Abstract
Microplastics (MPs) are ubiquitous pollutants found in marine, freshwater and terrestrial ecosystems. With so many MPs in aquatic systems, it is inevitable that they will be ingested by aquatic organisms and be transferred up through the food chain. However, to date, no study has considered whether MPs can be transmitted by means of ontogenic transference, i.e. between life stages that use different habitats. Here, we determine whether fluorescent polystyrene beads could transfer between Culex mosquito life stages and, particularly, could move into the flying adult stage. We show for the first time that MPs can be transferred ontogenically from a feeding (larva) into a non-feeding (pupa) life stage and subsequently into the adult terrestrial life stage. However, transference is dependent on particle size, with smaller 2 µm MPs transferring readily into pupae and adult stages, while 15 µm MPs transferred at a significantly reduced rate. MPs appear to accumulate in the Malpighian tubule renal excretion system. The transfer of MPs to the adults represents a potential aerial pathway to contamination of new environments. Thus, any organism that feeds on terrestrial life phases of freshwater insects could be impacted by MPs found in aquatic ecosystems.
Keywords: food chain, ontogeny, life stage, Malpighian tubules, microplastics, Culex pipiens
1. Introduction
Microplastics (MPs) are ubiquitous pollutants found in marine, freshwater and terrestrial ecosystems [1–3]. There is little doubt that plastic and MP pollution is a major environmental concern globally. Despite this, there is relatively little research into the impact of MPs on freshwater ecosystems, with most research concentrating on marine systems and organisms [2]. MPs have been defined as plastic particles smaller than 5 mm in size [4,5]. However, this simple description covers a wide range of types, including, among others, polypropylene, polyethylene and polystyrene MPs entering the environment in different shapes and sizes, including fibres, pellets and cosmetic beads [6,7]. MPs are categorized based on their origin as primary or secondary types, depending on whether they were released into the environment as MPs (primary) or have degraded to that size in the environment (secondary) [8,9]. MPs pass through terrestrial environments in household wastewater [2,10]. Rivers can subsequently deliver MPs into the sea and lakes, where they can be found in high concentrations [11–13].
MPs are ingested by aquatic organisms and can be transferred through the food chain in both freshwater and marine environments [14–18]. However, to date, no study has considered whether MPs can be transmitted by means of ontogenic transference, i.e. between life stages that use different habitats. Freshwater environments are inhabited by insects that spend their juvenile stages in water but their adult stages in the terrestrial environment. Such insects include mayflies, dragonflies, midges and mosquitoes, most of which are eaten by terrestrial vertebrates. This raises the potential for MPs to enter terrestrial ecosystems from freshwater habitats aerially via transference to adult invertebrate life stages. Here, we thus determine whether 2 and 15 µm fluorescent polystyrene beads could transfer between insect life stages and, particularly, could move into the flying adult stage. Fluorescent beads were selected to enable MPs to be easily detected in the non-feeding stages and also to allow an investigation of location within the body during metamorphosis. The Culex pipiens mosquito complex was selected as a model for this study given their worldwide distribution and broad habitat preference [19]. Mosquitoes develop through four feeding larval instars and a non-feeding pupal stage, and finally emerge into a flying adult.
2. Material and methods
For additional details of all methods and analyses, see the electronic supplementary material.
Two types of MPs were used: a 2 µm fluorescent yellow-green carboxylate-modified polystyrene (density 1.050 g cm−3, excitation 470 nm; emission 505 nm, Sigma-Aldrich, UK) and a 15.45 ± 1.1 µm fluorescent dragon green polystyrene (density 1.06 g cm−3 (5 × 106 particles ml−1), excitation 480 nm; emission 520 nm, Bangs Laboratories, Inc., USA). Four treatments were used: a control with no MPs, a treatment of 8 × 105 2 µm particles ml−1, a treatment of 8 × 102 15 µm particles ml−1 and a 1 : 1 mixture of both treatments. Each replicate (five per treatment) contained 10 third instar C. pipiens larvae in a 50 ml glass beaker filled with 50 ml of tap water. The control and all treatments contained 100 mg of pelleted guinea pig food. Treatments were assigned randomly to a position on the laboratory bench to reduce experimental error.
One random individual was removed from each beaker when every mosquito had moulted into the fourth instar, and again when they pupated or emerged as adults. All samples were then placed in separate 1.5 ml Eppendorf tubes and stored at −20°C prior to examination. MPs were extracted from mosquitoes by homogenization and filtration. The filter membrane was examined using an epi-fluorescent microscope (Zeiss Axioskop) under a 20× lens to count the number of fluorescent MPs. Adults were further dissected under a binocular stereo microscope (0.7×–4.5×) to extract the gut and quantify the numbers of MPs under the epi-fluorescent microscope [20].
All data were analysed using the statistical software R v. 3.4.2 [21]. MP counts were analysed using generalized linear models assuming a quasi-Poisson distribution. Uptake of MPs was examined with respect to ‘particle size’, ‘treatment’ and ‘life stage’. We performed model simplification via stepwise removal of non-significant effects. Tukey's tests were used post hoc for multiple comparisons.
3. Results
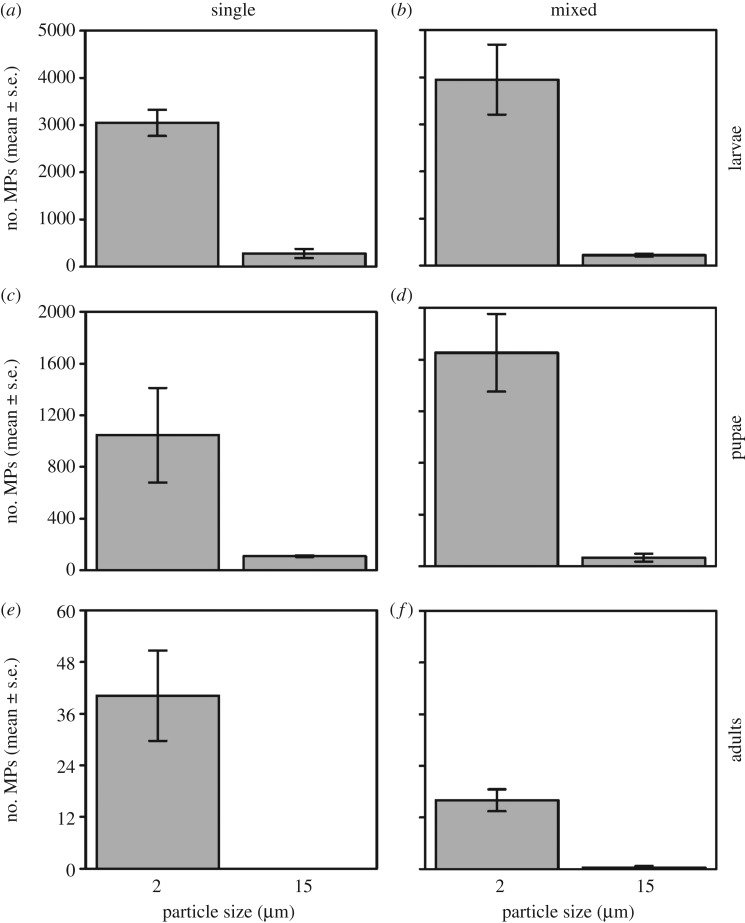
No MPs were found in control groups of any mosquito life stage. Densities of MPs were significantly different between life stages (F2,56 = 160.42, p < 0.001), with MP numbers significantly falling as mosquitoes moved between successive ontogenic levels (all p < 0.001) (figure 1; electronic supplementary material, table S1 and S2). MP transference to adults was confirmed by fluorescent microscopy where the beads were detected in the adult abdomen, specifically inside the Malpighian tubules (figure 2).
Figure 1.
Uptake counts of MPs across larval (a,b), pupal (c,d) and adult (e,f) Culex mosquito stages following single (a,c,e) and mixed (b,d,f) exposures to 2 and 15 µm beads. Means are ±s.e. (n = 5 per experimental group).
Figure 2.

Epi-fluorescent microscope images showing fluorescent MP particles within (a) the abdomen of an adult mosquito before dissection and (b) the abdominal Malpighian tubules following dissection.
Significantly more 2 µm particles were found in mosquito life stages than 15 µm particles overall (F1,58 = 303.98, p < 0.001). MPs uptake was also significantly greater overall in mixed exposure treatments (F1,55 = 6.00, p = 0.02). Although 2 µm particles were transferred to adults in all instances, we found no transference of 15 µm particles following single treatment exposures. However, in the mixed MPs treatment, transference to adults of both 2 and 15 µm particles was evidenced (figure 1).
4. Discussion
Here, we show for the first time that MPs can be transferred ontogenically from a feeding (larval) into a non-feeding (pupal) life stage and subsequently into the flying (adult) life stage. Transference through to adults was found in both MP sizes, although the larger 15 µm MPs were not ingested as readily as the 2 µm MPs. Dissection of mosquito adults showed that 2 µm MPs accumulated in the renal excretion system of Malpighian tubules which, unlike the gut, pass from larvae to adult stages without visible reorganization [22]. This has been demonstrated previously to provide a physical transport system between stages during metamorphosis for Pseudomonas bacteria and seems to be important for ontogenic transmission from larvae to adults [23].
Few 15 µm MPs were transferred into adults, suggesting that MP size is an important factor in ontogenic transfer which could be related to the transfer and accumulation of MPs in the Malpighian tubes. Although the translocation mechanism of MPs to the Malpighian tubules is unclear in mosquitoes, analysis of fish, fiddler crab and marine mussels has demonstrated that MPs can be translocated from gastrointestinal tracts into other tissues in a wide range of phyla [24–26]. Malpighian tubules have an entry point to the gut between the mid- and hindgut of mosquitoes, but the flow of fluid is from the Malpighian tubules to the hindgut [27]. Diptera are known to produce structures called concretions in the Malpighian tubules which have been shown to sequester heavy metals [28]. However, it is unlikely that this pathway would operate with a solid MP.
Our results have important implications because any aquatic life stage that is able to consume MPs and transfer them to their terrestrial life stage is a potential vector of MPs onto novel aerial and terrestrial habitats. Ingestion of MP-contaminated organisms by terrestrial organisms is not new [29]. Indeed, the widespread distribution of MPs in marine environments has meant that animals such as fish and shellfish sold for human consumption are contaminated with a range of plastics with a consequent transference of MPs between trophic levels [24]. Unlike MP fibres, which are common in the air and atmosphere, there has been no evidence for MP beads being transported into the air [30]. We have demonstrated here that species with aquatic and terrestrial life stages can harbour MPs through their life history. Adults are predated on emergence by many animals including dipteran flies Empididae and Dolichopodididae, while resting predominantly by spiders and in flight they are the prey of dragonflies, damselflies, birds (such as swallows and swifts) and bats [31]. Where many insects are emerging from a highly contaminated site, the possibility of contamination of these predators could be high. While mosquitoes were used here as a model organism, any freshwater insect that can ingest MPs will likely equally transmit plastics into a terrestrial adult stage. This has implications for organisms that feed on adult stages with aerial and terrestrial animals accordingly open to MP exposure and transference appearing to occur at a higher rate for smaller MPs.
Supplementary Material
Acknowledgements
We thank Natali Ortiz-Perea for assisting with mosquito colony rearing.
Data accessibility
Data files are available in the electronic supplementary material.
Authors' contribution
All authors provided substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; were involved in drafting the article or revising it critically for important intellectual content; approved the final version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests
We declare we have no competing interests.
Funding
A.C. is funded by the University of Reading. R.A.-J. is self-funded and R.N.C. is funded through the Department for the Economy, Northern Ireland.
References
- 1.Sighicelli M, Pietrelli L, Lecce F, Iannilli V, Falconieri M, Coscia L, Di Vito S, Nuglio S, Zampetti G. 2018. Microplastic pollution in the surface waters of Italian subalpine lakes. Environ. Pollut. 236, 645–651. ( 10.1016/j.envpol.2018.02.008) [DOI] [PubMed] [Google Scholar]
- 2.Wagner M, Lambert S. 2018. Freshwater microplastics. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 3.Mason SA, Welch V, Neratko J. 2018. Synthetic polymer contamination in bottled water. New York, NY: Fredonia State University. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eriksen M, Lebreton LCM, Carson HS, Thiel M, Moore CJ, Borerro JC, Galgani F, Ryan PG, Reisser J. 2014. Plastic pollution in the world's oceans: more than 5 trillion plastic pieces weighing over 250 000 tons afloat at sea. PLoS ONE 9, e111913 ( 10.1371/journal.pone.0111913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imhof HK, Ivleva NP, Schmid J, Niessner R, Laforsch C. 2013. Contamination of beach sediments of a subalpine lake with microplastic particles. Curr. Biol. 23, 1–15. ( 10.1016/j.cub.2013.09.001) [DOI] [PubMed] [Google Scholar]
- 6.Andrady AL, Neal MA. 2009. Applications and societal benefits of plastics. Phil. Trans. R. Soc. B 364, 1977–1984. ( 10.1098/rstb.2008.0304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rocha-Santos T, Duarte AC. 2014. A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. Trends Anal. Chem. 65, 47–53. ( 10.1016/j.trac.2014.10.011) [DOI] [Google Scholar]
- 8.Moore CJ. 2008. Synthetic polymers in the marine environment: a rapidly increasing, long-term threat. Environ. Res. 108, 131–139. ( 10.1016/j.envres.2008.07.025) [DOI] [PubMed] [Google Scholar]
- 9.Barnes DKA, Galgani F, Thompson RC, Barlaz M. 2009. Accumulation and fragmentation of plastic debris in global environments. Phil. Trans. R. Soc. B 364, 1985–1998. ( 10.1098/rstb.2008.0205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mason SA, Garneau D, Sutton R, Chu Y, Ehmann K, Barnes J, Fink P, Papazissimos D, Rogers DL. 2016. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 218, 1045–1054. ( 10.1016/j.envpol.2016.08.056) [DOI] [PubMed] [Google Scholar]
- 11.Eriksen M, Mason S, Wilson S, Box C, Zellers A, Edwards W, Farley H, Amato S. 2013. Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar. Pollut. Bull. 77, 177–182. ( 10.1016/j.marpolbul.2013.10.007) [DOI] [PubMed] [Google Scholar]
- 12.Fischer EK, Paglialonga L, Czech E, Tamminga M. 2016. Microplastic pollution in lakes and lake shoreline sediments—a case study on Lake Bolsena and Lake Chiusi (central Italy). Environ. Pollut. 213, 648–657. ( 10.1016/j.envpol.2016.03.012) [DOI] [PubMed] [Google Scholar]
- 13.Su L, Xue Y, Li L, Yang D, Kolandhasamy P, Li D, Shi H. 2016. Microplastics in Taihu Lake, China. Environ. Pollut. 216, 711–719. ( 10.1016/j.envpol.2016.06.036) [DOI] [PubMed] [Google Scholar]
- 14.Aljaibachi R, Callaghan A. 2018. Impact of polystyrene microplastics on Daphnia magna mortality and reproduction in relation to food availability. PeerJ 6, e4601 ( 10.7717/peerj.4601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, Moger J, Galloway TS. 2013. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 47, 6646–6655. ( 10.1021/es400663f) [DOI] [PubMed] [Google Scholar]
- 16.Scherer C, Brennholt N, Reifferscheid G, Wagner M. 2017. Feeding type and development drive the ingestion of microplastics by freshwater invertebrates. Sci. Rep. 7, 17006 ( 10.1038/s41598-017-17191-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sussarellu R, et al. 2016. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl Acad. Sci. USA 113, 2430–2435. ( 10.1073/pnas.1519019113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messinetti S, Mercurio S, Parolini M, Sugni M, Pennati R. 2018. Effects of polystyrene microplastics on early stages of two marine invertebrates with different feeding strategies. Environ. Pollut. 237, 1080–1087. ( 10.1016/j.envpol.2017.11.030) [DOI] [PubMed] [Google Scholar]
- 19.Dow JA, Maddrell SH, Görtz A, Skaer NJ, Brogan S, Kaiser K. 1994. The Malpighian tubules of Drosophila melanogaster: a novel phenotype for studies of fluid secretion and its control. J. Exp. Biol. 197, 421–428. [DOI] [PubMed] [Google Scholar]
- 20.Coleman J, Juhn J, James AAA. 2007. Dissection of midgut and salivary glands from Ae. aegypti mosquitoes. J. Vis. Exp. 5, e228 ( 10.3791/228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R Development Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 22.Clements AN. 1992. The biology of mosquitoes. Volume 1: development, nutrition and reproduction. London: Chapman and Hall. [Google Scholar]
- 23.Chavshin AR, Oshaghi MA, Vatandoost H, Yakhchali B, Zarenejad F, Terenius O. 2015. Malpighian tubules are important determinants of Pseudomonas transstadial transmission and longtime persistence in Anopheles stephensi. Parasit. Vectors 8, 1–7. ( 10.1186/s13071-015-0635-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Von Moos N, Burkhardt-Holm P, Köhler A. 2012. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ. Sci. Technol. 46, 11 327–11 335. [DOI] [PubMed] [Google Scholar]
- 25.Brennecke D, Ferreira EC, Costa TMM, Appel D, da Gama BAP, Lenz M. 2015. Ingested microplastics (>100μm) are translocated to organs of the tropical fiddler crab Uca rapax. Mar. Pollut. Bull. 96, 491–495. ( 10.1016/j.marpolbul.2015.05.001) [DOI] [PubMed] [Google Scholar]
- 26.Avio CG, Gorbi S, Regoli F. 2015. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: first observations in commercial species from Adriatic Sea. Mar. Environ. Res. 111, 18–26. ( 10.1016/j.marenvres.2015.06.014) [DOI] [PubMed] [Google Scholar]
- 27.Piermarini PM. 2016. Renal excretory processes in mosquitoes, 1st edn Wooster, OH: Elsevier Ltd. [Google Scholar]
- 28.Leonard EM, Pierce LM, Gillis PL, Wood CM, O'Donnell MJ. 2009. Cadmium transport by the gut and Malpighian tubules of Chironomus riparius. Aquat. Toxicol. 92, 179–186. ( 10.1016/j.aquatox.2009.01.011) [DOI] [PubMed] [Google Scholar]
- 29.Huerta Lwanga E, Gertsen H, Gooren H, Peters P, Salánki T, Van Der Ploeg M, Besseling E, Koelmans AA, Geissen V. 2016. Microplastics in the terrestrial ecosystem: implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ. Sci. Technol. 50, 2685–2691. ( 10.1021/acs.est.5b05478) [DOI] [PubMed] [Google Scholar]
- 30.Dris R, Gasperi J, Saad M, Mirande C, Tassin B. 2016. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Poll. Bull. 104, 290–293. [DOI] [PubMed] [Google Scholar]
- 31.Medlock JM, Snow KR. 2008. Natural predators and parasites of British mosquitoes: a review. Eur. Mosquito Bull. 25, 1–11. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data files are available in the electronic supplementary material.