Abstract
Background:
Remarkable discrepancy exists in outcomes between men/women for multiple malignancies. We sought to expose sex differences in using platelet count and neutrophil-to-lymphocyte ratio (NLR) to predict overall survival for select cancer types with focus on head and neck squamous cell carcinoma (HNSCC).
Experimental Design:
Peripheral blood samples from 9365 patients seen in a tertiary teaching hospital with nine different primary tumors were retrospectively examined. HNSCC RNA-sequencing data from The Cancer Genome Atlas were analyzed by two computational means (CIBERSORT and ESTIMATE) to extend our observations to the tumor microenvironment.
Results:
For HNSCC, platelet count was more predictive of overall survival for males (Log-rank test: HR = 1.809, 95% CI: 1.461–2.239 vs. HR = 1.287, 95% CI: 0.8901–1.861) while NLR was more predictive for females (HR = 2.627, 95% CI: 1.716–4.02 vs. HR = 1.261, 95% CI 0.998–1.593). For females, lymphocyte count was more associated with survival than neutrophil count (multivariate Cox regression: p = 0.0015 vs p = 0.7476). Both CIBERSORT (p = 0.0061) and ESTIMATE (p = 0.022) revealed greater immune infiltration in females. High tumor infiltration by T lymphocytes was more strikingly associated with survival in females (HR = 0.20, p = 0.0281) than in males (HR = 0.49, p = 0.0147).
Conclusions:
This is the first study to comprehensively demonstrate sex bias in the clinical utility of platelet, granulocyte and lymphocyte counts as biomarkers to prognosticate HNSCC patients.
Impact:
This work emphasizes the necessity to consider sex in appraising inflammatory markers for cancer risk stratification.
Keywords: sex, cancer, HNSCC, NLR, TCGA, CIBERSORT
Introduction
In 2017, cancer was estimated to be the cause of nearly 1 out of every 4 deaths in the United States. Head and neck squamous cell carcinoma (HNSCC), comprising of cancers of the oral cavity, pharynx, and larynx, accounts for 4% of all cancers, resulting in nearly 50,000 new cases and 10,000 deaths last year alone (1). Primary risk factors for HNSCC include smoking, alcohol use, and human papilloma virus (HPV) infection of the oropharynx. In fact, etiology of over 70% of oropharyngeal cancers diagnosed in the United States after the year 2000 could be attributed to HPV infection (2,3).
Interestingly, men have historically been much more likely to develop both HPV-unassociated and –associated HNSCC, with their risk being approximately 3 times as high as it is for women (4–9). Indeed, HNSCC is the 7th most common cancer in men but only the 13th most common in women in the US (10). This substantial male bias is insufficiently explained by sex differences in smoking and alcohol consumption (11). Furthermore, females present with lower rates of HPV infection despite not engaging with proportionally fewer oral sexual partners (8,9). Sex bias likely reflects fundamental biological differences between males and females as mediated by sex chromosomes and hormones. Yet, literature on their role in HNSCC is sparse (11,12).
Males and females share robust differences in their susceptibility to various autoimmune and infectious diseases, emphasizing that sex is an important biological variable affecting the immune system (13). However, potential implication of sex-based immunological differences on the pathogenesis of various malignancies has not been thoroughly investigated. Markers of subclinical inflammation (platelet, neutrophil, lymphocyte counts, and neutrophil-to-lymphocyte ratio [NLR]) have been proposed as prognostic indicators for both overall survival (OS) and recurrence-free-survival (RFS) in a variety of cancer types, including HNSCC. In general, both high platelet counts and high NLR are associated with decreased survival (14–24). In this study, we determined sex-specific prognostic value of peripheral immune/inflammatory responses in various malignancies with focus on HNSCC. Additionally, CIBERSORT (Cell-type Identification By Estimating Relative Subsets Of Rna Transcripts) and ESTIMATE (Estimation of Stromal and Immune cells in Malignant Tumor tissues using Expression data) were used to analyze RNA-sequencing data from The Cancer Genome Atlas (TCGA) to compare male and female immune cell infiltration in HNSCC tumors and to validate our findings at the level of the tumor microenvironment (25,26). This study offers an important insight that select cancers in males and females may have differential immune involvement and therefore provide support for stratification in the use of platelet count and NLR by patients’ sex in the era of precision oncology.
Materials and Methods
Data collection
The MUSC Institutional Review Board approved all study activities, and the studies were conducted in accordance with the U.S. Common Rule. For each case, we abstracted data on demographic characteristics, clinical, and pathological variables at diagnosis, and outcome using 2 different data sources: the Hollings Cancer Center cancer registry and the MUSC Clinical Data Warehouse. The registry is part of a state mandated data system that collects cancer incidence on all cases in South Carolina. The Clinical Data Warehouse is a single, secure, and integrated database extracted from the MUSC OACIS Clinical Data Repository, which includes patient demographics, International Classification of Disease-coded diagnoses, International Classification of Disease-coded procedures, medications, and laboratory test results. These databases were subsequently linked, in a blinded fashion, through an honest broker at the Clinical Data Warehouse, and entered into a secured study database.
Independent variables obtained from the Clinical Data Warehouse included pretreatment hematologic parameters (platelet count, red blood cell count, white blood cell count, absolute neutrophil count, absolute lymphocyte count, and hemoglobin level). Variables obtained from the registry included sociodemographic characteristics (age at diagnosis, sex, and race), lifestyle factors including smoking status (never, current, former) and alcohol use (never, current, former), location of the primary tumor, TNM pathologic staging, and the p16 (HPV) status. The p16 status, assayed by immunohistochemistry, was used as a surrogate marker for HPV. All values were collected at the time of diagnosis.
Baseline characteristics
The Cancer Registry at Hollings Cancer Center, Medical University of South Carolina (MUSC) was used to identify 9365 total patients with primary tumors from nine different anatomical locations (bladder, colorectal, esophageal, head and neck, renal, liver, lung, skin melanoma, and pancreas). Cancer types were defined by ICD-10-CM codes and histological subtypes were all included in analysis. The study population consisted of histologically-confirmed cases diagnosed between January 1, 2000 and June 30, 2012.
Data analysis
Overall survival (OS) was the primary measured outcome. Statistical analyses were performed using Kaplan-Meier (KM) curves, log-rank test, and Cox proportional hazards regression. Variables considered in statistical analyses included platelet count (continuous variable), absolute neutrophil count (continuous variable), absolute lymphocyte count (continuous variable), age (continuous variable), race (white, black, other), tobacco history (never, current, former), alcohol history (never, current, former), and combined AJCC pathologic stage (I, II, III, IV). For the nine cancer type screen, a platelet cutoff factor of 300 K/mm3 was used to separate patients into two groups (high and low). Patients with platelet counts lower than 150 K/mm3 or higher than 450 K/mm3 were not included in this screen to avoid the possible effect of primary or secondary hematological comorbidities on survival (18). Based on results from a previous meta-analysis, a NLR cutoff of 3 was used to separate patients into two groups (high and low) (27). Chi-square test was used to test homogeneity in distribution of high and low groups for males and females.
All hematological parameters at time of diagnosis were not known for all patients. However, all patients with known platelet count or NLR values were included in analyses. The total number of male and female patients with known hematological parameters for each cancer are shown in Supplementary Table S1.
TCGA data preparation for ESTIMATE and CIBERSORT
RNA-sequencing data from The Cancer Genome Atlas (TCGA, 01–28-16 release) were downloaded from the Broad Institute GDAC Firehose (http://firebrowse.org). RNA-sequencing data for the HNSCC cohort was used (n = 520) and we downloaded RSEM normalized values generated from the RNA-seqV2 platform.
To prepare the expression data for CIBERSORT analysis, genes with RSEMavg < 10 were removed. The expression matrix was then normalized using voom (from R package limma) to transform RSEM to a distribution resembling microarray data. The transformed values were then uploaded to the CIBERSORT web portal (http://cibersort.stanford.edu/). CIBERSORT’s LM22 signature matrix was used to analyze the expression matrix. Permutations were set to 1000 and quantile normalization was turned off. Cell categories were simplified by summing component percentages into four groups: T lymphocyte, B lymphocyte, macrophages, and granulocytes.
ESTIMATE scores (Immune, Stromal, and ESTIMATE) for the TCGA HNSCC cohort used in this paper were downloaded from the ESTIMATE portal website (http://bioinformatics.mdanderson.org/estimate/). Note that these scores were calculated based on the RNA-seqV2 platform, which we also used to calculate the CIBERSORT scores. Both ESTIMATE and CIBERSORT data were recombined with the clinical annotations (including patient gender and pathological stage) extracted with the rtcga package. Pearson correlation and Welch’s t-test were used to compare CIBERSORT and ESTIMATE derived values. All analyses were performed with R, using the packages rtcga, ggplot2, limma, and survival (28–31).
Kaplan-Meier analyses with CIBERSORT cell fractions
Overall survival data for the TCGA cohort were retrieved with rtcga. Only patients with CIBERSORT P-values less than 0.05 were included in this analysis. Optimal cutoff points for total T lymphocyte count and granulocyte count were determined by using an outcome-oriented method of maximizing Log-rank statistics. This computation was done using the R package survminer. The cutoff point determined for T lymphocyte and granulocyte counts were 36% infiltration and 6.3% infiltration respectively. Female and male patients were then separated into high and low groups prior to KM analysis.
Results
Tumor-bearing women have higher mean platelet counts and absolute lymphocyte counts for select cancer types
To determine whether sex differences in circulating platelet, lymphocyte, or neutrophil counts exist, such hematological parameters were compared in male and female patients for each of the nine cancer types (Fig. 1). All stages of cancers were included and the samples were collected at the time of diagnosis. As a group, females presented with statistically significantly higher platelet counts for most cancers, higher neutrophil counts for only one cancer (liver), and higher lymphocyte counts for six cancers (colorectal, head and neck, kidney, lung, melanoma, and pancreas). It is notable that patients with liver cancers presented with particularly lower platelet, lymphocyte, and neutrophil counts compared to patients with other cancer types.
Figure 1:
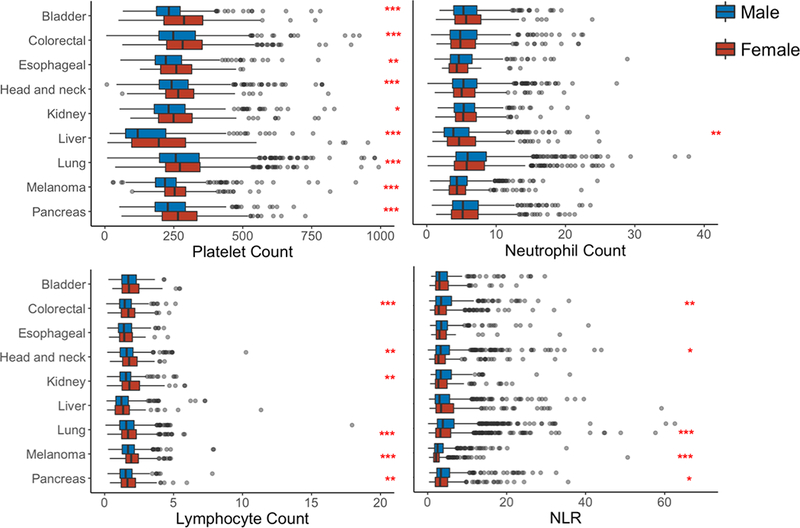
Platelet, absolute neutrophil, and absolute lymphocyte counts in peripheral blood were compared by sex at the time of diagnosis for nine cancer types
Platelet count (K/mm3), absolute lymphocyte count (K/mm3), absolute neutrophil count (K/mm3), and neutrophil-lymphocyte-ratio (NLR) were collected at the time of diagnosis. These values were plotted for male and female patients (for all stages combined) with primary bladder (n = 478), colorectal (840), esophageal (376), head and neck (1066), kidney (966), liver (601), lung (1902), melanoma (966), and pancreatic cancer (901). Malignancy type was determined by ICD-10-CM and retrieved from the Clinical Data Warehouse. Boxplots were generated for each hematological parameter and cancer type. Upper and lower limits of each box are 25th and 75th percentiles respectively, with whiskers spanning 1.5 IQR from these percentiles. Statistical comparisons between males and females were made by Mann-Whitney U test. P-value = * < 0.05 ** < 0.01 *** < 0.001
Multiple cancers show sex bias when using platelet count as a prognostic marker
To evaluate the association between platelet counts and OS for each sex separately, KM curves were generated, stratified by platelet count and sex (Fig. 2). The platelet cutoff value used to sort patients was 300 K/mm3 in order to be consistent with previously published studies (16). Additionally, the cutoff was numerically convenient for dividing the counts into quartiles within the range of physiologically normal platelet counts (150–450). Patients with platelet counts less than 150 K/mm3 or greater than 450 K/mm3 were not included in this preliminary screen in order to avoid confounding hematological comorbidities (18). Log-rank test results indicate that colorectal, esophageal, head and neck, and liver cancers showed the largest differences in prognoses between males and females. For these four cancers, high platelet count was associated with decreased OS in males but not in females. As for other cancer types, there was either no (renal, melanoma, pancreas) or non sex-specific (bladder, lung) relationship between platelet and OS.
Figure 2:
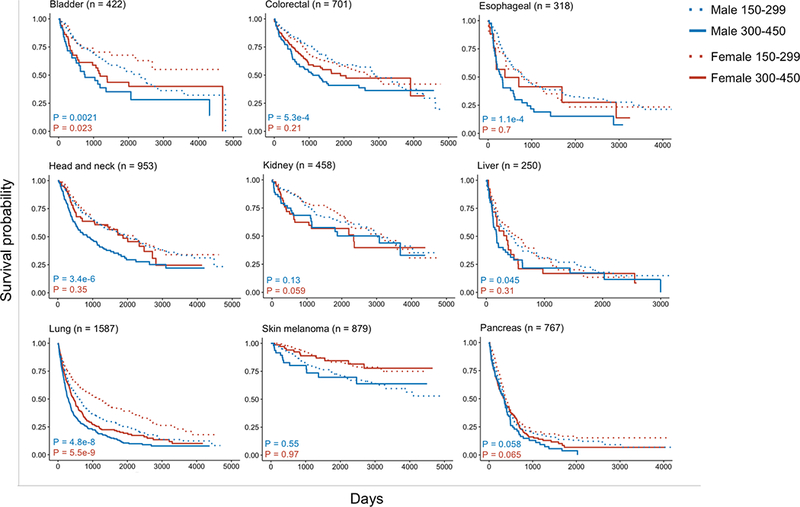
Kaplan-Meier curves of overall survival for male and female patients stratified by high and low platelet counts for multiple cancers
Patients were divided into four groups by sex (male or female) and platelet count (150–299 K/mm3 or 300–450 K/mm3). Patients with counts less than 150 or greater than 450 were not included in this analysis. Chi-square test showed that variation in the distribution of high and low platelet count was heterogeneous for males and females in all cancers except for colorectal, esophageal, liver, and lung (with α = 0.01). Kaplan-Meier curves were plotted for each cancer type (all stages) based on overall survival. P-values were computed by log-rank test for each sex separately.
Head and neck and bladder cancers show sex bias when using NLR as a prognostic marker
Based on results from a previous meta-analysis, a NLR cutoff of 3 was used to separate patients into two groups (27). KM curves were generated, stratified by NLR category and sex (Fig. 3). Log-rank tests demonstrated significant difference in OS distribution between patients with high and low NLR in both sexes for most cancers. However, head and neck cancers were remarkable for exhibiting a sex discrepancy in prognostic value. For head and neck cancer, NLR was not significantly associated with OS for males (HR = 1.261, 95% CI: 0.998 – 1.593) as compared to females (HR = 2.627, 1.716 – 4.02). Additionally, Cox regression results showed head and neck cancer was uniquely significant in that sex affects NLR’s contribution towards OS (β=0.702, P=0.0044) (Supplementary Table S2).
Figure 3:

Kaplan-Meier curves of overall survival for male and female patients stratified by high and low NLR for multiple cancers
Neutrophil-to-lymphocyte ratio (NLR) was calculated by dividing absolute neutrophil count by absolute lymphocyte count. Patients were separated into four groups by sex (male or female) and neutrophil-lymphocyte ratio (less than 3 or greater than 3). Chi-square test showed that variation in distribution of high and low NLR was homogenous for both males and females except for lung and skin melanoma (with α = 0.01). Kaplan-Meier curves were plotted for each cancer type based on overall survival. P-values were computed by log-rank tests for each sex separately.
Females were less likely smokers, drinkers, and presented at lower stages in the HNSCC cohort
Due to a great sex discrepancy in the association between platelet count/NLR and OS and adequate sample size for HNSCC, we focused subsequent analyses on this patient population (Table 1). When comparing by sex, females were more likely to be negative for smoking history and alcohol use. However, this could be influenced by response bias. They also tended to present with an earlier staged tumor. Additionally, primary lesion locations differed between males and females, where males presented with relatively fewer oral cavity tumors and more laryngeal tumors. In terms of hematological parameters, females had higher mean platelet counts, but there was no difference in absolute neutrophil count, absolute lymphocyte count, WBC count, or NLR value. As expected, females had lower RBC and hemoglobin counts (32). In this cohort of patients, the number of patients with known HPV status was insufficient (85 males, 21 females, n = 106) for us to make a statistical conclusion on the contributions of HPV status.
Table 1:
Demographics of HNSCC patients from MUSC Cancer Registry (2000–2012)
| Category | No. of patients (%) | Mean (SD) | Male | Female | P-value |
|---|---|---|---|---|---|
| Total | 1222 | 954 | 268 | ||
| Age | 59.9 (11.1) | 59.9 (10.6) | 60.0 (12.7) | 0.9 | |
| Race | 0.18 | ||||
| White | 904 (74) | 697 (73) | 207 (77) | ||
| African American | 298 (24) | 243 (25) | 55 (21) | ||
| Other | 20 (2) | 14 (1) | 6 (2) | ||
| Smoking | <0.0001 | ||||
| Never | 174 (15) | 108 (12) | 66 (25) | ||
| Former | 367 (31) | 297 (33) | 70 (27) | ||
| Current | 633 (54) | 507 (56) | 126 (48) | ||
| Unknown | 48 | 42 | 6 | ||
| Alcohol | <0.0001 | ||||
| Never | 306 (28) | 197 (23) | 109 (46) | ||
| Former | 176 (16) | 144 (17) | 32 (14) | ||
| Current | 613 (56) | 519 (60) | 94 (40) | ||
| Unknown | 27 | 94 | 33 | ||
| Stage | 0.002 | ||||
| I | 148 (18) | 102 (16) | 46 (26) | ||
| II | 73 (9) | 55 (9) | 18 (10) | ||
| III | 173 (21) | 129 (20) | 44 (25) | ||
| IV | 413 (51) | 344 (55) | 69 (39) | ||
| Unknown | 415 | 324 | 91 | ||
| Primary site | <0.0001 | ||||
| Lip | 15 (1) | 13 (1) | 2 (1) | ||
| Oral cavity | 495 (41) | 351 (37) | 144 (54) | ||
| Salivary gland | 21 (2) | 18 (2) | 3 (1) | ||
| Tonsils | 154 (13) | 130 (14) | 24 (9) | ||
| Oropharynx | 68 (6) | 60 (6) | 8 (3) | ||
| Nasopharynx | 18 (1) | 14 (1) | 4 (1) | ||
| Pyriform sinus | 31 (3) | 27 (3) | 4 (1) | ||
| Hypopharynx | 40 (3) | 35 (4) | 5 (2) | ||
| Nasal cavity | 67 (5) | 44 (5) | 23 (9) | ||
| Larynx | 267 (22) | 228 (24) | 39 (15) | ||
| Other | 46 (4) | 34 (4) | 12 (4) | ||
| Blood counts | |||||
| Platelets | 807:280 | 263.3 (96.2) | 257.9 (97.6) | 280 (89.8) | 0.0008 |
| NLR | 569:177 | 4.77 (5.12) | 4.92 (5.29) | 4.28 (4.51) | 0.11 |
| Neutrophils | 569:177 | 5.93 (3.29) | 5.96 (3.34) | 5.86 (3.15) | 0.73 |
| Lymphocytes | 569:177 | 1.77 (1.47) | 1.74 (1.61) | 1.87 (0.81) | 0.14 |
| WBC | 806:259 | 8.51 (3.84) | 8.6 (4.11) | 8.25 (2.84) | 0.12 |
| RBC | 806:259 | 4.39 (0.62) | 4.43 (0.63) | 4.26 (0.56) | <0.0001 |
| Hgb | 806:259 | 13.39 (1.82) | 13.53 (1.87) | 12.94 (1.59) | <0.0001 |
Baseline characteristics of patients with primary head and neck squamous cell carcinoma (HNSCC). Patients were stratified by sex and age, race, smoking/alcohol history, staging, location of primary tumor, and hematological parameters. Statistical comparisons were made with Welch’s T-test for age and blood counts, Fisher’s exact test for location of primary tumor (due to low counts in some cells), and Chi-square test for the remaining variables. “Unknown” groups were excluded in these statistical comparisons for smoking history, alcohol history, and staging. In the fourth and fifth columns, standard deviations are provided in parentheses for age and blood counts while percentages are provided in parentheses for the remaining variables.
Platelet count, but not neutrophils or lymphocyte count, is correlated with clinical staging
To see if we could reproduce findings from previous reports, we assessed whether platelet count and NLR were associated with OS in our cohort with both sexes combined (18,22,27,33). We also tested additional cutoff criteria, including separating patients into four subgroups (platelet count: 150–224, 225–299, 300–374, 375–450 K/mm3; NLR: quartiles) in order to gain more insight into their contributions towards survival. Indeed, when patients were not separated by sex, both platelet count (Log-rank test: P = 0.0011) and NLR (P = 0.00014) were significantly associated with OS (Fig. 4, Supplementary Fig. S1). However, when males and females were considered separately, platelet count was no longer associative for females, and NLR is no longer associative for males (Fig. 4).
Figure 4:
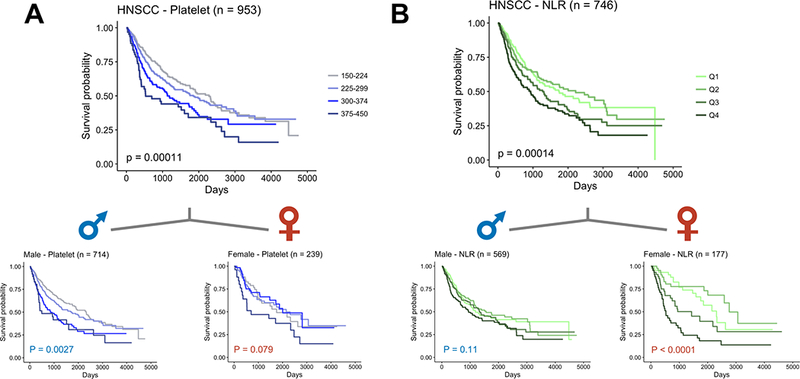
Sex-dependent survival based on platelet count and NLR for HNSCC
Patients were divided into four groups by (A) platelet counts within physiologically normal range (150–224, 225–299, 300–374, 375–450 K/mm3) or (B) neutrophil-to-lymphocyte ratio in quartiles. Chi-square test confirmed homogeneity for NLR but not platelet count (with α = 0.01). Kaplan-Meier curves were plotted for each of males,females, and both sexes. P-values were computed by log-rank tests comparing the four groups with equal weighting.
Furthermore, we postulated that both platelet count and NLR might be correlated with cancer staging. Platelet count did positively correlate with stage and female sex (ANOVA; Stage: P = 0.00162, Sex: P = 0.00033) but NLR (P = 0.096, P = 0.219), neutrophil counts (P = 0.099, P = 0.902), and lymphocyte counts (P = 0.335, P = 0.420) did not (Supplementary Fig. S2, Supplementary Table S3).
Multivariate analyses determine lymphocyte counts as the primary contributor to sex discrepancy in NLR prognostic value
To probe into the nuances of NLR predictive value, we tested whether neutrophils or lymphocytes were individually associated with OS. Log-rank tests revealed that neutrophil count was associated with worse prognosis for both males and females, but lymphocyte count was associated with better prognosis only for females (Supplementary Fig. S3). However, univariate analyses are unable to address the possibility of confounding interactions.
Therefore, multivariate analysis was performed with Cox proportional hazards regression. Regressions were modeled for each sex separately based on platelet, neutrophil, and lymphocytes count (all continuous variable), age, race, tobacco/alcohol history, and stage (Supplementary Table S4). In agreement with our findings above, platelet count was associated with worse survival for males exclusively. On the other hand, only higher lymphocyte count correlated with increased survival in females. Surprisingly, neutrophil count was no longer predictive of survival.
To test if the results hold for a homogenous anatomical subgroup of HNSCC, we performed the same Cox regression using only oropharyngeal cancers. The impact of neutrophil count and lymphocyte count on survival were consistent with that of the mixed group (data not shown). However, platelet count was now barely significant for females (P=0.050) but not for males (P=0.092). Therefore, we believe that platelet count as a continuous variable does not have a strong impact on survival, especially upon adjusting for stage.
Gene expression analyses suggest that females have higher immune infiltration in the tumor microenvironment
Thus far, we have demonstrated remarkable sex-specific differences in the prognostic value of peripheral hematological parameters. In order to validate our findings at the level of the tumor microenvironment, we performed unbiased analysis using RNA-sequencing data of bulk tumor samples from TCGA. We separated the patients by sex and attempted to ascertain if there were any differences in immune-signatures.
ESTIMATE and CIBERSORT methods were used in parallel to evaluate the immune profile of the TCGA HNSCC cohort. ESTIMATE uses single-sample gene set expression analysis to predict tumor purity and presence of infiltrating immune and stromal cells in bulk tumor data (26). Immune score measures the extent of immune cell infiltration, Stromal score measures stromal cell infiltration, and ESTIMATE score is a measurement of tumor purity. Females expressed significantly higher ESTIMATE scores and Immune scores, signifying higher expression of immune infiltration and tumor purity (Fig. 5A). There was no significant difference in stromal infiltration.
Figure 5:
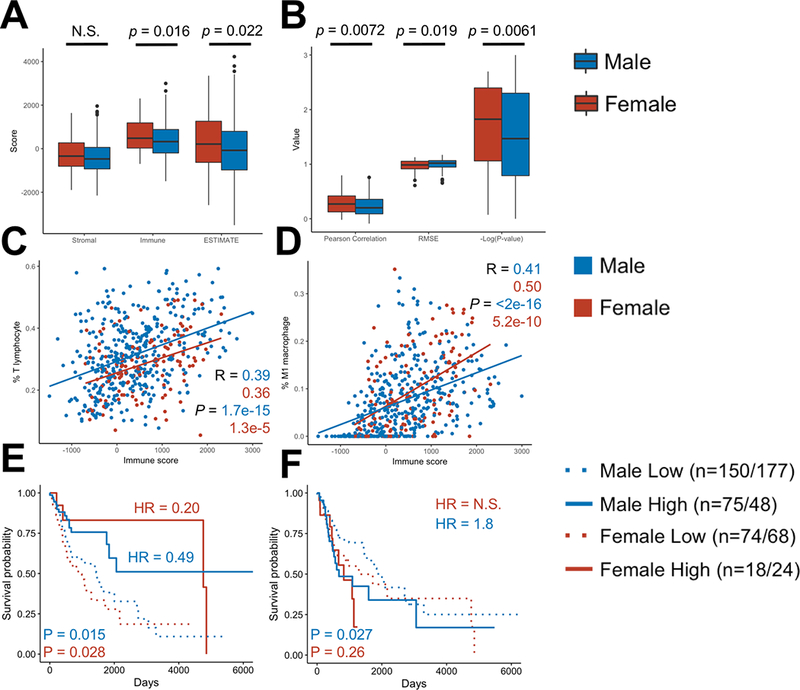
CIBERSORT and ESTIMATE analyses of TCGA RNA-sequencing data of HNSCC reveals higher immune activity in female bulk tumor samples
All patients in the TCGA’s Head and Neck Squamous Cell Carcinoma cohort (n = 520) were analyzed by ESTIMATE (Estimation of STromal and Immune cells in MAlignant Tumor tissues using Expression data) and CIBERSORT (Cell-type Identification By Estimating Relative Subsets Of Rna Transcripts).
(a, b) ESTIMATE score, Immune score, and Stromal score (A) and CIBERSORT minus log-transformed P-values, Pearson correlations, and RMSE (B) were compared between males and females. Comparison between male and female means were done by Welch’s T-test.
(C, D) CIBERSORT T lymphocyte count (C) and M1 macrophage count (D) were plotted with ESTIMATE Immune score, showing a positive association between increased percentage of these cell types and overall tumor infiltration. Statistics were computed by Pearson correlation test.
(E, F) Kaplan-Meier curves were generated to test the association between overall survival and each of CIBERSORT T lymphocyte (E) and granulocyte relative count (F). Groups were split by sex and an optimal cutoff point was determined by computing the maximally selected rank statistics. Hazard ratios (HR) were computed by log-rank test between high and low groups of each sex. N.S. is `not significant`. Cohort sizes are shown as T lymphocyte/granulocyte. Chi-square test confirmed homogeneity for both T-lymphocyte and granulocyte counts (with α = 0.01).
CIBERSORT is a method for characterizing the cellular composition using expression data from a heterogeneous sample (25). Moreover, the goodness-of-fit of the CIBERSORT algorithm offers an independent measurement of immune infiltration (34). In samples with higher immune cell infiltration, CIBERSORT is better at estimating the distribution of immune cells. As a result, lower P-values/RMSE or higher Pearson correlations are associated with greater immune infiltration. Analyzing the female samples with CIBERSORT resulted in lower P-values, lower RMSE, and higher Pearson correlations (Fig. 5B).
To test agreement between the two distinctive algorithmic methods, the Pearson correlation produced by CIBERSORT was plotted as a function of the ESTIMATE Immune score for all samples. There was a strong positive correlation (R = 0.59; P = 6 × 10−51; unpublished observations), suggesting that both methods were consistent in predicting immune infiltration.
T-lymphocyte and M0/M1 macrophages were responsible for increased tumor immune infiltration in both sexes
To determine which immune cell subsets were most responsible for increases in immune infiltration, we performed correlation analyses between CIBERSORT relative cell percentages and ESTIMATE Immune score. T-lymphocytes, as a group, and M1/M2 macrophages were the only cell types that were positively associated with Immune score (Fig. 5C–D, Supplementary Fig. S4F). On the other hand, M0 macrophages, B-lymphocyte, and granulocytes–including mast cells, eosinophils, and neutrophils–were negatively associated with immune score (Supplementary Fig. S4).
CIBERSORT T lymphocyte and granulocyte fractions are able to predict survival
KM curves were plotted to evaluate the association between CIBERSORT T lymphocyte/granulocyte counts and OS in patients with notable immune infiltration (CIBERSORT P-value < 0.05) (Fig. 5E–F). Log-rank tests showed that having a high T lymphocyte count was significantly correlated with longer survival in both sexes. However, the difference in survival between female high and low count groups (HR = 0.201, 0.048–0.842, p = 0.0281) was more than double that of male high and low count groups (HR = 0.495, 95% CI: 0.281–0.871, p = 0.0147). On the other hand, having a high granulocyte count was associated with worse survival in males only (HR = 1.80, 1.07–3.03, p = 0.0268). The effect of granulocyte count on female survival was not significant (HR = 1.54, 0.73–3.27, p = 0.256). We further implemented Chi-square test to check whether the difference in hazard ratio between males and females is affected by the differences in size of subgroups. The result indicates that there are no significant differences in distributions of the high and low groups between females and males (P = 0.021 and 0.44, for T-lymphocytes and granulocytes, respectively, with α = 0.01).
Discussion
Persistence of sexual dimorphism in select cancer incidence and survival even after statistical correction for occupational and behavioral factors implies the existence of cellular and molecular differences in disease progression between the two sexes. Indeed, sex chromosomes and hormones have been shown to regulate various steps in cancer pathogenesis via both tumor cell-intrinsic and -extrinsic mechanisms (35,36). Here, we examined the latter with focus on sex-biased involvement of various hematopoietic compartments primarily in patients with HNSCC. In males, both univariate and multivariate analyses indicated that use of circulating platelet and neutrophil counts as prognostic markers was more effective. In females, circulating lymphocyte counts, rather than neutrophils, were more correlated with overall survival. Interestingly, gene expression analyses using TCGA database suggested that females have higher immune cell infiltration into the tumor microenvironment and T lymphocyte signature can more strikingly predict their survival. To the best of our knowledge, this is the first study to comprehensively demonstrate sex bias in the clinical utility of platelet, granulocyte and lymphocyte counts as biomarkers to prognosticate HNSCC patients via analyses at both the level of periphery and the tumor microenvironment.
Our results provide a valuable starting point to encourage intense investigations for molecular mechanisms underlying sex-specificity in the predictive value of peripheral blood counts. Of note, studies have suggested the existence of sex bias in platelet activity, immunity, and hemostasis. For instance, a platelet proteome analysis detected higher levels of signaling and activation proteins in male platelets compared to female platelets (37). Platelet derived TGF-β has been shown to not only promote tumor growth and metastasis (38), but also play a role in converting peripheral naïve T cells to regulatory T cells, which can suppress anti-tumor immune function (39). Greater activity of male platelets may lend to its increased significance in cancer prognosis. Also, while we did not detect a sex difference in total lymphocyte count, higher peripheral percentages of overall and IL-2 producing T-lymphocytes have been reported in women compared to men (40). Higher peripheral T cell count in females may mediate more efficient immune surveillance and tumor infiltration. The latter has been associated with better prognosis in multiple cancers including – but not limited to – melanoma, colorectal, ovarian, and breast, many of which demonstrate sex bias in their incidence and survival (41–45). Furthermore, sex differences may exist in the function or subtype identities of immune cells that studies involving absolute peripheral counts fail to uncover. Indeed, both T regulatory and T helper cells would be considered as part of absolute lymphocyte count despite playing distinctive physiological roles. Due to mostly comparable neutrophil and lymphocyte counts between the sexes in our study cohort, observed disparity in NLR’s prognostic value could be due to differences in lymphocyte activity or cellular subtype ratios. Overall, our findings implicate that anti-tumor immunity may be largely different between males and females.
Our study corroborates other studies that highlight elevated circulating platelet count and NLR as biomarkers for poor prognosis in a variety of cancer types, association of which remains not universally accepted and yet established in the clinic (46,47). One important barrier to their clinical implementation is their lack of consistency and reproducibility (48–50). Our study shows that failing to account for sex may lead to confounding of these variables with cancer progression.
Our study limitations included the followings. First, the patient population came from a single study center (MUSC). Multi-centric prospective studies are required to reduce selection bias. Second, the anatomical distribution of HNSCC cases differed between males and females. Third, although we independently validated our study results using TCGA database, CIBERSORT and ESTIMATE methods are–as their names suggest–estimates of immune infiltration based on gene signature. Quantitative analysis by flow-cytometry or immunohistochemistry would be optimal for measuring infiltration of specific immune cell-subtypes.
There currently exists a revolution in sex biology, in which researchers are required to consider sex in all appropriate experimental designs and analyses. In accordance with its importance, we argue here that sex is a critical variable in our understanding of the role of circulating platelet, granulocyte and lymphocyte counts in cancer progression. Detailed knowledge will be highly relevant to clinical practice given that peripheral blood counts are easily obtainable and pharmacological tools to modulate the function of sex hormones are readily available.
Supplementary Material
Acknowledgments
This work was supported by multiple NIH grants P01 CA186866, R01 CA213290, R01 CA188419, R01 AI077283 (to Z.L.), R01 GM122078, and R21 CA209848 (to D.C.) and Hollings Cancer Center’s Cancer Center Support Grant P30 CA138313. H.K. was supported by scholarships from the Abney Foundation and Hollings Cancer Center. Z.L. is the Abney Scholar for Stem Cell Biology and Therapy and is supported by the South Carolina SmartState Center of Excellence.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.American Cancer Society. Cancer Facts & Figures 2017. Cancer Facts Fig 2017 [Internet]. 2017;1 Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html
- 2.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J. Clin. Oncol 2015. page 3235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marur S, Forastiere AA. Head and neck cancer: Changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008. page 489–501. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol [Internet]. 2008;26:612–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18235120 [DOI] [PubMed] [Google Scholar]
- 6.Gillison ML, D’Souza G, Westra W, Sugar E, Xiao W, Begum S, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–20. [DOI] [PubMed] [Google Scholar]
- 7.Smith EM, Rubenstein LM, Haugen TH, Hamsikova E, Turek LP. Tobacco and alcohol use increases the risk of both HPV-associated and HPV-independent head and neck cancers. Cancer Causes Control. 2010;21:1369–78. [DOI] [PubMed] [Google Scholar]
- 8.Ryerson AB, Peters ES, Coughlin SS, Chen VW, Gillison ML, Reichman ME, et al. Burden of potentially human papillomavirus-associated cancers of the oropharynx and oral cavity in the US, 1998–2003. Cancer. 2008;113:2901–9. [DOI] [PubMed] [Google Scholar]
- 9.Dahlstrom KR, Little JA, Zafereo ME, Lung M, Wei Q, Sturgis EM. Squamous cell carcinoma of the head and neck in never smoker–never drinkers: A descriptive epidemiologic study. Head Neck [Internet]. 2008;30:75–84. Available from: 10.1002/hed.20664 [DOI] [PubMed] [Google Scholar]
- 10.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin [Internet]. 2018;68:7–30. Available from: http://doi.wiley.com/10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 11.Langevin SM, Grandis JR, Taioli E. Female hormonal and reproductive factors and head and neck squamous cell carcinoma risk. Cancer Lett. 2011;310:216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nainani P, Paliwal A, Nagpal N, Agrawal M. Sex hormones in gender-specific risk for head and neck cancer: A review. [Internet]. J. Int. Soc. Prev. Community Dent 2014. page S1–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25452920%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4247543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein SL, Flanagan KL. Sex differences in immune responses. Nat. Rev. Immunol 2016. page 626–38. [DOI] [PubMed] [Google Scholar]
- 14.Ayhan A, Bozdag G, Taskiran C, Gultekin M, Yuce K, Kucukali T. The value of preoperative platelet count in the prediction of cervical involvement and poor prognostic variables in patients with endometrial carcinoma. Gynecol Oncol. 2006;103:902–5. [DOI] [PubMed] [Google Scholar]
- 15.Lin MS, Huang JX, Zhu J, Shen HZ. Elevation of platelet count in patients with colorectal cancer predicts tendency to metastases and poor prognosis. Hepatogastroenterology. 2012;59:1687–90. [DOI] [PubMed] [Google Scholar]
- 16.Nakahira M, Sugasawa M, Matsumura S, Kuba K, Ohba S, Hayashi T, et al. Prognostic role of the combination of platelet count and neutrophil--lymphocyte ratio in patients with hypopharyngeal squamous cell carcinoma. Eur Arch Oto-Rhino-Laryngology [Internet]. 2016;273:3863–7. Available from: 10.1007/s00405-016-3996-3 [DOI] [PubMed] [Google Scholar]
- 17.Brown KM, Domin C, Aranha GV., Yong S, Shoup M. Increased preoperative platelet count is associated with decreased survival after resection for adenocarcinoma of the pancreas. Am J Surg. 2005. page 278–82. [DOI] [PubMed] [Google Scholar]
- 18.Rachidi S, Wallace K, Day TA, Alberg AJ, Li Z. Lower circulating platelet counts and antiplatelet therapy independently predict better outcomes in patients with head and neck squamous cell carcinoma. J Hematol Oncol [Internet]. 2014;7:65 Available from: http://jhoonline.biomedcentral.com/articles/10.1186/s13045-014-0065-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Todenhöfer T, Renninger M, Schwentner C, Stenzl A, Gakis G. A new prognostic model for cancer-specific survival after radical cystectomy including pretreatment thrombocytosis and standard pathological risk factors. BJU Int. 2012;110. [DOI] [PubMed] [Google Scholar]
- 20.Deng Q, He B, Liu X, Yue J, Ying H, Pan Y, et al. Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. J Transl Med. 2015;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caruso RA, Bellocco R, Pagano M, Bertoli G, Rigoli L, Inferrera C. Prognostic value of intratumoral neutrophils in advanced gastric carcinoma in a high-risk area in Northern Italy. Mod Pathol. 2002;15:831–7. [DOI] [PubMed] [Google Scholar]
- 22.Valero C, Pardo L, López M, García J, Camacho M, Quer M, et al. Pretreatment count of peripheral neutrophils, monocytes, and lymphocytes as independent prognostic factor in patients with head and neck cancer. Head Neck [Internet]. 2017. [cited 2018 Jan 12];39:219–26. Available from: http://doi.wiley.com/10.1002/hed.24561 [DOI] [PubMed] [Google Scholar]
- 23.Panje C, Riesterer O, Glanzmann C, Studer G. Neutrophil-lymphocyte ratio complements volumetric staging as prognostic factor in patients treated with definitive radiotherapy for oropharyngeal cancer. BMC Cancer. 2017;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halazun KJ, Hardy MA, Rana AA, Woodland DC, Luyten EJ, Mahadev S, et al. Negative Impact of Neutrophil-Lymphocyte Ratio on Outcome After Liver Transplantation for Hepatocellular Carcinoma. Ann Surg [Internet]. 2009;250:141–51. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00000658-200907000-00022 [DOI] [PubMed] [Google Scholar]
- 25.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshihara K, Shahmoradgoli M, Martínez E, Vegesna R, Kim H, Torres-Garcia W, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin J, Qin Y, Luo Y, Feng M, Lang J. Prognostic value of neutrophil-to-lymphocyte ratio for nasopharyngeal carcinoma: A meta-analysis Yang H, editor. Medicine (Baltimore: ). 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosinski M, Biecek P. RTCGA: The Cancer Genome Atlas Data Intergration. 2016.
- 29.Wickham H Ggplot2 [Internet]. Elegant Graph. Data Anal. 2009. Available from: http://link.springer.com/10.1007/978-0-387-98141-3%5Cnpapers3://publication/doi/10.1007/978-0-387-98141-3
- 30.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin H, Zelterman D. Modeling Survival Data: Extending the Cox Model. Technometrics [Internet]. 2002;44:85–6. Available from: http://www.tandfonline.com/doi/abs/10.1198/tech.2002.s656 [Google Scholar]
- 32.Murphy WG. The sex difference in haemoglobin levels in adults - Mechanisms, causes, and consequences. Blood Rev. 2014. page 41–7. [DOI] [PubMed] [Google Scholar]
- 33.Rachidi S, Wallace K, Wrangle JM, Day T a, Alberg AJ, Li Z. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma. Head Neck [Internet]. 2015;1–21. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26040762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ali HR, Chlon L, Pharoah PDP, Markowetz F, Caldas C. Patterns of Immune Infiltration in Breast Cancer and Their Clinical Implications: A Gene-Expression-Based Retrospective Study. PLoS Med. 2016;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clocchiatti A, Cora E, Zhang Y, Dotto GP. Sexual dimorphism in cancer. Nat. Rev. Cancer 2016. page 330–9. [DOI] [PubMed] [Google Scholar]
- 36.Dunford A, Weinstock DM, Savova V, Schumacher SE, Cleary JP, Yoda A, et al. Tumor-suppressor genes that escape from X-inactivation contribute to cancer sex bias. Nat Genet. 2017;49:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eidelman O, Jozwik C, Huang W, Srivastava M, Rothwell SW, Jacobowitz DM, et al. Gender dependence for a subset of the low-abundance signaling proteome in human platelets. Hum Genomics Proteomics [Internet]. 2010;2010:164906 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2958630&tool=pmcentrez&rendertype=abstract%5Cnhttp://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=20981232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drabsch Y, Ten Dijke P. TGF-β signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev. 2012;31:553–68. [DOI] [PubMed] [Google Scholar]
- 39.Chen W, Jin W, Hardegen N, Lei K, Li L, Marinos N, et al. Conversion of Peripheral CD4+CD25- Naive T Cells to CD4+CD25+ Regulatory T Cells by TGF-β Induction of Transcription Factor Foxp3 J Exp Med [Internet]. Rockefeller University Press; 2003;198:1875–86. Available from: http://jem.rupress.org/content/198/12/1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bouman A, Schipper M, Heineman MJ, Faas MM. Gender difference in the non-specific and specific immune response in humans. Am J Reprod Immunol [Internet]. 2004;52:19–26. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15214938 [DOI] [PubMed] [Google Scholar]
- 41.Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol [Internet]. 2012;30:2678–83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22711850 [DOI] [PubMed] [Google Scholar]
- 42.Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med [Internet]. 2005;353:2654–66. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16371631 [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med [Internet]. 2003;348:203–13. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12529460 [DOI] [PubMed] [Google Scholar]
- 44.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. [DOI] [PubMed] [Google Scholar]
- 45.Mahmoud SMA, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AHS, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–55. [DOI] [PubMed] [Google Scholar]
- 46.Voutsadakis IA. Thrombocytosis as a prognostic marker in gastrointestinal cancers. World J Gastrointest Oncol [Internet]. 2014;6:34 Available from: http://www.wjgnet.com/1948-5204/full/v6/i2/34.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guthrie G, Roxburgh C, Farhan-Alanie O, Horgan P, McMillan D. Comparison of the prognostic value of longitudinal measurements of systemic inflammation in patients undergoing curative resection of colorectal cancer. Br J Cancer [Internet]. 2013;109:24–8. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3708558&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drucker E, Krapfenbauer K. Pitfalls and limitations in translation from biomarker discovery to clinical utility in predictive and personalised medicine. EPMA J [Internet]. 2013;4:7 Available from: http://link.springer.com/10.1186/1878-5085-4-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poste G Bring on the biomarkers. Nature. 2011. page 156–7. [DOI] [PubMed] [Google Scholar]
- 50.Pusztai L, Hatzis C, Andre F. Reproducibility of research and preclinical validation: Problems and solutions. Nat. Rev. Clin. Oncol 2013. page 720–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


