Abstract
Background/Aims
Chronic kidney disease is a pro-inflammatory condition where the interplay between different regulatory pathways and immune cells mediates an unfavorable remodeling of the vascular wall and myocardial hypertrophy. These mechanisms include the action of CXCL12. The aim of this study is to evaluate the association between serum CXCL12 with left ventricular hypertrophy (LVH) and blood pressure control in chronic kidney disease (CKD) patients.
Methods
This single-center observational study involved 90 stable CKD stage 1–5 patients (including 33 renal transplant recipients) and 25 healthy age- and sex-matched control subjects. CXCL12 was quantified by ELISA. 24-h ambulatory blood pressure monitoring was performed in 90 patients and 25 healthy controls. Left ventricular mass index (LVMI) was calculated based on the transthoracic echocardiography measurements in 27 patients out of the CKD population and in the whole control group.
Results
CXCL12 correlated significantly with LVMI by multivariate regression analysis (coefficient B = 0.33, p = 0.02) together with age (B = 0.30, p = 0.03) and gender (B = 0.41, p = 0.003). A positive correlation was observed between CXCL12 and average 24-h systolic blood pressure (SBP) (rho = 0.35, p = 0.001), daytime SBP (rho = 0.35, p = 0.001), and nocturnal SBP (rho = 0.30, p = 0.002). Nocturnal hypertension was frequent (46% of CKD patients).
Conclusions
The results of our study point towards a link between CXCL12 and LVH as well as blood pressure control among patients with CKD, supporting the thesis that CXCL12 may be regarded as a new potential uremic toxin.
Keywords: Left ventricular hypertrophy, Nocturnal hypertension, Renin-angiotensin-aldosterone, SDF-1alpha
Introduction
Uremic toxin is a compound that accumulates in the organism as a result of a decline in renal function and, in consequence, negatively influences the physiological processes of the organism [1]. A broader definition encompasses compounds, not only those excreted by kidneys (such as phosphate or potassium), but also those who accumulate due to ineffective molecular clearance. By this definition, uremic toxins also include cytokines such as IL-1β, a strong pro-inflammatory molecule, the levels of which increase in chronic kidney disease (CKD) patients and its action may be reversed by its receptor recombinant antagonist as described by Hung et al. [2]. This is not the only compound which rises in serum of patients with CKD as proposed by the European Uremic Toxin Work Group (EUTox). Among them, there are tumor necrosis factor-alpha (TNF-α) and IL-6, ∼5–20 kDa sized proteins that play a vital role in immune system activation [1]. A chemokine, that is a small chemoattractant cytokine, which is not listed in the proposed uremic toxin database is CXCL12, with its best described isoform SDF-1alpha, that acts on the CXCR4 receptor. The main role of CXLC12/SDF-1 is to regulate the trafficking of CXCR4+ hemato/lymphopoietic cells, their homing in main hemato/lymphopoietic organs, and accumulation in tissues affected by inflammation. CXCL12/SDF-1 is secreted by bone marrow-, lymph node-, muscle-, and lung-derived fibroblasts as well as some central nervous system cells [3]. Secretion of CXCL12 may be induced in the cardiomyocytes by the renin-angiotensin-aldosterone (RAA) system in the failing heart [4]. Importantly, its serum concentration was reported to increase in CKD patients [5].
In this study, we attempt to evaluate whether there is a link between serum CXCL12 and cardiovascular complications, such as left ventricular hypertrophy (LVH) and arterial hypertension.
Patients and Methods
Patient Cohorts
In this single-center cross-sectional study, we enrolled 90 CKD patients with estimated glomerular filtration rate (eGFR) calculated according to the CKD-EPI formula stable over the last 3 months, under the care of an outpatient clinic. Renal transplant patients were included, but no hemodialysis or peritoneal dialysis patients were enrolled in order to decrease the heterogeneity of the patient population. Patients with acute infection or other acute disease (myocardial infarction, stroke, acute heart failure, pulmonary embolism, acute transplant rejection, acute renal failure, surgery, trauma) within the last 90 days, active malignancy, chronic pulmonary disease, primary hyperaldosteronism, or liver cirrhosis were excluded. We enrolled 25 age- and sex-matched control subjects qualified as healthy based on the medical doctor's evaluation and basic laboratory tests with no history of renal or vascular disease.
Sample Collection and Laboratory Measurements
Blood samples were drawn into EDTA-containing tubes in the morning after a 12-h overnight fast in all participants. Serum samples were centrifuged according to a standardized protocol, aliquoted, and stored at −80°C until analysis. Quantification of serum CXCL-12 was performed via ELISA (Human CXCL-12/SDF-1alpha Quantikine ELISA Kit; R&D Systems, Minneapolis, MN, USA) in duplicates, according to the manufacturer's protocol. The coefficients of variation for ELISA were 2.00, 3.95, and 1.50% for the CXCL12 concentrations of 1.26, 2.57, and 4.94 ng/mL, respectively). A detailed description of the sample collection has previously been published [6].
Standardized Clinic Blood Pressure and 24-h Blood Pressure Measurement
The average of 2 standardized measurements (i.e., measured after at least 10 min of quiet resting, in a sitting position) was considered as the patients' clinic blood pressure. 24-h ambulatory blood pressure monitoring (ABPM) was performed in both the study and control groups (Spacelabs 90207, Redmond, WA, USA). ABPM recordings were carried out every 20 min during the day and 45 min during the night (defined according to the time of sleep provided by patients). Patients were instructed to maintain their usual level of activity and take medications at usual doses. The blood pressure readings were assessed according to recommendations of the European Society of Hypertension (ESH), which provide hypertension thresholds as follows: 24-h average ABPM, ≥130/80 mm Hg; daytime ABPM, ≥135/85 mm Hg; nighttime ABPM, ≥120/70 mm Hg. As an indicator of night-day arterial pressure fall, we chose the night-day systolic blood pressure (SBP) ratio (the ratio of the average value of SBP during the night and the average value of SBP during the day).
Echocardiographic Studies
Among the CKD population, 27 patients underwent a complete echocardiographic assessment per formed by a single echocardiographer blinded to the biochemical and gene expression results of the patients with the use of an Aplio 500 ultrasound system (Toshiba, Tokyo, Japan). Linear internal measurements of the left ventricle and its walls was performed in the parasternal long-axis view in the two-dimensional echocardiographic image perpendicular to the left ventricular (LV) long axis and measured at or immediately below the level of the mitral valve leaflet tips at the end diastole. Left ventricular mass index (LVMI) was calculated according to the Cube formula as recommended by the American Society of Echocardiography: LV mass = 0.8 × 1.04 × [(IVS + LVID + PWT)3 – LVID3] + 0.6 g. As LVH we defined 95 g/m2 in women and 115 g/m2 in men (IVS, interventricular septum; LVID, left ventricular internal diameter; PWT, posterior wall thickness). Left ventricular ejection fraction was calculated according to Simpson's method.
Statistical Analysis
Categorical values were assessed by 2-sided Fisher's exact test. Normally distributed parameters were assessed by Student's t test. In case of unequal variances, Cochran–Cox approximation was applied. Parameters with non-normal distribution were assessed by nonparametric Mann-Whitney U test. Pearson product moment correlation coefficient (R) and Spearman rank correlation (rho) were applied to assess the association between the variables. Normally distributed variables are reported as mean ± standard deviation. Non-normally distributed variables are reported as median and interquartile range and categorical data as percentage. Multivariate regression analysis with automatic backwards selection was performed. Two-tailed tests were applied. p < 0.05 was considered as statistically significant.
Results
Baseline Characteristics
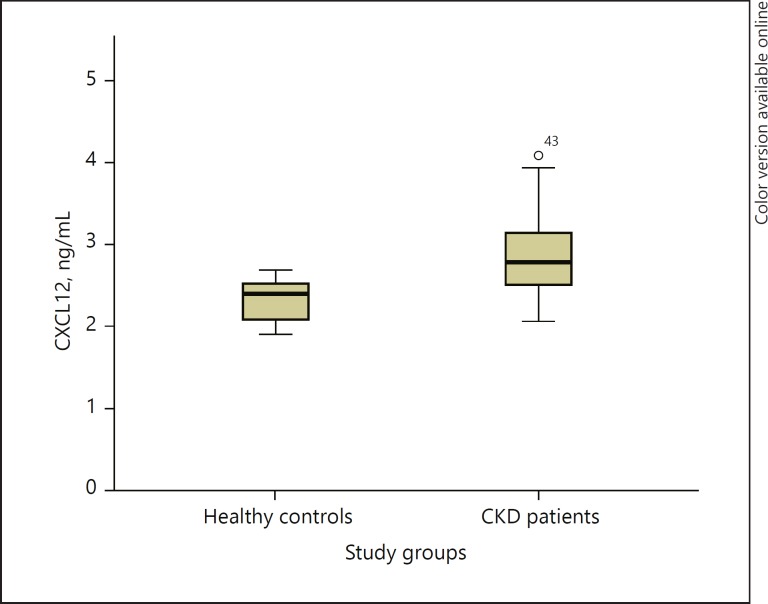
The baseline characteristics of the study groups are presented in Table 1. Decline in eGFR was associated with a weak to moderate rise in blood lipids (triglycerides [TG], rho = −0.45, p = 0.001), inflammatory parameters (white blood cells [WBC], rho = −0.28, p = 0.002; neu trophils, rho = −0.26, p = 0.004, C-reactive protein [CRP], rho = −0.33, p = 0.001), potassium (rho = −0.46, p = 0.001) and ABPM parameters including average nocturnal SBP (rho = −0.34, p = 0.001), average 24-h SBP (rho = −0.28, p = 0.003), and night-day SBP ratio (rho = −0.26, p = 0.008). Serum CXCL12 concentration was higher in the CKD group compared to healthy control participants (Fig. 1).
Table 1.
Baseline characteristics of the study group
| Control group (n = 25) | Study group (n = 90) | p value | |
|---|---|---|---|
| Age, years | 55±13 | 60±14 | 0.09 |
| Male gender | 12 (48) | 47 (52) | 0.56 |
| BMI | 26±4 | 27±7 | 0.42 |
| eGFR, mL/min/1.73 m2 | 84 (75 to 94) | 42 (30 to 58) | 0.001* |
| LDL, mg/dL | 114±22 | 99±40 | 0.02 |
| HDL, mg/dL | 63 (51 to 78) | 57 (46 to 73) | 0.22 |
| TG, mg/dL | 93 (66 to 133) | 133 (96 to 183) | 0.005* |
| TC, mg/dL | 200±22 | 181±40 | 0.02 |
| CRP, mg/dL | 1.1 (0.6 to 1.8) | 1.4 (0.7 to 3.0) | 0.14 |
| Fasting glucose, mg/dL | 92 (84 to 98) | 98 (89 to 107) | 0.04 |
| WBC, G/L | 5.64 (5.33 to 6.50) | 7.3 (5.8 to 8.8) | 0.002* |
| Neutrophils, G/L | 3.17 (2.91 to 3.98) | 4.3 (3.7 to 5.7) | 0.002* |
| Lymphocytes, G/L | 1.73 (1.44 to 2.02) | 1.9 (1.5 to 2.5) | 0.26 |
| NtoL | 1.90 (1.57 to 2.61) | 2.2 (1.6 to 3.2) | 0.20 |
| Platelets, G/L | 243 (219 to 254) | 224 (189 to 250) | 0.13 |
| Sodium, mmol/L | 141 (140 to 142) | 141 (139 to 143) | 0.78 |
| Potassium, mmol/L | 4.20 (4.00 to 4.40) | 4.6 (4.4 to 4.9) | 0.001* |
| CXCL12, ng/mL | 2.39 (2.09 to 2.54) | 2.79 (2.51 to 3.14) | 0.001* |
| Clinic SBP, mm Hg | 120 (112 to 124) | 129 (120 to 138) | 0.001* |
| Clinic DBP, mm Hg | 75 (70 to 81.5) | 80 (70 to 85) | 0.35 |
| 24-h SBP, mm Hg | 118 (112 to 125) | 126 (118 to 135) | 0.008* |
| 24-h DBP, mm Hg | 75 (70 to 82) | 74 (66 to 79) | 0.88 |
| Day SBP, mm Hg | 120±9 | 129±11 | 0.025 |
| Day DBP, mm Hg | 75 (68 to 77) | 77 (69 to 83) | 0.77 |
| Night SBP, mm Hg | 110±10 | 119±16 | 0.003* |
| Night DBP, mm Hg | 64±7 | 67±9 | 0.19 |
| ND systolic ratio | 0.88 (0.83 to 0.94) | 0.93 (0.86 to 0.99) | 0.07 |
| ND diastolic ratio | 0.82 (0.77 to 0.91) | 0.89 (0.82 to 0.95) | 0.05 |
| Ejection fraction, % | 64±7 | 66±11 | 0.27 |
| LVH | 9 (36) | 21 (77) | 0.001* |
| Impaired relaxation | 2 (8) | 5 (20) | 0.01* |
| Diastolic dysfunction | 0 (0) | 6 (24) | |
| Smoker | 1 (4) | 15 (17) | 0.11 |
| Heart failure | 0 (0) | 12 (13) | 0.05 |
| CAD | 0 (0) | 18 (20) | 0.01* |
| AF | 0 (0) | 9 ((10) | 0.09 |
| ACS | 0 (0) | 6 (7) | 0.18 |
| Stroke | 0 (0) | 6 (7) | 0.18 |
| DM | 0 (0) | 23 (26) | 0.007* |
| Insulin treatment | 0 (0) | 15 (17) | 0.03 |
| IFG | 5 (20) | 46 (51) | 0.04 |
Data are presented as mean ± SD, median (Q1–Q3), or number (percentage of population).
ACS, acute coronary syndrome; AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CRP, C-reactive protein; DM, diabetes mellitus; DPB, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; IFG, impaired fasting glycemia; LDL, low-density lipoprotein; LVH, left ventricular hypertrophy; ND, night-day; NtoL, neutrophil to lymphocyte ratio; SBP, systolic blood pressure; TG, triglycerides; TC, total cholesterol; WBC, white blood cells.
p values significant after controlling for false discovery rate at a level of 0.05 according to the Benjamini-Hochberg procedure.
Fig. 1.
Boxplot graph presenting mean, 25th percentile, 75th percentile, and outliers for the CKD group and the healthy control group.
Taking into consideration the relatively small group of patients with available echocardiographic data, for the purpose of the statistical analysis, we divided the whole study population into the following subgroups. The “normal LVMI” group included 5 patients with CKD (out of 27 with available echocardiography) and 16 control subjects (out of 25). The “LVH” group included 22 patients with CKD (out of 27 with available echocardiography) and 9 control subjects with calculated LVMI ≥95 g/m2 in women and ≥115 g/m2 in men. Subjects with LVH were older, presented worse renal function (eGFR 48 vs. 77 mL/min/1.73 m2), worse metabolic profile including lower median high-density lipoprotein (HDL), higher median TG, higher mean fasting glucose with a greater proportion of subjects with impaired fasting glycemia, as well as higher CXCL-12 and clinic and 24-h average SBP. All exact data and p values are presented in online supplementary Table 1 (for all online suppl. material, see www.karger.com/doi/10.1159/000490396). The bivariate correlations for LVMI and noc turnal SBP (including clinical and laboratory variables) are presented in online supplementary Tables 2 and 3, respectively.
Antihypertensive Medication
All hypertensive patients were treated with antihypertensive medication if they were already diagnosed with hypertension at the moment of inclusion. The exact percentage of patients treated with particular medication was as follows: beta-blockers, 56 (62%); RAA system antagonists, 49 (54%); diuretics, 48 (53%); calcium channel antagonists, 39 (43%); alpha-blockers, 11 (12%); clonidine, 5 (6%). Another frequently applied class of drugs were statins in 49 patients (54%). The medication applied had no significant impact on CXCL12 levels (data not shown).
CXCL12 Correlates
We observed a positive significant correlation between CXCL12 and LVMI (rho = 0.34, p = 0.02) and average ABPM parameters: 24-h SBP (rho = 0.35, p = 0.001), daytime SBP (rho = 0.35 p = 0.001), and nocturnal SBP (rho = 0.30, p = 0.002). CXLCL12 level was associated with the stage of CKD (online suppl. Fig. 1). Other significant correlates of CXCL12 included eGFR (rho = −0.61, p = 0.001), TG (rho = 0.34, p = 0.001), and HDL (rho = −0.29, p = 0.01).
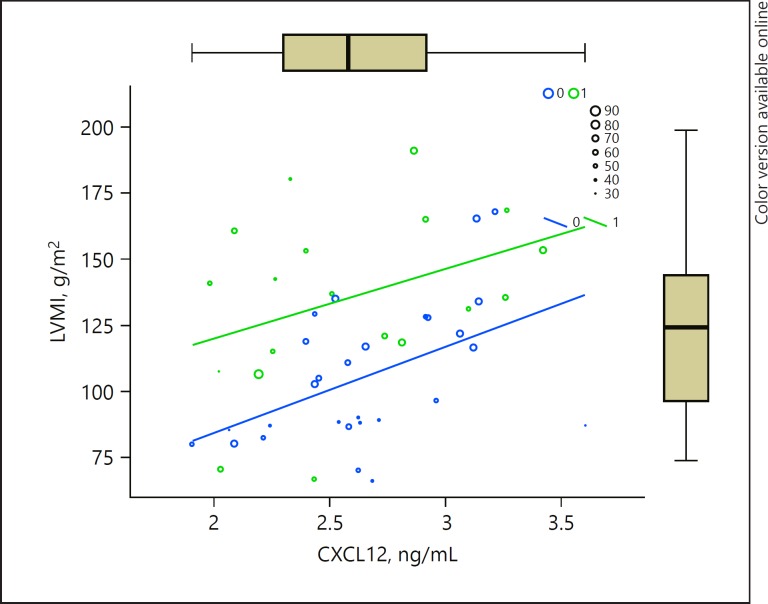
As a next step, we performed multivariate regression analysis that included the variables proven to correlate significantly with LVMI in the bivariate analysis (clinic SBP, eGFR, CXCL12, age) adding gender, diabetes mellitus, and number of hypotensive medications as the input parameters in the model derived with the automatic backward variable selection method. The results are presented in Table 2. According to the model, the only significant predictors of LVMI remain age, CXCL12, and gender. The multiple correlation coefficient R = 0.627 indicates that there is a strong correlation between the observed LVMIs and those predicted by the regression model. Our model accounts for 39.4 % of LVMI variability (or R2 = 0.394). The model fit assessment was significant (ANOVA, F(3,38) = 8.5, p = 0.001). The graphical presentation of the model is depicted in Figure 2.
Table 2.
Multiple linear regression model for the prediction of LVMI derived with the automatic backward variable selection
| Independent variable | Coefficient B | 95% CI for B | t | p value | Collinearity statistics |
|
|---|---|---|---|---|---|---|
| tolerance | VIF | |||||
| CXCL12 (ng/mL) | 24.17 | 4.81 to 43.53 | 2.53 | 0.02 | 0.91 | 1.10 |
| Age (years) | 0.66 | 0.08 to 1.24 | 2.30 | 0.03 | 0.92 | 1.08 |
| Gender | 25.32 | 9.37 to 41.3 | 3.21 | 0.003 | 0.98 | 1.02 |
R = 0.627, R2 = 0.394. CI, confidence interval; VIF, variance inflation factor.
Fig. 2.
Graph representing a multivariate regression model of LVMI in relation to age (represented as circles with size corresponding to the value, as shown in the legend), gender (1/green, male; 0/blue, female), and CXCL12 serum concentration (ng/mL, x axis). F(3,38) = 8.5, p = 0.001.
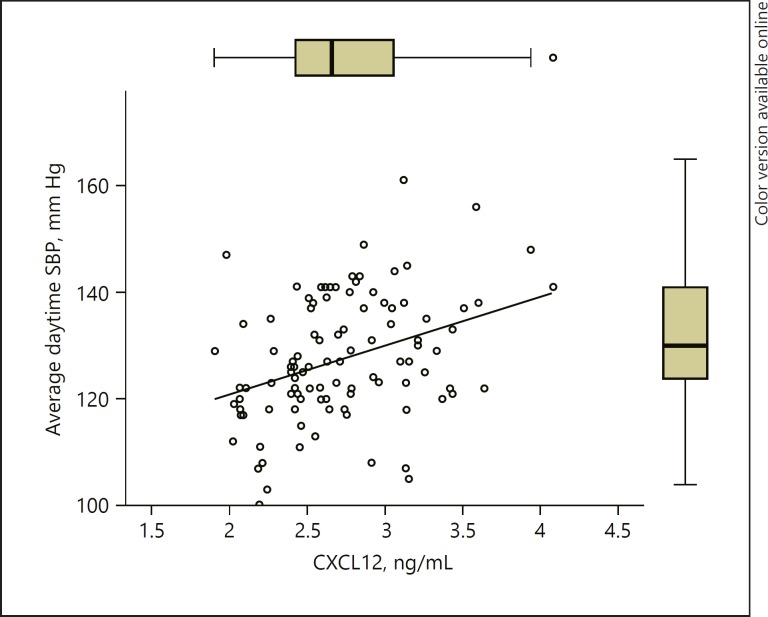
The multiple linear regression model derived with automatic backward variable selection based on the results of bivariate correlations of average daytime SBP revealed a trend in the case of CXCL12 and average daytime SBP correlation (p = 0.058; Fig. 3; Table 3).
Fig. 3.
Graph representing a multivariate regression model of average daytime SBP in relation to CXCL12 serum concentration. Rho = 0.35, p = 0.001.
Table 3.
Multiple linear regression model for the prediction of average daytime SBP derived with parameters that correlated in the bivariate analysis (a) and the automatic backward variable selection (b) based on the CKD population (n = 90)
a.
Parameters that correlated in the bivariate analysis1
| Independent variables | B | 95.0% CI for B | t | p value | Collinearity statistics |
|
|---|---|---|---|---|---|---|
| tolerance | VIF | |||||
| Constant | 93.293 | 62.2 to 124.4 | 6.031 | 0 | ||
| eGFR (mL/min/1.73m2) | 0.119 | −0.05 to 0.29 | 1.429 | 0.159 | 0.685 | 1.459 |
| Age (years) | 0.037 | −0.21 to 0.28 | 0.31 | 0.758 | 0.861 | 1.161 |
| CXCL12 (ng/mL) | 9.392 | 1.52 to 17.26 | 2.4 | 0.02 | 0.753 | 1.328 |
| CRP (mg/dL) | 0.59 | −0.53 to 1.71 | 1.059 | 0.295 | 0.548 | 1.824 |
b.
Automatic backward variable selection2
| Independent variable | Coefficient B | 95.0% CI for B | t | p value | ||
|---|---|---|---|---|---|---|
| Constant | 109.844 | 89.9 to 129.7 | 11.078 | 0 | ||
| CXCL12 (ng/mL) | 6.499 | −0.24 to 13.24 | 1.934 | 0.058 |
Input variables: CXCL12 (ng/mL), age, eGFR, CRP, number of antihypertensive medications. B, unstandardized coefficient B; CI, confidence intervals; VIF, variance inflation factor.
ANOVA for the model F = 1.26, p = 0.295.
ANOVA for the model F = 3.47, p = 0.058.
Discussion
In this first study aimed at verifying the role of CXCL12 in cardiovascular complications, the main findings can be summarized as follows:
An increased CXCL12 plasma concentration was confirmed among CKD patients that strongly correlated with a deterioration of kidney function.
CXCL12 correlated with LVMI and presented a trend towards a positive correlation with average daytime SBP by multivariate analysis.
Given the limited sample size, we regard the results of the study as preliminary; nevertheless, our observation is in line with the previously reported links between CXCL12 and pathologic remodeling within the myocardium as well as alterations to the vessel wall in a preclinical setting.
CXLC12 and Inflammation
Based on the available reports, progressive renal fibrosis activates RAA in the course of CKD. This in turn promotes further fibrosis not only within the kidneys but also the myocardium [7]. It has also been proven that following the activation of the RAA axis, mineralocorticoids activate inflammation within the cardiovascular system via CXLC12/SDF-1alpha [8]. By interaction with CXCR4, CXCL12 induces the recruitment of T cells and fibrocytes to the myocardium [4, 8]. Although CXCL12 may trigger the attraction of potentially beneficial tissue-committed stem/progenitor cells to the heart muscle, this remains only a hypothesis and a negative effect in form of an increase in fibrosis and myocardial mass is observed in vivo in the setting of mineralocorticoid increase associated with CKD [3, 8]. It has been demonstrated that the resulting LVH can be reversed by CXCR4 antagonism as demonstrated for example by Chu et al. on a murine renal failure model [8, 9]. It seems plausible that CXCL12 could stimulate the inflow of T cells to the vessel wall as well, but this hypothesis has not been confirmed so far.
CXCL12 in Cardiomyocyte Dysfunction and Apoptosis
Based on the study by Pyo et al. [10], it is known that inflammatory activation (and specifically CXCL12) promotes the progression of heart failure via a direct detrimental impact on cardiac myocytes. Activation of CXCR4 attenuates beta-adrenergic-mediated calcium mobilization resulting in a negative inotropic effect and reduced fractional shortening within cardiac myocytes [10]. Apart from these functional changes, high concentrations of CXCL12/SDF-1 induce apoptosis of cardiac myocytes via a TNF-α dependent pathway [11].
Of note, although fibrosis and lymphocyte influx was observed simultaneously in the heart and kidney, previous reports point out that increased CXCL12/SDF-1alpha mRNA is restricted to the myocardium and not observed in renal tissue which implies that the heart is a source of this chemokine [8]. It stays in accordance with the observation derived from a prospective cohort of CKD patients that SDF-1alpha/CXCL12 is an independent predictor of death or myocardial infarction [5]. In addition to its prohypertrophic effect on the myocardium, it stimulates LDL–oxLDL uptake into the platelets, resulting in pro-oxidative, thrombogenic effects in platelets and clot structure that favors adverse events [12, 13].
CXLC12 and the Vascular Wall
The weak correlations between CXCL12 and inflammatory parameters observed within the study cohort and the reported ability to traffic lymphocytes make us interpret its rise as a part of the CKD inflammatory milieu and name CXLC12 an inflammatory biomarker. Inflammation induced by RAA within the vascular wall that includes the infiltration of T lymphocytes has been described as an important component in the development of hypertension by Guzik et al. and Wei et al. [14-16]. This is in line with our observation that CXCL12 concentration (as lymphocyte-attractant) correlates with SBP assessed by ABPM. Additionally, it can also increase blood pressure by activating the sympathetic nervous system. [17].
Role of CXLC12 in CKD
Considering all the preclinical and clinical data, there is evidence supportive of the negative influence of CXCL-12 on the myocardium and vessels enabling us to call it a potential uremic toxin. There are data available on its protective role in ischemic conditions and reparative stem cell recruiting capabilities on animal models in the setting of myocardial infarction, which have not been translated into the improvement of the patients' outcome in human studies of heart failure [18-20]. Probably, the role of CXCL12 depends on the microenvironment in which it acts (including interaction with inflammatory and progenitor cells), timing (acute ischemic injury or chronic condition), and concentration (dose-dependent cardiac myocyte apoptosis as shown by Jarrah et al. [11]; beneficial effect of CXCL12 at lower concentrations that ceased at high concentration reported in a porcine model by Penn et al. [21]) as well as the mechanism that induces the remodeling (increased afterload vs. volume overload). It is possible that the proapoptotic influence exerted by CXCL12 directly on the cardiomyocytes cannot be balanced by CXLC12-driven influx of reparatory stem cells, which play a vital role in muscle regeneration [22]. This is in line with the hypothesis expressed by other researchers that ventricular hypertrophy and heart failure are cardiomyocyte deficiency disorders [23, 24]. Moreover, increased levels of pro-inflammatory cytokines, which can be observed in CKD patients, may contribute to decreased engraftment of reparatory stem cells [25].
Limitations
The limited sample size of the study group poses the greatest shortcoming of our study. It should also be taken into account that the evaluation of CXCL12 and blood pressure relationship was performed within a study of already treated patients and this relationship may be blunted by this pharmacologic intervention. Furthermore, we have accounted for fasting glucose level in our LVH model. It should be noticed that it would be interesting to include also glycated hemoglobin (HbA1c) in the model, as its concentration is not altered by acute factors (such as stress or exercise) and reflects long-term blood glucose concentration including postprandial hyperglycemia, which could confound our results. However, as the HbA1c assessments were not routinely performed at the time point of the blood sampling executed within the current study, this parameter could not be incorporated into the present model.
Conclusion
The results of our study point towards a link between CXCL12 and LVH as well as hypertension among patients with CKD, supporting the thesis that CXCL12 could be regarded as a uremic toxin. Preclinical data and studies on patients with heart failure and coronary artery disease are in concordance with our results. However, as we face some reports of a positive influence of CXLC12 on the myocardium in the context of reparatory stem cell functions and bearing in mind the limited sample size, further studies are warranted both based on larger populations and at the molecular level to further verify this link.
Statement of Ethics
This study protocol was approved by the Institutional Research Ethics Committee. The study was ethically conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients and controls.
Disclosure Statement
The authors declare no conflict of interest.
Author Contributions
D.K.-T. contributed to conception and design, collection, analysis, and interpretation of data, and wrote the manuscript. T.P. contributed to conception and design, interpreted and discussed the results. D.Z. ana lyzed and interpreted the data. D.S. contributed to data collection. M.J. discussed the results. L.P. contributed to conception and design, interpretation of data, and discussion of the results. M.K. contributed to conception and design, interpretation of data, and discussion of the results. All authors contributed to the revision of the draft and finally approved the manuscript.
Supplementary Material
Supplementary data
Supplementary data
References
- 1.Vanholder R, De Smet R, Glorieux G, et al. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–1943. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- 2.Hung AM, Ellis CD, Shintani A, Booker C, Ikizler TA. IL-1beta receptor antagonist reduces inflammation in hemodialysis patients. J Am Soc Nephrol. 2011;22:437–442. doi: 10.1681/ASN.2010070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, et al. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 4.Chu PY, Mariani J, Finch S, McMullen JR, Sadoshima J, Marshall T, et al. Bone marrow-derived cells contribute to fibrosis in the chronically failing heart. Am J Pathol. 2010;176:1735–1742. doi: 10.2353/ajpath.2010.090574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta NN, Matthews GJ, Krishnamoorthy P, Shah R, McLaughlin C, Patel P, et al. Higher plasma CXCL12 levels predict incident myocardial infarction and death in chronic kidney disease: findings from the Chronic Renal Insufficiency Cohort study. Eur Heart J. 2014;35:2115–2122. doi: 10.1093/eurheartj/eht481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klimczak D, Kuch M, Pilecki T, Zochowska D, Wirkowska A, Paczek L. Plasma microRNA-155–5p is increased among patients with chronic kidney disease and nocturnal hypertension. J Am Soc Hypertens. 2017;11:831–841. doi: 10.1016/j.jash.2017.10.008. e4. [DOI] [PubMed] [Google Scholar]
- 7.Nishioka T, Suzuki M, Onishi K, Takakura N, Inada H, Yoshida T, et al., Eplerenone attenuates myocardial fibrosis in the angiotensin II-induced hypertensive mouse involvement of tenascin-C induced by aldosterone-mediated inflammation. J Cardiovasc Pharmacol. 2007;49:261–268. doi: 10.1097/FJC.0b013e318033dfd4. [DOI] [PubMed] [Google Scholar]
- 8.Chu PY, Zatta A, Kiriazis H, Chin-Dusting J, Du XJ, Marshall T, et al. CXCR4 antagonism attenuates the cardiorenal consequences of mineralocorticoid excess. Circ Heart Fail. 2011;4:651–658. doi: 10.1161/CIRCHEARTFAILURE.110.960831. [DOI] [PubMed] [Google Scholar]
- 9.Muhlstedt S, Ghadge SK, Duchene J, Qadri F, Jarve A, Vilianovich L, et al. Cardiomyocyte-derived CXCL12 is not involved in cardiogenesis but plays a crucial role in myocardial infarction. J Mol Med. 2016;94:1005–1014. doi: 10.1007/s00109-016-1432-1. [DOI] [PubMed] [Google Scholar]
- 10.Pyo RT, Sui J, Dhume A, Palomeque J, Blaxall BC, Diaz G, et al. CXCR4 modulates contractility in adult cardiac myocytes. J Mol Cell Cardiol. 2006;41:834–844. doi: 10.1016/j.yjmcc.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarrah AA, Schwarskopf M, Wang ER, LaRocca T, Dhume A, Zhang S, et al. SDF-1 induces TNF-mediated apoptosis in cardiac myocytes. Apoptosis. 2018;23:79–91. doi: 10.1007/s10495-017-1438-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee M, Rath D, Schlotterbeck J, Rheinlaender J, Walker-Allgaier B, Alnaggar N, et al. Regulation of oxidized platelet lipidome: implications for coronary artery disease. Eur Heart J. 2017;38:1993–2005. doi: 10.1093/eurheartj/ehx146. [DOI] [PubMed] [Google Scholar]
- 13.Zabczyk M, Undas A. Plasma fibrin clot structure and thromboembolism: clinical implications. Pol Arch Intern Med. 2017;127:873–881. doi: 10.20452/pamw.4165. [DOI] [PubMed] [Google Scholar]
- 14.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei Z, Spizzo I, Diep H, Drummond GR, Widdop RE, Vinh A. Differential phenotypes of tissue-infiltrating T cells during angiotensin II-induced hypertension in mice. PLoS One. 2014;9 doi: 10.1371/journal.pone.0114895. e114895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Januszewicz A, Guzik T, Prejbisz A, Mikolajczyk T, Osmenda G, Januszewicz W. Malignant hypertension: new aspects of an old clinical entity. Polskie Archiwum Medycyny Wewnetrznej. 2016;126:86–93. [PubMed] [Google Scholar]
- 17.Wei SG, Zhang ZH, Yu Y, Felder RB. Central SDF-1/CXCL12 expression and its cardiovascular and sympathetic effects: the role of angiotensin II, TNF-alpha, and MAP kinase signaling. Am J Physiol Heart Circ Physiol. 2014;307:H1643–H1654. doi: 10.1152/ajpheart.00432.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ziff OJ, Bromage DI, Yellon DM, Davidson SM. Title: Therapeutic strategies utilising SDF-1alpha in ischaemic cardiomyopathy. Cardiovasc Res. 2018;114:358–367. doi: 10.1093/cvr/cvx203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larocca TJ, Jeong D, Kohlbrenner E, Lee A, Chen J, Hajjar RJ, et al. CXCR4 gene transfer prevents pressure overload induced heart failure. J Mol Cell Cardiol. 2012;53:223–232. doi: 10.1016/j.yjmcc.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jorbenadze R, Schleicher E, Bigalke B, Stellos K, Gawaz M. Expression of platelet-bound stromal-cell derived factor-1 (SDF-1) and number of CD34(+) progenitor cells in patients with congestive heart failure. Platelets. 2014;25:409–415. doi: 10.3109/09537104.2013.829913. [DOI] [PubMed] [Google Scholar]
- 21.Penn MS, Pastore J, Miller T, Aras R. SDF-1 in myocardial repair. Gene Ther. 2012;19:583–587. doi: 10.1038/gt.2012.32. [DOI] [PubMed] [Google Scholar]
- 22.Kulesza A, Burdzinska A, Szczepanska I, Zarychta-Wisniewska W, Pajak B, Bojarczuk K, et al. The Mutual Interactions between mesenchymal stem cells and myoblasts in an autologous co-culture model. PLoS One. 2016;11 doi: 10.1371/journal.pone.0161693. e0161693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiedler LR, Maifoshie E, Schneider MD. Mouse models of heart failure: cell signaling and cell survival. Curr Top Dev Biol. 2014;109:171–247. doi: 10.1016/B978-0-12-397920-9.00002-0. [DOI] [PubMed] [Google Scholar]
- 24.Chung ES, Miller L, Patel AN, Anderson RD, Mendelsohn FO, Traverse J, et al. Changes in ventricular remodelling and clinical status during the year following a single administration of stromal cell-derived factor-1 non-viral gene therapy in chronic ischaemic heart failure patients: the STOP-HF randomized Phase II trial. Eur Heart J. 2015;36:2228–2238. doi: 10.1093/eurheartj/ehv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gala K, Burdzinska A, Idziak M, Wilczek E, Paczek L. Transplantation of mesenchymal stem cells into the skeletal muscle induces cytokine generation. Cytokine. 2013;64:243–250. doi: 10.1016/j.cyto.2013.06.314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data