Abstract
Objective:
To test whether plasma interleukin-1 receptor antagonist (IL-1ra) or variants within the gene encoding for IL-1ra (IL1RN), or proteins involved in regulating IL-1β levels or IL-1β response, are associated with pediatric acute respiratory distress syndrome (PARDS) or outcomes in mechanically ventilated children with parenchymal lung disease.
Design:
Prospective cohort study.
Setting:
Twenty-two pediatric intensive care units participating in the multi-site clinical trial, Randomized Evaluation of Sedation Titration for Respiratory Failure (RESTORE; U01 HL086622).
Subjects:
Children 2 weeks to 17 years of age treated with invasive mechanical ventilation for acute airways and/or parenchymal lung disease.
Measurements and Main Results:
378 of 549 patients had PARDS; DNA and plasma were obtained from 523/549 and 480/549 patients, respectively. Plasma IL-1ra was highest on the day of intubation (Day 0) and decreased over the subsequent 3 days (p<0.0001). IL-1ra level was higher in patients with PARDS than those without PARDS (p<0.0001). Multivariable regression analysis of data across all days demonstrated a significant association of IL-1ra (OR= 1.30; 95% CI=1.10-1.52; p=0.002) and day (p<0.05) with PARDS, independent of age and PRISM-III score. Analysis on individual days indicated that plasma IL-1ra levels were associated with PARDS on Days 0 and 2, independent of age and PRISM-III score (p=0.04 and 0.003, respectively), however did not quite reach significance on Days 1 and 3 (p=0.06 and 0.07, respectively). IL-1ra was independently associated with mortality on Day 1 (p=0.02). IL-1ra also correlated with length of mechanical ventilation, measures of oxygenation and pediatric intensive care unit (PICU) length of stay. No genetic variants were associated with PARDS.
Conclusions:
Plasma IL-1ra is associated with PARDS, PICU length of stay, length of mechanical ventilation, and mortality in children with acute respiratory failure requiring mechanical ventilation.
Keywords: biomarkers, acute respiratory distress syndrome, genetic variants, critical illness, pediatrics, ARDS, PARDS, SNP
Introduction
Acute respiratory distress syndrome (ARDS) is characterized by disruption of the alveolar endothelial/epithelial permeability barrier and influx of protein rich fluid into the alveolar space (1). Other hallmarks of ARDS include dysregulation of the inflammatory response and coagulation/fibrinolysis. A number of studies have examined the association of plasma biomarkers and/or genetic variants with ARDS, or ARDS outcomes, in an attempt to better understand the pathological process and to identify markers that might be useful for diagnosis, prognostication or response to treatment. Early studies in adults demonstrated that interleukin-1 receptor antagonist (IL-1ra) is elevated in plasma and bronchoalveolar lavage fluid (BALF) in patients with ARDS and that levels may be associated with outcome (2–4). IL-1ra is a naturally occurring inhibitor of IL-1β that lacks agonist activity but competitively binds to the IL-1 receptor thereby blocking binding of IL-1β (5–8). IL-1β is an early pro-inflammatory cytokine activated in response to stressors like infection which induces the expression of other cytokines and chemokines involved in the inflammatory response. Recently analysis of three adult cohorts of patients either with ARDS, or at risk for ARDS, demonstrated that a specific variant in the gene for IL-1ra (IL1RN) is associated with reduced risk of ARDS and that the level of plasma IL-1ra appears to be associated with this variant (9).
Very few studies have examined the association of plasma or genetic markers with ARDS in children. While much of what has been learned in adults is likely to be applicable to children, differences in mortality rates and response to treatment (10, 11), together with changes in the developing immune system, suggest there may be differences between children and adults that impact the development, and outcomes of, pediatric ARDS (PARDS) (1, 12). Substantial differences in immune responsiveness between adults and children are well documented. Neonates have fewer neutrophils than adults and their polymorphonuclear cells (PMNs) have a decreased chemotactic response that may persist until 1–2 years of age (12). In addition, neonatal PMNs and monocytes release less cytokine in response to lipopolysaccharide (LPS) compared to adults (13–15). In one study examining temporal cytokine profiles in both severely burned adults and children, the cytokine profiles differed significantly with adults exhibiting a more hyper-inflammatory state (16). In healthy children substantial changes in lymphocyte subsets (17, 18) and cytokine response (19–21) are also known to occur during childhood. These data suggest that there are important maturational differences between adults and children in the development, pathophysiology and outcomes of ARDS that may be reflected in plasma biomarkers. This study tests whether plasma IL-1ra or genetic variants within IL1RN, or genes encoding proteins involved in regulating either IL-1β levels or response, are associated with PARDS in mechanically ventilated children with parenchymal lung disease. We hypothesized that both elevated IL-1ra and specific variants would be associated with PARDS.
Materials and Methods
Study Design
This study, Genetic Variation and Biomarkers in Children with Acute Lung Injury (BALI; R01HL095410), was a prospective ancillary study to the multi-site clinical trial, Randomized Evaluation of Sedation Titration for Respiratory Failure (RESTORE; U01 HL086622) (22). BALI was designed to examine the association of specific plasma protein and genetic biomarkers with PARDS among prospectively enrolled children with acute respiratory failure. Twenty-two of the 31 pediatric intensive care units (PICUs) participating in RESTORE volunteered to participate in this study. The study was approved by the Institutional Review Boards at all participating sites. After enrollment in RESTORE, parents or guardians were approached for consent for participation in BALI. In RESTORE, children 2 weeks to 17 years old treated with invasive mechanical ventilation for acute airways and/or parenchymal lung disease were eligible (22). Children for whom the length of mechanical ventilation was unlikely to be altered by the sedation management protocol being examined in RESTORE (i.e., children who were ventilator dependent on PICU admission or those expected to be extubated within 24 h) were excluded. There were no additional inclusion or exclusion criteria for the BALI study.
Screening for RESTORE and BALI was performed concurrently. Guardians for patients were approached for consent for RESTORE within 24 h of meeting RESTORE study criteria and were approached for consent for participation in BALI as soon as possible after consenting for RESTORE (often immediately afterwards). Blood samples were taken within 24 hours of consent for BALI and again 24 and 48 hours later. The primary analyses examined the association of plasma or genetic biomarkers with the presence of PARDS. PARDS was defined using the Pediatric Acute Lung Injury Consensus Conference (23) criteria for oxygenation index (OI) or oxygen saturation index (OSI) with the addition of the presence of bilateral infiltrates within 2 days before or 1 day after meeting OI or OSI criteria for PARDS. RESTORE and BALI were both designed when PARDS was defined using the American European Consensus Conference (AECC) definition (24). Consequently, chest radiograph data only included presence or absence of bilateral infiltrates. Individual chest radiographs were not collected as part of either study. Secondary analyses examined association of biomarkers with oxygenation defect (OI, PaO2/FiO2) and other relevant clinical outcomes including duration of mechanical ventilation, PICU length of stay in survivors and 90 day in-hospital mortality.
Assay of IL-1ra
Plasma IL-1ra was measured in duplicate by ELISA (#DRAOOB, R&D Systems, Inc., Minneapolis, MN). The reported limit of detection of the assay is 18.3 pg/ml; intra- and inter-assay coefficients of variations are 5.3% and 8.6%, respectively.
Genotyping
Selection of candidate genes and single nucleotide polymorphisms (SNPs) and methods for genotyping are described in the Supplement.
Statistical Analyses
Analyses related to plasma IL-1ra
Basic descriptive analyses of all demographic and key dependent variables (including frequency distributions or means, medians and standard deviations, and Pearson correlations as appropriate) were conducted on each of the four study days (0, 1, 2 and 3); differences between study days were evaluated for statistical significance using generalized estimating equations (GEE) to account for correlations within and between individuals. Development of PARDS, and secondary outcomes (death, duration of mechanical ventilation and length of PICU stay) were examined for changes over time. IL-1ra levels within each day (0-3) were investigated for association with all demographic and clinical measures (age, gender, race, ethnicity, past medical history (asthma, prematurity, seizure disorder, neurologic disorder, cancer, chromosomal abnormality), primary diagnosis (pneumonia, sepsis, bronchiolitis, asthma, aspiration), PRISM III score) using appropriate bivariate chi-square, t-tests or Wilcoxon Rank Sum Test analyses. The primary hypotheses were evaluated using binary logistic regression models for each binary outcome (PARDS, death) and Poisson regression models for the duration of mechanical ventilation and length of PICU stay outcomes. Multivariable models were developed based on the results of the bivariate analysis on association of IL-1ra with clinical and demographic variables and were adjusted for severity illness (PRISM III) and potential impact of age on IL-1ra level. As a result, for consistency across outcomes the final analyses were modeled longitudinally as a function of study day, age, PRISM III score and the log transformed IL-1ra level. All multivariable logistic regression models of binary outcomes accounted for individual clustering using GEE and adjusted for the covariates to assess changes in association with outcomes over days from Day 0 to Day 3. The explanatory measure of age was included in the final models (together with PRISM III score and IL-1ra) as fixed or time-varying covariates. We reported odds ratios with 95% confidence intervals. All statistical tests were two-tailed, with a significance level of 0.05. All statistical analyses were performed using SAS version 9.4.
Genetic association analyses
The association of genetic variants with PARDS was examined using PLINK, a statistical tool set designed specifically to perform analyses related to genome wide or family based genetic association studies or population based genetic linkage analysis (25, 26). For analysis results were stratified by race and ethnicity as the frequency of genetic polymorphisms and the linkage disequilibrium patterns differ between ethnicities and races (27, 28) and such groups should be analyzed separately (29). Non-Hispanic Caucasians, Hispanic Caucasians and African Americans (52%, 20%, and 17% of the cohort, respectively) were examined as these groups included 89% of enrolled patients. The frequency of each of these subgroups in patients with successfully genotyped DNA samples (n=477) was identical both to that of the group with DNA (n=523) and the total group enrolled (n=549). Because of the relatively small number of patients principal component analysis (PCA) was performed in the whole group using the admixture informative markers listed in Table S2. Results from the PCA are shown in Figure S1. Samples were identified as outliers within each of the three major subgroups if the genomic distance of any one of the five nearest neighbors within the subgroup had a Z score less than −2.326. Outliers identified were removed from further analysis. Twelve individuals were removed from the non-Hispanic Caucasian subgroup, two from the Hispanic Caucasians and three from the African Americans leaving 234, 96, and 82 individuals, respectively. For each of the three subpopulations principal components (PCs) 1 and 2 were then used to adjust for population structure in a multivariable model of association of SNPs with PARDS which also included age, gender, past medical history of asthma, and primary diagnosis of pneumonia and sepsis. A meta-analysis of the results from the association studies for each of the 3 subpopulations was performed with the computationally efficient statistical tool designed specifically for meta-analyses of genome wide association studies, METAL (30), using β-coefficients and standard errors determined from the individual association analyses. Significance was adjusted for multiple comparisons and defined as a p<1.25×10−4.
Results
There were 549 patients enrolled in BALI between August of 2009 and December of 2013; DNA and plasma samples were obtained from 480 patients with an additional 43 patients for which only DNA samples were available (Figure S2). Table 1 describes the demographic and clinical characteristics of the cohort. The demographic and clinical characteristics of those with samples are nearly identical to those of the total cohort (Table S3) and are also very similar to those reported for patients enrolled in the RESTORE clinical trial (22). A total of 69% (n=378) of the patients met the criteria for PARDS and 83% (n=312) of children with PARDS met criteria on the day of intubation (Study Day 0); another 11% (n=42) met criteria on Study Day 1 and the remaining 6% met criteria on Study Days 2–5. Although 82% of the patients with plasma samples had three consecutive samples taken approximately 24 h apart, only 13% (n=62) of the patients had samples taken on Day 0, as patients’ caregivers were only approached for consent for this study after patients were enrolled in RESTORE. Consequently, for most patients, blood sampling began on Day 1 (n=209, 44%) or Day 2 (n=155, 32%).
Table 1.
Characteristics of Patients with and without Pediatric Acute Respiratory Distress Syndrome (PARDS)
| Characteristics | All (n=549) |
No PARDS (n=171) |
PARDS (n=378) |
pa |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Female | 251 (46) | 84 (49) | 167 (44) | 0.28 |
| Non-Hispanic White | 287 (52) | 84 (49) | 203 (54) | 0.26 |
| Intervention site | 324 (60) | 105 (61) | 219 (58) | 0.46 |
| Primary diagnosis | ||||
| Pneumonia | 203 (37) | 52 (30) | 151 (40) | 0.03 |
| Bronchiolitis | 107 (20) | 37 (22) | 70 (19) | 0.39 |
| Acute respiratory failure related to sepsis | 104 (19) | 26 (15) | 78 (21) | 0.13 |
| Asthma or reactive airway disease | 56 (10) | 27 (16) | 29 (8) | 0.004 |
| Aspiration pneumonia | 34 (6) | 14 (8) | 20 (5) | 0.19 |
| Other | 45 (8) | 15 (9) | 30 (8) | 0.74 |
| Medical history of: | ||||
| Prematurity | 72 (13) | 19 (11) | 53 (14) | 0.35 |
| Asthma | 89 (16) | 36 (21) | 53 (14) | 0.04 |
| Seizure disorder | 56 (10) | 17 (10) | 39 (10) | 0.89 |
| Neurologic disorder | 45 (8) | 16 (9) | 29 (8) | 0.51 |
| Cancer | 37 (7) | 12 (7) | 25 (7) | 0.86 |
| Chromosomal abnormality | 39 (7) | 8 (5) | 31 (8) | 0.14 |
| Died | 47 (9) | 19 (11) | 28 (7) | 0.15 |
| PRISM III | 8 (4-13) | 8 (3-12) | 9 (4-14) | 0.03 |
| Age (y), median (IQR) | 3.8 (0.6-11.0) | 3.0 (0.5-10.9) | 4.0 (0.7-11.1) | 0.39 |
p value determined by Chi-square or Mann-Whitney test. PRISM= pediatric risk of mortality, y= year, IQR= interquartile range.
Plasma IL-1ra was most elevated on the day of intubation (Day 0) and decreased significantly (p<0.0001) over the subsequent three days (Figure 1). Plasma IL-1ra correlated significantly with OI on Days 0-3 (Table 2). Similarly, plasma IL-1ra correlated with the PaO2/FiO2 ratio on Days 0–2 (Table 2). IL-1ra was not associated with age, gender, race, past medical history of prematurity, immunodeficiency, cancer, aspiration, pneumonia, chromosomal abnormalities or seizures. Plasma IL-1ra was lower in patients with history of asthma (p< 0.05) and higher in patients with sepsis (p<0.001). Plasma IL-1ra was associated with PRISM-III on Days 1, 2 and 3 (correlation coefficients 0.24, 0.26, 0.28, all p< 0.001, respectively), but was not collinear.
Figure 1. Plasma interleukin-1 receptor antagonist (IL-1ra) levels decrease over time in children with acute respiratory failure.
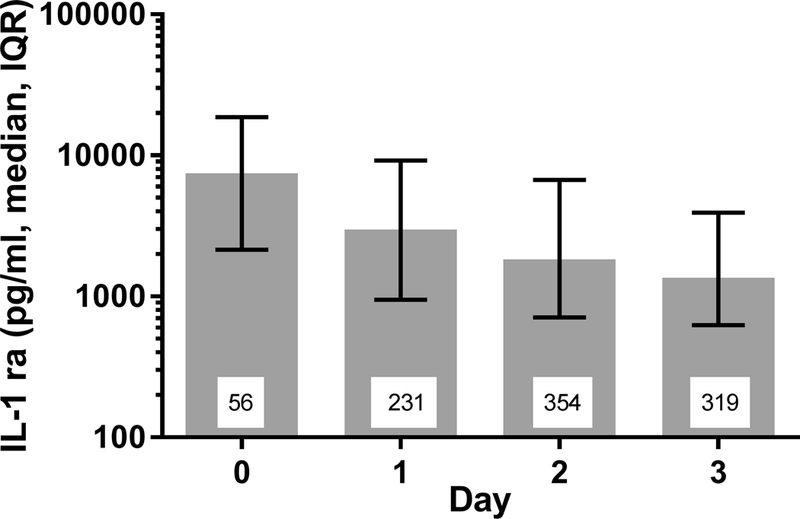
Day 0 is the day of intubation. All days are significantly different from the others (p<0.0001) as determined by linear modeling using generalized estimating equations methods as described in Methods. The number inside each bar indicates the number of plasma samples analyzed from the indicated days.
Table 2.
Plasma Interleukin-1 Receptor Antagonist Levels are Correlated with Indices of Oxygenation in Patients with Acute Respiratory Failure
| Day | N | Oxygenation Index Correlation Coefficient |
p | PaO2/FiO2 Correlation Coefficient |
p |
|---|---|---|---|---|---|
| 0 | 41 | 0.36 | 0.02 | −0.32 | 0.04 |
| 1 | 171 | 0.31 | <0.0001 | −0.21 | <0.005 |
| 2 | 249 | 0.27 | < 0.0001 | −0.16 | 0.01 |
| 3 | 215 | 0.18 | 0.008 | −0.12 | 0.07 |
N indicates the number of plasma samples analyzed from the indicated day with oxygenation index or PaO2/FiO2 available on that day.
Association of IL-1ra with the development of PARDS
The characteristics of patients with or without PARDS at any time were similar (Table 1), except that in patients with PARDS the frequency of patients with pneumonia was greater and the frequencies of patients with the primary diagnosis of asthma or with a history of asthma were lower. In addition, the severity of illness, as measured by PRISM III score, in patients with PARDS was slightly greater than in patients without PARDS. As shown in Figure 2A, the level of plasma IL-1ra was significantly greater at intubation through Day 3 in those with PARDS compared to those without PARDS (p<0.0001). Multivariable regression analysis of data across all days demonstrated a significant association of IL-1ra (OR= 1.30; 95% CI=1.10–1.52; p=0.002) and day (p<0.05) on presence of PARDS, independent of age and PRISM-III score. Analysis on each day (Table 3) indicated that on Days 0 and 2, plasma IL-1ra levels were independently associated with PARDS; however the association on Days 1 and 3 did not quite reach significance (p=0.06 and 0.07, respectively).
Figure 2. Plasma interleukin-1 receptor antagonist (IL-1ra) is higher in children with PARDS or in non-survivors.
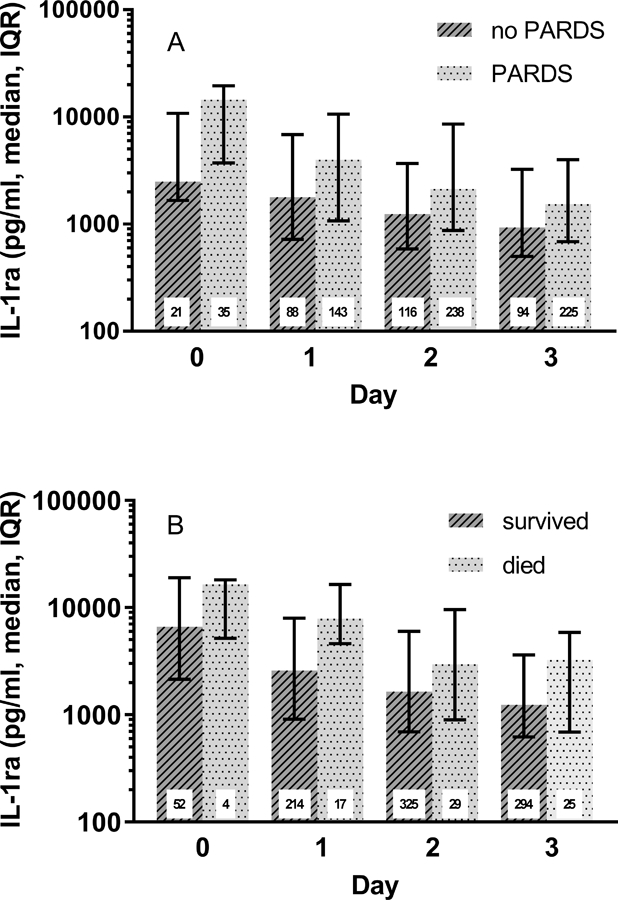
A) Plasma IL-1ra in children with or without pediatric acute respiratory distress syndrome (PARDS). If a child met the criteria for PARDS on the indicated day or any day before that they were considered to have PARDS. p<0.0001 using generalized estimating equations methods as described in Methods. B) Comparison of plasma IL-1ra in children who died or survived. Day 0 is the day of intubation. The number inside each bar indicates the number of plasma samples analyzed from the indicated days.
Table 3.
Multivariable Analysis of Association of Interleukin-1 Receptor Antagonist (IL-1ra) with Pediatric Acute Respiratory Distress Syndrome
| Variable | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Day 0 | |||
| Age | 0.96 | 0.87-1.06 | 0.43 |
| PRISM III | 1.04 | 0.96-1.12 | 0.38 |
| IL-1ra | 1.79 | 1.03-3.11 | 0.04 |
| Day 1 | |||
| Age | 1.01 | 0.96-1.06 | 0.68 |
| PRISM III | 1.02 | 0.99-1.06 | 0.23 |
| IL-1ra | 1.23 | 0.99-1.53 | 0.06 |
| Day 2 | |||
| Age | 1.00 | 0.96-1.04 | 0.92 |
| PRISM III | 1.01 | 0.98-1.05 | 0.42 |
| IL-1ra | 1.33 | 1.10-1.60 | 0.003 |
| Day 3 | |||
| Age | 1.00 | 0.96-1.04 | 0.85 |
| PRISM III | 1.02 | 0.99-1.06 | 0.27 |
| IL-1ra | 1.23 | 0.99-1.53 | 0.07 |
N for Days 0-3 is 56, 231, 354, and 319, respectively. PRISM= pediatric risk of mortality
Association of IL-1ra with other clinical outcomes in children with acute respiratory failure
Univariate analysis indicated for children with acute respiratory failure plasma IL-1ra levels on Days 0-3 were positively correlated with duration of mechanical ventilation and plasma IL-1ra levels on Days 1–3 correlated with PICU length of stay (Table S4). Multivariable analysis demonstrated that IL-1ra was associated with duration of mechanical ventilation on Days 1–3 even after adjustment for other relevant variables such as age and severity of illness (p<0.001, Days 1–3, Table 4). IL-1ra on Days 1–3 also remained associated with PICU length of stay in the multivariable analysis (p=0.009, p<0.001, p=0.002, Days 1–3, respectively, Table 5). In addition, plasma IL-1ra was elevated on all days in children with acute respiratory failure who died (Figure 2B). However, only IL-1ra on Day 1 was associated with death in both univariate (p=0.003) and multivariable regression analyses (Table S5), where plasma IL-1ra was associated with death independent of age and severity of illness (OR= 1.85, 95% CI=1.12 – 3.06; p=0.02).
Table 4.
Multivariable Analysis of Association of Interleukin-1 Receptor Antagonist (IL-1ra) with Duration of Mechanical Ventilation in Children with Acute Respiratory Failure
| Variable | Correlation Coefficient | 95% Confidence Interval | p |
|---|---|---|---|
| Day 0 | |||
| Age | −0.06 | −0.10—0.01 | 0.01 |
| PRISM III | 0.01 | −0.02 – 0.05 | 0.45 |
| IL-1ra | 0.24 | −0.09–0.57 | 0.15 |
| Day 1 | |||
| Age | 0.01 | −0.01–0.03 | 0.17 |
| PRISM III | 0.01 | −0.01–0.03 | 0.24 |
| IL-1ra | 0.18 | 0.08–0.28 | <0.001 |
| Day 2 | |||
| Age | 0.02 | 0.01–0.03 | 0.007 |
| PRISM III | 0.01 | −0.001–0.02 | 0.10 |
| IL-1ra | 0.15 | 0.09–0.21 | <0.001 |
| Day 3 | |||
| Age | 0.02 | 0.004–0.03 | 0.01 |
| PRISM III | 0.01 | −0.001–0.02 | 0.08 |
| IL-1ra | 0.13 | 0.07–0.20 | < 0.001 |
N for Days 0-3 is 56, 231, 354, and 319, respectively. PRISM= pediatric risk of mortality.
Table 5.
Multivariable Analysis of Association of Interleukin-1 Receptor Antagonist (IL-1ra) with PICU Length of Stay in Survivors
| Variable | Correlation Coefficient | 95% Confidence Interval | p |
|---|---|---|---|
| Day 0 | |||
| Age | −0.62 | −1.03—0.22 | 0.003 |
| PRISM III | 0.20 | −0.31–0.71 | 0.43 |
| IL-1ra | 0.59 | −2.31–3.48 | 0.69 |
| Day 1 | |||
| Age | 0.14 | −0.19–0.48 | 0.41 |
| PRISM III | 0.12 | −0.16–0.41 | 0.39 |
| IL-1ra | 2.26 | 0.63–3.89 | 0.007 |
| Day 2 | |||
| Age | 0.25 | −0.01–0.50 | 0.06 |
| PRISM III | 0.09 | −0.13–0.31 | 0.42 |
| IL-1ra | 2.17 | 0.88–3.46 | <0.001 |
| Day 3 | |||
| Age | 0.29 | 0.02–0.56 | 0.04 |
| PRISM III | 0.10 | −0.13–0.33 | 0.41 |
| IL-1ra | 2.59 | 1.05–4.13 | 0.001 |
N for Days 0-3 is 52, 214, 325, and 294, respectively. PICU= pediatric intensive care unit, PRISM= pediatric risk of mortality
Association of genetic variants with PARDS
Genetic variants in the gene for IL-1ra, IL1RN, as well as variants in genes whose protein products impact either IL-1β level, or response, were tested for association with PARDS. The cohort was stratified into non-Hispanic Caucasians, Hispanic Caucasians and African Americans for analyses which were done with adjustment for population structure (as described in the Supplement). None of the variants examined showed a significant association (defined as p<1.25×10−4) with PARDS within any of the subgroups or when the three subgroups were combined in the meta-analysis (n=412). However, the meta-analysis did identify a SNP, rs2287047, in the gene that encodes the IL-1 receptor 1 (IL1R1), for which the A allele came close to reaching a significant association with a decreased rate of PARDS using a recessive multivariable regression model including age, gender, history of asthma, and diagnosis of pneumonia or sepsis (p=7.4×10−4). None of the IL1RN SNPs were associated with level of plasma IL-1ra.
Discussion
The main findings of this study can be summarized as follows. IL-1ra levels are highest in children with acute respiratory failure requiring mechanical ventilation on the day of intubation. IL-1ra levels are associated with the development of PARDS independent of clinical and demographic factors, and Day 1 levels are independently associated with death. In addition, IL-1ra levels correlate with measures of oxygenation defect, days of mechanical ventilation and PICU length of stay. Genetic variants in either the gene for IL-1ra or in genes related to IL-1β level are not associated with development of PARDS.
In the cohort as a whole, plasma IL-1ra levels were highest on the day of intubation and dropped significantly over subsequent days. This observation suggests that therapies targeting inflammation for either prevention or mitigation of PARDS are likely to be most useful very early in the trajectory of illness, potentially in patients identified to be at risk for PARDS. IL-1ra levels are higher in patients with PARDS than in those without PARDS suggesting that those with higher IL-1ra levels have generated a more robust inflammatory response (though IL-1ra is an anti-inflammatory cytokine) and may be more susceptible to developing PARDS. The answer to this question will require a prospective study that enrolls patients from non-PICU environments before they develop PARDS, similar to the current, NIH-funded, adult Prevention & Early Treatment of Acute Lung Injury (PETAL) network (http://petalnet.org/). Examining the association of IL-1ra with PARDS is complicated by the fact that patients with a condition that causes an inflammatory response (i.e., infection, trauma, aspiration, sepsis) are at greater risk for the development of ARDS. However, better understanding this association is particularly important because a recent study examining the mortality benefit of treatment with recombinant IL-1ra from a clinical trial of adults with sepsis suggests that the response varies with plasma IL-1ra level with those with higher levels benefiting from treatment (31).
Plasma IL-1ra was significantly associated with oxygenation measures, length of mechanical ventilation, and PICU length of stay in the entire cohort (children with acute respiratory failure) on all days examined suggesting that IL-1ra levels are an indicator of severity of illness in mechanically ventilated children with acute airways or parenchymal lung disease. In addition, plasma IL-1ra on Day 1, but not Day 2 or 3, was independently associated with death. As expected the levels of IL-1ra on Days 2 and 3 while still high, are lower than that seen on Days 0 and 1, consistent with IL-1ra being triggered very early in the inflammatory cascade. This suggests that IL-1ra is only associated with death in samples taken early after intubation presumably early in the inflammatory response. The lack of association of IL-1ra on day of intubation with death is likely due to the paucity of plasma samples on Day 0 and the low mortality in the cohort. The association we observed between IL-1ra and oxygenation measures in children with acute respiratory failure is similar to findings in one other PARDS study where IL-1ra was also reported to be associated with oxygenation measures (32). The association of IL-1ra with death in children with acute respiratory failure contrasts with findings in children with PARDS where IL-1ra was not associated with death (32). It is unclear whether this difference is due to a difference in the cohorts, that is, children with acute respiratory failure, including those with PARDS, versus a cohort of exclusively PARDS patients, or a difference in number of patients enrolled in these studies. Interestingly plasma IL-1ra has been reported to be associated with death in a small study of adult patients at risk for ARDS [3].
There were no variants in the gene for IL-1ra, including rs315952, which were associated with risk of PARDS or IL-1ra levels. This is in contrast to a previous study in adults in which the SNP rs315952 was associated with reduced risk of ARDS and higher plasma IL-1ra levels (9). This difference may be due to the smaller numbers in our study. We did observe an association between a SNP in IL1R1, rs2287047, and protection from PARDS in the meta-analysis including non-Hispanic Caucasians, Hispanic Caucasians and African Americans, though the p value did not reach significance (p=7.4×10−4). IL1R1 encodes for the IL-1 receptor 1, which is critical for response to IL-1. rs2287047 is in a non-coding region of IL1R1 which is involved in regulating transcription in many tissues, including lung, as indicated by the presence of epigenetic modifications involved in enhanced expression on histones in this region when evaluated using HaploReg (33). In addition, rs2287047 has been reported to be an expression quantitative trait locus (eQTL) which is associated with expression of the IL-1 receptor 1 (33, 34). Future studies will be needed to determine if this variant is associated with level of IL-1R1 and/or ARDS.
This study describes not only the inflammatory milieu in mechanically ventilated children with acute respiratory failure but also the occurrence, timing and severity of PARDS in this group of children. The occurrence of PARDS was higher than expected with 69% of the cohort meeting the criteria for PARDS. This is particularly surprising as the parent RESTORE trial intended to enroll pediatric patients with acute respiratory failure and not a sicker subset with established PARDS, and children who were ventilator dependent on PICU admission were excluded. Of the 378 children with PARDS, 83% had PARDS on the day of intubation, 11% on day 1; only 6% met criteria more than 1 day after intubation. Recently a single site retrospective study reported the median time after intubation for diagnosis for PARDS in mechanically ventilated children was 0.1–0.6 days depending upon the definition used (35). Together these findings have major implications for future biomarker study design. Specifically, by the time the degree of respiratory failure is severe enough to require mechanical ventilation over half of children may already meet the diagnosis of PARDS, which is now accommodated for by the PALICC definition of PARDS. Consequently, future studies examining association of plasma biomarkers in samples taken before diagnosis of PARDS with the development of PARDS, or studies aimed at examining specific therapies for prevention of PARDS, will need to enroll patients at risk for PARDS before they require invasive mechanical ventilation.
One of the strengths of this study is that a larger number of at risk children were enrolled than any other pediatric ARDS study to date and the cohort also includes a larger number of PARDS patients than any study published thus far. To our knowledge, this is the first study demonstrating an association of IL-1ra with ARDS. The only study examining plasma IL-1ra and ARDS in adults reported no association on univariate analysis, though the study enrolled only 77 patients in total including 19 with ARDS [3]. This study is also the first to examine whether plasma IL-1ra or genetic variants in either the gene for IL-1ra or in genes related to IL-1β level or action are associated with development of PARDS.
This study has some limitations. The definition for PARDS changed during the study period. Consequently for the analysis, PARDS was defined using OI or OSI criteria as suggested in the most recent recommendation for PARDS (23). However, chest radiograph interpretation was subject to the constraints of the parent study data collection, which was limited to presence of new bilateral infiltrates on each study day. Also, while the majority of patients met the criteria for PARDS on the day of intubation (Day 0), only 13% of patients had blood drawn for plasma biomarkers on Day 0. Consequently, we were unable to determine whether IL-1ra levels are significantly higher in those destined to develop PARDS before they meet the criteria for PARDS. This is noteworthy as it limited our ability to assess whether plasma IL-1ra was higher before the onset of PARDS in patients who progressed to develop PARDS. Lastly, the lack of any significant association of genetic variants with PARDS has to be interpreted with caution as the number of patients was relatively small for a genetic association study and there are no other similar cohorts of children with acute respiratory failure with and without PARDS that can be used for validation.
Conclusions
In summary, elevated plasma levels of IL-1ra have value as a marker of early inflammatory cascade activation in children with acute respiratory failure and there is a significant relationship of plasma IL-1ra to the development of PARDS, oxygenation measures, and long-term patient outcomes. The lack of a more consistent association of plasma IL-1ra with either death or PARDS across all days may be because IL-1ra is not a specific marker of death or PARDS, because patients are at a different time points in their trajectory of illness, or because the number of samples on Day 0 is low and the samples obtained on Days 1–3 were sampled at a time that IL-1ra levels are falling. Future studies will be needed with samples obtained before PARDS develops to answer this question.
Supplementary Material
Acknowledgments
We would like to thank all the patients and guardians of those patients for their participation in the study. We would also like to acknowledge the contribution of the BALI study investigators at the sites that participated in the RESTORE study including: Scot T. Bateman (University of Massachusetts Memorial Children’s Medical Center, Worcester, MA), M. D. Berg (University of Arizona Medical Center, Tucson, AZ), Santiago Borasino (Children’s Hospital of Alabama, Birmingham, AL), G. Kris Bysani (Medical City Children’s Hospital, Dallas, TX), Allison S. Cowl (Connecticut Children’s Medical Center, Hartford, CT), Cindy Darnell Bowens (Children’s Medical Center of Dallas, Dallas, TX), E. Vincent S. Faustino (Yale-New Haven Children’s Hospital,New Haven, CT), Lori D. Fineman (University of California San Francisco Benioff Children’s Hospital at San Francisco, San Francisco, CA), A. J. Godshall (Florida Hospital for Children, Orlando, FL), Ellie Hirshberg (Primary Children’s Medical Center, Salt Lake City, UT), Aileen L. Kirby (Oregon Health & Science University Doernbecher Children’s Hospital, Portland, OR), Gwenn E. McLaughlin (Holtz Children’s Hospital, Jackson Health System, Miami,FL), Shivanand Medar (Cohen Children’s Medical Center of New York, Hyde Park, NY), Phineas P. Oren (St. Louis Children’s Hospital, St. Louis, MO), James B. Schneider (Cohen Children’s Medical Center of New York, Hyde Park, NY), Adam J. Schwarz (Children’s Hospital of Orange County, Orange, CA), Thomas P. Shanley (C. S. Mott Children’s Hospital at the University of Michigan, Ann Arbor, MI), Lauren R. Sorce (Ann & Robert H. Lurie, Children’s Hospital of Chicago, Chicago, IL), Edward J. Truemper (Children’s Hospital and Medical Center, Omaha, NE), Michele A. Vander Heyden (Children’s Hospital at Dartmouth, Dartmouth, NH), Kim Wittmayer (Advocate Hope Children’s Hospital, IL), Athena Zuppa (Children’s Hospital of Philadelphia, Philadelphia, PA) and the RESTORE data coordination center led by David Wypij, PhD (Department of Biostatistics, Harvard School of Public Health, Boston, Massachusetts; Department of Pediatrics, Harvard Medical School, Boston, Massachusetts; Department of Cardiology, Boston Children’s Hospital, Boston, Massachusetts).
Source of Funding: This study was funded by a grant from the NIH awarded to Drs. Dahmer, Flori and Quasney (R01HL095410). The parent study was supported by grants from the NIH awarded to Drs. Curley and Wypij (U01HL086622, U01 HL086649).
Footnotes
Article Tweet: Plasma IL-1ra is associated with pediatric ARDS and mortality in children with acute respiratory failure requiring mechanical ventilation.
Conflict of Interest: The authors declare that they have no conflict of interest.
Copyright form disclosure: Drs. Dahmer, Curley, Matthay, and Flori’s institution received funding from the National Heart, Lung, and Blood Institute. Drs. Dahmer, Sapru, Curley, Matthay, and Flori received support for article research from the National Institutes of Health (NIH). Dr. Matthay’s institution also received funding from the Department of Defense and Bayer Pharmaceuticals, and he received funding from Amgen (research grant), GlaxoSmithKline (research grant), Boehringer Ingelheim (consultancy), Roche-Genentec (Chair, DSMB), Qualigen (consultancy), and Cerus Therapeutics (consultancy). Dr. Flori received funding from Bode and Collins Law Firm (expert testimony) and Genentech Advisory Board (consulting). Dr. Gildengorin disclosed that she does not have any potential conflicts of interest.
References
- 1.Sapru A, Flori H, Quasney MW, et al. : Pathobiology of acute respiratory distress syndrome. Pediatr Crit Care Med 2015; 16:S41–50 [DOI] [PubMed] [Google Scholar]
- 2.Park WY, Goodman RB, Steinberg KP, et al. : Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2001; 164:1896–1903 [DOI] [PubMed] [Google Scholar]
- 3.Parsons PE, Moss M, Vannice JL, et al. : Circulating IL-1ra and IL-10 levels are increased but do not predict the development of acute respiratory distress syndrome in at-risk patients. Am J Respir Crit Care Med 1997; 155:1469–1473 [DOI] [PubMed] [Google Scholar]
- 4.Donnelly SC, Strieter RM, Reid PT, et al. : The association between mortality rates and decreased concentrations of interleukin-10 and interleukin-1 receptor antagonist in the lung fluids of patients with the adult respiratory distress syndrome. Ann Intern Med 1996; 125:191–196 [DOI] [PubMed] [Google Scholar]
- 5.Arend WP: The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev 2002; 13:323–340. [DOI] [PubMed] [Google Scholar]
- 6.Garlanda C, Dinarello CA, Mantovani A: The interleukin-1 family: back to the future. Immunity 2013; 39:1003–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garlanda C, Riva F, Bonavita E, et al. : Negative regulatory receptors of the IL-1 family. Semin Immunol 2013; 25:408–415 [DOI] [PubMed] [Google Scholar]
- 8.Palomo J, Dietrich D, Martin P, et al. : The interleukin (IL)-1 cytokine family--Balance between agonists and antagonists in inflammatory diseases. Cytokine 2015; 76:25–37 [DOI] [PubMed] [Google Scholar]
- 9.Meyer NJ, Feng R, Li M, et al. : IL1RN coding variant is associated with lower risk of acute respiratory distress syndrome and increased plasma IL-1 receptor antagonist. Am J Respir Crit Care Med 2013; 187:950–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flori HR, Glidden DV, Rutherford GW, et al. : Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med 2005; 171:995–1001 [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman JJ, Akhtar SR, Caldwell E, et al. : Incidence and outcomes of pediatric acute lung injury. Pediatrics 2009; 124:87–95 [DOI] [PubMed] [Google Scholar]
- 12.Smith LS, Zimmerman JJ, Martin TR: Mechanisms of acute respiratory distress syndrome in children and adults: a review and suggestions for future research. Pediatr Crit Care Med 2013; 14:631–643 [DOI] [PubMed] [Google Scholar]
- 13.Bortolussi R, Howlett S, Rajaraman K, et al. : Deficient priming activity of newborn cord blood-derived polymorphonuclear neutrophilic granulocytes with lipopolysaccharide and tumor necrosis factor-alpha triggered with formyl-methionyl-leucyl-phenylalanine. Pediatr Res 1993; 34:243–248 [DOI] [PubMed] [Google Scholar]
- 14.Lee SM, Suen Y, Chang L, et al. : Decreased interleukin-12 (IL-12) from activated cord versus adult peripheral blood mononuclear cells and upregulation of interferon-gamma, natural killer, and lymphokine-activated killer activity by IL-12 in cord blood mononuclear cells. Blood 1996; 88:945–954 [PubMed] [Google Scholar]
- 15.Peters AM, Bertram P, Gahr M, et al. : Reduced secretion of interleukin-1 and tumor necrosis factor-alpha by neonatal monocytes. Biol Neonate 1993; 63:157–162 [DOI] [PubMed] [Google Scholar]
- 16.Finnerty CC, Jeschke MG, Herndon DN, et al. : Temporal cytokine profiles in severely burned patients: a comparison of adults and children. Mol Med 2008; 14:553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shearer WT, Rosenblatt HM, Gelman RS, et al. : Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol 2003; 112:973–980 [DOI] [PubMed] [Google Scholar]
- 18.Shahabuddin S, Al-Ayed I, Gad El-Rab MO, et al. : Age-related changes in blood lymphocyte subsets of Saudi Arabian healthy children. Clin Diagn Lab Immunol 1998; 5:632–635 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Hartel C, Adam N, Strunk T, et al. : Cytokine responses correlate differentially with age in infancy and early childhood. Clin Exp Immunol 2005; 142:446–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Upham JW, Lee PT, Holt BJ, et al. : Development of interleukin-12-producing capacity throughout childhood. Infect Immun 2002; 70:6583–6588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood JH, Partrick DA, Johnston RB Jr.,: The inflammatory response to injury in children. Curr Opin Pediatr 2010; 22:315–320 [DOI] [PubMed] [Google Scholar]
- 22.Curley MA, Wypij D, Watson RS, et al. : Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. JAMA 2015; 313:379–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pediatric Acute Lung ICCG: Pediatric acute respiratory distress syndrome: consensus recommendations from th epediatric acute lung injury consensus conference. Ped Crit Care Med 2015; 16:428–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernard GR, Artigas A, Brigham KL, et al. : The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149:818–824 [DOI] [PubMed] [Google Scholar]
- 25.Chang CC, Chow CC, Tellier LC, et al. : Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 2015; 4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purcell S, Neale B, Todd-Brown K, et al. : PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81:559–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beaty TH, Fallin MD, Hetmanski JB, et al. : Haplotype diversity in 11 candidate genes across four populations. Genetics 2005; 171:259–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goddard KA, Hopkins PJ, Hall JM, et al. : Linkage disequilibrium and allele-frequency distributions for 114 single-nucleotide polymorphisms in five populations. Am J Hum Genet 2000; 66:216–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balding DJ: A tutorial on statistical methods for population association studies. Nat Rev Genetics 2006; 7:781–791 [DOI] [PubMed] [Google Scholar]
- 30.Willer CJ, Li Y, Abecasis GR: METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 2010; 26:2190–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer NJ, Reilly JP, Anderson BJ, et al. : Mortality Benefit of Recombinant Human Interleukin-1 Receptor Antagonist for Sepsis Varies by Initial Interleukin-1 Receptor Antagonist Plasma Concentration. Crit Care Med 2018; 46:21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zinter MS, Orwoll BE, Spicer AC, et al. : Incorporating inflammation into mortality risk in pediatric acute respiratory distress syndrome. Crit Care Med 2017; 45:858–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward LD, Kellis M: HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res 2012; 40:D930–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Consortium GT: Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 2015; 348:648–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parvathaneni K, Belani S, Leung D, et al. : Evaluating the performance of the pediatric acute lung injury consensus conference definition of acute respiratory distress syndrome. Pediatr Crit Care Med 2017; 18:17–25 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


