Abstract
Objective:
Whether older people living with HIV (PLWH) can achieve similar functional benefits with exercise as their uninfected peers and the ideal intensity of exercise needed for these benefits are not known.
Design:
Sedentary adults (50–75 years) with or without HIV were recruited for 24-weeks of supervised endurance/resistance exercise. After 12 weeks of moderate-intensity exercise, participants were randomized to continue moderate- or advance to high-intensity exercise for an additional 12 weeks.
Methods:
Outcomes by serostatus and exercise intensity (moderate, high) were compared using linear and mixed effects regression models and controlled for baseline values or week 12 values.
Results:
32 PLWH and 37 controls were enrolled; 27 PLWH (12 moderate/15 high) and 29 controls (15 moderate/14 high) completed 24 weeks. PLWH had significantly poorer physical function across nearly all baseline measures. Both groups had significant improvements in all function measures. From 0–12 weeks, PLWH had significantly greater percent improvements (mean, 95% CI) than controls on VO2 max (5 [0, 10]%); from 13–24 weeks, PLWH had significantly greater percent improvements on stair climb (−5 [−10, −1]%), and 400-MWT (−3 [−5, −0]%); all p<0.05. An interaction between exercise intensity and HIV serostatus was significant for measures of strength: PLWH randomized to high-intensity gained significantly more strength than moderate-intensity in bench and leg press (6 [0,12]% and 10 [2,17]% greater; both p<0.05); controls had similar gains regardless of intensity.
Conclusions:
Both moderate- and high-intensity exercise resulted in significant improvements in physical function; high-intensity exercise may impart greater strength benefits to PLWH.
Keywords: physical function, aging, HIV, exercise, exercise intensity, frailty
Background:
With a dramatic improvement in life expectancy, the majority of people living with HIV (PLWH) are now aged 50 years or older.[1] These older PLWH have an increased burden of age-related comorbidities, and, subsequently, impairments in physical function and the development of frailty.[2] Regular physical activity improves function in other older adult populations with multiple comorbidities and prevents functional decline in middle-aged and older adult populations. [3, 4] Similar to the general population, PLWH have high rates of sedentary behavior and low rates of regular physical activity, with a recent meta-analysis suggesting that only about one-half of PLWH meet recommended U.S. Department of Health and Human Services (DHHS) physical activity guidelines of at least 150 minutes of moderate-intensity activity weekly.[5, 6] Moreover, the lowest rate of physical activity is found in older PLWH with the greatest burden of comorbidities.[7]
Endurance or resistance exercise interventions in PLWH support the safety and efficacy of exercise to improve cardiovascular, metabolic, and functional measures in younger PLWH. [8, 9] Extrapolating from these findings and from studies of older uninfected persons, some investigators have proposed the standard DHHS exercise guidelines for older PLWH, including a combination of endurance and resistance exercise three times per week.[6, 10, 11] While meeting the standard DHHS guidelines for regular physical activity is sensible for most older adults, the added benefit or harm of high-intensity exercise in restoring and improving function in HIV has not been studied. For example, among older, frail adults, supervised high-intensity exercise appears as safe and may be more effective in improving functional and strength measures than low or moderate-intensity interventions.[12, 13]
Evaluation of high-intensity exercise in PLHW may be important, as this population appears to have greater impairment in cardiorespiratory capacity (as measured by maximal oxygen consumption, VO2 max) across the age-spectrum compared to age-matched uninfected controls,[14] with even greater impairments among older PLWH with non-AIDS comorbidities. [15]. Underlying mitochondrial and skeletal muscle impairments including impaired peripheral oxygen uptake,[16] impaired cardiac response to exercise, [17] or underlying impairments in oxygen diffusion capacity,[18] related to HIV or exposure to older antiretroviral therapy (ART), suggest that PLWH may require a different exercise dose to restore and achieve levels of cardiovascular fitness and physical function similar to HIV-uninfected peers. Conversely, these impairments may suggest greater difficulty in achieving or lower response to high compared to moderate-intensity exercise in older PLWH. To best inform the dose of exercise needed for maximal benefit in older PLWH, the goals of this study were to determine the impact of exercise on physical function and compare changes in physical function in response to two levels of exercise intensity among sedentary, older adults living with or without HIV infection.
Methods:
The Exercise for Healthy Aging Study enrolled PLWH and HIV-uninfected controls from April 2014 to May 2017 (Clinical Trials NCT02404792). All participants were aged 50 to 75 years, sedentary (<60 minutes of physical activity each week for 6 months preceding by self-report), had a body mass index (BMI) between 20 and 40 kg/m2, and had no contraindications to initiating an exercise regimen (e.g., severe mobility limitation, unstable angina, supplemental oxygen requirement, uncontrolled hypertension). Participants with diabetes had hemoglobin A1c of 7.5% or less; sex hormone supplementation was restricted to stable, physiologic doses for ≥3 months prior to study entry and intramuscular testosterone was excluded. PLWH were on stable ART with an undetectable HIV-1 RNA for >2 years and a CD4 T-cell count >200 cells/μL. The study procedures were approved by the Colorado Multiple Institutional Review Board. Written informed consent was obtained from all participants.
Study Procedures
All participants underwent a graded, treadmill, cardiovascular stress test and measurement of VO2. During an initial 5-min warm-up, walking speed was adjusted to elicit a heart rate (HR) ~70% of age-predicated maximum HR. Speed was held constant and the grade of the treadmill was increased by 2% every 2 minutes until volitional exhaustion or until the test was stopped. Testing was terminated if any of the American College of Sports Medicine absolute (and, in some cases, relative) stopping criteria were met.[11] The O2 and CO2 content of expired air were measured continuously by open circuit spirometry and averaged every 30 seconds using an automated online system (TrueMax 2400; ParvoMedics, Sandy, UT). Objective evidence that VO2 max was attained included at least 2 of the following: a plateau in VO2 despite an increased energy demand, a respiratory exchange ratio in excess of 1.10, and a maximal HR within 10 beats of the age-predicted value. In the absence of these benchmarks, the maximum measured VO2 was deemed VO2 peak. All participants had to reach 85% of age-predicted maximum HR for cardiac risk assessment prior to proceeding to the exercise intervention. Abnormal test results required further evaluation and risk assessment per a cardiologist’s recommendation prior to proceeding. Muscle strength was evaluated as the maximal weight that can be lifted only 1 time using correct lifting form through the full range of motion (1-repetition maximum, 1-RM).
Exercise intervention:
Each participant attended supervised exercise sessions 3 times/week for 24 weeks at the University of Colorado-Anschutz Medical Campus Exercise Research Laboratory. Participants began with a 2-week supervised, low-intensity exercise acclimation for machine familiarization consisting of 20–30 minutes of treadmill walking at 30–40% of VO2 max and 3 sets of 8 repetitions of 4 weight-assisted machine (Cybex) exercises (bench press, leg press, lateral pulldown, and a rotating 4th exercise) at low-intensity (40–50% of the 1-RM). After 2 weeks, participants increased cardiovascular endurance exercise intensity to 40–50% VO2 max and time by 5 minutes every week to achieve a goal of 50 minutes/session by the end of 12 weeks. Resistance exercise was increased to 60–70% of 1-RM; 1-RM was reassessed every 3 weeks and target weight loads adjusted as needed. At week 12, VO2 max measurements were repeated and participants were randomized to either continue moderate-intensity exercise or advance to high-intensity (60–70% of week 13 VO2 max and >80% 1-RM) for the remaining 12 weeks. Adherence to the intervention was calculated as the number of attended exercise sessions divided by the number of expected exercise sessions.
Outcome Measures:
We used a modified version of the Short Physical Performance Battery (mSPPB)[19] that improves discrimination of physical function at the higher end of the functional spectrum by using 10 repeat chair stands (increase from 5), a 4-m timed walk (usual speed), and a standing balance test for 30 seconds with a single leg stand. Split times for the chair stand and standing balance test were obtained for calculation of the standard SPPB score. Additional functional outcomes were the time to complete a 400-m walk (400 MWT) and time to climb a flight of 10 stairs. Frailty was assessed using criteria established by Fried, et al.[20] Weakness was assessed by the average of three dominant hand grip strength measurements and defined by applying previously-defined gender and body mass index (BMI) cutoffs using a Jamar dynamometer.[20] Slowness was defined by the average of 2 readings on a 4-m walk: men ≤173 cm and women ≤159 cm in height requiring ≥6.22 seconds or men >173 cm and women >159 cm requiring ≥5.33 seconds to complete the walk met the criterion for slowness.[20] Weight loss at study entry was defined by self-report of an unintentional weight loss of ≥10 pounds during the past year. Low activity was defined as being “limited a lot” in response to the Short Form (SF)-36 question, “does your health limit you in vigorous activities such as running, lifting heavy objects, or participating in strenuous sports?” Exhaustion was defined as experiencing at least 3–4 times per week feeling that “everything I do is an effort” or “sometimes I just cannot get going” from the Center for Epidemiologic Studies Depression scale. Participants were considered non-frail if they met 0 components, pre-frail if 1 or 2 components, and frail if 3–5 components were met. Repeat chair stands were measured every 2 weeks and 1-RM tests every 4 weeks. All other physical function measures and questionnaires were obtained prior to initiating exercise, at week 12, and at week 24 on a non-exercise day or prior to an exercise session.
Anticipated and unanticipated events were categorized as definitely, probably, possibly, or not related to the exercise intervention.
Statistical analyses
Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Colorado[21]. The primary outcome was change from baseline in 10-time chair rise time. Additional outcomes, including differences by exercise intensity, were considered secondary. The randomization was balanced by HIV serostatus, gender, and age. Log transformations were performed due to right skew, as appropriate, and results reported as geometric means. Baseline measures were compared via t- and chi-square tests.
Primary predictors included HIV serostatus and randomized intensity. All linear and mixed models adjusted for baseline (or week 12). Adjusted models also included both age and BMI (selected a priori), and interactions between HIV serostatus, randomized intensity, and time, as appropriate. Kaplan-Meier estimation was utilized for time to event outcomes. Two-sided tests are reported assuming a 0.05 significance level. Outcomes were considered complementary and reported without adjustment for multiple comparisons.[22, 23] All relevant analyses are reported to allow a post-hoc adjustment. Analyses utilized R (v. 3.4.2) and SAS (v.9.4).
Results:
A total of 89 PLWH and HIV-uninfected controls underwent screening and 69 participants began exercise (32 PLWH, 37 HIV-uninfected controls; Figure 1). Baseline demographic characteristics participants are shown in Table 1.
Figure 1.
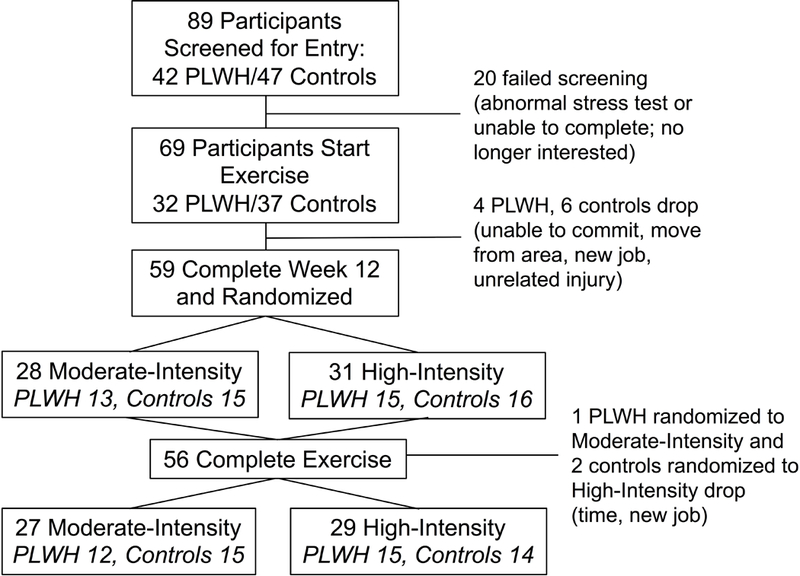
Study enrollment
Table 1:
Baseline Characteristics between People Living with HIV (PLWH) and Controls
| PLWH (n=32) | Controls (n=37) | P value | |
|---|---|---|---|
| Age (mean*, CI) | 56.6 (54.6, 58.6) | 58.3 (56.1, 60.6) | 0.25 |
| Men (%) | 28 (87.5) | 35 (94.6) | 0.54 |
| Race | 0.09 | ||
| Black | 9 (28) | 3 (8) | |
| White | 20 (63) | 31 (84) | |
| More than 1 race or other | 3 (9) | 3 (8) | |
| Hispanic/Latino | 4 (13) | 4 (11) | 1.0 |
| BMI (mean, SD) | 27.3 (4.3) | 29.9 (5.0) | 0.02 |
| Current smoker (%) | 5 (16) | 4 (11) | 0.72 |
| More than 2 alcohol drinks/d | 0 | 4 (11) | 0.12 |
| Illicit drug use in the past 2 years | 6 (19) | 0 | 0.008 |
| Sexual preference | <0.001 | ||
| Male, prefers sex with women | 1 (3) | 27 (73) | |
| Male, prefers sex with men | 25 (78) | 6 (16) | |
| Male, sex with women and men | 1 (3) | 1 (3) | |
| Marijuana use | 19 (59) | 5 (14) | <0.001 |
| Employment | <0.001 | ||
| Full/part-time | 8 (25) | 26 (70) | |
| Retired | 5 (16) | 9 (24) | |
| Disabled/unemployed | 19 (59) | 2 (6) | |
| High school education or less | 10 (31) | 4 (11) | 0.07 |
| Comorbidities | |||
| Hypertension | 17 (53) | 16 (43) | 0.56 |
| Hyperlipidemia | 15 (47) | 22 (60) | 0.42 |
| Diabetes | 2 (6) | 4 (11) | 0.68 |
| Cardiovascular disease | 1 (3) | 2 (6) | 1.0 |
| Depression, anxiety, or bipolar disorder | 17 (49) | 9 (26) | 0.03 |
| ≥3 comorbidities | 23 (72) | 19 (51) | 0.14 |
| Veterans Aging Cohort Study Index, % ≥ 20 points | 18 (56) | 9 (24) | 0.01 |
| Montreal Cognitive Assessment score | 25.25 (2.54) | 26.62 (2.49) | 0.03 |
| Current use of testosterone | 6 (19) | 1 (3) | 0.04 |
| Current use of statin | 13 (41) | 15 (41) | 1.0 |
| Integrase inhibitor-containing regimen | 21 (66) | ||
| Protease inhibitor-containing regimen | 13 (41) | ||
| NNRTI-containing regimen | 9 (28) | ||
| Any prior zidovudine treatment | 12 (38) | ||
| Any prior stavudine treatment | 14 (44) | ||
| Current CD4 count (mean*, CI) | 599 (512,699) | ||
| Time since HIV diagnosis (mean*, CI) | 18.9 (15.8, 22.7) | ||
| Estimated duration continuous ART | 11.1 (8.4, 14.6) |
geometric mean; NNRTI, non-nucleoside reverse transcriptase inhibitor
A majority of participants had 12 of 12 possible points on the SPPB (66% PLWH, 84% controls); 3 PLWH and 1 control had an SPPB score of 10 or less. While no participants met criteria for frailty prior to exercise, 75% of PLWH (N=24) and 49% of controls (N=18) were pre-frail. Weakness was the most common frailty criterion met (10 PLWH, 9 controls) followed by exhaustion (9 PLWH, 3 controls) and low activity (6 PLWH, 8 controls). Compared to controls, PLWH had significantly poorer performance on time to rise from a chair 10 times, 400-MWT, stair climb, bench press, leg press, and lateral pull-down (Table 2).
Table 2.
Baseline Measures of Physical Function
| Outcome | PLWH (n=32) | Controls (n=37) | P-Value |
|---|---|---|---|
| Frailty | 0.047 | ||
| Non-frail | 8 (25) | 19 (51) | |
| Pre-frail | 24 (75) | 18 (49) | |
| Short Physical Performance Battery Score | 0.14 | ||
| Score = 12 (no impairment) | 21 (66) | 31 (84) | |
| Score <12 | 11 (34) | 6 (16) | |
| Chair Rise (seconds) | 19.6 (17.8, 21.6) | 17.2 (15.8, 18.7) | 0.042 |
| 400-m walk (seconds) | 251.3 (241.2, 261.8) | 230.5 (220.6, 240.9) | 0.005 |
| Stair climb (seconds) | 4 (3.7, 4.3) | 3.5 (3.3, 3.7) | 0.006 |
| Grip strength (kg) | 32.4 (29.2, 36.1) | 34.2 (31.1, 37.6) | 0.45 |
| Bench press (kg) | 47.7 (42.2, 53.8) | 57.2 (51.4, 63.6) | 0.02 |
| Leg press (kg) | 122.7 (110.0, 136.9) | 146.3 (134.5, 159.2) | 0.012 |
| Lateral pull-down (kg) | 71.1 (66.4, 76.1) | 82.0 (76.8, 87.4) | 0.003 |
| VO2 max (mL/kg/min) | 25.2 (22.9, 27.8) | 26.3 (24.3, 28.5) | 0.49 |
Physical Function Changes by HIV Serostatus
Significant improvements in physical function were seen between weeks 0–12 and between weeks 13–24 in nearly all physical function measures, regardless of serostatus group (Figure 2A, Supplemental Table 1). Compared to controls, PLWH had significantly greater percent improvements in VO2 max between weeks 0–12. From weeks 13–24, PLWH (moderate and high-intensity combined) had significantly greater percent improvement in 400-MWT and stair climb compared to controls. Further adjustment for age and BMI yielded similar results with the exception that the differences in VO2 max and 400-MWT by HIV serostatus no longer reached statistical significance (p=0.06 and 0.08, respectively; Supplemental Table 2).
Figure 2.
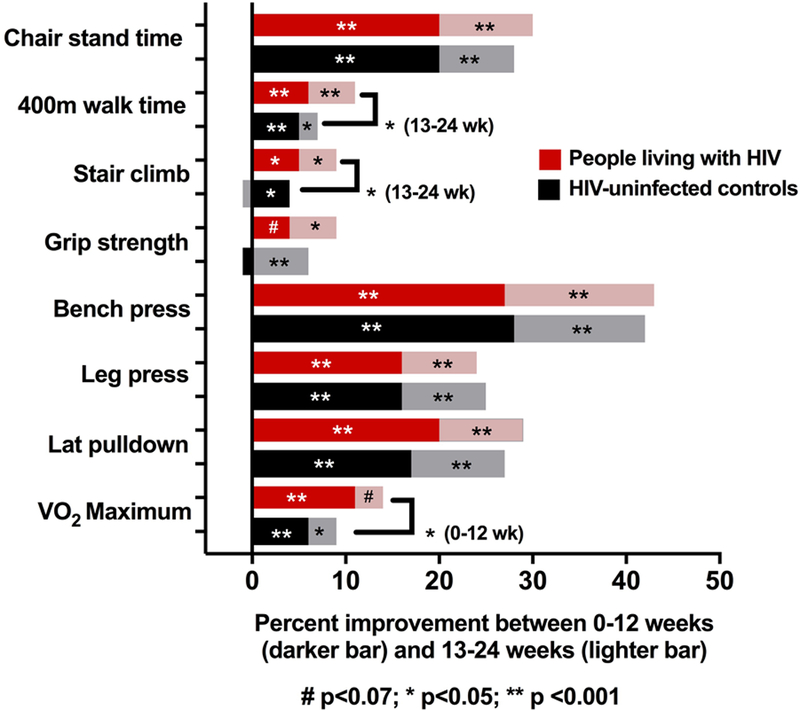
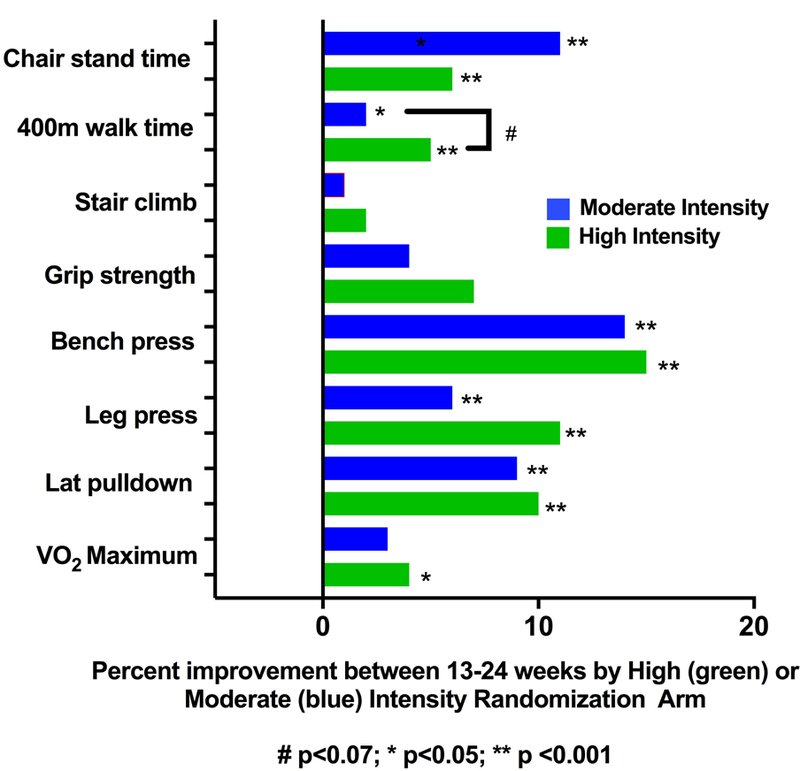
a) Percent Improvement in Functional Measures with 12 and 24 Weeks of Cardiovascular and Resistance Exercise (Moderate/High Intensity Combined). b) Percent Improvement in Functional Measures Between Weeks 13–24 with High or Moderate Intensity (PLWH/Controls Combined)
Between baseline and week 24, 8 PLWH and 2 controls had an improvement in SPPB score and no participants had a decline in SPPB score. Following 24 weeks of exercise, 13 PLHW and 7 controls had fewer frailty criteria, and 3 PLWH and 5 controls had more frailty criteria than at baseline.
Physical Function Changes by Exercise Intensity
Twenty-eight (13 PLWH, 15 controls) participants were randomized to continue moderate-intensity exercise and 31 (15 PLWH, 16 controls) to advance to high-intensity exercise. In secondary analyses, both moderate- and high-intensity exercise groups experienced significant improvements between weeks 13–24 on 1-RM measures, 400-MWT, and chair stand time (Figure 2B). The high-intensity group tended to have greater percent improvements in most functional outcomes; the only significant diffference between these groups at 24 weeks was a faster 400-MWT among high-intensity exercisers. Similar results were seen in analyses further adjusted for age and BMI except the difference in 400-MTW no longer reached statistical significance (p=0.06; Supplemental Table 2).
Among high-intensity exercisers from week 13 to 24: 5 had an improvement in frailty score and none worsened; 2 had an improvement in SPPB score and 2 had a decline. In contrast, 1 moderate intensity exerciser had fewer frailty criteria while 4 met more frailty criteria; no moderate intensity exercisers had an improvement in SPPB score and 1 declined.
Changes in Physical Function by HIV Serostatus and Exercise Intensity
In exploratory analyses, we assessed whether the effects of exercise intensity differed by HIV serostatus in measures repeated every 2–4 weeks (strength and chair rise) and outcomes assessed at weeks 0, 12, and 24 (VO2 max, grip strength, stair climb, 400-MWT). The effect of the exercise intensity differed by HIV status in bench (p=0.011) and leg press (p= 0.033), with a trend towards significant difference in lateral pulldown (p=0.056), Figure 3, Supplemental Table 3. Among PLWH, high-intensity exercisers had significantly greater gains than moderate-intensity exercisers in bench and leg press strength (p=0.049 and 0.016, respectively). No significant differences in exercise intensity were detected among controls (all p ≥ 0.11). Among PLWH, both moderate and high-intensity groups had significant improvements in chair rise time but the change did not differ by exercise intensity (p= 0.32). In functional outcomes assessed only at weeks 0, 12, and 24, no significant interactions between HIV and exercise intensity were detected (Figure 3) but, for most outcomes, greater percent improvements were found in PLWH who exercised at higher intensity compared to moderate intensity.
Figure 3.
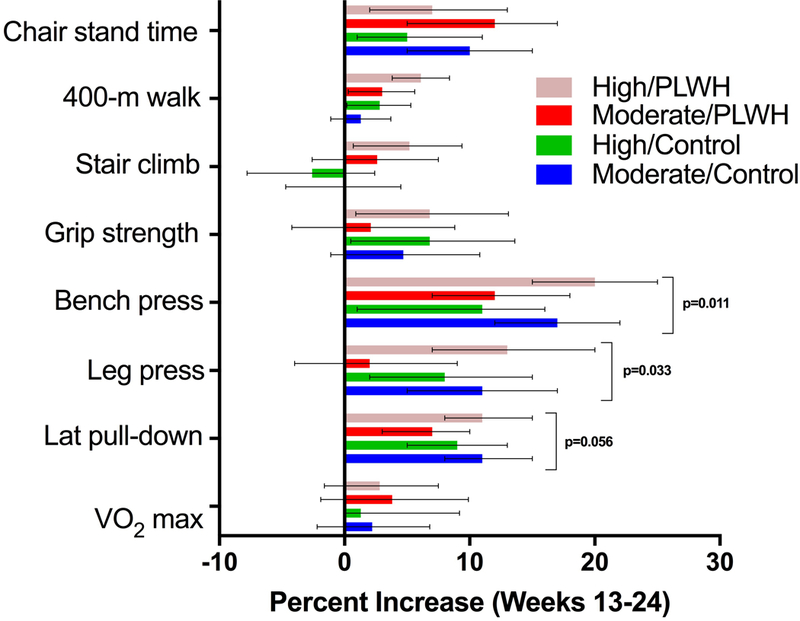
Interactions between Exercise Intensity and HIV Serostatus
The number of pre-frail participants decreased from week 12 to 24 among PLWH randomized to moderate (5 vs 4) and high-intensity (11 vs 8) exercise, and controls randomized to high-intensity (6 vs 5) exercise, but increased among moderate-intensity control exercisers (5 to 7).
Exercise Adherence
Ten (4 PLWH, 6 controls) participants withdrew from the study before 12 weeks, and 3 (1 PLWH, 2 controls) between weeks 13 and 24. For participants completing at least 1 exercise session, the median adherence during the first 12 weeks was 91.7% (IQR 86.1, 94.4%) among PLWH and 88.9% (83.3, 94.4) among controls. For participants completing at least 1 exercise session after 12 weeks, adherence from weeks 13–24 was 88.9% (73.6, 88.9%) among PLWH and 77.8% (73.6, 88.9%) among controls. Adherence was similar between the high-intensity (83.3 [77.1, 88.9%] for weeks 13–24) and moderate-intensity arms (83.3 [68.1, 88.9%]). Overall (week 0–24), the adherence was highest among PLWH randomized to moderate-intensity (90.3%) and lowest among controls randomized to high-intensity (82.7%); adherence was 87.5% in PLWH randomized to high-intensity and controls to moderate-intensity exercise.
Adverse Events
From baseline to week 12, PLWH reported 12 related events (definitely, probably, or possibly) to the intervention, and 5 events that were unrelated; controls reported 10 related and 2 unrelated events. Events between weeks 1–12 included musculoskeletal (16), followed by dizziness (3), hypotension (1), shortness of breath (1), and hernia (1). Between weeks 13–24, PLWH reported 13 related and 3 unrelated events; controls reported 2 related and 1 unrelated event. Events were most commonly musculoskeletal (13). Time to the first adverse event did not differ significantly by HIV serostatus (baseline to week 12; p=0.38), by exercise intensity (week 13–24; p=0.62), or by frailty group (baseline to week 12; p=0.22 and week 13–24; p=0.43).
Discussion:
Regular physical activity is recommended for all older adults for the well-described benefits on cardiovascular risk, obesity, diabetes, cancer, neurocognition, and physical function. Even among older adults with impaired physical function, moderate intensity exercise has been shown to delay the onset or reverse disability, as recently demonstrated in the large LIFE Trial. [24] As disability is increasingly more difficult to reverse with increasing impairment, returning older adults to the “normal” functional curve is key to maintaining function. We have shown that among older, sedentary PLWH, greater impairments in many measures of baseline physical function can significantly improve with 6 months of endurance and resistance exercise. This is one of few studies to compare functional outcomes from the same exercise protocol in older adults living with HIV and uninfected controls of similar ages. Lastly, the comparisons of two levels of exercise intensity in a randomized clinical trial provide data to formulate exercise recommendations and guidelines for older adults living with HIV.
Although low- or moderate-intensity exercise may have greater long-term adherence and patient acceptability, we hypothesized that a higher intensity exercise intervention would result in greater improvements in physical function within 6 months, delaying the onset of subsequent disability. Indeed, we found that both moderate- and high-intensity exercise resulted in significant improvements in nearly all physical function outcomes, including improvements in SPPB components, decrease in the proportion of pre-frail participants, and reduction in the number of frailty criteria. Not only were our participants adherent to the exercise regimen regardless of the intensity, PLWH randomized to higher intensity experienced significantly greater percent improvement in measures of strength compared to those randomized to moderate-intensity, an intensity-effect not observed among controls. Although we were underpowered to detect an interaction between HIV serostatus and exercise intensity, we did detect a trend towards greater gains in response to high-intensity exercise in PLWH.
To the best of our knowledge, no studies in the current ART era have compared the effects of exercise intensity on muscle strength, cardiovascular endurance, or physical function outcomes. Three pre- or early ART era exercise interventions suggested a dose-response of exercise intensity on outcomes of strength and endurance,[17, 25, 26] although studies were small with poor retention, and participants were younger, many with AIDS, and most with either no ART or with zidovudine-based regimens.
Most exercise interventions in PLWH have focused on strength and endurance outcomes among younger PLWH,[27] with few studies representative of the now majority of PLWH aged 50 and older experiencing multimorbidity and impairments in physical function. Recent Cochrane reviews of endurance and resistance exercise interventions among PLWH [8, 9] have highlighted a focus on strength, endurance, body composition and immune function, rather than physical function outcomes, with studies primarily focused on younger persons living with HIV. In one of the only other studies focused on older adults,[28] a 12-month supervised, twice weekly resistance exercise intervention among adults 60 years or older living with or without HIV allowed self-selection of either light, moderate, or heavy intensity (with 12, 10, or 8 repetitions per exercise, respectively). Both PLWH and controls had improvements in many functional measures. No comparison of the exercise intensity was provided.[28] A multidimensional 10-week center-based physiotherapy program that included moderate to vigorous endurance and resistance exercise, stretching, and goal setting, found significantly improved physical function among 37 middle and older-aged PLWH (mean age 51), but this study did not have an uninfected control group.[29]
Lower intensity or home-based interventions are often seen as more feasible interventions for larger-scale implementation. A physical activity counseling intervention combined with an exercise prescription among PLWH aged 45 or older led to a 15% greater improvement in chair rise time and 5% greater improvement in 6-minute walk distance compared to a non-exercising control group.[30] In comparison, our moderate to high intensity exercise intervention led to an overall 30% improvement in chair rise time and 11% improvement in a comparable 400-MWT. In summary, low or moderate-intensity exercise interventions can lead to improvements in function, but results may be less pronounced than those seen with high-intensity exercise.
Whether the benefits of exercise among older, sedentary PLWH with prolonged ART exposure differ from the exercise effects among uninfected controls is not well understood or studied. Impairment in exercise capacity despite similar age and physical activity,[14] and impairments in mitochondrial function may mitigate the benefits of exercise when compared to uninfected controls.[31] Furthermore, underlying fatigue, neuropathy, substance abuse, and mood disorders that tend to be more prevalent among HIV-infected participants (as we saw) may limit the ability to achieve similar gains as uninfected controls in response to a standardized intervention. However, PLWH in our study achieved greater gains in physical function across multiple parameters, suggesting that HIV serostatus does not attenuate the effects of exercise on physical function.
Several strengths of our study should be highlighted. We included an uninfected control group of similar age, and a cohort of both PLWH and controls representative of a community-dwelling population in Colorado in regards to diversity and substance use. Nearly 90% of our participants completed the intervention, exercise adherence was similar in PLWH and controls, and the supervised center-based intervention provided greater standardization than unsupervised (e.g., home-based) interventions. Per DHHS guidelines, our intervention included both endurance and resistance exercise, but was limited to three days/week, a feasible exercise goal for many older adults. Due to the intensive, center-based intervention, our recruitment was biased towards individuals that worked or lived near the research center and were able to commit to 6 months of exercise. These factors, in addition to limited hours of the research center and the targeted BMI range, may have limited the number of women we were able to enroll. Lastly, as highlighted on Table 1, our PLWH differed from controls by many demographic characteristics and comorbidities. Due to our sample size, we were unable to account for many of these factors that may have also contributed to the exercise response (i.e., attenuated responses among smokers).
In summary, among sedentary, older adults, the effects of exercise on physical function are not attenuated among PLWH. Both moderate- and high-intensity exercise result in significant improvements in physical function, endurance, and strength, and may delay the onset of disability and frailty. We found no reason to dissuade older PLWH from progressing to higher-intensity exercise following several weeks of moderate-intensity training. Adherence to at least the recommended 150 minutes/week of moderate-intensity or 75 minutes/week of high-intensity endurance exercise, and regular resistance exercise, should be encouraged for all older PLWH.
Supplementary Material
Acknowledgments
Funding:
This work was supported by the Gilead Sciences Research Scholars Program in HIV (to KME) the National Institute of Aging of the National Institutes of Health [K23AG050260] to KME, the National Institute of Allergy and Infectious Diseases [K24 AI120834] to TTB and NCATS Colorado CTSA Grant Number UL1TR002535. The funding sources had no a role in data collection, analysis, or interpretation; trial design; or patient recruitment. No payments were made in the writing of this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the U.S. Department of Veterans Affairs or the United States Government.
Conflicts of Interest:
KME has received research funding to the University of Colorado from Gilead Sciences and Merck, and has consulted for EMD Serono, Theratechnologies, and Gilead Sciences. TTB has served as a consultant to Gilead Sciences, Merck, Theratechnologies, and EMD-Serono,
References
- 1.Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, Sighem A, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 2015; 15(7):810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piggott DA, Erlandson KM, Yarasheski KE. Frailty in HIV: Epidemiology, Biology, Measurement, Interventions, and Research Needs. Curr HIV/AIDS Rep 2016; 13(6):340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc 2009; 41(7):1510–1530. [DOI] [PubMed] [Google Scholar]
- 4.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011; 43(7):1334–1359. [DOI] [PubMed] [Google Scholar]
- 5.Vancampfort D, Mugisha J, De Hert M, Probst M, Firth J, Gorczynski P, et al. Global physical activity levels among people living with HIV: a systematic review and meta-analysis. Disabil Rehabil 2018; 40(4):388–397. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans U.S. Department of Health and Human Services; 2008. ODPHP Publication No. U0036. Available at: http://www.health.gov/paguidelines 7/11/2018. [Google Scholar]
- 7.Vancampfort D, Mugisha J, Richards J, De Hert M, Probst M, Stubbs B. Physical activity correlates in people living with HIV/AIDS: a systematic review of 45 studies. Disabil Rehabil 2017:1–12. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien KK, Tynan AM, Nixon SA, Glazier RH. Effectiveness of aerobic exercise for adults living with HIV: systematic review and meta-analysis using the Cochrane Collaboration protocol. BMC Infect Dis 2016; 16:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Brien KK, Tynan AM, Nixon SA, Glazier RH. Effectiveness of Progressive Resistive Exercise (PRE) in the context of HIV: systematic review and meta-analysis using the Cochrane Collaboration protocol. BMC Infect Dis 2017; 17(1):268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yahiaoui A, McGough EL, Voss JG. Development of evidence-based exercise recommendations for older HIV-infected patients. J Assoc Nurses AIDS Care 2012; 23(3):204–219. [DOI] [PubMed] [Google Scholar]
- 11.American College of Sports Medicine, Riebe D, Ehrman JK, Liguori G, Magal M ACSM’s guidelines for exercise testing and prescription. Tenth edition ed. Philadelphia: Wolters Kluwer; 2018. [Google Scholar]
- 12.Theou O, Stathokostas L, Roland KP, Jakobi JM, Patterson C, Vandervoort AA, et al. The effectiveness of exercise interventions for the management of frailty: a systematic review. J Aging Res 2011; 2011:569194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan DH, Roberson PK, Smith ES, Price JA, Bopp MM. Effects of muscle strength training and megestrol acetate on strength, muscle mass, and function in frail older people. J Am Geriatr Soc 2007; 55(1):20–28. [DOI] [PubMed] [Google Scholar]
- 14.Oursler KK, Sorkin JD, Smith BA, Katzel LI. Reduced aerobic capacity and physical functioning in older HIV-infected men. AIDS Res Hum Retroviruses 2006; 22(11):1113–1121. [DOI] [PubMed] [Google Scholar]
- 15.Oursler KK, Katzel LI, Smith BA, Scott WB, Russ DW, Sorkin JD. Prediction of cardiorespiratory fitness in older men infected with the human immunodeficiency virus: clinical factors and value of the six-minute walk distance. Journal of the American Geriatrics Society 2009; 57(11):2055–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cade WT, Fantry LE, Nabar SR, Keyser RE. Decreased peak arteriovenous oxygen difference during treadmill exercise testing in individuals infected with the human immunodeficiency virus. Arch Phys Med Rehabil 2003; 84(11):1595–1603. [DOI] [PubMed] [Google Scholar]
- 17.Stringer WW, Berezovskaya M, O’Brien WA, Beck CK, Casaburi R. The effect of exercise training on aerobic fitness, immune indices, and quality of life in HIV+ patients. Med Sci Sports Exerc 1998; 30(1):11–16. [DOI] [PubMed] [Google Scholar]
- 18.Kristoffersen US, Lebech AM, Mortensen J, Gerstoft J, Gutte H, Kjaer A. Changes in lung function of HIV-infected patients: a 4.5-year follow-up study. Clin Physiol Funct Imaging 2012; 32(4):288–295. [DOI] [PubMed] [Google Scholar]
- 19.Simonsick EM, Newman AB, Nevitt MC, Kritchevsky SB, Ferrucci L, Guralnik JM, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci 2001; 56(10):M644–649. [DOI] [PubMed] [Google Scholar]
- 20.Newman AB, Gottdiener JS, McBurnie MA, Hirsch CH, Kop WJ, Tracy R, et al. Associations of subclinical cardiovascular disease with frailty. The journals of gerontology Series A, Biological sciences and medical sciences 2001; 56(3):M158–166. [DOI] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990; 1(1):43–46. [PubMed] [Google Scholar]
- 23.Saville DJ. Multiple Comparison Procedures: The Practical Solution. The American Statistician 1990; 44(2):174–180. [Google Scholar]
- 24.Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 2014; 311(23):2387–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terry L, Sprinz E, Ribeiro JP. Moderate and high intensity exercise training in HIV-1 seropositive individuals: a randomized trial. Int J Sports Med 1999; 20(2):142–146. [DOI] [PubMed] [Google Scholar]
- 26.MacArthur RD, Levine SD, Birk TJ. Supervised exercise training improves cardiopulmonary fitness in HIV-infected persons. Med Sci Sports Exerc 1993; 25(6):684–688. [PubMed] [Google Scholar]
- 27.Gomes Neto M, Conceicao CS, Oliveira Carvalho V, Brites C. Effects of Combined Aerobic and Resistance Exercise on Exercise Capacity, Muscle Strength and Quality of Life in HIV-Infected Patients: A Systematic Review and Meta-Analysis. PLoS One 2015; 10(9):e0138066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Souza PM, Jacob-Filho W, Santarem JM, Zomignan AA, Burattini MN. Effect of progressive resistance exercise on strength evolution of elderly patients living with HIV compared to healthy controls. Clinics (Sao Paulo) 2011; 66(2):261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown D, Claffey A, Harding R. Evaluation of a physiotherapy-led group rehabilitation intervention for adults living with HIV: referrals, adherence and outcomes. AIDS Care 2016; 28(12):1495–1505. [DOI] [PubMed] [Google Scholar]
- 30.Shah KN, Majeed Z, Yoruk YB, Yang H, Hilton TN, McMahon JM, et al. Enhancing physical function in HIV-infected older adults: A randomized controlled clinical trial. Health Psychol 2016; 35(6):563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ortmeyer HK, Ryan AS, Hafer-Macko C, Oursler KK. Skeletal muscle cellular metabolism in older HIV-infected men. Physiol Rep 2016; 4(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


