Abstract
Inhibitors of the cyclin-dependent kinases 4 and 6 (CDK4/6) were originally designed to block proliferation and cell cycle progression of cancer cells in which the activity of these kinases is dysregulated. CDK4/6 inhibitors have already been FDA-approved for the treatment of estrogen receptor (ER)-positive breast cancer and are being tested in numerous other cancer types. However, several recent studies have identified novel effects of CDK4/6 inhibitors on tumor growth, most notably an indirect effect resulting from the activation of immune surveillance. This Perspective discusses these recent observations, including the effects that CDK4/6 inhibitors may have on immune cells themselves. It is likely that CDK4/6 inhibitors will have a broader impact than their expected induction of cell cycle arrest in the treatment of human cancers.
Main Text
The RB pathway has been elucidated biochemically and genetically in the past 30 years. In the canonical pathway, complexes of Cyclin-dependent kinases (CDKs) and their partner Cyclins (Cyc) —specifically CycD/CDK4,6 and CycE/CDK2—phosphorylate RB (and its family members p107 and p130), resulting in RB inactivation. This allows for the activation of E2F transcription factors that promote the transcription of genes whose products are critical for cell cycle progression in G1/S (reviewed in (1–3)). Accumulating evidence indicates that the RB pathway is more complex than initially described and that non-canonical roles not only exist in cells but also play a role in cancer (3, 4). Nevertheless, inhibitors of CDK4/6 and CDK2 have been developed with the general idea of re-activating the canonical function of RB in cells in which the RB1 gene is not silenced, deleted, or mutated, thereby arresting cancer cells in G0/G1 and blocking tumor growth (5). In particular, a number of selective CDK4/6 inhibitors have been developed and FDA-approved or are in clinical trials in several types of human cancers (e.g. (6–10)). Recent observations indicate that these CDK4/6 inhibitors may have a broader anti-cancer role, beyond their direct anti-proliferative effects on cancer cells.
Immunostimulatory effects of CDK4/6 inhibitors on cancer cells and the tumor microenvironment
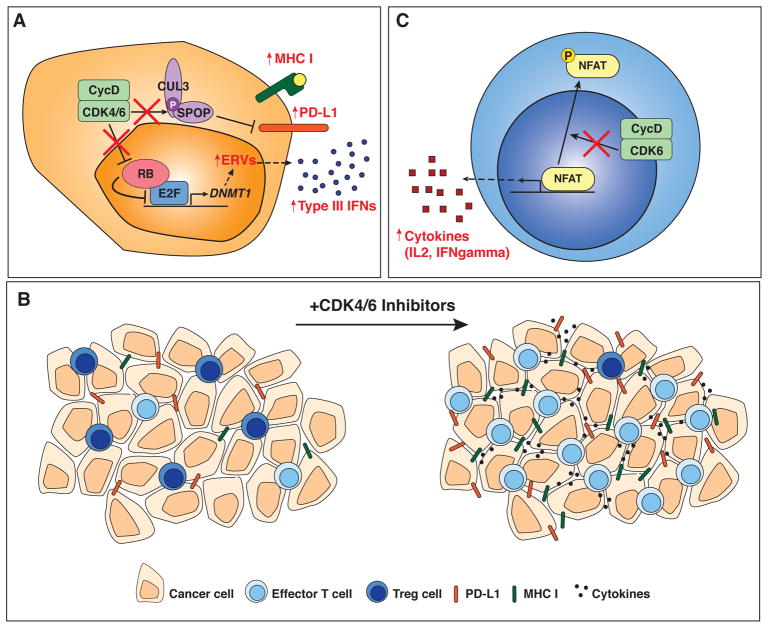
A first study by Goel, De Cristo, and colleagues showed that CDK4/6 inhibition promotes anti-tumor immunity through multiple mechanisms (11). In this study, inhibition of CDK4/6 by abemaciclib in breast cancer cells was associated with an increase in antigen presentation, in addition to the expected downregulation of cell cycle genes. These observations support early studies in which RB activity was connected to increased antigen presentation in response to interferon gamma (12, 13). Mechanistically, the authors of this new study noted an increase in type III interferon molecules upon abemaciclib treatment, which they linked to the induction of endogenous retroviral genes (ERVs) (Figure 1A). This induction of ERVs was mediated by the hypomethylation of their genomic loci due to the down-regulation of the DNA methyltransferase DNMT1, a direct E2F target whose expression is repressed when RB is activated upon CDK4/6 inhibition. Thus, abemaciclib treatment may induce a response similar to an anti-viral response in cancer cells that are RB wild-type. In addition, CDK4/6 inhibition led to a reduction in immunosuppressive regulatory T cells (Tregs) in the tumor microenvironment (Figure 1B), possibly due to an enhanced cell cycle inhibition specifically in these cells due to higher levels of cell cycle molecules such as RB itself. Thus, while this study was focused on RB wild-type cancer cells, some of the effects of CDK4/6 inhibition could lead to therapeutic benefit in RB-deficient tumors as well via the relative inhibition of Tregs versus anti-cancer immune cells.
Figure 1. Non-canonical anti-cancer effects of CDK4/6 inhibitors.
A. In response to CDK4/6 inhibition (indicated by red X’s and red text), cancer cells up-regulate MHC I at their surface, and, in an RB-dependent mechanism (see main text), produce interferons (IFNs). These effects may activate the anti-cancer activity of immune cells. CDK4/6 inhibition also leads to PD-L1 up-regulation, which suggests that CDK4/6 inhibitors may be combined effectively with PD-L1 blockade in the clinic.
B. At the cellular level, treatment with CDK4/6 inhibitors stimulate anti-cancer immune responses in the tumor microenvironment, including a decrease in Tregs, an increase in effector T cells, and higher levels of immunostimulatory molecules, concomitant with increased levels of PD-L1.
C. CDK4/6 inhibition in T cells results in NFAT activation and the production of cytokines that can also enhance the activity of immune cells against cancer cells.
A study by Teo and colleagues, investigating the combination of CDK4/6 and PI3Kalpha inhibitors in triple-negative breast cancer, also identified critical effects of CDK4/6 inhibition on the tumor microenvironment. The authors showed that combination of CDK4/6 and PI3Kalpha inhibitors is synergistic in RB wild-type triple-negative breast cancer cells, in part by promoting immune responses (14). In addition to enhancing the death of cancer cells, this combination therapy led to an increase in T-cell activation and a decrease in immunosuppressive cell populations, such as monocytic myeloid-derived suppressor cells (mMDSCs) and Tregs, through mechanisms that are not yet clear. These effects were observed mainly with combination treatment, and not with CDK4/6 inhibitor alone as reported by Goel et al., which may reflect differences among CDK4/6 inhibitors (ribociclib versus abemaciclib), breast cancer subtypes (triple-negative versus HER2-positive), or a combination of the two. Importantly, Teo et al. demonstrated that the addition of immune-checkpoint blockade (anti-PD1 and anti-CTLA4) to the combination of CDK4/6 and PI3Kalpha inhibitors resulted in a further enhancement of anti-tumor responses, providing additional support for the idea that CDK4/6 inhibition could be used in combination with strategies aiming at stimulating the immune system.
Novel roles for CDK4/6 in immune-related molecular pathways
In a related study, Deng, Wang, Jenkins, and colleagues investigated kinase inhibitors that enhance IL-2 secretion, an indicator of T cell activation, in Jurkat T cells (15). In this screen, they identified CDK4/6 inhibitors (palbociclib and trilaciclib) as activators of IL-2 production, and went on to show that CDK6 can directly phosphorylate and inhibit NFAT4, a known regulator of IL-2 secretion, in both Jurkat cells and primary human CD4+ T cells (Figure 1C). These data suggest a role for CDK6 in the regulation of NFAT activity in T cells, which could provide another mechanism by which CDK4/6 inhibition may enhance T cell activation. The authors also found that treatment with CDK4/6 inhibitors can lead to an increased percentage of effector cells within the tumor microenvironment in mouse models of lung cancer. This increase of effector T cell function occurred even in the presence of Tregs. Combination of PD-1 blockade and CDK4/6 inhibition was synergistic, and the effects of CDK4/6 inhibition were mediated at least in part by T cells, as depletion of CD4+ or CD8+ T cells abrogated this response. Similar to the previous studies, Tregs were shown to be more sensitive to the anti-proliferative effects of CDK4/6 inhibition than other immune cells. As discussed by the authors, it is likely that the increased secretion of IFNgamma from CD8+ T cells, even in the presence of Tregs, depends on properly timed doses of CDK4/6 inhibitors, which will be key for the use of these inhibitors in the clinic.
Similar observations were made by Schaer, Beckmann, and colleagues in colon cancer cells (16): abemaciclib treatment resulted in enhanced intra-tumoral T cell activation and inflammation, and a phased combination therapy regimen with abemaciclib and anti-PD-L1 therapy led to the greatest tumor regression. At the molecular level, this study found a direct effect in T cells that again involved the modulation of NFAT activity and increased expression of IL2 and TNFalpha after abemaciclib treatment. Interestingly, the combination therapy in this study led not only to the direct activation of T cells, but also to the modulation of innate immunity through mechanisms that enhance antigen presentation and T cell priming by dendritic cells. Together, these studies demonstrate that the anti-cancer effects of CDK4/6 inhibitors through T cells, and perhaps other immune cells, may apply to several cancer types, thereby extending the reach of CDK4/6 inhibitors in the clinic.
Zhang, Bu, Wang, and colleagues identified another novel function of CDK4/6 by exploring mechanisms that control PD-L1 expression and stability (17). The authors first showed that PD-L1 levels fluctuate with cell cycle progression, with higher levels in M and early G1 phases. They identified an inverse correlation between CDK4/6 activity and PD-L1 expression in cancer cells. Genetic ablation of D-type cyclins or CDK4/6 inhibition resulted in increased PD-L1 expression and a reduction in CD3+ tumor-infiltrating lymphocytes (TILs) in a breast cancer model in vivo. This reduction in TILs seems to contradict the increased anti-tumor immunity reported by other studies. However, because CD3 is expressed by both Tregs and effector T cells, the decrease in CD3+ cells may result largely from the decreased proliferation of Tregs. Other measures of effector T cell activity were not reported in this study. Mechanistically, the authors identified the Cullin 3-based E3 ligase and the adaptor Speckle-type POZ protein (SPOP) as key inhibitors of PD-L1 protein levels. CycD/CDK4/6 directly phosphorylates SPOP, which promotes the stability of SPOP itself. Palbociclib treatment reduces this phosphorylation, which destabilizes SPOP, and therefore stabilizes PD-L1 (Figure 1A). Importantly, tumors with mutations in the C-terminal PD-L1 degron are mutually exclusive with tumors with mutations in the substrate-binding MATH domain of SPOP, suggesting a functional link. It is thus possible that the enhanced tumorigenic effect of SPOP mutations are due to increased PD-L1 levels and attenuation of immune responses (Figure 1B). This work identifies an additional level of regulation of the immune system by CDK4/6 and another instance in which combination of CDK4/6 inhibition and PD-L1 blockade could result in increased anti-tumor responses.
Protecting immune cells from radiation or chemotherapy with CDK4/6 inhibitors
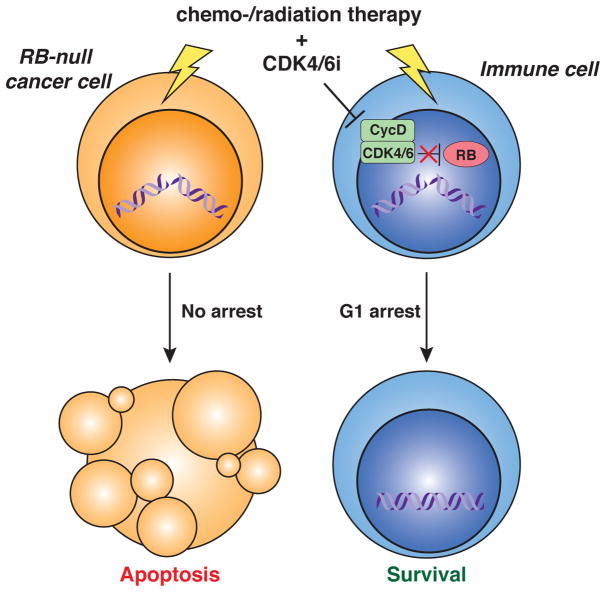
These studies are particularly timely given the recent clinical trials by G1 Therapeutics with their CDK4/6 inhibitor trilaciclib (also known as G1T28). In RB-deficient tumors, inhibition of CDK4/6 activity rarely affects the proliferation of the cancer cells themselves, but may stop the proliferation of T cells. While such an induction of cell cycle arrest can be detrimental in the long-term, it could prove beneficial in the short-term. Treatment with radiation therapy or chemotherapy kills proliferating cells, including T cells, and the addition of CDK4/6 inhibitors may protect immune cells from these effects by slowing their cell cycle and allowing more time for DNA repair (Figure 2). This elegant concept is supported by strong pre-clinical and clinical data (18–20), which show preservation of both lymphoid and myeloid lineages. Again, the dosing schedule will be critical for providing maximum benefit to the immune system while avoiding negative side effects, but the current clinical data suggest that CDK4/6 inhibitors could prove beneficial in a wider range of cancers than previously considered. While this strategy is promising, it is worth noting that prolonged treatment with CDK4/6 inhibitors may induce senescence in stromal cells, which, in some cases, may promote the growth of cancer cells (21).
Figure 2. Using canonical effects of CDK4/6 inhibitors in RB-deficient cancer.
In RB-deficient cancer cells, inhibition of CDK4/6 (CDK4/6i) cannot activate the G1 checkpoint and does not result in cell cycle arrest; in these cells, chemotherapy and/or radiation can induce DNA damage and subsequent cell death. In contrast, immune cells arrest in G1 after CDK4/6i (e.g. trilaciclib –see main text), which can provide some protection from the death-inducing effects of chemo-/radiation therapy, including by preventing DNA damage that would otherwise occur in S-phase or by providing more time to repair DNA damage.
Concluding remarks
In conclusion, CDK4/6 inhibitors – and perhaps CDK2 inhibitors once more specific compounds are developed – are certainly attractive molecules to slow or stop the growth of cancer cells that are wild-type for RB function. However, in these tumors, and possibly in RB mutant tumors, CDK4/6 inhibition may also enhance the anti-cancer effects of the immune system by increasing antigen presentation and cytokine secretion by cancer cells. While some level of immune system activation may be expected in cases where CDK4/6 inhibitors induce senescence, increased anti-cancer immunity is observed even in the absence of the senescence-associated secretory phenotype (SASP) (10,14). Furthermore, CDK4/6 inhibitors may have direct effects on non-cancer cells in the tumor microenvironment, particularly anti-tumor immune cells, which could lead to new combination therapies in a broad range of cancer patients. Of course, these effects may be complex and not always anti-cancer. First, RB activity may control the expression of genes involved in multiple immune response pathways (22). Second, CDK4/6 inhibitors may affect neutrophils and neutrophil extracellular traps (NETs) (23), which may promote or inhibit cancer depending on the context (24). Third, accumulating evidence shows that oncogenic pathways influence the interactions between cancer cells and immune cells, directly and indirectly (25). Many additional studies will be required to dissect the complex effects of CDK4/6 inhibitors on human tumors, including those recently reviewed by Klein and colleagues (26), but these recent findings support the idea that these inhibitors may be more potent anti-cancer molecules than previously anticipated.
Acknowledgments
The authors thank members of the Sage lab and Laurent Le Cam for critical comments on the manuscript. This work was supported by the NIH (CA114102 and CA213273 to J.S.) and the NSF (GRFP to A.C.C.). J.S. is the Harriet and Mary Zelencik Scientist in Children’s Cancer and Blood Diseases. The authors declare no financial competing interests.
References
- 1.Burkhart DL, Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer. 2008;8:671–82. doi: 10.1038/nrc2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dick FA, Rubin SM. Molecular mechanisms underlying RB protein function. Nat Rev Mol Cell Biol. 2013;14:297–306. doi: 10.1038/nrm3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dyson NJ. RB1: a prototype tumor suppressor and an enigma. Genes Dev. 2016;30:1492–502. doi: 10.1101/gad.282145.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dick FA, Goodrich DW, Sage J, Dyson NJ. Non-canonical functions of the RB protein in cancer. Nat Rev Cancer. 2018 doi: 10.1038/s41568-018-0008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: From Discovery to Therapy. Cancer Discov. 2016;6:353–67. doi: 10.1158/2159-8290.CD-15-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016;375:1925–36. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 7.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med. 2016;375:1738–48. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 8.Sledge GW, Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2− Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol. 2017;35:2875–84. doi: 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 9.Goldman JW, Shi P, Reck M, Paz-Ares L, Koustenis A, Hurt KC. Treatment Rationale and Study Design for the JUNIPER Study: A Randomized Phase III Study of Abemaciclib With Best Supportive Care Versus Erlotinib With Best Supportive Care in Patients With Stage IV Non-Small-Cell Lung Cancer With a Detectable KRAS Mutation Whose Disease Has Progressed After Platinum-Based Chemotherapy. Clin Lung Cancer. 2016;17:80–4. doi: 10.1016/j.cllc.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Dickler MN, Tolaney S, Rugo HS, Cortés J, Dieras V, Patt DA, et al. MONARCH 1, a phase 2 study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2-metastatic breast cancer. Clinical Cancer Research. 2017 doi: 10.1158/1078-0432.CCR-17-0754. clincanres. 0754.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548:471–5. doi: 10.1038/nature23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Y, Ussery GD, Muncaster MM, Gallie BL, Blanck G. Evidence for retinoblastoma protein (RB) dependent and independent IFN-gamma responses: RB coordinately rescues IFN-gamma induction of MHC class II gene transcription in noninducible breast carcinoma cells. Oncogene. 1994;9:1015–9. [PubMed] [Google Scholar]
- 13.Zhu X, Pattenden S, Bremner R. pRB is required for interferon-gamma-induction of the MHC class II abeta gene. Oncogene. 1999;18:4940–7. doi: 10.1038/sj.onc.1202876. [DOI] [PubMed] [Google Scholar]
- 14.Teo ZL, Versaci S, Dushyanthen S, Caramia F, Savas P, Mintoff CP, et al. Combined CDK4/6 and PI3Kalpha Inhibition Is Synergistic and Immunogenic in Triple-Negative Breast Cancer. Cancer Res. 2017;77:6340–52. doi: 10.1158/0008-5472.CAN-17-2210. [DOI] [PubMed] [Google Scholar]
- 15.Deng J, Wang ES, Jenkins RW, Li S, Dries R, Yates K, et al. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer Discov. 2018;8:216–33. doi: 10.1158/2159-8290.CD-17-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaer DA, Beckmann RP, Dempsey JA, Huber L, Forest A, Amaladas N, et al. The CDK4/6 Inhibitor Abemaciclib Induces a T Cell Inflamed Tumor Microenvironment and Enhances the Efficacy of PD-L1 Checkpoint Blockade. Cell reports. 2018;22:2978–94. doi: 10.1016/j.celrep.2018.02.053. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Bu X, Wang H, Zhu Y, Geng Y, Nihira NT, et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature. 2018;553:91–5. doi: 10.1038/nature25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bisi JE, Sorrentino JA, Roberts PJ, Tavares FX, Strum JC. Preclinical Characterization of G1T28: A Novel CDK4/6 Inhibitor for Reduction of Chemotherapy-Induced Myelosuppression. Mol Cancer Ther. 2016;15:783–93. doi: 10.1158/1535-7163.MCT-15-0775. [DOI] [PubMed] [Google Scholar]
- 19.He S, Roberts PJ, Sorrentino JA, Bisi JE, Storrie-White H, Tiessen RG, et al. Transient CDK4/6 inhibition protects hematopoietic stem cells from chemotherapy-induced exhaustion. Sci Transl Med. 2017:9. doi: 10.1126/scitranslmed.aal3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts P, Lai A, Sorrentino J, Malik R. 29IN Trilaciclib (G1T28), a CDK4/6 inhibitor, enhances the efficacy of combination chemotherapy and immune checkpoint inhibitor treatment in preclinical models. Annals of Oncology. 2018:29. mdy046. 13. [Google Scholar]
- 21.Guan X, LaPak KM, Hennessey RC, Yu CY, Shakya R, Zhang J, et al. Stromal Senescence By Prolonged CDK4/6 Inhibition Potentiates Tumor Growth. Mol Cancer Res. 2017;15:237–49. doi: 10.1158/1541-7786.MCR-16-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutcheson J, Witkiewicz AK, Knudsen ES. The RB tumor suppressor at the intersection of proliferation and immunity: relevance to disease immune evasion and immunotherapy. Cell Cycle. 2015;14:3812–9. doi: 10.1080/15384101.2015.1010922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amulic B, Knackstedt SL, Abu Abed U, Deigendesch N, Harbort CJ, Caffrey BE, et al. Cell-Cycle Proteins Control Production of Neutrophil Extracellular Traps. Dev Cell. 2017;43:449–62 e5. doi: 10.1016/j.devcel.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Erpenbeck L, Schon MP. Neutrophil extracellular traps: protagonists of cancer progression? Oncogene. 2017;36:2483–90. doi: 10.1038/onc.2016.406. [DOI] [PubMed] [Google Scholar]
- 25.Spranger S, Gajewski TF. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat Rev Cancer. 2018;18:139–47. doi: 10.1038/nrc.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein ME, Kovatcheva M, Davis LE, Tap WD, Koff A. CDK4/6 Inhibitors: The Mechanism of Action May Not Be as Simple as Once Thought. Cancer Cell. 2018 doi: 10.1016/j.ccell.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]