O4lacZ-PgyrAluc) and a topA strain FL1182 (VS111(DE3)ΔlacZ attTn7::PT7A1/O4lacZ-PgyrAluc). Due to technical difficulties, the four T1 transcription terminators were not included in these constructs. Similar to results for plasmid DNA templates as shown above, transcription by E. coli RNA polymerase can substantially inhibit transcription from PgyrA on the E. coli chromosome (Fig. 3A,B). For example, TCDS was able to inhibit 24% and 47% of PgyrA in FL1181 and FL1182, respectively. Interestingly, in the absence of IPTG, PgyrA in FL1182 is more active than that in FL1181 (Fig. 3B). As demonstrated previously43, in the absence of IPTG, PT7A1/O4 is much more active in the wildtype strain MG1655 that that in the topA strain VS111. Although the DNA templates may be more negatively supercoiled globally in VS111, the localized supercoiling around PgyrA in the wildtype strain MG1655 should be more negatively supercoiled than that in VS111 due to TCDS. In this way, the expression level of luciferase in VS111 should be higher than that in MG1655 in the absence of IPTG.
Figure 3.
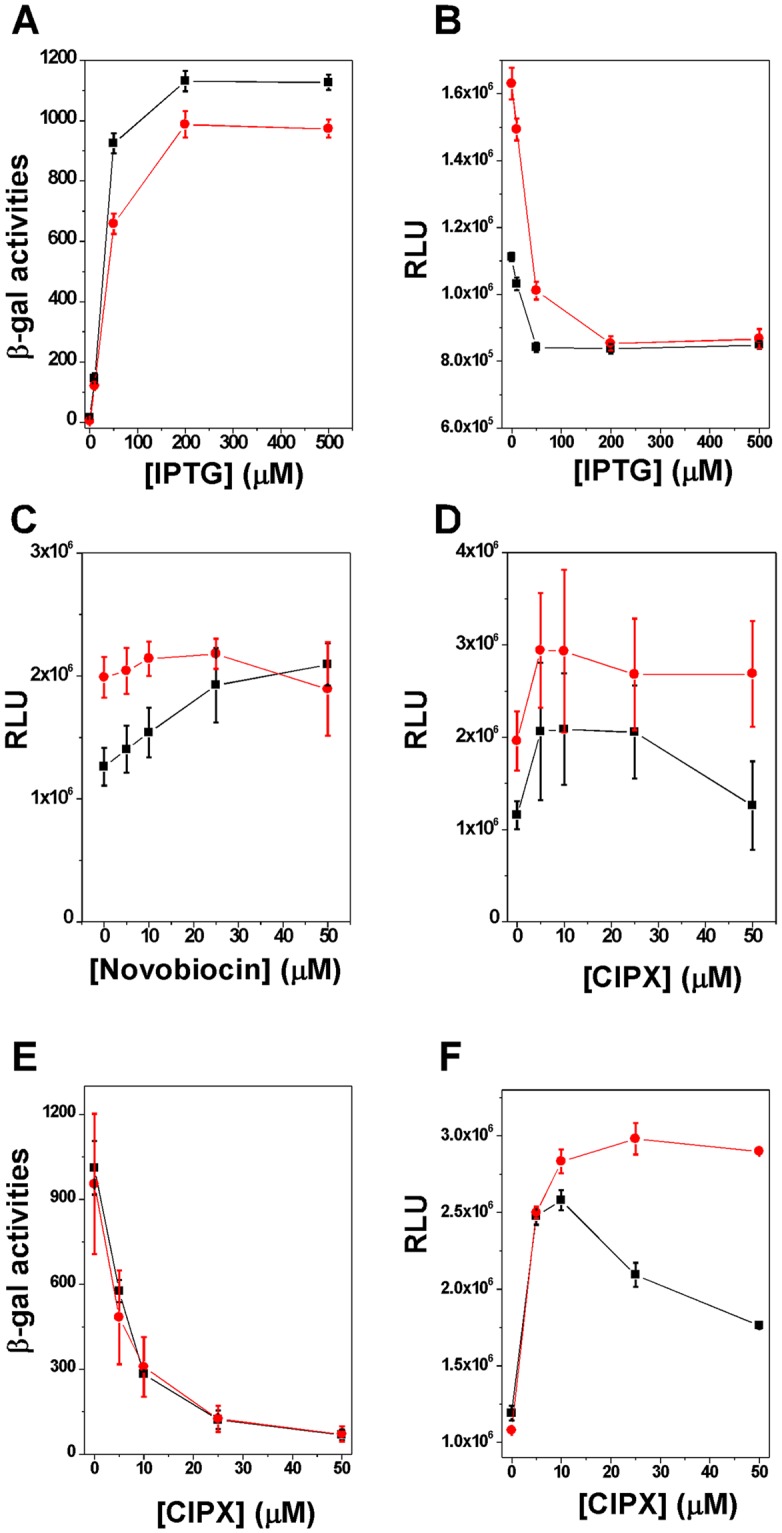
Strong inhibition of the supercoiling-sensitive PgyrA by TCDS on the chromosome. (A,B) TCDS assays for PgyrA on the chromosome. E. coli strains FL1181 (MG1655(DE3)ΔlacZ attTn7::PT7A1/O4lacZ-PgyrAluc; black squares and lines) and FL1182 (VS111(DE3)ΔlacZ attTn7::PT7A1/O4lacZ-PgyrAluc; red circles and lines) were used. The activities of β-galactosidase and firefly luciferase were determined as described under Methods and plotted versus the IPTG concentration. (C,D) Effects of novobiocin (C) and ciprofloxacin (D) on PgyrA of FL1181 (black squares and lines) and FL1182 (red circles and lines) in the absence of IPTG. (E,F) DNA gyrase inhibitors significantly enhanced the expression of firefly luciferase for FL1181 and FL1182 in the presence of IPTG. Overnight cell cultures were diluted 100-fold and grown until OD600 reached ~0.2. Then 0.5 mM of IPTG and various concentrations of ciprofloxacin or other antibiotics were added to the cell cultures. After 30 min incubation, the activities of β-galactosidase and firefly luciferase were determined described under Methods. (C) Ciprofloxacin (CIXP) inhibited the expression of β-galactosidase. (D) CIXP greatly enhanced the expression of firefly luciferase. The standard deviation (SD) was determined according to results from three independent experiments.
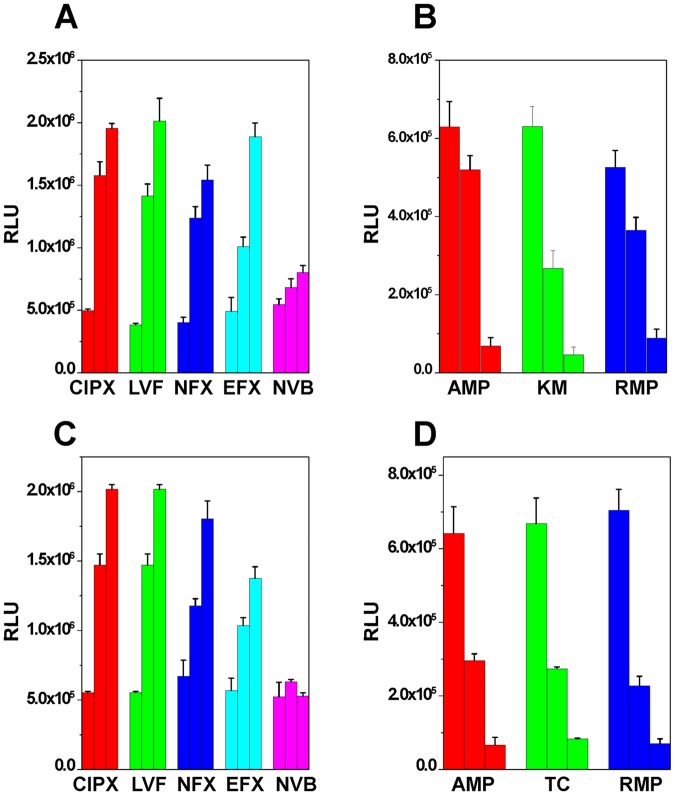
Since it was shown that gyrase inhibitors, such as coumermycin, quinolones, and novobiocin, are able to induce the expression of gyrA and gyrB in bacteria46,47, we also treated FL1181 and FL1182 with two gyrase inhibitors, novobiocin and ciprofloxacin, and examined whether these two gyrase inhibitors are able to increase the firefly luciferase expression under the control of PgyrA. At the early exponential stage, novobiocin slightly enhanced the expression of firefly luciferase in FL1181 (Fig. 3C) and did not have much effect on the expression of firefly luciferase in the topA strain FL1182 (Fig. 3C). Ciprofloxacin at low concentrations slightly stimulated the expression of firefly luciferase for both strains (Fig. 3D; the differences appear to be statistically insignificant) and inhibited the expression of firefly luciferase in FL1181 at 50 μM (Fig. 3D). Intriguingly, in the presence of IPTG, the stimulation of firefly luciferase expression by ciprofloxacin was significantly amplified (Fig. 3F) although ciprofloxacin at high concentrations completely inhibited the expression of β-galactosidase for both strains (Fig. 3E). We noticed some differences between these two E. coli strains. For the wild type strain FL1181, the stimulation of firefly luciferase expression by ciprofloxacin decreased at higher concentrations, i.e., 20 and 50 μM. For topA strain FL1182, however, the stimulation by ciprofloxacin plateaued at 10 μM and stayed high at 50 μM. These results suggest that topoisomerase I plays a role in the regulation of PgyrA activities in E. coli. We further tested several other gyrase inhibitors including levofloxacin, norfloxacin, enrofloxacin, and novobiocin, and found that only fluoroquinolones dramatically stimulated the expression of firefly luciferase in FL1181 and FL1182 in the presence of IPTG (Fig. 4A and C). Novobiocin’s effect on the expression of firefly luciferase is negligible for both strains (Fig. 4A and C). At the tested concentrations, i.e., 5 and 10 µM, these fluoroquinolones slightly inhibit the growth of the two E. coli strains (Fig. S2). We also tested several other types of antibiotics, such as transcription inhibitors (rifampicin), protein synthesis inhibitors (kanamycin and tetracycline), and cell wall synthesis inhibitors (ampicillin), and found that all these antibiotics inhibited the expression of firefly luciferase in FL1181 and FL1182 (Figs 4B,D and S3). These results suggest that the enhancement of the expression of firefly luciferase is specific for gyrase inhibitors, especially for fluoroquinolones. These results also suggest that this stimulation assay can be used to identify antibiotics targeting bacterial DNA gyrase.
Figure 4.
The stimulation of expression of firefly luciferase of FL1181 (A) and FL1182 (C) by fluoroquinolones in the presence of 0.5 mM IPTG. CIXP, LVF, EFX, NFX, and novobiocin represent ciprofloxacin, levofloxacin, enrofloxacin, norfloxacin, and novobiocin, respectively. Three bars from left to right represent luciferase activities in the presence of 0, 5, and 10 μM of fluoroquinolones, respectively. (B and D) The inhibition of expression of firefly luciferase by other antibiotics (none gyrase inhibitors) for FL1181 (B) and FL1182 (D). RMP, KM, AMP, and TC represent rifampicin, kanamycin, ampicillin, and tetracycline, respectively. The following are concentrations used in the experiments from left to right: AMP, 0, 150, 300 μM; KM, 0, 40, 80 μM; RMP, 0, 25, 50 μM; TC, 0, 10, 20 μM. The standard deviation (SD) was determined according to results from three independent experiments.
We believe that the twin supercoiled domain model of transcription24 can explain why gyrase inhibitors are able to stimulate the expression of firefly luciferase in FL1181 and FL1182. At the early exponential phase, RNA polymerase is actively transcribing genes along the E. coli chromosome, introducing localized DNA supercoiling around these genes, and remodeling the chromosome. For FL1181 and FL1182, the divergently coupled PgyrA and PT7A1/O4 promoters with the luc and lacZ genes are located at 84.2 min of the E. coli chromosome near the seven rRNA operons48. Since the E. coli RNA polymerase transcribes along these seven rRNA operons away from 84.2 min of the E. coli chromosome, transcription should introduce significant amounts of (−) supercoils to this region. As a result, PgyrA is repressed. For the wild type strain FL1181, in the presence of novobiocin, DNA gyrase is no longer capable of removing (+) supercoiled domain generated during transcription. Topoisomerase I, on the other hand, relaxes (−) supercoiled domain. In this way, DNA templates including the chromosome should be more relaxed, which resulted in the stimulation of the expression of firefly luciferase under the control of PgyrA (Fig. 3C). Since the topA strain FL1182 does not have DNA topoisomerase I to remove (−) supercoiled domain, the DNA supercoiling status in FL1182 will not fluctuate significantly in the presence of novobiocin. This is the reason why novobiocin did not greatly affect the expression of firefly luciferase in FL1182 (Fig. 3C). Ciprofloxacin is a different DNA gyrase inhibitor and forms gyrase-cipro-DNA complexes that cause the termination of transcription for both FL1181 and FL1182 (Fig. 3E). The (−) supercoiled domain should not be formed. As a result, ciprofloxacin was able to “stimulate” the expression of firefly luciferase for both strains (Fig. 3D).
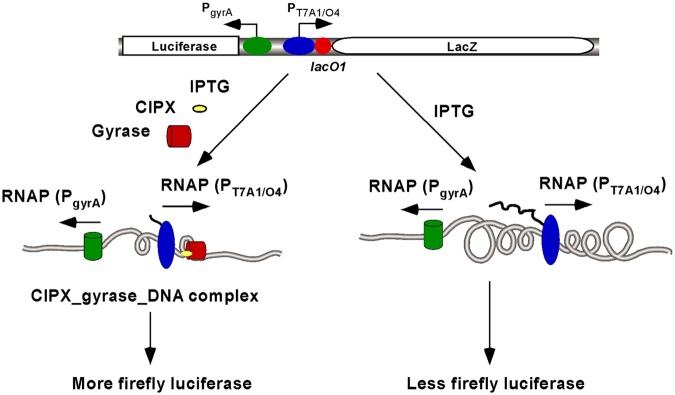
Regarding why fluoroquinolones, in the presence of IPTG, are able to enhance the expression of firefly luciferase (Fig. 4A and C), we favor the model depicted in Fig. 5 for explanation. In the presence of IPTG, transcription initiated from the strong PT7A1/O4 produces a significant amount of (−) supercoils behind the RNA polymerase and as a result inhibits the expression of firefly luciferase by PgyrA (Fig. 3B). However, ciprofloxacin stabilizes gyrase-cipro-DNA complexes for those DNA gyrases that remove the (+) supercoiled domain in front of RNA polymerase. As a result, transcription from PT7A1/O4 is terminated (Fig. 3E) and the (−) supercoiling domain behind the RNA polymerase is not formed. Because PgyrA is a weak promoter and transcription from PgyrA should not produce significant amounts of (+) supercoils in front of RNA polymerase, gyrase-cipro-DNA complexes are not formed. In this scenario, ciprofloxacin will not be able to inhibit the expression of firefly luciferase. In contrast, the (−) DNA supercoiled domain from the divergently coupled PT7A1/O4 is not formed, the expression of firefly luciferase is greatly “enhanced” (Fig. 3F). Because novobiocin only inhibits DNA gyrase activities and does not form gyrase-novobiocin-DNA complexes, it should not significantly enhance or inhibit the expression of firefly luciferase in FL1181 and FL1182 (Fig. 4A and C). Other antibiotics, due to not affecting DNA supercoiling status in vivo, should not be able to enhance the expression of firefly luciferase. In contrast, they inhibited the expression of firefly luciferase and β-glactosidase in FL1181 and FL1182.
Figure 5.
A possible mechanism to explain effects of ciprofloxacin on PgyrA in the presence of IPTG. In the presence of IPTG (right panel), transcription from PT7A1/O4 induces significant TCDS and inhibits the expression of firefly luciferase from PgyrA. However, in the presence of gyrase inhibitor ciprofloxacin, ciprofloxacin stabilizes gyrase-cipro-DNA complex that blocks transcription from PT7A1/O4. The (−) supercoils behind RNA polymerase are not formed. As a result, the expression of firefly luciferase is “enhanced.”
Summary
Here, using a unique in vivo system, we demonstrated that transient and localized (−) TCDS provided by E. coli RNA polymerase could inhibit the PgyrA at the plasmid and chromosomal levels. We also found that fluoroquinolones, such as ciprofloxacin, were able to substantially increase the expression of the firefly luciferase under the control of the PgyrA in the presence of IPTG. This unique property of TCDS can be effectively used to screen and identify antimicrobial compounds targeting bacterial DNA gyrase.
Methods
Materials
Kanamycin, lysozyme, and ortho-Nitrophenyl-β-galactoside (ONPG) were purchased from Sigma-Aldrich Corporation (St. Louis, MO). Ampicillin and bovine serum albumin (BSA) were bought from Fisher Scientific (Fairlawn, NJ). Isopropyl-β-D-thiogalactopyranoside (IPTG) was obtained from Anatrace, Inc (Maumee, Ohio). All restriction enzymes, T4 DNA ligase, and T4 polynucleotide kinase were purchased from New England Biolabs (Beverly, MA). Pfu DNA polymerase was obtained from Stratagene, Inc. (La Jolla, CA). All synthetic oligonucleotides were purchased from Eurofins Genomics (Huntsville, AL). Plasmid and DNA fragment cleaning kits including QIAprep Spin Miniprep Kit, QIAquick Gel Extraction Kit, and QIAquick Nucleotide Removal Kit were obtained from QIAGEN, Inc. (Valencia, CA). Luciferase Assay System was bought from Promega Corporation (Madison, WI).
Plasmid DNA templates
Circular plasmid pZXD133, a derivative of pBR322, was described previously43. Plasmid pZXD144 was constructed by inserting a 70 bp synthetic oligomer harboring a PgyrA into the BamHI and HindIII sites of pZXD133. In this case, PgyrA is divergently coupled to PT7A1/O4 (Fig. 1). Linear plasmid pZXD150 was described previously38.
Bacterial strains
E. coli strains MG1655(DE3) and VS111(DE3) were described previously22,23,43. E. coli strains FL1181 (MG1655(DE3)ΔlacZ attTn7::PT7A1/O4lacZ-PgyrAluc) and FL1182 (VS111(DE3)ΔlacZ attTn7::PT7A1/O4lacZ-PgyrAluc) were created by utilizing a Tn7-based site-specific recombination system45 as follows. A 5.1 kb DNA fragment harboring the divergently coupled PgyrA and PT7A1/O4 promoters controlling the luc and lacZ genes, respectively, was inserted into the attTn7 site of the E. coli chromosome44 (84 min) to yield FL1181 and FL1182 in which the IPTG-inducible PT7A1/O4 controls the expression of β-galactosidase.
The expression of β-galactosidase
The expression level of β-galactosidase was measured as described in previous publications38,49. Briefly, 100 mL of LB was inoculated with 1 mL of overnight bacterial cell culture at ratio of 1:100 until OD600 = ~0.2. 100 μL of bacterial cell culture was added to 900 μL of Z-buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, and 50 mM β-mercaptoethanol). Then, 60 μL of chloroform and 30 μL of 0.1% SDS were added to lyse the cells. After cell lysates were incubated at 30 °C for 5 minutes, 200 μL of ONPG (4 mg/mL) was added. After another 15 min of incubation at 30 °C, 500 μL of 1 M Na2CO3 was added to stop the reaction. After cell debris was removed by centrifugation at 13,000 rpm for 1 min, the OD420 and OD550 values were measured in a Cary 50 spectrophotometer. β-Galactosidase activities (E) were calculated using equation:
| 1 |
where t and v, respectively, represent reaction time and cell culture volume.
Luciferase assay
The expression of the firefly luciferase in E. coli were monitored by using the luciferase assay as described in our previous publication38.
Electronic supplementary material
Acknowledgements
We thank Drs Nikolai V. Ravin and Nancy L. Craig for providing us with the linear plasmid pG591 and the circular plasmid pGRG36, respectively. This work was supported by Grants 1R15GM109254-01A1 and 1R21AI125973-01A1 from the National Institutes of Health (to F.L.).
Author Contributions
F.L. designed research; S.D., K.D., and X.Z. performed research; F.L. analyzed data; F.L. wrote the paper.
Competing Interests
A US patent has been awarded to authors related to this manuscript. Patent title: Materials and Methods for identifying gyrase inhibitors. US Patent Number: 10000807.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-33089-4.
References
- 1.Bates, A. D. & Maxwell, A. DNA Topology (Oxford University Press, Oxford, UK, 2005).
- 2.James, C. Wang Untangling the Double Helix: DNA Entanglement and the Action of the DNA Topoisomerases (Cold Spring Harbor Laboratory Press, 2008).
- 3.Cozzarelli, N. R. & Wang, J. C. DNA Topology and Its Biological Effects (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 1990).
- 4.Snoep JL, van der Weijden CC, Andersen HW, Westerhoff HV, Jensen PR. DNA supercoiling in Escherichia coli is under tight and subtle homeostatic control, involving gene-expression and metabolic regulation of both topoisomerase I and DNA gyrase. Eur. J. Biochem. 2002;269:1662–1669. doi: 10.1046/j.1432-1327.2002.02803.x. [DOI] [PubMed] [Google Scholar]
- 5.Zechiedrich EL, et al. Roles of topoisomerases in maintaining steady-state DNA supercoiling in Escherichia coli. J. Biol. Chem. 2000;275:8103–8113. doi: 10.1074/jbc.275.11.8103. [DOI] [PubMed] [Google Scholar]
- 6.Lockshon D, Morris DR. Positively supercoiled plasmid DNA is produced by treatment of Escherichia coli with DNA gyrase inhibitors. Nucleic Acids Res. 1983;11:2999–3017. doi: 10.1093/nar/11.10.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menzel R, Gellert M. Regulation of the genes for E. coli DNA gyrase: homeostatic control of DNA supercoiling. Cell. 1983;34:105–113. doi: 10.1016/0092-8674(83)90140-X. [DOI] [PubMed] [Google Scholar]
- 8.Pruss GJ. DNA topoisomerase I mutants. Increased heterogeneity in linking number and other replicon-dependent changes in DNA supercoiling. J. Mol. Biol. 1985;185:51–63. doi: 10.1016/0022-2836(85)90182-2. [DOI] [PubMed] [Google Scholar]
- 9.Blot N, Mavathur R, Geertz M, Travers A, Muskhelishvili G. Homeostatic regulation of supercoiling sensitivity coordinates transcription of the bacterial genome. EMBO Rep. 2006;7:710–715. doi: 10.1038/sj.embor.7400729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sobetzko P. Transcription-coupled DNA supercoiling dictates the chromosomal arrangement of bacterial genes. Nucleic Acids Res. 2016;44:1514–1524. doi: 10.1093/nar/gkw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong KS, Ahn J, Khodursky AB. Spatial patterns of transcriptional activity in the chromosome of Escherichia coli. Genome Biol. 2004;5:R86. doi: 10.1186/gb-2004-5-11-r86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peter BJ, et al. Genomic transcriptional response to loss of chromosomal supercoiling in Escherichia coli. Genome Biol. 2004;5:R87. doi: 10.1186/gb-2004-5-11-r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lal A, et al. Genome scale patterns of supercoiling in a bacterial chromosome. Nat. Commun. 2016;7:11055. doi: 10.1038/ncomms11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu HY, Shyy SH, Wang JC, Liu LF. Transcription generates positively and negatively supercoiled domains in the template. Cell. 1988;53:433–440. doi: 10.1016/0092-8674(88)90163-8. [DOI] [PubMed] [Google Scholar]
- 15.Tsao YP, Wu HY, Liu LF. Transcription-driven supercoiling of DNA: direct biochemical evidence from in vitro studies. Cell. 1989;56:111–118. doi: 10.1016/0092-8674(89)90989-6. [DOI] [PubMed] [Google Scholar]
- 16.Drolet M, Bi X, Liu LF. Hypernegative supercoiling of the DNA template during transcription elongation in vitro. J. Biol. Chem. 1994;269:2068–2074. [PubMed] [Google Scholar]
- 17.Leng F, McMacken R. Potent stimulation of transcription-coupled DNA supercoiling by sequence-specific DNA-binding proteins. Proc. Natl. Acad. Sci. USA. 2002;99:9139–9144. doi: 10.1073/pnas.142002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leng F, Amado L, McMacken R. Coupling DNA supercoiling to transcription in defined protein systems. J. Biol. Chem. 2004;279:47564–47571. doi: 10.1074/jbc.M403798200. [DOI] [PubMed] [Google Scholar]
- 19.Lodge JK, Kazic T, Berg DE. Formation of supercoiling domains in plasmid pBR322. J. Bacteriol. 1989;171:2181–2187. doi: 10.1128/jb.171.4.2181-2187.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cook DN, Ma D, Pon NG, Hearst JE. Dynamics of DNA supercoiling by transcription in Escherichia coli. Proc. Natl. Acad. Sci. USA. 1992;89:10603–10607. doi: 10.1073/pnas.89.22.10603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynch AS, Wang JC. Anchoring of DNA to the bacterial cytoplasmic membrane through cotranscriptional synthesis of polypeptides encoding membrane proteins or proteins for export: a mechanism of plasmid hypernegative supercoiling in mutants deficient in DNA topoisomerase I. J. Bacteriol. 1993;175:1645–1655. doi: 10.1128/jb.175.6.1645-1655.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samul R, Leng F. Transcription-coupled hypernegative supercoiling of plasmid DNA by T7 RNA polymerase in Escherichia coli topoisomerase I-deficient strains. J. Mol. Biol. 2007;374:925–935. doi: 10.1016/j.jmb.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhi X, Leng F. Dependence of transcription-coupled DNA supercoiling on promoter strength in Escherichia coli topoisomerase I deficient strains. Gene. 2013;514:82–90. doi: 10.1016/j.gene.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu LF, Wang JC. Supercoiling of the DNA template during transcription. Proc. Natl. Acad. Sci. USA. 1987;84:7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen D, Bowater R, Dorman CJ, Lilley DM. Activity of a plasmid-borne leu-500 promoter depends on the transcription and translation of an adjacent gene. Proc. Natl. Acad. Sci. USA. 1992;89:8784–8788. doi: 10.1073/pnas.89.18.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan J, Shu L, Wu HY. Activation of the leu-500 promoter by adjacent transcription. J. Bacteriol. 1994;176:1077–1086. doi: 10.1128/jb.176.4.1077-1086.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El HD, Bossi L. Activation and silencing of leu-500 promoter by transcription-induced DNA supercoiling in the Salmonella chromosome. Mol. Microbiol. 2000;37:583–594. doi: 10.1046/j.1365-2958.2000.02015.x. [DOI] [PubMed] [Google Scholar]
- 28.Rhee KY, et al. Transcriptional coupling between the divergent promoters of a prototypic LysR-type regulatory system, the ilvYC operon of Escherichia coli. Proc. Natl. Acad. Sci. USA. 1999;96:14294–14299. doi: 10.1073/pnas.96.25.14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Peso, S. T. & Shingler,V. Inter-sigmulon communication through topological promoter coupling. Nucleic Acids Res (2016). [DOI] [PMC free article] [PubMed]
- 30.Mukai FH, Margolin P. Analysis of Unlinked Suppressors of an O Degrees Mutation in Salmonella. Proc. Natl. Acad. Sci. USA. 1963;50:140–148. doi: 10.1073/pnas.50.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubnau E, Margolin P. Suppression of promoter mutations by the pleiotropic supx mutations. Mol. Gen. Genet. 1972;117:91–112. doi: 10.1007/BF00267607. [DOI] [PubMed] [Google Scholar]
- 32.Lilley DM, Higgins CF. Local DNA topology and gene expression: the case of the leu-500 promoter. Mol. Microbiol. 1991;5:779–783. doi: 10.1111/j.1365-2958.1991.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 33.Wu HY, Tan J, Fang M. Long-range interaction between two promoters: activation of the leu-500 promoter by a distant upstream promoter. Cell. 1995;82:445–451. doi: 10.1016/0092-8674(95)90433-6. [DOI] [PubMed] [Google Scholar]
- 34.Fang M, Wu HY. A promoter relay mechanism for sequential gene activation. J. Bacteriol. 1998;180:626–633. doi: 10.1128/jb.180.3.626-633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang M, Wu HY. Suppression of leu-500 mutation in topA+ Salmonella typhimurium strains. The promoter relay at work. J. Biol. Chem. 1998;273:29929–29934. doi: 10.1074/jbc.273.45.29929. [DOI] [PubMed] [Google Scholar]
- 36.Chen CC, Wu HY. Transcription-driven DNA supercoiling and gene expression control. Front Biosci. 2003;8:d430–d439. doi: 10.2741/968. [DOI] [PubMed] [Google Scholar]
- 37.Wu HY, Fang M. DNA supercoiling and transcription control: a model from the study of suppression of the leu-500 mutation in Salmonella typhimurium topA- strains. Prog. Nucleic Acid Res. Mol. Biol. 2003;73:43–68. doi: 10.1016/S0079-6603(03)01002-X. [DOI] [PubMed] [Google Scholar]
- 38.Zhi X, et al. Transient and dynamic DNA supercoiling potently stimulates the leu-500 promoter in Escherichia coli. J. Biol. Chem. 2017;292:14566–14575. doi: 10.1074/jbc.M117.794628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menzel R, Gellert M. Modulation of transcription by DNA supercoiling: a deletion analysis of the Escherichia coli gyrA and gyrB promoters. Proc. Natl. Acad. Sci. USA. 1987;84:4185–4189. doi: 10.1073/pnas.84.12.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Straney R, Krah R, Menzel R. Mutations in the −10 TATAAT sequence of the gyrA promoter affect both promoter strength and sensitivity to DNA supercoiling. J. Bacteriol. 1994;176:5999–6006. doi: 10.1128/jb.176.19.5999-6006.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Unniraman S, Nagaraja V. Axial distortion as a sensor of supercoil changes: a molecular model for the homeostatic regulation of DNA gyrase. J. Genet. 2001;80:119–124. doi: 10.1007/BF02717907. [DOI] [PubMed] [Google Scholar]
- 42.Deneke J, Ziegelin G, Lurz R, Lanka E. The protelomerase of temperate Escherichia coli phage N15 has cleaving-joining activity. Proc. Natl. Acad. Sci. USA. 2000;97:7721–7726. doi: 10.1073/pnas.97.14.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fulcrand G, et al. DNA supercoiling, a critical signal regulating the basal expression of the lac operon in Escherichia coli. Sci. Rep. 2016;6:19243. doi: 10.1038/srep19243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waddell CS, Craig NL. Tn7 transposition: recognition of the attTn7 target sequence. Proc. Natl. Acad. Sci. USA. 1989;86:3958–3962. doi: 10.1073/pnas.86.11.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKenzie GJ, Craig NL. Fast, easy and efficient: site-specific insertion of transgenes into enterobacterial chromosomes using Tn7 without need for selection of the insertion event. BMC. Microbiol. 2006;6:39. doi: 10.1186/1471-2180-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neumann S, Quinones A. Discoordinate gene expression of gyrA and gyrB in response to DNA gyrase inhibition in Escherichia coli. J. Basic Microbiol. 1997;37:53–69. doi: 10.1002/jobm.3620370109. [DOI] [PubMed] [Google Scholar]
- 47.Menzel R, Gellert M. Fusions of the Escherichia coli gyrA and gyrB control regions to the galactokinase gene are inducible by coumermycin treatment. J. Bacteriol. 1987;169:1272–1278. doi: 10.1128/jb.169.3.1272-1278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nomura M. Engineering of bacterial ribosomes: replacement of all seven Escherichia coli rRNA operons by a single plasmid-encoded operon. Proc. Natl. Acad. Sci. USA. 1999;96:1820–1822. doi: 10.1073/pnas.96.5.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller, J. H. Experiments in Molecular Genetics (Cold Spring HarborLaboratory, Cold Spring Harbor, NY, 1972).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.