Abstract
Extinction risk assessments of marine invertebrate species remain scarce, which hinders effective management of marine biodiversity in the face of anthropogenic impacts. To help close this information gap, in this paper we provide a metric of relative extinction risk that combines palaeontological data, in the form of extinction rates calculated from the fossil record, with two known correlates of risk in the modern day: geographical range size and realized thermal niche. We test the performance of this metric—Palaeontological Extinction Risk In Lineages (PERIL)—using survivorship analyses of Pliocene bivalve faunas from California and New Zealand, and then use it to identify present-day hotspots of extinction vulnerability for extant shallow-marine Bivalvia. Areas of the ocean where concentrations of bivalve species with higher PERIL scores overlap with high levels of climatic or anthropogenic stressors should be considered of most immediate concern for both conservation and management.
Keywords: extinction vulnerability, extinction rate, geographical range size, thermal niche, conservation, biodiversity
1. Background
Anthropogenic impacts, ranging from climate change to resource exploitation and urbanization, are evidently driving an increasing number of species to the brink of extinction [1–3]. Documented biodiversity losses attributable to human impacts have been much sparser in the oceans than on land but are likely to increase [4–7]. Managing marine biodiversity in the face of such pressures requires reliable estimates of extinction vulnerabilities of individual species and clades, information that is lacking for the vast majority of marine species [8,9]. Formal assessments of extinction vulnerability by the International Union for the Conservation of Nature (IUCN) are widely available for terrestrial vertebrates and are valuable for conservation science, business and policy [10–14]. However, only a minute fraction of invertebrate species (2%) have been evaluated by the IUCN [7,8,15]; as of 2014, only 13% of marine species (mostly vertebrates) have been assessed, of which 25% are Data Deficient (DD) [16]. Here, we address this information gap using a new metric of extinction vulnerability that integrates palaeontological data on the extinction history of lineages with the present-day geographical range sizes and realized thermal niches of individual species, factors which have repeatedly been shown to be associated with extinction risk [17–20]. We test the performance of this metric during a past extinction event using two widely separated and well-resolved Pliocene bivalve faunas, and then use it to assess the extinction vulnerabilities of 5681 shallow-marine living bivalve species globally, to identify vulnerabilities of extant species and geographical hotspots of potential extinction risk.
2. Material and methods
(a). Extinction metric
Extinction vulnerabilities of species and higher taxa are determined by a variety of ecological and life-history traits, many of which can be species/clade specific [17,18,21,22]. Identifying trait combinations that are reliable predictors of extinction vulnerability for a particular group is a prerequisite for developing effective metrics of extinction risk. One approach for identifying such traits is to analyse information from past extinctions. Recent studies have used a logistic regression approach using fossil datasets to identify traits that are the best correlates of extinction risk in past extinctions and used those to develop predictions of future vulnerability [9]. While this can be a powerful method, it does rely on a central assumption—that relative contributions of each parameter to extinction risk from anthropogenic impacts are the same as those during past extinctions. Yet, it is increasingly evident that many of the impacts faced by species today [23] differ strongly from those in the geological past [1]. For example, size-selective harvesting is recognized as having a negative impact on many marine fish and invertebrates [24,25], but such human predation has no natural analogue [26,27]. The ability of parameters chosen solely using past extinctions to predict future anthropogenic losses remains unclear. Accordingly, we formulated a vulnerability metric using parameters known to be reliable correlates of vulnerability in the current extinction crisis. Following the same reasoning, we chose to assign equal weights to each parameter in our metric, rather than using a weighting scheme using past extinctions [9]. This approach is the most parsimonious where the relative contributions of factors are unknown. However, our metric could be easily adapted to incorporate a weighting scheme if desired for future analyses (see discussion in the electronic supplementary material).
The vulnerability metric used here, termed PERIL (Palaeontological Extinction Risk In Lineages), is a measure of relative risk that can be stated as 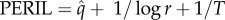 , where
, where 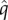 is the extinction rate of genera within a clade (here, a taxonomic family), r is the geographical range occupied by the species (km2) and T is the range of sea-surface temperatures (SST) occupied by the species (°C).
is the extinction rate of genera within a clade (here, a taxonomic family), r is the geographical range occupied by the species (km2) and T is the range of sea-surface temperatures (SST) occupied by the species (°C).
Extinction rates are calculated from the fossil record of genus durations within families (electronic supplementary material). Such rates are phylogenetically conserved in marine bivalves [28], specifically at the family level, as used here and other groups, such as mammals and birds [21], angiosperms [29] and ammonites [30], and thus should be relevant for assessing species-level vulnerabilities; we view this as a measure of clade volatility, and thus sensitivity to stresses in general, rather than to any specific pressure. Geographical ranges are represented by the area of the convex hull of species occurrences. A large geographical range increases the probability of surviving a perturbation, providing that the reach of that event is smaller than the range of the species (electronic supplementary material). Realized thermal niches of species are the range of satellite-derived SST (data from MARSPEC [31] encountered by the convex hull of the species). Thermal niches are likely to play a significant role in mediating species' responses to anthropogenic warming [19,20] (electronic supplementary material), and so we include them in our vulnerability metric. We treat geographical distributions and realized thermal ranges as separate parameters because recent studies show that geographical range size can be a poor predictor of the thermal niches of marine species, most notably in the tropics [32]. Unfortunately, direct measurements of physiological tolerances are available for only a handful of marine bivalve species, and estimates of realized thermal niches of species can only approximate their true thermal tolerances, but these estimates are likely to be conservative, especially for widely distributed species [20].
All three parameters are range-scaled between 0 and 1 to equalize their contribution to the final score. Geographical range is logged because the distribution is heavily skewed, and geographical range and thermal niche are inverted so that large raw values correspond to low scores, because larger ranges and wider thermal tolerances are known to provide resilience to extinction. Parameters are summed and the result is also range-scaled between 0 and 1 as a final step, so that PERIL scores of 0 indicate the least and 1 the most risk among the analysed set of species. The parameters are thus equally weighted in our formulation.
(b). Data
We use the PERIL metric to evaluate relative vulnerabilities of extant marine bivalve species (n = 5681), from the intertidal zone to the edge of the continental shelf (taken as 200 m), using a global distributional database of shallow-water marine bivalves [33]. Bivalves are a highly biodiverse group which provide important ecosystem services and support key fisheries. Species distributions of marine bivalves across the world's oceans are well documented [34,35], and the group has an excellent fossil record, which allows for robust estimates of the present-day and palaeontological parameters of the PERIL metric.
(c). Test of metric in the fossil record
Because PERIL cannot be tested using living species, we used two well-documented regional extinctions during the Pliocene—California [36] and New Zealand (NZ) (using the late Pliocene (Mangapanian) local stage) fauna [37]—instead. These faunas have relatively high levels of species extinctions (table 1) and well-resolved taxonomies including many genera with extant representatives (electronic supplementary material), making them ideal for testing the effectiveness of PERIL. The Pliocene faunas are on opposite sides of the Pacific Ocean, with just one species in common (Hiatella arctica) = 0.2% of the combined total (n = 444), and so can be treated as independent tests. (We calculate a separate regional convex hull for H. arctica in each region, to preserve independence of the faunas.) We chose to use well-resolved regional faunas rather than an aggregation of global records because of concerns regarding the taxonomic standardization and spatial coverage of publicly available global Pliocene data [38]. Comparison of our datasets to corresponding downloads of Pliocene data from the Paleobiology Database (PBDB) [39] confirms this: after taxonomic standardization, the PBDB records 108 (43%) of the 254 bivalve species in the Pliocene California dataset used here and contains no Late Pliocene locality records at all from NZ; there are only 24 Pliocene NZ bivalve species in the PBDB, of which five range into our Late Pliocene dataset of 191 species. We computed the PERIL scores for each species in each of the Pliocene assemblages (see electronic supplementary material for methods) and then used generalized linear models to conduct a survivorship analysis for each parameter alone, and the complete PERIL metric, using ΔAICc to compare the performance of these models (table 1 and figure 1).
Table 1.
Number of species, median range size (in number of bins), mean-scaled extinction rate and mean PERIL score for the extinct and extant components of the Pliocene-Recent datasets.
| n | median range size (km2) | mean-scaled extinction rate | mean thermal niche (range in °C) | mean PERIL | |
|---|---|---|---|---|---|
| California extant | 152 | 122 932.852 | 0.37 | 5.5 | 0.48 |
| California extinct | 102 | 40 490.069 | 0.61 | 4.8 | 0.64 |
| NZ extant | 80 | 11 547.402 | 0.36 | 5.39 | 0.52 |
| NZ extinct | 111 | 1227.353 | 0.49 | 5.45 | 0.63 |
Figure 1.
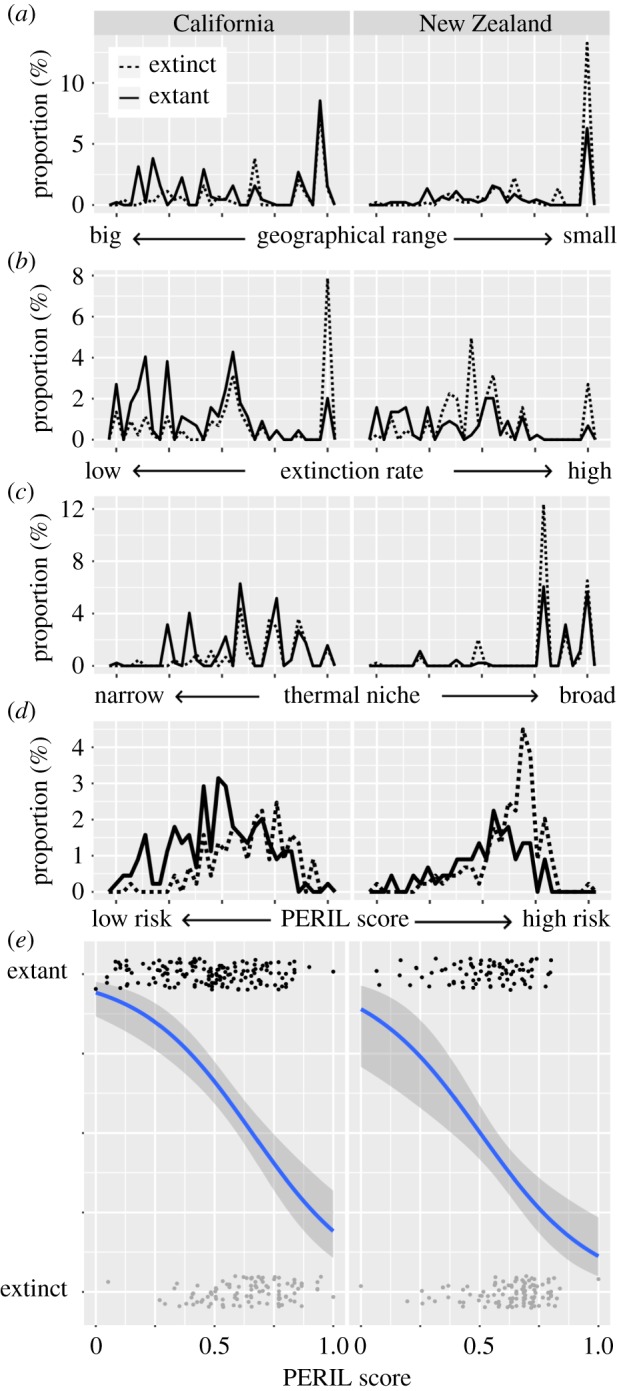
Post-Pliocene extinctions for two regional bivalve faunas. Dashed line indicates extinct species and solid line extant species. (a) Scaled geographical range of species. (b) Scaled extinction rate for genera in families. (c) Resulting PERIL score. (d) Survivorship for each fauna. (e) Black points are species that survived to present day, and grey points are extinct species. All panels share the same x-axis scale (from 0 to 1). (Online version in colour.)
(d). Palaeontological extinction risk in lineages' hotspots in present-day oceans
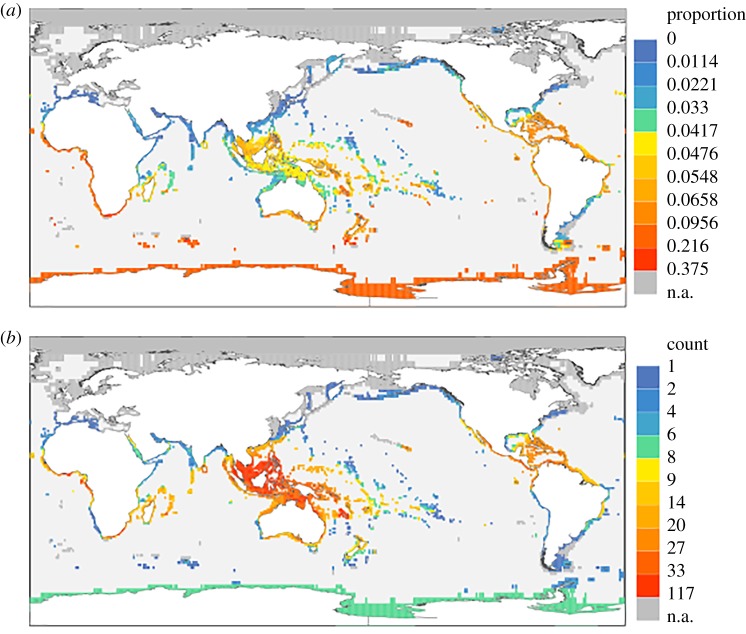
To identify global hotspots of PERIL, we use an approach analogous to the Red List and map distributions of at-risk taxa in the global oceans. Unlike the Red List, PERIL scores are not inherently categorical, so we have chosen the 80th percentile as a ‘high’ risk threshold, and mapped proportions of species in each grid-cell that score over that threshold (figure 2a). For comparison, we also map raw counts of species over the 80th percentile threshold in each grid-cell (figure 2b), with robustness of findings under alternative thresholds documented in electronic supplementary material, figure S3.
Figure 2.
Hotspots of extinction risk in marine bivalves as identified by the PERIL metric. (a) Proportion of species in grid-cell scoring over the 80th percentile. (b) Raw number of species in grid-cell scoring over the 80th percentile. Breaks are quantiles. The tropical west Pacific has the greatest number of species at risk (as in (b)), but they constitute a smaller proportion of the fauna than the at-risk species in the much more depauperate Antarctic fauna (as in (a)).
3. Results and discussion
(a). Test of metric in the fossil record
In both fossil faunas, the model incorporating the PERIL score predicts the likelihood of extinction better than any parameter alone (table 2). The consistency of these results between two faunas with different stratigraphic frameworks and a single shared species validates PERIL as an effective index of extinction risk. The performance of the PERIL model also suggests that equal weighting of parameters can validly predict past extinctions.
Table 2.
ΔAICc scores for competing models in Pliocene-Recent survivorship analyses. Geographical range is a better predictor than the extinction rate, but the addition of extinction rate in the computation of PERIL results in higher [40] model support in all regions.
| ΔAICc |
null deviance |
residual deviance |
|||||
|---|---|---|---|---|---|---|---|
| d.f. | California | NZ | California | NZ | California | NZ | |
| intercept-only (null model) | 1 | 54.3 | 31.9 | 61.04 | 46.49 | 61.04 | 46.49 |
| geographical range | 2 | 29.3 | 6.8 | 342.2 | 259.7 | 333.6 | 247.1 |
| extinction rate | 2 | 2.9 | 6.5 | 342.2 | 259.7 | 307.3 | 246.8 |
| thermal niche | 2 | 27.8 | 19.1 | 342.2 | 259.7 | 332.1 | 259.4 |
| PERIL | 2 | 0 | 0 | 342.2 | 259.7 | 292.7 | 221.5 |
(b). Palaeontological extinction risk in lineages' hotspots in present-day oceans
Although the most biodiverse regions of the ocean can have large numbers of at-risk bivalve species in absolute terms, our results show that they do not necessarily harbour proportionately more at-risk species than areas with lower species richness (figure 2a). For example, the tropical Indo-West Pacific (IWP) region harbours proportionately fewer at-risk bivalve species per cell than NZ. This is because a significant fraction of the IWP species have broad geographical ranges, and although these ranges are primarily achieved by tracking isotherms within the tropics [32,33], many of these species also extend into neighbouring temperate zones [41], and thus have larger realized thermal niches overall than species restricted to smaller provinces. The high proportion of at-risk species in NZ reflects the relatively small amount of shelf habitat and high levels of endemism [42], resulting in both small geographical ranges and narrow realized thermal niches. This decoupling of species richness and extinction risk in bivalves differs from that observed for reef corals, where ecoregions with the highest species richness also contain the highest proportion of threatened species [43]. Our maps qualitatively resemble maps of risk in other clades produced from available IUCN data (e.g. www.biodiversitymapping.org), suggesting congruence between our approach to risk assessment and that of the Red List.
Our results also reveal a striking contrast between the two poles—the Southern Ocean harbours proportionately more species with high PERIL scores than does the Arctic (figure 2a). This reflects the fact that many Arctic taxa range outside the polar region and thus have larger realized thermal niches than taxa in Antarctica, which can often have large circumantarctic geographical ranges but small realized thermal niches reflecting the narrow temperature range in the polar Southern Ocean (electronic supplementary material, figure S4).
A previous study [9] identified risk hotspots for marine bivalves using grid-cell means of their intrinsic risk measure. Their study highlights, for bivalves, NZ, NE Australia to Fiji, Sumatra and the Caribbean, with the rest of the IWP, Madagascar and the eastern coast of Africa also being relatively high risk. PERIL finds high risk in the Caribbean and NZ regions using a proportion of species over the 80th percentile, but also finds very high risk in Antarctica, Hawaii, the tropical E Pacific and the West coast of Africa (figure 2a), which are not identified by Finnegan et al. [9]. Differences are not surprising given the differences in analytical approach and underlying data. Moreover, Finnegan et al. focused on extinction risk for genera, whereas our results are at the species level, which can produce different spatial trends. What is striking is that, despite these differences in methodology and data, two of the strongest hotspots in terms of proportion of the biota at risk—the Caribbean and NZ—are the same in both studies. This strongly suggests that bivalve taxa in these regions may be particularly vulnerable to anthropogenic threats.
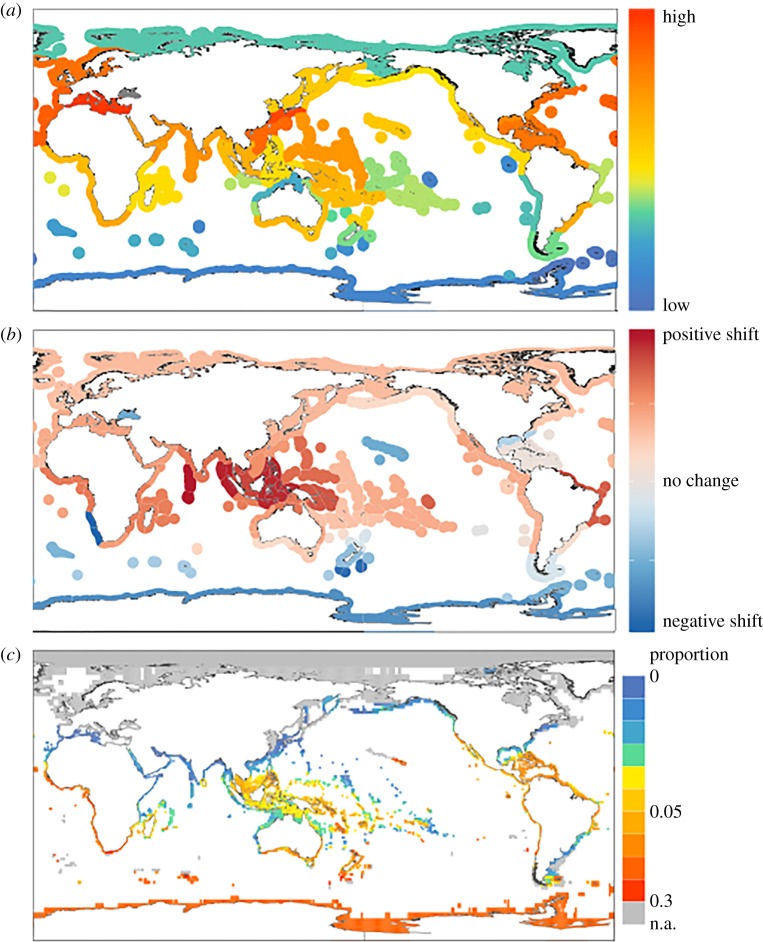
The PERIL metric allows us to rank extinction risks, but modelling future vulnerabilities of species also requires information about the magnitude of threats they are likely to encounter. We investigated potential overlaps of PERIL and threat hotspots by comparing the proportion of high PERIL score species in marine provinces (sensu Spalding et al. [44]) with the human impact scores of Halpern et al. [45–47] and climate change velocities calculated for the same provinces [19] (figure 3). Multiple marine provinces show overlap (figure 3), e.g. those within the Central Indo-Pacific, Temperate Australasia (including NZ) and Tropical Northwest Atlantic (including the Caribbean) realms. Similar hotspots of biodiversity and risk around the Indo-Pacific, Australasia and the Caribbean have been identified for vertebrates [48], and for corals, gastropods and lobsters [49]. Some of these areas are tropical, harbouring high species richness, while others are in temperate waters with lower species richness but distinctive bivalve assemblages. However, the lack of such overlap, by itself, does not mean that other PERIL hotspots are of less concern, especially given that the two metrics of impact used here do not cover all aspects of global change. A case in point is the high-latitude Southern Ocean. This region harbours a substantial concentration of bivalve species with high PERIL scores (figure 2a) and its aragonite saturation state is expected to decrease substantially by mid-century owing to anthropogenic ocean acidification [50]. Such a decrease could negatively affect Antarctic bivalve species [51] and other calcifying organisms.
Figure 3.
Comparison of hotspots of human impact, projected velocity of climate change and PERIL scores. (a) Mean human impact scores (Halpern scores, see [9] and SOM) in Spalding et al. [44] provinces. (b) Mean climate velocity (Burrows scores, see [9] and electronic supplementary material) in Spalding provinces and (c) PERIL scores: proportion of taxa in grid-cells scoring higher than the 80th percentile (as in figure 2(a)).
The PERIL index is a relative metric and is calculated very differently from the absolute vulnerability metric of the Red List and results in a continuous metric as opposed to categorical assignments. Nevertheless, the expectation is that species with high relative vulnerability scores should, in general, also score high on an absolute vulnerability scale. Testing the correspondence between PERIL and IUCN categories is difficult because the threat status of so few marine bivalve species has been assessed by the IUCN. Only 10% of molluscs (the majority of them from freshwater) have been assessed, with a total of 29 species of marine bivalves, of which 15 are classed as DD [15]. This sample size is not sufficient for strong statistical conclusions, but a Spearman rank-order correlation test shows a weakly positive relationship between PERIL and Red List categories if DD species are excluded (n = 14, ρ = 0.25, p = 0.38) and a moderately positive relationship if DD species are included and ranked between LR and VU as displayed in IUCN publications (n = 29, ρ = 0.4, p = 0.02). On the balance of available evidence, the IUCN designations and the PERIL index are largely concordant.
We do not have a full inventory of all living bivalve species, and the same is true for many other clades. Discovery of new species might affect the results reported here in terms of spatial patterns of proportional risk to faunas, but the raw score of a species is not affected by the presence or absence of another species. Additions or subtractions of species will require the rescaling of the dataset, but the impact of new species discoveries on the mapping of risk hotspots will depend on existing knowledge of the clade, and the number and location of new discoveries. For bivalves, a well-studied clade, a recent quantitative model of species discovery [52] shows that undescribed species are unlikely to alter large-scale present-day diversity patterns and represent only small proportions of regional faunas, suggesting that large-scale spatial patterns of PERIL should be relatively robust to sampling. Just as Red List assessments are periodically revisited, PERIL scores for clades should be regularly updated in light of new taxonomic work. Shifts in geographical distributions of marine species in response to anthropogenic transport and climate change have already been documented [53] and are predicted to intensify in the future [20]. Such changes will, of course, also affect PERIL scores—introduced species established on a second coastline, or expanding along a single coastline as they track warming temperatures, should be less vulnerable to global extinction than those remaining in place; species whose ranges contract owing to climate change, pollution, overfishing, anoxia and other factors [45–47] will become more vulnerable. The dynamic nature of the PERIL metric can be used to monitor how relative vulnerabilities of species change as anthropogenic effects intensify.
How anthropogenic extinctions will impact trait and functional diversity of marine species also remains poorly known; the decline in the functional diversity of marine bivalves with the latitudinal decline in taxonomic diversity is a poor predictor of extinction effects [54]. Body size has featured prominently in analyses of extinctions, both in the geological past and in the present day [13,18,55–57], and a recent study [58] suggested that future extinctions of marine invertebrates are likely to be size-selective, where large-bodied species are often disproportionately at risk. For marine bivalves, however, we find the opposite: PERIL is weakly negatively correlated with body size (Pearson's product–moment correlation = −0.13, p = <2.2 × 10−6) and overall, bivalves above the 80th percentile PERIL score threshold are significantly smaller than those below it (two-sample Kolmogorov–Smirnov test, p = <2.2 × 10−6; electronic supplementary material, figure S5), and when families are tested independently, where there is a significant difference it is the smaller species that are more at risk (electronic supplementary material, table S2 and figure S5). Our results most likely differ from those of Payne et al. [58] because that study included only three fully marine genera or subgenera of Bivalvia with IUCN risk assessments (out of a total of 2561 molluscan genera).
Our comprehensive analysis of marine bivalves suggests that anthropogenic extinctions are likely to preferentially affect small-bodied species of marine bivalves, a pattern opposite to that seen for terrestrial vertebrates [59], which are often put disproportionately at risk at larger body sizes [60]. A subset of large-bodied bivalve taxa are exploited commercially, but these and many other large species tend to be sufficiently widespread to avoid the potentially bimodal size-distribution of extinction risk recently reported in vertebrates [61]. The pattern for bivalves observed here, given the majority of wild bivalve species are not exploited commercially, makes sense in terms of the positive relationship of body size with geographical range size, fecundity and other factors [24,62,63].
4. Conclusion
A major obstacle to conservation and management policy-making efforts globally is the patchy availability of extinction risk estimates for living taxa, particularly marine species. The PERIL metric provides relative estimates of vulnerability for species that are otherwise difficult to evaluate, and the inclusion of data on prior extinction rates within lineages adds an under-used dimension to risk assessment. By integrating extinction histories from the fossil record with present-day biodistributional data, PERIL scores for regions or clades can usefully be mapped alongside stressors, to pinpoint situations of most immediate concern for conservation and management.
Supplementary Material
Acknowledgements
We thank Sarah Berke for her initial work on this study and Roma Klapaukh for his help with the R code for calculating extinction rate. We acknowledge the use of data from the New Zealand Fossil Record Electronic Database (www.fred.org.nz). We are grateful to the many malacologists who kindly provided taxonomic advice, assistance and/or access to collections in their care, and to the members of the joint Price-Jablonski lab for comments. Moriaki Yasuhara and two anonymous reviewers provided feedback that much improved the manuscript.
Data accessibility
Data files are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.n8f2q8g [64]. Dataset S1: [file: ‘PERIL_rawdata.csv’] Modern Bivalvia—5681 currently valid species with range sizes (raw convex hulls and convex hulls clipped to water depths of 200 m (i.e. the generalized shelf-slope break), realized thermal niches and extinction rates, downloaded on 29-6-2018. Dataset S2: [file: ‘paleoPERILscores.csv’] Pliocene Bivalvia from California [36] and NZ [37]; a total of 444 species from two faunas, with range sizes, temperature ranges, extinction rates, PERIL scores and taxonomic notes. R code [files: PERIL_metric.R, paleoPERIL_metric.R] is also provided for calculation of PERIL scores and reproduction of non-map figures.
Authors' contributions
D.J., K.R. and G.H. conceived the study and all authors participated in study design. K.S.C., S.M.E. and G.H. carried out analyses and K.S.C. drafted the manuscript with substantial input from all other authors.
Competing interests
We declare we have no competing interests.
Funding
We thank the National Aeronautics and Space Administration (EXOB08-0089) and the National Science Foundation (NSF) Sedimentary Geology and Paleobiology Program (EAR-0922156) (to D.J.) and the NSF Graduate Research Fellowship Program (to S.M.E.) and the NSF Doctoral Dissertation Improvement Grant Program (DEB-1501880) (to S.M.E.) for support.
References
- 1.Wake DB, Vredenburg VT. 2008. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl Acad. Sci. USA 105 (Suppl 1), 11 466–11 473. ( 10.1073/pnas.0801921105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnosky AD, et al. 2011. Has the Earth's sixth mass extinction already arrived? Nature 471, 51–57. ( 10.1038/nature09678) [DOI] [PubMed] [Google Scholar]
- 3.Ceballos G, Ehrlich PR, Dirzo R. 2017. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl Acad. Sci. USA 114, e6089–e6096. ( 10.1073/pnas.1704949114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dulvy NK, Sadovy Y, Reynolds JD. 2003. Extinction vulnerability in marine populations. Fish. Fish. 4, 25–64. ( 10.1046/j.1467-2979.2003.00105.x) [DOI] [Google Scholar]
- 5.Briggs JC. 2011. Marine extinctions and conservation. Mar. Biol. 158, 485–488. ( 10.1007/s00227-010-1596-0) [DOI] [Google Scholar]
- 6.Webb TJ, Mindel BL. 2015. Global patterns of extinction risk in marine and non-marine systems. Curr. Biol. 25, 506–511. ( 10.1016/j.cub.2014.12.023) [DOI] [PubMed] [Google Scholar]
- 7.McCauley DJ, Pinsky ML, Palumbi SR, Estes JA, Joyce FH, Warner RR. 2015. Marine defaunation: animal loss in the global ocean. Science 347, 1255641 ( 10.1126/science.1255641) [DOI] [PubMed] [Google Scholar]
- 8.Harnik PG, et al. 2012. Extinctions in ancient and modern seas. Trends Ecol. Evol. 27, 608–617. ( 10.1016/j.tree.2012.07.010) [DOI] [PubMed] [Google Scholar]
- 9.Finnegan S, et al. 2015. Paleontological baselines for evaluating extinction risk in the modern oceans. Science 348, 567–570. ( 10.1126/science.aaa6635) [DOI] [PubMed] [Google Scholar]
- 10.Rödder D, et al. 2009. Global amphibian extinction risk assessment for the panzootic chytrid fungus. Diversity 1, 52–66. ( 10.3390/d1010052) [DOI] [Google Scholar]
- 11.Lee TM, Jetz W. 2011. Unravelling the structure of species extinction risk for predictive conservation science. Proc. R. Soc. B 278, 1329–1338. ( 10.1098/rspb.2010.1877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keith DA, et al. 2014. Detecting extinction risk from climate change by IUCN Red List criteria. Conserv. Biol. 28, 810–819. ( 10.1111/cobi.12234) [DOI] [PubMed] [Google Scholar]
- 13.Plotnick RE, Smith FA, Lyons SK. 2016. The fossil record of the sixth extinction. Ecol. Lett. 19, 546–553. ( 10.1111/ele.12589) [DOI] [PubMed] [Google Scholar]
- 14.Bennun L, et al. 2018. The value of the IUCN Red List for business decision-making. Conserv. Lett. 11, e12353 ( 10.1111/conl.12353) [DOI] [Google Scholar]
- 15.International Union for the Conservation of Nature. 2018. See http://www.iucnredlist.org/ (accessed 5 June 2018).
- 16.Global Marine Species Assessment. 2018. See https://sites.wp.odu.edu/GMSA/ (accessed 26 July 2018).
- 17.McKinney ML. 1997. Extinction vulnerability and selectivity: combining ecological and paleontological views. Annu. Rev. Ecol. Syst. 28, 495–516. ( 10.1146/annurev.ecolsys.28.1.495) [DOI] [Google Scholar]
- 18.O'Grady JJ, Reed DH, Brook BW, Frankham R. 2004. What are the best correlates of predicted extinction risk? Biol. Conserv. 118, 513–520. ( 10.1016/j.biocon.2003.10.002) [DOI] [Google Scholar]
- 19.Burrows MT, et al. 2011. The pace of shifting climate in marine and terrestrial ecosystems. Science 334, 652–655. ( 10.1126/science.1210288) [DOI] [PubMed] [Google Scholar]
- 20.Molinos JG, et al. 2015. Climate velocity and the future global redistribution of marine biodiversity. Nat. Clim. Change 6, 83–90. [Google Scholar]
- 21.Purvis A, Agapow PM, Gittleman JL, Mace GM. 2000. Nonrandom extinction and the loss of evolutionary history. Science 288, 328–330. ( 10.1126/science.288.5464.328) [DOI] [PubMed] [Google Scholar]
- 22.Jones KE, Purvis A, Gittleman JL. 2003. Biological correlates of extinction risk in bats. Am. Nat. 161, 601–614. ( 10.1086/368289) [DOI] [PubMed] [Google Scholar]
- 23.Young HS, McCauley DJ, Galetti M, Dirzo R. 2016. Patterns, causes, and consequences of anthropocene defaunation. Ann. Rev. Ecol. Evol. Syst. 47, 333–358. ( 10.1146/annurev-ecolsys-112414-054142) [DOI] [Google Scholar]
- 24.Fenberg PB, Roy K. 2008. Ecological and evolutionary consequences of size-selective harvesting: how much do we know? Mol. Ecol. 17, 209–220. ( 10.1111/j.1365-294X.2007.03522.x) [DOI] [PubMed] [Google Scholar]
- 25.Darimont CT, Carlson SM, Kinnison MT, Paquet PC, Reimchen TE, Wilmers CC. 2009. Human predators outpace other agents of trait change in the wild. Proc. Natl Acad. Sci. USA 106, 952–954. ( 10.1073/pnas.0809235106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vermeij GJ. 2012. The limits of adaptation: humans and the predator–prey arms race. Evolution 66, 2007–2014. ( 10.1111/j.1558-5646.2012.01592.x) [DOI] [PubMed] [Google Scholar]
- 27.Darimont CT, Fox CH, Bryan HM, Reimchen TE. 2015. The unique ecology of human predators. Science 349, 858–860. ( 10.1126/science.aac4249) [DOI] [PubMed] [Google Scholar]
- 28.Roy K, Hunt G, Jablonski D. 2009. Phylogenetic conservatism of extinctions in marine bivalves. Science 325, 733–737. ( 10.1126/science.1173073) [DOI] [PubMed] [Google Scholar]
- 29.Vamosi JC, Wilson JRU. 2008. Nonrandom extinction leads to elevated loss of angiosperm evolutionary history. Ecol. Lett. 11, 1047–1053. ( 10.1111/j.1461-0248.2008.01215.x) [DOI] [PubMed] [Google Scholar]
- 30.Hardy C, Fara E, Laffont R, Dommergues J-L, Meister C, Neige P, Laudet V. 2012. Deep-time phylogenetic clustering of extinctions in an evolutionarily dynamic clade (Early Jurassic Ammonites). PLoS ONE 7, e37977 ( 10.1371/journal.pone.0037977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sbrocco EJ, Barber PH, 2013. MARSPEC: ocean climate layers for marine spatial ecology: ecological Archives E094-086. Ecology 94, 979–979. ( 10.1890/12-1358.1) [DOI] [Google Scholar]
- 32.Tomašových A, Jablonski D, Berke SK, Krug AZ, Valentine JW. 2015. Nonlinear thermal gradients shape broad-scale patterns in geographic range size and can reverse Rapoport's rule. Global Ecol. Biogeogr. 24, 157–167. ( 10.1111/geb.12242) [DOI] [Google Scholar]
- 33.Jablonski D, et al. 2013. Out of the tropics, but how? Fossils, bridge species, and thermal ranges in the dynamics of the marine latitudinal diversity gradient. Proc. Natl Acad. Sci. USA 110, 10 487–10 494. ( 10.1073/pnas.1308997110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belanger CL, Jablonski D, Roy K, Berke SK, Krug AZ, Valentine JW. 2012. Global environmental predictors of benthic marine biogeographic structure. Proc. Natl Acad. Sci. USA 109, 14 046–14 051. ( 10.1073/pnas.1212381109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomašových A, et al. 2016. Unifying latitudinal gradients in range size and richness across marine and terrestrial systems. Proc. R. Soc. B 283, 20153027 ( 10.1098/rspb.2015.3027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall CA. 2002. Nearshore marine paleoclimate regions, increasing zoogeographic provinciality, molluscan extinctions, and paleoshorelines, California: Late Oligocene (27 Ma) to Late Pliocene (2.5 Ma) Geol. Soc. Am. Spec. 357, 1–489. [Google Scholar]
- 37.New Zealand Fossil Record Electronic Database. 2018. www.fred.org.nz (accessed 7 June 2018).
- 38.Valentine JW, Jablonski D, Krug AZ, Berke SK. 2013. The sampling and estimation of marine paleodiversity patterns: implications of a Pliocene model. Paleobiology 39, 1–20. ( 10.1666/0094-8373-39.1.1) [DOI] [Google Scholar]
- 39.Paleobiology Database. 2018. See https://paleobiodb.org (accessed 4 July 2018).
- 40.Burnham KP, Anderson DR, Huyvaert KP. 2011. AIC model selection and multimodal inference in behavioural ecology: some background, observations, and comparisons. Behav. Ecol. Sociobiol. 65, 23–35. ( 10.1007/s00265-010-1029-6) [DOI] [Google Scholar]
- 41.Jablonski D, Huang S, Roy K, Valentine JW. 2017. Shaping the latitudinal diversity gradient: new perspectives from a synthesis of paleobiology and biogeography. Am. Nat. 189, 1–12. ( 10.1086/689739) [DOI] [PubMed] [Google Scholar]
- 42.Spencer HG, et al. 2009. Phylum Mollusca. In New Zealand inventory of biodiversity: volume 1. Kingdom Animalia: Radiata, Lophotrochozoa, Deuterostomia (ed. Gordon DP.), pp. 161–254. Christchurch, New Zealand: Canterbury University Press. [Google Scholar]
- 43.Huang D, Roy K. 2015. The future of evolutionary diversity in reef corals. Phil. Trans. R. Soc. B 370, 20140010 ( 10.1098/rstb.2014.0010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spalding MD, et al. 2007. Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. BioScience 57, 573–583. ( 10.1641/B570707) [DOI] [Google Scholar]
- 45.Halpern BS, et al. 2008. A global map of human impact on marine ecosystems. Nature 319, 948–952. ( 10.1126/science.1149345) [DOI] [PubMed] [Google Scholar]
- 46.Halpern BS, et al. 2012. An index to assess the health and benefits of the global ocean. Nature 488, 615–620. ( 10.1038/nature11397) [DOI] [PubMed] [Google Scholar]
- 47.Halpern BS, et al. 2015. Spatial and temporal changes in cumulative human impacts on the world's ocean. Nat. Commun. 6, 7615 ( 10.1038/ncomms8615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramírez F, Afán I, Davis LS, Chiaradia A. 2017. Climate impacts on global hot spots of marine biodiversity. Sci. Adv. 3, e1601198 ( 10.1126/sciadv.1601198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts CM, et al. 2002. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 295, 1280–1284. ( 10.1126/science.1067728) [DOI] [PubMed] [Google Scholar]
- 50.McNeil BI, Matear RJ. 2008. Southern Ocean acidification: a tipping point at 450-ppm atmospheric CO2. Proc. Natl Acad. Sci. USA 105, 18 860–18 864. ( 10.1073/pnas.0806318105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cummings V, et al. 2011. Ocean acidification at high latitudes: potential effects on functioning of the Antarctic bivalve Laternula elliptica. PLoS ONE 6, e16069 ( 10.1371/journal.pone.0016069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edie SM, Smits PD, Jablonski D. 2017. Probabilistic models of species discovery and biodiversity comparisons. Proc. Natl Acad. Sci. USA 114, 3666–3671. ( 10.1073/pnas.1616355114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poloczanska ES, et al. 2016. Responses of marine organisms to climate change across oceans. Front. Mar. Sci. 3, 62 ( 10.3389/fmars.2016.00062) [DOI] [Google Scholar]
- 54.Edie SM, Jablonski D, Valentine JW. 2018. Contrasting responses of functional diversity to major losses in taxonomic diversity. Proc. Natl Acad. Sci. USA 115, 732.–. ( 10.1073/pnas.1717636115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Owens IPF, Bennett PM. 2000. Ecological basis of extinction risk in birds: habitat loss versus human persecution and introduced predators. Proc. Natl Acad. Sci. USA 97, 12 144–12 148. ( 10.1073/pnas.200223397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cardillo M, Bromham L. 2001. Body size and risk of extinction in Australian mammals. Conserv. Biol. 15, 1435–1440. ( 10.1046/j.1523-1739.2001.00286.x) [DOI] [Google Scholar]
- 57.Cardillo M, et al. 2005. Multiple causes of high extinction risk in large mammal species. Science 309, 1239–1241. ( 10.1126/science.1116030) [DOI] [PubMed] [Google Scholar]
- 58.Payne JL, Bush AM, Heim NA, Knope ML, McCauley DJ. 2016. Ecological selectivity of the emerging mass extinction in the oceans. Science 353, 1284–1286. ( 10.1126/science.aaf2416) [DOI] [PubMed] [Google Scholar]
- 59.Fritz SA, Purvis A. 2010. Selectivity in mammalian extinction risk and threat types: a new measure of phylogenetic signal strength in binary traits. Conserv. Biol. 24, 1042–1051. ( 10.1111/j.1523-1739.2010.01455.x) [DOI] [PubMed] [Google Scholar]
- 60.Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJB, Collen B. 2014. Defaunation in the Anthropocene. Science 345, 401–406. ( 10.1126/science.1251817) [DOI] [PubMed] [Google Scholar]
- 61.Ripple WJ, Wolf C, Newsome TM, Hoffmann M, Wirsing AJ, McCauley DJ. 2017. Extinction risk is most acute for the world's largest and smallest vertebrates. Proc. Natl Acad. Sci. USA 114, 10 678–10 683. ( 10.1073/pnas.1702078114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jablonski D, Roy K, Valentine JW. 2003. Evolutionary macroecology and the fossil record? In Macroecology: concepts and consequences (eds Blackburn TM, Gaston KJ), pp. 368–390. Oxford, UK: Blackwell Science. [Google Scholar]
- 63.Harnik PG, Fitzgerald PC, Payne JL, Carlson SJ. 2014. Phylogenetic signal in extinction selectivity in Devonian terebratulide brachiopods. Paleobiology 40, 675–692. ( 10.1666/14006) [DOI] [Google Scholar]
- 64.Collins KS, Edie SM, Hunt G, Roy K, Jablonski D. 2018. Data from: Extinction risk in extant marine species integrating paleontological and biodistributional data Dryad Digital Repository. ( 10.5061/dryad.n8f2q8g) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- New Zealand Fossil Record Electronic Database. 2018. www.fred.org.nz (accessed 7 June 2018).
- Collins KS, Edie SM, Hunt G, Roy K, Jablonski D. 2018. Data from: Extinction risk in extant marine species integrating paleontological and biodistributional data Dryad Digital Repository. ( 10.5061/dryad.n8f2q8g) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data files are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.n8f2q8g [64]. Dataset S1: [file: ‘PERIL_rawdata.csv’] Modern Bivalvia—5681 currently valid species with range sizes (raw convex hulls and convex hulls clipped to water depths of 200 m (i.e. the generalized shelf-slope break), realized thermal niches and extinction rates, downloaded on 29-6-2018. Dataset S2: [file: ‘paleoPERILscores.csv’] Pliocene Bivalvia from California [36] and NZ [37]; a total of 444 species from two faunas, with range sizes, temperature ranges, extinction rates, PERIL scores and taxonomic notes. R code [files: PERIL_metric.R, paleoPERIL_metric.R] is also provided for calculation of PERIL scores and reproduction of non-map figures.