Main Text
The vast majority of the synapses in our brain are built by en passant (French for “in passing”) boutons, in which axons enlarge their diameter from ∼200 nm to ∼1 μm. Action potentials propagating along the axon trigger the opening of voltage-gated Ca2+ channels, and within a few hundred μs, ∼10,000 Ca2+ ions rush into the bouton, driven by a 70-mV electrical gradient and a 20,000-fold concentration gradient (due to 50 nM intra- and 1 mM extracellular [Ca2+]). In the vicinity of the Ca2+ channels, the intracellular Ca2+ concentration therefore rises rapidly to ∼10 μM and triggers the fusion of neurotransmitter-filled vesicles (1). After the Ca2+ channels close, diffusion of Ca2+ ions into the bouton cytoplasm leads to the collapse of local Ca2+ microdomains and an increase in the average (residual) Ca2+ concentration ranging from 100 nM to 1 μM.
But what happens to all of these Ca2+ ions in the bouton? This question is important because accumulation of Ca2+ within the bouton controls the probability and synchronicity of neurotransmitter release (2). Furthermore, Ca2+ accelerates vesicle recruitment (3), influences endocytosis (4), and controls various forms of presynaptic long-term plasticity (5).
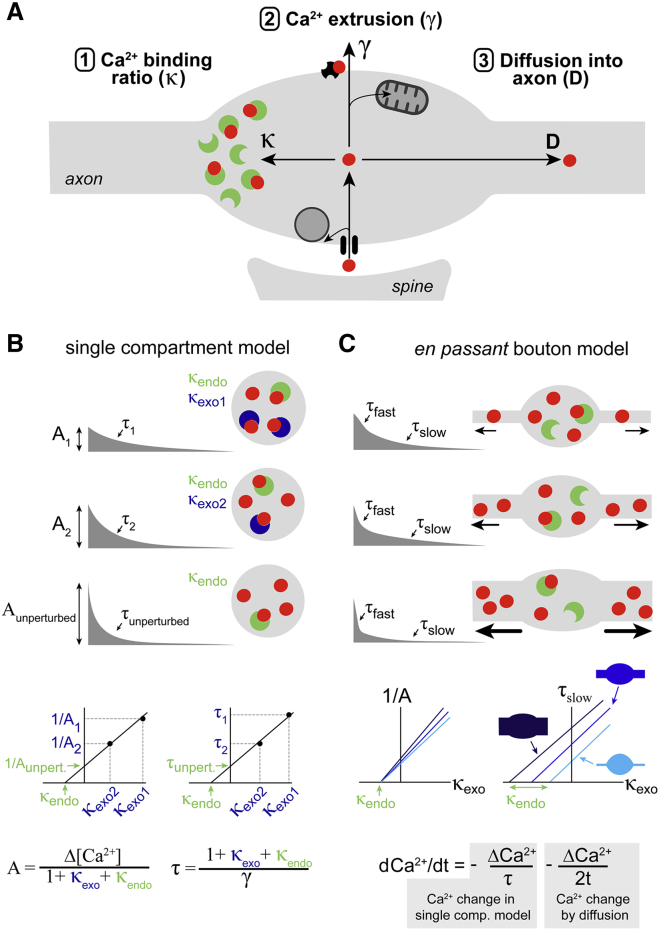
The fate of Ca2+ inside the bouton is determined by at least three processes (Fig. 1 A). First, the majority of Ca2+ ions will bind to endogenous Ca2+ buffers. The Ca2+ binding ratio κ is defined as the ratio of Ca2+ ions that, upon entering the bouton, bind to buffers to the ones that remain free (6). For example, κ = 4 means that four of five entering Ca2+ ions are buffer-bound and one remains free (cf. Fig. 1 A). In a single-compartment spherical cell, κ can be determined by perfusing the cell with different concentrations of fluorescent Ca2+ indicator dyes and measuring the action potential-evoked Ca2+ transients (6; Fig. 1 B). The dye acts as an exogenous buffer (dark blue) that adds to the endogenous buffer (green). With increasing dye concentration, the Ca2+ transients become smaller and slower. Plotting the inverse of the Ca2+ transient amplitude (1/A) or the decay time constant (τ) against the buffer-binding ratio of the dye (κexo) yields a linear relationship (see equations in Fig. 1 B). The endogenous Ca2+ binding ratio (κendo) can be extrapolated from the x-intercepts and the amplitude and the time constant in the unperturbed bouton from the y-intercepts of the relevant graphs.
Figure 1.
Diffusion into the axon shapes presynaptic Ca2+ signals. (A) An illustration of the three major determinants of Ca2+ signals in en passant presynaptic boutons is shown. Tran and Stricker (8) found that, besides buffering and extrusion, diffusion into the axon significantly impacts Ca2+ transients. (B) In a single-compartment model, the endogenous Ca2+ dynamics can be back extrapolated from amplitudes and time constants of Ca2+ dye signals. (C) With increasing axon diameter, the Ca2+ decay accelerates, becomes biexponential, and contaminates τ back extrapolation of κ.
The second factor determining the Ca2+ time course is the removal of Ca2+ from the cytoplasm. This is described by the extrusion rate γ (Fig. 1 A) and can be mediated by transport across the plasma membrane and/or by uptake into organelles. Mitochondrial uptake appears to be important when intracellular Ca2+ concentration is strongly increased, e.g., during trains of action potentials (7).
In this issue of Biophysical Journal, Tran and Stricker (8) provide an elegant analysis and convincingly demonstrate the importance of a third mechanism that controls presynaptic Ca2+ dynamics at small en passant boutons, namely, the diffusion of Ca2+ into the axon (Fig. 1 A). By thoroughly investigating boutons of neocortical layer 5 pyramidal neurons in acute brain slices, they found that diffusion into the axon significantly accelerates the decay of Ca2+ concentration. When recording presynaptic Ca2+ signals, the authors observed biexponential decays of Ca2+ transients (τfast and τslow). Furthermore, they found a low value of κendo = 10 from 1/A back extrapolation but larger values from τslow back extrapolation (Fig. 1 C). They then excluded nonlinear Ca2+ removal, saturation of fast buffers, and release from internal Ca2+ stores as potential underlying processes. However, they resolved axonal Ca2+ signals with a delay depending on the distance from the bouton. When extending a computational model of a bouton with an axon of varying diameter, they could quantitatively explain the biexponential decay kinetics and the differential κendo estimates observed experimentally. With a large diameter at the upper border of experimentally determined values (9), the model nicely reproduced the experimental findings. Finally, the authors derived an analytical equation of the contribution of diffusion into the axon that explains the biexponential decay kinetics (see equation in Fig. 1 C). These results convincingly demonstrate that the efflux of Ca2+ into the axon critically affects the Ca2+ transient in small en passant boutons.
In a previous seminal study published in Biophysical Journal, the Ca2+ transients at a large presynaptic terminal, the calyx of Held, could be described without considering diffusion into the axon (10). This difference can be readily explained by the large volume of these terminals in relation to the corresponding axon. Consistently, a previous study at small en passant boutons of layer 2/3 pyramidal neurons observed biexponential Ca2+ transients in a subset of boutons (11). However, in contrast to Tran and Stricker (8), prominent Ca2+ signals could not be resolved in adjacent axons. The large κendo of 140 obtained from τ back extrapolation (11) is probably an overestimation because Tran and Stricker show that axonal diffusion contaminates the τ back extrapolation but less so the 1/A back extrapolation (cf. Fig. 1 C). Interestingly, the washout of a slow endogenous buffer also contaminates the τ back extrapolation, although in the opposite direction (12). Thus, 1/A back extrapolation of κ seems to be more robust but requires quantitative Ca2+ measurements. Both τ and 1/A back extrapolation critically depend on the exact binding constants of the dyes, as beautifully demonstrated by Stricker and colleagues in a recent study (13). Finally, presynaptic whole-cell patch-clamp recordings from small en passant boutons have recently become possible (14, 15) and may provide the tools for a more robust quantification of Ca2+ concentration and a better control over the washout of endogenous buffers. It will therefore be interesting to see if such recordings confirm the low κendo and the large contribution of diffusion to Ca2+ dynamics at en passant boutons.
To explain presynaptic function and modifications during short- and long-term plasticity, a detailed knowledge of presynaptic Ca2+ dynamics is required (16). Tran and Stricker have now revealed the relevance of the passage of presynaptic Ca2+ through the axon. The study represents an important step toward a quantitative understanding of presynaptic Ca2+ signaling in the 1014 small boutons of our brain.
Editor: Jane Dyson.
References
- 1.Schneggenburger R., Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- 2.Jackman S.L., Regehr W.G. The mechanisms and functions of synaptic facilitation. Neuron. 2017;94:447–464. doi: 10.1016/j.neuron.2017.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipstein N., Sakaba T., Brose N. Dynamic control of synaptic vesicle replenishment and short-term plasticity by Ca2+-calmodulin-Munc13-1 signaling. Neuron. 2013;79:82–96. doi: 10.1016/j.neuron.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Yamashita T., Eguchi K., Takahashi T. Developmental shift to a mechanism of synaptic vesicle endocytosis requiring nanodomain Ca2+ Nat. Neurosci. 2010;13:838–844. doi: 10.1038/nn.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korte M., Schmitz D. Cellular and system biology of memory: timing, molecules, and beyond. Physiol. Rev. 2016;96:647–693. doi: 10.1152/physrev.00010.2015. [DOI] [PubMed] [Google Scholar]
- 6.Neher E., Augustine G.J. Calcium gradients and buffers in bovine chromaffin cells. J. Physiol. 1992;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim M.H., Korogod N., Lee S.H. Interplay between Na+/Ca2+ exchangers and mitochondria in Ca2+ clearance at the calyx of Held. J. Neurosci. 2005;25:6057–6065. doi: 10.1523/JNEUROSCI.0454-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran V., Stricker C. Diffusion of Ca2+ from small boutons en passant into the axon shapes AP-evoked Ca2+ transients. Biophys J. 2018;115:1344–1356. doi: 10.1016/j.bpj.2018.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rollenhagen A., Ohana O., Lübke J.H.R. Structural properties of synaptic transmission and temporal dynamics at excitatory layer 5B synapses in the adult rat somatosensory cortex. Front. Synaptic Neurosci. 2018;10:24. doi: 10.3389/fnsyn.2018.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helmchen F., Borst J.G., Sakmann B. Calcium dynamics associated with a single action potential in a CNS presynaptic terminal. Biophys. J. 1997;72:1458–1471. doi: 10.1016/S0006-3495(97)78792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koester H.J., Sakmann B. Calcium dynamics associated with action potentials in single nerve terminals of pyramidal cells in layer 2/3 of the young rat neocortex. J. Physiol. 2000;529:625–646. doi: 10.1111/j.1469-7793.2000.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delvendahl I., Jablonski L., Hallermann S. Reduced endogenous Ca2+ buffering speeds active zone Ca2+ signaling. Proc. Natl. Acad. Sci. USA. 2015;112:E3075–E3084. doi: 10.1073/pnas.1508419112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran V., Park M.C.H., Stricker C. An improved measurement of the Ca2+-binding affinity of fluorescent Ca2+ indicators. Cell Calcium. 2018;71:86–94. doi: 10.1016/j.ceca.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Kawaguchi S.Y., Sakaba T. Fast Ca2+ buffer-dependent reliable but plastic transmission at small CNS synapses revealed by direct bouton recording. Cell Reports. 2017;21:3338–3345. doi: 10.1016/j.celrep.2017.11.072. [DOI] [PubMed] [Google Scholar]
- 15.Novak P., Gorelik J., Korchev Y.E. Nanoscale-targeted patch-clamp recordings of functional presynaptic ion channels. Neuron. 2013;79:1067–1077. doi: 10.1016/j.neuron.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neher E. Some subtle lessons from the calyx of Held synapse. Biophys. J. 2017;112:215–223. doi: 10.1016/j.bpj.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]