Abstract
Heteropoly acids were used as catalysts for cyclodehydration of various 1,n-diols. Cyclodehydration of butane-1,4-diol, pentane-1,5-diol and hexane-1,6-diol catalysed by H3PW12O40 gave tetrahydrofuran, tetrahydropyran and oxepane, respectively. Cyclodehydration of diethylene glycol, triethylene glycol, diethylene glycol monomethyl ether and polyethylene glycol 200 catalysed by H3PW12O40 gave 1,4-dioxane. In particular, cyclodehydration of hexane-1,6-diol gave an excellent yield of oxepane (80%). The selectivity exhibited by the H3PW12O40 catalyst was even better than that exhibited by other reported catalyst systems for similar cyclodehydration reactions.
Keywords: heteropoly acid, cyclic ether, cyclodehydration, catalyst, synthesis
1. Introduction
Many natural compounds, such as inostamycins, isosorbide and polyether antibiotics, incorporate cyclic ethers as structural subunits and have significant biological activity [1–3]. Additionally, some cyclic ethers have distinctive aromas and are used as flavours [4]; (−)-ambrox is a typical representative of this type of cyclic ether [5]. Many of the commonly used synthetic approaches for the formation of cyclic ethers, including cycloaddition and cyclization, involve chlorine chemistry or heavy metals at different levels [6,7]. Additionally, cyclization reactions are often conducted under acidic conditions [8,9]. Cyclodehydration of 1,n-diols to cyclic ethers is an industrially important reaction [4]. These reactions are usually carried out using inorganic and organic acids, solid acid catalysts (such as clays), group (IV) metal halides, metallocenes, sulfated zirconia, zeolite or calcium phosphate. There is a strong interest in the use of solid acid catalysts to replace conventional homogeneous catalysts, such as inorganic and organic acids [10]. Although conventional catalysts are very effective, they produce highly corrosive media and chemically reactive waste streams [11]. However, using heteropoly acids (HPAs) for the general operation of large chemical processes is ecofriendly and safe [12–14].
HPA catalysts provide several advantages that make them economically and environmentally attractive [15]. HPAs can be viewed as versatile catalysts because they contain multiple active sites, including metals, protons and oxygen atoms. Protons can act as Brønsted acids to promote acid-catalysed reactions [16]. HPA catalysts can contain one or two types of acidic sites, acidic protons and/or Lewis-acidic metals [17]. Both types of acidic sites can work as active sites in acid catalysis. HPA catalysts can improve many classical acid catalysis reactions, such as cracking, condensation, isomerization, Friedel–Crafts and amination reactions [18–21]. Therefore, there has been considerable interest in the use of HPAs as catalysts.
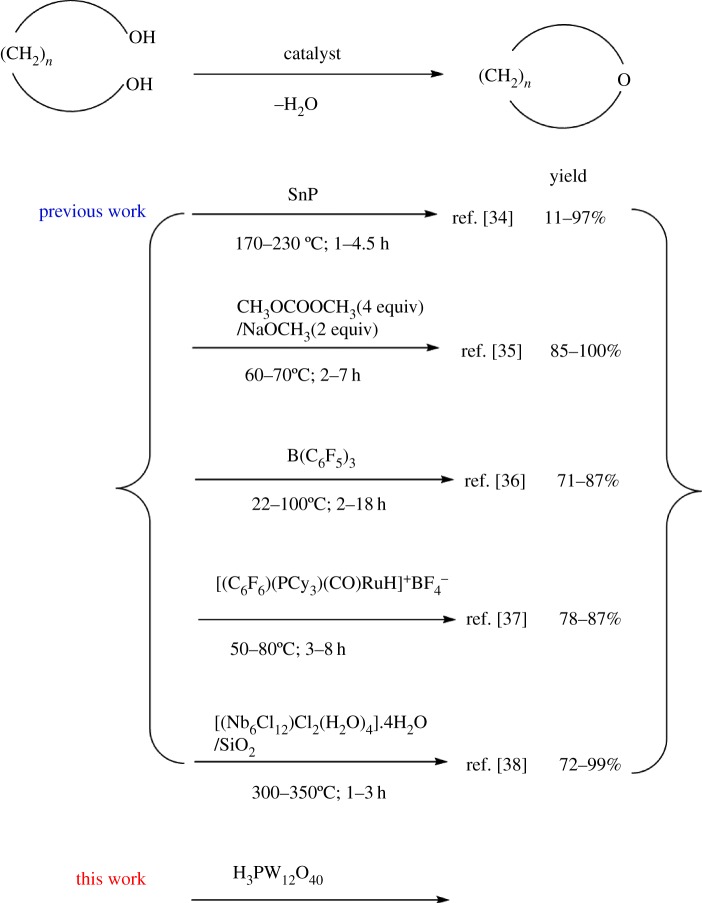
HPAs are active and selective for tetrahydrofuran (THF) production. One commercial THF production method is based on a well-known technology that uses strongly inorganic acids as catalysts for cyclodehydration of butane-1,4-diol [22–27]. Recent advances in the synthesis of substituted THF rings have been achieved using different synthetic methods with novel catalysts and alternative starting materials [28–33]. Additionally, reports of cyclodehydration of 1,n-diols to cyclic ethers have been published; this is essentially a dehydration reaction between two hydroxy groups to yield an ether (figure 1) [34–38]. The present paper reports the application of HPAs as solid acid catalysts for cyclodehydration of 1,n-diols to their corresponding cyclic ethers with high yield and selectivity (figure 2).
Figure 1.
Approaches to the synthesis of cyclic ethers by cyclodehydration of 1,n-diols.
Figure 2.
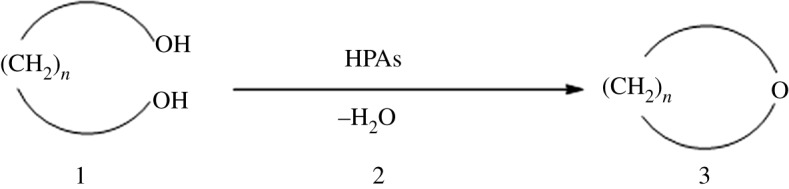
Cyclodehydration of 1,n-diols catalysed by HPAs.
2. Material and methods
2.1. Materials
All the chemicals were purchased commercially. All reagents were of analytical grade and were used directly.
2.2. The synthetic method of catalyst 2a–2d
All the catalysts (2a–2d) were synthesized using the same approach. The following method is provided for catalyst 2a as an example.
Concentrated H3PO4 was added to a boiling aqueous solution of Na2WO4·2H2O in a 4 : 1 acid/salt ratio and boiling was maintained in a reflux system for 8 h. The salt was precipitated by the addition of KCl, then purified by recrystallization and cooled overnight to 5°C. The product was filtered, washed and then vacuum-dried for 8 h. The product was treated with ether and a concentrated HCl (37%) solution. The released Dawson acid formed an additional compound with the ether, which allowed it to be separated from the solution. After obtaining the ether solution with the acid, the ether was eliminated and the remaining solution was placed in a vacuum-desiccator until crystallization.
2.3. Upscaling with 2a as a catalyst
A mixture of butane-1,4-diol (900.12 g, 10 mol) and catalyst 2a (49.04 g, 0.01 mol) was placed in a round-bottom flask fitted with a distillation unit. The mixture was stirred and heated in an oil bath. When the temperature reached 100°C, THF and water began to distil from the mixture. Stirring and heating at this temperature were continued until the distillation stopped (8 h). THF (664.60 g, 9.23 mol; 92.3% yield) was obtained after drying over molecular sieves and filtering.
2.4. Synthesis of compounds 3a–3g
Compounds 3a–3g were all synthesized using the same approach. The following method is provided for the synthesis of THF (3a) as an example.
A mixture of butane-1,4-diol (200 mmol) and an HPA (H3PW12O40) catalyst (0.2 mmol) was added to a round-bottom flask fitted with a distillation unit. The mixture was stirred magnetically and heated in an oil bath. When the temperature reached 100°C, THF and water began to distil from the mixture. The mixture was continuously stirred and heated at this temperature until the distillation was complete. THF was obtained by drying the distillate over CaCl2 and filtering. Reactions were performed with various 1,n-diols and the products were identified using 1H NMR, 13C NMR and mass spectrometry.
3. Results and discussion
The cyclodehydration reaction conditions were optimized under solvent-free conditions using butane-1,4-diol (1a) as the reagent.
As with all catalysis, the first step in using HPAs for selective catalytic dehydration of butane-1,4-diol to THF was to choose an appropriate HPA catalyst. The metals of the HPA catalysts were the selected focus because they are the active sites in the acid-catalysed reactions [18,19]. Accordingly, a series of HPA catalysts were prepared, as summarized in table 1, and a set of standard experimental conditions employed (100°C for 3 h) to assess the relative utility of the synthetic catalysts. The catalytic activities decreased in the following order: 2a (H3PW12O40) > 2c (H4SiW12O40) > 2b (H3PMo12O40) > 2d (H4SiMo12O40). Catalysts 2a and 2c provided higher yields than the other catalysts. Moreover, although Mo and W belong to the same group, they displayed different catalytic activities in this reaction; the order of the catalytic activities was in accordance with that of the Brønsted acidity of the HPAs [16]. Tungsten HPA 2a was the catalyst of choice because of its stronger acidity, higher thermal stability and lower oxidation potential than 2b, 2c and 2d. Overall, catalyst 2a (H3PW12O40) gave the best yield (98%).
Table 1.
The efficiencies of various HPAs catalysts used in the synthesis of THF.
| entry | catalyst | chemical composition of catalyst | yield (%) |
|---|---|---|---|
| 1 | 2a | H3PW12O40 | 98 |
| 2 | 2b | H3PMo12O40 | 87 |
| 3 | 2c | H4SiW12O40 | 91 |
| 4 | 2d | H4SiMo12O40 | 82 |
The effect of the amount of catalyst 2a (H3PW12O40) on the reaction yield was thoroughly investigated. The yield of THF (3a, structure shown in table 3) progressively increased from 70 to 99% with increased catalyst loading (table 2). Notably, a catalyst loading of 0.1 mol% was highly effective for the model reaction. The effect of the temperature and reaction time was subsequently investigated, both of which significantly affected the reaction. The yield of THF progressively increased from 62 to 98% with increased reaction time. A yield of 98% was obtained under optimum conditions of 0.1 mol% catalyst loading at 100°C for 3 h reaction. THF was obtained as the only product.
Table 3.
Cyclodehydration of 1,n-diols catalysed by H3PW12O40.
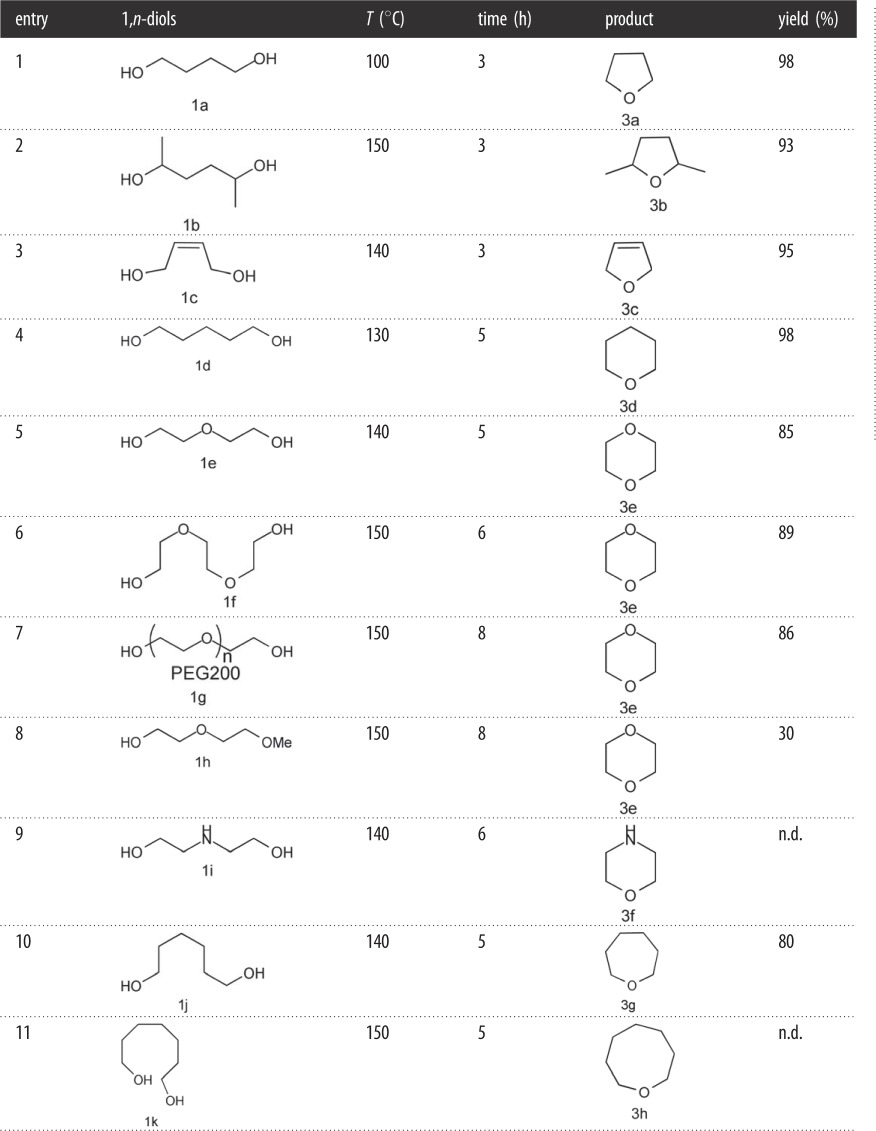 |
n.d.: not detected.
Table 2.
Optimization of the synthesis of THF catalysed by H3PW12O40a.
| entry | cat. loading (mmol) (%) | T (°C) | time (h) | yield (%) |
|---|---|---|---|---|
| 1 | 0.05 | 100 | 3 | 70 |
| 2 | 0.1 | 100 | 3 | 98 |
| 3 | 0.2 | 100 | 2.5 | 99 |
| 4 | 0.1 | 90 | 10 | 60 |
| 5 | 0.1 | 120 | 3 | 98 |
| 6 | 0.1 | 100 | 1 | 62 |
| 7 | 0.1 | 100 | 2 | 80 |
aReaction conditions: butane-1,4-diol (200 mmol).
Based upon the above optimum reaction conditions, amplification experiments were performed. The upscaling experiment with 2a as a catalyst was described in ‘Material and methods'. The generally good yields (664.60 g, 9.23 mol; 92.3% yield) enabled the production of THF in the order of 100 g. Therefore, this method has prospective applications in the production of THF.
Next, the optimum reaction conditions were used in the synthesis of cyclic ethers by cyclodehydration of 1,n-diols catalysed by catalyst 2a (table 3). Cyclodehydration of butane-1,4-diol gave a 98% yield of THF in 3 h. As shown in table 2, cyclodehydration of hexane-2,5-diol (1b) required a higher temperature to afford 2,5-dimethyltetrahydrofuran (3b) in lower yield; (Z/E)-but-2-ene-1,4-diol (1c) to afford 2,5-dihydrofuran (3c) also required higher temperature and provided a lower yield; and pentane-1,5-diol (1d) gave tetrahydropyran (3d) in near-quantitative yield at a slightly higher temperature (table 2). As shown in table 3, diethylene glycol (1e) gave 1,4-dioxane (3e) in 85% yield, and triethylene glycol (1f) under these conditions gave 1,4-dioxane (3e) in 89% yield. These were significantly higher yields of cyclic ether 3e from diethylene glycol (1e) and triethylene glycol (1f) than previously obtained using other catalyst systems [34–38]. Similarly, polyethylene glycol 200 (1g) gave 1,4-dioxane (3e) in an excellent yield of 86% (table 3). Cyclodehydration of diethylene glycol monomethyl ether (1h) and diethanolamine (1i) also afforded cyclic ether 3f in slightly lower yields (table 3). Cyclodehydration of hexane-1,6-diol (1j) required a higher temperature and longer reaction time to yield oxepane (3g) in higher yield (80%; table 3) than previously obtained using other catalyst systems [34–38]. Cyclodehydration of heptane-1,7-diol (1k) to form 3h did not occur because the catalyst was too far from the diol group to play a direct role.
Compounds 3a–3g (table 3) were all synthesized using the same approach according to the synthetic method described for 3a in ‘Material and methods’. Products 3a–3g were identified using 1H NMR, 13C NMR and mass spectrometry.
3.1. Compound 3a
1H NMR (300 MHz; CDCl3) δ: 3.58–3.62 (4H, m), 1.67–1.76 (4H, m). 13C NMR (75 MHz; CDCl3) δ: 67.6, 25.3. MS, m/z: 72 (M+).
3.2. Compound 3b
1H NMR (300 MHz; CDCl3) δ: 4.02–4.12 (2H, m), 3.82–3.93 (2H, m), 1.98–2.02 (2H, m), 1.87–1.92 (2H, m), 1.40–1.43 (4H, m), 1.18 (6H, d, J = 6.3 Hz), 1.14 (6H, d, J = 6.3 Hz). 13C NMR (75 MHz; CDCl3) δ: 75.2, 74.4, 34.1, 32.9, 21.3. MS, m/z: 100 (M+).
3.3. Compound 3c
1H NMR (300 MHz; CDCl3) δ: 5.85 (2H, s), 4.62 (4H, d, J = 0.6 Hz). 13C NMR (75 MHz; CDCl3) δ: 126.1, 75.3. MS, m/z: 70 (M+).
3.4. Compound 3d
1H NMR (300 MHz; CDCl3) δ: 3.60 (4H, t, J = 5.0 Hz), 1.49–1.64 (6H, m). 13C NMR (75 MHz; CDCl3) δ: 68.6, 26.7, 23.3. MS, m/z: 86 (M+).
3.5. Compound 3e
1H NMR (300 MHz; CDCl3) δ: 3.64 (4H, s). 13C NMR (75 MHz; CDCl3) δ: 66.9. MS, m/z: 88 (M+).
3.6. Compound 3g
1H NMR (300 MHz; CDCl3) δ: 3.65 (4H, t, J = 5.7 Hz), 1.67 (4H, t, J = 4.2 Hz), 1.59 (4H, q, J = 3.0 Hz). 13C NMR (75 MHz; CDCl3) δ: 69.9, 30.9, 26.7. MS, m/z: 100 (M+).
4. Conclusion
Cyclic ethers were obtained by the reaction of 1,n-diols using HPA catalyst H3PW12O40 (2a), an inexpensive and simply prepared catalyst. The selectivity exhibited by catalyst H3PW12O40 was better than that of several reported catalyst systems for similar cyclodehydration reactions. Similar catalysts also catalysed the conversion of polyethylene glycols and triethylene glycol to 1,4-dioxane. Cyclodehydration of hexane-1,6-diol gave oxepane in excellent yield. With H3PW12O40 as a catalyst, the upscaling experiment provided generally good yields, enabling THF production in the order of 100 g; this method has prospective applications in the production of THF. Therefore, the HPA catalyst H3PW12O40 is a promising solid acid catalyst on which further research will be undertaken.
Supplementary Material
Acknowledgements
We acknowledge China Agricultural University for providing a necessary facility for carrying out the spectral analysis of compounds.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
Y.S. and B.F. conceived of the project and designed the experiments. Y.S. drafted the manuscript. Y.H., M.L., J.L. and N.J. carried out the experiments and analysed the data. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This work was supported by the National Natural Science Foundation of China (31501683) and the Special Fund for Supervision of Quality and Safety of Agricultural Products (Risk Assessment) from the Chinese Ministry of Agriculture (GJFP 2017011).
References
- 1.Alkhathlan HZ, Khan M, Abdullah MMS, AlMayouf AM, Yacine Badjah-HadjAhmed A, AlOthman ZA, Mousa AA. 2015. Anticorrosive assay-guided isolation of active phytoconstituents from Anthemis pseudocotula extracts and a detailed study of their effects on the corrosion of mild steel in acidic media. RSC Adv. 5, 54 283–54 292. ( 10.1039/c5ra09154c) [DOI] [Google Scholar]
- 2.Nalla V, Shaikh A, Bapat S, Vyas R, Karthikeyan M, Yogeeswari P, Sriram D, Muthukrishnan M. 2018. Identification of potent chromone embedded [1,2,3]-triazoles as novel antitubercular agents. R. Soc. open sci. 5, 1–15. ( 10.1098/rsos.171750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lei P, Ding Y, Zhang X, Adijiang A, Li H, Ling Y, An J. 2018. A practical and chemoselective ammonia-free birch reduction. Org. Lett. 20, 3439–3442. ( 10.1021/acs.orglett.8b00891) [DOI] [PubMed] [Google Scholar]
- 4.Fráter G, Bajgrowicz JA, Kraft P. 1998. Fragrance chemistry. Tetrahedron 54, 7633–7703. ( 10.1016/S0040-4020(98)00199-9) [DOI] [Google Scholar]
- 5.Rossiter KJ. 1996. Structure-odor relationships. Chem. Rev. 96, 3201–3240. ( 10.1021/cr950068a) [DOI] [PubMed] [Google Scholar]
- 6.Panda B, Sarkar TK. 2008. A one-pot tandem oxidation–reduction protocol for the synthesis of cyclic ethers from their diols. Tetrahedron Lett. 46, 6701–6703. ( 10.1016/j.tetlet.2005.07.132) [DOI] [Google Scholar]
- 7.Zhao H, Holladay JE, Brown H, Zhang ZC. 2007. Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science 316, 1597–1600. ( 10.1126/science.1141199) [DOI] [PubMed] [Google Scholar]
- 8.Zheng T, Narayan RS, Schomaker JM, Borhan B. 2005. One-pot regio- and stereoselective cyclization of 1, 2, n-triols. J. Am. Chem. Soc. 127, 6946–6947. ( 10.1021/ja043002i) [DOI] [PubMed] [Google Scholar]
- 9.Aquino M, Cardani S, Fronza G, Fuganti C, Fernandez RP, Tagliani A. 1991. Baker's yeast reduction of arylalkyl and arylalkenil γ- and δ-keto acids. Cheminform 47, 7887–7896. ( 10.1016/S0040-4020(01)81944-X) [DOI] [Google Scholar]
- 10.Enthaler S, Trautner A. 2013. Iron-catalyzed ring-closing depolymerization of poly (tetrahydrofuran). Chem. Sus. Chem. 6, 1334–1336. ( 10.1002/cssc.201300380) [DOI] [PubMed] [Google Scholar]
- 11.Tomczyk KM, Guńka PA, Parzuchowski PG, Zachara J, Rokicki G. 2012. Intramolecular etherification of five-membered cyclic carbonates bearing hydroxyalkyl groups. Green. Chem. 14, 1749–1758. ( 10.1039/c2gc35265f) [DOI] [Google Scholar]
- 12.Takagaki A. 2016. Kinetic analysis of aqueous-phase cyclodehydration of 1, 4-butanediol and erythritol over a layered niobium molybdate solid acid. Catal. Sci. Technol. 6, 791–799. ( 10.1039/c5cy01126d) [DOI] [Google Scholar]
- 13.Costa VV, Rocha KADS, Oliveira LCA, Kozhevnikova EF, Kozhevnikov IV, Gusevskaya EV. 2016. Heteropoly acid catalysts for the synthesis of fragrance compounds from bio-renewables: acetylation of nopol and terpenic alcohols. RSC Adv. 6, 43 217–43 222. ( 10.1039/c6ra02266a) [DOI] [Google Scholar]
- 14.Sartori G, Maggi R. 2006. Use of solid catalysts in Friedel–Crafts acylation reactions. Chem. Rev. 106, 1077–1104. ( 10.1021/cr040695c) [DOI] [PubMed] [Google Scholar]
- 15.Wang SS, Yang GY. 2015. Recent advances in polyoxometalate-catalyzed reactions. Chem. Rev. 115, 4893–4962. ( 10.1021/cr500390v) [DOI] [PubMed] [Google Scholar]
- 16.Kozhevnikov IV. 1998. Catalysis by heteropoly acids and multicomponent polyoxometalates in liquid-phase reactions. Chem. Rev. 98, 171–198. ( 10.1021/cr960400y) [DOI] [PubMed] [Google Scholar]
- 17.Rüther T, Hultgren VM, Timko BP, Bond AM, Jackson WR, Wedd AG. 2003. Electrochemical investigation of photooxidation processes promoted by sulfo-polyoxometalates: coupling of photochemical and electrochemical processes into an effective catalytic cycle. J. Am. Chem. Soc. 125, 10 133–10 143. ( 10.1021/ja029348f) [DOI] [PubMed] [Google Scholar]
- 18.Yang SX, Tian HY, Sun BG, Liu YG, Hao YF, Lv YY. 2016. One-pot synthesis of (−)-ambrox. Sci. Rep. 6, 32 650 ( 10.1038/srep32650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang SX, Hao YF, Wang JL, Wang H, Zheng YM, Tian HY, Liu YG, Sun BG. 2017. Selective catalytic dehydration of furfuryl alcohol to 2,2′-difurfuryl ether using a polyoxometalate catalyst. Sci. Rep. 7, 12 954 ( 10.1038/s41598-017-13472-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei YH, Cao XH. 2014. Preparation, characterization and catalytic application for the synthesis of tetrahydrofuran. Adv. Mater. Res. 838, 2322–2325. ( 10.4028/www.scientific.net/AMR.838-841.2322) [DOI] [Google Scholar]
- 21.Alharbi W, Kozhevnikova EF, Kozhevnikov IV. 2015. Dehydration of methanol to dimethyl ether over heteropoly acid catalysts: the relationship between reaction rate and catalyst acid strength. ACS Catal. 5, 7186–7193. ( 10.1021/acscatal.5b01911) [DOI] [Google Scholar]
- 22.Baronetti G, Briand L, Sedran U, Thomas H. 1998. Heteropolyacid-based catalysis: Dawson acid for MTBE synthesis in gas phase. Appl. Catal. A-Gen. 172, 265–272. ( 10.1016/S0926-860X(98)00134-3) [DOI] [Google Scholar]
- 23.Romanelli GP, Thomas HJ, Baronetti GT, Autino JC. 2003. Solvent-free catalytic preparation of 1, 1-diacetates from aldehydes using a Wells–Dawson acid (H6P2W18O62· 24H2O). Tetrahedron Lett. 44, 1301–1303. ( 10.1016/S0040-4039(02)02817-4) [DOI] [Google Scholar]
- 24.Romanelli GP, Bennardi D, Ruiz DM, Baronetti G, Thomas HJ, Autino JC. 2004. A solvent-free synthesis of coumarins using a Wells–Dawson heteropoly acid as catalyst. Tetrahedron Lett. 45, 8935–8939. ( 10.1016/j.tetlet.2004.09.183) [DOI] [Google Scholar]
- 25.Firouzabadi H, Iranpoor N, Nowrouzi F. 2004. Aluminum dodecatungstophosphate (AlPW12O40) as a non-hygroscopic Lewis acid catalyst for the efficient Friedel–Crafts acylation of aromatic compounds under solvent-less conditions. Tetrahedron 60, 10 843–10 850. ( 10.1016/j.tet.2004.09.049) [DOI] [Google Scholar]
- 26.Firouzabadi H, Iranpoor N, Jafari AA. 2005. Facile preparation of symmetrical and unsymmetrical ethers from their corresponding alcohols catalyzed by aluminumdodecatangstophosphate (AlPW12O40), as a versatile and a highly water tolerant Lewis acid. J. Mol. Catal. A-Chem. 227, 97–100. ( 10.1016/j.molcata.2004.09.078) [DOI] [Google Scholar]
- 27.Firouzabadi H, Iranpoor N, Jafari AA. 2005. An efficient and chemoselective method for protection of thiols catalyzed by aluminumdodecatungstophosphate (AlPW12O40), as a highly water tolerant Lewis acid catalyst. Tetrahedron Lett. 46, 2683–2686. ( 10.1016/j.tetlet.2005.02.071) [DOI] [Google Scholar]
- 28.Air V, Deepthi A. 2007. Cerium (IV) ammonium nitrate a versatile single-electron oxidant. Chem. Rev. 107, 1862–1891. ( 10.1021/cr068408n) [DOI] [PubMed] [Google Scholar]
- 29.Shinde VM, Patil GN, Katariya A, Mahajan YS. 2015. Production of tetrahydrofuran by dehydration of 1, 4-butanediol using Amberlyst-15: batch kinetics and batch reactive distillation. Chem. Eng. Process 95, 241–248. ( 10.1016/j.cep.2015.06.016) [DOI] [Google Scholar]
- 30.Mizugaki T, Togo K, Maeno Z, Mitsudome T, Jitsukawa K, Kaneda K. 2016. One-pot transformation of levulinic acid to 2-methyltetrahydrofuran catalyzed by Pt–Mo/H-β in water. ACS Sustain. Chem. Eng. 4, 682–685. ( 10.1021/acssuschemeng.6b00181) [DOI] [Google Scholar]
- 31.Wang JL, Hao YF, Wang H, Yang SX, Tian HY, Sun BG, Liu YG. 2017. Rapidly responsive and highly selective fluorescent probe for bisulfite detection in food. J. Agric. Food. Chem. 65, 2883–2887. ( 10.1021/acs.jafc.7b00353) [DOI] [PubMed] [Google Scholar]
- 32.Wang H, Wang JL, Yang SX, Tian HY, Liu YG, Sun BG. 2018. Highly selective and rapidly responsive fluorescent probe for hydrogen sulfide detection in wine. Food Chem. 257, 150–154. ( 10.1016/j.foodchem.2018.02.130) [DOI] [PubMed] [Google Scholar]
- 33.Alinezhad H, Soleymani E, Zare M. 2017. Facile method for the synthesis of 2, 3-dihydroquinazolin-4(1H)-ones catalyzed by SiO2–H3PW12O40 in water. Res. Chem. Intermediat. 43, 457–466. ( 10.1007/s11164-016-2634-4) [DOI] [Google Scholar]
- 34.Patel SM, Chudasama UV, Ganeshpure PA. 2001. Metal (IV) phosphates as solid acid catalysts for selective cyclodehydration of 1,n-diols. Green Chem. 3, 143–145. ( 10.1039/B100503K) [DOI] [Google Scholar]
- 35.Aricò F, Tundo P, Maranzana A, Tonachini G. 2012. Synthesis of five-membered cyclic ethers by reaction of 1,4-diols with dimethyl carbonate. ChemSusChem 5, 1578–1586. ( 10.1002/cssc.201100755) [DOI] [PubMed] [Google Scholar]
- 36.Hellal M, Falk FC, Wolf E, Dryzhakov M, Moran J. 2014. Breaking the dichotomy of reactivity vs. chemoselectivity in catalytic SN1 reactions of alcohols. Org. Bio. Chem. 12, 5990–5994. ( 10.1039/C4OB01265H) [DOI] [PubMed] [Google Scholar]
- 37.Kim J, Lee DH, Kalutharage N, Yi CS. 2014. Selective catalytic synthesis of unsymmetrical ethers from the dehydrative etherification of two different alcohols. ACS Catal. 4, 3881–3885. ( 10.1021/cs5012537) [DOI] [Google Scholar]
- 38.Nagashima S, Sasaki T, Kamiguchi S, Chihara T. 2015. Synthesis of common-sized heterocyclic compounds by intramolecular cyclization over halide cluster catalysts. Chem. Lett. 44, 764–766. ( 10.1246/cl.150134) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.