Abstract
Pain feels different in different social contexts, yet the mechanisms behind social pain modulation remain poorly understood. To elucidate the impact of social context on pain processing, we investigated how group membership, one of the most important social context factors, shapes pain relief behaviourally and neurally in humans undergoing functional neuroimaging. Participants repeatedly received pain relief from a member of their own group (ingroup treatment) or a member of a disliked outgroup (outgroup treatment). We observed a decrease in pain ratings and anterior insula (AI) pain responses after outgroup treatment, but not after ingroup treatment. Moreover, path analyses revealed that the outgroup treatment induced a stronger relief learning in the AI, which in turn altered pain processing, in particular if the participant entered the treatment with a negative impression toward the outgroup individual. The finding of enhanced analgesia after outgroup treatment is relevant for intergroup clinical settings. More generally, we found that group membership affects pain responses through neural learning and we thus elucidate one possible mechanism through which social context impacts pain processing.
Keywords: reinforcement, ingroup bias, outgroup support, classical conditioning, expectation, anterior insula
1. Introduction
Pain is one of the most salient negative physiological experiences. Thus, it is crucial for us to understand the psychological and neural mechanisms that underpin pain reduction. Previous research has shown a reduction of subjective and physiological pain responses (pain relief) as a result of learning [1–8]. We understand relatively well how pain relief learning affects pain processing. Specifically, learning to anticipate pain relief corresponds to a build-up of conditioned responses to cues or individuals that are associated with pain relief [2,3]. Moreover, social context variables are known to influence pain processing and induce analgesia [9–13]. However, we do not know how social context affects pain-related learning processes.
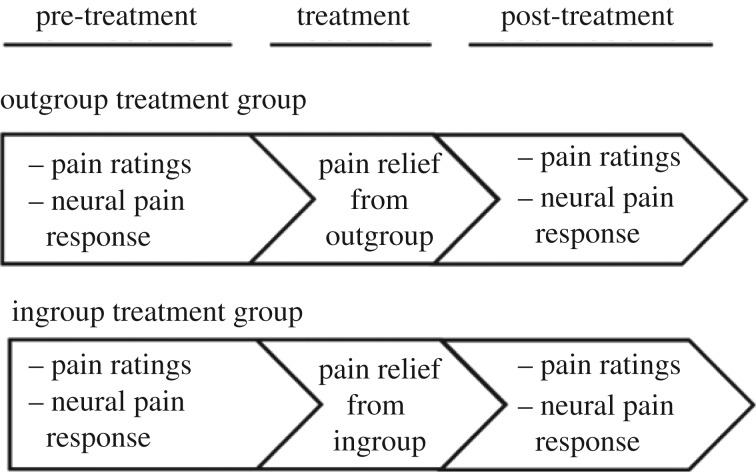
In the present research, we investigated how the anticipation of pain relief is influenced by one of the most important social factors: group membership. While undergoing functional magnetic resonance imaging (fMRI), participants experienced a pain-relieving treatment from an individual of their own group (ingroup treatment group) or from an individual of a disliked outgroup (outgroup treatment group; figure 1; see electronic supplementary material). Before and after the treatment, participants received painful stimulation and we recorded their pain ratings and pain-related brain responses. In more detail, before the treatment, participants learned to associate a cue (green arrow) with painful stimulation to be delivered to the back of their left hand. During the treatment, the same pain cue (green arrow) was presented. Importantly, now it was associated in the majority (75%) of trials with pain relief provided by an ingroup or an outgroup individual (see electronic supplementary material). Classical learning theory predicts that the repeated experience of pain relief gradually changes the value of the cue. Instead of predicting pain, it becomes a predictor of pain relief in the majority of trials. Such learning-related change in predicted pain commonly results in a reduction of subjective and physiological pain responses and forms the basis for learned analgesia [1–8]. One key prediction of classical learning theory is that learning is stronger the less individuals expect the outcome they actually receive, because more unexpected outcomes elicit larger prediction errors [14]. Thus, the impact of learning on pain processing should be stronger the less participants expected to experience pain relief.
Figure 1.
Experimental design. Except for the group membership of the treatment provider, the outgroup treatment group and the ingroup treatment group received identical pain relief treatment.
We considered two possible pathways through which social context in the form of ingroup or outgroup treatment could affect pain processing. It is known that social context alters learning [15–17], because it invokes social priors that affect individual expectations regarding outcomes such as pain [2]. Given that learning shapes pain processing as outlined above, it is possible that social context modulates pain processing indirectly via its impact on learning to anticipate pain relief during the treatment. Evidence from social psychology research shows that most people expect to be treated badly by outgroup individuals [18–20]. Based on these findings, it is plausible to assume that participants might enter the treatment with a more negative prior towards the outgroup treatment provider as compared to participants receiving treatment from the ingroup treatment provider. The positive experiences of pain relief from an outgroup treatment provider contradict this negative prior. Consequently, pain relief from an outgroup treatment provider should elicit large prediction errors that in turn elicit strong learning. By contrast, positive experiences with an ingroup member should confirm the positive ingroup prior, and consequently elicit relatively small prediction errors and little learning. As a result, we should find a learning-related pre versus post reduction of pain ratings and neural pain responses after outgroup treatment, but not after ingroup treatment.
Alternatively, treatment may affect subsequent pain processing directly, independently of learning [12]. This assumption is inspired by previous evidence showing that social support can buffer psychological and physiological reactions to negative events directly [21–24]. There is some evidence that the impact of social support increases with social closeness ([21,22] for review), and thus may be stronger in the ingroup as compared to the outgroup condition. In this case, we should find a stronger pre-to-post decrease in pain ratings and neural pain responses for ingroup treatment as compared to outgroup treatment. However, other studies have shown that receiving active or passive support from a stranger was as good as receiving it from a close person such as a friend [24]. Based on this evidence, a pre-to-post decrease in pain ratings and neural pain responses should be found independently of the group membership of the treatment provider, i.e. to a comparable extent in the ingroup and the outgroup treatment group.
On the neural level, pain reduction in the absence of analgesic drugs is commonly related to a reduction of neural responses in pain-responsive regions such as the insular cortex, anterior cingulate cortex, thalamus, and somatosensory cortex [5,25–27]. The modulation of pain responses by psychological factors such as social context and learning has been particularly linked to the anterior portion of the insular cortex (AI; [28,29]). The AI is known to track mismatches between pain anticipation and actual outcomes, which is crucial for pain-related learning [6,17,29,30]. Moreover, it flexibly connects to brain regions that are involved in the emotional and evaluative processing of social information [31,32], and has been related to social categorization [33–36]. In light of this evidence, the AI cortex is a plausible neural candidate for the construction of pain experiences that are shaped by learning and social context [28,29]. Accordingly, either pathway (direct impact of social context or indirect impact of social context via learning) would predict that the effects of treatment are tracked by the AI cortex and expressed in an ingroup-related or outgroup-related pre-to-post-treatment reduction of AI pain responses and pain ratings.
2. Material and methods
(a). Participants
Forty healthy men (mean age = 22.7, SE = 0.41) participated in the study and were randomly assigned to an ingroup and outgroup treatment group. There were no age differences between the groups, t38 = −0.34, p = 0.73. Four datasets had to be discarded, two because of motion artefacts and two due to technical problems with the response box, resulting in groups of 18 (ingroup treatment) and 18 (outgroup treatment). Four confederates served as an ingroup or outgroup treatment provider, counterbalanced across participants (electronic supplementary material for details).
(b). Social context manipulation
Ingroup treatment providers were individuals who ostensibly shared the participant's nationality (Swiss), while outgroup treatment providers were ostensible of Balkan descent. Individuals of Balkan descent constitute one of the largest minority groups in our country (Switzerland), whose presence is often portrayed as problematic. Prior to the treatment, participants rated their impression of the ingroup and outgroup treatment provider on a well-established impression scale (see electronic supplementary material for details).
(c). Pain delivery
Participants received painful electrical stimulation (bipolar, monophasic, maximum duration: 1 000 ms, input range: 5 V, output range: 50 mA) via an electrode (diameter of 0.5 cm) that was attached to the back of their left hand from a single-current stimulator (DS5, Digitimer Ltd., Hertfordshire, UK). The individual pain thresholds were determined using a standard procedure in which participants received shocks with slowly increasing intensity and rated the intensity of each shock on a scale from 1 (not painful) to 10 (extremely painful). We used a subjective threshold of eight for pain stimulation during the study.
(d). Experimental design
Participants were randomly assigned to two different groups, an ingroup treatment group or an outgroup treatment group. Participants from the ingroup treatment group were informed that they would receive treatment from a person of their nationality (Swiss, introduced with a Swiss name), participants from the outgroup treatment group were informed that they would be treated by a person of a different nationality (introduced with a Balkan name). Apart from the name of the treatment provider, the outgroup and the ingroup conditions were identical. The treatment provider and a member from the other group sat on a chair next to the scanner such that the participant could see their hands. To hold overall social context constant, an outgroup and an ingroup member were present in all parts of the study (i.e. during pre-treatment, treatment, and post-treatment). However, after the treatment session, the respective treatment provider and the member from the other group were replaced by a different individual from the same group (i.e. the remaining two confederates representing an ingroup and an outgroup member) for the post-treatment session. This measure was taken to reduce demand effects that might occur if the participant were to rate pain in the presence of the same person that had provided the pain treatment. As a result, all participants interacted with two confederates representing ingroup members, and two confederates representing outgroup members. The roles of the confederates were counterbalanced across participants, such that each confederate acted as an ingroup or outgroup member equally often during the pre-treatment and treatment session, and during the post-treatment session. Participants were informed that their ratings were confidential and that they would not meet the treatment providers or the other people after the experiment. The details of the pre- and post-treatment sessions (pain processing) and the treatment session are provided in the electronic supplementary material.
(e). Behavioural and neural data analyses
(i). Reinforcement learning model
To test for neural learning signals reflecting trial-by-trial changes in pain relief anticipation during the treatment session, we used a standard reinforcement learning model ([14,37]; see electronic supplementary material).
(ii). Regression analyses
To identify the impact of neural learning signals on pre- versus post-treatment changes in pain ratings, we conducted an ANOVA based on an ordinary least-squares (OLS) regression model (electronic supplementary material for details).
(iii). Path analyses
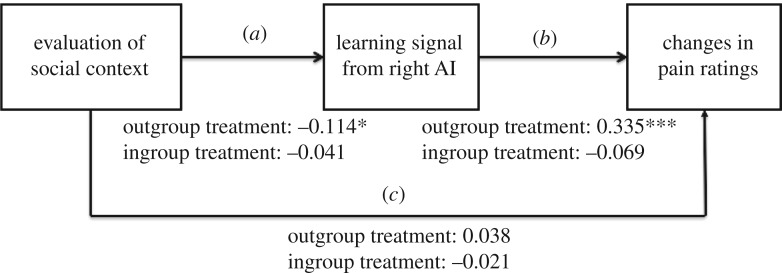
To specifically assess whether the degree to which group manipulation invoked social priors affects pain processing directly (figure 3, Path c) or indirectly via its impact on learning (figure 3, Paths a and b), we estimated a mediation model excluding the treatment group variable. Next, we performed a moderated mediation model that included the grouping variable treatment group using the Lavaan package in R [38]. Follow-up tests were then performed within each treatment group to characterize the path strength for each group separately (electronic supplementary material for details).
Figure 3.
Path analysis shows that group membership moderates analgesia through learning. We used participants’ impression ratings for the ingroup and outgroup treatment provider, which reflect individualized social priors, as a predictor variable (a). The individual learning signals from the right AI served as a mediator variable (b) and the individual pre- versus post-treatment differences in pain ratings entered as a dependent variable (c). In addition, we included treatment group (ingroup versus outgroup treatment) as a moderator variable to assess between-group model differences. The direct Path (c) from social context evaluation to pre- versus post-treatment changes in pain ratings was not significant. In the outgroup treatment group, social context evaluation affected the pain ratings indirectly via its impact on learning, as reflected by significant indirect path coefficients (Paths a and b). * p < 0.05; *** p < 0.001.
(iv). Effect sizes
For analyses comparing means between groups (t-tests) we computed Cohen's d [39], for Analyses of Variance, Cohen's f and for multiple regression models with continuous predictor variables, Cohen's f2, which is more appropriate as it allows evaluation of local effects [38,39]. For χ2-statistics used for model comparisons, Cohen's w was computed. To reflect effect size in structural equation models, we computed standardized coefficients reflecting the magnitude of change in the outcome variable (in s.d. units) in response to a one s.d. increase in the predictor variable [40]. For imaging results, we determined the respective effect sizes based on the average beta estimates extracted from all the voxels within significant activation clusters to reflect the statistics reported in the paper, and based on betas from all voxels within the anatomical ROI for unbiased effect size estimates, as suggested recently [41].
Details about Image acquisition, preprocessing, first and second model analyses of imaging data are provided in the electronic supplementary material. Two separate first-level models were estimated for each participant, one that captured the neural correlates of learning during the treatment session (learning model) and one that captured pain-related activations in the pre-treatment and post-treatment sessions (pain model). The second-level analyses were based on contrast images that resulted from linearly contrasting parameter estimates for the regressors of interest in the learning and pain models. To test the hypothesis that AI cortex activation reflects pain-related learning and resulting pre- versus post-treatment differences in pain processing, we analysed our data in bilateral anatomical masks of the insular cortex [42], using small-volume family-wise error (SV FWE) correction (p < 0.05). Moreover, we conducted exploratory whole brain analyses (uncorrected, p < 0.001, k = 5; electronic supplementary material, tables S1, S3, and S4).
3. Results
(a). Social context manipulation impacts group evaluation
We tested whether our participants distinguished between the two social contexts using their impression ratings of the ingroup and outgroup treatment providers (see electronic supplementary material for a description of the scale). The ratings were collected before the treatment and thus reflect participants' prior perception of the ingroup and outgroup member. We entered impression ratings into a mixed ANOVA with treatment group (ingroup/outgroup treatment group) as a between-subjects factor and social context (ingroup/outgroup treatment provider) as a within-subjects factor. The results revealed a significant main effect of social context, F1,34 = 10.85, p = 0.002, f = 0.565, indicating that ingroup members were perceived as significantly more favourable (ingroup rating = 28.92, outgroup rating = 24.5). The main effect of treatment group was not significant, F1,34 = 3.31, p = 0.078, f = 0.437, nor was the interaction between treatment group and social context, F1,34 = 0.097, p = 0.76, f = 0.053.
Follow-up tests showed that in both treatment groups, the impression of the outgroup compared to the ingroup treatment provider was significantly more negative, ingroup treatment group, t17 = −2.37, p = 0.03, d = 0.58; outgroup treatment group, t17 = −2.3, p = 0.039, d = 0.56. Thus, before the treatment, the participants perceived the outgroup member more negatively than the ingroup member, which indicates that our manipulation of social context was successful.
(b). Behavioural learning during the treatment
We modelled the individual learning rates based on emotion ratings during the ingroup and the outgroup treatment. The results revealed average learning rates of 0.35; 95% CI = [0.24 0.45] for the outgroup treatment group and 0.39; 95% CI = [0.28 0.49] for the ingroup treatment group with no significant differences between the treatment groups, t34 = −0.44, p = 0.66, d = −0.07. Moreover, the estimated learning rates did not significantly differ from the assumed learning rate of 0.3 that is commonly found in learning studies [43], outgroup treatment group, t17 = 0.78, p = 0.44, d = 0.18; ingroup treatment group, t17 = 1.26, p = 0.22, d = 0.30. These results provide behavioural evidence for learning in both treatment groups.
(c). Pain relief learning in the anterior insula
Next, we obtained a neural model of pain relief learning during the ingroup and the outgroup treatment. To do so, we regressed participants’ neural responses to the pain-predicting cue of each treatment trial against model estimates from a reinforcement learning model [14]. In this model, we estimated how participants' prior anticipation of pain relief (conservatively initialized at zero) changed as a function of the prediction errors elicited by preceding treatment experiences (i.e. pain relief or no pain relief). Specifically, the prediction errors were weighted by the learning rate and induced trial-to-trial changes in ‘pain relief values’ by being combined with previous pain relief anticipation into current pain relief anticipation (Vt). Vt served as a parametric modulator of the neural responses in the pain anticipation window of each trial.
The result of the parametric regression analysis for the entire sample (i.e. both ingroup and outgroup treatment group) showed that learned pain relief anticipation mainly correlated with the activation of the right anterior insula (AI), Z = 3.7, P (SV FWE-corrected) = 0.04, P (whole brain cluster level FWE-corrected) = 0.013, d (cluster) = 0.76, d (anatomical insula mask) = 0.318 (electronic supplementary material, figure S1, red; electronic supplementary material, table S1). Contrasting the respective neural learning signals between the treatment groups did not reveal significant results, even at a liberal uncorrected threshold of p < 0.05. These results indicate that, in both treatment groups, learning-induced pain relief anticipation is captured by the neural response of the AI, most prominently in the right AI (electronic supplementary material for an additional parametric regression with the classical prediction error (δt)).
(d). Analgesic effects of social context and learning
To identify the analgesic effects of social context, we first tested whether the type of learning context leads to differential changes in pain ratings before compared to after pain relief treatment. To this end, we entered the pain ratings into an ANOVA with the factors time (pre-/post-treatment) and treatment group (ingroup/outgroup). Results showed a significant main effect of time, F1,34 = 7.106, p = 0.012, f = 0.457, which was modulated by treatment group as indicated by a significant interaction between time and treatment group, F1,34 = 6.13, p = 0.019, f = 0.425. Figure 2a specifies the interaction between social context and learning by showing a significant pre-to-post-treatment reduction in the outgroup treatment group, t17 = −3.1, p = 0.006, d = 0.74, but not in the ingroup treatment group, t17 = −0.17, p = 0.87, d = 0.03.
Figure 2.
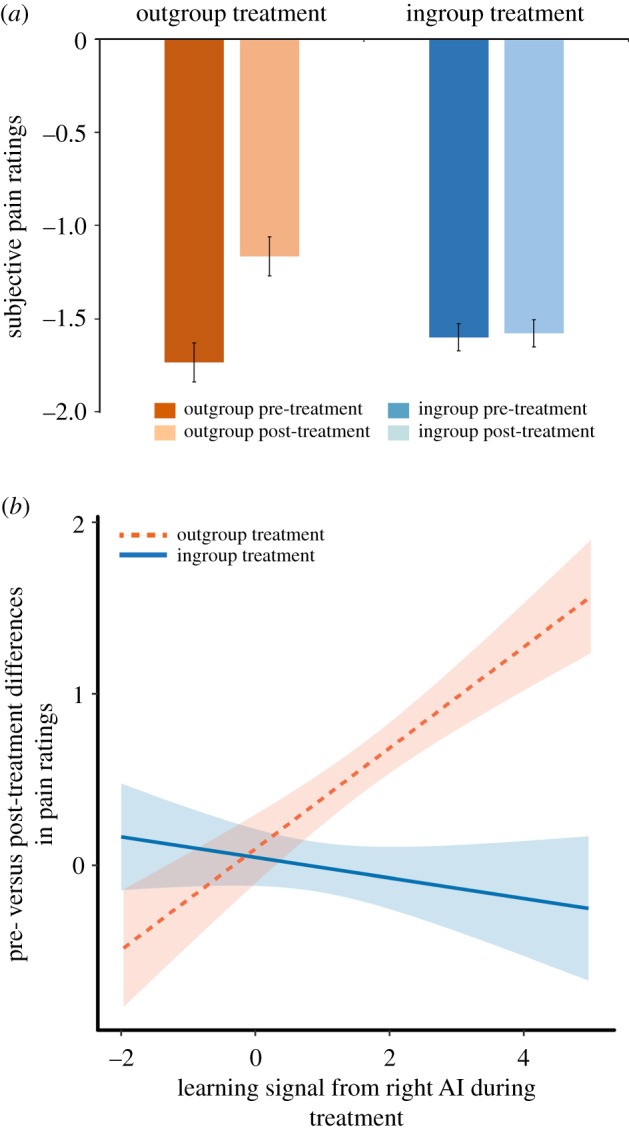
Pain ratings and interaction between social context manipulation (ingroup versus outgroup treatment) and learning during the treatment. (a) Average ratings of subjective pain experience (the scale ranged from −4 to +4 with more negative numbers corresponding to higher pain) before and after ingroup and outgroup treatment. Participants' pain ratings were reduced after they received pain relief from an outgroup treatment provider, but not after they received pain relief from an ingroup treatment provider. (b) Interaction between social context manipulation (ingroup versus outgroup treatment) and individual learning signals from the right AI. After outgroup treatment, the pre- versus post-treatment difference in pain ratings was predicted by learning, while there was no such relationship after ingroup treatment (linear regression; see electronic supplementary material, table S2, right panel, c). (Online version in colour.)
While the assignment of confederates to the different roles in the experiment was fully counterbalanced, it is still possible that specific confederates can influence the analgesic effects of the treatment. A first ANOVA showed that the effect reported above did not change if the individual treatment provider (i.e. a variable coding the identity of the helper) was added as a control variable, time, F1,28 = 6.699, p = 0.015, f = 0.489, time (pre/post-treatment) × treatment group (ingroup/outgroup), F1,28 = 5.776, p = 0.023, f = 0.454. Second, we conducted a three-way ANOVA with the factors time (pre/post-treatment), treatment group (ingroup/outgroup), and individual treatment provider. The results showed no significant effect of treatment provider on the pre versus post differences in pain ratings, F3,28 = 0.680, p = 0.567, f = 0.272, with no differences between treatment groups, F3,28 = 0.038, p = 0.99, f = 0.256. Moreover, there was no significant main effect of treatment provider, F3,28 = 1.12, p = 0.358, f = 1.39, and no interaction with any other variable (all interaction terms Ps > 0.56). These results show that the observed effects are independent of individual features of the treatment providers.
Our findings so far indicate that pain-relieving treatment effectively reduced sensitivity to pain in participants treated by the outgroup but not in participants treated by the ingroup. Note that these effects occurred even though both treatments were identical with respect to the objective probabilities and experienced frequencies of pain relief.
Next, we tested whether social context and learning jointly influence the impact of pain relief treatment on pain perception. To this end, we used the difference between ratings before compared to after pain relief treatment as the dependent variable. As a learning variable, we included the individual beta estimates extracted from a 6 mm sphere around the SV FWE-corrected activation peak in the right AI reported above (using Marsbar; [44]). Social context was coded categorically (ingroup or outgroup). The ANOVA showed a significant interaction between learning and social context, F1,30 = 7.421, p = 0.0107, f = 0.497 (figure 2b; electronic supplementary material, table S2, left panel), but no significant main effects for learning and social context. In sequential regression analyses, we also tested for the effects of social context and learning separately. These analyses yielded significant effects for both factors, learning, t = 2.23, p = 0.03, f = 0.409 (electronic supplementary material, table S2, right panel a) and social context, t = 2.3, p = 0.03, f = 0.425 (electronic supplementary material table S2, right panel b) when the interaction term was not modelled, and a significant interaction effect with no main effects for the full model (electronic supplementary material, table S2, right panel c). In line with previous findings, these results show that pain processing is affected by social context [9–13] and learning [1–8]. Extending this previous evidence, we find that social context and learning interact and account for pre- versus post-treatment differences in pain ratings.
(e). Path analysis reveals that the analgesic mechanism is learning based
To elucidate the mechanism driving the analgesic effect shown in figure 2b, we next conducted path analyses. In these analyses, we aimed to use a more specific measure of how each participant perceived the ingroup or the outgroup member prior to the treatment, and thereby to more precisely capture the impact of social context. To achieve this aim, we included the individual impression ratings for the ingroup and outgroup member as a social context variable. First, to assess whether individualized social priors affect pain processing directly (figure 3, Path c) or indirectly (figure 3, Paths a and b) via an impact on learning, we submitted the whole dataset (N = 36) to a path model. The results showed significant indirect effects (Path a coefficient = −0.086, p = 0.007, standardized coefficient = −0.409, Path b coefficient = 0.140, p = 0.049, standardized coefficient = 0.330), but no significant direct effects (Path c coefficient = −0.011, p = 0.470, standardized coefficient = −0.121). These results indicate that social context affects analgesia largely via its influence on the neural correlates of learning.
Given the significant differences between the ingroup and outgroup treatment that we observed above, it is important to estimate the effect of treatment group. To do so, we added treatment group (ingroup versus outgroup treatment) as a moderator variable and estimated a moderated mediation model [45] (see Material and methods for details). Results showed that the significant indirect path is moderated by treatment group, 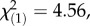 p = 0.033, w = 0.356. Follow-up analyses testing the effects for each group on each indirect path separately revealed that the significant effect of treatment group reflects a significant indirect path in the outgroup treatment group, but not in the ingroup treatment group, Path a coefficient outgroup treatment = −0.114, p = 0.03, standardized coefficient = −0.453, ingroup treatment = −0.041, p = 0.42, standardized coefficient = 0.19; Path b coefficient outgroup treatment = 0.34, p < 0.001, standardized coefficient = 0.772, ingroup treatment = −0.069, p = 0.38, standardized coefficient = −0.0194. The significant negative relationship between social prior and learning in the outgroup treatment group (figure 3, Path a) indicates that the learning signal increased with increasingly negative evaluations of outgroup members. The significant positive link between learning and pre- versus post-treatment changes in pain ratings (figure 3, Path b) indicates that the increases in the AI learning signals of the outgroup treatment group predicted larger reductions in the subjective pain experience. Together, the results support the model of an indirect impact of social priors (induced by group manipulation) on pain processing by means of neural learning.
p = 0.033, w = 0.356. Follow-up analyses testing the effects for each group on each indirect path separately revealed that the significant effect of treatment group reflects a significant indirect path in the outgroup treatment group, but not in the ingroup treatment group, Path a coefficient outgroup treatment = −0.114, p = 0.03, standardized coefficient = −0.453, ingroup treatment = −0.041, p = 0.42, standardized coefficient = 0.19; Path b coefficient outgroup treatment = 0.34, p < 0.001, standardized coefficient = 0.772, ingroup treatment = −0.069, p = 0.38, standardized coefficient = −0.0194. The significant negative relationship between social prior and learning in the outgroup treatment group (figure 3, Path a) indicates that the learning signal increased with increasingly negative evaluations of outgroup members. The significant positive link between learning and pre- versus post-treatment changes in pain ratings (figure 3, Path b) indicates that the increases in the AI learning signals of the outgroup treatment group predicted larger reductions in the subjective pain experience. Together, the results support the model of an indirect impact of social priors (induced by group manipulation) on pain processing by means of neural learning.
(f). Impact on neural pain responses
So far, our results have revealed a reduction of subjective pain ratings after outgroup treatment (figure 2a) that is driven by learning (figure 2b and path analysis). In our final analyses, we tested for a link between these effects on the neural level. First, we contrasted pain-related activations before the outgroup treatment with pain-related activations after the outgroup treatment. The results showed a pre versus post difference in the bilateral AI cortex, left AI, Z = 3.68, p (SV FWE-corrected) = 0.05, d(cluster) = 0.8, d(anatomical mask) = 0.38 (electronic supplementary material, table S3).
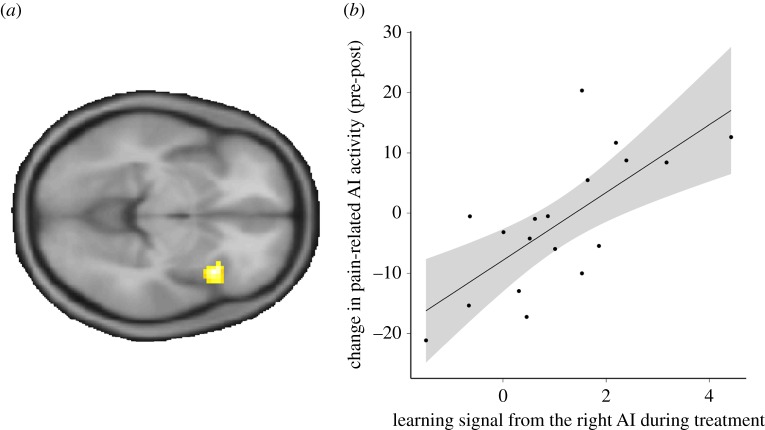
Next, we tested if the pre versus post outgroup treatment reduction in the AI cortex was driven by learning. To do so, we conducted a second-level regression with the learning signal from the right AI as the independent variable, and the pre versus post differences in pain-related activation as the dependent variable. We found that individual learning was associated with pre- versus post-treatment differences in the right AI, Z = 4.4, p (SV FWE-corrected) = 0.006, FWE-corrected (whole brain cluster level) = 0.02, f2(cluster) = 0.36, f2(anatomical insula mask) = 0.42 (figure 4; electronic supplementary material, table S4). Thus, neural learning during outgroup treatment was positively related to reductions in pain-induced activation.
Figure 4.
Individual learning signals from the right AI during outgroup treatment predict neural analgesia. (a) Second-level regression between the individual magnitude of neural learning during the treatment and the pre- versus post-treatment changes in pain-related activation, FWE-corrected (whole brain at cluster level) = 0.02, visualized at p < 0.001 (uncorrected). (b) Illustration of the results in (a). Extraction of contrast estimates from a 6 mm sphere around the SV FWE-corrected activation peak in AI shows a clear relationship between neural learning and pain-related signals for the experimental group. Specifically, a stronger learning signal in the insula during outgroup treatment predicts a larger pre-to-post-treatment reduction in pain-related activation.
A final control analysis showed that the correlation between the AI in different parts of the study cannot be explained by potential imaging artefacts (for details see electronic supplementary material).
4. Discussion
Our study investigated how one of the most important social context factors, namely, group membership, interacts with learning to shape subjective and neural responses to pain. We found that group membership modulates pain processing in an intriguing way by inducing stronger subjective and neural analgesia after outgroup than after ingroup treatment. Importantly, the enhanced outgroup analgesia occurred even though our participants received identical pain relief treatment in the ingroup and outgroup context. Path analysis revealed that group membership had no direct effect on pain processing. Instead, it affected pain processing via its impact on learning.
The effect of learning on pain processing observed in our study is in line with previous studies that documented reduced sensitivity to pain after repeated experience of pain relief, both on the subjective and the neural level [1–8]. Extending these findings, our results show that learning reduces pain sensitivity conditional on a negative social prior, here captured by participants' impression ratings towards the outgroup member. The more negatively a person evaluated the outgroup treatment provider prior to the treatment, the stronger was the impact of learning on pain perception. This implies that the same type of learning (here a classical learning mechanism with a comparable learning rate) has a fundamentally different impact when it takes place in the context of a negative social prior (i.e. in the outgroup treatment group), compared to a positively evaluated social context (i.e. in the ingroup treatment group). Our results show that learning affects pain processing more strongly when it contradicts and updates a negative prior (regarding the outgroup treatment provider) than when it confirms a positive prior (regarding the ingroup treatment provider).
The observed impact of social priors on learning is in line with previous social learning studies [46–48]. However, these studies investigated learning while observing others, whereas our study focused on learning while experiencing treatment by others that had an actual impact on participants' pain. Our neural and behavioural results emphasize the importance of social priors induced by different social treatment contexts. Based on that it could be argued that the reduction of pain responses after outgroup treatment simply reflects threat- or stress-related analgesia, produced by the presence of an outgroup member. Given that an outgroup member was also present in the pre-treatment pain session, this view predicts that already in that first session pain processing should be reduced in the outgroup, compared to the ingroup, condition. Contrary to this prediction, we find that pre-treatment pain levels are similar in the presence of outgroup and ingroup members (figure 2a) and that learning, indexed by current pain relief anticipation and cue-related AI responses, plays a central role for post-treatment analgesia (figures 2b, 3, and 4).
The mechanistic insights gained from the path analyses with pain ratings (figure 3) were confirmed by the neural data. Participants showed a stronger decline in neural pain responses after outgroup treatment as compared to ingroup treatment. The individual extent of the pre versus post changes in neural pain responses was predicted by the individual neural learning signal during outgroup treatment. The neural learning signal reflecting learned anticipation of pain relief correlated with changes of activation in the right AI, the same structure that showed the strongest pre- versus post reduction in pain-related responses. It is well known that the AI plays an important role in social categorization [33–36] and is among those regions that are sensitive to social rewards and punishments [49,50]. Falk et al. [33] proposed that brain regions that are sensitive to social rewards and punishments, such as the AI, might mediate the relationship between social influences and behavioural or physiological responses. Our results support this model by showing that the AI tracks a learning signal that is associated with a specific social category (here the outgroup), and, at the same time, uses this learning signal to update subjective and physiological pain responses. Together, these results point to the AI cortex as a plausible neural basis of the interplay between social categorization, learning, and pain processing.
Our analysis on learning-related changes in pain value and neural pain processing also revealed other brain regions, most consistently the supplementary motor area (SMA) and the middle temporal gyrus (electronic supplementary material, tables S1 and S4). The SMA has been associated with the pain-related priming of movements [51,52] and the middle temporal gyrus has been linked to the emotional processing of threat and pain [53,54]. We had no a priori assumptions with regard to these regions, and thus can only speculate about their functions in the current paradigm. In-keeping with the literature, the activation in SMA might reflect learning-related motor preparation when pain might occur [51,52], while the temporal gyrus could support emotional learning in the context of pain [53,54]. In any case, the AI appears to be part of a larger network involved in learning about pain relief.
Given that the AI is involved in social categorization processes and learning, the pre versus post decrease in pain responses could reflect a reduction of category-related prejudices or a learning-related reduction of pain value. In our path analysis, we included the individual impression ratings for the ingroup and outgroup member that were collected before the study and thus capture individual differences in outgroup prejudices invoked by the group manipulation. The results showed that these individual differences in prior prejudices had no direct impact on pre versus post differences in pain responses, but predicted them via their impact on learning (i.e. learning-related changes in pain values). These findings suggest that neither social priors (i.e. prejudices) nor learning-related changes in pain value alone can account for the observed pre versus post decrease in pain responses. Instead, the observed effects in pain processing result from the interaction of social factors and learning. That said, future research may want to assess changes in prejudices potentially arising from the treatment. One previous study [17] indeed showed that positive outgroup experiences increase empathy with outgroup members.
At first glance, the finding that a manipulation of social context (here, ingroup versus outgroup treatment provision) has little direct effect on the processing of aversive events (here, pain stimulation) might seem to contrast with reports of a direct beneficial effect of social support on stress and pain responses, in particular, if support is provided by a similar or familiar individual [12,21–23]. However, social support can have a positive impact irrespective of similarity or closeness [24], corroborating the notion that the potential benefit of social support varies depending on the framing of the social interaction [55,56] and relationship characteristics [57]. It is also worth keeping in mind that in our study, participants more positively evaluated the ingroup than the outgroup member, but did not establish a personal relationship with this person. We chose this setting to control for the effects of other social variables besides group membership (e.g. warmth, attractiveness, perceived empathy, etc.), but it is possible that a personal interaction is required to unleash analgesic effects of ingroup support. Considering this possibility, our results should not be interpreted as evidence against the beneficial impact of social support on psychological and physiological well-being, but rather show that the limited reliance on ingroup support in impersonal intergroup settings can be overcome by alternative mechanisms. Indeed, our results suggest that the benefits of learning from positive outgroup experiences can outweigh the benefits of ingroup support if participants interact with unfamiliar treatment providers.
Our study revealed converging evidence from different analyses on the behavioural and neural level, with medium to large effect sizes. However, the relatively small sample size (N = 40, 36 included in all analyses) remains a limitation of this study. Owing to the complex experimental design (social group manipulation, pain thresholding, coordination between four confederates and the participants), it was not possible to record data from more than 40 subjects for the current experiment. However, we strongly encourage future research to focus on specific aspects of the current results and adapt the experimental design accordingly. One suggestion would be to use a simpler group intervention or perform group intervention outside the scanner, in an effort to save time during the scanning session.
Together, our results indicate that important social context variables such as group membership affect pain processing via their impact on learning. Specifically, classical learning mechanisms can turn outgroup negativity into a treatment benefit and thus potentially dissolve it. On a conceptual level, our findings demonstrate the manner in which the combination of different psychological factors can enhance analgesia. We have gained these insights because we considered the potential interplay between important psychosocial factors, here, group membership and learning. Thus, our work highlights the importance of a multi-factorial approach to human pain processing that takes into account the interactions between internal and external pain-modulating factors.
Supplementary Material
Acknowledgements
We thank Marius Vollberg and Karl Treiber for their help with data collection, and Björn Lindström, Alexander Soutschek, and Tamara Herz for helpful feedback on the manuscript.
Ethics
The study was approved by the Research Ethics Committee of the Canton of Zurich (EK: E-24/2008) and all participants gave informed consent.
Data accessibility
Behavioural data and scripts are available at github.com: https://github.com/AffectiveNeuroeconomics/Social_Analgesia
Imaging data are available at neurovault.org [58].
https://neurovault.org/collections/3496/; https://neurovault.org/collections/3499/; https://neurovault.org/collections/3500/.
Authors' contributions
Conceptualization: G.H., J.B.E., P.N.T.; Methodology: G.H., J.B.E., P.N.T.; Formal Analysis: G.H., J.B.E.; Investigation: G.H., J.B.E.; Writing of original draft: G.H.; writing – review and editing: G.H., J.B.E., P.N.T.; Funding acquisition: P.N.T., G.H. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by the Swiss National Science Foundation (grant nos PP00P1 150739, 100014_165884, and 100019_176016) and the German Research Foundation (grant nos. HE 4566/2-1 and HE 4566/3-1). We also acknowledge the support of the Neuroscience Center Zurich.
References
- 1.Au YST, Colagiuri B, Lovibond PF, Colloca L. 2014. Partial reinforcement, extinction, and placebo analgesia. Pain. 155, 1110–1117. ( 10.1016/j.pain.2014.02.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchel C, Geuter S, Sprenger C, Eippert F. 2014. Placebo analgesia: a predictive coding perspective. Neuron. 81, 1223–1239. ( 10.1016/j.neuron.2014.02.042) [DOI] [PubMed] [Google Scholar]
- 3.Colloca L, Miller FG. 2011. How placebo responses are formed: a learning perspective. Proc. Soc. B 366, 1859–1869. ( 10.1098/rstb.2010.0398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egorova N, Park J, Orr SP, Kirsch I, Gollub RL, Kong J. 2015. Not seeing or feeling is still believing: conscious and non-conscious pain modulation after direct and observational learning. Sci. Rep. 5, 16809 ( 10.1038/srep16809) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lui F, Colloca L, Duzzi D, Anchisi D, Benedetti F, Porro CA. 2010. Neural bases of conditioned placebo analgesia. Pain. 151, 816–824. ( 10.1016/j.pain.2010.09.021) [DOI] [PubMed] [Google Scholar]
- 6.Seymour B, O'Doherty JP, Koltzenburg M, Wiech K, Frackowiak R, Friston K, Dolan R. 2005. Opponent appetitive-aversive neural processes underlie predictive learning of pain relief. Nat. Neurosci. 8, 1234–1240. ( 10.1038/nn1527) [DOI] [PubMed] [Google Scholar]
- 7.Jensen K, Kirsch I, Odmalm S, Kaptchuk TJ, Ingvar M. 2015. Classical conditioning of analgesic and hyperalgesic pain responses without conscious awareness. Proc. Natl Acad. Sci. USA 112, 7863–7867. ( 10.1073/pnas.1504567112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jepma M, Wager TD. 2015. Conceptual conditioning: mechanisms mediating conditioning effects on pain. Psychol. Sci. 26, 1728–1739. ( 10.1177/0956797615597658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wager TD, Atlas LY. 2015. The neuroscience of placebo effects: connecting context, learning and health. Nat. Rev. Neurosci. 16, 403–418. ( 10.1038/nrn3976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz KA, Pfister R, Buchel C. 2016. Rethinking explicit expectations: connecting placebos, social cognition, and contextual perception. Trends Cogn. Sci. 20, 469–480. ( 10.1016/j.tics.2016.04.001) [DOI] [PubMed] [Google Scholar]
- 11.Leonard MT, Cano A, Johansen AB. 2006. Chronic pain in a couples context: a review and integration of theoretical models and empirical evidence. J. Pain Off. J. Am. Pain Soc. 7, 377–390. ( 10.1016/j.jpain.2006.01.442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krahe C, Springer A, Weinman JA, Fotopoulou A. 2013. The social modulation of pain: others as predictive signals of salience – a systematic review. Front. Hum. Neurosci. 7, 386 ( 10.3389/fnhum.2013.00386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlino E, Frisaldi E, Benedetti F. 2014. Pain and the context. Nat. Rev. Rheumatol. 10, 348–355. ( 10.1038/nrrheum.2014.17) [DOI] [PubMed] [Google Scholar]
- 14.Rescorla RA, Wagner AR. 1972. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In Classical conditioning II: current research and theory (eds AH Black & WF Prokasy), pp. 64–99. New York, NY: Appleton-Century-Crofts. [Google Scholar]
- 15.Seid-Fatemi A, Tobler PN. 2015. Efficient learning mechanisms hold in the social domain and are implemented in the medial prefrontal cortex. Soc. Cogn. Affect. Neurosci. 10, 735–743. ( 10.1093/scan/nsu130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindstrom B, Selbing I, Molapour T, Olsson A. 2014. Racial bias shapes social reinforcement learning. Psychol. Sci. 25, 711–719. ( 10.1177/0956797613514093) [DOI] [PubMed] [Google Scholar]
- 17.Hein G, Engelmann JB, Vollberg MC, Tobler PN. 2016. How learning shapes the empathic brain. Proc. Natl. Acad. Sci. USA 113, 80–85. ( 10.1073/pnas.1514539112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moy J, Ng SH. 1996. Expectation of outgroup behaviour: can you trust the outgroup? Eur. J. Soc. Psychol. 26, 333–340. ( 10.1002/(SICI)1099-0992(199603)26:2%3C333::AID-EJSP747%3E3.0.CO;2-1) [DOI] [Google Scholar]
- 19.Vivian JE, Berkowitz NH. 1993. Anticipated outgroup evaluations and intergroup bias. Eur. J. Soc. Psychol. 23, 513–524. ( 10.1002/ejsp.2420230508) [DOI] [Google Scholar]
- 20.Vanbeselaere N. 1993. Ingroup bias in the minimal group situation: an experimental test of the inequity prevention hypothesis. Basic Appl. Soc. Psychol. 14, 385–400. ( 10.1207/s15324834basp1404_1) [DOI] [Google Scholar]
- 21.Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. 1996. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychol. Bull. 119, 488–531. ( 10.1037/0033-2909.119.3.488) [DOI] [PubMed] [Google Scholar]
- 22.Kikusui T, Winslow JT, Mori Y. 2006. Social buffering: relief from stress and anxiety. Proc. Soc. B 361, 2215–2228. ( 10.1098/rstb.2006.1941) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Blasi Z, Harkness E, Ernst E, Georgiou A, Kleijnen J. 2001. Influence of context effects on health outcomes: a systematic review. Lancet 357, 757–762. ( 10.1016/S0140-6736(00)04169-6) [DOI] [PubMed] [Google Scholar]
- 24.Brown SL, Nesse RM, Vinokur AD, Smith DM. 2003. Providing social support may be more beneficial than receiving it: results from a prospective study of mortality. Psychol. Sci. 14, 320–327. ( 10.1111/1467-9280.14461) [DOI] [PubMed] [Google Scholar]
- 25.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. 2004. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science 303, 1162–1167. ( 10.1126/science.1093065) [DOI] [PubMed] [Google Scholar]
- 26.Schafer SM, Geuter S, Wager TD. 2018. Mechanisms of placebo analgesia: a dual-process model informed by insights from cross-species comparisons. Prog. Neurobiol. 160, 101–122. ( 10.1016/j.pneurobio.2017.10.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geuter S, Eippert F, Hindi Attar C, Buchel C. 2013. Cortical and subcortical responses to high and low effective placebo treatments. Neuroimage. 67, 227–236. ( 10.1016/j.neuroimage.2012.11.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiech K. 2016. Deconstructing the sensation of pain: the influence of cognitive processes on pain perception. Science 354, 584–587. ( 10.1126/science.aaf8934) [DOI] [PubMed] [Google Scholar]
- 29.Geuter S, Boll S, Eippert F, Buchel C. 2017. Functional dissociation of stimulus intensity encoding and predictive coding of pain in the insula. eLife. 6, e24770 ( 10.7554/eLife.24770) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. 2006. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 442, 1042–1045. ( 10.1038/nature05051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. 2011. Flexible cerebral connectivity patterns subserve contextual modulations of pain. Cereb. Cortex 21, 719–726. ( 10.1093/cercor/bhq146) [DOI] [PubMed] [Google Scholar]
- 32.Taylor KS, Seminowicz DA, Davis KD. 2009. Two systems of resting state connectivity between the insula and cingulate cortex. Hum. Brain Mapp. 30, 2731–2745. ( 10.1002/hbm.20705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falk E, Way B, Jasinska A. 2012. An imaging genetics approach to understanding social influence. Front. Hum. Neurosci. 6, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amodio DM. 2014. The neuroscience of prejudice and stereotyping. Nat. Rev. Neurosci. 15, 670 ( 10.1038/nrn3800) [DOI] [PubMed] [Google Scholar]
- 35.Chang LW, Krosch AR, Cikara M. 2016. Effects of intergroup threat on mind, brain, and behavior. Curr. Opin. Psychol. 11, 69–73. ( 10.1016/j.copsyc.2016.06.004) [DOI] [Google Scholar]
- 36.Kaplan JT, Freedman J, Iacoboni M. 2007. Us versus them: political attitudes and party affiliation influence neural response to faces of presidential candidates. Neuropsychologia. 45, 55–64. ( 10.1016/j.neuropsychologia.2006.04.024) [DOI] [PubMed] [Google Scholar]
- 37.Dayan P, Abbott L. 2003. Theoretical neuroscience: computational and mathematical modeling of neural systems. J. Cogn. Neurosci. 15, 154–155. ( 10.1162/089892903321107891) [DOI] [Google Scholar]
- 38.Selya AS, Rose JS, Dierker LC, Hedeker D, Mermelstein RJ. 2012. A practical guide to calculating Cohen's f2, a measure of local effect size, from PROC MIXED. Front. Psychol. 3, 111 ( 10.3389/fpsyg.2012.00111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen J. 1992. A power primer. Psychol. Bull. 112, 155 ( 10.1037/0033-2909.112.1.155) [DOI] [PubMed] [Google Scholar]
- 40.Kline RB. 2015. Principles and practice of structural equation modeling. New York, NY: The Guilford Press. [Google Scholar]
- 41.Geuter S, Qi G, Welsh RC, Wager TD, Lindquist MA. 2018. Effect size and power in fMRI group analysis. bioRxiv.
- 42.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 15, 273–289. ( 10.1006/nimg.2001.0978) [DOI] [PubMed] [Google Scholar]
- 43.Gershman SJ. 2015. Do learning rates adapt to the distribution of rewards? Psychon. Bull. Rev. 22, 1320–1327. ( 10.3758/s13423-014-0790-3) [DOI] [PubMed] [Google Scholar]
- 44.Brett M, Anton J-L, Valabregue R, Poline J-B. 2002. Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage. 16, S497. [Google Scholar]
- 45.Preacher KJ, Rucker DD, Hayes AF. 2007. Addressing moderated mediation hypotheses: theory, methods, and prescriptions. Multivariate Behav. Res. 42, 185–227. ( 10.1080/00273170701341316) [DOI] [PubMed] [Google Scholar]
- 46.Lockwood PL, Apps MA, Valton V, Viding E, Roiser JP. 2016. Neurocomputational mechanisms of prosocial learning and links to empathy. Proc. Natl Acad. Sci. USA 113, 9763–9768. ( 10.1073/pnas.1603198113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burke CJ, Tobler PN, Baddeley M, Schultz W. 2010. Neural mechanisms of observational learning. Proc. Natl Acad. Sci. USA 107, 14431–14436. ( 10.1073/pnas.1003111107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Apps MA, Lesage E, Ramnani N. 2015. Vicarious reinforcement learning signals when instructing others. J. Neurosci. 35, 2904–2913. ( 10.1523/JNEUROSCI.3669-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Molenberghs P, Bosworth R, Nott Z, Louis WR, Smith JR, Amiot CE, Vohs KD, Decety J. 2014. The influence of group membership and individual differences in psychopathy and perspective taking on neural responses when punishing and rewarding others. Hum. Brain Mapp. 35, 4989–4999. ( 10.1002/hbm.22527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruff CC, Fehr E. 2014. The neurobiology of rewards and values in social decision making. Nat. Rev. Neurosci. 15, 549 ( 10.1038/nrn3776) [DOI] [PubMed] [Google Scholar]
- 51.Misra G, Coombes SA. 2015. Neuroimaging evidence of motor control and pain processing in the human midcingulate cortex. Cereb. Cortex 25, 1906–1919. ( 10.1093/cercor/bhu001) [DOI] [PubMed] [Google Scholar]
- 52.Coombes SA, Wang WE, Roy A, Ho RLM. 2018. Neurophysiological evidence of the dynamic and adaptive pain-motor interaction. J. Physiol. 596, 2639–2640. ( 10.1113/JP276325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geng H, Wang Y, Gu R, Luo YJ, Xu P, Huang Y, Li X. 2018. Altered brain activation and connectivity during anticipation of uncertain threat in trait anxiety. Hum. Brain Mapp. 39, 3898–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ballotta D, Lui F, Porro CA, Nichelli PF, Benuzzi F. 2018. Modulation of neural circuits underlying temporal production by facial expressions of pain. PLoS ONE 13, e0193100 ( 10.1371/journal.pone.0193100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schaefer C, Coyne JC, Lazarus RS. 1981. The health-related functions of social support. J. Behav. Med. 4, 381–406. ( 10.1007/BF00846149) [DOI] [PubMed] [Google Scholar]
- 56.Barrera M. 1986. Distinctions between social support concepts, measures, and models. Am. J. Community Psychol. 14, 413–445. ( 10.1007/BF00922627) [DOI] [Google Scholar]
- 57.Hennessy MB, Kaiser S, Sachser N. 2009. Social buffering of the stress response: diversity, mechanisms, and functions. Front. Neuroendocrinol. 30, 470–482. ( 10.1016/j.yfrne.2009.06.001) [DOI] [PubMed] [Google Scholar]
- 58.Gorgolewski KJ, et al. 2015. NeuroVault.org: a web-based repository for collecting and sharing unthresholded statistical maps of the human brain Front. Neuroinform. 9, 8 ( 10.3389/fninf.2015.00008) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Behavioural data and scripts are available at github.com: https://github.com/AffectiveNeuroeconomics/Social_Analgesia
Imaging data are available at neurovault.org [58].
https://neurovault.org/collections/3496/; https://neurovault.org/collections/3499/; https://neurovault.org/collections/3500/.