Abstract
Objective:
Among people with HIV, there are few long-term studies of non-invasive ultrasound-based measurements of the carotid artery predicting major health events. We hypothesized that such measurements are associated with 10-year mortality in the Women’s Interagency HIV Study (WIHS) and Multicenter AIDS Cohort Study (MACS), and that associations differ by HIV serostatus.
Design:
Nested cohort study.
Methods:
Participants without coronary heart disease underwent B-mode carotid artery ultrasound, with measurement of common carotid artery intima-media thickness (CCA-IMT); carotid artery plaque (focal IMT>1.5 mm) at six locations; and Young’s modulus of elasticity, a measure of arterial stiffness. We examined all-cause mortality using Cox models, controlling for demographic, behavioral, cardiometabolic, and HIV-related factors.
Results:
Among 1,722 women (median age 40 years, 90% non-white, 71% HIV-positive) and 1,304 men (median age 50, 39% non-white, 62% HIV-positive), 11% died during follow-up. Mortality was higher among HIV-positive women (19.9 deaths/1,000 person-years, 95% CI 14.7–28.8) than HIV-positive men (15.1/1,000, 95% CI 8.3–26.8). In adjusted analyses, plaque was associated with mortality (HR 1.44, 95% CI 1.10–1.88) regardless of HIV serostatus, and varied by sex (among women, HR 1.06, 95% CI 0.74–1.52; among men; HR 2.19, 95% CI 1.41–3.43). The association of plaque with mortality was more pronounced among HIV-negative (HR 3.87, 95% 1.95–7.66) than HIV-positive participants (HR 1.35, 95% CI 1.00–1.84). Arterial stiffness was also associated with mortality (HR 1.43 for highest versus lowest quartile, 95% CI 1.02–2.01). Greater CCA-IMT was not associated with mortality.
Conclusions:
Carotid artery plaque was predictive of mortality, with differences observed by sex and HIV serostatus.
Keywords: HIV, atherosclerosis, intima-media thickness, plaque, arterial stiffness, mortality
INTRODUCTION
B-mode ultrasound-based measures of the carotid artery predict future risk of cardiovascular disease (CVD) and all-cause mortality in the general population.[1, 2] Carotid artery ultrasound can be used to image non-invasively various stages of arteriosclerosis, including arterial stiffening, wall thickening, plaque formation, and luminal narrowing, before cerebrovascular and cardiac disease manifest clinically.[3, 4] These measures are also used to characterize subclinical CVD burden in people living with HIV, a population with increased risks of myocardial infarction,[5] stroke,[6] heart failure,[7] and CVD mortality,[8] as well as a preponderance of CVD risk factors.[9, 10]
Different features of subclinical CVD have been found to have variable correlations in populations and also have independent associations with CVD risk.[11–15] In addition, metabolic variables and other CVD risk factors vary in their strengths of association with different arterial features, suggesting that multiple pathways studied through imaging may be responsible for disease.[16] Furthermore, elevated levels of immune activation, inflammation, and coagulation attributable to HIV infection may lead to greater CVD risk,[17, 18] but few studies of people with HIV have corroborated findings in the general population linking subclinical carotid artery disease measures with major health events. Therefore, we examined associations of three carotid artery ultrasound measurements – common carotid artery intima-media thickness (CCA-IMT), presence of plaque, and arterial stiffness – with all-cause and non-AIDS mortality over 10 years in the Women’s Interagency HIV Study (WIHS) and Multicenter AIDS Cohort Study (MACS). We hypothesized that more subclinical disease would be associated with poorer survival, and that associations would differ by HIV serostatus.
METHODS
Setting and participants.
We conducted a nested cohort study within the female WIHS and the male MACS cohorts, two prospective multicenter studies of individuals with or at risk for HIV infection.[19, 20] Each cohort involves semi-annual follow-up visits with detailed examinations, specimen collection, and structured interviews (Supplement). WIHS and MACS participants enrolled in a vascular substudy beginning in 2004.[21, 22] The primary substudy exclusion criterion in MACS was self-reported history of coronary heart disease, including angina, myocardial infarction, or coronary revascularization; this exclusion was not implemented in WIHS but was applied to this analysis. All individuals provided informed consent, and studies were approved by each site’s Institutional Review Board.
Carotid artery data.
Most participants attended a baseline substudy visit between 2004 and 2006. Some MACS men (29%) had their baseline visit between 2010 and 2013.[23] All participants underwent high-resolution B-mode carotid artery ultrasound to image six locations in the right carotid artery according to published procedures:[24] the near and far walls of the common carotid artery, carotid bifurcation, and internal carotid artery. A standardized protocol was used at all sites,[21] and measurements were obtained at a centralized reading center.
Three carotid artery ultrasound parameters were selected for the current study because they are common non-invasive measurements of arteriosclerosis, and they have been associated with mortality in other populations.[25, 26] The first carotid artery ultrasound parameter was IMT measured in the far wall of the common carotid artery (CCA). Mean CCA-IMT was assessed from standardized ultrasound images in a plaque-free area by automated computerized edge detection.[27] We choose to measure IMT at the CCA because this segment is almost always free from lesions. These measurements provide a “clean” wall thickness measure whereas measurements in other segments such as the internal carotid artery typically include IMT and lesion measurements combined. The second measure was presence of focal plaque, defined as an area with localized IMT >1.5 mm measured in at least one of the six aforementioned artery locations.[28] Lastly, Young’s modulus of elasticity, an index of arterial stiffness,[27] was estimated in units of 105×N/m2, as: PP/DD×0.5×DD/IMTD, where PP is pulse pressure measured at the time of the ultrasound, DD is percent arterial dilation over the cardiac cycle, DD is carotid diameter at diastole, and IMTD is CCA-IMT at diastole. Higher Young’s modulus values indicate stiffer arteries. Coefficients of variation of CCA-IMT, carotid diameters, and blood pressures suggest high reproducibility.[27]
Outcomes.
The primary outcome was all-cause mortality, ascertained using both active surveillance of participants and linkages with the National Death Index (NDI).[29] For this analysis, the NDI was searched through 2014, except for two sites that completed searches through 2016. Participants were followed from the date of the baseline carotid scan to the earlier of the date of death or most recent NDI search (maximum 11.7 years).
A secondary outcome was death due to non-HIV-related causes (excluding external causes), based on the underlying cause of death derived from the death certificate. A non-HIV-related cause was defined as any death that did not include an ICD-10 code for HIV disease as the underlying cause (B20-B24), with a further exclusion of deaths due to external causes including accidents, intentional self-harm, or assault (V01-Y89), or use of psychoactive substances (F11-F16, F18-F19);[30] this was done to focus on naturally occurring deaths. We did not examine CVD deaths separately, because death certificate data alone may be insufficient to determine etiology and only a subset of deaths were adjudicated based on medical record review.
Other variables.
HIV infection was ascertained by ELISA and confirmed by Western blot. Potential confounders were measured at the core study visit closest in time to the baseline carotid scan (median time between core study visit and baseline carotid scan: 16 days, IQR 1–57). Demographic and behavioral confounders included age, self-reported race/ethnicity (black non-Hispanic, Hispanic, white non-Hispanic or other), education, income, study site, history of injection drug use, current crack/cocaine and alcohol use, current smoking, and hepatitis C virus (HCV) infection. Cardiometabolic risk factors included body mass index (BMI), systolic blood pressure, total and high-density lipoprotein cholesterol, current use of antihypertensive and lipid-lowering medications, and diabetes mellitus. HIV-related variables included current CD4+ T-lymphocyte cell count, current virologic suppression, current ART use, and history of clinical AIDS. We also examined cumulative use of protease inhibitors and abacavir in sensitivity analyses, because some prior studies have reported associations with CVD risk.[31, 32]
Statistical methods.
Within each cohort, we compared characteristics by HIV serostatus and examined Spearman correlations among ultrasound measurements. We determined death rates per 1,000 person-years, age-standardized to the 2000 U.S. Standard Population.
We constructed cumulative mortality curves by levels of each carotid measurement for a person aged 45 years, which was the average age at baseline. For time to all-cause mortality, we used Cox regression to model the association with carotid measurements. For time to non-HIV mortality, we used competing risks regression to account for the competing risk of death due to HIV disease or external causes.[33] For both outcomes, we developed nested models to serially adjust for HIV serostatus, age, demographic and behavioral characteristics, cardiometabolic risk factors, and HIV-related factors. Because associations were qualitatively similar with each successively nested model, we report only the fully-adjusted results. We examined the three carotid measurements in separate models as well as simultaneously, to assess their isolated and independent associations with mortality.
Models were first developed within each cohort, and we combined cohorts when results were qualitatively similar. We ran stratified models among men and women, and among HIV-positive and negative participants, and assessed effect modification using two-way multiplicative interaction terms. Sensitivity analyses evaluated assumptions regarding study variables (Supplement). Analyses were conducted using R 3.3.2 (R Foundation for Statistical Computing), including the packages epitools, survival, and survminer. We determined statistical significance based on a two-sided P-value <0.05. We used IVEware 0.3 (University of Michigan) to implement multiple imputation (5 datasets) to account for <1% of values that we assumed were missing at random.[34]
RESULTS
We studied 1,722 women (71% HIV-positive) and 1,304 men (62% HIV-positive), contributing 26,800 person-years of observation (Table 1). Men were on average 10 years older than women at the baseline vascular study visit (median 50 versus 40). HIV-positive and negative groups were generally similar, although participants with HIV were more likely to have previously injected drugs and be co-infected with HCV. Among HIV-positive individuals, two-thirds reported using ART at baseline. Women were more likely to be non-white than men (90% versus 39%). Women were more likely to smoke and have higher BMI. Men had higher systolic blood pressures and total cholesterol and were more likely to be taking anti-hypertensive or lipid-lowering medications.
Table 1.
Study population characteristics, baseline carotid artery ultrasound measures, follow-up, and mortality, by cohort and HIV serostatus.
| Women’s Interagency HIV Study (WIHS) women | Multicenter AIDS Cohort Study (MACS) men | |||
|---|---|---|---|---|
| HIV+ (N=1,231) | HIV- (N=491) | HIV+ (N=807) | HIV- (N=497) | |
| Demographic characteristics | ||||
| Age at baseline vascular study visit, years (median, IQR) | 41 (35–47) | 37 (29–44) | 48 (44–54) | 53 (46–59) |
| Race/ethnicity (No., %) | ||||
| Black (non-Hispanic) | 713 (58) | 302 (62) | 251 (31) | 122 (25) |
| Hispanic | 357 (29) | 136 (28) | 89 (11) | 31 (6) |
| White (non-Hispanic) or other | 161 (13) | 53 (11) | 467 (58) | 344 (69) |
| Income (No., %) | ||||
| <$30,000 per year | 1013 (82) | 399 (81) | 417 (52) | 180 (37) |
| $30,000+ per year | 218 (18) | 92 (19) | 388 (48) | 313 (63) |
| Education (at study entry) (No., %) | ||||
| Did not complete high school | 499 (41) | 161 (33) | 59 (7) | 24 (5) |
| Completed high school | 356 (29) | 159 (33) | 128 (16) | 56 (11) |
| Some college or completed college | 353 (29) | 161 (33) | 427 (53) | 233 (47) |
| Attended/complete graduate school | 22 (2) | 7 (1) | 193 (24) | 184 (37) |
| Behavior-related characteristics | ||||
| History of injection drug use (No., %) | 323 (28) | 98 (22) | 127 (17) | 38 (8) |
| Current crack/cocaine use (No., %) | 80 (7) | 52 (11) | 97 (13) | 40 (8) |
| Current alcohol use (No., %) | ||||
| Abstainer | 661 (54) | 179 (36) | 171 (21) | 64 (13) |
| Light (<3 drinks/week, WIHS; 1–3, MACS) | 433 (35) | 219 (45) | 426 (53) | 250 (51) |
| Moderate (3–13, WIHS; 4–13 MACS) | 106 (9) | 67 (14) | 160 (20) | 134 (27) |
| Heavier (14+ drinks/week) | 30 (2) | 25 (5) | 42 (5) | 46 (9) |
| History of hepatitis C virus infection (No., %) | 382 (31) | 90 (18) | 130 (16) | 37 (7) |
| Metabolic risk factors | ||||
| Current smoker (No., %) | 535 (43) | 247 (50) | 264 (33) | 123 (25) |
| Body mass index, kg/m2 (median, IQR) | 27.0 (23.5–31.6) | 29.3 (24.5–35.3) | 25.1 (22.7–27.9) | 26.3 (24.1–29.3) |
| Systolic blood pressure, mmHg (median, IQR) | 114 (106–126) | 115 (106–126) | 125 (117–135) | 129 (120–139) |
| Total cholesterol, mg/dL (median, IQR) | 171 (144–198) | 173 (150.5–203) | 190 (162–217) | 195 (170–222) |
| HDL cholesterol, mg/dL (median, IQR) | 45 (36–56) | 52 (43–65) | 43.4 (36.0–52.4) | 49.6 (41.6–58.2) |
| Current use of anti-hypertensive medications (No., %) | 238 (19) | 69 (14) | 208 (26) | 134 (27) |
| Current use of lipid-lowering medications (No., %) | 77 (6) | 8 (2) | 226 (28) | 111 (23) |
| History of diabetes (No., %) | 134 (11) | 59 (12) | 82 (11) | 48 (10) |
| Menopause (self-reported) (No., %) | 280 (23) | 59 (12) | N/A | N/A |
| HIV-specific characteristics | ||||
| Baseline CD4+ T-cell count, cells/mm3 (median, IQR) | 431 (262–631) | N/A | 527 (364–717) | N/A |
| Baseline HIV-1 viral load, copies/mL (median, IQR) | 250 (80–8550) | N/A | 40 (40–613.5) | N/A |
| Undetectable baseline HIV-1 viral load (No., %) | 538 (44) | N/A | 354 (45) | N/A |
| History of clinical AIDS (No., %) | 449 (36) | N/A | 111 (14) | N/A |
| Potent ART use in past 6 months (No., %) | 763 (62) | N/A | 606 (76) | N/A |
| Protease inhibitor use in past 6 months (No., %) | 451 (37) | 337 (42) | ||
| Abacavir use in past 6 months (No., %) | 227 (18) | 62 (8) | ||
| Cumulative exposure to potent ARTa, years (median, IQR) | 3.5 (2.5–6.5) | N/A | 5.9 (3.4–8.2) | N/A |
| … to protease inhibitorsa, years (median, IQR) | 2.5 (0.5–4.5) | N/A | 4.0 (0.5–7.1) | N/A |
| … to abacavira, years (median, IQR) | 0.0 (0.0–2.0) | N/A | 0.0 (0.0–2.1) | N/A |
| Nadir CD4+ T-cell count before ART usea, cells/mm3 (median, IQR) | 272 (153–395) | N/A | 280.5 (156–401) | N/A |
| Carotid artery ultrasound measures at baseline | ||||
| Presence of focal plaque (No.,%) | 129 (10) | 32 (7) | 221 (27) | 145 (29) |
| CCA-IMT, mm (median, IQR) | 0.71 (0.65–0.78) | 0.70 (0.64–0.77) | 0.72 (0.66–0.80) | 0.74 (0.68–0.83) |
| Young’s modulus, 105*N/m2 (median, IQR) | 5.48 (4.01–7.95) | 4.91 (3.56–7.16) | 6.91 (5.43–9.00) | 7.03 (5.45–9.17) |
| Follow-up and mortality data | ||||
| Follow-up time, years (median, IQR) | 10.3 (9.8–10.6) | 10.4 (10.1–10.7) | 9.6 (4.5–10.3) | 9.5 (3.8–10.2) |
| Deaths, any cause (No.,%) | 219 (18) | 21 (4) | 72 (9) | 31 (6) |
| Age-standardized rate per 1,000 person-years, 95% confidence interval | 19.9 (14.7–28.8) | 14.7 (2.3–56.4) | 15.1 (8.3–26.8) | 7.7 (4.3–13.4) |
| Deaths, non-HIV causes (excluding external causes) (No.,%) | 90 (7) | 18 (4) | 37 (5) | 24 (5) |
| Age-standardized rate per 1,000 person-years, 95% confidence interval | 10.2 (5.6–19.1) | 14.2 (2.0–56.0) | 5.8 (2.5–14.3) | 4.9 (2.6–9.5) |
| Deaths, external causesb (No.,%) | 29 (2) | 3 (0.6) | 8 (1) | 5 (1) |
| Deaths, unknown cause (No.,%) | 1 (0.08) | 0 | 8 (1) | 2 (0.4) |
AIDS = acquired immunodeficiency syndrome, ART = antiretroviral therapy, CCA-IMT = common carotid artery intima-media thickness, HIV = human immunodeficiency virus, IQR = interquartile range, NNRTI = non-nucleoside reverse transcriptase inhibitor, NRTI = nucleoside reverse transcriptase inhibitor, N/A = not applicable, PI = protease inhibitor.
All characteristics assessed at baseline unless otherwise noted.
Among those with a history of ART use.
71% of deaths due to an external cause were attributed to substance use.
Carotid artery ultrasound measurements.
More subclinical carotid artery disease was detected among MACS men than WIHS women (Table 1). Median CCA-IMT was 0.728 mm in men (interquartile range [IQR] 0.668–0.815) and 0.707 mm in women (IQR 0.648–0.774, p<0.001). Plaque was observed in 28% of men, compared with 8% of women (p<0.001). Arterial stiffness, as assessed by Young’s modulus, was also significantly greater among men (median 6.94 units, IQR 5.44–9.05) compared with women (median 5.35 units, IQR 3.86–7.78, p<0.001).
Spearman correlation coefficients among the carotid artery parameters by cohort are shown in Supplementary Table 1. Correlations were highest between plaque and CCA-IMT (range of coefficients: 0.24–0.27). Correlations between plaque and Young’s modulus and between CCA-IMT and Young’s modulus were minimal (range: −0.07–0.09). All three measures correlated moderately with age (range: 0.12–0.49), systolic blood pressure (range: 0.10–0.27), and pulse pressure (range: 0.05–0.19).
Follow-up and deaths.
Median follow-up time was 10.3 years (IQR 9.9–10.6) for women and 9.5 years (IQR 4.3–10.3) for men. Of 343 deaths reported (11% of participants, 240 women and 103 men, median time to death 4.7 years [IQR 2.2–7.6]), 169 had a non-HIV-related cause of death, after excluding 45 who died of an external cause (Table 1). The age-standardized death rate was 17.6/1,000 person-years (95% confidence interval [CI] 12.5–25.9) among women and 10.9/1,000 (95% CI 7.8–15.0) among men. Death rates were two-fold higher among HIV-positive compared with HIV-negative participants (18.3/1,000 [95% CI 13.9–24.7] versus 7.7/1,000 [95% CI 4.9–11.9]). Similar trends were observed for non-HIV-related deaths.
Associations of carotid artery measures with all-cause mortality.
We examined cumulative mortality in age-adjusted analyses stratified by cohort (Figure 1). For plaque and stiffness, a gradient was observed in which participants with more subclinical disease had greater mortality. Similar relationships were observed when we limited analyses to HIV-positive participants.
Figure 1. Cumulative all-cause mortality in the Women’s Interagency HIV Study (WIHS) (red) and Multicenter AIDS Cohort Study (MACS) (blue), based on carotid artery ultrasound measurement levels among (a) all participants, and (b) HIV-positive participants only.
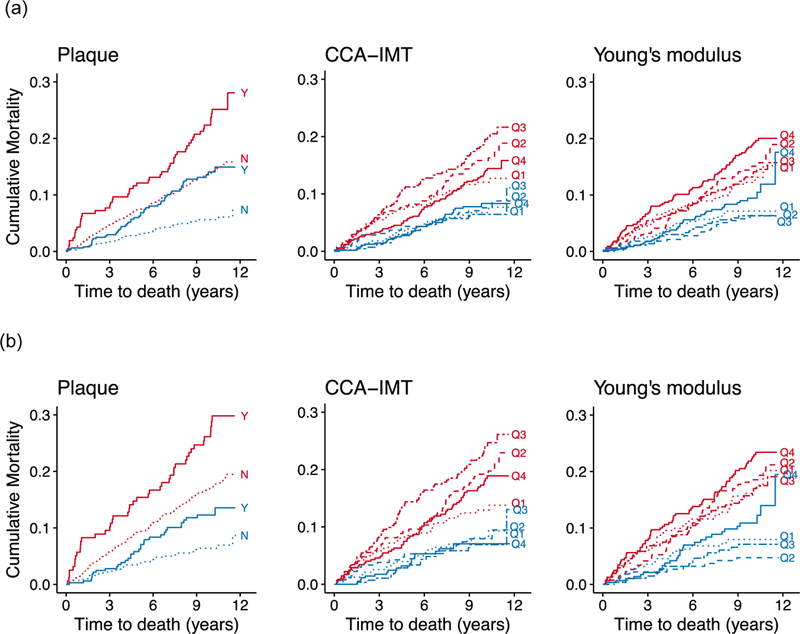
CCA-IMT = common carotid artery intima-media thickness. Solid line = most severe phenotype (plaque, highest quartile of CCA-IMT/Young’s modulus); dotted line = least severe phenotype (no plaque, lowest quartile of CCA-IMT/Young’s modulus); dashed lines = intermediate phenotypes. Cumulative mortality curves are predicted for a participant at the average age of the study population (45 years) at baseline.
Successive adjustments for potential demographic, behavioral, cardiometabolic, and HIV-related confounders attenuated these findings (data not shown), but most relationships remained statistically significant despite control for these multiple risk factors. Table 2 shows the fully adjusted associations of each carotid artery measure with all-cause mortality in separate models. Differences by sex were assessed using a test for interaction (p<0.05). Among men, having plaque at baseline was associated with a two-fold increased hazard of death (hazard ratio [HR] 2.19, 95% CI 1.41–3.43, p<0.001), whereas this association was not apparent in women (HR 1.06, 95% CI 0.74–1.52, p=0.75; p for interaction 0.006). When we combined women and men, the association of plaque with all-cause mortality remained statistically significant (HR 1.44, 95% CI 1.10–1.88, p=0.007). We also found significant associations between Young’s modulus and all-cause mortality in women but not men. Women in the highest quartile had a 71% increased hazard of death compared with those in the lowest quartile (HR 1.71, 95% CI 1.11–2.61, p=0.01), whereas the same relationship was not apparent among men (HR 1.08, 95% CI 0.61–1.89, p=0.79; p for interaction 0.99). We did not observe an increase in all-cause mortality with greater CCA-IMT. Instead, individuals in the highest quartile of CCA-IMT appeared to have better survival than those in the lowest quartile (HR 0.71, 95% CI 0.48–1.05, p=0.08), with a statistically significant trend of lower mortality risk with increasing CCA-IMT (p=0.03). Including all three carotid artery ultrasound measurements in the same model resulted in similar associations.
Table 2.
Adjusted association of carotid artery measures with time to death due to any cause, HIV-positive and HIV-negative combined.
| WIHS women (N=1,722) | MACS men (N=1,304) | WIHS and MACS (N=3,026) | P for interaction by cohort | P for interaction by HIV serostatus | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |||
| Carotid artery measures in separate models | ||||||||
| Plaque (ref. = no plaque) | 1.06 (0.74–1.52) | 0.75 | 2.19 (1.41–3.43) | <0.001 | 1.44 (1.10–1.88) | 0.007 | 0.008 | <0.001 |
| CCA-IMT (ref. = Quartile 1) | ||||||||
| Quartile 2 | 1.03 (0.66–1.61) | 0.90 | 1.08 (0.58–1.99) | 0.81 | 1.07 (0.75–1.52) | 0.72 | 0.12 | 0.15 |
| Quartile 3 | 1.06 (0.67–1.66) | 0.81 | 0.71 (0.37–1.35) | 0.29 | 0.93 (0.65–1.33) | 0.70 | ||
| Quartile 4 | 0.66 (0.40–1.08) | 0.10 | 0.79 (0.42–1.49) | 0.47 | 0.71 (0.48–1.05) | 0.08* | ||
| Young’s modulus (ref. = Quartile 1) | ||||||||
| Quartile 2 | 0.98 (0.64–1.48) | 0.92 | 0.76 (0.41–1.39) | 0.37 | 0.93 (0.67–1.31) | 0.70 | 0.99 | 0.27 |
| Quartile 3 | 1.09 (0.71–1.67) | 0.68 | 0.64 (0.34–1.17) | 0.15 | 0.97 (0.68–1.36) | 0.84 | ||
| Quartile 4 | 1.71 (1.11–2.61) | 0.01** | 1.08 (0.61–1.89) | 0.79 | 1.43 (1.02–2.01) | 0.04* | ||
| Carotid artery measures in the same model | ||||||||
| Plaque (ref. = no plaque) | 1.20 (0.83–1.73) | 0.32 | 2.26 (1.44–3.54) | <0.001 | 1.54 (1.18–2.01) | 0.002 | 0.02 | 0.001 |
| CCA-IMT (ref. = Quartile 1) | ||||||||
| Quartile 2 | 1.10 (0.71–1.72) | 0.67 | 1.08 (0.58–2.00) | 0.82 | 1.11 (0.78–1.58) | 0.57 | 0.37 | 0.22 |
| Quartile 3 | 1.12 (0.71–1.76) | 0.63 | 0.66 (0.34–1.27) | 0.21 | 0.94 (0.66–1.36) | 0.76 | ||
| Quartile 4 | 0.71 (0.43–1.18) | 0.19 | 0.70 (0.36–1.36) | 0.29 | 0.72 (0.48–1.07) | 0.11* | ||
| Young’s modulus (ref. = Quartile 1) | ||||||||
| Quartile 2 | 0.97 (0.64–1.47) | 0.88 | 0.79 (0.42–1.46) | 0.45 | 0.91 (0.64–1.28) | 0.58 | 0.89 | 0.23 |
| Quartile 3 | 1.10 (0.72–1.68) | 0.61 | 0.62 (0.33–1.16) | 0.13 | 0.93 (0.66–1.32) | 0.68 | ||
| Quartile 4 | 1.63 (1.06–2.51) | 0.02* | 1.00 (0.55–1.81) | 0.99 | 1.32 (0.94–1.87) | 0.11 | ||
All models adjusted for HIV serostatus, age, race/ethnicity, education, income, study site, history of injection drug use, crack/cocaine use, smoking, alcohol, hepatitis C virus infection, body mass index, systolic blood pressure, total and high-density lipoprotein cholesterol, use of anti-hypertensive or cholesterol medications, diabetes, baseline CD4 T-cell count, antiretroviral therapy use, and history of AIDS.
CCA-IMT = common carotid artery intima-media thickness, CI = confidence interval, HR = hazard ratio, MACS = Multicenter AIDS Cohort Study, WIHS = Women’s Interagency HIV Study.
p<0.05 for trend,
p<0.01 for trend.
When we stratified analyses by HIV serostatus, we found that associations limited to HIV-positive participants were similar to those in the combined analyses, with some significant associations becoming marginally significant (Table 3). The ability of carotid artery ultrasound measures to predict death appeared to be stronger among HIV-negative than HIV-positive participants, although associations had wide confidence intervals (Supplementary Table 2). Accordingly, few of the relationships showed significant multiplicative effect modification by HIV serostatus. The exception was the association of plaque with mortality, which was significantly more pronounced in HIV-negative (HR 3.87, 95% CI 1.95–7.66, p<0.001) compared with HIV-positive participants (HR 1.35, 95% CI 1.00–1.84, p=0.05) and showed evidence of both multiplicative (p<0.001) and additive effect modification (data not shown).
Table 3.
Adjusted association of carotid artery measures with time to death due to any cause, HIV-positive only.
| WIHS women (N=1,231) | MACS men (N=807) | WIHS and MACS (N=2,038) | P for interaction by cohort | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Carotid artery measures in separate models | |||||||
| Plaque (ref. = no plaque) | 1.01 (0.69–1.48) | 0.95 | 1.63 (0.95–2.83) | 0.08 | 1.24 (0.91–1.67) | 0.17 | 0.06 |
| CCA-IMT (ref. = Quartile 1) | |||||||
| Quartile 2 | 1.16 (0.73–1.85) | 0.53 | 0.93 (0.46–1.90) | 0.85 | 1.05 (0.72–1.53) | 0.80 | 0.70 |
| Quartile 3 | 1.18 (0.73–1.90) | 0.51 | 0.59 (0.28–1.21) | 0.15 | 0.94 (0.64–1.38) | 0.74 | |
| Quartile 4 | 0.75 (0.45–1.27) | 0.29 | 0.61 (0.29–1.28) | 0.19 | 0.67 (0.44–1.02) | 0.06* | |
| Young’s modulus (ref. = Quartile 1) | |||||||
| Quartile 2 | 0.92 (0.60–1.42) | 0.72 | 0.55 (0.25–1.20) | 0.13 | 0.83 (0.58–1.21) | 0.34 | 0.64 |
| Quartile 3 | 1.01 (0.65–1.56) | 0.97 | 0.62 (0.30–1.28) | 0.19 | 0.95 (0.66–1.37) | 0.78 | |
| Quartile 4 | 1.47 (0.94–2.28) | 0.09 | 0.99 (0.56–1.93) | 0.99 | 1.36 (0.95–1.96) | 0.10 | |
| Carotid artery measures in the same model | |||||||
| Plaque (ref. = no plaque) | 1.15 (0.78–1.71) | 0.47 | 1.65 (0.93–2.91) | 0.09 | 1.35 (1.00–1.84) | 0.05 | 0.048 |
| CCA-IMT (ref. = Quartile 1) | |||||||
| Quartile 2 | 1.22 (0.76–1.94) | 0.41 | 0.93 (0.45–1.90) | 0.83 | 1.08 (0.74–1.57) | 0.69 | 0.85 |
| Quartile 3 | 1.23 (0.76–2.00) | 0.39 | 0.57 (0.27–1.18) | 0.13 | 0.95 (0.64–1.40) | 0.80 | |
| Quartile 4 | 0.80 (0.47–1.36) | 0.40 | 0.55 (0.25–1.20) | 0.14 | 0.67 (0.44–1.04) | 0.08* | |
| Young’s modulus (ref. = Quartile 1) | |||||||
| Quartile 2 | 0.92 (0.60–1.41) | 0.71 | 0.59 (0.26–1.31) | 0.19 | 0.81 (0.56–1.18) | 0.28 | 0.80 |
| Quartile 3 | 1.02 (0.66–1.59) | 0.91 | 0.64 (0.30–1.34) | 0.24 | 0.93 (0.64–1.35) | 0.70 | |
| Quartile 4 | 1.43 (0.92–2.24) | 0.11 | 0.90 (0.45–1.82) | 0.78 | 1.26 (0.87–1.83) | 0.22 | |
All models adjusted for age, race/ethnicity, education, income, study site, history of injection drug use, crack/cocaine use, smoking, alcohol, hepatitis C virus infection, body mass index, systolic blood pressure, total and high-density lipoprotein cholesterol, use of anti-hypertensive or cholesterol medications, diabetes, baseline CD4 T-cell count, antiretroviral therapy use, and history of AIDS.
CCA-IMT = common carotid artery intima-media thickness, CI = confidence interval, HR = hazard ratio, MACS = Multicenter AIDS Cohort Study, WIHS = Women’s Interagency HIV Study.
p<0.05 for trend.
Associations with non-HIV-related mortality.
Because many deaths were due to HIV disease, we further examined associations of carotid artery measurements with mortality by limiting the analyses to naturally occurring non-HIV-related deaths (Table 4). In these analyses, which accounted for competing risks and excluded deaths due to HIV as well as external causes (accidents, assaults, overdoses), associations of focal plaque with mortality were strengthened, with the hazard ratio among women and men combined increasing from 1.54 (95% CI 1.18–2.01) to 1.85 (95% CI 1.24–2.74). In women alone the hazard ratio increased from 1.20 to 1.51, while in men alone, the hazard ratio increased from 2.26 to 2.44. In contrast, associations of Young’s modulus with mortality became negligible when focusing on non-HIV deaths.
Table 4.
Adjusted association of carotid artery measures with time to non-HIV-related death, HIV-positive and HIV-negative combined.
| Time to non-HIV related death | Time to death due to any cause (base case) | |||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | P-value for interaction by HIV serostatus | HR (95% CI) | P-value | P-value for interaction by HIV serostatus | |
| WIHS women | ||||||
| Plaque (ref. = no plaque) | 1.51 (0.85–2.68) | 0.16 | 0.02 | 1.20 (0.83–1.73) | 0.32 | 0.02 |
| CCA-IMT (ref. = Quartile 1) | ||||||
| Quartile 2 | 0.67 (0.34–1.34) | 0.26 | 0.32 | 1.10 (0.71–1.72) | 0.67 | 0.88 |
| Quartile 3 | 0.64 (0.32–1.24) | 0.19 | 1.12 (0.71–1.76) | 0.63 | ||
| Quartile 4 | 0.48 (0.23–1.00) | 0.05 | 0.71 (0.43–1.18) | 0.19 | ||
| Young’s modulus (ref. = Quartile 1) | ||||||
| Quartile 2 | 0.78 (0.41–1.48) | 0.45 | 0.01 | 0.97 (0.64–1.47) | 0.88 | 0.02 |
| Quartile 3 | 1.13 (0.59–2.16) | 0.71 | 1.10 (0.72–1.68) | 0.61 | ||
| Quartile 4 | 1.17 (0.62–2.20) | 0.63 | 1.63 (1.06–2.51) | 0.02* | ||
| MACS men | ||||||
| Plaque (ref. = no plaque) | 2.44 (1.34–4.59) | 0.006 | 0.09 | 2.26 (1.44–3.54) | <0.001 | 0.16 |
| CCA-IMT (ref. = Quartile 1) | ||||||
| Quartile 2 | 2.12 (0.89–5.04) | 0.09 | 0.10 | 1.08 (0.58–2.00) | 0.82 | 0.27 |
| Quartile 3 | 1.46 (0.61–3.49) | 0.40 | 0.66 (0.34–1.27) | 0.21 | ||
| Quartile 4 | 1.48 (0.61–3.63) | 0.39 | 0.70 (0.36–1.36) | 0.29 | ||
| Young’s modulus (ref. = Quartile 1) | ||||||
| Quartile 2 | 0.78 (0.35–1.74) | 0.54 | 0.13 | 0.79 (0.42–1.46) | 0.45 | 0.87 |
| Quartile 3 | 0.92 (0.43–1.95) | 0.82 | 0.62 (0.33–1.16) | 0.13 | ||
| Quartile 4 | 0.88 (0.39–2.00) | 0.76 | 1.00 (0.55–1.81) | 0.99 | ||
| WIHS and MACS | ||||||
| Plaque (ref. = no plaque) | 1.85 (1.24–2.74) | 0.002 | 0.01 | 1.54 (1.18–2.01) | 0.002 | 0.001 |
| CCA-IMT (ref. = Quartile 1) | ||||||
| Quartile 2 | 1.07 (0.63–1.80) | 0.88 | 0.31 | 1.11 (0.78–1.58) | 0.57 | 0.22 |
| Quartile 3 | 0.91 (0.54–1.55) | 0.64 | 0.94 (0.66–1.36) | 0.76 | ||
| Quartile 4 | 0.79 (0.45–1.39) | 0.33 | 0.72 (0.48–1.07) | 0.11* | ||
| Young’s modulus (ref. = Quartile 1) | ||||||
| Quartile 2 | 0.81 (0.50–1.33) | 0.41 | 0.02 | 0.91 (0.64–1.28) | 0.58 | 0.23 |
| Quartile 3 | 1.02 (0.63–1.65) | 0.92 | 0.93 (0.66–1.32) | 0.68 | ||
| Quartile 4 | 1.02 (0.64–1.64) | 0.93 | 1.32 (0.94–1.87) | 0.11 | ||
All models adjusted for HIV serostatus, age, race/ethnicity, education, income, study site, history of injection drug use, crack/cocaine use, smoking, alcohol, hepatitis C virus infection, body mass index, systolic blood pressure, total and high-density lipoprotein cholesterol, use of anti-hypertensive or cholesterol medications, diabetes, baseline CD4 T-cell count, antiretroviral therapy use, history of AIDS, and the other carotid artery measures.
CCA-IMT = common carotid artery intima-media thickness, CI = confidence interval, HR = hazard ratio, MACS = Multicenter AIDS Cohort Study, WIHS = Women’s Interagency HIV Study.
p<0.05 for trend.
Because of a statistically significant (p=0.01) interaction between plaque and HIV serostatus with respect to mortality, we performed analyses of naturally occurring deaths (excluding those due to HIV) stratified by HIV serostatus. Effect estimates had wide confidence intervals due to more limited power. In analyses restricted to HIV-positive participants (Supplementary Table 3), associations by focal plaque presence were strengthened, with the hazard ratio among women and men combined increasing from 1.35 (95% CI 1.00–1.84) to 1.48 (95% CI 0.91–2.41). Among HIV-negative participants, associations by focal plaque presence also strengthened, with the hazard ratio increasing from 3.87 (95% CI 1.95–7.66) to 4.98 (95% CI 2.21–11.25) (Supplementary Table 4). Results of additional sensitivity analyses are presented in the Appendix.
DISCUSSION
We found that carotid artery plaque detected by B-mode ultrasound was independently associated with mortality in adults living with HIV who were free of clinical CVD. Subgroup analyses suggested that this association was stronger among the primarily white, highly educated male MACS cohort than the poorer, largely minority female WIHS cohort. Differences by cohort in the strength of associations between subclinical CVD and all-cause mortality may be attributable to differences between cohorts in the distribution of causes of death, although we had limited ability to evaluate this due to incompleteness of etiology on the death certificate. Nonetheless, it was notable that associations between carotid plaque and mortality were reproduced across two HIV-positive cohorts that are demographically very different, although studied using nearly identical methods. Arterial stiffness was also associated with mortality.
To our knowledge, ours is the first study to show that carotid artery plaque predicts mortality in adults with HIV. This is important because few studies to date have linked subclinical atherosclerosis with major health outcomes in HIV-positive people, although B-mode carotid artery ultrasound measurements are widely used markers of subclinical CVD. One electronic medical record-based study found that among 209 HIV-positive patients who underwent neck computed tomography with contrast, the presence of carotid plaque was associated with a three-to-four-fold increased risk of atherosclerotic CVD events and stroke.[35] In contrast, our analysis was conducted among persons free of clinical CVD and without a clinical indication for specialized imaging. This allowed for broader generalizability and assessment of pre-clinical disease indicators. Our key finding that HIV-positive individuals showed weaker associations between plaque and mortality, rather than stronger associations, warrants further investigation into the reasons for this disparity.
The association that we found between carotid artery plaque and mortality was also more pronounced in men than in women. It is unclear whether this is a consequence of differences in the underlying biology, differences in risk factor distributions, or a combination of the two. While the cohorts vary by age, race/ethnicity, socioeconomic status, and behavioral risk factors, we controlled extensively for these factors; nonetheless, there may be residual confounding. In support of a biological explanation, differences related to sex hormones, adaptive or innate immunity, and drug responses are increasingly postulated as modulators of carotid artery atherosclerosis and CVD risk in women versus men.[36, 37] The composition of plaques may differ between men and women, owing to differences in inflammatory and histologic features.[38–40] More detailed assessments of plaque are beyond the scope of the current paper but may explain in part our results.
An unexpected finding was that greater CCA-IMT seemed to be associated with lower mortality risk, which was observed consistently in HIV-positive women and men. We cannot rule out survival bias as an explanation, as many study participants had lived with HIV for many years, and some with greater IMT could have died prior to this substudy. A pooled analysis of 5 cohorts (including WIHS and MACS) found that compared to HIV-negative individuals, CCA-IMT was lower among HIV-positive individuals even after confounder adjustment.[41] This finding was particularly noted among those not on ART with high levels of circulating HIV RNA. Thus, an emerging picture suggests that CCA-IMT may have a paradoxical association with disease in the context of HIV. We speculate that chronic arterial remodeling, altered metabolism, or other phenomena affecting the vasculature may account for the presence of thinned arterial walls among persons with long-standing HIV infection, as has been observed in patients with cancer and rheumatoid arthritis.[42–44]
Two smaller studies conducted among HIV-positive persons found significant associations between greater IMT and mortality. Mangili detected an association using IMT measured at the CCA,[45] while Hsu found an association using IMT measured at the internal carotid artery (ICA) but not at the CCA.[46] Variations in protocols may partially explain why these findings differ from ours. We limited CCA-IMT assessment to the far wall, where measurements are known to be more reproducible[47], whereas Mangili assessed CCA-IMT at both the near and far walls. While we defined plaque based on focal thickening >1.5 mm as measured in carotid sections including the ICA, we did not conduct a standardized assessment of plaque-free IMT in the ICA region.
We are not aware of studies linking arterial stiffness to mortality among HIV-positive persons. A meta-analysis of 10 non-HIV studies found that greater arterial stiffness was associated with a 22% increased risk of all-cause mortality.[2] Here, we found that women with greater arterial stiffness had higher mortality risk, but this association was not apparent in men. While this finding is consistent with chance, it may suggest sex-based differences. The Multi-Ethnic Study of Atherosclerosis found that men and women had similar rates of change in Young’s modulus over ten years; however, a slower rate of increase over time associated with higher education level and lipid-lowering medication use was only observed among women.[48] In our study, women had lower education levels and were less likely to be receiving lipid-lowering medication, suggesting that ongoing arterial stiffening related to these factors could have contributed to their higher mortality risk. While our study was limited to baseline measurements of stiffness, examination of longitudinal patterns by sex may clarify whether disparities in disease management or other factors may contribute to the variation.
We believe that this is the largest study of health outcomes to date involving HIV-associated subclinical atherosclerosis. The analysis is nested within two well-established cohorts that involve standardized longitudinal collection of HIV and non-HIV risk factors, with HIV-negative participants who are similar demographically and behaviorally to their HIV-positive counterparts. Another major strength is that women comprise over half of the study population. As a result, we were able to identify sex-specific differences that suggest potentially different underlying disease mechanisms. However, the men and women in our studies have distinct sociodemographic and behavioral profiles that likely contribute to broader mortality differences,[29, 49] and therefore more work is needed to disentangle these factors with respect to cause-specific mortality. One limitation is that we did not have complete information on incident CVD events such as myocardial infarction and stroke. Although the death certificate-based underlying cause of each death was recorded, we limited cause-specific analyses of mortality to non-HIV-related deaths as a category instead of a more focused group of CVD deaths, to avoid potential misclassification. While we measured atherosclerosis via B-mode ultrasound, assessments in other arterial beds or via other modalities could provide additional prognostic information.[12, 50, 51] Finally, our study population was highly ART-experienced. Therefore, our findings may not be generalizable to more recently-infected individuals.
Our study supports the validity of subclinical CVD markers to assess future health risks in HIV-positive adults. Our findings also suggest differences in mortality risk associated with atherosclerosis markers by HIV serostatus, across carotid artery features, and by sex that warrant further investigation into their underlying mechanisms. More specialized methods of analyzing the complex interactions that are present, such as machine learning, may be needed to help better isolate disease pathways and glean information from multiple vascular assessments.[52] This is especially true in the context of HIV infection, where existing CVD risk prediction equations may be inadequate.[53] Such work may help to identify appropriately tailored interventions to prevent and treat CVD in the growing number of people aging with HIV.
Supplementary Material
ACKNOWLEDGMENTS
D.B.H. conceived and designed the work, and drafted the paper. J.M. and D.B.H. performed statistical analyses. J.M., S.A.H., A.L.F., F.J.P, L.A.K., W.S.P., R.C.K., H.N.H., and K.A. contributed to design of the work. S.A.H., A.L.F., F.J.P, S.J.G., M.D.W., S.K., J.M.L., P.C.T., L.A.K., W.S.P., R.C.K., H.N.H., and K.A. contributed to acquisition of data. K.A. conceived the work. D.B.H., J.M., S.A.H., A.L.F., F.J.P, S.J.G., M.D.W., S.K., J.M.L., P.C.T., M.J.F., L.A.K., W.S.P., R.C.K., H.N.H., and K.A. contributed to the interpretation of data for the work, revised the work critically for important intellectual content, approved the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy of integrity of any part of the work are appropriately investigated and resolved.
WIHS (Principal Investigators): Bronx WIHS (Kathryn Anastos and Anjali Sharma), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye), U01-AI-034994; Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I – WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID) at the National Institutes of Health, with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA).
MACS (Principal Investigators): Johns Hopkins University Bloomberg School of Public Health (Joseph Margolick), U01-AI35042; Northwestern University (Steven Wolinsky), U01-AI35039; University of California, Los Angeles (Roger Detels), U01-AI35040; University of Pittsburgh (Charles Rinaldo), U01-AI35041; the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson), UM1-AI35043. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID) at the National Institutes of Health, with additional co-funding from the National Cancer Institute (NCI). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR000424 (JHU CTSA).
This work was also supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health [K01-HL-137557 to D.B.H.; R01-HL-083760, R01-HL-095140, and R01-HL-126543 to R.C.K.; and R01-HL-095129 to W.S.P.].
The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) and Multicenter AIDS Cohort Study (MACS).
Footnotes
Conflicts of interest and sources of funding: No conflicts of interest are declared.
REFERENCES
- 1.Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging 2014; 7(10):1025–1038. [DOI] [PubMed] [Google Scholar]
- 2.van Sloten TT, Sedaghat S, Laurent S, London GM, Pannier B, Ikram MA, et al. Carotid stiffness is associated with incident stroke: a systematic review and individual participant data meta-analysis. J Am Coll Cardiol 2015; 66(19):2116–2125. [DOI] [PubMed] [Google Scholar]
- 3.Blankenhorn DH, Hodis HN. George Lyman Duff Memorial Lecture. Arterial imaging and atherosclerosis reversal. Arterioscler Thromb 1994; 14(2):177–192. [DOI] [PubMed] [Google Scholar]
- 4.Peters SA, Grobbee DE, Bots ML. Carotid intima-media thickness: a suitable alternative for cardiovascular risk as outcome? Eur J Cardiovasc Prev Rehabil 2011; 18(2):167–174. [DOI] [PubMed] [Google Scholar]
- 5.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173(8):614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr 2012; 60(4):351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butt AA, Chang CC, Kuller L, Goetz MB, Leaf D, Rimland D, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med 2011; 171(8):737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanna DB, Ramaswamy C, Kaplan RC, Kizer JR, Anastos K, Daskalakis D, et al. Trends in cardiovascular disease mortality among persons with HIV in New York City, 2001–2012. Clin Infect Dis 2016; 63(8):1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan RC, Kingsley LA, Sharrett AR, Li X, Lazar J, Tien PC, et al. Ten-year predicted coronary heart disease risk in HIV-infected men and women. Clin Infect Dis 2007; 45(8):1074–1081. [DOI] [PubMed] [Google Scholar]
- 10.Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med 2015; 162(5):335–344. [DOI] [PubMed] [Google Scholar]
- 11.Mack WJ, LaBree L, Liu C, Selzer RH, Hodis HN. Correlations between measures of atherosclerosis change using carotid ultrasonography and coronary angiography. Atherosclerosis 2000; 150(2):371–379. [DOI] [PubMed] [Google Scholar]
- 12.Karim R, Hodis HN, Detrano R, Liu CR, Liu CH, Mack WJ. Relation of Framingham risk score to subclinical atherosclerosis evaluated across three arterial sites. Am J Cardiol 2008; 102(7):825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blaha MJ. The future of CV risk prediction: multisite imaging to predict multiple outcomes. JACC Cardiovasc Imaging 2014; 7(10):1054–1056. [DOI] [PubMed] [Google Scholar]
- 14.Riley WA, Evans GW, Sharrett AR, Burke GL, Barnes RW. Variation of common carotid artery elasticity with intimal-medial thickness: The ARIC Study. Atherosclerosis Risk in Communities. Ultrasound Med Biol 1997; 23(2):157–164. [DOI] [PubMed] [Google Scholar]
- 15.Jogestrand T, Eiken O, Nowak J. Relation between the elastic properties and intima-media thickness of the common carotid artery. Clin Physiol Funct Imaging 2003; 23(3):134–137. [DOI] [PubMed] [Google Scholar]
- 16.Spence JD, Hegele RA. Noninvasive phenotypes of atherosclerosis: similar windows but different views. Stroke 2004; 35(3):649–653. [DOI] [PubMed] [Google Scholar]
- 17.Stein JH, Hsue PY. Inflammation, immune activation, and CVD risk in individuals with HIV infection. Jama 2012; 308(4):405–406. [DOI] [PubMed] [Google Scholar]
- 18.Schechter ME, Andrade BB, He T, Richter GH, Tosh KW, Policicchio BB, et al. Inflammatory monocytes expressing tissue factor drive SIV and HIV coagulopathy. Sci Transl Med 2017; 9(405). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, et al. The Women’s Interagency HIV Study: An observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005; 12(9):1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR Jr. The Multicenter AIDS Cohort Study: Rationale, organization, and selected characteristics of the participants. Am J Epidemiol 1987; 126(2):310–318. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan RC, Kingsley LA, Gange SJ, Benning L, Jacobson LP, Lazar J, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. Aids 2008; 22(13):1615–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanna DB, Post WS, Deal JA, Hodis HN, Jacobson LP, Mack WJ, et al. HIV infection is associated with progression of subclinical carotid atherosclerosis. Clin Infect Dis 2015; 61(4):640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Post WS, Budoff M, Kingsley L, Palella FJ Jr., Witt MD, Li X, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med 2014; 160(7):458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2001; 135(11):939–953. [DOI] [PubMed] [Google Scholar]
- 25.Stork S, van den Beld AW, von Schacky C, Angermann CE, Lamberts SW, Grobbee DE, et al. Carotid artery plaque burden, stiffness, and mortality risk in elderly men: a prospective, population-based cohort study. Circulation 2004; 110(3):344–348. [DOI] [PubMed] [Google Scholar]
- 26.Polak JF, Szklo M, Kronmal RA, Burke GL, Shea S, Zavodni AE, et al. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2013; 2(2):e000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selzer RH, Mack WJ, Lee PL, Kwong-Fu H, Hodis HN. Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis 2001; 154(1):185–193. [DOI] [PubMed] [Google Scholar]
- 28.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis 2012; 34(4):290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hessol NA, Kalinowski A, Benning L, Mullen J, Young M, Palella F, et al. Mortality among participants in the Multicenter AIDS Cohort Study and the Women’s Interagency HIV Study. Clin Infect Dis 2007; 44(2):287–294. [DOI] [PubMed] [Google Scholar]
- 30.Zimmerman R, Li W, Begier E, Davis K, Gambatese M, Kelley D, et al. Summary of Vital Statistics, 2011: Appendix A: Supplemental Population, Mortality and Pregnancy Outcome Data Tables. http://www.nyc.gov/html/doh/downloads/pdf/vs/vs-appendix-a-2011.pdf, 17 Jul 2013. [Google Scholar]
- 31.Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte A, El-Sadr W, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007; 356(17):1723–1735. [DOI] [PubMed] [Google Scholar]
- 32.Sabin CA, Worm SW, Weber R, Reiss P, El-Sadr W, Dabis F, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D Study: A multi-cohort collaboration. Lancet 2008; 371(9622):1417–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assn 1999; 94:496–509. [Google Scholar]
- 34.Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodol 2001; 27(1):85–95. [Google Scholar]
- 35.Janjua SA, Staziaki PV, Szilveszter B, Takx RAP, Mayrhofer T, Hennessy O, et al. Presence, characteristics, and prognostic associations of carotid plaque among people living with HIV. Circ Cardiovasc Imaging 2017; 10(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raghavan A, Rimmelin DE, Fitch KV, Zanni MV. Sex differences in select non-communicable HIV-associated comorbidities: Exploring the role of systemic immune activation/inflammation. Curr HIV/AIDS Rep 2017; 14(6):220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rexrode K Sex differences in sex hormones, carotid atherosclerosis, and stroke. Circ Res 2018; 122(1):17–19. [DOI] [PubMed] [Google Scholar]
- 38.Halvorsen DS, Johnsen SH, Mathiesen EB, Njolstad I. The association between inflammatory markers and carotid atherosclerosis is sex dependent: The Tromso Study. Cerebrovasc Dis 2009; 27(4):392–397. [DOI] [PubMed] [Google Scholar]
- 39.Sangiorgi G, Roversi S, Biondi Zoccai G, Modena MG, Servadei F, Ippoliti A, et al. Sex-related differences in carotid plaque features and inflammation. J Vasc Surg 2013; 57(2):338–344. [DOI] [PubMed] [Google Scholar]
- 40.Wendorff C, Wendorff H, Pelisek J, Tsantilas P, Zimmermann A, Zernecke A, et al. Carotid plaque morphology Is significantly associated with sex, age, and history of neurological symptoms. Stroke 2015; 46(11):3213–3219. [DOI] [PubMed] [Google Scholar]
- 41.Hanna DB, Guo M, Buzkova P, Miller TL, Post WS, Stein JH, et al. HIV infection and carotid artery intima-media thickness: Pooled analyses across 5 cohorts of the NHLBI HIV-CVD Collaborative. Clin Infect Dis 2016; 63(2):249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wanscher O, Clemmesen J, Nielsen A. Negative correlation between atherosclerosis and carcinoma. Br J Cancer 1951; 5(2):172–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cancer Juhl S. and atherosclerosis: negative correlation. Acta Pathol Microbiol Scand 1955; 37(2):167–181. [PubMed] [Google Scholar]
- 44.Roman MJ, Moeller E, Davis A, Paget SA, Crow MK, Lockshin MD, et al. Preclinical carotid atherosclerosis in patients with rheumatoid arthritis. Ann Intern Med 2006; 144(4):249–256. [DOI] [PubMed] [Google Scholar]
- 45.Mangili A, Polak JF, Quach LA, Gerrior J, Wanke CA. Markers of atherosclerosis and inflammation and mortality in patients with HIV infection. Atherosclerosis 2011; 214(2):468–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu DC, Ma YF, Hur S, Li D, Rupert A, Scherzer R, et al. Plasma IL-6 levels are independently associated with atherosclerosis and mortality in HIV-infected individuals on suppressive antiretroviral therapy. Aids 2016; 30(13):2065–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wikstrand J Methodological considerations of ultrasound measurement of carotid artery intima-media thickness and lumen diameter. Clin Physiol Funct Imaging 2007; 27(6):341–345. [DOI] [PubMed] [Google Scholar]
- 48.Stern R, Tattersall MC, Gepner AD, Korcarz CE, Kaufman J, Colangelo LA, et al. Sex differences in predictors of longitudinal changes in carotid artery stiffness: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 2015; 35(2):478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wada N, Jacobson LP, Cohen M, French A, Phair J, Munoz A. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome-infected and human immunodeficiency syndrome-negative individuals followed simultaneously in long-term cohort studies, 1984–2008. Am J Epidemiol 2013; 177(2):116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stein JH, Currier JS, Hsue PY. Arterial disease in patients with human immunodeficiency virus infection: What has imaging taught us? JACC Cardiovasc Imaging 2014; 7(5):515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Guallar E, Qiao Y, Wasserman BA. Is carotid intima-media thickness as predictive as other noninvasive techniques for the detection of coronary artery disease? Arterioscler Thromb Vasc Biol 2014; 34(7):1341–1345. [DOI] [PubMed] [Google Scholar]
- 52.Deo RC. Machine learning in medicine. Circulation 2015; 132(20):1920–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feinstein MJ, Nance RM, Drozd DR, Ning H, Delaney JA, Heckbert SR, et al. Assessing and refining myocardial infarction risk estimation among patients with human immunodeficiency virus. JAMA Cardiol 2017; 2(2):155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


