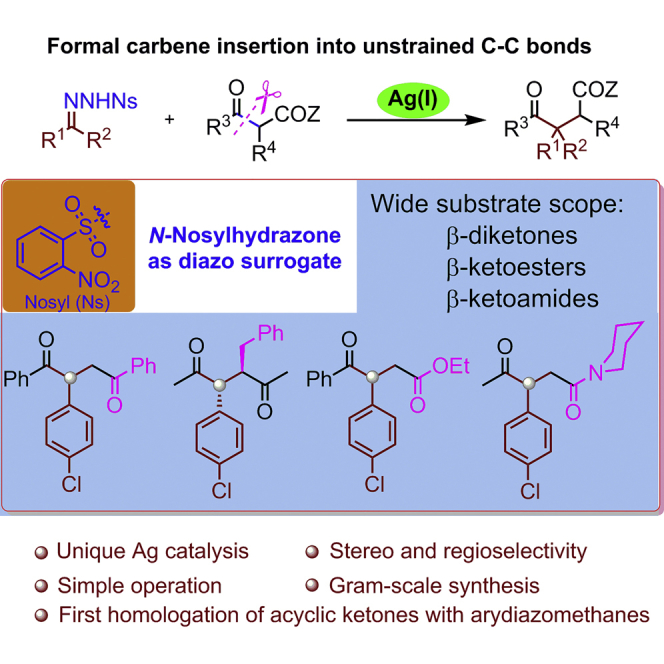
Summary
A regio- and stereoselective silver-catalyzed formal carbene insertion into 1,3-dicarbonyls has been developed, using N-nosylhydrazones as diazo surrogates. Two new C−C bonds are constructed at the carbenic carbon center through the selective cleavage of the C−C(=O) σ-bond of acyclic 1,3-dicarbonyls, enabling the preparation of various synthetically useful polysubstituted γ-diketones, γ-ketoesters, and γ-ketoamides in high yields. The in situ formation of a donor-acceptor cyclopropane, via reaction of the enolate of the 1,3-dicarbonyl with an electrophilic silver carbenoid, is proposed as a key process in the catalytic cycle.
Subject Areas: Chemistry, Catalysis, Organic Synthesis
Graphical Abstract
Highlights
-
•
Regio- and stereoselective carbene insertion into unstrained C−C bonds
-
•
Homologation of acyclic ketones with arydiazomethanes
-
•
N-nosylhydrazones as room temperature decomposable diazo surrogates
Chemistry; Catalysis; Organic Synthesis
Introduction
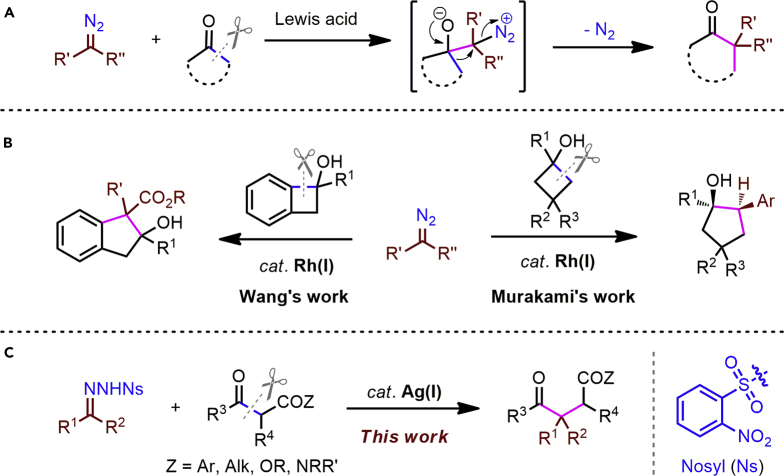
Selective one-carbon insertion into C−C σ-bonds is a highly desirable strategy to homologate organic molecules (Candeias et al., 2016). However, this process is, in general, highly challenging, due to the difficulty of cleaving the relatively inert C−C σ-bonds (Murakami et al., 2016, Fumagalli et al., 2017, Souillart and Cramer, 2015, Chen et al., 2014). Aside from reactions in strained systems, very few efficient strategies are available (Chen et al., 2014); among these, the homologation of ketones with diazo compounds represents one of the most explored strategies, whereby the diazo compound acts as ambiphilic species in sequential nucleophilic addition/1,2-rearrangement cascades (Candeias et al., 2016, Moebius and Kingsbury, 2009, Hashimoto et al., 2009, Hashimoto et al., 2011, Li et al., 2013) (Figure 1A). Diazo compounds have been widely explored as a source of carbenoids under transition metal catalysis (Xia et al., 2017, Ford et al., 2015, Davies and Manning, 2008, Doyle et al., 1998); in the context of C–C insertions, Wang and co-workers have described the formal insertion of diazo-derived rhodium carbenoids into the cyclic C−C bonds of strained benzocyclobutenols (Xia et al., 2014), whereas Murakami's group reported a related enantioselective insertion using N-tosylhydrazones as carbene precursors (Yada et al., 2014) (Figure 1B). In both reports, strain release provides a crucial thermodynamic driving force (Fumagalli et al., 2017). In sharp contrast, the selective one-carbon insertion of diazo-derived carbenoids into unstrained acyclic C−C σ-bonds is a formidable challenge (Brogan and Zercher, 1996). Very recently, we have also realized a silver-catalyzed formal carbene insertion into acyclic C-C bonds, affording 1,4-dicarbonyl products bearing an all-carbon quaternary center (Liu et al., 2018). However, the process still has certain deficiencies like the need to synthesize and handle potentially toxic and explosive diazo compounds and the fact that the C-C bond cleavage is limited to 1,3-diketones. As part of our continued interest in the silver-catalyzed activation of diazo compounds (Liu et al., 2017a, Liu et al., 2017b, Liu et al., 2018), we here report the silver-catalyzed formal carbene insertion into the unstrained C−C(=O) bonds of 1,3-dicarbonyls (Xi et al., 2014), using N-nosylhydrazones (Xia and Wang, 2017, Xiao et al., 2013, Shao and Zhang, 2012, Barluenga and Valdés, 2011) as diazo surrogates (Figure 1C). This represents the first example of the homologation of acyclic ketones with aryldiazomethanes (Candeias et al., 2016, Xia et al., 2013) and offers a straightforward route to construct synthetically useful polysubstituted 1,4-dicarbonyls, which can be difficult to synthesize by other approaches (DeMartino et al., 2008, Yoo et al., 2010, Liu et al., 2011).
Figure 1.
One-Carbon Insertion of Diazo Compounds into C−C σ-Bonds
(A) Lewis acid-promoted nucleophilic addition/1,2-rearrangement.
(B) Rh(I)-catalyzed formal carbene insertion into strained C-C bonds.
(C) This work: Ag(I)-catalyzed formal carbene insertion into unstrained C-C bonds.
Results and Discussion
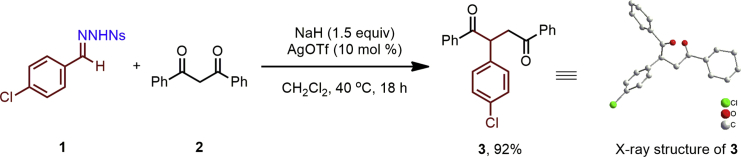
As shown in Scheme 1, initial efforts to achieve the optimal conditions for this insertion process used the reaction of 4-chlorobenzaldehyde N-nosylhydrazone 1 and 1,3-diphenylpropane-1,3-dione 2 as the model, AgOTf (10 mol %) and NaH (1.5 equiv) in CH2Cl2 at 40°C (please see Table S1 for details), under which the one-carbon insertion product 3 was obtained in 92% yield. The product structure was unambiguously established by single-crystal X-ray analysis (please see Table S2 for details).
Scheme 1.
Optimization of N-Nosylhydrazone 1 Insertion into 1,3-Diketone 2
See also Figures S1 and S2.
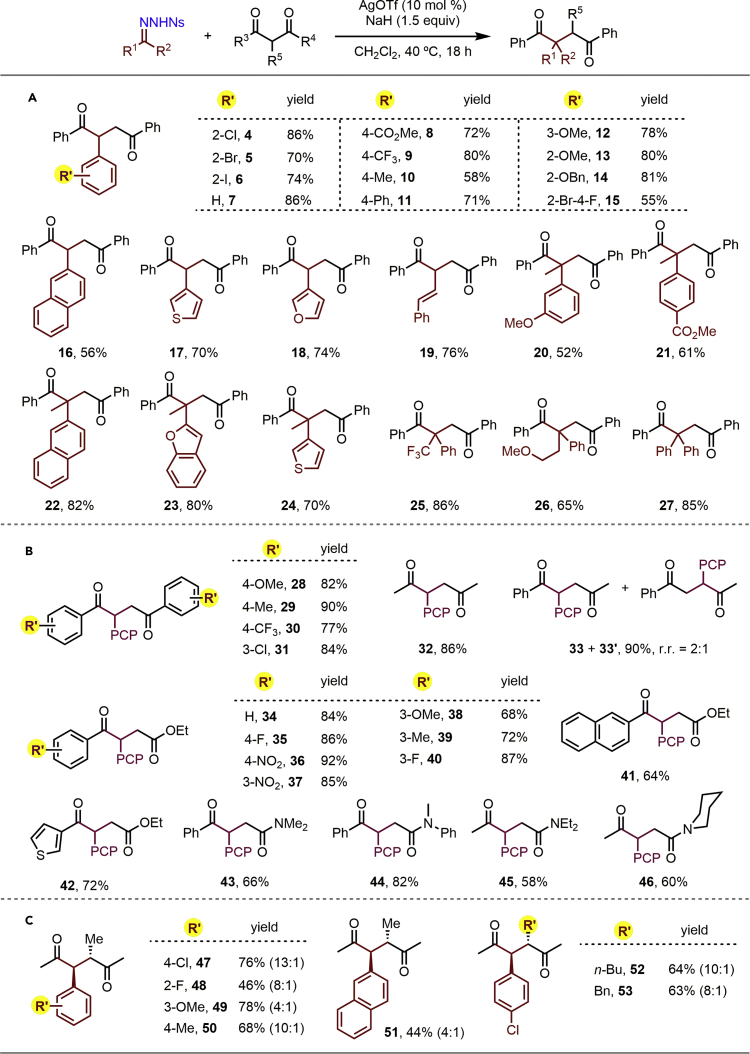
With these conditions in hand, the reaction scope was investigated, first focusing on the N-nosylhydrazone substrate (Figure 2A). Pleasingly, a wide variety of aldehyde- and ketone-derived N-nosylhydrazones proved to be suitable substrates. Benzaldehydes substituted with halogens, and a range of other electron-withdrawing (CO2Me and CF3), electron-neutral, and electron-donating groups (Me, OMe, and OBn) at the para, meta, or ortho positions of the phenyl ring were all well-tolerated, affording the desired 1,4-dicarbonyls 4–14 in 58%–86% yield. Notably, a potentially reactive benzylic C–H bond in substrate remained intact (Soldi et al., 2014), giving the desired C−C insertion product 14 in 81% yield. More complex aldehyde-derived N-nosylhydrazones were also compatible, such as disubstituted arenes, naphthyl, 3-thienyl, 3-furyl, and even cinnamyl groups, leading to the corresponding products 15–19 in good yields. To our delight, ketone-derived N-nosylhydrazones also underwent successful C–C insertion to give 1,4-dicarbonyls containing an all-carbon α-quaternary stereocenter (Quasdorf and Overman, 2014, Zeng et al., 2016, Feng et al., 2017), requiring only a slight increase of the reaction temperature to 50°C. The scope of these substrates was again broad, with both electron-rich and electron-poor aryl, naphthyl, and heteroaryl methyl ketone-based hydrazones all undergoing smooth reaction with 2 to give 1,4-diketones (20–27) in 52%–86% yield. N-nosylhydrazones bearing a trifluoromethyl group and a 2-methoxyethyl group were also compatible with the reaction conditions, giving α-functionalized 1,4-dicarbonyls 25 and 26 in 86% and 65% yield, respectively. Even the sterically hindered diphenyl N-nosylhydrazone delivered the desired product 27 in excellent yield (85%). Collectively, these results demonstrate the breadth of N-nosylhydrazones that can be employed in this insertion chemistry.
Figure 2.
Substrate Scope Investigation
(A) C-C insertion reaction of N-nosylhydrazones.
(B) C-C insertion reaction of 1,3-dicarbonyls.
(C) Diastereoselective insertion into α-substituted 1,3-dicarbonyls.
Reaction conditions: N-nosylhydrazones (0.3 mmol), NaH (0.45 mmol), and CH2Cl2 (6.0 mL) were stirred at room temperature for 1 hr, then 1,3-dicarbonyls (0.45 mmol) and AgOTf (0.03 mmol) were added, after which the mixture was stirred at 40°C for 18 hr; yields are isolated yields. The reaction was performed at 50°C for compounds 20–27. PCP, p-chlorophenyl for compounds 28–46. See also Figures S3–S111.
Next, a variety of 1,3-dicarbonyl compounds, including β-diketones, β-ketoesters, and β-ketoamides, were examined in reactions with 4-chlorophenyl N-nosylhydrazone 1 (Figure 2B). Various symmetrical β-diketones with different substituted aryl/alkyl groups were all excellent substrates, affording the corresponding insertion products 28–32 in excellent yields (77%–90%). In the case of unsymmetrical phenyl methyl 1,3-diketone, a 2:1 mixture of products 33 and 33′ was obtained in 90% combined yield. This regioselectivity was dramatically enhanced on turning our attention to β-ketoesters: both electron-rich and electron-poor aryl, 2-naphthyl, and 3-thienyl β-ketoesters all smoothly reacted with N-nosylhydrazone 1 to deliver the corresponding γ-ketoesters (34–42) in 62%–92% yield as single regioisomers. The catalytic protocol could also be extended to β-ketoamides, affording γ-ketoamides (43–46) in moderate to good yields (58–82%), with these one-carbon insertions once again proceeding in a regioselective manner, i.e., into the C(=O)−C bond.
Inspired by the above results, we sought to challenge the scope of the chemistry by using various α-substituted 1,3-diketones (Figure 2C), which have the potential to install two adjacent stereocentres in the product 1,4-dicarbonyls. In the event, 1,3-diketones featuring α-methyl, n-butyl, and benzyl groups all participated efficiently in the C−C insertion reaction with a variety of N-nosylhydrazones, giving 2,3-disubstituted 1,4-diketones 47–53 in moderate to high yields (44%–78%) and, to our delight, with good to excellent diastereoselectivity (4:1–13:1 dr) (Dittrich et al., 2016). Such 2,3-disubstituted 1,4-diketones are widespread in biologically active natural products (DeMartino et al., 2008, Yoo et al., 2010) but are not easily accessed in a stereoselective manner by other methods (Liu et al., 2011).
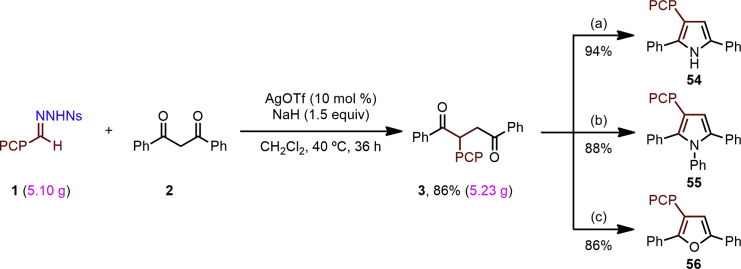
Finally, the robustness of the insertion was tested by performing a multigram-scale synthesis (Scheme 2). We were pleased to find that the reaction could readily be carried out on 15 mmol scale (5.10 g of 1), affording 3 in high yield (86%, 5.23 g). The synthetic utility of the product 1,4-diketone 3 was briefly explored through its conversion to a 2,3,5-trisubstituted pyrrole 54 on treatment with magnesium nitride (94%) (Veitch et al., 2008); to a 1,2,3,5-tetrasubstituted pyrrole (55) on refluxing with aniline in acetic acid (88%); and to a 2,3,5-trisubstituted furan (56) on treatment with TsOH in toluene at 110°C (86%) (Liu et al., 2011).
Scheme 2.
Multigram-Scale Synthesis of 3 and Further Transformations
Reaction conditions: (a) Mg3N2, MeOH, 90°C, 24 hr; (b) aniline (1.5 equiv), AcOH, reflux, 18 hr; (c) TsOH (50 mol %), toluene, 110°C, 8 hr.
See also Figures S112–S117.
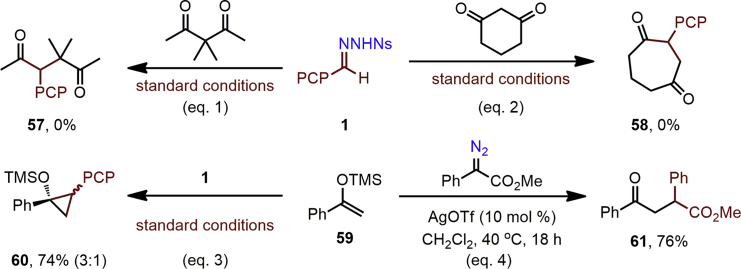
Insight into the possible mechanism of this formal C–C insertion process was obtained through a series of control experiments (Scheme 3, see also Scheme S1). First, attempted reaction of 3,3-dimethylpentane-2,4-dione (Scheme 3, Equation 1) met with failure, suggesting the ability of the dicarbonyl to form an enol or enolate to be important. Cyclohexane-1,3-dione also proved unsuccessful (Scheme 3, Equation 2); this substrate can clearly undergo enolization but cannot form an enolate capable of chelating to a metal ion through both oxygen atoms (Curini et al., 2006). Under the standard silver catalysis, the reaction of N-nosylhydrazone 1 and silylenol ether 59, which has no an electron-withdrawing group, resulted in cyclopropane 60, without ring-opening (Scheme 3, Equation 3). In contrast, reaction of methyl phenyldiazoacetate with 59 afforded C-C coupling product 61, which can be explained by in situ formation and ring-opening of a donor-acceptor cyclopropane intermediate (Scheme 3, Equation 4). The intermediacy of a silver enolate was then probed through the reactions of silver acetylacetonate and sodium acetylacetonate with 1 under the standard conditions. Only the former was successful, with product 32 isolated in 40% yield (Scheme S1). This may imply that the silver ion plays a dual role not only in the formation of a silver carbenoid from a diazo compound generated in situ from the N-nosylhydrazone but also in the delivery of this carbenoid to the dicarbonyl through formation of a bidentate-complexed silver enolate.
Scheme 3.
Mechanistic Studies
See also Figures S118–S123.
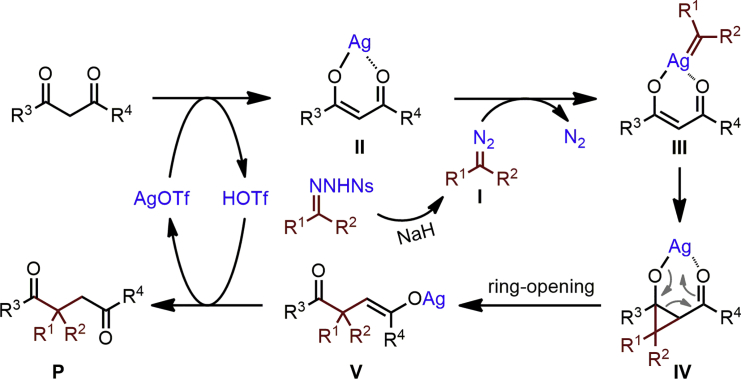
On the basis of these observations and related precedents (Brogan and Zercher, 1996, Liu et al., 2018), a plausible mechanism for this formal C−C insertion process is proposed in Scheme 4. Initially, NaH promotes decomposition of the N-nosylhydrazone 1 to generate an unstable donor-type diazo species I, which then reacts with the silver enolate II formed from reaction of AgOTf with 1,3-dicarbonyls to give a key intermediate silver carbenoid III (Thompson and Davies, 2007, Caballero et al., 2011, Luo et al., 2015). Intramolecular cyclopropanation of the enolate alkene by a carbene transfer-insertion affords cyclopropane IV, which undergoes a ring-opening retro-aldol process to generate intermediate V (Cavitt et al., 2014). Protonation of V produces the 1,4-dicarbonyl product P and regenerates the silver(I) catalyst.
Scheme 4.
Proposed Mechanism
Conclusion
In summary, a silver-catalyzed formal carbene insertion into the unstrained C−C σ-bonds of 1,3-dicarbonyls has been developed using N-nosylhydrazones as diazo surrogates. In this process, two new C−C bonds are formed to the carbenic carbon atom, along with the regioselective cleavage of a C−C σ-bond. This protocol enables the assembly of synthetically important 1,4-dicarbonyls possessing tertiary/quaternary stereocenters in high yields and with excellent regio- and stereoselectivities. The dual role of the silver catalyst—as a Lewis acid in the formation of a silver enolate and as a transition metal in forming a silver carbenoid—is crucial. Given the mild reaction conditions, broad substrate scope, excellent functional group tolerance, and ready availability of the hydrazone substrates, this one-carbon insertion chemistry has much potential for the construction of useful polysubstituted 1,4-dicarbonyls.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
X.B. thanks NSFC (21522202, 21502017), and E.A.A. thanks the EPSRC for support (EP/M019195/1).
Author Contributions
Z.L., and X.Z. performed the synthetic experiments and analyzed the experimental data; Z.L., M.V., and G.Z. wrote the original draft; E.A.A. and X.B. performed investigations and prepared the manuscript; X.B. supervised.
Declaration of Interests
The authors declare no competing interests.
Published: October 26, 2018
Footnotes
Supplemental Information includes Transparent Methods, 123 figures, 1 scheme, 2 tables, and 1 data file and can be found with this article online at https://doi.org/10.1016/j.isci.2018.09.006.
Data and Software Availability
The data for the X-ray crystallographic structure of 3 has been deposited in the Cambridge Crystallographic Data Center under accession number CCDC: 1563346 (also see Table S1 in Supplemental Information).
Supplemental Information
References
- Barluenga J., Valdés C. Tosylhydrazones: new uses for classic reagents in palladium-catalyzed cross-coupling and metal-free reactions. Angew. Chem. Int. Ed. 2011;50:7486–7500. doi: 10.1002/anie.201007961. [DOI] [PubMed] [Google Scholar]
- Brogan J.B., Zercher C.K. Zinc-mediated conversion of β-keto esters to γ-keto esters. J. Org. Chem. 1996;62:6444–6446. [Google Scholar]
- Caballero A., Despagnet-Ayoub E., Díaz-Requejo M.M., Díaz-Rodríguez A., González-Núñez M.E., Mello R., Muñoz B.K., Ojo W.S., Asensio G., Etienne M., Pérez P.J. Silver-catalyzed C-C bond formation between methane and ethyl diazoacetate in supercritical CO2. Science. 2011;332:835–838. doi: 10.1126/science.1204131. [DOI] [PubMed] [Google Scholar]
- Candeias N.R., Paterna R., Gois P.M.P. Homologation reaction of ketones with diazo compounds. Chem. Rev. 2016;116:2937–2981. doi: 10.1021/acs.chemrev.5b00381. [DOI] [PubMed] [Google Scholar]
- Cavitt M.A., Phun L.H., France S. Intramolecular donor-acceptor cyclopropane ring-opening cyclizations. Chem. Soc. Rev. 2014;43:804–818. doi: 10.1039/c3cs60238a. [DOI] [PubMed] [Google Scholar]
- Chen F., Wang T., Jiao N. Recent advances in transition-metal-catalyzed functionalization of unstrained carbon- carbon bonds. Chem. Rev. 2014;114:8613–8661. doi: 10.1021/cr400628s. [DOI] [PubMed] [Google Scholar]
- Curini M., Epifano F., Genovese S. Ytterbium triflate catalyzed synthesis of β-keto enol ethers. Tetrahedron Lett. 2006;47:4697. [Google Scholar]
- Davies H.M.L., Manning J.R. Catalytic C-H functionalization by metal carbenoid and nitrenoid insertion. Nature. 2008;451:417–424. doi: 10.1038/nature06485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartino M.P., Chen K., Baran P.S. Intermolecular enolate heterocoupling: scope, mechanism, and application. J. Am. Chem. Soc. 2008;130:11546–11560. doi: 10.1021/ja804159y. [DOI] [PubMed] [Google Scholar]
- Dittrich N., Jung E.K., Davidson S.J., Barker D. An Acyl-Claisen/Paal-Knorr approach to fully substituted pyrroles. Tetrahedron. 2016;72:4676–4689. [Google Scholar]
- Doyle M.P., McKervey M.A., Ye T. Wiley-Interscience; 1998. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds. [Google Scholar]
- Feng J., Holmes M., Krische M.J. Acyclic quaternary carbon stereocenters via enantioselective transition metal catalysis. Chem. Rev. 2017;117:12564–12580. doi: 10.1021/acs.chemrev.7b00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford A., Miel H., Ring A., Slattery C.N., Maguire A.R., McKervey M.A. Modern organic synthesis with α-diazocarbonyl compounds. Chem. Rev. 2015;115:9981–10080. doi: 10.1021/acs.chemrev.5b00121. [DOI] [PubMed] [Google Scholar]
- Fumagalli G., Stanton S., Bower J.F. Recent methodologies that exploit C-C single-bond cleavage of strained ring systems by transition metal complexes. Chem. Rev. 2017;117:9404–9432. doi: 10.1021/acs.chemrev.6b00599. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Naganawa Y., Maruoka K. Stereoselective construction of seven-membered rings with an all-carbon quaternary center by direct Tiffeneau−Demjanov-type ring expansion. J. Am. Chem. Soc. 2009;131:6614–6617. doi: 10.1021/ja900941k. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Naganawa Y., Maruoka K. Desymmetrizing asymmetric ring expansion of cyclohexanones with α-diazoacetates catalyzed by chiral aluminum Lewis acid. J. Am. Chem. Soc. 2011;133:8834–8837. doi: 10.1021/ja202070j. [DOI] [PubMed] [Google Scholar]
- Li W., Liu X., Tan F., Hao X., Zheng J., Lin L., Feng X. Catalytic asymmetric homologation of α-ketoesters with α-diazoesters: synthesis of succinate derivatives with chiral quaternary centers. Angew. Chem. Int. Ed. 2013;52:10883–10886. doi: 10.1002/anie.201305478. [DOI] [PubMed] [Google Scholar]
- Liu C., Deng Y., Wang J., Yang Y., Tang S., Lei A. Palladium-catalyzed C-C bond formation to construct 1,4-diketones under mild conditions. Angew. Chem. Int. Ed. 2011;50:7337–7341. doi: 10.1002/anie.201101638. [DOI] [PubMed] [Google Scholar]
- Liu Z., Li Q., Liao P., Bi X. Silver-catalyzed [2+1] cyclopropenation of alkynes with unstable diazoalkanes: N-nosylhydrazones as room-temperature decomposable diazo surrogates. Chem. Eur. J. 2017;23:4756–4760. doi: 10.1002/chem.201605335. [DOI] [PubMed] [Google Scholar]
- Liu Z., Li Q., Yang Y., Bi X. Silver(I)-promoted insertion into X–H (X = Si, Sn, and Ge) bonds with N-nosylhydrazones. Chem. Commun. 2017;53:2503–2506. doi: 10.1039/c6cc09650f. [DOI] [PubMed] [Google Scholar]
- Liu Z., Sivaguru P., Zanoni G., Anderson E.A., Bi X. Catalyst-dependent chemoselective formal insertion of diazo compounds into C-C or C-H bonds of 1,3-dicarbonyl compounds. Angew. Chem. Int. Ed. 2018;57:8927–8931. doi: 10.1002/anie.201802834. [DOI] [PubMed] [Google Scholar]
- Luo H., Wu G., Zhang Y., Wang J. Silver(I)-catalyzed N-trifluoroethylation of anilines and O-trifluoroethylation of amides with 2,2,2-trifluorodiazoethane. Angew. Chem. Int. Ed. 2015;54:14503–14507. doi: 10.1002/anie.201507219. [DOI] [PubMed] [Google Scholar]
- Moebius D.C., Kingsbury J.S. Catalytic homologation of cycloalkanones with substituted diazomethanes. mild and efficient single-step access to α-tertiary and α-quaternary carbonyl compounds. J. Am. Chem. Soc. 2009;131:878–879. doi: 10.1021/ja809220j. [DOI] [PubMed] [Google Scholar]
- Murakami M., Amii H., Ito Y., Chatani N. Wiley-VCH; 2016. Cleavage of Carbon-Carbon Single Bonds by Transition Metals. [Google Scholar]
- Quasdorf K.W., Overman L.E. Catalytic enantioselective synthesis of quaternary carbon stereocentres. Nature. 2014;516:181–191. doi: 10.1038/nature14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z., Zhang H. N-Tosylhydrazones: versatile reagents for metal-catalyzed and metal-free cross-coupling reactions. Chem. Soc. Rev. 2012;41:560–572. doi: 10.1039/c1cs15127d. [DOI] [PubMed] [Google Scholar]
- Soldi C., Lamb K.N., Squitieri R.A., González-López M., Di Maso M.J., Shaw J.T. Enantioselective intramolecular C-H insertion reactions of donor-donor metal carbenoids. J. Am. Chem. Soc. 2014;136:15142–15145. doi: 10.1021/ja508586t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souillart L., Cramer N. Catalytic C–C bond activations via oxidative addition to transition metals. Chem. Rev. 2015;115:9410–9464. doi: 10.1021/acs.chemrev.5b00138. [DOI] [PubMed] [Google Scholar]
- Thompson J.L., Davies H.M.L. Enhancement of cyclopropanation chemistry in the silver-catalyzed reactions of aryldiazoacetates. J. Am. Chem. Soc. 2007;129:6090–6091. doi: 10.1021/ja069314y. [DOI] [PubMed] [Google Scholar]
- Veitch G.E., Bridgwood K.L., Rands-Trevor K., Ley S.V. Magnesium nitride as a convenient source of ammonia: preparation of pyrroles. Synlett. 2008;10:2597–2600. [Google Scholar]
- Xi Y., Su Y., Yu Z., Dong B., McClain E.J., Lan Y., Shi X. Chemoselective carbophilic addition of α-diazoesters through ligand-controlled gold catalysis. Angew. Chem. Int. Ed. 2014;53:9817–9821. doi: 10.1002/anie.201404946. [DOI] [PubMed] [Google Scholar]
- Xia Y., Wang J. N-Tosylhydrazones: versatile synthons in the construction of cyclic compounds. Chem. Soc. Rev. 2017;46:2306–2362. doi: 10.1039/c6cs00737f. [DOI] [PubMed] [Google Scholar]
- Xia Y., Liu Z., Liu Z., Ge R., Ye F., Hossain M., Zhang Y., Wang J. Formal carbene insertion into C-C bond: Rh(I)-catalyzed reaction of benzocyclobutenols with diazoesters. J. Am. Chem. Soc. 2014;136:3013–3015. doi: 10.1021/ja500118w. [DOI] [PubMed] [Google Scholar]
- Xia Y., Qiu D., Wang J. Transition-metal-catalyzed cross-couplings through carbene migratory insertion. Chem. Rev. 2017;117:13810–13889. doi: 10.1021/acs.chemrev.7b00382. [DOI] [PubMed] [Google Scholar]
- Xia Y., Qu P., Liu Z., Ge R., Xiao Q., Zhang Y., Wang J. Catalyst-free intramolecular formal carbon insertion into σ-C-C bonds: a new approach toward phenanthrols and naphthols. Angew. Chem. Int. Ed. 2013;52:2543–2546. doi: 10.1002/anie.201209269. [DOI] [PubMed] [Google Scholar]
- Xiao Q., Zhang Y., Wang J. Diazo compounds and N-tosylhydrazones: novel cross-coupling partners in transition-metal-catalyzed reactions. Acc. Chem. Res. 2013;46:236–247. doi: 10.1021/ar300101k. [DOI] [PubMed] [Google Scholar]
- Yada A., Fujita S., Murakami M. Enantioselective insertion of a carbenoid carbon into a C-C bond to expand cyclobutanols to cyclopentanols. J. Am. Chem. Soc. 2014;136:7217–7220. doi: 10.1021/ja502229c. [DOI] [PubMed] [Google Scholar]
- Yoo E.J., Wasa M., Yu J.-Q. Pd(II)-catalyzed carbonylation of C(sp3)-H bonds: a new entry to 1,4-dicarbonyl compounds. J. Am. Chem. Soc. 2010;132:17378–17380. doi: 10.1021/ja108754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Cao Z., Wang Y., Zhou F., Zhou J. Catalytic enantioselective desymmetrization reactions to all-carbon quaternary stereocenters. Chem. Rev. 2016;116:7330–7396. doi: 10.1021/acs.chemrev.6b00094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.