Abstract
Background
We sought to determine the recommended phase II dose (RP2D) and schedule of GSK2141795, an oral pan-AKT kinase inhibitor.
Patients and Methods
Patients with solid tumors were enrolled in the dose-escalation phase. Pharmacokinetic (PK) analysis after a single dose (Cycle 0) informed dose escalation using accelerated dose titration. Once one grade 2 toxicity or dose-limiting toxicity was observed in Cycle 1, the accelerated dose titration was terminated and a 3+3 dose escalation was started. Continuous daily dosing was evaluated along with two intermittent regimens (7 days on/7 days off and 3 times per week). In the expansion phase at RP2D, patients with endometrial or prostate cancer, as well as those with select tumor types with a PIK3CA mutation, AKT mutation or PTEN loss, were enrolled. Patients were evaluated for adverse events (AEs), PK parameters, blood glucose and insulin levels, and tumor response.
Results
The RP2D of GSK2141795 for once-daily dosing is 75 mg. The most common (>10%) treatment-related AEs included diarrhea, fatigue, vomiting, and decreased appetite. Most AEs were low grade. The frequency of hyperglycemia increased with dose; however, at the RP2D, grade 3 hyperglycemia was only reported in 4% of patients and no grade 4 events were observed. PK characteristics were favorable, with a prolonged half-life and low peak-to-trough ratio. There were two partial responses at the RP2D in patients with either a PIK3CA mutation or PTEN loss.
Conclusion
GSK2141795 was safe and well-tolerated, with clinical activity seen as monotherapy at the RP2D of 75 mg daily. NCT00920257
Keywords: GSK2141795, Akt, PIK3, PTEN, endometrial cancer
INTRODUCTION
AKT is a serine/threonine protein kinase with three isoforms (AKT1, AKT2, and AKT3) that participate in multiple pathways regulating several cellular processes, including survival, proliferation, tissue invasion, and metabolism [1,2]. Aberrant activation of the AKT pathway has been reported in several malignancies, and hyperactivation of the AKT pathway can correlate with chemotherapy resistance and poor prognosis [3,4]. Preclinical data suggest that blocking AKT1 and AKT2 can inhibit tumor cell proliferation and either induce apoptosis or sensitize tumors to undergo apoptosis in response to other cytotoxic agents [5]. AKT2 is also an integral part of the insulin signaling pathway, therefore hyperglycemia and hyperinsulinemia may result from AKT2 inhibition [3,4].
GSK2141795 is a potent, ATP-competitive, reversible pan-AKT inhibitor (AKT1 Ki*=0.066nM, AKT2 Ki*=1.4nM, and AKT3 Ki*=1.5nM) that decreases phosphorylation of various AKT substrates and inhibits signaling in multiple cancer cell lines [6]. GSK2141795 inhibits cell proliferation in vitro, with an EC50<1µM in cell lines with PI3K or phosphatase and tensin homolog (PTEN) alterations, presumably from a blockade of proliferation and/or an induction of apoptosis. This first-in-human study evaluated the safety and efficacy of GSK2141795 in patients with solid tumors. The primary objectives were to evaluate the safety of GSK2141795, including adverse events (AEs) and changes in laboratory values, with particular attention to blood glucose and insulin levels, and to evaluate pharmacokinetic (PK) parameters following single and repeat dosing. The secondary objective was to evaluate tumor response defined by the Response Evaluation Criteria in Solid Tumors (RECIST). The translational objective was to assess relationships between markers of AKT and RAS/RAF pathway activation in tumors and anticancer activity and safety of GSK2141795.
PATIENTS AND METHODS
Study population
For the dose-escalation cohorts, patients with a solid tumor malignancy non-responsive to standard therapies, or for which there was no approved or curative therapy, were enrolled. Additional eligibility criteria included an Eastern Cooperative Oncology Group performance status of 0 or 1, a fasting serum glucose <126 mg/dL, and adequate hematologic, hepatic, and renal function. Patients were required to have a left ventricular ejection fraction ≥50% based on transthoracic echocardiogram (TTE) or multigated acquisition (MUGA) scan. Patients previously diagnosed with type 1 or 2 diabetes were excluded.
The dose-expansion cohort required that patients have a diagnosis of endometrial cancer, prostate cancer, or a tumor with specific prospectively defined biomarkers (histology independent group [HIG]). Endometrial cancer patients were required to have relapsed or metastatic disease treated with ≤2 prior chemotherapy regimens in the metastatic setting. Prostate cancer patients were required to have castration-resistant, locally advanced or metastatic disease treated with ≤2 prior cytotoxic chemotherapy regimens in the relapsed or metastatic setting.
Patients in the HIG had a solid tumor malignancy with one of the following genetic aberrations: PIK3CA mutation, AKT mutation, or loss of PTEN protein. Tumor types were also limited as follows: ovarian cancer, colorectal or pancreatic/biliary cancer (KRAS and BRAF wild type), melanoma (BRAF and NRAS wild type), non-small cell lung cancer (KRAS wild type), or thyroid cancer (BRAF wild type). Mutation status was determined using WAVE-Surveyor denaturing high-performance liquid chromatography (DHPLC) technology, Transgenomic, Inc., Omaha, NE. PTEN loss was assessed by immunohistochemistry (IHC) using the PTEN (138G6) rabbit monoclonal antibody from Cell Signaling, Beverly, MA, catalog #9559. Samples were scored by an anatomic pathologist for relative intensity of staining 0 to 3+ for both the tumor and stroma. Samples were considered negative if the PTEN stroma score was 1+ or greater and the PTEN tumor score was 0. IHC was performed at Quintiles Laboratories Ltd. for 38 patients and at MD Anderson Cancer Center for 9 patients.
Type 2 diabetes was allowed in the expansion cohort if diagnosed ≥6 months prior to enrollment with a hemoglobin A1C ≤8% at screening. Patients were excluded if they had previously diagnosed type 1 diabetes mellitus; human immunodeficiency virus or solid organ transplant; a history of an allogeneic stem cell transplant; conditions that could affect gastrointestinal absorption; symptomatic or untreated central nervous system metastases or leptomeningeal involvement; Class III or IV heart failure as defined by the New York Heart Association (NYHA) functional classification system; corrected QT (QTc) interval ≥470 msec or other clinically significant electrocardiogram (ECG) abnormalities; history of hepatitis B or C; or a currently unresolved toxicity grade ≥2 from previous anticancer therapy.
Human investigations were performed after approval by an institutional review board and in accordance with an assurance filed with and approved by the U.S. Department of Health and Human Services. Informed consent was obtained from each subject or subject's guardian.
Study design
The dose-escalation phase began using an accelerated dose titration regimen. In each cohort, patients were administered a single dose of GSK2141795 followed by serial blood sampling for PK analyses collected predose through 72 hours post-dose (Cycle 0). Once PK sampling was complete, patients began repeat dosing in Cycle 1. The dose for subsequent cohorts was determined after analysis of the PK and safety data from the previous cohort and may have been up to twice the dose of the previous cohort. Dosing began in Cohort 1 with GSK2141795 10 mg orally once daily (qd). Doubling of doses of GSK2141795 continued until either one grade 2 toxicity (with the exception of alopecia, nausea, vomiting, diarrhea, hemoglobin, lymphopenia, taste changes, or alkaline phosphatase elevation in the presence of bony metastases) or one dose-limiting toxicity (DLT) was observed in cycle 1 of the cohort. At that point, the accelerated dose titration procedure was terminated and 3+3 dose escalation started.
GSK2141795 capsules were administered under fasting conditions. The starting dose was 10 mg qd, with additional dose levels including 20, 40, 75, 100 and 150 mg qd until the maximum tolerated dose (MTD) was reached. Two alternative dosing regimens were evaluated at the 75 mg and 100 mg dose levels: 7 days of continuous qd dosing of GSK2141795 followed by 7 days off (7-on/7-off) and an intermittent dosing schedule building to three times a week (t.i.w.) dosing by cycle 1 week 3 (dosing on day 1 in week 1, days 8 and 12 in week 2, and t.i.w. in week 3 and all subsequent weeks). Cycle length was 21 days for the continuous daily dosing regimen and 28 days for the alternative dosing regimens. The expansion cohort began once a recommended phase II dose (RP2D) was identified using continuous daily dosing.
Safety assessments
Clinical and laboratory assessments were performed weekly during the first cycle of treatment, at the start of each subsequent cycle, and at the final study visit. AEs were graded using Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Laboratory assessments included routine hematology and chemistry panels, urinalysis, fasting lipid panel, hemoglobin A1C, thyroid function, and coagulation parameters. All patients monitored fingerstick blood glucose at home daily during the first cycle and in subsequent cycles if clinically warranted. An ECHO or MUGA scan was required at screening. Twelve lead ECGs were obtained at screening, weekly in cycle 1, at the start of each subsequent cycle, and at the final study visit. A DLT was defined as a grade ≥3 hematological or non-hematological toxicity, or a treatment delay of ≥14 days due to unresolved toxicity occurring within the first cycle of therapy.
Pharmacokinetic Assessments
Serial blood samples for PK analysis were obtained pre-dose and 0.5, 1, 2, 3, 4, 5, 8, 10–12, 14–22, 24, 48, and 72 hours following the initial dose of GSK2141795. Time points up to 24 hours were collected to evaluate steady state on day 8 (10 and 20 mg cohorts) or day 15 (40, 75, 100, and 150 mg cohorts) for the continuous daily dosing regimen, day 7 for the 7-on/7-off regimen, and day 22 for the t.i.w. regimen. For patients dose-reduced after day 1, steady state PK data were evaluated at their new dose level. Analysis was performed by Worldwide Bioanalysis, Drug Metabolism and Pharmacokinetics (DMPK), GlaxoSmithKline in Upper Merion, PA. Plasma concentrations of GSK2141795 were quantified using a validated analytical method (range: 1–1000 ng/mL) based on liquid-liquid extraction, followed by high-performance liquid chromatography-mass spectrometry (HPLC-MS/MS) analysis. The lower limit of quantification was 1.00 ng/mL.
Pharmacodynamic Assessments
Serial blood glucose levels were collected during screening and in cycle 1 pre-dose and at 2, 4 and 8 hours following the first dose and the day 8 or 15 dose. Selected patients in the dose-escalation portion of the study were monitored for glucose and insulin levels following a standardized meal at screening and on cycle 1 day 15. Pre-dose levels were drawn prior to GSK2141795 dosing and at 2 hours post-dose immediately prior to the meal and at 2.5, 3, 3.5, 4, and 5 hours post dosing. As a potential marker of glucose fluctuations, 1, 5-anhydroglucitol (1,5-AG) levels were also measured at screening and steady state. Paired tumor biopsies were optional at screening and post-dose (days 15–21). Markers of AKT and MAPK pathway activation, cell proliferation, and apoptosis were assessed by IHC, performed by Mosaic Laboratories, LLC.
Statistical analysis
No formal statistical analysis was performed on safety data. Safety data were listed and summarized by cohort. PK parameters were calculated by standard non-compartmental methods Model 200 of WinNonlin Professional Edition version 5.2 (Pharsight Corporation, Mountain View, CA). Exploratory graphical analyses were performed to assess the potential relationship between GSK2141795 exposure and PD endpoints (1,5-AG) using linear regression analysis.
RESULTS
Patients
Seventy-seven patients were enrolled across 10 treatment cohorts: 10, 20, 40, 75, 100, and 150 mg qd; 75 and 100 mg 7-on/7-off; and 75 and 100 mg t.i.w. The expansion phase included 32 patients at the 75 mg qd dose. The study was initiated on 06/16/2009 and completed (data cutoff) on 04/17/2013. Table 1 lists baseline demographic and clinical characteristics. At study completion, 52 patients (68%) had withdrawn due to disease progression, 13 patients (17%) had withdrawn as a result of an AE. Eight patients (10%) had withdrawn due to investigator discretion, and 4 (5%) patients had withdrawn consent.
Table 1.
Baseline patient demographic and clinical characteristics
| Characteristic | Patients (N = 77) |
|---|---|
| Median age, years (range) | 62 (25 to 89) |
| Sex, n (%) | |
| Female | 51 (66) |
| Male | 26 (34) |
| Ethnicity, n (%) | |
| White | 66 (85) |
| Black | 6 (8) |
| American Indian | 1 (1) |
| Asian | 4 (6) |
| Median height, cm (range) | 167.0 (152.0 to 197.0) |
| Median weight, kg (range) | 68.2 (42.0 to 119.0) |
| ECOG PS, n (%) | |
| 0 | 50 (65) |
| 1 | 27 (35) |
| Tumor type, n (%) | |
| Endometrium/uterus | 12 (16) |
| Prostate | 10 (13) |
| Ovary | 9 (12) |
| Colon/rectum | 9 (12) |
| Breast | 6 (8) |
| Neuroendocrine | 4 (5) |
| Head and neck | 4 (5) |
| Other | 23 (29) |
| Prior treatment regimens, n (%) | |
| Chemotherapy | 69 (90) |
| Biologic therapy (monoclonal antibodies) | 39 (51) |
| Hormonal therapy | 20 (26) |
| Immunotherapy | 4 (5) |
| No. of prior chemotherapy regimens, n (%) | |
| 1 | 12 (16) |
| 2 | 15 (19) |
| 3 | 12 (16) |
| 4 | 9 (12) |
| >4 | 21 (27) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status.
Dose escalation and dose-limiting toxicities
DLTs were reported in 7 patients. The first DLT of grade 3 hypoglycemia was observed in the 75 mg qd dosing cohort. The dose of 150 mg qd exceeded the MTD, with two DLTs of grade 3 hyperglycemia. The intermediate dose of 100 mg qd also exceeded the MTD, with two DLTs, including grade 1 stomatitis that did not resolve, leading to a treatment delay of >14 days, as well as grade 4 hypoglycemia. Two DLTs, grade 3 hyperglycemia and grade 3 rash, were observed in the 100 mg qd for 7-on/7-off dosing cohort. No DLTs were reported in the 75 mg qd for 7-on/7-off, 75 mg t.i.w., or 100 mg t.i.w. dosing cohorts. The study defined the MTD as 75 mg for both the qd dosing and the alternative schedule of 7-on/7-off. An MTD for a schedule of t.i.w. was not established.
Safety
The most common AEs (>25%, any cause) were diarrhea (64%), nausea (61%), fatigue (49%), vomiting (43%), and decreased appetite (39%), the majority of which were grade 1 or 2 (Table 2). Treatment-related AEs leading to permanent discontinuation of study treatment for 6 patients were as follows: esophagitis (two grade 3, 75 mg qd and 150 mg qd), ALT increase (grade 3, 100 mg 7-on/7-off), nausea (grade 2, 75 mg qd), decreased appetite (grade 2, 40 mg qd), and rash (grade 1, 75 mg qd). AEs led to a dose reduction for 8 patients at the RP2D of 75 mg qd and included the following: rash (1 grade 3, 2 grade 2), diarrhea (1 grade 3, 1 grade 2), mucosal inflammation (grade 2), dysphagia (grade 2), and fatigue (grade 2). The frequency of treatment-related hyperglycemia increased with increasing doses on the qd schedule (21% at 75 mg, 50% at 100 mg, and 100% at 150 mg). The most frequently reported treatment-related grade 3 or 4 AE across all treatment cohorts was hyperglycemia (8%). However, there were no events of diabetic ketoacidosis or additional toxicity associated with the hyperglycemia. Three patients required dose reductions for grade 3 hyperglycemia at doses above the RP2D (2 at 150 mg daily and 1 at 100 mg 7-on/7-off). Grade 3 hyperglycemia was reported in only 4% of patients at the RP2D; no events of grade 4 hyperglycemia occurred at the RP2D; and no dose reductions were required for hyperglycemia at the RP2D.
Table 2.
Most commonly reported (≥10%) AEs (any cause)a
| AE | Grade 1, n (%) |
Grade 2, n (%) |
Grade 3, n (%) |
Grade 4, n (%) |
Total, n (%) |
|---|---|---|---|---|---|
| Diarrhea | 33 (43) | 13 (17) | 3 (4) | 0 | 49 (64) |
| Nausea | 42 (55) | 4 (5) | 1 (1) | 0 | 47 (61) |
| Fatigue | 21 (27) | 16 (21) | 0 | 1 (1) | 38 (49) |
| Vomiting | 29 (38) | 3 (4) | 1 (1) | 0 | 33 (43) |
| Decreased appetite | 23 (30) | 6 (8) | 0 | 1 (1) | 30 (39) |
| Abdominal pain | 13 (17) | 4 (5) | 3 (4) | 0 | 20 (26) |
| Constipation | 14 (18) | 3 (4) | 0 | 0 | 17 (22) |
| Hyperglycemia | 2 (3) | 9 (12) | 5 (6) | 1 (1) | 17 (22) |
| Rash | 8 (10) | 8 (10) | 1 (1) | 0 | 17 (22) |
| Weight decreased | 12 (16) | 5 (6) | 0 | 0 | 17 (22) |
| Back pain | 11 (14) | 3 (4) | 1 (1) | 0 | 15 (19) |
| Dyspnea | 10 (13) | 3 (4) | 2 (3) | 0 | 15 (19) |
| Dizziness | 12 (16) | 0 | 0 | 0 | 12 (16) |
| Pyrexia | 10 (13) | 2 (3) | 0 | 0 | 12 (16) |
| Urinary tract infection | 6 (8) | 6 (8) | 0 | 0 | 12 (16) |
| Cough | 9 (12) | 0 | 0 | 0 | 9 (12) |
| Peripheral edema | 9 (12) | 0 | 0 | 0 | 9 (12) |
| Headache | 7 (9) | 0 | 1 (1) | 1 (1) | 9 (12) |
| Dysgeusia | 7 (9) | 1 (1) | 0 | 0 | 8 (10) |
| Mucosal inflammation | 7 (9) | 1 (1) | 0 | 0 | 8 (10) |
Abbreviations: AE, adverse event.
By Common Terminology Criteria for Adverse Events (version 3.0).
Pharmacokinetics
Single dose PK of GSK2141795 was evaluated after oral administration of GSK2141795 capsules under fasting conditions (Table 3). GSK2141795 was absorbed with a median time to maximal concentration (Tmax) of 3.0–8.0 hours after oral intake across all doses and regimens, majority between 2.0 and 4.0 hours. The AUC(0–24) and Cmax increased in a generally dose-proportional manner after single-dose administration of doses ranging between 10 and 150 mg with a mean slope (90% confidence interval [CI]) of the values slightly greater than 1.14 (0.97, 1.32) and 1.13 (0.90, 1.37) respectively, with slope of 1 signifying proportionality. Mean variability (CV%) in exposure for patients receiving the 75 mg dose in the continuous daily regimen was 37% for AUC(0–24) and 62% for Cmax.
Table 3.
Pharmacokinetic parameters following single dose administration of GSK2141795
| Dose Group (mg) |
AUC(0–24) (ng*hr/mL) |
Cmax (ng/mL) |
Tmax (hours) |
|---|---|---|---|
|
| |||
| Once-daily dosing | |||
|
| |||
| 10 (n = 2) | (125.0, 162.0) | (9.0, 10.5) | (3.0, 4.0) |
|
| |||
| 20 (n = 1) | 221.0 | 14.6 | 3.13 |
|
| |||
| 40 (n = 4) | |||
|
| |||
| Mean | 629.0 | 46.2 | 3.7 |
| [CV, %] | [21] | [28] | |
| (range) | (500.0 to 782.0) | (32.7 to 59.9) | (2.0 to 8.0) |
|
| |||
| 75 (n = 15) | |||
|
| |||
| Mean | 1,262.0 | 94.6 | 3.0 |
| [CV, %] | [37] | [62] | |
| (range) | (549.0 to 2,307.0) | (37.9 to 325.0) | (2.0 to 23.8) |
|
| |||
| 100 (n = 4) | |||
|
| |||
| Mean | 2,210.0 | 159.0 | 4.0 |
| [CV, %] | [45] | [73] | |
| (range) | (1,410.0 to 3,864.0) | (101.0 to 391.0) | (3.0 to 8.0) |
|
| |||
| 150 (n = 3) | |||
|
| |||
| Mean | 2,593.0 | 154.0 | 8.0 |
| [CV, %] | [31] | [61] | |
| (range) | (1,901.0 to 3,541.0) | (94.2 to 292.0) | (3.0 to 10.7) |
|
| |||
| 7 days on/7 days off | |||
|
| |||
| 75 (n = 4) | |||
|
| |||
| Mean | 1,155.0 | 84.8 | 3.0 |
| [CV, %] | [41] | [49] | |
| (range) | (547.0 to 1,777.0) | (29.1 to 142.0) | (3.0 to 4.1) |
|
| |||
| 100 (n = 5) | |||
|
| |||
| Mean | 1,526.0 | 103.0 | 3.0 |
| [CV, %] | [28] | [50] | |
| (range) | (1,047.0 to 2,073.0) | (52.1 to 203.0) | (2.0 to 3.0) |
|
| |||
| t.i.w. | |||
|
| |||
| 75 (n = 4) | |||
|
| |||
| Mean | 1,443.0 | 106.0 | 3.2 |
| [CV, %] | [11] | [18] | |
| (range) | (1,316.0 to 1,633.0) | (83.0 to 130.0) | (2.0 to 8.0) |
|
| |||
| 100 (n = 3) | |||
|
| |||
| Mean | 1,809.0 | 114.0 | 3.0 |
| [CV, %] | [27] | [43] | |
| (range) | (1,349.0 to 2,361.0) | (80.8 to 180.0) | (1.0 to 8.0) |
Abbreviations: AUC(0–24), area under plasma concentration –time curve over 0 to 24 hours; Cmax, maximum observed plasma; Tmax, time of maximum observed plasma; CV, coefficient of variation.
Repeat dose PK show that GSK2141795 is absorbed with median Tmax generally occurring within 2.0 to 4.0 hours after administration (Table 4). Increases in day 15 AUC(0–24) and Cmax were slightly greater than proportional after continuous daily repeat dosing between doses of 10 and 150 mg. The mean slope (90% CI) of the power model was 1.29 (1.07, 1.51) for AUC(0–24) and 1.28 (1.07, 1.49) for Cmax. CV% in exposure for patients receiving the 75 mg dose in the continuous daily regimen was 52% for AUC(0–24) and 50% for Cmax. GSK2141795 accumulates with repeat daily dosing. The accumulation ratio was used to calculate a mean effective t1/2 of approximately 2.8 days for continuous qd dosing suggesting it would take approximately 14 days to reach steady-state.
Table 4.
Pharmacokinetic parameters following repeat dose administration of GSK2141795 (days 7, 8, 15, or 22)
| Dose Group (mg) |
AUC(0–24) (ng*hr/mL) |
Cmax (ng/mL) |
Tmax (hours) |
C24 (ng/mL) |
AR | t1/2, eff (hours) |
|---|---|---|---|---|---|---|
|
| ||||||
| Once-daily dosing | ||||||
|
| ||||||
| 10 (n = 2) | (387.0, 555.0) | (24.3, 32.7) | (2.0, 2.0) | (23.8, 33.6) | (3.1, 3.4) | (42.7, 48.1) |
|
| ||||||
| 20 (n = 1) | 1,238 | 90.3 | 3.0 | 42.1 | 5.6 | 84.7 |
|
| ||||||
| 40 (n = 4) | ||||||
| Mean | 3,260.0 | 230.0 | 2.6 | 119.0 | 5.18 | 75.6 |
| [CV, %] | [54] | [36] | [57] | [60] | [66] | |
| (range) | (1,670.0 to 5,464.0) | (128.0 to 339.0) | (2.0 to 5.0) | (72.7 to 234.0) | (2.3 to 10.7) | (29.8 to 169.0) |
|
| ||||||
| 75 (n = 15) | ||||||
| Mean | 6,383.0 | 409.0 | 2.1 | 247.0 | 4.38 | 63.7 |
| [CV, %] | [52] | [50] | [54] | [29] | [33] | |
| (range) | (3,064.0 to 17,060.0) | (170.0 to 1,008.0) | (0.0 to 8.0) | (100.0 to 603.0) | (2.5 to 7.1) | (31.7 to 109.0) |
|
| ||||||
| 100 (n = 3) | ||||||
| Mean | 10,940.0 | 764.0 | 2.0 | 350.0 | 4.91 | 72.6 |
| [CV, %] | [17] | [17] | [26] | [32] | [36] | |
| (range) | (9,354.0 to 13,090.0) | (677.0 to 920.0) | (1.0–2.0) | (253.0 to 430.0) | (3.4 to 6.6) | (47.6 to 102.0) |
|
| ||||||
| 150 (n = 2) | (15,037 to 15,745) | (901 to 932) | (0.0 to 4.0) | (381, 648) | (5.8 to 8.3) | (87.9 to 129) |
| (range) | ||||||
|
| ||||||
| 7 Days on/7 Days off | ||||||
|
| ||||||
| 75 (n = 3) | ||||||
| Mean | 8,723.0 | 574.0 | 2.0 | 304.0 | 7.90 | NA |
| [CV, %] | [25] | [16] | [36] | |||
| (range) | (6,429.0 to 10,746.0) | (482.0 to 659.0) | (1.05 to 3.0) | (191.0 to 406.0) | (2.52 to 24.74) | |
|
| ||||||
| 100 (n = 5) | ||||||
| Mean | 7,342.0 | 439.0 | 4.0 | 281.0 | 4.14 | NA |
| [CV, %] | [63] | [62] | [50] | |||
| (range) | (4,521.0 to 16,262) | (230 to 933) | (0.0 to 7.75) | (180.0 to 482.0) | (3.14 to 5.45) | |
|
| ||||||
| t.i.w. | ||||||
|
| ||||||
| 75 (n = 3) | ||||||
| Mean | 3,143.0 | 191.0 | 3.0 | 113.0 | 2.11 | NA |
| [CV, %] | [31] | [50] | [59] | |||
| (range) | (2,222.0 to 4,256.0 | (115.0 to 322.0) | (0.5 to 3.0) | (76.4 to 212.0) | (1.36 to 3.27) | |
|
| ||||||
| 100 (n = 3) | ||||||
| Mean | 4,502.0 | 268 | 3.0 | 160.0 | 2.49 | NA |
| [CV, %] | [35] | [73] | [32] | |||
| (range) | (3,043.0 to 6,373.0) | (147.0, 583.0) | (1.0 to 5.0) | (112.0 to 220.0) | (2.13 to 2.90) | |
Abbreviations: AUC(0−τ), area under plasma concentration–time curve over 0 to 24 hours; Cmax, maximum observed plasma; Tmax, time of maximum observed plasma; C24, plasma at 24 hours; AR, accumulation ratio; t½, eff, effective half-life; CV, coefficient of variation; NA, not available.
Pharmacodynamics
PD was assessed using serial blood glucose levels drawn at screening, post-dose on day 1 and then post-dose on day 8 or 15. Home glucose monitoring was performed for safety only and results were not included in the PD analysis. Only 5 patients had matched insulin samples following a standardized meal at screening and on cycle 1 day 15. Three of the five patients had two-fold greater insulin levels on day 15 compared with screening following the meal. Decreased 1,5-AG was not consistently reported when an AE of hyperglycemia was reported. Decreased 1,5-AG may be associated with higher concentrations of GSK2141795; however, this association was not consistent across the study or across tumor types. PD assessments of optional paired tumor biopsies were obtained from two patients (both in the 75 mg qd cohort) and did not show notable changes in markers of the AKT or ERK pathways (phospho-AKT; PRAS40; phospho-PRAs40; ERK; phospho-ERK), cell proliferation (Ki67), or apoptosis (cleaved caspase-3). Due to the limited sample availability, no conclusions can be drawn.
Biomarkers
Tumor mutation results for patients treated at 75 mg qd are shown in Table 5. The frequency of PTEN loss in the endometrial and prostate cancer patient populations were 7 of 10 patients (70%) and 3 of 8 patients (38%), respectively. The highest frequency of PIK3CA mutations was observed in the endometrial population with 6 of 10 patients (60%), followed by colon cancer with 2 of 4 patients (50%). Two instances of rare PIK3CA mutations, N1044K and Y1021C, were observed in two endometrial cancer patients. No mutations in AKT or BRAF were detected in tumor tissues screened. Two samples were positive for a KRAS mutation. One of 5 tissue samples was positive for an NRAS mutation.
Table 5.
Mutation results for patients treated at 75mg daily
| Tumor type | PTEN loss |
PIK3CA mutation |
AKT mutation |
BRAF mutation |
KRAS mutation |
NRAS mutation |
PR or SD |
|---|---|---|---|---|---|---|---|
| Endometrium | Yes | Yes | No | Unk | Unk | Unk | SD |
| Endometrium | Yes | Yes | No | Unk | Unk | Unk | |
| Endometrium | Yes | Yes | Unk | No | Unk | Yes | SD |
| Endometrium | Yes | No | No | Unk | Unk | Unk | SD |
| Endometrium | Yes | No | No | Unk | Unk | Unk | SD |
| Endometrium | Yes | No | No | Unk | Unk | Unk | |
| Endometrium | Yes | No | Unk | No | No | No | |
| Endometrium | No | Yes | No | Unk | Unk | Unk | PR |
| Endometrium | Unk | Yes | Unk | No | No | Unk | |
| Endometrium | Unk | Yes | Unk | No | Yes | No | |
| Prostate | Yes | No | Unk | Unk | Unk | Unk | SD |
| Prostate | Yes | No | Unk | Unk | Unk | Unk | SD |
| Prostate | Yes | No | Unk | Unk | Unk | Unk | |
| Prostate | No | No | Unk | Unk | Unk | Unk | SD |
| Prostate | No | No | Unk | Unk | Unk | Unk | |
| Prostate | No | No | Unk | Unk | Unk | Unk | |
| Prostate | Unk | No | Unk | Unk | Unk | Unk | |
| Prostate | Unk | No | Unk | Unk | Unk | Unk | |
| Ovary | Yes | No | Unk | No | No | Unk | PR |
| Ovary | Yes | No | Unk | No | No | Unk | |
| Ovary | No | No | Unk | No | No | Unk | |
| Ovary | No | No | Unk | No | No | Unk | |
| Ovary v unknown primary | Yes | No | Unk | No | Yes | Unk | |
| Colon/rectum | Yes | Yes | No | No | No | Unk | |
| Colon/rectum | Yes | No | Unk | Unk | Unk | Unk | |
| Colon/rectum | Yes | No | Unk | No | Unk | Unk | |
| Colon/rectum | No | Yes | Unk | No | No | Unk | |
| Cervix | Yes | No | Unk | Unk | Unk | Unk | |
| Cervix | Yes | No | Unk | No | No | No | |
| Head/neck | Yes | No | Unk | Unk | Unk | Unk | |
| Head/neck | Unk | Yes | Unk | No | No | No | |
| Breast | No | No | No | Unk | Unk | Unk | |
| Hepatocellular | Yes | No | Unk | Unk | Unk | Unk | |
| Leiomyosarcoma | Yes | No | Unk | Unk | Unk | Unk |
Abbreviation: Unk, unknown; PR, partial response; SD, stable disease >6 months
Clinical Activity
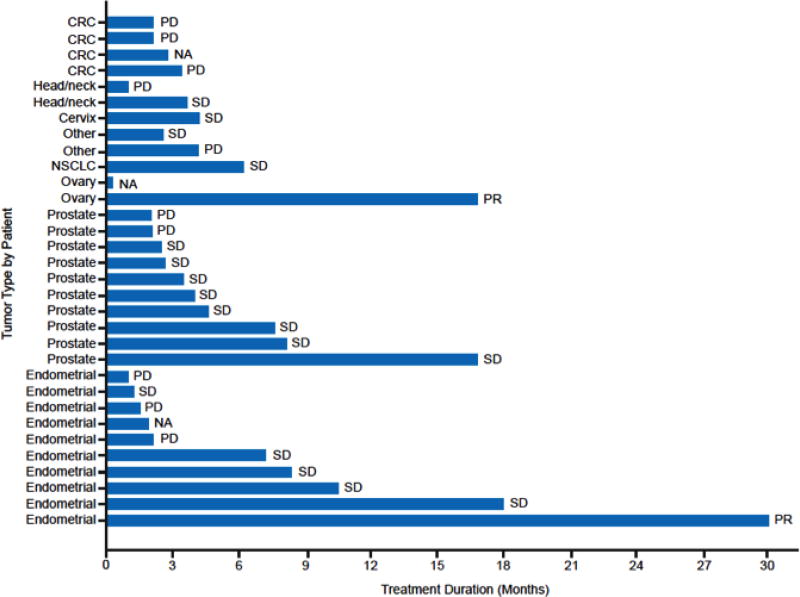
Two PRs, lasting approximately 18 and 30 months, were reported at the RP2D dose of 75 mg qd (Fig. 1 and Table 5). One PR was reported in a patient with ovarian cancer with PTEN loss by IHC and the other PR in a patient with endometrial cancer with a PIK3CA mutation (H1047R). In addition, there were 3 prostate cancer patients and 4 endometrial cancer patients with stable disease (SD) remaining on therapy for more than 6 months (Fig. 1 and Table 5). No correlation between clinical activity and particular tumor type and/or genetic aberrations could be established.
Figure 1.
Duration of Treatment by Tumor Type in the Dose-Expansion Cohort
Abbreviations: CRC, colorectal cancer; NA, not applicable; NSCLC, non-small cell lung cancer; PD, progressive disease; PR, partial response; SD, stable disease
DISCUSSION
This first-in-human study of the oral pan-AKT kinase inhibitor GSK2141795 defined the MTD and RP2D for daily dosing as 75 mg. In an effort to optimize the side effect profile of GSK2141795, which was found to have a mean effective half-life of 2.8 days, an alternative dose schedule of 7-on/7-off was evaluated but found to also have an MTD of 75 mg and similar AEs. This is consistent with other AKT and PIK3CA inhibitors, with which alternative schedules were found to have similar tolerability to daily dosing [7–10].
The most common AEs in the study were diarrhea, nausea, fatigue, vomiting, and decreased appetite. Hyperglycemia and rash were less common, both seen in 22% of patients. This is consistent with other AKT and PI3K inhibitors, with which the most common DLTs and AEs reported are gastrointestinal related, rash, fatigue, or hyperglycemia [7–13]. The majority of AEs in this study were grade 1 or 2 and were manageable with standard supportive care. At the RP2D, dose reductions were required most commonly for rash (3 patients) and diarrhea (2 patients). Dose modifications were not necessary for hyperglycemia. Hyperglycemia may reflect target inhibition of AKT and did correlate with dose in this study.
Unfortunately, despite efforts to identify patients most likely to respond to an AKT inhibitor, the presence of PIK3CA mutations or PTEN loss in the setting of wild-type BRAF and KRAS was not shown to confer sensitivity to GSK2141795 in this study. Of the 2 patients with partial responses, one had PTEN loss and the other had a PIK3CA mutation. However, patients with similar genetic profiles (Table 5) did not respond. This is also consistent with other phase I studies of AKT and PI3K inhibitors, in which the presence or absence of genetic aberrations thought to activate the pathway do not correlate with clinical response. In fact, it is notable that while activation of the Ras pathway has been hypothesized to confer resistance, responders in other phase I trials have actually had KRAS mutations [7,12]. In this study, one of the prolonged SD patients had an NRAS mutation. However, the data in this and other studies are limited by the degree of unknown genetic information among the patients and their tumors.
GSK2141795 has a favorable exposure profile that allows a narrow range within an individual given the small peak-to-trough ratios. Because AKT is essential in many non-tumor cell types, AKT inhibitors may cause toxicity due to pAKT suppression in normal cells, thereby creating a narrow therapeutic index [4]. The PK characteristics of GSK2141795, which include a prolonged half-life and small peak-to-trough ratios, may minimize the risk of potential Cmax-related toxicities, maximize time of target inhibition, and allow for dosing schedules other than daily.
Dual MEK/AKT inhibition with trametinib and GSK2141795 has been explored in metastatic NRAS-mutant and wild-type melanoma and uveal melanoma, without clinical benefit [14,15]. Preliminary results of a clinical trial of trametinib in relapsed/refractory multiple myeloma, which included the sequential addition of GSK2141795 at the time of disease progression, showed modest results. The results, however, suggest an improved overall response rate [16]. Currently, GSK2141795 is not in active development.
Acknowledgments
Editorial assistance was provided by SciMentum and funded by GlaxoSmithKline. Investigators performed the human investigations after approval by a local Human Investigations Committee and in accord with an assurance filed with and approved by the Department of Health and Human Services. The investigators obtained informed consent from each participant or each participant's guardian.
Financial support: This study was funded by GlaxoSmithKline (NCT number: NCT00920257). Drs. Aghajanian and Bell-McGuinn (while at MSK) are supported in part by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Disclosure of potential conflicts of interest
C. Aghajanian declares she participated on a Focus Study Steering Committee for Mateon Therapeutics and has served on the Advisory Boards of Clovis, Cerulean Pharma, Bayer, and VentiRx at various time points since July 2015. K. M. Bell-McGuinn declares she is currently an employee and stockholder of Eli Lilly. H. A. Burris III declares he has no conflict of interest. L. L. Siu declares she has received research funding from GlaxoSmithKline. L. Stayner declares she has no conflict of interest. J. J. Wheler declares she has no conflict of interest. D. S. Hong declares he has received research funding from Adaptimmune, Abbvie, Amgen, AstraZeneca, Bayer, BMS, Daiichi-Sanko, Eisai, Genentech, Genmab, Ignyta, Infinity, Kite, Kyowa, Eli Lilly, LOXO, Mirati, Merck, Medimmune, Molecular Template, Novartis, Pfizer, and Takeda; D. S. Hong has also received consulting fees from Bayer, Baxter, Guidepoint Global, and Janssen; D. S. Hong has also received travel accommodations from LOXO and Mirna, and has ownership interest in Molecular Match and Oncoresponse. C. Kurkjian declares she has no conflict of interest. S. Pant declares he has no conflict of interest. A. Santiago-Walker declares she has received personal fees from GlaxoSmithKline. J. L. Gauvin declares she has received personal fees and has stock in GlaxoSmithKline. J.M. Antal declares she holds stock in GlaxoSmithKline and is an employee and holds stock in G1 Therapeutics. J.B. Opalinska declares she was an employee and owns stock in GlaxoSmithKline. S.R. Morris declares she was an employee of and has patent interest with GlaxoSmithKline. J.R. Infante declares he is an employee of Johnson & Johnson.
Previous presentation of the manuscript: Presented as an oral abstract at the 2011 ASCO Annual Meeting. Citation: J Clin Oncol 29: 2011 (suppl; abstr 3003)
References
- 1.Morgensztern D, McLeod HL. PI3K/AKT/Mtor pathway as a target for cancer therapy. Anti-cancer Drugs. 2005;16:797–803. doi: 10.1097/01.cad.0000173476.67239.3b. [DOI] [PubMed] [Google Scholar]
- 2.Tanno S, Tanno S, Mitsuuchi Y, et al. AKT activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res. 2001;61:589–593. [PubMed] [Google Scholar]
- 3.Bellacosa A, Testa JR, Moore R, et al. A portrait of AKT kinases: human cancer and animal models depict a family with strong individualities. Cancer Biol Ther. 2004;3:268–275. doi: 10.4161/cbt.3.3.703. [DOI] [PubMed] [Google Scholar]
- 4.Altomare DA, Testa JR. Pertubations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 5.DeFeo-Jones D, Barnett SF, Fu S, et al. Tumor cell sensitization to apoptotic stimuli by selective inhibition of specific AKT/PKB family members. Mol Cancer Ther. 2005;4:271–279. [PubMed] [Google Scholar]
- 6.Dumble M, Crouthamel MC, Zhang SY, et al. Discovery of novel AKT inhibitors with enhanced anti-tumor effects in combination with the MEK inhibitor. PLoS ONE. 2014;9:e100880. doi: 10.1371/journal.pone.0100880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yap TA, Yan L, Patnaik A, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 8.Yap TA, Yan L, Patnaik A, et al. Interrogating two schedules of the AKT inhibitor MK-2206 in patients with advanced solid tumors incorporating novel pharmacodynamic and functional imaging biomarkers. Clin Cancer Res. 2014;20:5672–5685. doi: 10.1158/1078-0432.CCR-14-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong DS, Bowles DW, Falchook GS, et al. A multicenter phase I trial of PX-866, an oral irreversible phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:4173–4182. doi: 10.1158/1078-0432.CCR-12-0714. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro GI, Rodon J, Bendell C, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of SAR245408 (XL147), an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2013;20:233–245. doi: 10.1158/1078-0432.CCR-13-1777. [DOI] [PubMed] [Google Scholar]
- 11.Spencer A, Yoon S, Harrison SJ, et al. The novel AKT inhibitor afuresertib shows favorable safety, pharmacokinetics, and clinical activity in multiple myeloma. Blood. 2014;124:2190–2195. doi: 10.1182/blood-2014-03-559963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2011;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 13.Britten C, Adjei AA, Millham R, et al. Phase I study of PF-04691502, a small molecule, oral, dual inhibitor of PI3K and mTOR, in patients with advanced cancer. Invest New Drugs. 2014;32:510–517. doi: 10.1007/s10637-013-0062-5. [DOI] [PubMed] [Google Scholar]
- 14.Algazi AP, Esteve-Puig R, Nosrati A, et al. Dual MEK/AKT inhibition with trametinib and GSK2141795 does not yield clinical benefit in metastatic NRAS-mutant and wild-type melanoma. Pigment Cell Melanoma Res. 2018;31:110–114. doi: 10.1111/pcmr.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoushtari AN, Kudchadkar RR, Panageas K, et al. A randomized phase 2 study of trametinib with or without GSK2141795 in patients with advanced uveal melanoma. J Clin Oncol. 2016;34(suppl) Abstract 9511. [Google Scholar]
- 16.Trudel S, Bahlis NJ, Venner CP, et al. Biomarker driven phase II clinical trial of trametinib in relapsed/refractory multiple myeloma with sequential addition of the AKT inhibitor GSK2141795 at time of disease progression to overcome treatment failure: a trial of the Princess Margaret Phase II Consortium. Blood. 2016;128(22):4526. (abstract) [Google Scholar]