Abstract
The extracellular matrix (ECM) is an intricate network that provides structural and anchoring support to cells in order to stabilize cell morphology and tissue architecture. The ECM also controls many aspects of the cell’s dynamic behavior and fate through its ongoing, bidirectional interaction with cells. These interactions between the cell and components of the surrounding ECM are implicated in several biological processes, including development and adult tissue repair in response to injury, throughout the lifespan of multiple species. The present review gives an overview of the growing evidence that cell-matrix interactions play a pivotal role in the aging process. The focus of the first part of the article is on recent studies using cell-derived decellularized ECM, which strongly suggest that age-related changes in the ECM induce cellular senescence, a well-recognized hallmark of aging. This is followed by a review of findings from genetic studies indicating that changes in genes involved in cell–ECM adhesion and matrix-mediated intracellular signaling cascades affect longevity. Finally, mention is made of novel data proposing an intricate interplay between cell-matrix interactions and the renin-angiotensin system that may have a significant impact on mammalian arterial stiffness with age.
Keywords: Integrin, fibronectin, cellular senescence, reactive oxygen species, arterial stiffness
1. Introduction
Human average life expectancy in developed countries has increased dramatically in the last century through reductions in infant mortality and improvements in public health, nutrition, and general living standards (Crimmins, 2015). A continued increase in life expectancy is also predicted over the next 10 years in many industrialized countries (Kontis et al., 2017). However, the rise in life expectancy has been accompanied by an increase in prevalence of chronic disorders, such as cancer and Type 2 diabetes, which are the major causes of disability and mortality (Crimmins, 2015). This phenomenon poses major economic and social challenges for nations and families (Harper, 2014), and more research initiatives that have the potential to increase healthspan and compress morbidity have been recently proposed to address these concerns (Kennedy et al., 2014). Are disease and disability an inescapable result of aging in humans? The observation that a minority of individuals are able to reach a very advanced age in good clinical conditions indicates that healthy aging is possible (Franceschi and Bonafe, 2003). Over the last three decades, numerous studies have been performed in both model systems and humans to reveal the underlying mechanisms of aging and longevity assurance (reviewed recently in (Aunan et al., 2016; Carmona and Michan, 2016; Khan et al., 2017)). These studies have clearly shown that the ability to live a long and healthy life depends on highly interconnected and evolutionary conserved mechanisms of aging affecting mitochondrial function, nutrient-sensing/growth signaling pathways, chromatin silencing, telomere integrity, protein homeostasis, immune response, and the ability to maintain a balance between formation and detoxification of reactive oxygen species. The identification of cellular and molecular hallmarks of aging indicates the potential for lifestyle-behavioral and/or pharmacological interventions to improve health and extend longevity in humans (Kennedy et al., 2014). Promising strategies to slow human aging, such as the development of caloric restriction mimetics that may enhance nutrient sensing, have been recently summarized (Longo et al., 2015). In this review I would like to draw attention to the growing literature suggesting that cell-matrix interactions are major contributors to the aging process. Over the past few years, there has also been an upsurge of interest in the role played by the renin–angiotensin systems (RAS) in tissue degeneration and functional decline occurring with advancing age. In this regard, the final section of this review summarizes recent studies suggesting an intricate interplay between cell-matrix interactions and RAS that might promote vascular aging. Pharmacological and lifestyle strategies targeting vascular aging processes that are influenced by this interplay may have great potential to improve healthspan.
2. Dynamic interactions between the cell and ECM
2.1 Overview of cell adhesion to ECM and mechanosensing
The ECM is the non-cellular component present within all tissue and organs that provides structural and anchoring support to the cells in order to stabilize cell morphology and tissue architecture (Frantz et al., 2010). Most normal tissue-derived cells require adhesion to the ECM for growth and survival, a phenomenon known as anchorage dependence (Stoker et al., 1968). Cell to matrix adhesion occurs through structures, called focal adhesions, that link the ECM to the actin cytoskeleton. Focal adhesions are dynamic protein complexes that consist of 150 different molecules, including vinculin, talin, paxillin, and mechanosensors signaling molecules, such as integrin receptors and focal adhesion kinase (FAK) (Zhou et al., 2015). It is through the mechanosensors signaling pathways that cells exert actin cytoskeleton-mediated traction forces to sense their physical surrounding, such as matrix “stiffness” (e.g. the mechanical resistivity of the ECM), and respond to it. ECM stiffness, which plays a prominent role in the maintenance of mechanically-stressed tissues, such as skeletal muscle, bone, blood vessels, and adipose, is now emerging as a crucial regulator of cell behavior and thereby tissue formation and homeostasis (Zhou et al., 2015). This is not intended to be an in-depth review of this area, and the reader is directed to excellent reviews that have been published recently for a comprehensive information on ECM molecular composition and function, cell-matrix attachment, and mechanotransduction cellular processes (Cox and Erler, 2011; Jaalouk and Lammerding, 2009; Kular et al., 2014; Seong et al., 2013).
2.2 ECM composition and remodeling
The ECM is an intricate network composed of two main classes of macromolecules, soluble proteoglycans and fibrous structural proteins, such as collagens, fibronectins, laminins, and elastins, produced and secreted by resident fibroblasts (reviewed by (Daley et al., 2008; Frantz et al., 2010; Iozzo and Schaefer, 2015). Specialized sheets of ECM are represented by the basement membranes (BM) that surround epithelial, endothelial, muscle, fat, and Schwann cells (Jayadev and Sherwood, 2017). The major components of the BM are type IV collagens, nidogen, heparan sulfate proteoglycans (HSPG), and laminins.
The secretion of ECM components starts at the earliest stages of embryogenesis; however, it is constantly rebuilded and remodeled during normal physiological processes, such as tissue repair (the fibrotic response) (Lu et al., 2011). ECM remodeling is mediated by specific proteinases that are responsible for ECM degradation, such as matrix metalloproteinases (MMPs), A Disintegrin and Metalloproteinases (ADAM), ADAMs with thrombospondin motifs (ADAMTS), and their inhibitors (tissue inhibitor of MMPs or TIMPs) (Cox and Erler, 2011). The properties of the ECM are also controlled by post-translational modifications of ECM components that occur through enzymatic cross-linking processes catalyzed by lysyl oxidases, and non-enzymatic cross-linking processes involving glycation and transglutamination (Erler and Weaver, 2009). Furthermore, growing evidence suggests that cell-ECM attachment influences the cell production of reactive oxygen species (ROS), which, in turn, affect the production, assembly, and turnover of the ECM during matrix remodeling (recently reviewed by (Eble and de Rezende, 2014).
Excess deposition of ECM components without reciprocally balanced degradation (e.g. pathological fibrosis) can lead to organ dysfunction as a consequence of the perturbation of signals mediated by ECM (Lu et al., 2011). Together with excess ECM deposition, increased cross-linking contributes to increased tissue stiffness, which is associated with a diverse array of pathologies (Lampi and Reinhart-King, 2018).
2.3 Cell-matrix interactions and mechanochemical signaling
As mentioned above, the ECM profoundly influences many aspects of the cell’s dynamic behavior, including cell proliferation and growth, cell shape, migration, and differentiation (Zhou et al., 2015), and cell fate choices (Trappmann et al., 2012). This is achieved through the binding of ECM components to cell surface receptors, such as integrins and syndecans.
Integrins are heterodimeric molecules composed of two transmembrane subunits, α and β. In mammals, there are 18 α-subunits and 8 β-subunits and to date 24 different αβ heterodimers have been identified at the protein level, with each of them having specificity for different ECM ligands, including fibronectins (e.g., α5β1, αvβ3, α4β1), collagens (e.g., α1β1, α2β1), and laminins (e.g., α2β1, α3β1, α6β1) (Humphries et al., 2006). A dynamic cooperation exists between ECM components and integrins to regulate several growth factors signaling pathways. This interplay is crucial in the establishment of stem cell niches and in the maintenance and differentiation of stem cells (Brizzi et al., 2012).
Syndecans belong to the HSPGs, a remarkable family of glycosaminoglycans (GAGs), that also include glycosylphosphatidylinositol-anchored glypicans and secreted proteoglycans found in BMs (agrin, collagen XVIII, and perlecan) (Sarrazin et al., 2011). Across species, all HSPGs are characterized by an extracellular domain with attachment sites for GAGs that mediate interactions with a wide array of ligands, including soluble growth factors, morphogens, chemokines, and cytokines (Sarrazin et al., 2011). However, unlike the other HSPGs, syndecans also contain highly conserved transmembrane domains and short cytoplasmic tails (Sarrazin et al., 2011). Because of their cytoplasmic domain, which includes binding sites for cytoskeletal proteins and protein kinases, members of the syndecan family can function independently and/or in synergy with the integrin-mediated signaling to control cell adhesion and ECM stiffness (Morgan et al., 2007; Xian et al., 2010). Although invertebrates have only one Syndecan (Sdc) gene and protein, there are four genes (SDC1, SDC2, SDC3, and SDC4) and corresponding proteins in vertebrates (Chakravarti and Adams, 2006). Syndecan-4 is widely expressed in most adult tissues and is the only mammalian syndecan that promotes focal adhesion assembly around pre-existing integrin clusters on fibronectin (Woods and Couchman, 2001). In addition, novel functions have recently been suggested for syndecan-4 as regulator of cytosolic cation influx, through the control of transient receptor potential canonical (TRPC) calcium channels (Gopal et al., 2015), and as “linker” between cell-cell and cell ECM interactions (Gopal et al., 2017).
Engagement of ECM components to integrins and/or syndecans triggers mechanical and biochemical alterations (mechanotransduction), such as changes in intracellular calcium concentration or activation of the steroid receptor coactivator-FAK complex, the phosphatidylinositol 3-kinase (PI3K)/Akt cascade, and the mitogen-activated protein kinase (MAPK)/extracellular-signal-related kinase (ERK) pathway (Jaalouk and Lammerding, 2009; Schwartz and Assoian, 2001). The mechanochemical pathways then convey a signal from the cell membrane through the cytoskeleton to the nucleus. This leads to chromatin remodeling and changes in expression of genes (some of which are regulated by circadian clocks) that in turn reorganize and remodel the ECM (Dupont et al., 2011; Maya-Mendoza et al., 2016). Therefore, an ongoing, bidirectional interaction (“dynamic reciprocity”) exists between the cell and the ECM through which the cellular information can markedly impact the tissue microenvironment and vice versa (Xu et al., 2009). This close relationship enables cells to adapt to ongoing stresses imparted upon them and ultimately maintain correct tissue homeostasis under normal conditions. For instance, lack of ECM contact through loss of integrin engagement or the engagement with inappropriate ECM has been shown to inhibit both ERK and PI3K/Akt signaling and to stimulate “apoptotic activators” leading to anoikis execution (a specialized form of apoptosis) (Le Gall et al., 2000; Qi et al., 2006). However, when a persistent imbalance in the relationship between the cell and ECM occur, essential cellular functions become disrupted, significantly altering the way in which cells can respond to their environment.
3. The role of cell-ECM interactions in aging and longevity
3.1 Cellular senescence is influenced by age-related ECM alterations
There are several publications indicating that the biochemical composition and mechanical properties of ECM are altered with age. The reader is referred to (Phillip et al., 2015) for a comprehensive and critical review of the primary biophysical changes occurring in cells and tissues that accompany the aging process.
Collagen is the largest component of the ECM. Several studies have reported age-related changes in BM collagen synthesis and MMP-mediated degradation rate in both animal models and humans (Mays et al., 1991; Stephens and Grande-Allen, 2007). Together with ECM remodeling, the proportion of collagen subtypes also changes with age across species (Gazoti Debessa et al., 2001; Mays et al., 1988). Moreover, it is well established that an increase in collagen fibers cross-linking occurs in aging tissues (Snedeker and Gautieri, 2014).
First proposed by Johan Björkstein in 1942, the cross-linking theory of aging postulates that aging results from accumulation of intra-intermolecular covalent bond (cross-links”) between molecules with a slow turnover, such as collagen and elastin (Bjorksten and Tenhu, 1990). This accumulation produces alterations of the physical, chemical, and biological properties of the cell that can ultimately lead to significant dysfunction of multiple organ systems and thereby increased risk of death (Bjorksten and Tenhu, 1990). Cross-linking between molecules occurs slowly with normal aging (Snedeker and Gautieri, 2014), but it is accelerated under hyperglycaemic stress due to the formation of advanced glycation end products (AGEs) that occur from a reaction (Maillard reaction or glycation) between reducing sugars and matrix proteins (Miyata et al., 1997). In recent years, AGEs have received particular attention in the context of skin aging and the idea of using skin AGEs as biomarkers of aging has been proposed (Gkogkolou and Bohm, 2012). Support for the cross-linking theory of aging lately came from findings of a solid and comprehensive literature review (Semba et al., 2010). However, compelling evidence from culture decellularized ECM (DECM) studies (see below) suggest that perturbations of cell-ECM interactions and matrix-mediated mechanochemical signaling networks could be the culprit behind the aging process. This idea is further supported by data mining/systems biology studies performed to identify proteins highly connected (“hubs”) in interaction networks underlying both human longevity and multiple age-related diseases (Wolfson et al., 2009). Among the most enriched pathways in the common networks revealed by these studies, there are those involved in growth signaling and cell-ECM interactions, such as focal adhesion and regulation of actin cytoskeleton, with hubs that include FAK, fibronectin, paxillin, and vinculin (Wolfson et al., 2009). Notably, focal adhesion is also among the most enriched pathways in the common networks for longevity, age-related diseases, and cellular senescence (Tacutu et al., 2011).
Cellular senescence is an evolutionary conserved tissue damage response in adult individuals that also plays an important role during mammalian embryonic development (Munoz-Espin et al., 2013; Storer et al., 2013). It consists in a state of irreversible growth arrest that is used by the cell to limit its proliferation (Campisi, 2013; Neves et al., 2015). Senescent cells remain metabolically active, but are resistant to mitogenic stimulation (Shelton et al., 1999). While the growth arrest is achieved via either p16INK4A or p21CIP1 as key executers of cell-cycle arrest, senescent cells are characterized by increased production of ROS and ECM-modifying proteases, as well as widespread changes in chromatin organization and gene expression that ultimately lead to the secretion of numerous pro-inflammatory cytokines, chemokines, and growth factors (a phenotype termed the “senescence associated secretory phenotype” or SASP) (Campisi, 2013). The number of senescent cells increases exponentially with age (Campisi, 2013) and signals from the ECM can induce cellular senescence. This has been shown by a very elegant study from Choi et al. (2011) who were the first to use the DECM technology (in which the ECM is removed from its natural cells to produce a scaffold for other cells) to investigate this issue. They reported that seeding aged, senescent cells onto DECM derived from young human fibroblasts was sufficient to induce a youthful state in the senescent cells (Choi et al., 2011). Consistent results were also found by Sun et al. (2011) who revealed that exposure of aged mesenchymal stem cells from female mice to DECM from young donors generated a significant increase in the expression of the gene encoding the cellular reverse transcriptase telomerase and a decrease in ROS levels.
Various studies have reported that ECM affects ROS production through integrins and vice versa (Boin et al., 2014; Eble and de Rezende, 2014), it is, therefore, plausible that persistent changes in cell-ECM interactions occurring with advanced age may increase ROS production and deregulate redox-sensitive transcription factors and signaling pathways that, ultimately, contribute to cellular senescence (reviewed in (Davalli et al., 2016). Senescent cells, in turn, produce and secrete active MMPs and the ECM molecule fibronectin (Coppe et al., 2010). In this regard, there is plenty of evidence suggesting that aging is associated with increased activity of MMPs in several tissues/organs (Geng et al., 2017; Lopez-Luppo et al., 2017; Oelusarz et al., 2013). Furthermore, an increased expression of fibronectin has been shown in human aging fibroblasts and vascular endothelial cells (Kumazaki et al., 1993) as well as in the vasculature of old mice (Yoon et al., 2016) and skeletal muscle of human elderly subjects (Parker et al., 2017).
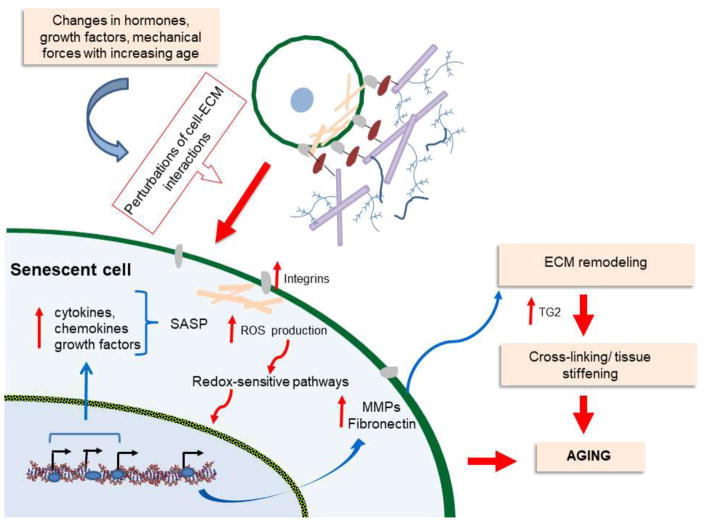
As a result of the dynamic reciprocity between the cell and ECM components, the enhanced senescence-induced secretion of MMPs and fibronectin is likely to produce ECM reorganization and changes in cell behavior of surrounding cells that can lead to cell stiffness. Given that the protein cross-linking transglutaminase 2 supports the association of fibronectin and integrin on the cell surface (Oh et al., 2017), increased transglutaminase 2 may then contribute to the stiffening of aged tissues (Oh et al., 2017; Steppan et al., 2017). The proposed process (Figure 1) can potentially promote normal age-related changes in function of tissues and organs, but also frailty phenotypes and chronic disorders if factors, such as hypertension, hyperglycemia-induced accumulation of AGEs, and lipid peroxidation, exacerbate ECM remodeling and sustain the fibrotic process, thereby causing irreversible tissue dysfunction (Ingles et al., 2014; Konstam et al., 2011; Phillip et al., 2015). In fact, advanced age is associated with an increased incidence of pathological fibrosis in many human organs (Harvey et al., 2016; Raghu et al., 2006). The mechanisms behind this association remain mostly elusive, but recent work revealed that sustained fibrosis in the lungs of 18-month-old mice leads to accumulation of senescent myofibroblasts (Hecker et al., 2014). This study also showed that the senescent phenotype was elicited by alterations in cellular redox homeostasis resulting from enhanced expression of the nicotinamide adenine dinucleotide phosphatase (NADPH) oxidase-4 (Nox-4) and an impaired capacity of the nuclear factor erythroid 2 (NF2)-related factor 2 (Nrf2)-antioxidant response element system (Hecker et al., 2014).
Figure 1. Schematic depicting the proposed mechanism through which cell-matrix interactions contribute to aging.
Changes in hormones, growth factors, or other physiological signals occurring with advanced age in the tissue microenvironment stimulate alterations in cell-ECM interactions. The increases in ROS production deregulate redox-sensitive transcription factors and signaling pathways that promote cellular senescence. The enhanced levels of matrix metalloproteinases (MMPs) and fibronectin induced by the senescent cell result in ECM reorganization and changes in cell behavior of surrounding cells, leading to cell stiffness. Increased levels of transglutaminase 2 (TG2) contribute to cross-linking and stiffening of aged tissues. The inflammatory Senescence-Associated Secretory Phenotype (SASP) can reinforce the aging process.
The regenerative capacity of multiple tissues also declines with age, thereby leading to tissue degeneration, malfunction, and potentially pathology (Yun, 2015). Altered ECM architecture and matrix stiffness can influence this process. This has been recently suggested by Stearns-Reider et al. (2017) who reported that muscle stem cells seeded on DECM scaffolds from 24 month old mice acquired an increased tendency to differentiate into a fibrogenic lineage, at the expense of myogenicity
3.2 Genetic perturbations in cell-matrix interactions influence longevity
Several genetic studies in animal models and humans suggest that genes encoding ECM components and molecules involved in cell-matrix interactions influence longevity. Experiments in both Caenorhabditis elegans and Drosophila melanogaster have revealed that loss-of function mutations and knockdown of genes encoding components of the integrin-signaling complex improve performance in functional activities impaired with aging and extend lifespan (Goddeeris et al., 2003; Hansen et al., 2005; Kumsta et al., 2014). Furthermore, in a recent study performed in C. elegans, Ewald et al. (2015) found that a decline in collagen expression, mediated by the Protein skinhead-1/Nrf2 stress response pathway, occurs during aging in adult worms. This work further showed that various genetic, nutritional, and pharmacological longevity-promoting interventions can delay this decline, thereby suggesting that ECM remodeling is a longevity-assurance mechanism (Ewald et al., 2015). Significant associations of variants in the COL5A1 (encoding the collagen type V alpha 1 chain), ITGA1 (encoding the integrin subunit alpha 1), and PTK2 (encoding FAK1) genes with longevity have also been described in humans (Garatachea et al., 2014; Sebastiani et al., 2012; Yashin et al., 2010).
In line with the studies depicted above, we and others reported that hypomorphic homozygous mutations in the Sdc gene reduce lifespan in D. melanogaster (De Luca et al., 2010; Wilson et al., 2013). Our work in flies also showed that the Sdc mutation perturbed the whole-body metabolism of young flies, and a parallel human genetic study found American children homozygous for the minor G-allele of the SDC4 rs1981429 polymorphism having more intra-abdominal fat than those homozygous for the T-allele (De Luca et al., 2010). Remarkably, in a follow-up study, the rs1981429 G-allele was also observed to be significantly associated with higher levels of fasting plasma triglycerides (a risk factor for cardiovascular disease) and a lower likelihood of becoming a centenarian in a cohort of healthy elderly Italian subjects (age 64 to 107 years) (Rose et al., 2015). Syndecan GAG chains interact with laminin α chains (Horiguchi et al., 2012). Thus, it is exciting that in an independent analysis performed in the same cohort of healthy elderly Italian subjects, we identified a significant association of the rs4925386-T allele in the LAMA5 gene (encoding the Laminin subunit, alpha 5) with longevity. Moreover, the rs4925386-T allele turned out to be correlated with shorter stature (De Luca et al., 2016). Given that the alternate allele of LAMA5 rs4925386 (G) is a risk factor for colorectal cancer (Houlston et al., 2010), our findings are in agreement with evidence suggesting that shorter stature is related to extended lifespan and reduced risk of multiple cancers (Samaras, 2012). It is also important to point out that a decline in height occurs with age due to changes in the bones, muscles, and joints (Fernihough and McGovern, 2015). In this regard, a recent study performed in a Canadian cohort of more than more 20,000 subjects of age 40–70 years reported that elderly hypertensive individuals were shorter and displayed a lower bone mass density and a higher rate of fractures than elderly normotensive individuals (El-Bikai et al., 2015). We did not see any significant correlation between variants in the LAMA5 and/or SDC4 genes and hypertension in our cohort of healthy elderly subjects. But, the SDC4 rs1981429 polymorphism was found significantly associated with hypertension and increased prevalence of coronary artery disease in a cohort of middle-aged subjects from the Tampere adult population cardiovascular risk study (Kunnas and Nikkari, 2014). Overall, these observations indicate that variants in genes involved in the interaction between the cell and the ECM may be among the factors linking adult height, cancer, hypertension, and aging.
4. The interplay between cell-matrix interactions and RAS and its role in the aged vasculature
4.1 RAS as a nexus between hypertension and aging
A steady increase in both systolic and diastolic blood pressure occurs with advanced age (Conti et al., 2012). This increase in blood pressure arises in part as a result of chronic activation of the circulating RAS, which is a systemic hormonal system whose primary function is to regulate arterial pressure as well as water and sodium homeostasis (Griendling et al., 1993). The main effector of RAS is Angiotensin (Ang) II that is produced by enzymatic sequential cleavage of peptides derived from the liver-produced angiotensinogen. Angiotensinogen is converted by renin to Ang I, which in turn is converted to the octapeptide Ang II by the action of the angiotensin-converting enzyme (ACE) (Griendling et al., 1993). Ang II exerts its actions by binding with equal affinity to two main G protein-coupled receptors, type 1 receptor (AT1R) and type 2 receptor (AT2R), which have different tissue distribution and opposite effects on vascular tone. While the activation of AT1R is associated with vasoconstriction, inflammation, and cellular growth and migration, AT2R activation leads to vasodilatory, antiproliferative, and proapoptotic actions (Abadir, 2011). The pleiotropic effects of Ang II are due to the fact that it induces multiple signaling pathways through activation of its receptors. For instance, depending on cell types, Ang-II stimulates MAPKs, various protein kinase C isoforms, small G-proteins (such as Ras, Rho, and Rac), and NADH/NADPH through activation of AT1R. On the other hand, it activates protein phosphatases, the nitric oxide-cGMP system, and phospholipase A2 through AT2R (Nouet and Nahmias, 2000). Two drug classes inhibit RAS by directly targeting Ang II. They are the inhibitors of the ACE enzyme (ACEi) and the angiotensin receptor blockers (ARBs) that antagonize the binding of Ang II to AT1R. ACEi and ARBs are widely used in clinical practice to manage hypertension and chronic kidney disease (Abadir, 2011). In addition to the circulating system, several RAS components exist in almost every organ (local RAS), where they exert diverse organ-specific physiological functions through the action of de novo synthesized Ang II. Local RASs operate in concert with the systemic RAS, but also independently (Brenner et al., 2000; Viberti et al., 2002).
A large literature points to RAS as an important contributor to the aging process (reviewed in (Abadir, 2011; Basso et al., 2005; Carter et al., 2005; Heudes et al., 1994; Simon et al., 2015). For instance, it has been known for several years that ACEi or ARB administration reduces the risk of new-onset T2D and improves cardiovascular disorders via the ability of the drugs to attenuate Ang II signaling (Abuissa et al., 2005). More recent evidence suggests that ARB therapy can also improve episodic memory and cognitive decline in the elderly (Stuhec et al., 2017; Trofimiuk et al., 2017). Furthermore, administration of ACEi, ARB, or both has been shown to reduce age-associated fracture risk (Solomon et al., 2011), and improve physical function in older individuals with impairment of daily activities (Sumukadas et al., 2007) and in physically independent elderly people (Coelho et al., 2016).
Genetic and pharmacological studies in rodents and C. elegans have also shown that RAS influences longevity. Specifically, Benigni et al. (2009) were the first to report that AT1R knockout mice lived 26% longer than genetically matched wild-type mice. In an independent study in rats, long-term treatment with the ACEi enalapril also led to a significant increase in lifespan, despite the fact that the animals were fed palatable hyperlipidic diets (Santos et al., 2009). Finally, work from Kumar et al. (2016) showed that reducing the activity of the acn-1 gene (encoding the C. elegans homolog of mammalian ACE) by RNA interference resulted in mean and maximum lifespan extension in worms of 21% and 18%, respectively. Pharmacological inhibition of acn-1/ACE also extended the worm lifespan (Kumar et al., 2016).
The exact mechanism(s) by which pharmacological blockade of RAS provides benefits on the age-related functional decline of organs and tissues are still unclear. However, one current hypothesis is that the age-retarding effects associated with ACEi or ARB therapy are partly due to the capacity of these drugs to reduce ROS production and thereby preserve the physiological phosphorylation state of the mitochondria. This hypothesis is based on the fact that many of the pathways through which Ang II/AT1R signals involve activation of the Nox family enzymes, which have the capacity to generate superoxide and other downstream ROS (Dikalov, 2011). Ang II- induced ROS, in turn, target downstream redox-sensitive pathways as well as mitochondrial function and ROS production creating a vicious feed-forward cycle of ROS accumulation that disrupts redox homeostasis (Dikalov, 2011). The reader is referred to excellent review articles by (Benigni et al., 2010; Conti et al., 2012; de Cavanagh et al., 2011; Onder et al., 2006; Vajapey et al., 2014) for further details on this topic.
4.2 Ang II and cell-matrix interactions in the vasculature
A remodeling of the arterial system progressively occurs with advancing age. The age-associated changes in arterial structure and function entail activation of the Ang-II signaling, changes in the functional properties of the vascular smooth muscles cells (VSMCs) (reviewed in (Lacolley et al., 2012). The VSMCs are the cellular components of the normal blood vessel wall that synthesize and secrete insoluble ECM molecules and provide structural integrity to the vessel (Louis and Zahradka, 2010). The adult VSMCs primary function is to maintain vascular homeostasis through active contraction and relaxation. However, mature contractile VSMCs show high plasticity (e.g. switching from a contractile to a noncontractile/synthetic phenotype) and can acquire the ability to migrate and proliferate in order to promote vessel repair and remodeling processes initiated by vascular wall injury. Failure of the migrating/proliferating VSMCs to return to the contractile phenotype can induce pathogenic vascular remodeling, thereby contributing to vascular lesion formation and diverse vascular diseases (Louis and Zahradka, 2010). It has been known for some time that binding of Ang II to AT1R contributes to the age-associated increase in migration and proliferation capacity of VSMC, with chronic Ang II exposure inducing pro-fibrotic and pro-inflammatory responses. Specifically, the Ang-II-induced formation of molecules, such as transforming growth factor-β1 (TGF-β1), monocyte chemotactic protein-1 (MCP-1), and MMPs, by VSMCs leads to inflammation, accumulation of fibronectin and collagen, decrease in elastin content, and ultimately to arterial stiffness (reviewed in (Wang et al., 2010). Moreover, the increased levels of fibronectin and calcium-dependent tissue transglutaminase 2 induced by the Ang II signaling may promote the calcification of vascular cells that is responsible for age-induced changes in vessel mechanics (Ding et al., 2006; Johnson et al., 2008). The pivotal role of Ang II in vascular stiffness is further corroborated by the fact that inhibition of ACE activity can reduce arterial stiffness in old people, independently of changes in blood pressure (Hayashi et al., 2006).
Besides the well-recognized pro-fibrotic activity of Ang II in the vasculature, some in vitro studies have also revealed a role of cell-matrix interactions in the Ang II-induced migration and proliferation of VSMCs. Tamura et al. (2001) first reported that the integrin/FAK signaling pathway interacts synergistically with the Ang II signaling to activate the ERK cascade in VSMCs. This finding has since been corroborated by more recent work showing that α1β1 integrin and integrin-linked kinase, an intracellular serine/threonine kinase involved in cell-matrix interactions, modulate the Ang II effects on VSMC (Bunni et al., 2011; Moraes et al., 2015). Ang II controls vascular cell production of ROS through various Noxs, including Nox-1 and Nox-2 (Nguyen Dinh Cat et al., 2013). Interestingly, studies by Moers and collaborators (Moraes et al., 2015, 2016) have provided evidence that Ang II produces ROS in the VSMC in two stages, the second of which is mediated by the α1β1 integrin signaling. Based on their data, the authors propose that Ang II signaling briefly stimulates Nox-1 activity leading to the early phase of ROS production. This is followed by activation of the α1β1 integrin/ILK signaling that, in turn, induces the second phase of ROS production via Nox-2 activation (Moraes et al., 2015, 2016).
5. Concluding remarks
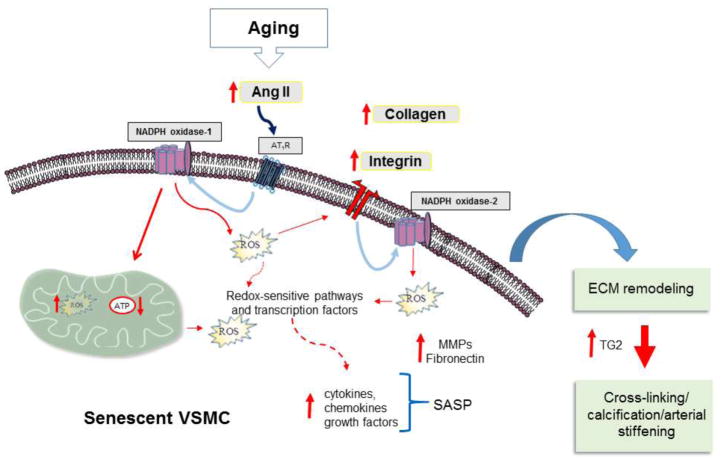
A variety of changes in biological factors, such as hormones, growth factors and cytokines, occur in the connective tissue surrounding the cell with advancing age. These changes can perturb the mechanisms of communication between the cell and ECM, thereby impacting cell behavior and fate choice, such as stimulating cellular senescence. In the vasculature, Ang II is known to induce cellular senescence of VSMC by eliciting Nox-1 and mitochondrial ROS production (Tsai et al., 2016). Thus, the increase in activation of the local Ang II/AT1R signaling seen with age (Yoon et al., 2016) could be one of the cues responsible for perturbing cell-matrix interactions through the ROS-induced stimulation of collagen and integrin synthesis (Boin et al., 2014; Moraes et al., 2015). The subsequent changes in redox-sensitive intracellular signaling pathways and transcription factors may then lead to cellular senescence, ECM remodeling, and arterial thickness (Figure 2). There is no evidence yet that the crosstalk between Ang-II/Nox and integrin/ILK signaling influences ROS production in aged VSMC and evidence is necessary to prove this claim. Nevertheless, the data summarized in this review make it a compelling area for further investigation. In addition, the focus here has been specifically on the vasculature system. However, given the many roles that the cell-matrix interactions and local RASs have in maintaining connective tissue homeostasis, it is likely that the effect of their intricate interplay may impact the age-associated degeneration of multiple tissues.
Figure 2. Schematic depicting the proposed mechanism through which the interplay between cell-matrix interactions and angiotensin II signaling influences vascular aging.
The age-associated increase in activation of the local Ang II/AT1R induces ROS production through NADPH oxidase-1 activity. The enhanced ROS levels stimulate the synthesis of collagen and integrins, which in turn activate NADPH oxidase-2 activity. Activation of redox-sensitive intracellular signaling pathways, such as mitogen-activated protein kinase cascade, and mitochondrial homeostasis impairment induce cellular senescence. This in turn leads to increased levels of metalloproteinases (MMPs) and fibronectin and thereby ECM remodeling. Increased levels of transglutaminase 2 (TG2) contribute to cross-linking, calcification, and arterial thickness. SASP: Senescence-Associated Secretory Phenotype.
Highlights.
Cell-matrix interactions play a role in development, adult tissue repair, and aging.
Cellular senescence is induced by age-related ECM alterations.
Genetic studies indicate that cell-matrix interactions influence longevity.
The crosstalk between cell-matrix interactions and angiotenin II signaling has a role in the aged vasculature.
Acknowledgments
The author would like to thank Prof. Giuseppina Rose for insightful discussions and critically reviewing the manuscript. This work was in part supported by U.S. National Institutes of Health Grant R01DK084219.
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abadir PM. The frail renin-angiotensin system. Clin Geriatr Med. 2011;27:53–65. doi: 10.1016/j.cger.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuissa H, Jones PG, Marso SP, O’Keefe JH., Jr Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: a meta-analysis of randomized clinical trials. J Am Coll Cardiol. 2005;46:821–826. doi: 10.1016/j.jacc.2005.05.051. [DOI] [PubMed] [Google Scholar]
- Aunan JR, Watson MM, Hagland HR, Soreide K. Molecular and biological hallmarks of ageing. Br J Surg. 2016;103:e29–46. doi: 10.1002/bjs.10053. [DOI] [PubMed] [Google Scholar]
- Basso N, Paglia N, Stella I, de Cavanagh EM, Ferder L, del Rosario Lores Arnaiz M, Inserra F. Protective effect of the inhibition of the renin-angiotensin system on aging. Regul Pept. 2005;128:247–252. doi: 10.1016/j.regpep.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2:247–257. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, Conti S, Rottoli D, Longaretti L, Cassis P, Morigi M, Coffman TM, Remuzzi G. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorksten J, Tenhu H. The crosslinking theory of aging--added evidence. Exp Gerontol. 1990;25:91–95. doi: 10.1016/0531-5565(90)90039-5. [DOI] [PubMed] [Google Scholar]
- Boin F, Erre GL, Posadino AM, Cossu A, Giordo R, Spinetti G, Passiu G, Emanueli C, Pintus G. Oxidative stress-dependent activation of collagen synthesis is induced in human pulmonary smooth muscle cells by sera from patients with scleroderma-associated pulmonary hypertension. Orphanet J Rare Dis. 2014;9:123. doi: 10.1186/s13023-014-0123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner BM, Cooper ME, de Zeeuw D, Grunfeld JP, Keane WF, Kurokawa K, McGill JB, Mitch WE, Parving HH, Remuzzi G, Ribeiro AB, Schluchter MD, Snavely D, Zhang Z, Simpson R, Ramjit D, Shahinfar S Investigators RS. The losartan renal protection study--rationale, study design and baseline characteristics of RENAAL (Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan) J Renin Angiotensin Aldosterone Syst. 2000;1:328–335. doi: 10.3317/jraas.2000.062. [DOI] [PubMed] [Google Scholar]
- Brizzi MF, Tarone G, Defilippi P. Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr Opin Cell Biol. 2012;24:645–651. doi: 10.1016/j.ceb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Bunni MA, Kramarenko II, Walker L, Raymond JR, Garnovskaya MN. Role of integrins in angiotensin II-induced proliferation of vascular smooth muscle cells. Am J Physiol Cell Physiol. 2011;300:C647–656. doi: 10.1152/ajpcell.00179.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona JJ, Michan S. Biology of Healthy Aging and Longevity. Rev Invest Clin. 2016;68:7–16. [PubMed] [Google Scholar]
- Carter CS, Onder G, Kritchevsky SB, Pahor M. Angiotensin-converting enzyme inhibition intervention in elderly persons: effects on body composition and physical performance. J Gerontol A Biol Sci Med Sci. 2005;60:1437–1446. doi: 10.1093/gerona/60.11.1437. [DOI] [PubMed] [Google Scholar]
- Chakravarti R, Adams JC. Comparative genomics of the syndecans defines an ancestral genomic context associated with matrilins in vertebrates. BMC Genomics. 2006;7:83. doi: 10.1186/1471-2164-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HR, Cho KA, Kang HT, Lee JB, Kaeberlein M, Suh Y, Chung IK, Park SC. Restoration of senescent human diploid fibroblasts by modulation of the extracellular matrix. Aging Cell. 2011;10:148–157. doi: 10.1111/j.1474-9726.2010.00654.x. [DOI] [PubMed] [Google Scholar]
- Coelho VA, Probst VS, Nogari BM, Teixeira DC, Felcar JM, Santos DC, Gomes MV, Andraus RA, Fernandes KB. Angiotensin-II blockage, muscle strength, and exercise capacity in physically independent older adults. J Phys Ther Sci. 2016;28:547–552. doi: 10.1589/jpts.28.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti S, Cassis P, Benigni A. Aging and the renin-angiotensin system. Hypertension. 2012;60:878–883. doi: 10.1161/HYPERTENSIONAHA.110.155895. [DOI] [PubMed] [Google Scholar]
- Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM. Lifespan and Healthspan: Past, Present, and Promise. Gerontologist. 2015;55:901–911. doi: 10.1093/geront/gnv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121:255–264. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- Davalli P, Mitic T, Caporali A, Lauriola A, D’Arca D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid Med Cell Longev. 2016;2016:3565127. doi: 10.1155/2016/3565127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cavanagh EM, Inserra F, Ferder L. Angiotensin II blockade: a strategy to slow ageing by protecting mitochondria? Cardiovasc Res. 2011;89:31–40. doi: 10.1093/cvr/cvq285. [DOI] [PubMed] [Google Scholar]
- De Luca M, Crocco P, De Rango F, Passarino G, Rose G. Association of the Laminin, Alpha 5 (LAMA5) rs4925386 with height and longevity in an elderly population from Southern Italy. Mech Ageing Dev. 2016;155:55–59. doi: 10.1016/j.mad.2016.03.003. [DOI] [PubMed] [Google Scholar]
- De Luca M, Klimentidis YC, Casazza K, Chambers MM, Cho R, Harbison ST, Jumbo-Lucioni P, Zhang S, Leips J, Fernandez JR. A conserved role for syndecan family members in the regulation of whole-body energy metabolism. PLoS One. 2010;5:e11286. doi: 10.1371/journal.pone.0011286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med. 2011;51:1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding HT, Wang CG, Zhang TL, Wang K. Fibronectin enhances in vitro vascular calcification by promoting osteoblastic differentiation of vascular smooth muscle cells via ERK pathway. J Cell Biochem. 2006;99:1343–1352. doi: 10.1002/jcb.20999. [DOI] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Eble JA, de Rezende FF. Redox-relevant aspects of the extracellular matrix and its cellular contacts via integrins. Antioxid Redox Signal. 2014;20:1977–1993. doi: 10.1089/ars.2013.5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Bikai R, Tahir MR, Tremblay J, Joffres M, Seda O, Sedova L, Awadalla P, Laberge C, Knoppers BM, Dumas P, Gaudet D, Ste-Marie LG, Hamet P. Association of age-dependent height and bone mineral density decline with increased arterial stiffness and rate of fractures in hypertensive individuals. J Hypertens. 2015;33:727–735. doi: 10.1097/HJH.0000000000000475. [DOI] [PubMed] [Google Scholar]
- Erler JT, Weaver VM. Three-dimensional context regulation of metastasis. Clin Exp Metastasis. 2009;26:35–49. doi: 10.1007/s10585-008-9209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald CY, Landis JN, Porter Abate J, Murphy CT, Blackwell TK. Dauer-independent insulin/IGF-1-signalling implicates collagen remodelling in longevity. Nature. 2015;519:97–101. doi: 10.1038/nature14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernihough A, McGovern ME. Physical stature decline and the health status of the elderly population in England. Econ Hum Biol. 2015;16:30–44. doi: 10.1016/j.ehb.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M. Centenarians as a model for healthy aging. Biochem Soc Trans. 2003;31:457–461. doi: 10.1042/bst0310457. [DOI] [PubMed] [Google Scholar]
- Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garatachea N, Fuku N, He ZH, Tian Y, Arai Y, Abe Y, Murakami H, Miyachi M, Yvert T, Venturini L, Santiago C, Santos-Lozano A, Rodriguez G, Ricevuti G, Pareja-Galeano H, Sanchis-Gomar F, Emanuele E, Hirose N, Lucia A. PTK2 rs7460 and rs7843014 polymorphisms and exceptional longevity: a functional replication study. Rejuvenation Res. 2014;17:430–438. doi: 10.1089/rej.2014.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazoti Debessa CR, Mesiano Maifrino LB, Rodrigues de Souza R. Age related changes of the collagen network of the human heart. Mech Ageing Dev. 2001;122:1049–1058. doi: 10.1016/s0047-6374(01)00238-x. [DOI] [PubMed] [Google Scholar]
- Geng X, Hwang J, Ye J, Shih H, Coulter B, Naudin C, Jun K, Sievers R, Yeghiazarians Y, Lee RJ, Boyle AJ. Aging is protective against pressure overload cardiomyopathy via adaptive extracellular matrix remodeling. Am J Cardiovasc Dis. 2017;7:72–82. [PMC free article] [PubMed] [Google Scholar]
- Gkogkolou P, Bohm M. Advanced glycation end products: Key players in skin aging? Dermatoendocrinol. 2012;4:259–270. doi: 10.4161/derm.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddeeris MM, Cook-Wiens E, Horton WJ, Wolf H, Stoltzfus JR, Borrusch M, Grotewiel MS. Delayed behavioural aging and altered mortality in Drosophila beta integrin mutants. Aging cell. 2003;2:257–264. doi: 10.1046/j.1474-9728.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- Gopal S, Multhaupt HAB, Pocock R, Couchman JR. Cell-extracellular matrix and cell-cell adhesion are linked by syndecan-4. Matrix Biol. 2017;60–61:57–69. doi: 10.1016/j.matbio.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Gopal S, Sogaard P, Multhaupt HA, Pataki C, Okina E, Xian X, Pedersen ME, Stevens T, Griesbeck O, Park PW, Pocock R, Couchman JR. Transmembrane proteoglycans control stretch-activated channels to set cytosolic calcium levels. J Cell Biol. 2015;210:1199–1211. doi: 10.1083/jcb.201501060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling KK, Murphy TJ, Alexander RW. Molecular biology of the renin-angiotensin system. Circulation. 1993;87:1816–1828. doi: 10.1161/01.cir.87.6.1816. [DOI] [PubMed] [Google Scholar]
- Hansen M, Hsu AL, Dillin A, Kenyon C. New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS genetics. 2005;1:119–128. doi: 10.1371/journal.pgen.0010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper S. Economic and social implications of aging societies. Science. 2014;346:587–591. doi: 10.1126/science.1254405. [DOI] [PubMed] [Google Scholar]
- Harvey A, Montezano AC, Lopes RA, Rios F, Touyz RM. Vascular Fibrosis in Aging and Hypertension: Molecular Mechanisms and Clinical Implications. Can J Cardiol. 2016;32:659–668. doi: 10.1016/j.cjca.2016.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Miyagawa K, Sato K, Ueda R, Dohi Y. Temocapril, an Angiotensin converting enzyme inhibitor, ameliorates age-related increase in carotid arterial stiffness in normotensive subjects. Cardiology. 2006;106:190–194. doi: 10.1159/000093024. [DOI] [PubMed] [Google Scholar]
- Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, Meldrum E, Sanders YY, Thannickal VJ. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med. 2014;6:231ra247. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heudes D, Michel O, Chevalier J, Scalbert E, Ezan E, Bariety J, Zimmerman A, Corman B. Effect of chronic ANG I-converting enzyme inhibition on aging processes. I. Kidney structure and function. Am J Physiol. 1994;266:R1038–1051. doi: 10.1152/ajpregu.1994.266.3.R1038. [DOI] [PubMed] [Google Scholar]
- Horiguchi K, Kouki T, Fujiwara K, Tsukada T, Ly F, Kikuchi M, Yashiro T. Expression of the proteoglycan syndecan-4 and the mechanism by which it mediates stress fiber formation in folliculostellate cells in the rat anterior pituitary gland. J Endocrinol. 2012;214:199–206. doi: 10.1530/JOE-12-0156. [DOI] [PubMed] [Google Scholar]
- Houlston RS, Cheadle J, Dobbins SE, Tenesa A, Jones AM, Howarth K, Spain SL, Broderick P, Domingo E, Farrington S, Prendergast JG, Pittman AM, Theodoratou E, Smith CG, Olver B, Walther A, Barnetson RA, Churchman M, Jaeger EE, Penegar S, Barclay E, Martin L, Gorman M, Mager R, Johnstone E, Midgley R, Niittymaki I, Tuupanen S, Colley J, Idziaszczyk S, Thomas HJ, Lucassen AM, Evans DG, Maher ER, Maughan T, Dimas A, Dermitzakis E, Cazier JB, Aaltonen LA, Pharoah P, Kerr DJ, Carvajal-Carmona LG, Campbell H, Dunlop MG, Tomlinson IP Consortium C, Group CC, Group CC, Consortium C. Meta-analysis of three genome-wide association studies identifies susceptibility loci for colorectal cancer at 1q41, 3q26.2, 12q13.13 and 20q13.33. Nat Genet. 2010;42:973–977. doi: 10.1038/ng.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingles M, Gambini J, Carnicero JA, Garcia-Garcia FJ, Rodriguez-Manas L, Olaso-Gonzalez G, Dromant M, Borras C, Vina J. Oxidative stress is related to frailty, not to age or sex, in a geriatric population: lipid and protein oxidation as biomarkers of frailty. J Am Geriatr Soc. 2014;62:1324–1328. doi: 10.1111/jgs.12876. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayadev R, Sherwood DR. Basement membranes. Curr Biol. 2017;27:R207–R211. doi: 10.1016/j.cub.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Polewski M, Terkeltaub RA. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ Res. 2008;102:529–537. doi: 10.1161/CIRCRESAHA.107.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SS, Singer BD, Vaughan DE. Molecular and physiological manifestations and measurement of aging in humans. Aging Cell. 2017;16:624–633. doi: 10.1111/acel.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. 2011;4:98–108. doi: 10.1016/j.jcmg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet. 2017;389:1323–1335. doi: 10.1016/S0140-6736(16)32381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kular JK, Basu S, Sharma RI. The extracellular matrix: Structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J Tissue Eng. 2014;5:2041731414557112. doi: 10.1177/2041731414557112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Dietrich N, Kornfeld K. Angiotensin Converting Enzyme (ACE) Inhibitor Extends Caenorhabditis elegans Life Span. PLoS Genet. 2016;12:e1005866. doi: 10.1371/journal.pgen.1005866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazaki T, Kobayashi M, Mitsui Y. Enhanced expression of fibronectin during in vivo cellular aging of human vascular endothelial cells and skin fibroblasts. Exp Cell Res. 1993;205:396–402. doi: 10.1006/excr.1993.1103. [DOI] [PubMed] [Google Scholar]
- Kumsta C, Ching TT, Nishimura M, Davis AE, Gelino S, Catan HH, Yu X, Chu CC, Ong B, Panowski SH, Baird N, Bodmer R, Hsu AL, Hansen M. Integrin-linked kinase modulates longevity and thermotolerance in C. elegans through neuronal control of HSF-1. Aging cell. 2014;13:419–430. doi: 10.1111/acel.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnas T, Nikkari ST. Contribution of syndecan-4 genetic variants to hypertension, the TAMRISK study. BMC Res Notes. 2014;7:815. doi: 10.1186/1756-0500-7-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacolley P, Regnault V, Nicoletti A, Li Z, Michel JB. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res. 2012;95:194–204. doi: 10.1093/cvr/cvs135. [DOI] [PubMed] [Google Scholar]
- Lampi MC, Reinhart-King CA. Targeting extracellular matrix stiffness to attenuate disease: From molecular mechanisms to clinical trials. Sci Transl Med. 2018:10. doi: 10.1126/scitranslmed.aao0475. [DOI] [PubMed] [Google Scholar]
- Le Gall M, Chambard JC, Breittmayer JP, Grall D, Pouyssegur J, Van Obberghen-Schilling E. The p42/p44 MAP kinase pathway prevents apoptosis induced by anchorage and serum removal. Mol Biol Cell. 2000;11:1103–1112. doi: 10.1091/mbc.11.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, Curiel TJ, de Cabo R, Franceschi C, Gems D, Ingram DK, Johnson TE, Kennedy BK, Kenyon C, Klein S, Kopchick JJ, Lepperdinger G, Madeo F, Mirisola MG, Mitchell JR, Passarino G, Rudolph KL, Sedivy JM, Shadel GS, Sinclair DA, Spindler SR, Suh Y, Vijg J, Vinciguerra M, Fontana L. Interventions to Slow Aging in Humans: Are We Ready? Aging Cell. 2015;14:497–510. doi: 10.1111/acel.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Luppo M, Nacher V, Ramos D, Catita J, Navarro M, Carretero A, Rodriguez-Baeza A, Mendes-Jorge L, Ruberte J. Blood Vessel Basement Membrane Alterations in Human Retinal Microaneurysms During Aging. Invest Ophthalmol Vis Sci. 2017;58:1116–1131. doi: 10.1167/iovs.16-19998. [DOI] [PubMed] [Google Scholar]
- Louis SF, Zahradka P. Vascular smooth muscle cell motility: From migration to invasion. Exp Clin Cardiol. 2010;15:e75–85. [PMC free article] [PubMed] [Google Scholar]
- Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maya-Mendoza A, Bartek J, Jackson DA, Streuli CH. Cellular microenvironment controls the nuclear architecture of breast epithelia through beta1-integrin. Cell Cycle. 2016;15:345–356. doi: 10.1080/15384101.2015.1121354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays PK, Bishop JE, Laurent GJ. Age-related changes in the proportion of types I and III collagen. Mech Ageing Dev. 1988;45:203–212. doi: 10.1016/0047-6374(88)90002-4. [DOI] [PubMed] [Google Scholar]
- Mays PK, McAnulty RJ, Campa JS, Laurent GJ. Age-related changes in collagen synthesis and degradation in rat tissues. Importance of degradation of newly synthesized collagen in regulating collagen production. Biochem J. 1991;276(Pt 2):307–313. doi: 10.1042/bj2760307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T, Maeda K, Kurokawa K, van Ypersele de Strihou C. Oxidation conspires with glycation to generate noxious advanced glycation end products in renal failure. Nephrol Dial Transplant. 1997;12:255–258. doi: 10.1093/ndt/12.2.255. [DOI] [PubMed] [Google Scholar]
- Moraes JA, Frony AC, Dias AM, Renovato-Martins M, Rodrigues G, Marcinkiewicz C, Assreuy J, Barja-Fidalgo C. Alpha1beta1 and integrin-linked kinase interact and modulate angiotensin II effects in vascular smooth muscle cells. Atherosclerosis. 2015;243:477–485. doi: 10.1016/j.atherosclerosis.2015.09.026. [DOI] [PubMed] [Google Scholar]
- Moraes JA, Frony AC, Dias AM, Renovato-Martins M, Rodrigues G, Marcinkiewicz C, Assreuy J, Barja-Fidalgo C. Data in support of alpha1beta1 and integrin-linked kinase interact and modulate angiotensin II effects in vascular smooth muscle cells. Data Brief. 2016;6:330–340. doi: 10.1016/j.dib.2015.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 2007;8:957–969. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Espin D, Canamero M, Maraver A, Gomez-Lopez G, Contreras J, Murillo-Cuesta S, Rodriguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, Serrano M. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155:1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Neves J, Demaria M, Campisi J, Jasper H. Of flies, mice, and men: evolutionarily conserved tissue damage responses and aging. Dev Cell. 2015;32:9–18. doi: 10.1016/j.devcel.2014.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Dinh Cat A, Montezano AC, Burger D, Touyz RM. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid Redox Signal. 2013;19:1110–1120. doi: 10.1089/ars.2012.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouet S, Nahmias C. Signal transduction from the angiotensin II AT2 receptor. Trends Endocrinol Metab. 2000;11:1–6. doi: 10.1016/s1043-2760(99)00205-2. [DOI] [PubMed] [Google Scholar]
- Oelusarz A, Nichols LA, Grunz-Borgmann EA, Chen G, Akintola AD, Catania JM, Burghardt RC, Trzeciakowski JP, Parrish AR. Overexpression of MMP-7 Increases Collagen 1A2 in the Aging Kidney. Physiol Rep. 2013;1 doi: 10.1002/phy2.90. pii: e00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh YJ, Pau VC, Steppan J, Sikka G, Bead VR, Nyhan D, Levine BD, Berkowitz DE, Santhanam L. Role of tissue transglutaminase in age-associated ventricular stiffness. Amino Acids. 2017;49:695–704. doi: 10.1007/s00726-016-2295-z. [DOI] [PubMed] [Google Scholar]
- Onder G, Vedova CD, Pahor M. Effects of ACE inhibitors on skeletal muscle. Curr Pharm Des. 2006;12:2057–2064. doi: 10.2174/138161206777442137. [DOI] [PubMed] [Google Scholar]
- Parker L, Caldow MK, Watts R, Levinger P, Cameron-Smith D, Levinger I. Age and sex differences in human skeletal muscle fibrosis markers and transforming growth factor-beta signaling. Eur J Appl Physiol. 2017;117:1463–1472. doi: 10.1007/s00421-017-3639-4. [DOI] [PubMed] [Google Scholar]
- Phillip JM, Aifuwa I, Walston J, Wirtz D. The Mechanobiology of Aging. Annu Rev Biomed Eng. 2015;17:113–141. doi: 10.1146/annurev-bioeng-071114-040829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XJ, Wildey GM, Howe PH. Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. J Biol Chem. 2006;281:813–823. doi: 10.1074/jbc.M505546200. [DOI] [PubMed] [Google Scholar]
- Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- Rose G, Crocco P, De Rango F, Corsonello A, Lattanzio F, De Luca M, Passarino G. Metabolism and successful aging: Polymorphic variation of syndecan-4 (SDC4) gene associate with longevity and lipid profile in healthy elderly Italian subjects. Mech Ageing Dev. 2015;150:27–33. doi: 10.1016/j.mad.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Samaras TT. How height is related to our health and longevity: a review. Nutr Health. 2012;21:247–261. doi: 10.1177/0260106013510996. [DOI] [PubMed] [Google Scholar]
- Santos EL, de Picoli Souza K, da Silva ED, Batista EC, Martins PJ, D’Almeida V, Pesquero JB. Long term treatment with ACE inhibitor enalapril decreases body weight gain and increases life span in rats. Biochem Pharmacol. 2009;78:951–958. doi: 10.1016/j.bcp.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Assoian RK. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. Journal of cell science. 2001;114:2553–2560. doi: 10.1242/jcs.114.14.2553. [DOI] [PubMed] [Google Scholar]
- Sebastiani P, Solovieff N, Dewan AT, Walsh KM, Puca A, Hartley SW, Melista E, Andersen S, Dworkis DA, Wilk JB, Myers RH, Steinberg MH, Montano M, Baldwin CT, Hoh J, Perls TT. Genetic signatures of exceptional longevity in humans. PLoS One. 2012;7:e29848. doi: 10.1371/journal.pone.0029848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol A Biol Sci Med Sci. 2010;65:963–975. doi: 10.1093/gerona/glq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong J, Wang N, Wang Y. Mechanotransduction at focal adhesions: from physiology to cancer development. J Cell Mol Med. 2013;17:597–604. doi: 10.1111/jcmm.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton DN, Chang E, Whittier PS, Choi D, Funk WD. Microarray analysis of replicative senescence. Curr Biol. 1999;9:939–945. doi: 10.1016/s0960-9822(99)80420-5. [DOI] [PubMed] [Google Scholar]
- Simon CB, Lee-McMullen B, Phelan D, Gilkes J, Carter CS, Buford TW. The renin-angiotensin system and prevention of age-related functional decline: where are we now? Age (Dordr) 2015;37:9753. doi: 10.1007/s11357-015-9753-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedeker JG, Gautieri A. The role of collagen crosslinks in ageing and diabetes - the good, the bad, and the ugly. Muscles Ligaments Tendons J. 2014;4:303–308. [PMC free article] [PubMed] [Google Scholar]
- Solomon DH, Mogun H, Garneau K, Fischer MA. Risk of fractures in older adults using antihypertensive medications. J Bone Miner Res. 2011;26:1561–1567. doi: 10.1002/jbmr.356. [DOI] [PubMed] [Google Scholar]
- Stearns-Reider KM, D’Amore A, Beezhold K, Rothrauff B, Cavalli L, Wagner WR, Vorp DA, Tsamis A, Shinde S, Zhang C, Barchowsky A, Rando TA, Tuan RS, Ambrosio F. Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion. Aging Cell. 2017;16:518–528. doi: 10.1111/acel.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens EH, Grande-Allen KJ. Age-related changes in collagen synthesis and turnover in porcine heart valves. J Heart Valve Dis. 2007;16:672–682. [PubMed] [Google Scholar]
- Steppan J, Bergman Y, Viegas K, Armstrong D, Tan S, Wang H, Melucci S, Hori D, Park SY, Barreto SF, Isak A, Jandu S, Flavahan N, Butlin M, An SS, Avolio A, Berkowitz DE, Halushka MK, Santhanam L. Tissue Transglutaminase Modulates Vascular Stiffness and Function Through Crosslinking-Dependent and Crosslinking-Independent Functions. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004161. pii: e004161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M, O’Neill C, Berryman S, Waxman V. Anchorage and growth regulation in normal and virus-transformed cells. Int J Cancer. 1968;3:683–693. doi: 10.1002/ijc.2910030517. [DOI] [PubMed] [Google Scholar]
- Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J, Keyes WM. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155:1119–1130. doi: 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- Stuhec M, Keuschler J, Serra-Mestres J, Isetta M. Effects of different antihypertensive medication groups on cognitive function in older patients: A systematic review. Eur Psychiatry. 2017;46:1–15. doi: 10.1016/j.eurpsy.2017.07.015. [DOI] [PubMed] [Google Scholar]
- Sumukadas D, Witham MD, Struthers AD, McMurdo ME. Effect of perindopril on physical function in elderly people with functional impairment: a randomized controlled trial. CMAJ. 2007;177:867–874. doi: 10.1503/cmaj.061339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Li W, Lu Z, Chen R, Ling J, Ran Q, Jilka RL, Chen XD. Rescuing replication and osteogenesis of aged mesenchymal stem cells by exposure to a young extracellular matrix. FASEB J. 2011;25:1474–1485. doi: 10.1096/fj.10-161497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacutu R, Budovsky A, Yanai H, Fraifeld VE. Molecular links between cellular senescence, longevity and age-related diseases - a systems biology perspective. Aging (Albany NY) 2011;3:1178–1191. doi: 10.18632/aging.100413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Okazaki M, Tamura M, Kanegae K, Okuda H, Abe H, Nakashima Y. Synergistic interaction of integrin and angiotensin II in activation of extracellular signal-regulated kinase pathways in vascular smooth muscle cells. J Cardiovasc Pharmacol. 2001;38(Suppl 1):S59–62. doi: 10.1097/00005344-200110001-00013. [DOI] [PubMed] [Google Scholar]
- Trappmann B, Gautrot JE, Connelly JT, Strange DG, Li Y, Oyen ML, Cohen Stuart MA, Boehm H, Li B, Vogel V, Spatz JP, Watt FM, Huck WT. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11:642–649. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- Trofimiuk E, Wielgat P, Braszko JJ. Candesartan, angiotensin II type 1 receptor blocker is able to relieve age-related cognitive impairment. Pharmacol Rep. 2017;70:87–92. doi: 10.1016/j.pharep.2017.07.016. [DOI] [PubMed] [Google Scholar]
- Tsai IC, Pan ZC, Cheng HP, Liu CH, Lin BT, Jiang MJ. Reactive oxygen species derived from NADPH oxidase 1 and mitochondria mediate angiotensin II-induced smooth muscle cell senescence. J Mol Cell Cardiol. 2016;98:18–27. doi: 10.1016/j.yjmcc.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Vajapey R, Rini D, Walston J, Abadir P. The impact of age-related dysregulation of the angiotensin system on mitochondrial redox balance. Front Physiol. 2014;5:439. doi: 10.3389/fphys.2014.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viberti G, Wheeldon NM MicroAlbuminuria Reduction With VSI. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure-independent effect. Circulation. 2002;106:672–678. doi: 10.1161/01.cir.0000024416.33113.0a. [DOI] [PubMed] [Google Scholar]
- Wang M, Khazan B, Lakatta EG. Central Arterial Aging and Angiotensin II Signaling. Curr Hypertens Rev. 2010;6:266–281. doi: 10.2174/157340210793611668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RH, Lai CQ, Lyman RF, Mackay TF. Genomic response to selection for postponed senescence in Drosophila. Mech Ageing Dev. 2013;134:79–88. doi: 10.1016/j.mad.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson M, Budovsky A, Tacutu R, Fraifeld V. The signaling hubs at the crossroad of longevity and age-related disease networks. Int J Biochem Cell Biol. 2009;41:516–520. doi: 10.1016/j.biocel.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Woods A, Couchman JR. Syndecan-4 and focal adhesion function. Curr Opin Cell Biol. 2001;13:578–583. doi: 10.1016/s0955-0674(00)00254-4. [DOI] [PubMed] [Google Scholar]
- Xian X, Gopal S, Couchman JR. Syndecans as receptors and organizers of the extracellular matrix. Cell Tissue Res. 2010;339:31–46. doi: 10.1007/s00441-009-0829-3. [DOI] [PubMed] [Google Scholar]
- Xu R, Boudreau A, Bissell MJ. Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev. 2009;28:167–176. doi: 10.1007/s10555-008-9178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashin AI, Wu D, Arbeev KG, Ukraintseva SV. Joint influence of small-effect genetic variants on human longevity. Aging (Albany NY) 2010;2:612–620. doi: 10.18632/aging.100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HE, Kim EN, Kim MY, Lim JH, Jang IA, Ban TH, Shin SJ, Park CW, Chang YS, Choi BS. Age-Associated Changes in the Vascular Renin-Angiotensin System in Mice. Oxid Med Cell Longev. 2016;2016:6731093. doi: 10.1155/2016/6731093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun MH. Changes in Regenerative Capacity through Lifespan. Int J Mol Sci. 2015;16:25392–25432. doi: 10.3390/ijms161025392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Aponte-Santamaria C, Sturm S, Bullerjahn JT, Bronowska A, Grater F. Mechanism of Focal Adhesion Kinase Mechanosensing. PLoS Comput Biol. 2015;11:e1004593. doi: 10.1371/journal.pcbi.1004593. [DOI] [PMC free article] [PubMed] [Google Scholar]