Abstract
Aim
The aim of this study was to assess the combined effects of chronic kidney disease (CKD) and diabetes on the extent and developmental pattern of coronary artery disease (CAD).
Methods
A total of 3,017 self-referred asymptomatic individuals without known CAD who underwent 64-channel dual-source coronary computed tomography angiography between 2006 and 2010 were enrolled. The patients were divided into six groups based on their diabetes status (nondiabetic or diabetic) and estimated glomerular filtration rate (eGFR) (eGFR > 90 mL/min/1.73 m2, normal renal function; eGFR 60–89, mild CKD; or eGFR 30–59, moderate CKD). We compared the coronary artery calcium score (CACS), segment stenosis score (SSS), and ≥50% obstructive CAD among the groups.
Results
In nondiabetics, whereas SSS and ≥50% obstructive CAD were not different as renal function deteriorated, after adjusting for cardiovascular risk factors, CACS showed a unique developmental pattern: no CACS increase until mild CKD, but abrupt increase from the stage of moderate CKD (moderate vs. normal renal function, adjusted OR 5.118, 95% CI 1.293–20.262, p = 0.020). In diabetics, patients from the stage of mild CKD were more likely to have ≥50% obstructive CAD (p = 0.004), higher CACS (p = 0.020), and SSS (p = 0.001) in multivariable analysis.
Conclusions
The presence of CKD did not have a significant impact on the development of coronary atherosclerosis, but affected the progression of coronary calcification more markedly from the stage of moderate CKD in nondiabetics. However, in diabetics, the deterioration of renal function was significantly associated with the development of coronary atherosclerosis and calcification from the stage of mild CKD.
Keywords: Chronic kidney disease, Diabetes, Computed tomography, Calcification, Atherosclerosis
Introduction
Patients with chronic kidney disease (CKD) and/or diabetes have significantly increased cardiovascular morbidity and mortality in comparison with the general population. As diseases progress, CKD and diabetes have increased extent and severity of coronary atherosclerosis and increased prevalence of coronary calcification [1-3]. Since the majority of included patients in CKD studies had diabetes, the solitary effect of CKD on the genesis of coronary artery calcification and atherosclerosis has not been clarified. In addition, no studies have investigated the incremental or synergistic effect of CKD and diabetes on the development of coronary calcification and atherosclerosis.
Coronary computed tomography angiography (CCTA) has recently emerged as a noninvasive imaging modality for the detection or exclusion of coronary artery disease (CAD) with high accuracy [4, 5]. Prior studies using CCTA have demonstrated that patients with diabetes or CKD have a higher prevalence, extent, and severity of CAD compared with nondiabetics or those with normal renal function [6]. The aim of the present study was to evaluate the solitary effect of CKD and synergistic effect of CKD and diabetes on the developmental pattern of coronary calcification and atherosclerosis in asymptomatic individuals according to the stage of CKD and the presence of diabetes using a large CCTA registry.
Methods
Study Population
We retrospectively enrolled 3,138 consecutive asymptomatic self-referred individuals who had undergone 64-slice dual-source CCTA at 2 hospitals affiliated with the Catholic University of Korea between January 2006 and December 2010; 2,203 asymptomatic individuals underwent CCTA for general routine health evaluation in Seoul St. Mary's Hospital, and 935 asymptomatic patients with type 2 diabetes were enrolled for their vascular evaluation from the outpatient clinics of both Seoul St. Mary's Hospital and St. Vincent's Hospital of Suwon, Korea. Patients were eligible for this study if they were 30 years of age or older and had no angina or angina-equivalent symptoms. We excluded patients with type 1 diabetes (n = 2), known or suspected CAD (n = 49), prior myocardial infarction (n = 3), coronary revascularization (n = 8), treatment with antianginal medication (n = 5), ventricular or supraventricular arrhythmias (n = 1), or contraindications for the use of iodinated contrast media. To identify the effect of mild and moderate CKD on the genesis and progression of coronary calcification and atherosclerosis, we further excluded 2 patients with severe CKD (those with an estimated glomerular filtration rate [eGFR] of < 30 mL/min/1.73 m2). Fifty-one patients were excluded for the absence of serum creatinine data. A structured interview was performed by a physician or allied health professional before the CCTA examination to document demographic and clinical data. There was no industry involvement in the study design, conduct, or analysis. The institutional review boards at each hospital approved the study, and all of the patients provided written informed consent.
eGFR was calculated using the following equation from the Modification of Diet in Renal Disease (MDRD) study: eGFR (mL/min/1.73 m2) = 186.3 × (serum creatinine [mg/dL]–1.154) × (age [years]–0.203) × (0.742, if female). Patients were divided into 3 groups according to their renal function status: normal renal function (eGFR ≥90 mL/min/1.73 m2), mild CKD (eGFR 60–89 mL/min/1.73 m2), and moderate CKD (eGFR 30–59 mL/min/1.73 m2) [7]. The diagnosis of type 2 diabetes mellitus was made using the criteria of the American Diabetes Association. Subjects with a fasting blood glucose ≥126 mg/dL, glycated hemoglobin ≥6.5%, and/or a postchallenge blood glucose (2 h after a 75 g oral glucose load) ≥200 mg/dL were diagnosed with diabetes.
Scanning Protocol and Image Reconstruction
All patients were in normal sinus rhythm and were able to hold their breath as required for CCTA. Patients with a heart rate > 70 beats per minute (bpm) were administered intravenous esmolol at a dose of 3 mg at 5-min intervals, up to a total dose of 15 mg. If the patient's heart rate did not drop to < 70 bpm, CCTA was performed at the lowest heart rate. Imaging was performed using a 64-slice, dual-source CT scanner (SOMATOM Definition, Siemens, Forchheim, Germany). Each patient underwent an unenhanced, prospectively triggered coronary calcium scan prior to CCTA. During the CCTA acquisition, 80–110 mL of iodinated contrast (Iomeron 350, iomeprol; Bracco, Milan, Italy) was injected. Timing of contrast administration was optimized for uniform enhancement of the coronary arteries. A standard, retrospectively ECG-gated scanning protocol was applied with a slice collimation of 0.6 mm, gantry rotation time of 330 ms, tube voltage of 120 kVp, and maximum tube current of 400 mAs. All of the scans were performed using ECG-controlled tube current modulation. Estimated radiation doses ranged from 5 to 14 mSv. Images were reconstructed immediately after scan completion to identify coronary artery images that were free of motion artifact. Reconstructed CT image data were transferred to a computer workstation for postprocessing, including axial, multiplanar reformatting, maximum intensity projection, and short-axis cross-sectional views. Irrespective of the image quality, every arterial segment in each patient was scored in an intent-to-diagnose fashion.
CCTA Analysis
All scans were analyzed by 2 radiologists with experience of interpreting several thousand CCTA scans. In direct accordance with the Society of Cardiovascular Computed Tomography guidelines, coronary segments were visually scored for the presence of coronary plaque using a 16-segment coronary artery model in an intent-to-diagnose fashion [8]. Only segments with a diameter of > 1.5 mm were included in the analysis. Coronary plaques were considered when structures > 1 mm2 were detected within or adjacent to the coronary artery lumen, which could be clearly distinguished from the vessel lumen and the surrounding pericardial tissue.
The severity of stenosis of a coronary artery lumen was scored as none (0% luminal stenosis), nonobstructive (plaques with narrowing of the coronary artery lumen < 50%), or obstructive (plaques with a maximal stenosis ≥50%). CAD was diagnosed based on the maximum intraluminal stenosis in any segments of the major epicardial coronary arteries, with a threshold of ≥50% stenosis. Obstructive (≥50% luminal stenosis) CAD in the diagonal branches, obtuse marginal branches, and posterolateral branches were considered to be part of the left anterior descending artery, left circumflex (LCX) artery, and right coronary artery (RCA) systems, respectively. The posterior descending artery was considered to be part of the RCA or LCX system, depending on the coronary artery dominance. For each patient, we calculated the number of diseased vessels as 0, 1, 2, 3, or left main CAD.
The severity and extent of CAD was measured by several CCTA scores, including the coronary artery calcium score (CACS), segment involvement score (SIS), and segment stenosis score (SSS) [9]. The CACS was assessed using dedicated software (Siemens Calcium Score; Siemens). Coronary artery calcification was identified as a dense area in the coronary artery that exceeded a threshold of 130 HU. An overall Agatston score was recorded for each patient. The SIS was calculated as the total number of coronary artery segments harboring plaque, irrespective of the degree of luminal stenosis within each segment (minimum = 0; maxi mum = 16). The SSS was used as a measure of the overall coronary artery plaque extent. Each coronary segment was graded as having absent to severe plaque (i.e., scores from 0 to 3) based on the extent of obstruction of the coronary luminal diameter. The extent scores of each of the 16 individual segments were summed to yield a total score that ranged from 0 to 48.
Statistical Analysis
The continuous variables were expressed as the mean ± standard deviation and were compared using ANOVA and post hoc Bonferroni analysis. The categorical variables were compared using the χ2 test or Fisher's exact test. To reduce the impact of treatment selection bias and the potential confounding factors in an observational study, we performed a rigorous adjustment for differences in the baseline characteristics of patients using the Cox proportional hazards regression models. Adjusted covariates were traditional cardiovascular risk factors including age, sex, hypertension, dyslipidemia, and smoking. All the analyses were two tailed, with clinical significance defined as p values < 0.05. SAS software, version 9.2 (SAS Institute Inc., Cary, NC, USA) was used for the statistical analyses.
Results
Patient Characteristics
We finally enrolled 3,017 asymptomatic individuals who underwent CCTA. Their mean age was 57.3 ± 10.5 years, and 63.3% were male. 1,625 subjects had diabetes. In a total of 1,392 nondiabetic populations, 44.5% showed normal renal function, 53% had mild CKD and 2.5% had moderate CKD (Table 1). In the diabetic population, 38.6% showed normal renal function, 54.6% had mild CKD, and 6.8% had moderate CKD (Table 2). The calculated eGFR values (mL/min/1.73 m2) were 102.7, 79.3, and 52.9 according to the renal function status in the nondiabetic population, and 103.6, 78.0, and 52.5 in the diabetic population. Patients with impaired renal function were more likely to be older and have hypertension and dyslipidemia in both nondiabetic and diabetic populations.
Table 1.
Baseline characteristics of the nondiabetic patients in the study population
| Normal renal function (n = 619) | Mild CKD (n = 738) | Moderate CKD (n = 35) | p | |
|---|---|---|---|---|
| Age, years | 53.1±9.9 | 55.5±9.3 | 64.6±8.9 | <0.001 |
| Male | 280 (45.2) | 462 (62.6) | 23 (65.7) | <0.001 |
| Body mass index | 24.1±3.5 | 24.9±3.2 | 25.6±3.7 | <0.001 |
| SBP, mm Hg | 122.4±14.5 | 124.7±14.9 | 126.7±12.2 | 0.015 |
| DBP, mm Hg | 73.2±10.7 | 76.9±30.4 | 73.8±6.9 | 0.020 |
| Hypertension | 150 (26.3) | 234 (36.3) | 22 (68.8) | <0.001 |
| Smoking | 61 (33.0) | 92 (34.3) | 4 (33.3) | 0.956 |
| Dyslipidemia | 213 (34.5) | 322 (43.8) | 14 (41.2) | 0.002 |
| Hemoglobin, mg/dL | 14.1±1.5 | 14.7±1.5 | 14.2±1.7 | <0.001 |
| eGFR | 102.7±11.7 | 79.3±7.3 | 52.9±6.4 | <0.001 |
| Total cholesterol, mL/dL | 201.8±36.8 | 208.5±36.2 | 203.7±42.2 | 0.004 |
| Triglyceride, mL/dL | 109.2±81.7 | 114.5±77.5 | 143.8±141.6 | 0.043 |
| HDL cholesterol, mL/dL | 53.5±17.0 | 51.7±12.4 | 49.5±9.1 | 0.043 |
| LDL cholesterol, mL/dL | 122.8±32.0 | 128.8±32.6 | 123.2±38.8 | 0.003 |
| HbA1C, % | 5.5±0.4 | 5.6±0.3 | 5.6±4.1 | <0.001 |
| hs-CRP, mg/L | 1.5±3.1 | 1.8±5.4 | 1.8±5.9 | 0.349 |
Data are given as the mean ± SD or n (%). CKD, chronic kidney disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HbA1C, glycosylated hemoglobin; hs-CRP, high-sensitivity C-reactive protein.
Table 2.
Baseline characteristics of the diabetic patients in the study population
| Normal renal function (n = 627) | Mild CKD (n = 887) | Moderate CKD (n = 111) | p | |
|---|---|---|---|---|
| Age, years | 56.3±10.3 | 60.6±10.2 | 69.1±8.6 | <0.001 |
| Male | 451 (71.9) | 637 (71.8) | 56 (50.5) | <0.001 |
| Body mass index | 24.7±3.6 | 25.0±3.3 | 25.0±2.9 | 0.145 |
| SBP, mm Hg | 124.8±14.6 | 125.4±13.9 | 130.0±16.3 | 0.003 |
| DBP, mm Hg | 76.1±10.4 | 75.4±10.0 | 75.1±9.1 | 0.355 |
| Hypertension | 239 (40.1) | 404 (47.8) | 87 (79.1) | <0.001 |
| Smoking | 167 (42.4) | 195 (37.1) | 19 (22.4) | 0.002 |
| Dyslipidemia | 288 (45.9) | 460 (51.9) | 72 (64.9) | 0.001 |
| Hemoglobin, mg/dL | 14.4±1.5 | 14.3±1.7 | 13.0±1.7 | <0.001 |
| eGFR | 103.6±13.0 | 78.0±7.7 | 52.5±7.1 | <0.001 |
| Total cholesterol, mL/dL | 183.1±39.4 | 185.3±40.2 | 169.5±43.2 | 0.001 |
| Triglyceride, mL/dL | 139.1±107.6 | 134.3±88.3 | 139.1±66.8 | 0.606 |
| HDL cholesterol, mL/dL | 49.1±12.2 | 48.2±11.5 | 46.2±11.4 | 0.042 |
| LDL cholesterol, mL/dL | 107.7±34.1 | 112.6±35.7 | 100.7±40.4 | 0.003 |
| HbA1C, % | 9.1±3.7 | 9.5±4.0 | 8.7±3.5 | 0.037 |
| hs-CRP, mg/L | 3.4±1.2 | 3.6±1.4 | 6.5±3.4 | 0.287 |
Data are given as the mean ± SD or n (%). CKD, chronic kidney disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HbA1C, glycosylated hemoglobin; hs-CRP, high-sensitivity C-reactive protein.
CCTA Analysis
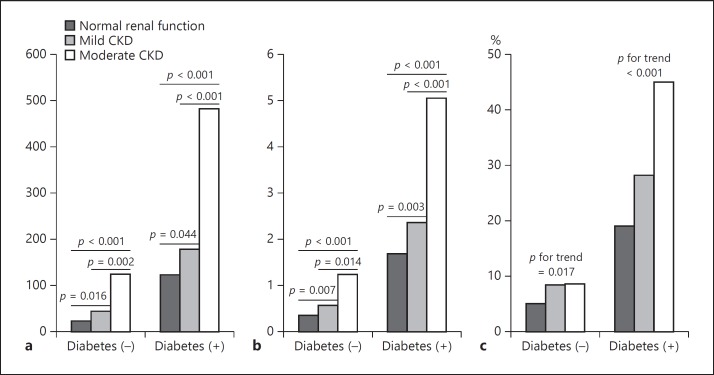
The mean CACS, SIS, and SSS values in the study population were 112.0 ± 345.5, 0.93 ± 1.83, and 1.42 ± 3.17, respectively. Of overall populations, CACS and coronary atherosclerotic burden indicators, including SSS and ≥50% obstructive CAD, were higher in the diabetics compared with the nondiabetics (Fig. 1). There was a stepwise increase in CACS, SSS, and the proportion of obstructive CAD according to the status of renal impairment. In univariate analysis, CACS, SIS, SSS, and the proportion of obstructive CAD were proportionately increased as renal function progressively deteriorated irrespective of the presence of diabetes (Tables 3, 4). Figure 1 shows mean values of CACS and SSS, and proportion of obstructive CAD according to diabetes and CKD stage. Regardless of the presence of diabetes, CACS and SSS were increasing in accordance with the stage of CKD. There was a positive relationship between coronary atherosclerosis and CKD stage. However, multivariate analysis adjusting for multiple cardiovascular risk factors showed that whereas SSS and ≥50% obstructive CAD were not different in nondiabetics as renal function deteriorated, CACS showed a unique developmental pattern: no CACS increase until mild CKD, but abrupt increase from the stage of moderate CKD (moderate vs. normal renal function, adjusted OR 5.118, 95% CI 1.293–20.262, p = 0.020) (Table 5). In diabetics, patients from the stage of mild CKD were more likely to have ≥50% obstructive CAD (OR 1.525; 95% CI 1.143–2.034; p = 0.004), higher CACS (OR 1.423, 95% CI 1.056–1.917; p = 0.020), and SSS (OR 1.856, 95% CI 1.282–2.687; p = 0.001).
Fig. 1.
Coronary artery calcification and atherosclerosis as assessed by coronary CT angiography according to diabetes status and chronic kidney disease stage. a Coronary artery calcification score. b Segment stenosis score. c ≥50% obstructive coronary artery disease (%).
Table 3.
CT coronary angiography findings for the nondiabetic patients in the study population
| Normal renal function (n = 619) | Mild CKD (n = 738) | Moderate CKD (n = 35) | p | |
|---|---|---|---|---|
| Normal | 464 (75.0) | 486 (65.9) | 18 (51.4) | <0.001 |
| CAD by stenosis ≥50% | 31 (5.0) | 62 (8.4) | 3 (8.6) | 0.045 |
| CAD by stenosis ≥70% | 11 (1.8) | 15 (2.0) | 2 (5.7) | 0.271 |
| Vessels affected | <0.001 | |||
| 1-vessel disease | 23 (3.7) | 47 (6.4) | 1 (2.9) | |
| 2-vessel disease | 6 (1.0) | 13 (1.8) | 1 (2.9) | |
| 3-vessel disease or left main CAD | 2 (0.3) | 2 (0.3) | 1 (2.9) | |
| Involved artery | <0.001 | |||
| Left main coronary artery | 1 (0.2) | 1 (0.1) | 0 (0) | |
| Left anterior descending artery | 22 (3.6) | 39 (5.3) | 2 (5.7) | |
| Left circumflex artery | 10 (1.6) | 15 (2.0) | 2 (5.7) | |
| Right coronary artery | 7 (1.1) | 23 (3.1) | 2 (5.7) | |
| Calcium score (Agatston) | 23.2±100.9 | 44.9±155.5 | 124.4±348.2 | <0.001 |
| Calcium score = 0 | 435 (70.6) | 444 (60.4) | 12 (35.3) | <0.001 |
| Calcium score ≥100 | 35 (5.7) | 73 (9.9) | 6 (17.6) | 0.002 |
| Calcium score ≥400 | 9 (1.5) | 22 (3.0) | 3 (8.8) | 0.010 |
| Segment involvement score | 0.27±0.79 | 0.43±0.98 | 0.83±1.80 | <0.001 |
| Segment involvement score >5 | 4 (0.6) | 5 (0.7) | 1 (2.9) | 0.315 |
| Segment stenosis score | 0.35±1.12 | 0.57±1.40 | 1.23±2.95 | <0.001 |
| Segment stenosis score >5 | 9 (1.5) | 17 (2.3) | 2 (5.7) | 0.155 |
Data are given as the mean ± SD or n (%). CKD, chronic kidney disease; CAD, coronary artery disease.
Table 4.
CT coronary angiography findings for the diabetic patients in the study population
| Normal renal function (n = 627) | Mild CKD (n = 887) | Moderate CKD (n = 111) | p | |
|---|---|---|---|---|
| Normal | 258 (41.1) | 331 (37.4) | 22 (19.8) | <0.001 |
| CAD by stenosis ≥50% | 119 (19.o) | 250 (28.2) | 50 (45.0) | <0.001 |
| CAD by stenosis ≥70% | 31 (4.9) | 69 (7.8) | 22 (19.8) | <0.001 |
| Vessels affected | <0.001 | |||
| 1-vessel disease | 69 (11.o) | 144 (16.3) | 17 (15.3) | |
| 2-vessel disease | 25 (4.o) | 62 (7.0) | 13 (11.7) | |
| 3-vessel disease or left main CAD | 25 (4.o) | 44 (5.0) | 18 (16.2) | |
| Involved artery | <0.001 | |||
| Left main coronary artery | 7 (1.1) | 11 (1.2) | 11 (9.9) | |
| Left anterior descending artery | 91 (14.5) | 177 (20.0) | 43 (38.7) | |
| Left circumflex artery | 45 (7.2) | 104 (11.8) | 20 (18.0) | |
| Right coronary artery | 51 (8.1) | 100 (11.3) | 27 (24.3) | |
| Calcium score (Agatston) | 123.5±372.3 | 178.6±431.7 | 482.0±719.5 | <0.001 |
| Calcium score = 0 | 283 (46.2) | 319 (37.0) | 13 (12.4) | <0.001 |
| Calcium score ≥100 | 134 (21.9) | 245 (28.5) | 59 (56.2) | <0.001 |
| Calcium score ≥400 | 45 (7.3) | 107 (12.4) | 35 (33.3) | <0.001 |
| Segment involvement score | 1.12±1.96 | 1.52±2.27 | 2.88±2.87 | <0.001 |
| Segment involvement score >5 | 3o (4.8) | 69 (7.8) | 20 (18.0) | <0.001 |
| Segment stenosis score | 1.69±3.4o | 2.37±3.98 | 5.05±6.06 | <0.001 |
| Segment stenosis score >5 | 53 (8.5) | 135 (15.3) | 35 (31.5) | <0.001 |
Data are given as the mean ± SD or n (%). CKD, chronic kidney disease; CAD, coronary artery disease.
Table 5.
Multivariate logistic regression for coronary calcification and atherosclerosis according to renal function status with or without diabetes
| Normal renal function | Mild CKD | Moderate CKD | ||
|---|---|---|---|---|
| Nondiabetics | ||||
| Coronary artery calcium | OR (95% CI) | 1.000 (Ref.) | 1.663 (0.885–3.126) | 5.118 (1.293–20.262) |
| score ≥100 | p | - | 0.114 | 0.020 |
| ≥50% obstructive CAD | OR (95% CI) p |
1.000 (Ref.) – |
1.251 (0.667–2.346) 0.486 |
1.574 (0.333–7.447) 0.567 |
| Diabetics | ||||
| Coronary artery calcium | OR (95% CI) | 1.000 (Ref.) | 1.423 (1.056–1.917) | 3.203 (1.877–5.467) |
| score ≥100 | p | - | 0.020 | <0.001 |
| ≥50% obstructive CAD | OR (95% CI) p |
1.000 (Ref.) – |
1.525 (1.143–2.034) 0.004 |
1.766 (1.061–2.939) 0.029 |
OR, odds ratio; CI, confidence interval; CAD, coronary artery disease; CKD, chronic kidney disease. OR was adjusted for the following variables: age, sex, hypertension, dyslipidemia, and smoking.
Discussion
In this two-center imaging study, we observed a synergistic effect of impaired renal function and diabetes on the extent and developmental pattern of coronary calcification and atherosclerosis in asymptomatic individuals using CCTA. The major findings of the present study were as follows: (1) the combination of type 2 diabetes and impaired renal function was associated with a greater extent of coronary calcification and atherosclerosis; (2) increased coronary calcification and atheroma burden in CCTA were noted as early as the mild CKD stage in diabetic patients; (3) coronary calcification abruptly increased from the stage of moderate CKD in nondiabetic patients; (4) in contrast to the expectation, the severity and extent of coronary atherosclerosis were not associated with the degree of renal impairment in nondiabetic patients. To our knowledge, this study is the first to examine the combined effect of impaired renal function and diabetes on the extent and developmental pattern of CAD in asymptomatic patients without prior known CAD using CCTA.
The influence of diabetes on the development of coronary calcification, atherosclerosis, and obstructive CAD has long been investigated. Large epidemiologic studies demonstrated that patients with diabetes had higher CACS compared with those without diabetes [10, 11]. The mechanism for advanced atherosclerosis and calcification in patients with diabetes is almost certainly multifactorial. The mechanisms causing the extensive accumulation of calcium in the arterial system of patients affected by diabetes mellitus are largely unknown but are likely multifactorial. Diabetes is associated with a larger atherosclerotic plaque burden, likely explaining the observed larger amount of coronary calcium [12, 13]. Our observational study resembles the previous studies.
CKD was known to be highly correlated with coronary artery calcium, coronary atherosclerosis, and revascularization rates [14, 15]. Several studies have demonstrated a correlation between the CACS determined by CCTA and CKD severity in the general population [16, 17]. Although it remains unclear whether impaired renal function is associated with coronary calcification independent of traditional cardiovascular risk factors, the development and progression of calcification seem to be linked to disrupted mineral metabolism in end-stage renal disease [18]. Conversely, other studies demonstrate that decreased renal function is not associated with greater plaque volume or growth, and luminal stenosis [19-21]. However, most studies included diabetic patients and did not distinguish diabetic from nondiabetic CKD.
CKD patients with concomitant type 2 diabetes present a higher CACS compared with nondiabetics or patients with normal renal function [6]. In addition to the coronary calcium score, there are limited data regarding coronary plaque and obstruction scores in asymptomatic diabetes and CKD patients. Our results demonstrated that moderate CKD patients had more coronary calcification compared with those with normal renal function and mild CKD in an asymptomatic nondiabetes group, although there was no difference between the CKD stage and coronary plaque burden. However, in asymptomatic diabetic patients, decreased renal function was associated with higher coronary calcification and plaque burden. These findings could help to explain the mechanisms of CAD development in diabetes and CKD.
Our study has several potential limitations. First, this study consists of observational registry data. Unmeasured confounders and selection bias might have affected the results. Our findings should be confirmed through large randomized clinical trials with long-term follow-ups. Second, there was no data about patients' clinical outcomes. The relationship between the status of diabetes of CKD patients, CCTA findings, and clinical outcomes was not available. Third, our data represented only a small number of patients (n = 541, 17.9%) who had microalbuminuria, which is also associated with increased risk of cardiovascular disease and atherosclerosis. Fourth, whether contrast-induced nephropathy developed after CCTA implementation was not evaluated.
Conclusions
The present study demonstrated that among nondiabetics, coronary calcification is more severe in those with moderate CKD compared with those with normal renal function and mild CKD, although the association with renal function and coronary atherosclerosis is not significant. However, in diabetics, the deterioration of renal function was significantly associated with the development of coronary atherosclerosis and calcification from the stage of mild CKD, suggesting a synergistic effect of CKD and diabetes on the progression of CAD.
Disclosure Statement
The authors declare that there is no conflict of interest.
Statement of Ethics
All patients provided written informed consent, and the study protocol was approved by the ethics committee at each participating center.
References
- 1.Goraya TY, Leibson CL, Palumbo PJ, Weston SA, Killian JM, Pfeifer EA, et al. Coronary atherosclerosis in diabetes mellitus: a population-based autopsy study. J Am Coll Cardiol. 2002;40:946–953. doi: 10.1016/s0735-1097(02)02065-x. [DOI] [PubMed] [Google Scholar]
- 2.Fox CS, Larson MG, Keyes MJ, Levy D, Clouse ME, Culleton B, et al. Kidney function is inversely associated with coronary artery calcification in men and women free of cardiovascular disease: the Framingham Heart Study. Kidney Int. 2004;66:2017–2021. doi: 10.1111/j.1523-1755.2004.00973.x. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Scholte AJ, Schuijf JD, Kharagjitsingh AV, Jukema JW, Pundziute G, van der Wall EE, et al. Prevalence of coronary artery disease and plaque morphology assessed by multi-slice computed tomography coronary angiography and calcium scoring in asymptomatic patients with type 2 diabetes. Heart. 2008;94:290–295. doi: 10.1136/hrt.2007.121921. [DOI] [PubMed] [Google Scholar]
- 5.Kamimura M, Moroi M, Isobe M, Hiroe M. Role of coronary CT angiography in asymptomatic patients with type 2 diabetes mellitus. Int Heart J. 2012;53:23–28. doi: 10.1536/ihj.53.23. [DOI] [PubMed] [Google Scholar]
- 6.Rana JS, Dunning A, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, et al. Differences in prevalence, extent, severity, and prognosis of coronary artery disease among patients with and without diabetes undergoing coronary computed tomography angiography: results from 10,110 individuals from the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes): an InteRnational Multicenter Registry. Diabetes Care. 2012;35:1787–1794. doi: 10.2337/dc11-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.K/DOQI clinical practice guidelines for chronic kidney disease evaluation, classification, and stratification. Am J Kidney Dis. 2002;39((suppl 1)):S1–S266. [PubMed] [Google Scholar]
- 8.Raff GL, Abidov A, Achenbach S, Berman DS, Boxt LM, Budoff MJ, et al. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr. 2009;3:122–136. doi: 10.1016/j.jcct.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50:1161–1170. doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 10.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43:1663–1669. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 11.Schurgin S, Rich S, Mazzone T. Increased prevalence of significant coronary artery calcification in patients with diabetes. Diabetes Care. 2001;24:335–338. doi: 10.2337/diacare.24.2.335. [DOI] [PubMed] [Google Scholar]
- 12.Goraya TY, Leibson CL, Palumbo PJ, Weston SA, Killian JM, Pfeifer EA, et al. Coronary atherosclerosis in diabetes mellitus: a population-based autopsy study. J Am Coll Cardiol. 2002;40:946–953. doi: 10.1016/s0735-1097(02)02065-x. [DOI] [PubMed] [Google Scholar]
- 13.de Araujo Goncalves P, Garcia-Garcia HM, Carvalho MS, Dores H, Sousa PJ, Marques H, et al. Diabetes as an independent predictor of high atherosclerotic burden assessed by coronary computed tomography angiography: the coronary artery disease equivalent revisited. Int J Cardiovasc Imaging. 2013;29:1105–1114. doi: 10.1007/s10554-012-0168-4. [DOI] [PubMed] [Google Scholar]
- 14.Ix JH, Katz R, Kestenbaum B, Fried LF, Kramer H, Stehman-Breen C, et al. Association of mild to moderate kidney dysfunction and coronary calcification. J Am Soc Nephrol. 2008;19:579–585. doi: 10.1681/ASN.2007070765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parikh NI, Hwang SJ, Larson MG, Hoffmann U, Levy D, Meigs JB, et al. Indexes of kidney function and coronary artery and abdominal aortic calcium (from the Framingham Offspring Study) Am J Cardiol. 2008;102:440–443. doi: 10.1016/j.amjcard.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho I, Min HS, Chun EJ, Park SK, Choi Y, Blumenthal RS, et al. Coronary atherosclerosis detected by coronary CT angiography in asymptomatic subjects with early chronic kidney disease. Atherosclerosis. 2010;208:406–411. doi: 10.1016/j.atherosclerosis.2009.08.040. [DOI] [PubMed] [Google Scholar]
- 17.Budoff MJ, Rader DJ, Reilly MP, Mohler ER, 3rd, Lash J, Yang W, et al. Relationship of estimated GFR and coronary artery calcification in the CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis. 2011;58:519–526. doi: 10.1053/j.ajkd.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 19.Gruberg L, Rai P, Mintz GS, Canos D, Pinnow E, Satler LF, et al. Impact of renal function on coronary plaque morphology and morphometry in patients with chronic renal insufficiency as determined by intravascular ultrasound volumetric analysis. Am J Cardiol. 2005;96:892–896. doi: 10.1016/j.amjcard.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 20.Joosen IA, Schiphof F, Versteylen MO, Laufer EM, Winkens MH, Nelemans PJ, et al. Relation between mild to moderate chronic kidney disease and coronary artery disease determined with coronary CT angiography. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047267. e47267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo D, Morrone LF, Imbriaco M, Pota A, Russo L, Scognamiglio B, et al. Coronary artery calcification and outcomes in diabetic patients with and without chronic kidney disease. Blood Purif. 2013;36:17–20. doi: 10.1159/000350580. [DOI] [PubMed] [Google Scholar]