Abstract
Fungal infections are a continuously increasing problem in modern health care. Understanding the complex biology of the emerging pathogens and unraveling the mechanisms of host defense may form the basis for the development of more efficient diagnostic and therapeutic tools. Neutrophils play a pivotal role in the defense against fungal pathogens. These phagocytic hunters migrate towards invading fungal microorganisms and eradicate them by phagocytosis, oxidative burst and release of neutrophil extracellular traps (NETs). In the last decade, the process of NET formation has received unparalleled attention, with numerous studies revealing the relevance of this neutrophil function for control of various mycoses. Here, we describe NET formation and summarize its role as part of the innate immune defense against fungal pathogens. We highlight factors influencing the formation of these structures and molecular mechanisms employed by fungi to impair the formation of NETs or subvert their antifungal effects.
Keywords: Neutrophil, neutrophil extracellular trap (NET), immunology, Candida, Aspergillus
1. Introduction
The innate immune system comprises an effective shield against fungal organisms that may otherwise invade tissues of our bodies. Physical, chemical, and microbial barriers cooperate with myeloid immune cells to recognize and eliminate fungal pathogens. Among these cells, polymorphonuclear leukocytes (neutrophils) play a decisive role. The importance of neutrophils for prevention and clearance of invasive fungal infections is widely recognized (1, 2). Patients with neutropenia are at high risk for contracting fatal fungal infections and prolonged neutropenia is associated with poor outcome. However, aspects of how neutrophils control the growth of this diverse group of pathogens, which are often too large to be phagocytosed, remained somewhat of a mystery until the discovery of neutrophil extracellular traps (NETs) (3). NET formation provides a means for neutrophils to kill fungi extracellularly, and the significance of this process in host immunity has been described for a variety of fungal infections, including candidiasis and aspergillosis (4–6). In this article, we review the neutrophil response to fungal pathogens, with a focus on the role of NETs. We describe the current understanding of factors stimulating NET release and mechanisms employed by fungi to resist killing by NETs.
2. Neutrophils and NETs
Neutrophils outnumber other white blood cells in circulation and are recruited in large numbers to sites of infection by chemokine gradients, where they serve as a first line of defense (7). In infected tissues, neutrophils activate their granule-stored weaponry via pattern recognition and cytokine receptor engagement, either by secretion of granule contents extracellularly, or by fusion of granules with pathogen-containing vesicles, so called phagosomes (8). The granules contain a variety of antimicrobial substances, including short antimicrobial peptides (AMPs) and proteolytic or nucleolytic enzymes (9). Upon stimulation of pattern recognition receptors, downstream kinase activation and Ca2+-mediated signaling trigger neutrophils to assemble a large protein complex known as Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase or phagocyte oxidase (Phox) (10). Components of this complex are both cytoplasmic (p40, p47 and p67) and membrane-bound (gp91 and p22). Assembly occurs on plasma and granular membranes, resulting in a functional enzymatic multimeric protein that reduces molecular oxygen to superoxide anion (11). The neutrophil enzymes superoxide dismutase and myeloperoxidase (MPO) further convert these highly reactive radicals to hydrogen peroxide and hypochloric acid, respectively. Collectively known as reactive oxygen species (ROS), the mix of these intermediates act both as efficient antimicrobials and as short-lived signaling molecules. Reactive oxygen species actively support the elimination of ingested microbes and promote the activation of pro-inflammatory processes (12). Although transcriptionally less active than other myeloid cells, neutrophils launch specific pathogen-tailored transcriptional responses upon microbial contact. Transcriptional regulation of cellular transport and cytokine production prepares neutrophils for battle and prompts the recruitment of additional immune cells (13).
In addition to degranulation, phagocytosis, ROS generation, and cytokine production, pathogen-induced activation of neutrophils also initiates cellular processes to expel chromatin to the exterior for NET release. Before release, these chromatin threads are decorated with antimicrobial components from granules and the cytoplasm (4). The cationic nature of histones and other antimicrobial proteins promote their attachment to DNA. The NET fibers then bind to the anionic surfaces of microbes, trapping and entangling them (Figure 1). Neighboring antimicrobials can also arrest growth or kill captured microbes (14). This extracellular chromatin meshwork has been implicated as an extracellular defense mechanism (3).
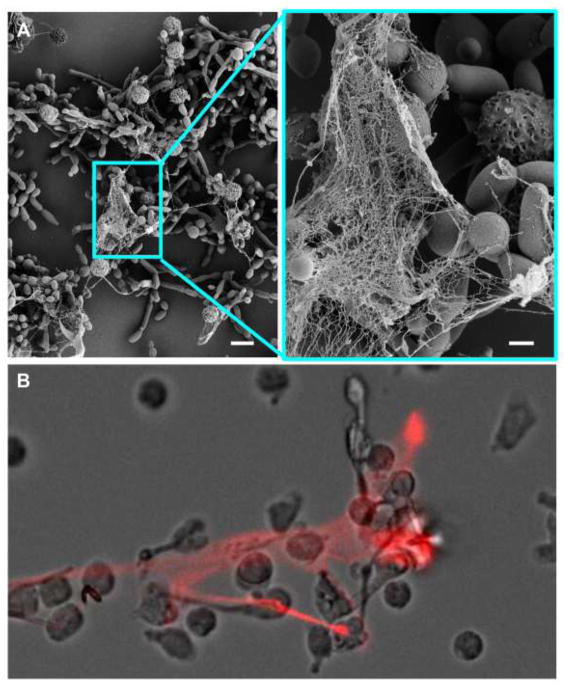
Figure 1. Neutrophils release NETs in response to C. albicans.
A) Scanning electron microscopy images reveal the formation of NETs following a 4 h incubation of human neutrophils with C. albicans. Measurement bars represent 10 μm and 2 μm for images obtained at 2,000 (left) and 10,000x (right), respectively. (B) Propidium iodide (red) staining shows the extracellular DNA of NETs released by human neutrophils upon exposure of human neutrophils to C. albicans.
Since the discovery of NETs in 2004, several release mechanisms have been described. The first reported general mechanism involves chromatin decondensation accompanied by disassembly of the nuclear envelope, which is followed by release of NETs upon plasma membrane rupture (15). This process generally lasts several hours and, as it results in neutrophil death, is referred to as “suicidal NETosis” (16). This form of NETosis is dependent on a functional Phox, as neutrophils from immune-deficient chronic granulomatous disease patients lacking functional Phox do not release NETs to either bacteria or phorbol 12-myristate 13-acetate (PMA), an activator of Protein Kinase C (15). As a consequence of kinase activation and Phox assembly, granules become leaky and allow serine protease neutrophil elastase (NE) to enter the nucleus, where it cleaves histones (17). This cleavage in turn supports chromatin decondensation, which is further propelled by MPO entering the nucleus. A membrane-associated, MPO-containing complex of cationic granule proteins enables NE release from granular vesicles in the absence of membrane fusion. In the cytoplasm, NE degrades actin filaments to arrest cellular movement and facilitate NE’s nuclear translocation (18).
Later, a mechanism of NET formation by living, rather than dead neutrophils, was described (19). During this process, neutrophils expel their mitochondrial DNA by a catapult-like mechanism which had earlier been described for eosinophil DNA trap release (20). Upon priming with GM-CSF and stimulation with LPS or anaphylatoxin, mitochondrial DNA traps are released rapidly, within 15 minutes of exposure. Similar to suicidal NETosis, this process appears to be Phox-dependent (19). In contrast, a ROS-independent mechanism of NET release occurs upon stimulation with certain bacteria. Here, nuclear DNA is packaged into vesicles, which fuse with the plasma membrane and release their DNA content to the exterior (21). In doing so, remaining neutrophil ghosts can still migrate and phagocytose (22). Bacterial toxins that create pores in host cell membranes can also induce NET-like structures. This mechanism appears to be independent of ROS (23, 24), and similar to induction of NETs by the activation of calcium channels. Consecutive calcium influx induces NETs in the absence of a functional Phox system (25). In contrast to PMA-induced NETosis, fewer mechanistic studies have shed light on how the process of fast DNA release, whether ROS-dependent or not, may occur. However, downstream of ROS-dependent and – independent mechanisms, enzymatic histone modification appears to be essential for chromatin decondensation and subsequent release of NETs (26). The protein arginine deaminase 4 (PAD4) catalyzes the conversion of arginine residues to citrulline, mainly on core histones. The importance of PAD4 for NET release has been implicated in several models of infection and other diseases (27). A recent review article, however, suggests that several cellular processes that expel DNA may only mimic NET release. Proposed mimics include expelled mitochondrial DNA following cytokine or anaphylotoxin stimulation and excreted nuclear DNA resulting from pore-forming toxins or calcium ionophors (28). The authors suggest that pore-forming toxins induce calcium influx and subsequent activation of PAD4 leading to hypercitrunillation. As a consequence of histone citrullination, chromatin undergoes decondensation and is excreted. Conversely, ROS-dependent NETosis and release of mitochondrial DNA do not appear to involve PAD4 activation or histone citrullination.
Other myeloid cells, including eosinophils and mast cells, have also been described to release DNA traps. However, neutrophils seem to be able to release the structures more efficiently than other cell types, arguing for a specific relevance of NETs among the neutrophil defense mechanisms (20, 29, 30). Given the versatility of neutrophils and the wide range of host niches where these granulocytes are required to function flawlessly, it is likely that they respond differently to a variety of stimuli. After all, the existence of redundant yet differentially-triggered pathways may ensure full neutrophil functionality in any possible host milieu.
3. Relevance of NETs during mycoses
3.1 Candidiasis
Candida spp. are microbial commensals of the gastrointestinal tract that can colonize the genitourinary tract and skin (31, 32). However, in the face of immunosuppression, Candida spp. frequently can cause mucosal disease or more severe invasive infection. Neutropenia, often due to chemotherapy or hematologic malignancy, places patients at particularly high risk for life-threatening disseminated candidiasis (33). Candida spp. rank as the third most common bloodstream infection in hospital setting, with C. albicans as the predominant pathogen (31). A role for NETs in the response to fungi was first described for this model pathogen in vitro, with subsequent investigations revealing the importance of this process for control of candidiasis in vivo (Figure 1) (4, 34).
Neutrophils release NETs in response to C. albicans in murine models of localized and disseminated candidiasis (4). In a subcutaneous abscess model of infection, neutrophils are recruited to the site of infection and align on the periphery of fungal foci. Imaging reveals the presence of web-like structures of DNA that co-localize with NET-associated proteins, including MPO, histones, and calprotectin. Similar structures can also be found in the lungs of mice with disseminated candidiasis (4, 6). Much of the importance of this neutrophil process for control of Candida resides in the delivery of the antimicrobial protein calprotectin (4). This protein complex (S100A8/A9) is a divalent metal ion chelator with potent activity against a variety of fungal pathogens, including C. albicans, C. neoformans, and Aspergillus spp. (4, 35, 36). Once in close proximity, calprotectin exerts antifungal activity through depletion of Zn2+ and/or Mn2+, which are essential for proliferation of these pathogens (4). Mice deficient in the production of calprotectin fail to deliver NET-associated calprotectin and exhibit a more rapid progression of subcutaneous C. albicans abscesses. The phenotype of calprotectin-deficiency is even more pronounced in a disseminated candidiasis model, where mice succumb to disseminated candidiasis twice as quickly as wildtype mice. These findings highlight the necessity of NETs for control of both superficial and invasive candidiasis, through their role in calprotectin release and delivery.
In the host, C. albicans displays multiple morphotypes, including yeast, hyphae, and pseudohyphae. While yeast forms are actively engulfed by phagocytosis, NETs appear to be critical for the killing and containment of the larger hyphal forms (6, 37). In one model of disseminated candidiasis, NETs are released in response to wildtype C. albicans, which produces filamentous forms during the course of pulmonary infection (6). However, NETs are not produced during infection with a yeast-locked mutant (hcg1Δ). Furthermore, NET production is a requirement for immune control of filamentous growth in vivo. Mice deficient in NET production through disruption of either MPO or Phox succumb to invasive candidiasis. In contrast, neutrophil attack against yeast morphotypes appears to function independent of NETosis, as MPO-deficient mice are capable of clearing infection caused by the yeast-locked mutant, presumably through phagocytosis. In addition, NETs are able to remodel the cell wall composition of C. albicans upon contact, leading to unmasking of β-glucan and enhanced recognition by Dectin-1-positive immune cells (38). In a model of disseminated candidiasis, these neutrophil-induced cell wall changes appear to be governed by activation of the C. albicans MAP kinase signaling pathway. Together, these studies demonstrate neutrophil-C. albicans interactions are influenced by fungal morphology and that subsequent neutrophil responses provoke fungal cell wall alterations, which in turn influence immunity.
While the majority of studies have focused on C. albicans, work with several non-albicans Candida spp. has revealed a likely role for NETs during candidiasis caused by these pathogens as well (39, 40). Although C. dubliniensis is capable of inducing NETs, the degree of NET induction is greatly reduced from that observed for C. albicans (39). This difference may be attributed to a difference in filamentation, as C. dubliniensis generates fewer hyphal forms. Surprisingly, C. glabrata, which lacks the ability to filament, is capable of eliciting NETs through a phagocytosis-dependent pathway (40). The mechanism underpinning why neutrophils respond differently to yeast forms of C. albicans and C. glabrata in vitro remains a mystery. Given the distinct neutrophil responses to these pathogens, further investigation of the role of NETs for control of candidiasis caused by non-albicans spp. would be of great interest.
3.2 Aspergillosis
Aspergillus spp. are ubiquitous environmental fungi that release spores, which are continuously inhaled and cleared by people with healthy immunity (41). However, patients with impaired immunity who do not efficiently eradicate these spores from their lungs develop invasive aspergillosis, a life-threatening angioinvasive infection (41–43). A large cohort at risk includes patients with neutropenia or hematologic malignancy, for whom A. fumigatus represents the most common pathogen (41). A second group of patients at risk are those who suffer from chronic granulomatous disease (CGD) (44). These patients have impaired Phox function, resulting in poor NET production and reduced neutrophil activity (15). In patients with this inherited disorder, A. nidulans emerges as a major pathogen, often resulting in refractory, disseminated disease (45).
Clinical studies and investigation with animal models of aspergillosis have shed light on the significance of NETs for containment and clearance of both of these pathogenic species (5, 36, 46, 47). In a clinical study involving a patient with CGD suffering from refractory invasive A. nidulans infection, Bianchi et al. linked the production of NETs to the resolution of invasive aspergillosis (46). In vitro, the Phox-deficient neutrophils lacked activity against A. nidulans conidia and hyphae. Restoration of Phox function by genetic complementation restored both NET production and antifungal activity. Furthermore, administration of gene therapy providing Phox activity rapidly cured the patient with treatment-refractory A. nidulans infection. Subsequent investigation revealed calprotectin (S100A8/A9) as the key antifungal component of NETs accounting for the activity against A. nidulans (36). By chelating Zn2+, calprotectin inhibits A. nidulans growth and can induce irreversible zinc starvation at higher concentrations.
In a murine model of pulmonary aspergillosis, neutrophils induce NETs upon encounter with A. fumigatus (Figure 2) (5). Observations made by 2-photon microscopy show that NETs form in conjunction with developing clusters of fungi with outgrowing hyphae. NETs are produced by newly recruited neutrophils, approximately 3–4 hours after migration to the site of infection. In contrast, conidia are engulfed by neutrophils. The formation of NETs in vivo to the larger hyphal forms of A. fumigatus is consistent with in vitro studies demonstrating a more robust NET release to hyphae over conidia, which would presumably be adequately cleared by phagocytosis (5, 35). In vitro, NETs exhibit fungistatic activity and are hypothesized to prevent fungal dissemination (5, 35). Similar to A. nidulans, A. fumigatus is inhibited by the NET-associated calprotectin (35). NETs also appear to modulate host immunity to A. fumigatus through release of long pentraxin (PTX) 3, a pattern recognition receptor that activates complement and facilitates pathogen recognition (48). The triggering of NETs in response to A. fumigatus is Phox-dependent (47). In a murine model of pulmonary aspergillosis, p47phox−/− mice deficient in Phox fail to generate NETs and ultimately develop progressive pneumonia. In vitro studies similarly show a requirement for Phox during induction of NETs by A. fumigatus.
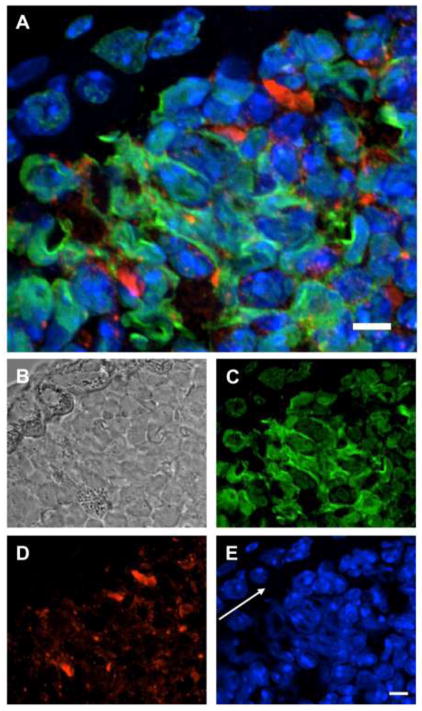
Figure 2. Neutrophils release NETs during A. fumigatus infection.
Immunohistochemistry was performed on bronchioles of mice with invasive pulmonary aspergillosis induced by nasal infection with hyphal filaments. MPO (red) and histone (green) were stained with specific antibodies and fluorescently-labelled secondary antibodies. The nuclear contents of cells are stained with DAPI (blue). In patchy areas NET-associated proteins MPO, histone and DAPI co-localize extracellularly as depicted by superimposition of all three fluorescent channels (A), phase contrast (B), histone (C), MPO (D) and DAPI (E). White arrow indicates direction of epithelial layer. Size bars represent 5 μM.
3.3 Other mycoses
While the majority of investigations examining the relevance of NETs in fungal mycoses have focused on candidiasis and aspergillosis, NETs are anticipated to be a player in other fungal infections as well. For example, NETs can be visualized in the corneal scrapings from patients with fungal keratitis, a sight-threatening infection caused by a variety of fungal species, including Aspergillus, Fusarium, Candida, and Alternaria (49). The degree of NET release appears to vary with regard to the infecting pathogen and may correlate with clinical cure. However, little is yet known about the influence of NET release on inflammation and the clinical course of fungal keratosis.
Recently, investigations have been performed to determine the role of NETs in the host response to paracoccidioidomycosis (50–52). Paracoccidioides spp. are dimorphic environmental pathogens endemic to many areas of Latin America. After inhalation of spores, Paracoccidioides spp. can propagate as yeast, establishing pulmonary infection which may progress to disseminated disease (53). One of the hallmarks of dissemination is cutaneous paracoccidioidomycosis. Using histopathological samples collected from patients with these cutaneous lesions, Della Coletta et al. revealed the formation of NETs (52). However, the importance of NETs for the clearance of paracoccidioidomycosis remains unclear. While both conidia and yeast induce NETs in vitro, P. brasiliensis is not susceptible to attack by NETs (50–52). Thus, it seems plausible that NETs function to contain P. brasiliensis during infection, preventing dissemination.
A role for NETs in the control of cryptococcosis has also been explored (4, 54, 55). This environmental pathogen propagates in the host as a yeast capable of causing pulmonary disease and disseminated disease with meningitis (56). Patients at particular risk include those with immunity impaired by either human immunodeficiency virus (HIV) or transplant-related immunosuppressant medications (56). While NETs can be induced in response to C. neoformans under some conditions, the capsule of C. neoformans is a potent inhibitor of NETosis (54). In contrast, C. gattii appears to trigger the release of NETs, but possesses virulence factors that resist killing by neutrophils (55). Whether NETs form in response to Cryptococcus spp. in vivo and the importance of this neutrophil process in cryptococcosis are areas of interest.
4. NETs and fungal pathogens
4.1 Induction of NETs by fungi
There have been many attempts to describe induction of NETs by different fungal pathogens (table 1). However, a one ligand-to-one pattern recognition receptor concept seems unlikely given the diversity of reports. NET induction by a fungus was first observed for C. albicans, a multi-morphic fungal pathogen (34). While relatively little is known about chlamydospore and pseudohyphal development in C. albicans, the transition from yeast growth to hyphal filamentation has been extensively studied. Invasion of and damage inflicted on epithelium and other host cells largely correlates with hyphal growth of C. albicans (57). Both yeast and hyphal forms induced NETs and were susceptible to inhibition by NETs (34). It is tantalizing to assume that the hyphal filaments, often too large to be ingested, may constitute a main target of NETs, in an attempt to compensate for the less efficient phagocytic uptake. Indeed, hyphae are more prone to trigger NET release (Figure 3) (58). This observation seems to be dependent on ROS, since hyphae induce increased ROS production by neutrophils when compared to yeast forms. This is in line with other reports showing that C. albicans yeasts can suppress ROS production by phagocytes (59) and that large amounts of yeasts are more efficient in ROS scavenging than hyphae (60).
Table 1.
Summary of fungi inducing / inhibiting NETs and their respective susceptibility to NET attack.
| Fungal organism | NET induction | Susceptibility to NETs | Reference | |
|---|---|---|---|---|
| Potency | Comment | |||
| Aspergillus fumigatus | ++ | Hyphae better inducers than conidia, GAG on cell surface shields off NETs | − | (5, 35, 89) |
| Aspergillus nidulans | ++ | Hyphae better inducers than conidia | ++ | (36, 46) |
| Arthroderma benhamiae | ++ | Conidia and hyphae induce NETs to similar extent | unknown | (122) |
| Candida albicans | ++ | Hyphae better inducers than yeast Yeast-locked mutants fail to induce Biofilms inhibit NETs | ++ | (6, 34, 58, 65) |
| Candida dubliniensis | + | Decreased NET induction compared to C. albicans | ++ | (39) |
| Candida glabrata | ++ | Yeast induce NETs more than biofilms | + | (40) |
| Cryptococcus neoformans | − | Capsule prevents NET formation | ++ | (54) |
| Cryptococcus gattii | ++ | Extracellular fibrils induce NETs and prevent NET attack | − | (55) |
| Paracoccidioides brasiliensis | ++ | Both conidia and yeast induce NETs | − | (52) |
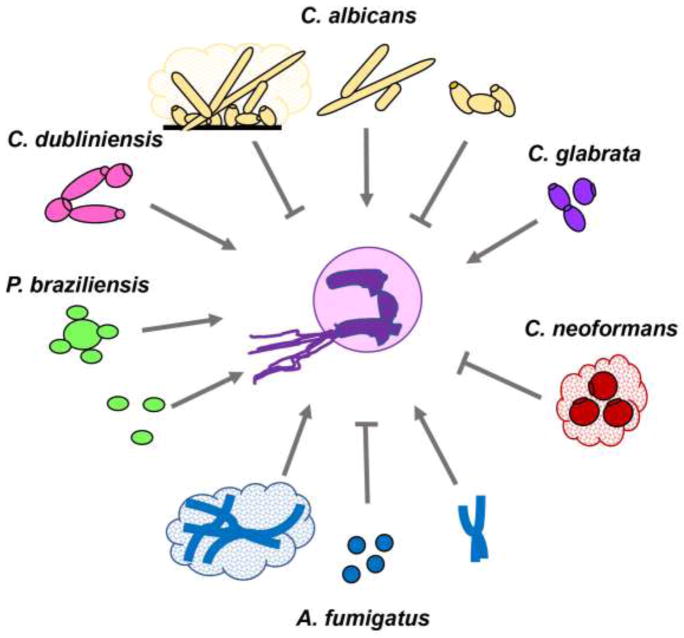
Figure 3. Induction and inhibition of NET release in response to fungal pathogens based on morphology.
C. albicans, C. dubliniensis, and C. glabrata induce NET formation. Yeast forms of C. albicans can inhibit NET release through engagement of Dectin-1 and subsequent induction of phagocytosis. In addition, yeast efficiently scavenge ROS which is required for NET induction. The capsule of C. neoformans inhibits NET release. A. fumigatus hyphae and biofilms trigger NET release. However, A. fumigatus conidia inhibit formation and are engulfed by phagocytosis. Both conidia and yeast forms of P. braziliensis induce NETs.
A recent report shed light on neutrophil signaling pathways involved in the more robust NET release observed in response to C. albicans hyphae when compared to yeast forms (6). Examination of a yeast-locked mutant strain revealed that one mechanism underlying this size-sensing programming involves the engagement of Dectin-1 and the initiation of phagocytosis. This process downregulates NE translocation to the nucleus, which prevents chromatin decondensation and NET release. The pathway is suggested to inhibit NET release when organisms can be effectively eliminated by phagocytosis, ultimately preventing uncontrolled NETosis and associated tissue damage. The relevance of cell size for NET induction has also been shown for A. nidulans (46) and A. fumigatus (5). Similar to C. albicans, long Aspergillus filaments readily induce NET release, whereas the relatively small conidia are poor inducers (5).
While numerous investigations have considered the influence of fungal morphology on NET induction, the neutrophil receptors triggered by fungal ligands and subsequent signaling pathways that initiate NET release remain more obscure. In the presence of the extracellular matrix protein fibronectin, neutrophils respond faster to C. albicans and release NETs more rapidly as compared to the suicidal NETosis induced by PMA (61). This fast induction can be induced by β-glucan particles and soluble β-glucan molecules via complement receptor 3 (CR3) mediated signaling as elucidated using blocking anti-CR3 antibodies. Additionally, this form of NET formation appears to be independent of Phox-derived ROS. Subsequent study has confirmed that particulate β-glucan triggers NETs and that this induction is dependent on Syk kinase, as demonstrated by the use of pharmacological inhibition (62). However, diverging engagement of neutrophil signaling pathways upon glucan particles and the various C. albicans morphotypes is likely to be observed, considering the influence of fungal size and morphology on NET production.
A recent study has explored the identities of neutrophil cell surface receptors that might be involved in the triggering of NETosis following fungal recognition. As may be expected, numerous C. albicans ligands exhibit the potential to induce NET formation. According to Zawrotniak and colleagues, C. albicans derived β-glucan, mannan, and certain cell wall proteins are all individually capable of inducing NETs (63). In this investigation, the β-glucan triggering of NETs could be partially blocked by specific antibodies directed against the cell surface lectin Dectin-1, the prime receptor for β-glucan. This is somewhat in contradiction to the size-sensing mechanism described by Branzk et al. (6), which reported that Dectin-1 activation during phagocytosis inhibited NET formation, whereas Dectin-1 blockage induced NETs. The reason why Dectin-1 inhibition results in differing neutrophil responses under these experimental conditions remains unclear.
In addition to cell wall polysaccharides, secreted aspartic proteases (SAPs) of C. albicans are capable of triggering NETs (63). C. albicans produces ten different SAPs, with expression patterns dependent on environmental conditions. All SAPs, with the exception of SAP3, 5 and 7, can induce NETosis. In contrast to β-glucans, for which Dectin-1 mediates the signal for NET triggering, SAPs appear to engage mainly the β-2 integrin CR3 (63) in accordance with the previously mentioned study (61). However, little is known about how these SAPs may trigger NETosis and if they play a role in immunity through NET induction in vivo. Further investigation in this area will be of interest.
Recently, Moyes et al. discovered the first cytolytic peptide toxin produced by C. albicans, which has been termed candidalysin (64). As bacterial cell-lytic toxins induce NET-like structures when exposed to human neutrophils (23), it seems logical that candidalysin may contribute to the NET release we observe when neutrophils and C. albicans are co-cultured. To date, however, it remains to be determined whether candidalysin exposure can trigger NET release in neutrophils. Furthermore, it is unclear if other pathogenic fungi produce similar cell-lytic toxins that may influence NET production.
4.2 Fungal strategies for modulation and inhibition of NET induction
Although NETs can provide targeted delivery of calprotectin and exhibit potent antifungal activity, a variety of fungi have developed strategies which can either modulate or block the release of NETs. C. albicans, C. neoformans, and A. fumigatus possess virulence traits to suppress NET release upon neutrophil encounter (5, 54, 65). Impairment of NET production by C. neoformans correlates with capsule production, which is typically observed during infection (54). However, for C. albicans and A. fumigatus, NET inhibition is growth phase-dependent (biofilm for C. albicans, conidia for A. fumigatus) (5, 65). Both C. neoformans and C. albicans engage inhibitory pathways that are not rescued by potent stimulators of NETosis, such as PMA. The mechanisms underpinning the impairment of NET release by these pathogens are discussed below.
In vitro investigation shows that neutrophils fail to release NETs in response to C. neoformans (54). This inhibition is linked to the production of a polysaccharide capsule, as a cap67Δ mutant defective in capsular production triggers NET release (54). The capsule of C. neoformans is largely comprised of a unique polysaccharide, glucuronoxylomannan, a key virulence factor with multiple immunomodulatory activities (66–69). The purified polymer alone, which consists of linear α-(1–3)-mannan substituted with β-(1–2)-glucopyranosyluronic acid and β-(1–4)-xylopyranosyl, suppresses NET release in response to PMA, consistent with a role for this capsule polysaccharide in NET impairment (54). NET inhibition may contribute to immune evasion in vivo. Neutrophils induced to form NETs, by stimulation with either PMA or the acapsular mutant, exhibit antifungal activity against the wildtype C. neoformans. This suggests NETs could be an effective method of killing if their formation were not otherwise inhibited.
Many fungal infections, including candidiasis, involve the formation of biofilms, communities of adherent cells growing within an extracellular matrix (70–77). Biofilms commonly propagate on surfaces of medical devices, such as vascular catheters, urinary catheters, and ventricular fluid shunts (76–79), as well as mucosal surfaces (80, 81). Given the large size of these aggregates, NETs would appear to be an ideal method to control these infections. However, very few NETs form upon exposure to C. albicans biofilms (65, 82). Inhibitory pathways induced by C. albicans biofilm persist in the presence of potent inducers, such as PMA (65). This impairment of NET release is thought to account for the resilience of biofilms to neutrophil attack, as C. albicans biofilms are up to 5-fold more resistant to killing by neutrophils when compared to free-floating planktonic organisms (65, 83–85). However, neutrophils pre-induced to form NETs (by PMA) are capable of inhibiting biofilms, suggesting NET release may be an effective mechanism of controlling biofilm infections, if the process were not otherwise blocked by the mature biofilm (65).
Impairment of NET release by C. albicans biofilms requires the presence of an intact extracellular matrix (65). Physical disruption of the biofilm both induces NET release and increases susceptibility to killing by neutrophils (65, 85). Inhibition of NETs appears to correlate with the production of matrix polysaccharides that are distinct from cell wall polysaccharides (65). A genetic screen identified a mutant strain (pmr1Δ/Δ) capable of inducing NETs while growing as a biofilm. PMR1 encodes a transporter required for cell wall mannosylation during planktonic growth. During biofilm growth, this enzyme is critical for production of α-mannans of the matrix, which assemble with β-glucans extracellularly to form a mannan-glucan complex (86, 87). This unique polysaccharide complex is postulated to contribute to NET inhibition during C. albicans biofilm formation. C. glabrata biofilms also appear to inhibit NET formation, although to lesser extent when compared to C. albicans biofilms (40). However, it is unknown if a similar mechanism of impairment by extracellular matrix is employed by C. glabrata biofilms.
Conidia of A. fumigatus suppress NET formation. In vivo imaging of the neutrophils in a murine model of pulmonary aspergillosis reveals distinct neutrophil responses to hyphal and conidial forms (5). While time-lapse imaging shows that NETs are released in response to hyphal forms, resting or swollen conidida trigger fewer NETs. Modulation of NET release is linked to hydrophobin RodA, a major conidial surface component that also impairs adaptive immunity (88). Conidia of a rodAΔ mutant, which lack this surface component, trigger NETosis, even to a greater level than that observed for hyphal forms (5). The specific mechanism underlying the ability of RodA to impair NET production is unknown, but one theory is that it exerts its activity by masking pathogen-associated molecular patterns of the conidia cell wall.
4.3 Mechanisms employed by fungi to escape NETs
A variety of fungi have developed defenses to resist killing by NETs. For example, A. fumigatus C. gattii and P. braziliensis induce NET release, but the NETs exhibit minimal fungicidal activity against these pathogens (51, 55, 89). While NETs may not be capable of eradicating these fungi, the structures may function to prevent dissemination. Several studies have shed light on the factors contributing to the resistance to NET attack for A. fumigatus and C. gattii. The most well-defined mechanism of protection from NETs has been described for hyphae in A. fumigatus biofilms.
During pulmonary infection, A. fumigatus forms microcolonies of hyphae encased in an extracellular matrix (75). One of the most abundant and well-described components is galactosaminogalactan (GAG), an α-1,4-linked linear heteroglycan composed of variable combinations of galactose and N-acetyl-galactosamine (GalNAc) (75, 90, 91). Disruption of GAG attenuates both biofilm formation and virulence (92, 93). While GAG exhibits multifactorial influence on immunity, one of its key roles is providing protection from killing by neutrophils, shielding A. fumigatus from the antifungal activity of NETs (89).
While Aspergillus spp. commonly produce GAG, the relative proportion of galactose and GalNAc varies among species and strains (94–97). Lee et al. capitalized on the differences in GAG composition between A. fumigatus and A. niger to further characterize the activity of GAG (89). While A. fumigatus produces GalNAc-rich GAG, A. niger produces GalNAc-poor GAG with 5-fold higher levels of galactose. In comparison to A. fumigatus, A niger is less virulent and more susceptible to neutrophil attack. To test a role for GalNAc-rich GAG in virulence, A. niger was induced to produce GalNAc-rich GAG by heterologous expression of Uge3, an A. fumigatus gene encoding an epimerase required for its synthesis. The A. niger strain manipulated to produce GalNAc-rich GAG exhibited enhanced virulence and increased resistance to neutrophil attack, similar to A. fumigatus. Therefore, protection from NETs links closely to GalNAc-rich GAG. The protective effect of GAG, a partially deacetylated and polycationic glycan, likely resides in its positive charge, which is theorized to inhibit the binding of the cationic antimicrobial peptides or histones in NETs.
C. gattii, an emerging cause of cryptococcosis, is another pathogen protected from NETs. The basidiomycete inhabits tropical and temperate regions in association with trees (98). To investigate fungal-plant interactions, Springer et al. utilized an Arabidopsis thaliana wound model and discovered that C. gattii produces unique extracellular fibrils when growing on plants or plant-derived media (55). Fibril production is dependent on capsule formation, as a capsular mutant (cap59Δ) lacks these structures (55, 99). Wildtype C. gattii initially grown in the wound model or on plant-derived media demonstrates hypervirulence when used in murine models of both pulmonary and disseminated cryptococcosis, as compared to yeast that had initially been grown in standard conditions. This difference in virulence correlates with decreased susceptibility to neutrophil attack, with C. gattii growing in plant-derived media exhibiting an approximately 2-fold higher resistance to neutrophil killing. As C. gattii grown under these conditions induces NETs, the extracellular fibrils produced by C. gattii are proposed to physically impair fungal entrapment and killing.
In addition to evading killing by NETs, fungi may also combat NETs through degradation of their DNA backbone. Numerous bacterial pathogens escape NETs through release of extracellular endonucleases capable of destroying NETs (100–105). Recent investigation of several C. albicans isolates revealed secretion of DNase into their culture supernatants (106). While these enzymes are hypothesized to degrade NETs and prevent fungal death, further studies are needed to define the role of secreted DNases in the protection of C. albicans from neutrophil killing.
4.4 Possible host-derived regulators of NET formation in the context of fungal infections
Neutrophils keep a potentially dangerous arsenal which can effectively eradicate intruders, but at the same time can harm the host’s own tissue (7). Therefore, neutrophil activity is tightly controlled. Given the fact that NETs expose membrane-damaging peptides, proteolytic enzymes and pro-inflammatory molecules directly to host tissue, it seems obvious that NET induction and half-life would need to be meticulously regulated. It is believed that blood-borne host-derived DNases significantly contribute to degradation of NETs both in circulation as well as in tissue (107). Smaller NET debris and neutrophil remnants are then likely ingested by macrophages and cleared by the liver. Presumably, there may be additional host factors that negatively regulate NET induction. Extensive studies of C. albicans in a whole blood model system did not reveal signs of NET formation, such as extracellular DNA or neutrophil cell death, during incubation times of several hours (108). These findings argue for the existence of factors circulating in blood that may limit the release of NETs, presumably to ensure blood flow and to avoid premature clotting of vessels, which may have fatal consequences for the host. In extreme scenarios, such as bloodstream infection with sepsis, the regulatory circuits seem to be overruled, as reports reveal extensive NET release in septic animal models (as reviewed by (109, 110)). In these extreme situations, thrombosis, which is also promoted by NETs (111), may be employed as a last resort to confine pathogen spread.
Recent studies have begun to uncover host-derived modulators of NET release for several infection models. Endothelial p33, a kininogen-binding and complement-related protein, has been shown to downregulate NET formation caused by danger-associated molecular pattern signaling (112). This protein is detectable in patients with fasciitis caused by Streptococcus pyogenes, where it co-localizes with MPO, an inducer and constituent of NETs. Another example is human immunodeficiency virus-1 (HIV-1) infection, where IL-10 released by dendritic cells contributes to the downregulated release of NETs, which are important for binding and neutralizing virus particles (113). In response to leishmanial parasites, neutrophils can release NETs capable of modulating immunity. During a protective Th1 response, IL-4 and GM-CSF drive monocytes to differentiate into dendritic cells. However, in the presence of NETs, this differentiation is skewed, leading to the development of anti-inflammatory macrophages which in turn release high amounts of IL-10 (114). While we have only scarce information about how NET formation is controlled during viral, bacterial and parasitic infections, knowledge about host-derived, negative regulators of NET release during fungal infections is virtually absent.
5. Outlook
Since their discovery in 2004, NETs have received increasing attention. Investigations have revealed diverse stimuli, signaling pathways, and release mechanisms for these DNA traps. In addition to their importance for control of numerous pathogens, their impact appears to reach far beyond antimicrobial immunity. NETs are suggested to be key players in autoimmune diseases, such as lupus erythematosus (107) and small vessel vasculitis (115). In addition, they can influence cancer pathology by promoting metastases (116) or aggravating cancer-associated thrombosis (117). Even possible contributions to brain-degenerative diseases, such as Alzheimer’s disease, have been attributed to NETs (118). It seems likely that the involvement of NETs in even more inflammatory disorders will be uncovered, as a myriad of these diseases exist. When reporting about inflammation, immunologists are unable to pass over neutrophils, since these granulocytes are recruited in large numbers to virtually any inflamed site and serve as a hallmark for an inflammatory milieu (119).
As neutrophils have the potential to trigger and release NETs, it is logical to assume that these structures fulfill a task and are relevant for the outcome of infection. However, we should keep in mind that neutrophils are efficient hunters with several loaded weapons, and NETs are just one piece of a bigger puzzle. Undoubtedly, neutrophils play a central role in the defense against many fungal pathogens (120) and thus one may mistakenly assume that NETs are equally relevant for eradication of all mycoses. As an example, A. fumigatus hyphae trigger NETs, but are not susceptible to NET attack (5). Nevertheless, people with healthy immunity do not acquire aspergillosis despite the inhalation of hundreds of A. fumigatus spores every day. Instead, neutrophils efficiently eradicate these inhaled spores primarily by ingestion and ROS-dependent triggering of apoptosis-like cell death in the fungus (121), indicating that NETs are less important in this particular situation. However, while NETs may be dispensable to prevent A. fumigatus infection in the immunocompetent host, they have been demonstrated to be critical for control of infection caused by the closely related pathogen A. nidulans (46). These differences convincingly illustrate that more studies are required to understand individual contributions of neutrophil weaponry to eradicate diverse types of mycoses. Ultimately, detailed knowledge should promote development of more efficient diagnostic and therapeutic tools which are urgently required to cure emerging fungal infections.
Highlights.
Neutrophils release extracellular DNA traps to ensnare invading microbes
Neutrophil extracellular traps (NETs) contribute to innate immune responses in mycoses
Many human fungal pathogens induce NETs and are susceptible to their attack
Release of NETs is regulated by size and morphology of fungal pathogens
Acknowledgments
Funding: C.F.U. is supported by grants of the Swedish Research Council (VR-MH 2014-02281) and the Kempe Foundation (SMK 1453). J.E.N. is supported by the National Institutes of Health (K08 AI108727), the Burroughs Wellcome Fund (1012299), and the Doris Duke Charitable Foundation (112580130).
Abbreviations
- AMP
antimicrobial peptide
- CGD
chronic granulomatous disease
- CR3
complement receptor 3
- GAG
galactosaminogalactan
- GalNAc
N-acetyl-galactosamine
- GM-CSF
granulocyte-macrophage colony stimulating factor
- HIV
human immunodeficiency virus
- IL
interleukin
- LPS
Lipopolysaccharide
- MPO
myeloperoxidase
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NE
neutrophil elastase
- NET
neutrophil extracellular trap
- PAD4
protein arginine deaminase 4
- PMA
phorbol 12-myristate 13-acetate
- Phox
phagocyte oxidase
- PTX3
pentraxin 3
- ROS
reactive oxygen species
- SAP
secreted aspartic protease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lehrer RI, Cline MJ. Interaction of Candida albicans with human leukocytes and serum. Journal of bacteriology. 1969;98(3):996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mansour MK, Levitz SM. Interactions of fungi with phagocytes. Current opinion in microbiology. 2002;5(4):359–65. doi: 10.1016/s1369-5274(02)00342-9. [DOI] [PubMed] [Google Scholar]
- 3.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 4.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5(10):e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruns S, Kniemeyer O, Hasenberg M, Aimanianda V, Nietzsche S, Thywissen A, et al. Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog. 2010;6(4):e1000873. doi: 10.1371/journal.ppat.1000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol. 2014;15(11):1017–25. doi: 10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33(5):657–70. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–89. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 9.Hager M, Cowland JB, Borregaard N. Neutrophil granules in health and disease. J Intern Med. 2010;268(1):25–34. doi: 10.1111/j.1365-2796.2010.02237.x. [DOI] [PubMed] [Google Scholar]
- 10.Cross AR, Jones OT, Harper AM, Segal AW. Oxidation-reduction properties of the cytochrome b found in the plasma-membrane fraction of human neutrophils. A possible oxidase in the respiratory burst. Biochem J. 1981;194(2):599–606. doi: 10.1042/bj1940599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segal AW. NADPH oxidases as electrochemical generators to produce ion fluxes and turgor in fungi, plants and humans. Open Biol. 2016;6(5) doi: 10.1098/rsob.160028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winterbourn CC, Kettle AJ, Hampton MB. Reactive Oxygen Species and Neutrophil Function. Annu Rev Biochem. 2016;85:765–92. doi: 10.1146/annurev-biochem-060815-014442. [DOI] [PubMed] [Google Scholar]
- 13.Niemiec MJ, Grumaz C, Ermert D, Desel C, Shankar M, Lopes JP, et al. Dual transcriptome of the immediate neutrophil and Candida albicans interplay. BMC Genomics. 2017;18(1):696. doi: 10.1186/s12864-017-4097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brinkmann V, Zychlinsky A. Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol. 2012;198(5):773–83. doi: 10.1083/jcb.201203170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–41. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yipp BG, Kubes P. NETosis: how vital is it? Blood. 2013;122(16):2784–94. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 17.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191(3):677–91. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metzler KD, Goosmann C, Lubojemska A, Zychlinsky A, Papayannopoulos V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 2014;8(3):883–96. doi: 10.1016/j.celrep.2014.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16(11):1438–44. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 20.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14(9):949–53. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 21.Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185(12):7413–25. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 22.Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18(9):1386–93. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjornsdottir H, Dahlstrand Rudin A, Klose FP, Elmwall J, Welin A, Stylianou M, et al. Phenol-Soluble Modulin alpha Peptide Toxins from Aggressive Staphylococcus aureus Induce Rapid Formation of Neutrophil Extracellular Traps through a Reactive Oxygen Species-Independent Pathway. Front Immunol. 2017;8:257. doi: 10.3389/fimmu.2017.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirschfeld J, Roberts HM, Chapple IL, Parcina M, Jepsen S, Johansson A, et al. Effects of Aggregatibacter actinomycetemcomitans leukotoxin on neutrophil migration and extracellular trap formation. J Oral Microbiol. 2016;8:33070. doi: 10.3402/jom.v8.33070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douda DN, Khan MA, Grasemann H, Palaniyar N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc Natl Acad Sci U S A. 2015;112(9):2817–22. doi: 10.1073/pnas.1414055112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207(9):1853–62. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohrbach AS, Slade DJ, Thompson PR, Mowen KA. Activation of PAD4 in NET formation. Front Immunol. 2012;3:360. doi: 10.3389/fimmu.2012.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konig MF, Andrade F. A Critical Reappraisal of Neutrophil Extracellular Traps and NETosis Mimics Based on Differential Requirements for Protein Citrullination. Front Immunol. 2016;7:461. doi: 10.3389/fimmu.2016.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Kockritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M, et al. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111(6):3070–80. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 30.Lopes JP, Stylianou M, Nilsson G, Urban CF. Opportunistic pathogen Candida albicans elicits a temporal response in primary human mast cells. Sci Rep. 2015;5:12287. doi: 10.1038/srep12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCarty TP, Pappas PG. Invasive Candidiasis. Infectious disease clinics of North America. 2016;30(1):103–24. doi: 10.1016/j.idc.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Lewis RE, Cahyame-Zuniga L, Leventakos K, Chamilos G, Ben-Ami R, Tamboli P, et al. Epidemiology and sites of involvement of invasive fungal infections in patients with haematological malignancies: a 20-year autopsy study. Mycoses. 2013;56(6):638–45. doi: 10.1111/myc.12081. [DOI] [PubMed] [Google Scholar]
- 34.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8(4):668–76. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 35.McCormick A, Heesemann L, Wagener J, Marcos V, Hartl D, Loeffler J, et al. NETs formed by human neutrophils inhibit growth of the pathogenic mold Aspergillus fumigatus. Microbes Infect. 2010;12(12–13):928–36. doi: 10.1016/j.micinf.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Bianchi M, Niemiec MJ, Siler U, Urban CF, Reichenbach J. Restoration of anti-Aspergillus defense by neutrophil extracellular traps in human chronic granulomatous disease after gene therapy is calprotectin-dependent. J Allergy Clin Immunol. 2011;127(5):1243–52e7. doi: 10.1016/j.jaci.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 37.Gazendam RP, van Hamme JL, Tool AT, van Houdt M, Verkuijlen PJ, Herbst M, et al. Two independent killing mechanisms of Candida albicans by human neutrophils: evidence from innate immunity defects. Blood. 2014;124(4):590–7. doi: 10.1182/blood-2014-01-551473. [DOI] [PubMed] [Google Scholar]
- 38.Hopke A, Nicke N, Hidu EE, Degani G, Popolo L, Wheeler RT. Neutrophil Attack Triggers Extracellular Trap-Dependent Candida Cell Wall Remodeling and Altered Immune Recognition. PLoS Pathog. 2016;12(5):e1005644. doi: 10.1371/journal.ppat.1005644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Svobodova E, Staib P, Losse J, Hennicke F, Barz D, Jozsi M. Differential interaction of the two related fungal species Candida albicans and Candida dubliniensis with human neutrophils. J Immunol. 2012;189(5):2502–11. doi: 10.4049/jimmunol.1200185. [DOI] [PubMed] [Google Scholar]
- 40.Johnson CJ, Kernien JF, Hoyer AR, Nett JE. Mechanisms involved in the triggering of neutrophil extracellular traps (NETs) by Candida glabrata during planktonic and biofilm growth. Sci Rep. 2017;7(1):13065. doi: 10.1038/s41598-017-13588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patterson TF, Thompson GR, 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feldmesser M. Role of neutrophils in invasive aspergillosis. Infection and immunity. 2006;74(12):6514–6. doi: 10.1128/IAI.01551-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerson SL, Talbot GH, Hurwitz S, Strom BL, Lusk EJ, Cassileth PA. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Annals of internal medicine. 1984;100(3):345–51. doi: 10.7326/0003-4819-100-3-345. [DOI] [PubMed] [Google Scholar]
- 44.Seger RA. Modern management of chronic granulomatous disease. British journal of haematology. 2008;140(3):255–66. doi: 10.1111/j.1365-2141.2007.06880.x. [DOI] [PubMed] [Google Scholar]
- 45.Segal BH, DeCarlo ES, Kwon-Chung KJ, Malech HL, Gallin JI, Holland SM. Aspergillus nidulans infection in chronic granulomatous disease. Medicine. 1998;77(5):345–54. doi: 10.1097/00005792-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Bianchi M, Hakkim A, Brinkmann V, Siler U, Seger RA, Zychlinsky A, et al. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood. 2009;114(13):2619–22. doi: 10.1182/blood-2009-05-221606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rohm M, Grimm MJ, D'Auria AC, Almyroudis NG, Segal BH, Urban CF. NADPH oxidase promotes neutrophil extracellular trap formation in pulmonary aspergillosis. Infection and immunity. 2014;82(5):1766–77. doi: 10.1128/IAI.00096-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaillon S, Peri G, Delneste Y, Fremaux I, Doni A, Moalli F, et al. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med. 2007;204(4):793–804. doi: 10.1084/jem.20061301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin X, Zhao Y, Zhang F, Wan T, Fan F, Xie X, et al. Neutrophil extracellular traps involvement in corneal fungal infection. Mol Vis. 2016;22:944–52. [PMC free article] [PubMed] [Google Scholar]
- 50.Bachiega TF, Dias-Melicio LA, Fernandes RK, de Almeida Balderramas H, Rodrigues DR, Ximenes VF, et al. Participation of dectin-1 receptor on NETs release against Paracoccidioides brasiliensis: Role on extracellular killing. Immunobiology. 2016;221(2):228–35. doi: 10.1016/j.imbio.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Mejia SP, Cano LE, Lopez JA, Hernandez O, Gonzalez A. Human neutrophils produce extracellular traps against Paracoccidioides brasiliensis. Microbiology (Reading, England) 2015;161(Pt 5):1008–17. doi: 10.1099/mic.0.000059. [DOI] [PubMed] [Google Scholar]
- 52.Della Coletta AM, Bachiega TF, de Quaglia e Silva JC, Soares AM, De Faveri J, Marques SA, et al. Neutrophil Extracellular Traps Identification in Tegumentary Lesions of Patients with Paracoccidioidomycosis and Different Patterns of NETs Generation In Vitro. PLoS Negl Trop Dis. 2015;9(9):e0004037. doi: 10.1371/journal.pntd.0004037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marques SA. Paracoccidioidomycosis: epidemiological, clinical, diagnostic and treatment updating. An Bras Dermatol. 2013;88(5):700–11. doi: 10.1590/abd1806-4841.20132463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rocha JD, Nascimento MT, Decote-Ricardo D, Corte-Real S, Morrot A, Heise N, et al. Capsular polysaccharides from Cryptococcus neoformans modulate production of neutrophil extracellular traps (NETs) by human neutrophils. Sci Rep. 2015;5:8008. doi: 10.1038/srep08008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Springer DJ, Ren P, Raina R, Dong Y, Behr MJ, McEwen BF, et al. Extracellular fibrils of pathogenic yeast Cryptococcus gattii are important for ecological niche, murine virulence and human neutrophil interactions. PLoS One. 2010;5(6):e10978. doi: 10.1371/journal.pone.0010978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2010;50(3):291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hofs S, Mogavero S, Hube B. Interaction of Candida albicans with host cells: virulence factors, host defense, escape strategies, and the microbiota. J Microbiol. 2016;54(3):149–69. doi: 10.1007/s12275-016-5514-0. [DOI] [PubMed] [Google Scholar]
- 58.Ermert D, Urban CF, Laube B, Goosmann C, Zychlinsky A, Brinkmann V. Mouse neutrophil extracellular traps in microbial infections. J Innate Immun. 2009;1(3):181–93. doi: 10.1159/000205281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wellington M, Dolan K, Krysan DJ. Live Candida albicans suppresses production of reactive oxygen species in phagocytes. Infect Immun. 2009;77(1):405–13. doi: 10.1128/IAI.00860-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hosseinzadeh A, Urban CF. Novel insight into neutrophil immune responses by dry mass determination of Candida albicans morphotypes. PLoS One. 2013;8(10):e77993. doi: 10.1371/journal.pone.0077993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Byrd AS, O'Brien XM, Johnson CM, Lavigne LM, Reichner JS. An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans. J Immunol. 2013;190(8):4136–48. doi: 10.4049/jimmunol.1202671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nani S, Fumagalli L, Sinha U, Kamen L, Scapini P, Berton G. Src family kinases and Syk are required for neutrophil extracellular trap formation in response to beta-glucan particles. J Innate Immun. 2015;7(1):59–73. doi: 10.1159/000365249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zawrotniak M, Bochenska O, Karkowska-Kuleta J, Seweryn-Ozog K, Aoki W, Ueda M, et al. Aspartic Proteases and Major Cell Wall Components in Candida albicans Trigger the Release of Neutrophil Extracellular Traps. Front Cell Infect Microbiol. 2017;7:414. doi: 10.3389/fcimb.2017.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532(7597):64–8. doi: 10.1038/nature17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnson CJ, Cabezas-Olcoz J, Kernien JF, Wang SX, Beebe DJ, Huttenlocher A, et al. The extracellular matrix of Candida albicans biofilms impairs formation of neutrophil extracellular traps. PLoS pathogens. 2016;12(9):e1005884. doi: 10.1371/journal.ppat.1005884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Monari C, Casadevall A, Retini C, Baldelli F, Bistoni F, Vecchiarelli A. Antibody to capsular polysaccharide enhances the function of neutrophils from patients with AIDS against Cryptococcus neoformans. AIDS (London, England) 1999;13(6):653–60. doi: 10.1097/00002030-199904160-00005. [DOI] [PubMed] [Google Scholar]
- 67.Vecchiarelli A. Immunoregulation by capsular components of Cryptococcus neoformans. Medical mycology. 2000;38(6):407–17. doi: 10.1080/mmy.38.6.407.417. [DOI] [PubMed] [Google Scholar]
- 68.Martinez LR, Casadevall A. Specific antibody can prevent fungal biofilm formation and this effect correlates with protective efficacy. Infection and immunity. 2005;73(10):6350–62. doi: 10.1128/IAI.73.10.6350-6362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martinez LR, Christaki E, Casadevall A. Specific antibody to Cryptococcus neoformans glucurunoxylomannan antagonizes antifungal drug action against cryptococcal biofilms in vitro. The Journal of infectious diseases. 2006;194(2):261–6. doi: 10.1086/504722. [DOI] [PubMed] [Google Scholar]
- 70.Nett JE. The Host's Reply to Candida Biofilm. Pathogens. 2016;5(1) doi: 10.3390/pathogens5010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nobile CJ, Johnson AD. Candida albicans biofilms and human disease. Annual review of microbiology. 2015;69:71–92. doi: 10.1146/annurev-micro-091014-104330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fanning SMA. Fungal biofilms. PLoS pathogens. 2012;8(4) doi: 10.1371/journal.ppat.1002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramage G, Mowat E, Jones B, Williams C, Lopez-Ribot J. Our current understanding of fungal biofilms. Critical reviews in microbiology. 2009;35(4):340–55. doi: 10.3109/10408410903241436. [DOI] [PubMed] [Google Scholar]
- 74.Kumamoto CA, Vinces MD. Alternative Candida albicans lifestyles: growth on surfaces. Annual review of microbiology. 2005;59:113–33. doi: 10.1146/annurev.micro.59.030804.121034. [DOI] [PubMed] [Google Scholar]
- 75.Loussert C, Schmitt C, Prevost MC, Balloy V, Fadel E, Philippe B, et al. In vivo biofilm composition of Aspergillus fumigatus. Cellular microbiology. 2010;12(3):405–10. doi: 10.1111/j.1462-5822.2009.01409.x. [DOI] [PubMed] [Google Scholar]
- 76.Walsh TJ, Schlegel R, Moody MM, Costerton JW, Salcman M. Ventriculoatrial shunt infection due to Cryptococcus neoformans: an ultrastructural and quantitative microbiological study. Neurosurgery. 1986;18(3):373–5. doi: 10.1227/00006123-198603000-00025. [DOI] [PubMed] [Google Scholar]
- 77.Donlan RM. Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis. 2001;33(8):1387–92. doi: 10.1086/322972. [DOI] [PubMed] [Google Scholar]
- 78.Ramage G, Martinez JP, Lopez-Ribot JL. Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS yeast research. 2006;6(7):979–86. doi: 10.1111/j.1567-1364.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- 79.Kojic EM, Darouiche RO. Candida infections of medical devices. Clinical microbiology reviews. 2004;17(2):255–67. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dongari-Bagtzoglou A. Mucosal biofilms: challenges and future directions. Expert review of anti-infective therapy. 2008;6(2):141–4. doi: 10.1586/14787210.6.2.141. [DOI] [PubMed] [Google Scholar]
- 81.Harriott MM, Lilly EA, Rodriguez TE, Fidel PL, Jr, Noverr MC. Candida albicans forms biofilms on the vaginal mucosa. Microbiology (Reading, England) 2010;156(Pt 12):3635–44. doi: 10.1099/mic.0.039354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kernien JF, Johnson CJ, Nett JE. Conserved Inhibition of neutrophil extracellular trap release by clinical Candida albicans biofilms. Journal of Fungi. 2017;3(49) doi: 10.3390/jof3030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xie Z, Thompson A, Sobue T, Kashleva H, Xu H, Vasilakos J, et al. Candida albicans biofilms do not trigger reactive oxygen species and evade neutrophil killing. The Journal of infectious diseases. 2012;206(12):1936–45. doi: 10.1093/infdis/jis607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Katragkou A, Simitsopoulou M, Chatzimoschou A, Georgiadou E, Walsh TJ, Roilides E. Effects of interferon-gamma and granulocyte colony-stimulating factor on antifungal activity of human polymorphonuclear neutrophils against Candida albicans grown as biofilms or planktonic cells. Cytokine. 2011;55(3):330–4. doi: 10.1016/j.cyto.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 85.Katragkou A, Kruhlak MJ, Simitsopoulou M, Chatzimoschou A, Taparkou A, Cotten CJ, et al. Interactions between human phagocytes and Candida albicans biofilms alone and in combination with antifungal agents. The Journal of infectious diseases. 2010;201(12):1941–9. doi: 10.1086/652783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mitchell KF, Zarnowski R, Sanchez H, Edward JA, Reinicke EL, Nett JE, et al. Community participation in biofilm matrix assembly and function. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(13):4092–7. doi: 10.1073/pnas.1421437112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zarnowski R, Westler WM, Lacmbouh GA, Marita JM, Bothe JR, Bernhardt J, et al. Novel entries in a fungal biofilm matrix encyclopedia. mBio. 2014;5(4):e01333–14. doi: 10.1128/mBio.01333-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, Elluru SR, et al. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature. 2009;460(7259):1117–21. doi: 10.1038/nature08264. [DOI] [PubMed] [Google Scholar]
- 89.Lee MJ, Liu H, Barker BM, Snarr BD, Gravelat FN, Al Abdallah Q, et al. The Fungal Exopolysaccharide Galactosaminogalactan Mediates Virulence by Enhancing Resistance to Neutrophil Extracellular Traps. PLoS Pathog. 2015;11(10):e1005187. doi: 10.1371/journal.ppat.1005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fontaine T, Delangle A, Simenel C, Coddeville B, van Vliet SJ, van Kooyk Y, et al. Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus. PLoS pathogens. 2011;7(11):e1002372. doi: 10.1371/journal.ppat.1002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee MJ, Gravelat FN, Cerone RP, Baptista SD, Campoli PV, Choe SI, et al. Overlapping and distinct roles of Aspergillus fumigatus UDP-glucose 4-epimerases in galactose metabolism and the synthesis of galactose-containing cell wall polysaccharides. The Journal of biological chemistry. 2014;289(3):1243–56. doi: 10.1074/jbc.M113.522516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gravelat FN, Beauvais A, Liu H, Lee MJ, Snarr BD, Chen D, et al. Aspergillus galactosaminogalactan mediates adherence to host constituents and conceals hyphal beta-glucan from the immune system. PLoS pathogens. 2013;9(8):e1003575. doi: 10.1371/journal.ppat.1003575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee MJ, Geller AM, Bamford NC, Liu H, Gravelat FN, Snarr BD, et al. Deacetylation of fungal exopolysaccharide mediates adhesion and biofilm formation. mBio. 2016;7(2) doi: 10.1128/mBio.00252-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ruperez P, Leal JA. Extracellular galactosaminogalactan from Aspergillus parasiticus. Transactions of the British Mycological Society. 1981;77(3):621–5. [Google Scholar]
- 95.Bardalaye PC, Nordin JH. Galactosaminogalactan from cell walls of Aspergillus niger. Journal of bacteriology. 1976;125(2):655–69. doi: 10.1128/jb.125.2.655-669.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gorin PA, Eveleigh DE. Extracellular 2-acetamido-2-deoxy-D-galacto-D-galactan from Aspergillus nidulans. Biochemistry. 1970;9(25):5023–7. doi: 10.1021/bi00827a029. [DOI] [PubMed] [Google Scholar]
- 97.Leal JA, Rupérez P. Extracellular polysaccharide production by Aspergillus nidulans. Transactions of the British Mycological Society. 1978;70(1):115–20. [Google Scholar]
- 98.Springer DJ, Billmyre RB, Filler EE, Voelz K, Pursall R, Mieczkowski PA, et al. Cryptococcus gattii VGIII isolates causing infections in HIV/AIDS patients in Southern California: identification of the local environmental source as arboreal. PLoS pathogens. 2014;10(8):e1004285. doi: 10.1371/journal.ppat.1004285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang YC, Kwon-Chung KJ. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Molecular and cellular biology. 1994;14(7):4912–9. doi: 10.1128/mcb.14.7.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Buchanan JT, Simpson AJ, Aziz RK, Liu GY, Kristian SA, Kotb M, et al. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol. 2006;16(4):396–400. doi: 10.1016/j.cub.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 101.Berends ET, Horswill AR, Haste NM, Monestier M, Nizet V, von Kockritz-Blickwede M. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J Innate Immun. 2010;2(6):576–86. doi: 10.1159/000319909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Midon M, Schafer P, Pingoud A, Ghosh M, Moon AF, Cuneo MJ, et al. Mutational and biochemical analysis of the DNA-entry nuclease EndA from Streptococcus pneumoniae. Nucleic acids research. 2011;39(2):623–34. doi: 10.1093/nar/gkq802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sumby P, Barbian KD, Gardner DJ, Whitney AR, Welty DM, Long RD, et al. Extracellular deoxyribonuclease made by group A Streptococcus assists pathogenesis by enhancing evasion of the innate immune response. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(5):1679–84. doi: 10.1073/pnas.0406641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol. 2006;16(4):401–7. doi: 10.1016/j.cub.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 105.Seper A, Hosseinzadeh A, Gorkiewicz G, Lichtenegger S, Roier S, Leitner DR, et al. Vibrio cholerae evades neutrophil extracellular traps by the activity of two extracellular nucleases. PLoS Pathog. 2013;9(9):e1003614. doi: 10.1371/journal.ppat.1003614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang X, Zhao S, Sun L, Li W, Wei Q, Ashman RB, et al. Different virulence of Candida albicans is attributed to the ability of escape from neutrophil extracellular traps by secretion of DNase. Am J Transl Res. 2017;9(1):50–62. [PMC free article] [PubMed] [Google Scholar]
- 107.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107(21):9813–8. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hunniger K, Lehnert T, Bieber K, Martin R, Figge MT, Kurzai O. A virtual infection model quantifies innate effector mechanisms and Candida albicans immune escape in human blood. PLoS Comput Biol. 2014;10(2):e1003479. doi: 10.1371/journal.pcbi.1003479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ma AC, Kubes P. Platelets, neutrophils, and neutrophil extracellular traps (NETs) in sepsis. J Thromb Haemost. 2008;6(3):415–20. doi: 10.1111/j.1538-7836.2007.02865.x. [DOI] [PubMed] [Google Scholar]
- 110.O'Brien XM, Biron BM, Reichner JS. Consequences of extracellular trap formation in sepsis. Curr Opin Hematol. 2017;24(1):66–71. doi: 10.1097/MOH.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107(36):15880–5. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Neumann A, Papareddy P, Westman J, Hyldegaard O, Snall J, Norrby-Teglund A, et al. Immunoregulation of Neutrophil Extracellular Trap Formation by Endothelial-Derived p33 (gC1q Receptor) J Innate Immun. 2017 doi: 10.1159/000480386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T, et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. 2012;12(1):109–16. doi: 10.1016/j.chom.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 114.Guimaraes-Costa AB, Rochael NC, Oliveira F, Echevarria-Lima J, Saraiva EM. Neutrophil Extracellular Traps Reprogram IL-4/GM-CSF-Induced Monocyte Differentiation to Anti-inflammatory Macrophages. Front Immunol. 2017;8:523. doi: 10.3389/fimmu.2017.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15(6):623–5. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Najmeh S, Cools-Lartigue J, Rayes RF, Gowing S, Vourtzoumis P, Bourdeau F, et al. Neutrophil extracellular traps sequester circulating tumor cells via beta1-integrin mediated interactions. Int J Cancer. 2017;140(10):2321–30. doi: 10.1002/ijc.30635. [DOI] [PubMed] [Google Scholar]
- 117.Demers M, Wagner DD. Neutrophil extracellular traps: A new link to cancer-associated thrombosis and potential implications for tumor progression. Oncoimmunology. 2013;2(2):e22946. doi: 10.4161/onci.22946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pietronigro EC, Della Bianca V, Zenaro E, Constantin G. NETosis in Alzheimer's Disease. Front Immunol. 2017;8:211. doi: 10.3389/fimmu.2017.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173–82. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 120.Lionakis MS. New insights into innate immune control of systemic candidiasis. Med Mycol. 2014;52(6):555–64. doi: 10.1093/mmy/myu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shlezinger N, Irmer H, Dhingra S, Beattie SR, Cramer RA, Braus GH, et al. Sterilizing immunity in the lung relies on targeting fungal apoptosis-like programmed cell death. Science. 2017;357(6355):1037–41. doi: 10.1126/science.aan0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Heddergott C, Bruns S, Nietzsche S, Leonhardt I, Kurzai O, Kniemeyer O, et al. The Arthroderma benhamiae hydrophobin HypA mediates hydrophobicity and influences recognition by human immune effector cells. Eukaryot Cell. 2012;11(5):673–82. doi: 10.1128/EC.00037-12. [DOI] [PMC free article] [PubMed] [Google Scholar]