Abstract
Background
There is paucity in the literature regarding gallbladder cancer in Saudi Arabia, possibly because it is not among the top 10 cancers diagnosed nationwide according to the Saudi Cancer Registry. Moreover, national or regional data on gallbladder cancer in Saudi Arabia have not been analyzed. The purpose of this study was to describe the presentation, disease stage, histology, and survival rates for gallbladder cancer in Saudi patients at a single institution between January 1, 2010 and December 31, 2017.
Materials and methods
This was a retrospective study of 76 patients who presented to our hospital between January 1, 2010 and December 31, 2017, with established diagnosis of gallbladder carcinoma. The diagnosis was made either histopathologically following simple laparoscopic cholecystectomy or biopsy from metastatic liver lesion in patients with gallbladder mass, or the high suspicion of gallbladder carcinoma based on incidental radiological findings. Presentation, disease stage, histology, and treatment modalities were analyzed using descriptive statistics and frequency distributions. Survival rates were analyzed and presented using Kaplan-Meier curves.
Results
Based on initial analyses the disease was more frequent among women (62.0%) than men (39.0%). Surgical resection was attempted in 40.8% patients. The average age at presentation and diagnosis of gallbladder carcinoma was 62.4 years. The disease had two peaks, one at 51.0 years and the other between 66.0 and 70.0 years. The median survival time for the overall at-risk patients was only 1.0 year, while for stage IVB patients was 7.2 months. Adenocarcinoma not otherwise specified (NOS) was the most common histopathology type (75.0%), with most patients presenting with stage IVB disease (75.0%). Gallbladder carcinoma was incidentally detected in 42.1%, including three cases (3.9%) diagnosed at our hospital.
Conclusions
Gallbladder cancer is a rare type of cancer in Saudi Arabia, and most patients are treated surgically, despite being mostly diagnosed at the advanced stage of the disease.
Keywords: Gallbladder cancer, Presentation, Disease stage, Histology, Resectability rate
Highlights
-
•
Gallbladder cancer is more prevalent in women in their mid-60s with BMI >30 kg/m2.
-
•
Age is a major risk factor for gallbladder cancer.
-
•
Adenocarcinoma not otherwise specified was most common histopathologic type.
1. Introduction
Based on autopsy studies, gallbladder cancer (GBC)1 is highly common among the biliary tract cancers, accounting for about 80.0%–90.0% of the biliary tract cancers globally, and is the third most common among the gastrointestinal cancers [1,2], with high prevalence in South America and South-east Asia [1]. However, there is significant regional and ethnic variability in the prevalence of GBC, with much lower prevalence in Europe and India [2]. In the Middle East, GBC is a rare neoplasm. It does not rank among the top 10 cancers in the Kingdom of Saudi Arabia (KSA), according to the Saudi Cancer Registry [3].
The silent nature of GBC makes it difficult to diagnose. Besides, most symptoms (including abdominal pain, fever, nausea, and jaundice) can be attributed to other more common conditions, such as hepatitis, stones of the biliary tract, and pancreatitis [4]. GBC typically has a very poor prognosis with a 5‐year survival rate of 29.0% for stage II and less than 2.0% for stage IVB [5]. The low survival rate is usually because of late presentation in most patients, with close to 37.0% of patients presenting with advanced stage disease (IVB) due to unspecific early symptoms [6].
The regional and ethnic variability in GBC prevalence in Saudi Arabia is a potential cause for the continuing dearth in literature in this topic. Further, national and regional data on GBC in Saudi Arabia have not been analyzed. Even though it is not among the more prevalent cancer types, the difficulty in diagnosing GBC presents the need to understand this disease in the context of the Saudi population.
Being a referral hospital, patients either diagnosed or suspected to have GBC are referred to our hospital for highly advanced treatment. The purpose of this study was to describe the characteristics of GBC in Saudi patients at a single institution and provide insight into their clinical profile, disease stage, histology, and survival rates.
2. Materials and methods
2.1. Patients
Data on gallbladder carcinoma patients, treated at our hospital between January 1, 2010 and December 31, 2017 were obtained from the institution's database. Data on patient characteristics including age, presence of gallstones, diabetes, and hypertension were collected. Stage at diagnosis and other cancer characteristics were also included. The 7th edition of the American Joint Committee on cancer (AJCC) staging system was used for clinical and/or surgical (pathological) staging. In patients with incidental gallbladder carcinoma, pTNM values were based on the first pathological report. Patient treatment strategies were decided by a team of medical specialists and included surgical removal, chemotherapy, or palliative care if other options were not feasible. The study was approved by the Institutional Review Board of our hospital. We report the results of this analysis in accordance with the STROCCS reporting statements [7].
2.2. Study design
Closed cohort and case study designs were employed. The occurrence of death among the same group of patients at various points of time over the 7-year study interval was reviewed, and the end point occurrence was correlated with patients' demographic characteristics, co-morbidities, operative procedures, and related factors.
2.3. Statistical analysis
Univariate frequency tables and charts were used alongside cross-tabulations to understand the presentations of various cancer-related symptoms and outcomes as well as the administration of various treatment modalities. Survival analysis was performed using the Kaplan-Meier method to assess overall survival rate, and determine the effectiveness of surgical treatment in survival. The log-rank Chi square test was used to compare relative survival rates between patients who underwent a resection and those who did not undergo any procedure. The survival rate was defined as the percentage of patients still alive at the end of the study period of 5-year after censoring had been accounted for.
3. Results
3.1. Patient characteristics
A total of 76 patients with GBC were included in the study. The average age at presentation and diagnosis was 62.4 years (range 38–82 years). The disease was most prevalent at 55 years of age and the most patients with GBC were 66–70 years old (Table 1). Further, most patients with GBC were female (n = 47, 62.0%). Only three patients (3.9%) were diagnosed at our hospital, all being incidental cases. The remainder of cases were referrals from other hospitals either upon suspicion of GBC (n = 44, 57.9%) or were incidental cases sent for further investigation and management (n = 29, 38.2%) (Table 2).
Table 1.
Descriptive statistics for patient data.
| Descriptive statistic | BMI | Age at diagnosis |
|---|---|---|
| Mean | 29.3 | 62.4 |
| Standard Error | 1.1 | 1.3 |
| Median | 27.4 | 62.5 |
| Mode | 24.5 | 55 |
| Standard Deviation | 9.5 | 10.9 |
| Kurtosis | 4.2 | −0.6 |
| Skewness | 1.5 | −0.2 |
| Range | 57 | 44 |
| Minimum | 14.7 | 38 |
| Maximum | 71.7 | 82 |
| Count | 76 | 76 |
| Confidence Level (95.0%) | 2.2 | 2.5 |
Table 2.
Clinicopathologic features of 76 patients with gallbladder cancer enrolled in this study.
| Observed clinicopathologic features | Count (Percentage) |
|---|---|
| Comorbidities and risk factors | n (%) |
| Diabetes | 38 (50) |
| Hypertension | 38 (50) |
| Smoking | 12 (16) |
| Gallstones | 57 (75) |
| Size of gallstones: | |
| Multiple, ≤1 cm | 43 (57.9) |
| Between 1 and 2 cm | 10 (13.2) |
| > 2 cm | 6 (7.9) |
| Presenting symptom | n (%) |
| Obstructive jaundice | 1 (1) |
| Right upper quadrant pain | 64 (84) |
| Epigastric pain | 4 (5) |
| Right upper quadrant and epigastric pain | 2 (3) |
| Vomiting and epigastric pain | 2 (3) |
| Painless obstructive jaundice | 2 (3) |
| Anorexia with weight loss and constipation | 1 (1) |
| Primary clinical diagnosis | n (%) |
| Biliary colic | 33 (43.4) |
| Acute cholecystitis | 24 (31.6) |
| Biliary pancreatitis | 7 (9.2) |
| Painless obstructive jaundice secondary to malignancy | 1 (1.3) |
| Chronic calcular cholecystitis | 10 (13.2) |
| Irritable bowel syndrome | 1 (1.3) |
| Imaging findings | n (%) |
| Metastatic GBC | 38 (50) |
| Gallbladder mass | 8 (10.5) |
| Gallbladder mucocele | 1 (1.3) |
| Adenomyomatosis of gallbladder | 1 (1.3) |
| No incidental findings (gallstones) | 27 (36.8) |
| Intraoperative diagnosis | n (%) |
| Gallstones | 24 (31.6) |
| Gallstones with polyp | 3 (3.9) |
| Metastatic GBC | 7 (9.2) |
| GBC | 9 (11.8) |
| Adenomyomatosis of gallbladder | 1 (1.3) |
| None | 32 (42.1) |
| Procedure of staging | n (%) |
| Surgical | 30 (39) |
| Clinical | 46 (61) |
| Pathological staging post-cholecystectomy | n (%) |
| Stage I | 3 (3.9) |
| Stage II | 13 (17.1) |
| Stage IIB | 1 (1.3) |
| Stage IIIB | 2 (2.6) |
| Stage IVB | 57 (75) |
| GBC type | n (%) |
| Adenocarcinoma (NOS) | 57 (75) |
| Papillary adenocarcinoma | 7 (9.2) |
| Mucinous adenocarcinoma | 3 (3.9) |
| Carcinosarcoma | 3 (3.9) |
| Papillary carcinoma | 1 (1.3) |
| Squamous cell carcinoma | 1 (1.3) |
| Neuroendocrine carcinoma | 1 (1.3) |
| Sarcomatoid carcinoma | 1 (1.3) |
| Adenosquamous carcinoma | 1 (1.3) |
| Poorly differentiated carcinoma | 1 (1.3) |
| Resectability | n (%) |
| Yes | 31 (40.8) |
| No | 47 (61.8) |
| Surgical resection attempted | n (%) |
| Yes | 31 (40.8) |
| Successful | 21 (67.7) |
| Unsuccessful | 10 (32.3) |
| No | 45 (59.2) |
| Lymphadenectomy | n (%) |
| Yes | 24 (31.6) |
| No | 52 (68.4) |
| Excision site | n (%) |
| Bile duct | 20 (26.3) |
| Port site | 13 (17.1) |
| Survival to the end of study period | n (%) |
| No | 44 (57.9) |
| Yes | 20 (26.3) |
| Lost follow up (censored) | 12 (15.8) |
| Recurrence | n (%) |
| Yes | 8 (10.5) |
| No | 14 (18.4) |
| Unresectable disease or lost follow up | 54 (71.0) |
| Disease stage upon recurrence | n (%) |
| Stage II | 4 (50.0) |
| Stage IVB | 4 (50.0) |
Using the obesity classifications of the Center of Disease and Control, 38.7% of the patients were either underweight or had normal weight, with 21.0% being overweight and 42.1% classified as obese. The body mass index (BMI) of the patients ranged between 14.7 kg/m2, and 71.7 kg/m2 with a mean of 29.3 kg/m2. Overall 50.0% (n = 38) of patients were diabetic and 50% suffered hypertension. Most patients (84.0%) were non-smokers.
3.2. Clinicopathologic findings
The chief complaint by most patients (n = 64, 84.0%) was right upper quadrant pain. Only 3.0% of patients reported both right upper quadrant and epigastric pain as their first symptom. The primary clinical diagnosis was biliary colic in 43.4% of patients followed by acute cholecystitis (31.6%). 51.3% (n = 39) of the patients presented with jaundice. Gallstones were present in 75.0% (n = 57) patients of which 57.9% had multiple small gallstones less than 1.0 cm in size (57.9%) (Table 2).
On imaging, 50.0% of the incidental cases were of metastatic gallbladder cancer. However, 36.8% of patients showed no pre-op incidental findings except gallstones. Table 2 gives a complete list of imaging findings in patients enrolled in this study.
The tumor was considered inoperable in 42.1% (n = 32) of patients since metastatic gallbladder cancer was found incidentally on imaging; hence, no definite diagnosis was reached. GBC was confirmed by frozen biopsy in 11.8% of patients intraoperatively, while 9.2% of patients had metastatic GBC. Table 2 shows the complete list of final diagnoses for the 76 enrolled patients. Lymphadenectomy during surgery was performed in only 24 patients. Bile duct excision and port site excision were not performed in most patients either due to unresectable metastatic disease, the presence of gallbladder mass or incidental finding of GBC after laparoscopic cholecystectomy with evidence of metastatic disease (Table 2).
The cancer was pathologically diagnosed in only 39.0% of patients. In the other 61.0%, clinical staging was employed due to unresectability. Overall most patients were classified as stage IVB, based on the AJCC staging system for gallbladder carcinoma (Table 2). Only 3.9% of the patients were diagnosed at stage I of the disease. Adenocarcinoma not otherwise specified (NOS) was the most common histopathology type (75.0%) followed by papillary adenocarcinoma (9.2%) and mucinous adenocarcinoma (3.9%). Carcinosarcoma was the pathological diagnosis in 3.9% of the patients.
As presented in detail in Table 2, 10.5% of the patients suffered recurrence of the cancer after surgical resection with disease-free margin. Fifty percent of the recurrent cases were diagnosed as stage II disease, while the remaining 50.0% as stage IVB cancer. No recurrence was reported in 18.4% of patients, while 71.1% of patients were either diagnosed with unresectable tumor or became censored.
3.3. Survival rates
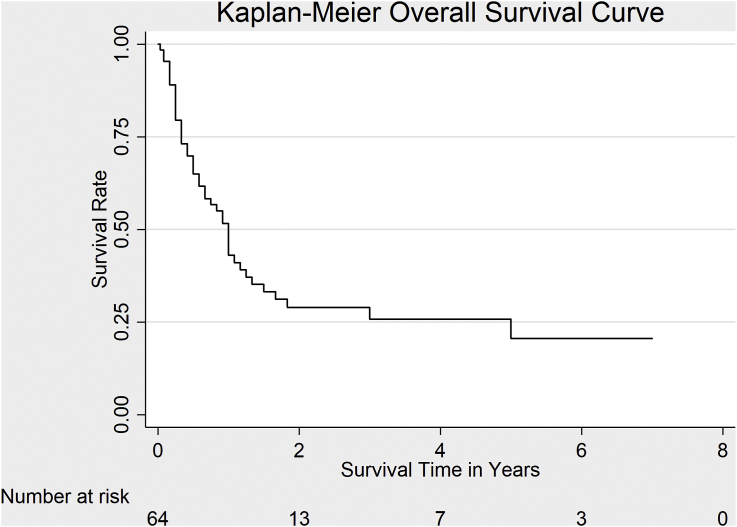
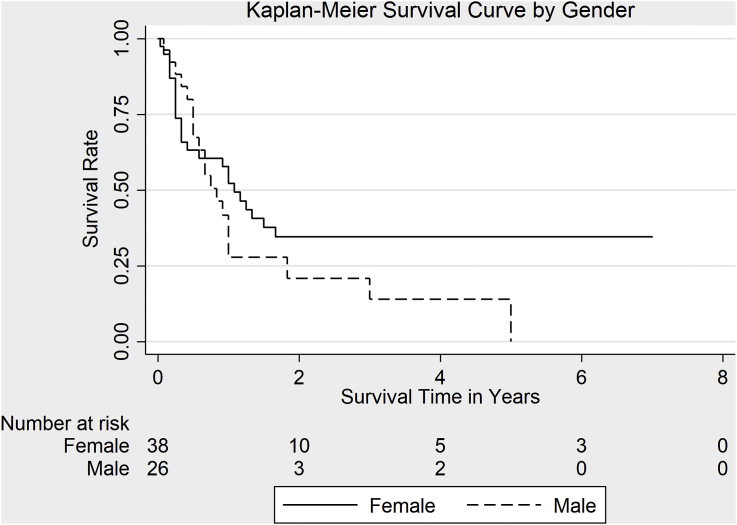
A total of 64 out of the original 76 patients (84.2%) patients were followed up in the study till the end while the rest were censored; hence, survival analysis was only performed on 64 patients. The survival probability at 3 months was 79.4%. The percentage of patients remaining alive 1.0 year after admission to the hospital was 42.9%. About 71% of deaths occurred within 2 years (Table 3). The overall median survival time for at-risk patients was estimated to be 1.0 year (Fig. 1). Estimated median survival time was 13.0 months in women and 10.0 months in men (Fig. 2). There was no statistically significant difference in survival rates for men and women, (χ2 (1) = 1.86, p = 0.17).
Table 3.
Survival rates using the Kaplan Meier Estimator.
| Time | Beginning Total | Net Fail | Lost | Survivor Function | SE | [95% CI] |
|---|---|---|---|---|---|---|
| 3 Months | 56 | 6 | 0 | 0.7943 | 0.0509 | 0.6722–0.8750 |
| 6 Months | 43 | 3 | 1 | 0.6495 | 0.0603 | 0.5180–0.7535 |
| 1 Year | 30 | 5 | 3 | 0.4294 | 0.0637 | 0.3034–0.5492 |
| 5 Years | 5 | 1 | 1 | 0.2054 | 0.0678 | 0.0924–0.3493 |
Fig. 1.
Overall survival rate in 76 patients with gallbladder cancer enrolled in this study.
Fig. 2.
Survival rate in men and women with gallbladder cancer enrolled in this study.
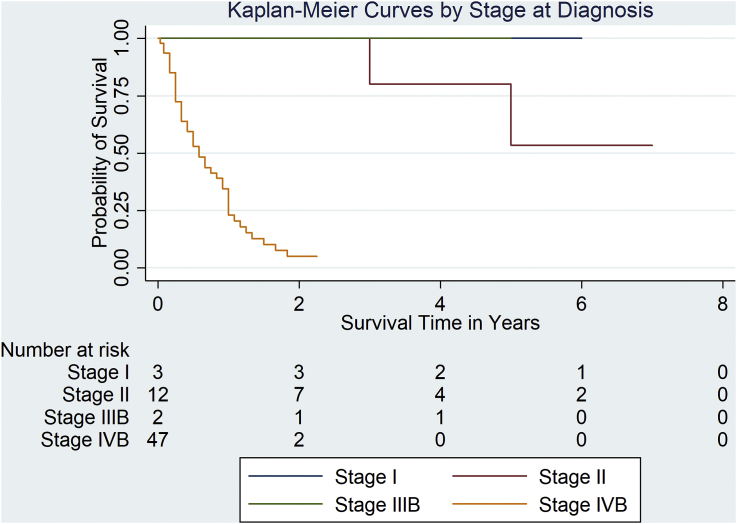
The estimated median survival time for Stage IVB patients was 7.0 months. The median survival times for patients in stages I, II, and IIIB were indeterminable because of either censoring of patients within each stage or the survival rate being less than 50.0% by the end of the study period (Fig. 3).
Fig. 3.
Predicted survival time based on stage of disease on diagnosis.
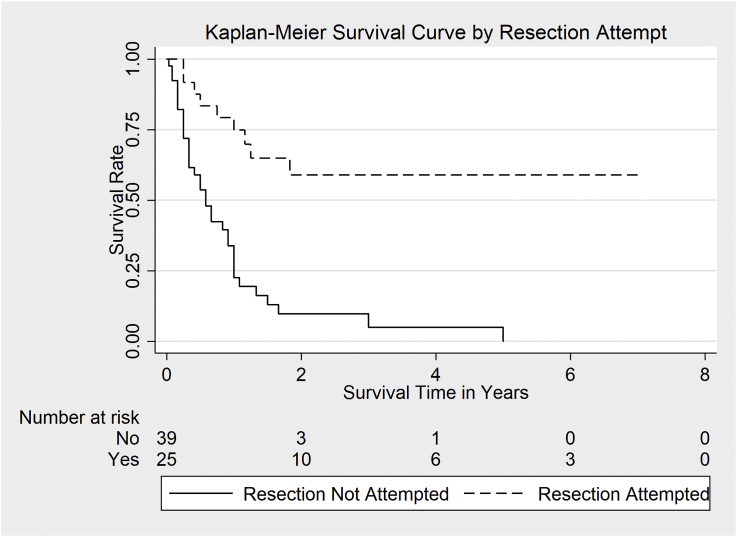
The 5-year survival probability in the enrolled patients was 20.5% (95% CI: 9.2%–34.9%). Survival rate was significantly higher in patients undergoing surgical resection (58.9% CI: 35.2%–76.5%, n = 25) (χ2 (1) = 21.6, p < 0.00) than in those for whom resection was not attempted (0.0%, n = 39) (Fig. 4).
Fig. 4.
Survival in patients who underwent surgical resection of the tumor vs those who did not.
Overall, the outcome for all study patients enrolled in our study was poor with 57.9% of the cases classified as fatal upon diagnosis. 26.3% of the total enrolled patients either survived beyond the study period or were lost to follow up for various reasons and 15.8% (n = 12) did not attend follow-ups from the onset leading to their exclusion from survival analysis (Table 3). This explains why the initial number at risk in the K-M survival curves was 64 as opposed to 76.
Overall, recurrence was observed in 10.5% of the patients after surgical resection with disease-free margin, of which 50.0% were diagnosed with stage II disease and the rest with stage IVB. No recurrence was reported in 18.4% of patients, while 71.0% (n = 54) of patients were diagnosed with unresectable tumor or were lost on follow-up (Table 2).
4. Discussion
This study sought to determine the incidence rate and survival rates associated with GBC at our institution between 2010 and 2017. GBC was more prevalent in women with a median age of 61 years. The high incidence of GBC in women and the median age for prevalence found in this study are consistent with the findings of Kumar and Choudhary [8] in India and Butte et al. [9] in USA, Japan, and Chile. Similarly, Dorobisz [10] reported that 75.0% of GBC patients were female and in their mid-60s [[11], [12], [13], [14]].
GBC is an aggressive disease with high mortality rate [1]. The incidence of the disease differs greatly based on heritable genetic traits and the level of environmental exposure to risk factors [2,14]. The disease is mainly asymptomatic until it reaches an advanced phase. In symptomatic patients, symptoms are often non-specific [2,15,16]. The same was observed in the cases enrolled in our study, with non-specific right upper quadrant pain being the most noted complaint in this cohort. Besides, diagnosis is also challenging [12] leading to a high chance for spread and later recurrence. In our study, most patients were diagnosed with GBC stage IVB. Indeed, about 10.5% of the patients in this study experienced a recurrence of the disease following a successful surgical resection.
Carcinomas are the most common malignancies of the gallbladder. Adenocarcinomas are reported in 90.0% of GBC cases [13]. We also observed that adenocarcinoma NOS was present in 75.0% of the patients in this cohort, followed by papillary adenocarcinoma and mucinous adenocarcinoma.
Incidental detection of GBC can occur in up to 70.0% of cases [12]. Such finding might occur during surgical treatment of the patient for suspicion of other gallbladder diseases [2]. In the present study, about 43.0% of the cases were diagnosed as incidental cases of gallbladder carcinoma, with the primary clinical diagnosis in over 40.0% being biliary colic followed by acute cholecystitis. Fortunately, the introduction of laparoscopic surgeries has improved the frequency of performing surgical removal of the gallbladder and increasing the rate of diagnosis for GBC [[17], [18], [19], [20]].
Advanced disease is reportedly followed by low survival rate [21], and most GBC cases do not survive more than 6 months after diagnosis [22], with only 5.0% of patients surviving 5 years [23]. In this study, most patients presented with fatal disease and all patients with stage IVB disease died within 1 year of diagnosis. Furthermore, in previous reports, the disease stages in 55.0% of study participants were reported to be classified as stages IA and IB upon diagnosis of GBC [24], while only 3.9% of the patients enrolled in this study were classified as having stage I disease.
Gallstones are considered risk factors for GBC. Indeed, studies have demonstrated that 85% of individuals with GBC had a positive history for gallstones [2,25,26], which is close to the 75% incidence of gallstones in our cohort.
There is reportedly a low probability that patients with gallstones will end with GBC, with only 31.6% of patients with a history of cholelithiasis having experienced GBC. Hence, ethnic predilections for the illness should be considered [[25], [26], [27]]. The size of the gallstones might also play a role in determining the frequency at which GBC develops. Reportedly, people with stones that are bigger than 3 cm in diameter are up to ten times more likely to suffer from GBC compared with patients who have smaller stones [26,28]. In the current study, 57.9% had several small stones less than 1.0 cm in size, which is contradictory to previous findings. Since, the type gallstones often matter in the development of GBC, with cholesterol gallstones being more common in GBC patients, the discrepancy observed in this study might be due to a major difference in the type of gallstone in the Saudi population.
Previous studies showed that obesity is a predisposition for the development of GBC [29]. The likelihood of developing the disease increases by 1.1 for men and 1.6 for women with every 5-point rise in BMI [30,31]. It is known that metabolic syndrome exacerbates serious co-morbidities, such as diabetes, in people with a BMI of >30 kg/m2, and diabetes is a strong risk factor for the formation of gallstones [32]. In this study, about 32% of cases had a BMI between 31.0 and 40.0 kg/m2. Whether the link between obesity and diabetes mellitus is strong enough to lead to GBC is not well understood and it may be hypothesized that obesity results in a compromised endocrine state that increases the risk GBS. Hence, with the variability in the geographical and ethnic prevalence of obesity, it is possible that the prevalence of GBC follows a similar pattern. However, evidence in this area is lacking and requires further studies.
The treatment of choice in cases of advanced disease is extended radical resections, which reportedly increases survival rates [[33], [34], [35], [36], [37]]. Adjuvant chemoradiotherapy (CRT), though less effective than surgical resection, has been recommended in cases where surgery is not feasible [38]. However, adjuvant CRT should be included alongside surgical therapy as a means of improving survival [39], with reportedly significant improvement in survival rate for patients with pT2-T3N0 disease. The biggest improvement was reported in patients with pT4 or pN + disease [40]. Adjuvant chemotherapy alone provided little improvement for patients with pT4 or pN + disease. However, this was significantly less than the advantages seen with adjuvant CRT [40]. Overall, surgical treatment remains the most effective treatment modality, irrespective of adjuvant CRT [41].
Surgical resection was attempted in 40.8% of the patients in this study, with port sites excision in 17.0% and bile duct excision in approximately 26.0% of the participants. Lymphadenectomy was also performed in 31.6% of patients. It should be noted that despite employing surgical resection as the treatment of choice in this study, the survival rate for most participants was 1 year, which was similar to that reported in previous studies [[42], [43], [44], [45], [46], [47], [48]].
The present study reinforces that the low prevalence of GBC in Saudi Arabia could be a manifestation of the low diagnostic rates of the condition because of its subtle and non-specific symptoms. It is important for researchers to assess risk factors associated with GBC to further the understanding of the pathogenesis of the disease, which might reveal the reason for the ethnic and geographic variances and provide clues for earlier diagnosis, improved treatment and prevention methods.
5. Strengths and limitations
Lower prevalence in Saudi Arabia and small sample size have often limited studies on this subject [49]. For this reason, the present study included patient data for a period of 7 years to enable as large a sample population as possible. Another strength of the study was the inclusion of multiple risk factors to GBC like diabetes, smoking, and the presence of gallstones. This allowed greater insights into the potential risk factors that might have a role in the variability in the prevalence of this disease.
One of the major limitations of the study is that it is restricted to patients from a single clinical site, which, while reducing differences in diagnostic criteria and methods, negatively affect the generalizability of the results. Larger, multi-centric studies are required to obtain a clear picture about the current prevalence of GBC in Saudi Arabia. Although we might have mentioned some of the factors that might increase the risk of GBC, we did not analyze the potential links between these factors and the prevalence of GBC, similar to a previous study [50]. Such analyses could provide possible etiological and mechanistic clues to explain the variable prevalence of the disease.
6. Conclusion
The present study found that the prevalence of GBC in Saudi Arabia is low, and is higher in women, with diabetes, hypertension, and presence of gallstones being the most common co-morbidities present in patients with GBC. Symptom profiles remain non-specific with right upper quadrant abdominal pain being the most common. Surgery remains the most common treatment strategy for patients with GBC. Nonetheless, associated diagnoses and survival, both before and after surgical interventions remain poor. The findings in the present study further reinforce the need for quicker and more efficient diagnostic methods for GBC and suggest that the low prevalence could be a manifestation of diagnostic challenges inherent to this condition.
Declarations
Ethical approval
Ethical approval was obtained from the Institutional Review Board (IRB) of the King Fahad Specialist Hospital, Dammam, Saudi Arabia. The ethical approval was signed on 19th July 2017 and was issued an IRB Study Number – SUR0314.
Funding sources
This study did not receive any funding from governmental or private organizations.
Authors' contributions
All authors MYD, AAA, SQ, SSA, MN, MSQ participated in concept, study design, data collection, and literature search. Data was analyzed by MYD. Writing of the paper were made by MYD and MSQ. All authors read and approved the final manuscript.Guarantor.
Conflicts of interest
The authors declare no conflict of interest.
Trial registry number
Uin = 4090.
Guarantor
Mohammed Yousef Aldossary.
Acknowledgements
Not applicable.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Provenance and peer review
Not commissioned, externally peer reviewed.
Footnotes
List of abbreviations: GBC, gallbladder cancer; KSA, Kingdom of Saudi Arabia; AJCC, the American Joint Committee on cancer.
Supplementary data to this article can be found online at http://doi.org/10.1016/j.amsu.2018.09.020.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Lazcano-Ponce E.C., Miquel J.F., Muñoz N. Epidemiology and molecular pathology of gallbladder cancer. CA A Cancer J. Clin. 2001;51:349–369. doi: 10.3322/canjclin.51.6.349. [DOI] [PubMed] [Google Scholar]
- 2.Cavallaro A., Piccolo G., Di Vita M. Managing the incidentally detected gallbladder cancer: algorithms and controversies. Int. J. Surg. 2014;12:S108–S119. doi: 10.1016/j.ijsu.2014.08.367. [DOI] [PubMed] [Google Scholar]
- 3.Chsgovsa. (2018). Available at: http://www.chs.gov.sa/Ar/HealthCenters/NCC/CancerRegistry/CancerRegistryReports/2014.pdf (accessed 24 Feb. 2018).
- 4.Kanthan R., Senger J.L., Ahmed S. Gallbladder cancer in the 21st century. J. Oncol. 2015;160(2):134–141. doi: 10.1155/2015/967472. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edge S.B., Byr D.R., Compton C.C. seventh ed. Springer; New York, NY: 2010. AJCC Cancer Staging Manual. [Google Scholar]
- 6.Duffy A., Capanu M., Abou‐Alfa G.K. Gallbladder cancer (GBC): 10 year experience at memorial sloan‐kettering cancer centre (MSKCC) J. Surg. Oncol. 2008;98:485–489. doi: 10.1002/jso.21141. [DOI] [PubMed] [Google Scholar]
- 7.Agha R.A., Borrelli M.R., Vella-Baldacchino M., Thavayogan R., Orgill D.P., for the STROCSS Group The STROCSS statement: strengthening the reporting of cohort studies in surgery. Int. J. Surg. 2017;46:198–202. doi: 10.1016/j.ijsu.2017.08.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar D., Choudhary P. Clinical profile and outcome of gall bladder carcinoma. Int. J. Adv. Multidiscip. Res. 2017;4:14–26. [Google Scholar]
- 9.Butte J.M., Matsuo K., Gonen M. Gallbladder cancer: differences in presentation, surgical treatment, and survival in patients treated at centers in three countries. J. Am. Coll. Surg. 2011;212:50–61. doi: 10.1016/j.jamcollsurg.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Dorobisz T., Dorobisz K., Chabowski M. Incidental gallbladder cancer after cholecystectomy: 1990 to 2014. OncoTargets Ther. 2016;9:4913–4916. doi: 10.2147/OTT.S106580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henley S.J., Weir H.K., Jim M.A., Watson M., Richardson L.C. Gallbladder cancer incidence and mortality, United States 1999-2011. Cancer Epidemiol. Biomark. Prev. 2015;24:1319–1326. doi: 10.1158/1055-9965.EPI-15-0199. [DOI] [PubMed] [Google Scholar]
- 12.Marks E.I., Yee N.N. Molecular genetics and targeted therapeutics in biliary tract carcinoma. World J. Gastroenterol. 2016;22:1335–1347. doi: 10.3748/wjg.v22.i4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma A., Saharma K.L., Gupta A., Yadav A., Kumar A. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: recent update. World J. Gastroenterol. 2017;23:3978–3998. doi: 10.3748/wjg.v23.i22.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cariati A., Piromalli E., Cetta F. Gallbladder cancers: associated conditions, histological types, prognosis, and prevention. Eur. J. Gastroenterol. Hepatol. 2014;26:562–569. doi: 10.1097/MEG.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 15.Magge R., Diamond E.L. Neurologic complications of gastrointestinal cancer. Cancer Neurology in Clinical Canc. Neurol. Clin. Pract. 2018:471–484. [Google Scholar]
- 16.Sujata J., S R., Sabina K., Mj H., Jairaipuri Z.S. Incidental gall bladder carcinoma in laparoscopic cholecystectomy: a report of 6 cases and a Review of the literature. J. Clin. Diagn. Res. 2013;7:85–88. doi: 10.7860/JCDR/2012/5001.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Aretxabala X.A., Roa I.S., Mora J.P. Laparoscopic cholecystectomy: its effect on the prognosis of patients with gallbladder cancer. World J. Surg. 2004;28:544–547. doi: 10.1007/s00268-004-6886-6. [DOI] [PubMed] [Google Scholar]
- 18.Tran T.B., Nissen N.N. Surgery for gallbladder cancer in the US: a need for greater lymph node clearance. J. Gastroenterol. Oncol. 2015;6:452–458. doi: 10.3978/j.issn.2078-6891.2015.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S.E., Jang J.Y., Lim C.S., Kang M.J., Kim S.W. Systematic Review on the surgical treatment for T1 gallbladder cancer. World J. Gastroenterol. 2011;17:174–180. doi: 10.3748/wjg.v17.i2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aloia T.A., Jarufe N., Javle M. Gallbladder cancer: expert consensus statement. HPB. 2015;17:681–690. doi: 10.1111/hpb.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rakić M., Patrlj L., Kopljar M. Gallbladder cancer. Hepatobiliary Surg. Nutr. 2014;3:221–226. doi: 10.3978/j.issn.2304-3881.2014.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y.C., Chiou C., Lin M.N., Lin C.L. The prevalence and risk factors for gallstone disease in taiwanese vegetarians. PLoS One. 2014;9:e115145. doi: 10.1371/journal.pone.0115145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hundal R., Shaffer E.A. Gallbladder cancer: epidemiology and outcome. J. Clin. Epidemiol. 2014;6:99–109. doi: 10.2147/CLEP.S37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian Y.H., Ji X., Liu B., Yang G.Y., Meng X.F., Xia H.T., Wang J., Huang Z.Q., Dong J.H. Surgical treatment of incidental gallbladder cancer discovered during or following laparoscopic cholecystectomy. World J. Surg. 2015;39(3):746–752. doi: 10.1007/s00268-014-2864-9. [DOI] [PubMed] [Google Scholar]
- 25.Lau C.S.M., Zywot A., Mahendraraj K., Chamberlain R.S. Gallbladder carcinoma in the United States: a population based clinical outcomes study involving 22,343 patients from the surveillance, epidemiology, and end result database (1973–2013) HPB Surg. 2017;2017:1532835. doi: 10.1155/2017/1532835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stinton L.M., Shaffer E.A. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut. Liver. 2012;6:172–187. doi: 10.5009/gnl.2012.6.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnelldorfer T. Porcelain gallbladder: a benign process or concern for malignancy? J. Gastrintest. Surg. 2013;17:1161–1168. doi: 10.1007/s11605-013-2170-0. [DOI] [PubMed] [Google Scholar]
- 28.Raina A., Bajpai M., Indubhushan Incidental gallbladder carcinoma - a retrospective study. IOSR J. Dent. Med. Sci. 2016;2:114–116. [Google Scholar]
- 29.Azvolinsky A. The obesity–cancer link: a growing connection. J. Natl. Cancer Inst. 2016;108:243. doi: 10.1093/jnci/djw243. [DOI] [PubMed] [Google Scholar]
- 30.Hariharan D., Saied A., Kocher H.M. Analysis of mortality rates for gallbladder cancer across the world. HPB. 2008;10:327–331. doi: 10.1080/13651820802007464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolin K.Y., Carson K., Colditz G.A. Obesity and cancer. Oncologist. 2010;15:556–565. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai H.C., Chang S.N., Lin C.C. Does diabetes mellitus with or without gallstones increase the risk of gallbladder cancer? Results from a population-based cohort study. J. Gastroenterol. 2013;48:856–865. doi: 10.1007/s00535-012-0683-z. [DOI] [PubMed] [Google Scholar]
- 33.Miyazaki M., Itoh H., Ambiru S., Shimizu H., Togawa A., Gohchi E., Nakajima N., Suwa T. Radical surgery for advanced gallbladder carcinoma. Br. J. Surg. 1996;83:478–481. doi: 10.1002/bjs.1800830413. [DOI] [PubMed] [Google Scholar]
- 34.Kai M., Chijiiwa K., Ohuchida J., Nagano M., Hiyoshi M., Kondo K. A curative resection improves the postoperative survival rate even in patients with advanced gallbladder carcinoma. J. Gastrointest. Surg. 2007;11:1025–1032. doi: 10.1007/s11605-007-0181-4. [DOI] [PubMed] [Google Scholar]
- 35.Chan S.Y., Poon R.T., Lo C.M., Ng K.K., Fan S.T. Management of carcinoma of the gallbladder: a single‐institution experience in 16 years. J. Surg. Oncol. 2008;97:156–164. doi: 10.1002/jso.20885. [DOI] [PubMed] [Google Scholar]
- 36.Yang X., Yuan J., Chen J., Yang J., Gao Q., Yan X., Zhang B., Feng S., Wu M. The prognostic importance of jaundice in surgical resection with curative intent for gallbladder cancer. BMC Canc. 2014;14:652. doi: 10.1186/1471-2407-14-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Misra S., Chaturvedi A., Misra N., Sharma I. Carcinoma of the gallbladder. Lancet Oncol. 2003;4:167–176. doi: 10.1016/s1470-2045(03)01021-0. [DOI] [PubMed] [Google Scholar]
- 38.Ghnnam W.M., Elbeshry T.M.A., Malek J.R. Incidental gallbladder carcinoma in laparoscopic cholecystectomy: five years local experience. Elective Med. J. 2014;2:47–67. [Google Scholar]
- 39.Todoroki T., Kawamoto T., Takahashi H., Takada Y., Koike N., Otsuka M., Fukao K. Treatment of gallbladder cancer by radical resection. Br. J. Surg. 1999;86:622–627. doi: 10.1046/j.1365-2168.1999.01085.x. [DOI] [PubMed] [Google Scholar]
- 40.Wang S.J., Lemieux A., Kalpathy-Cramer J. Nomogram for predicting the benefit of adjuvant chemoradiotherapy for resected gallbladder cancer. J. Clin. Oncol. 2011;29:4627–4632. doi: 10.1200/JCO.2010.33.8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogura Y., Mizumoto R., Isaji S., Kusuda T., Matsuda S., Tabata M. Radical operations for carcinoma of the gallbladder: present status in Japan. World J. Surg. 1991;15:337–343. doi: 10.1007/BF01658725. [DOI] [PubMed] [Google Scholar]
- 42.Mishra P.K., Sundeep S.S., Prithiviraj N., Varshney V., Goel N., Patil N. Predictors of curative resection and long term survival of gallbladder cancer - a retrospective analysis. Am. J. Surg. 2017;214:278–286. doi: 10.1016/j.amjsurg.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Memon M.A., Anwar S., Shiwani M.H., Memon B. Gallbladder carcinoma: a retrospective analysis of twenty-two years experience of a single teaching hospital. Int. Semin. Surg. Oncol. 2005;2:6. doi: 10.1186/1477-7800-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodrigues Santos C., Monteiro M., Castro L., Gonçalves R., Guedon G., Neto J., Salas H., Meton F., Albagli R. Gallbladder cancer: morbimortality and survival in patients treated at the Brazilian National Cancer Institute – INCA. HPB. 2016;18:473–474. [Google Scholar]
- 45.Grobmyer S.R., Lieberman M.D., Daly J.M. Gallbladder cancer in the twentieth century: single institution's experience. World J. Surg. 2004;28:47–49. doi: 10.1007/s00268-003-7131-4. [DOI] [PubMed] [Google Scholar]
- 46.Singh S.K., Talwar R., Kannan N., Tyagi A.K., Jaiswal P., Kumar A. Aggressive surgical approach for gallbladder cancer: a single-center experience from northern India. J. Gastrointest. Canc. 2015;46:399–407. doi: 10.1007/s12029-015-9766-4. [DOI] [PubMed] [Google Scholar]
- 47.Lilic N., Addison B., Hammodat H. Gallbladder carcinoma: a New Zealand centre's 10‐year experience with presentation, ethnic diversity and survival rate. ANZ J. Surg. 2015;85:260–263. doi: 10.1111/ans.12503. [DOI] [PubMed] [Google Scholar]
- 48.D'Hondt M., Lapointe R., Benamira Z. Carcinoma of the gallbladder: patterns of presentation, prognostic factors and survival rate. An 11-year single center experience. Eur. J. Surg. Oncol. 2013;39:548–553. doi: 10.1016/j.ejso.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 49.Mohamed A., Emran F., Ghanem N., Mohamed A. Gallbladder carcinoma, improving diagnosis and outcome. Internet J. Surg. 2009;23:1–11. [Google Scholar]
- 50.Zahrani I.H., Mansoor I. Gallbladder pathologies and cholelithiasis. Saudi Med. J. 2001;22:885–889. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.