Abstract
Glioblastoma (GBM) represents the most common, malignant and lethal primary brain tumour in adults. The primary cilium is a highly conserved and dynamic organelle that protrudes from the apical surface of virtually every type of mammalian cell. There is increasing evidence that abnormal cilia are involved in cancer progression, since primary cilia regulate cell cycle and signalling transduction. In this review, we summarize the role of primary cilium specifically with regard to GBM, where there is evidence postulating it as a critical mediator of GBM tumorigenesis and progression. This opens the way to the application of cilia-targeted therapies (‘ciliotherapy’) as a new approach in the fight against this devastating tumour.
Keywords: ciliogenesis, glioblastoma, glioma stem cells, molecular mechanism, primary cilium
Introduction
Glioblastoma (GBM) is a grade IV diffuse glioma according to the current criteria established by the World Health Organization (WHO)1 and represents the most common, malignant and lethal primary brain tumour in adults. Indeed, GBM accounts for about 50% of all gliomas and 45–50% of malignant primary brain and central nervous system (CNS) tumours, with an age-adjusted incidence ranging from 0.59 to 3.69 cases per 100,000 individuals.2,3 Because of its highly invasive nature, rapidly infiltrating the surrounding brain parenchyma, GBM has poor prognostic and survival rates with a median overall survival of 15 months and a 5-year survival rate of less than 5%,2 despite aggressive treatment combining surgery, radiotherapy and temozolomide (TMZ)-based chemotherapy.4 From a clinical perspective, two major GBM subtypes can be defined: primary or de novo GBMs comprise around 90% of total GBMs and mainly affect patients over 55 years old without any prior finding, whereas secondary GBMs develop in younger individuals from a previous lower-grade glioma and carry better prognosis.5 Nonetheless, the genomic and transcriptomic data obtained by The Cancer Genome Atlas Network (TCGA)6,7 have led to a molecular stratification of GBM based on genetic abnormalities and signalling pathways affected, establishing four subtypes: classical, mesenchymal, proneural and neural.7,8 Thus, classical GBMs are mainly defined by high-level EGFR amplification and overexpression, PTEN and CDKN2A deletion, absence of TP53 mutations, high expression of the neural/stem cell marker NES and activation of Sonic hedgehog (Shh) and Notch pathways. On its part, the mesenchymal subtype shows loss of NF1, mutations in PTEN and NF1, expression of mesenchymal markers such as CHI3L1 and MET, activation of mitogen-activated protein kinase (MAPK) signalling and high expression of genes involved in tumour necrosis factor (TNF) and nuclear factor kappa B (NF-κB) pathways. Proneural GBMs are characterized by amplification, overexpression and mutation of PDGFRA, mutations in IDH1 and TP53, expression of genes involved in proneural (such as SOX gene family) and oligodendrocytic development, and activation of the phosphatidylinositol-3-kinase (PI3K) pathway. Finally, neural GBM lacks a particular genetic profile but distinctively expresses neuronal markers such as NEFL, GABRA1, SYT1 and SLC12A5. Although clinical and molecular subtypes do not fully overlap, a significant correlation has been established between primary GBMs and the classical subtype, on the one hand, and secondary GBMs with the proneural subtype, on the other;2,7,8 however, the presence or absence of IDH1/2 mutations is the only diagnostic molecular marker considered by WHO to sub-classify GBM, so that primary GBMs are IDH-wildtype while IDH-mutated tumours correspond closely to secondary GBM.1,5 Furthermore, differences in prognosis and response to treatment have been described for these molecular subtypes, with proneural GBM displaying better prognosis and improved survival that are not related to the first-line multimodal treatment, which correlates with a delayed mortality in both classical and mesenchymal subtypes.7,8 The better outcome observed in proneural GBM is probably due to the presence of widespread hypermethylation across the genome, the so-called glioma-CpG island methylator phenotype or G-CIMP, which is tightly associated with the presence of IDH1/2 mutations.7,9 In this regard, MGMT promoter methylation has also been established as a good predictive marker for treatment response to alkylating chemotherapeutic agents such as TMZ,10,11 but recent work links this finding only to the classical GBM subtype.7
Unfortunately, GBM is currently considered incurable due to several intrinsic factors. First, the high morphological, cellular, molecular and genetic heterogeneity reported, mostly at intratumoral level, is believed to be a crucial factor for therapy failure and tumour relapse in GBM.12–15 Second, and even more importantly, GBMs harbour a small population of highly tumorigenic, self-renewing glioma stem cells (GSCs) that decisively contribute to tumour initiation, maintenance and recurrence, and also to radio- and chemotherapy resistance.16–18 There is therefore an urgent need to develop further research in GBM pathogenesis in order to identify new potential targets for treatment. In this sense, several recent works have addressed the role of primary cilium and ciliogenesis in GBM, which could represent an important niche scarcely explored until now.
The primary cilium, also known as immotile or sensorial cilium, is a highly conserved and dynamic organelle that protrudes from the apical surface of virtually every type of mammalian cell in a single copy.19,20 Primary cilia are composed of nine microtubule doublets without a central pair (9 + 0 structure) that form the ciliary axoneme, which is surrounded by a bilayer lipid membrane that is continuous with the plasma membrane but enriched in specific proteins and lipids required for ciliary activity; this particular composition is maintained through a specialized region at the proximal axoneme named the transition zone, which acts as a ciliary gate (see Figure 1).21,22
Figure 1.
Ciliary structure and trafficking processes at the primary cilium.
Cilia-targeted proteins are transported in Golgi-derived vesicles to primary cilia, where these vesicles can fuse with the ciliary pocket to enter the cilium, or alternatively with the plasma membrane from which they will then be transported to the ciliary pocket. Once into the base of the cilium, the ciliary cargoes are assembled into IFT particles and move along the axoneme to the tip (anterograde transport) or to the base (retrograde transport) to fulfil their specific roles in cilia, in a process mediated by the kinesin-2 and cytoplasmic dynein-2 motors. Importantly, the ciliary pocket represents an active site for exo- and endocytosis of membrane receptors, from which they can be recycled via endosomal recycling pathways to regulate signalling. In addition, primary cilia are able to release ciliary extracellular vesicles that could modulate signalling processes.
IFT, intraflagellar transport.
The axoneme is nucleated by the centrosomal mother centriole, which migrates to the ciliary assembly site and turns into the basal body of the mature primary cilium.23,24 The success of a correct cilia elongation and function relies on a bidirectional transport system called intraflagellar transport (IFT), which carries cilia-targeted cargoes in and out of the cilium by two large protein complexes (IFT particles).25,26 Once these particles are assembled, they move onto the microtubules along the axoneme either to the tip (anterograde transport) or to the base (retrograde transport) of the cilium in a process mediated by kinesin-2 and cytoplasmic dynein-2 motors, respectively (Figure 1). The primary cilium is widely considered the sensorial antenna of the cell, acting as a central hub for coordinating most of cellular signalling pathways such as Shh, Wingless/int (Wnt), transforming growth factor beta (TGF-β), Notch and mechanistic target of rapamycin kinase (mTOR), as well as signalling cascades mediated by receptor tyrosine kinases (RTKs), G-protein coupled receptors (GPCRs), purinergic receptors, ion channels or receptors for extracellular matrix proteins.27,28 Thus, by means of a receptor-enriched ciliary membrane, primary cilia are able to capture extracellular signals and integrate them to elaborate biological responses that involve cell cycle control, development and differentiation processes, migration and polarity, or proliferation and maintenance of stem cells.20 Focusing on the brain, the primary cilium activity is pivotal to regulate several stages of neurogenesis including early brain patterning, proliferation and differentiation of neural progenitor/stem cells, and neuronal maturation and maintenance.29,30 In fact, neuronal cilia are abundant and have a widespread distribution in the brain, which combined with the neuronal ciliary membrane enrichment in signalling elements such as GPCRs [somatostatin receptor 3 (SSTR3), melanin concentrating hormone receptor 1 (MCHR1), 5-hydroxytryptamine receptor 6 (HTR6), among others] and downstream signalling molecules [adenylate cyclase 3 (ADCY3)], suggests neuronal cilia can mediate neurotransmitter and hormone-mediated signalling to modulate neural activity.28,31 However, little is known about the role of primary cilia in glial cells (see below).
Defects in structure and/or function of cilia is the common aetiology of an expanding and heterogeneous group of inherited disorders collectively termed ciliopathies, mainly affecting primary cilium.32,33 Remarkably, many ciliopathies display neurological features (e.g. cognitive impairment, developmental delay, ataxia, brain malformations)34 and obesity, which have been related to cilia loss and mislocalization of ciliary receptors in hypothalamic neurons;35 these findings highlight that defects in neuronal cilia could lead to disturbed signalling pathways that disrupt brain homeostasis and result in disease phenotypes.31 Furthermore, there is increasing evidence that abnormal cilia are likely involved in cancer progression, since primary cilia regulate cell cycle and signalling transduction.36–38 On the one hand, ciliogenesis is tightly coupled to the cell cycle, so ciliary disassembly is a requisite to liberate the centriole and thereby promote mitotic spindle formation;39 primary cilium is in fact considered as a structural checkpoint for cell cycle re-entry. In this sense, loss or decrease of cilia is a commonly reported feature in different malignant tumours such as breast cancer, pancreatic adenocarcinoma, melanoma or renal cell carcinoma, so primary cilium has been proposed to act as a tumour suppressor organelle.37,38 On the other hand, many cancers display alterations in different signalling cascades, especially involving the upregulation of Shh signalling,36,40 which in turn constitutes the best-characterized cilia-regulated pathway.41 Finally, the emerging interplay between cilia and autophagia, a proteolytic mechanism essential to maintain homeostasis that is deregulated in cancer, represents additional evidence that strongly links cilia and cancer.42,43
Linking primary cilium and glioblastoma: current evidence
The role of primary cilium in glial cells, and more specifically with regard to astrocytes as GBM is a high-grade astrocytic malignancy, has been addressed in several works over the last years. Although much less is known compared with neuronal primary cilia, there is widespread evidence that glial cells possess a functional primary cilium, both in vivo and in cultured glial cells, which has been recently characterized.44–47 Structurally, only small populations of astrocytic cilia are ADCY3-positive, unlike neuronal primary cilia, which strongly associate with ADCY3 expression; conversely, the primary cilium assembled by astrocytes is better marked by ADP ribosylation factor-like GTPase 13B (ARL13B),47,48 a well-known ciliary protein of the Arf-like small GTPase family involved in ciliogenesis and ciliary trafficking.49 Concerning the functional implications of astrocytic primary cilia, these organelles have been mainly involved in controlling the expansion of radial astrocytes, considered the adult neural stem cells. Thus, radial astrocytes develop a primary cilium that is required to mediate the Shh-dependent proliferation and/or maintenance of these progenitors in adult hippocampus,50,51 as well as in the ventricular–subventricular zone, the major CNS postnatal germinal niche.52 Furthermore, primary cilium was described to act as the central hub that integrates and transduces the Shh signalling in astrocytes in order to regulate cell survival under stress conditions.46 Remarkably, key components of the Shh machinery such as the transducer Smoothened, frizzled class receptor (SMO) or the membrane receptor Patched 1 (PTCH1) localize to astrocytic primary cilia.46
Early work describing ciliated cells in human astrocytoma biopsies dates from the early 1970s,53 though it was not until 2009 that Moser and colleagues54 reported the first comprehensive comparison of ciliary expression profiles between normal human astrocytes and five human astrocytoma/GBM cell lines (U-87 MG, T98G, U-251 MG, U-373 MG and U-138 MG). This pioneering study proposed for the first time that aberrant ciliogenesis is a common feature in GBM-derived cells and it may contribute to developing malignant phenotypes. In detail, primary cilia formation was disrupted at early stages so that fully formed cilia were either completely absent, extremely rare or abnormal after incomplete ciliogenesis.54 In this sense, significant differences depending on the specific cell line were reported – for example, only U-87 MG cells were able to assemble an immature axoneme (with a frequency less than 1%) while the T98G cell line was never documented to initiate ciliogenesis, with the remaining lines displaying cilia in intermediate stages.54 In addition, centriolar findings such as abnormally shorter or longer centrioles as well as structural anomalies in distal/sub-distal appendages were also reported.54 Further work by the same authors corroborated these results in human GBM biopsies, with six of the seven patients analysed showing the previously reported defects in ciliogenesis, although extensive patient heterogeneity was noted.55 Based on these two descriptive studies, Moser and colleagues pointed out that abnormal primary cilia constitute a hallmark of GBM pathology. In contrast to the latter studies, a larger analysis of 23 GBM biopsies and five primary human GBM cell lines showed that small subpopulations in all cells/biopsies have mature primary cilia (8–25% of the total in each cell line/biopsy), but non-ciliated cells with abnormal centrioles were also identified.56 Moreover, cilia were observed all across the tumour microenvironment in the case of GBM tumours, including necrotic areas and the vasculature vicinity.56 Remarkably, these primary cilia are likely functional since well-known ciliary trafficking proteins such as intraflagellar transport 88 (IFT88) and ARL13B were localized to axonemes, indicating transport activity.56 Taken together, it seems that GBM cells, from both cell lines and tumour tissue, are not generally able to extend primary cilia; this observation is consistent with serum starvation experiments (common protocol to arrest cultured cells in G0 and promote primary cilia assembly) that did not show an enhanced ciliogenesis in GBM cells.54,56 However, further experiments are needed to confirm these ideas, especially in patient-derived primary cultures, as cell lines do not precisely reflect human GBM characteristics, and also in longer human cohorts. Interestingly, the downregulation of cilia-related genes in GBM recently reported by Shpak and colleagues57 based on TCGA gene expression annotations may give support to the decreased ciliation observed in this malignancy.
But apart from descriptive data, what is known about the functional significance of primary cilia in GBM initiation and progression? There is currently little evidence on whether GBM development is cilia-dependent, but several recent works have made substantial progress to determine if primary cilia can affect GBM proliferation. It is necessary to note that cilia can either induce or suppress tumorigenesis, depending on the oncogenic driver event,58,59 and that both roles are likely to take place in GBM. Thus, Yang and colleagues60 established for the first time that primary cilia loss mediated by cyclin-dependent kinase 20 (CDK20; formerly named as cell-cycle related kinase or CCRK) overexpression promotes GBM proliferation in the U-251 MG cell line; this abnormal growth was inhibited when depleting CDK20/CCRK and subsequently ciliogenesis was restored. Notably, this protein was reported to be highly expressed in glioma patients.61 This result, together with the reduced ciliogenesis observed in GBM cells and biopsies,54–56 suggests that inhibition of ciliogenesis and subsequent primary cilia loss is a mechanism that enhances unrestrained growth in GBM. In this sense, a recent work by Loskutov and colleagues62 also supports that ciliary loss is necessary to maintain a highly proliferative phenotype in GBM. On the other hand, subpopulations of GBM cells that extend mature primary cilia also seem to contribute to tumour growth since they express Ki-67, a well-known marker of actively dividing cells.56 This indicates that primary cilia loss could be independent of high proliferation rates and may occur due to alterations in (an)other mechanism(s) related to ciliogenesis, as previously suggested for pancreatic cancer cells.63 Despite the evidence pointing out that GBM progression is cilia-dependent, other studies targeting kinesin family member 3A (KIF3A), a component of the kinesin-2 motor that is required for ciliogenesis and has key roles in other CNS cancers such as medulloblastoma,58,64 yielded inconclusive results in GBM cells.65 As further explained below, when KIF3A expression was disrupted using a lentiviral vector in three patient-derived GBM cell lines, ciliogenesis was almost entirely ablated but opposite, cell-line-specific effects were observed regarding GBM proliferation and tumour-associated survival,65 in agreement with a previous study on U-251 MG cells that reported minimal effects.60 However, several reports focused on the role of centrosomal proteins, which play pivotal roles in ciliogenesis and cell division,24 showed a clearer effect on GBM pathogenesis. Thus, depletion of pericentriolar material 1 (PCM1) through CRISPR/Cas9 genome editing in primary GBM cells lines, a component of centriolar satellites surrounding centrioles that is expressed in GBM cell lines and biopsies, reduced ciliogenesis and significantly decreased cell proliferation due to high apoptosis levels.66 In support of this, centrosomal protein 55 (CEP55), a recent member of the centrosome-related protein family that is both upregulated and associated with a shorter survival rate in glioma patients, was also shown to inhibit proliferation in U-87 MG, U-251 MG and T98G cells when depleted;67,68 it should be noted that, to our knowledge, neither a link between CP55 and primary cilia has been established, nor was the ciliary status of these three astrocytoma/GBM cell lines before and after CP55 depletion evaluated.
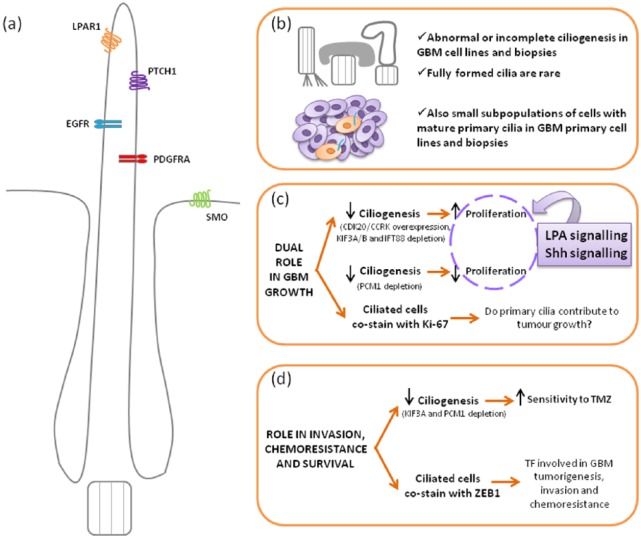
Furthermore, it has been proposed that primary cilia could play key roles in common features of GBM such as chemoresistance and tumour invasion. In this sense, while it is true that the functional significance of the ciliated GBM cell subpopulations still remains elusive, new evidence provides exciting avenues for further research. Intriguingly, GBM ciliated cells are known to express zinc finger E-box binding homeobox 1 (ZEB1),56 which is a transcription factor recently characterized as the central inducer of the epithelial–mesenchymal transition in GBM, leading to tumorigenesis, invasion and chemoresistance.69 In detail, ZEB1 has been proposed as a good candidate for tumour recurrence since it is mainly expressed in invasive GBM cells that also display poor sensitivity to TMZ mediated by MGMT, in addition to regulating the expression of glioma stemness-related factors such as sex determining region Y (SRY)-box 2 (SOX2) and oligodendrocyte transcription factor 2 (OLIG2); these results were confirmed in both primary GBM cell lines and xenograft models.69 Remarkably, about 45% of tumours analysed were ZEB1-positive and that correlated with worse prognosis (shorter survival rates and poorer response to TMZ treatment).69 In relation to this, the lack of primary cilium in PCM1-depleted and KIF3A-depleted primary GBM cells has been suggested to underlie the increased sensitivity to TMZ observed in such cells;66 therefore, ciliary proteins that mediate ciliogenesis, for example PCM1 and KIF3A, may modulate GBM survival. Moreover, Sarkisian and colleagues56 have pointed out that ARL13B, an astrocytic cilia marker also expressed in GBM ciliated cells as stated earlier, might play a guiding role for tumour cells to mediate GBM invasion since interneuronal migration in the developing brain is cilia-dependent and indeed requires ARL13B.70 Figure 2 summarizes the main roles of primary cilia in GBM.
Figure 2.
Schematic view of the main roles of the primary cilium in glioblastoma.
(a) Cartoon illustrating the typical mature primary cilium observed in human astrocytes and GBM samples, with large ciliary pockets; those membrane receptors that have been involved in GBM and localized to cilia are also represented. (b–d) Scheme of the current evidences of ciliary involvement in GBM focusing on three aspects: ciliary structure and distribution (b), role of primary cilia in GBM growth (c) and role in survival, chemoresistance and invasion properties (d).
CDK20/CCRK, cyclin-dependent kinase 20/cell-cycle related kinase; EGFR, epidermal growth factor receptor; GBM, glioblastoma; IFT88, intraflagellar transport 88; KIF3A/B, kinesin family member 3A/B; LPA, lysophosphatidic acid; LPAR1, lysophosphatidic acid receptor 1; PCM1, pericentriolar material 1; PDGFRA, platelet-derived growth factor receptor alpha; PTCH1, Patched 1; Shh, Sonic hedgehog; SMO, Smoothened, frizzled class receptor; TF, transcription factor; TMZ, temozolomide; ZEB1, zinc finger E-box binding homeobox.
On the other hand, it is worth considering the role of the regulatory factor X (RFX) family of transcription factors in GBM since they are well-established, key regulators of ciliary gene expression in animals.71,72 Thus, RFX1 is silenced in GBM cell lines and tumours due to differential intronic hypermethylation,73 and was also shown to inhibit GBM proliferation when overexpressed both in vitro and in vivo.73,74 RFX1 could therefore be a new tumour suppressor gene in GBM that would further impact cell invasion and survival, since it was demonstrated to inhibit CD44 expression and this led to a decrease in proliferation, invasion and survival of GBM cells.74 Interestingly, RFX1, RFX2 and RFX3 cooperate to modulate the maintenance of neural progenitors and GSCs through regulating fibroblast growth factor 1 (FGF1) promoter activity, an important mitogen for neurogenesis.75,76 It would be necessary to explore whether RFX1 mediates GBM progression through primary cilia, since this possibility was not addressed in the abovementioned studies. In this sense, although the biological role of RFX1 is not well defined and includes regulation of ciliary and non-ciliary targets,72 RFX1 and RFX2 were recently demonstrated to regulate the transcription level of ALMS1 gene, which encodes a protein localized to basal bodies and centrosomes that is mutated in Alström syndrome, a ciliopathy.77 It is also worth mentioning that some of the most well-characterized transcription factors in relation to GBM and GSCs such as signal transducer and activator of transcription 3 (STAT3) and several members of the SOX family have been linked to primary cilia; however, a direct regulation by primary cilium in GBM has not yet been reported. With regard to STAT3, it represents a convergence point for most of the oncogenic signalling pathways involved in GBM and is a pivotal promoter of GSC maintenance, tumour invasion, angiogenesis and immune evasion.78 Remarkably, mistrafficking of leptin receptor and other receptor complexes in hypothalamic neurons due to primary cilia dysfunction has been found to inactivate STAT3 signalling and lead to obesity phenotypes.79 Moreover, SOX factors are important mediators of tumorigenesis in a number of cancers such as GBM, where SOX2, SOX4 and SOX9 act as oncogenes that play central roles in the maintenance of GSCs to sustain cell stemness and tumorigenicity, and promote tumour proliferation, in addition to being associated with poor clinical outcomes.80 Intriguingly, combined knockout of Sox4 and Sox9 was reported to inhibit ciliogenesis in cholangiocytes, which could be related to the TGF-β and Notch deregulation observed in the deleted mice analysed.81
Primary cilium as potential mediator of signalling pathways involved in glioblastoma
The crucial role of primary cilium in mediating virtually all the cellular signalling cascades could anticipate an important but still scarcely explored function in GBM. In fact, deregulation of signalling pathways is a common feature underlying most tumours that decisively contributes to initiating tumorigenesis, as stated above. Moreover, primary cilia responsiveness to changing extracellular conditions could represent a strong adaptive mechanism to modulate tumour progression.38 It is also of interest to highlight that most of the altered signalling cascades related to GBM are well defined as cilia-regulated pathways. Thus, signal transduction mediated by RTKs such as the epidermal growth factor receptor (EGFR) and especially the platelet-derived growth factor receptor alpha (PDGFRA),27,82 as well as Shh,41 Wnt,83 TGF-β27,84 and Notch85,86 pathways, among others, are known to be coordinated by the primary cilium as sustained by a large body of evidence. It is therefore reasonable to think that primary cilia could be involved in the cellular signalling deregulation observed in many GBM patients. Since EGFR and PDGFRA are key drivers of classical and proneural GBMs, these subtypes might be more affected by cilia-related alterations.
Despite this, there is very little evidence on whether cilia-dependent regulation of signalling cascades is taking place in GBM, as detailed below. On the one hand, receptors of several pathways that significantly contribute to GBM have been localized to the primary cilium of astrocytes, such as PTCH1,46 EGFR and PDGFRA.87 In addition, the primary cilium assembled by human astrocytes was reported to have an invagination of the plasma membrane that surrounds the proximal axoneme and contains endocytic vesicles, called the “cilium-pit” [see Figure 2(a)].54 Remarkably, this membrane domain at the ciliary base was also detected in primary cilia from GBM tumours in both intact and abnormal form,55,56 as well as in GBM primary cell lines.56,65 This specialized structure corresponds to the one currently known as the ciliary pocket, which is well-characterized as an active site for exo- and endocytosis of receptors and other ciliary proteins that play a key role in cilia-mediated signalling.88,89 In this sense, endocytic control of receptor availability is required for modulating signal transduction in all the pathways listed above,90 a process that is tightly coupled to the ciliary pocket.89 Taken together, all these findings represent indirect evidence that primary cilia may actually be contributing to signalling disturbances found in GBM.
Turning now to functional studies, it is surprising that only three studies have so far investigated potential links between cilia and signalling in the pathological context of GBM, as will be described below. Of the three core pathways frequently altered in GBM – retinoblastoma (RB) signalling, tumour protein 53 (p53) signalling and RTK/RAS/PI3K signalling6 – only the latter has been shown to have some connection with primary cilia in GBM models. Thus, Yang and colleagues60 reported that the PI3K pathway, which is altered in nearly 90% of GBM,7 could be related to primary cilia loss via CDK20/CCRK. In this work, U-251 MG cells treated with LY294002, a broad-spectrum inhibitor of PI3Ks, were described to have increased ciliogenesis and reduced CDK20/CCRK mRNA levels.60 In the same study, the authors had demonstrated that CDK20/CCRK overexpression in U-251 MG cells led to primary cilia loss and subsequent increase in GBM proliferation, which is consistent with those findings after PI3K inhibition.60 Aberrant activation of the PI3K pathway could thereby impact cilia maintenance in GBM by promoting CDK20/CCRK expression,60 which would be particularly relevant for the proneural GBM subtype as it was reported to have increased activation of this signalling pathway.7 There is, however, no current evidence that primary cilium has any role in coordinating EGFR and PDGFRA-mediated signalling in GBM, although PDGFRA has long been associated with primary cilia27,82 and EGFR has recently been shown to suppress ciliogenesis in RPE1 cells, involving primary cilia in EGFR-mediated cell proliferation.91
On the other hand, Hoang-Minh and colleagues65 conducted a more comprehensive work focused on the Shh pathway and showed for the first time that some patient-derived GBM cell lines are able to transduce Shh-mediated signalling through primary cilia to promote cell proliferation in vitro. In detail, proliferation of L0 control cells (derived from a 43-year-old male patient) was increased after exogenous stimulation of the Shh pathway, whereas this effect was reversed with a cycloplamine pretreatment (a well-known inhibitor of Shh signalling by blocking SMO entry into cilia) and also by inhibition of ciliogenesis through KIF3A disruption, suggesting that unrestrained growth in GBM is Shh-dependent and mediated by primary cilia.65 Remarkably, ciliary recruitment of endogenous SMO and GLI family zinc finger 3 (GLI3) was observed following Shh stimulation.65 Moreover, mice xenografted with L0-KIF3A disrupted cells displayed longer survival rates than those transplanted with L0 control cells.65 Nevertheless, these effects were highly cell-line specific so that the S3 line (from a 75-year-old male) was completely unaffected by all the assayed experimental conditions, whereas the S2 line (from a 50-year-old male) behaved in the opposite way compared with the L0 cell line, showing non-response to Shh treatment in control cells but decreased cell proliferation after Shh stimulation in cells lacking KIF3A protein.65 In addition, xenografts with S2-KIF3A disrupted cells showed shorter survival times, possibly due to an increased tumorigenic activity of unmodified S2 cells as supported by the higher baseline proliferation rate observed in the absence of KIF3A/cilia in vitro.65 Primary cilia could therefore also mediate proliferation restraint in GBM, so it seems that the primary cilium is able to modulate GBM growth by Shh signalling, both in vitro and in vivo, playing a dual role in GBM tumorigenesis. This finding might have greater impact on the classical GBM subtype since it was described to highly express several components of this pathway,8 although a specific role for Shh signalling in IDH-mutated GBMs, which mostly correspond to the proneural subtype, has also been proposed.92 However, it is important to emphasize that, in comparison with medulloblastoma, where cilia and Shh pathway play a relevant role, GBM has few Shh or Shh-downstream target-dependent cases.
Finally, a very recent work by Loskutov and colleagues62 provides highly valuable information about how lysophosphatidic acid (LPA)-mediated signalling can significantly impact GBM proliferation via primary cilium. LPA is a recognized, lipid-based mitogen that is highly enriched in the brain and has a well-established role in GBM, where it mediates proliferation, invasion and angiogenesis properties.93 Importantly, LPA signals through binding on LPA receptors (LPARs), of which lysophosphatidic acid receptor 1 (LPAR1) is the predominant member in the CNS and is moreover highly expressed in GBM and GSCs.93 LPARs belong to the family of GPCRs, many of which are well-known to be specifically targeted to cilia;94,95 in this sense, endogenous LPAR1 was also recently found to localize to the astrocytic primary cilium.62 In this study, Loskutov and colleagues62 demonstrated that loss of primary cilium is enough to stimulate proliferation of human primary astrocytes as well as that this increased growth is LPA-dependent in a cilia-mediated manner. Thus, the authors described a cellular compartmentalization of the LPA signalling machinery in ciliated cells, which restricts LPA signalling and prevents unlimited proliferation in unmodified astrocytes, so that LPAR1 is confined to the cilium, whereas its downstream effectors, Gα12 and Gαq, are restricted to cytoplasm.62 When primary cilia are lost by IFT88 or KIF3B depletion, this physical barrier disappears and LPAR1 redistributes to the plasma membrane, where it associates with Gα12/Gαq to transduce LPA-mediated signalling, thereby promoting the unlimited growth of astrocytes and GBM patient-derived cells.62 Moreover, inhibition of the LPA pathway with Ki16425 (a LPAR1/3 antagonist) dramatically decreased cell growth rate in deciliated highly proliferative astrocytes, GBM patient-derived cells and xenografts.62 Even more relevant, intracranial injections of Ki16425 loaded into PEG-PLGA nanoparticles showed a great ability to inhibit tumoural growth in GBM patient-derived xenografts,62 which represents a promising therapeutic strategy.
Concluding remarks
The emerging role of primary cilia in tumorigenesis that has been reported over the last decade is bringing us a plethora of outstanding findings that are decisively contributing to a more in-depth understanding of cancer pathogenesis and also to development of novel, cilia-targeted therapeutic strategies. Thus, the key role of primary cilium in both cell cycle control and cellular signalling coordination anticipates an important contribution to cancer that is actually evidenced in its involvement in tumour initiation, invasion and migration, chemoresistance and cancer stem cell maintenance for many different tumours such as medulloblastoma or basal cell carcinoma as two relevant examples. Concerning GBM, considered the most aggressive primary brain tumour, several studies have started to address the potential role of this organelle in GBM progression, so the current evidence presented in this review represents a promising background that justifies further research in this field (summarized in Table 1). Briefly, it seems that most GBM cells fail to properly assemble primary cilia54,55 and only small subpopulations of ciliated cells can indeed be found in GBM tumours.56 Functionally, primary cilium is likely to play a dual role in GBM since ciliary loss was associated with unrestrained growth60,62 but also with decreased proliferation,66 whereas GBM ciliated cells might contribute to tumour growth since they co-stain with the cell proliferation marker Ki-67.56 In addition, primary cilium can modulate GBM proliferation through Shh65 and LPA62 signalling as well as the sensitivity to TMZ66 and possibly invasion and chemoresistance properties by ZEB1 expression in GBM ciliated cells.56 However, a number of challenging questions can be raised.
Table 1.
Main findings on the role of the primary cilium in glioblastoma.
| Year | Finding | Reference(s) |
|---|---|---|
| 2009 | First report of aberrant/failed ciliogenesis in human GBM cell lines | Moser et al.54 |
| 2013 | Primary cilia loss via CDK20/CCRK overexpression promotes GBM proliferation in U-251 MG cells, which could be partially mediated by aberrant activation of the PI3K pathway | Yang et al.60 |
| 2014 | Ciliogenesis is also disrupted in human GBM tumours | Moser et al.55 |
| 2014 | Small subpopulations of cells in GBM tumours are ciliated and co-stain with Ki-67 and ZEB1 | Sarkisian et al.56 |
| 2014 | Ciliary gene expression patterns are downregulated in GBM | Shpak et al.57 |
| 2016 | Primary cilium is able to modulate GBM proliferation through Shh signalling in patient-derived GBM cell lines, in a cell-line specific manner | Hoang-Minh et al.65 |
| 2016 | Reduced ciliogenesis via PCM1 depletion decreases proliferation and increases sensitivity to TMZ in patient-derived GBM cell lines | Hoang-Minh et al.66 |
| 2018 | Primary cilia loss is required to maintain highly proliferative phenotypes in patient-derived GBM cell lines by modulating LPA-mediated signalling | Loskutov et al.62 |
CDK20/CCRK, cyclin-dependent kinase 20/cell-cycle related kinase; GBM, glioblastoma; LPA, lysophosphatidic acid; PCM1, pericentriolar material 1; PI3K, phosphatidylinositol-3-kinase; Shh, Sonic hedgehog; TMZ, temozolomide; ZEB1, zinc finger E-box binding homeobox.
Is primary cilia loss a cause or a consequence of malignant transformation in GBM? When and how are primary cilia lost? Why do only certain GBM cell subpopulations remain ciliated? Are specific GBM subtypes more affected by changes in primary cilia? The available reports so far suggest that GBM cells display mechanisms to inhibit ciliogenesis;56,60 in fact, although only small percentages of ciliated cells are observed in GBM samples, most clones from patient-derived GBM cell cultures were shown to be able to form cilia.65 However, nothing is known about if ciliary loss occurs early in GBM tumorigenesis or whether primary cilia formation depends on the stage of differentiation of GBM cells. In this sense, it would be especially intriguing to investigate whether primary cilia could be associated with GSCs, as has been shown for mammary tumour-initiating cells.96 This is particularly important as most studies have been developed in glioma conventional cell lines. Does the primary cilium mediate other signalling pathways involved in the pathogenesis of GBM, apart from Shh and LPA signalling? Has the ciliary pocket any role in this regulation? Given the well-established role of primary cilium in coordinating many of the signalling pathways that influence GBM formation and progression, such as PDGF, TGF-β or Notch signalling, a ciliary coordination of more signalling cascades and their crosstalk in GBM is quite likely to exist and should be promptly addressed. In addition, abnormalities in ciliary length and morphology are known to disturb signal transduction mediated by the primary cilium,97–99 so it may be worth comparing cilia lengths between GBM and normal samples and its potential impact on signalling. On this point, a recent work by Jenks and colleagues100 revealed that drug-resistant cancer cells show increased cilia frequency and length, cilia tip fragmentation and also enhanced Shh activation, which undoubtedly reinforces this line of research in GBM. On the other hand, it is tempting to speculate on the following issue: Does the primary cilium release extracellular vesicles in the context of GBM? Have these vesicles any bioactive role in GBM initiation and progression? Over the last years, an emerging body of evidence points out that primary cilia are able to release extracellular vesicles to maintain ciliary composition, modulate signal transduction and contribute to cell-to-cell communication in different physiological and pathological conditions.101,102 In turn, extracellular vesicles are emerging as key mediators within the tumour microenvironment to promote tumour growth and metastasis for many cancers,103 including GBM, where they have been involved in tumour initiation, proliferation, invasiveness and chemoresistance.104–106 A potential connection between extracellular vesicles and cilia in GBM may therefore be worthy of further investigation.
Taken together, all the evidence presented here reinforces the potential role of the primary cilium as a critical mediator of GBM tumorigenesis and progression, which opens the way to the application of cilia-targeted therapies (‘ciliotherapy’) as a new approach in the fight against this devastating tumour. In this sense, restoration of primary cilia is an increasing strategy to restrain tumour growth in cancer,38,107 and also for GBM; therefore, further strategies based on targeting specific ciliary proteins and/or modulating cilia-dependent signalling in the context of GBM are expected to gain momentum in a few years. This ‘ciliary approach’ to GBM treatment could be especially relevant considering that clinical trials with targeted therapies, especially involving RTK targeting, have failed to improve patient outcome.15 Thus, treatment failures resulting from the extensive GBM intratumoral heterogeneity, with a mixture of cells that show different RTK coactivation throughout time and space,15 might be overcome if targeting a structural component such as the primary cilium. The recent report that manipulating ciliary length/integrity in different drug-resistant cancer cell lines re-sensitizes them to appropriate RTK inhibitors could represent a promising strategy to explore in the context of GBM.100 Finally, it might be interesting to consider strategies based on inhibiting histone deacetylase 6 (HDAC6) to restore primary cilia in GBM, since HDAC6 activation and ciliary loss seem to be common events in a variety of cancers38 and HDAC inhibitors are emerging as a promising group of epigenetic agents for GBM treatment.108,109 We expect that the outbreak of primary cilium as a key mediator of GBM will bring exciting new findings over the coming years that positively impact patient survival.
Footnotes
Funding: This work was supported by grants from the Carlos III Institute of Health and the European Regional Development Fund (PI13/02277, CP16/00039, DTS16/084 and PI16/01580); and Industry and Health Departments of the Basque Country.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: María Álvarez-Satta  https://orcid.org/0000-0003-2938-5357
https://orcid.org/0000-0003-2938-5357
Contributor Information
María Álvarez-Satta, Cellular Oncology group, Biodonostia Health Research Institute, San Sebastian, Spain.
Ander Matheu, Cellular Oncology group, Biodonostia Health Research Institute, Paseo Dr. Beguiristain s/n, CP 20014 San Sebastian, Spain CIBER de Fragilidad y Envejecimiento Saludable (CIBERfes), Madrid, Spain IKERBASQUE, Basque Foundation, Bilbao, Spain.
References
- 1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 2016; 131: 803–820. [DOI] [PubMed] [Google Scholar]
- 2. Ostrom QT, Bauchet L, Davis FG, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol 2014; 16: 896–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thakkar JP, Dolecek TA, Horbinski C, et al. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev 2014; 23: 1985–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352: 987–996. [DOI] [PubMed] [Google Scholar]
- 5. Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res 2013; 19: 764–772. [DOI] [PubMed] [Google Scholar]
- 6. The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008; 455: 1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell 2013; 155: 462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010; 17: 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noushmehr H, Weisenberger DJ, Diefes K, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 2010; 17: 510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med 2000; 343: 1350–1354. [DOI] [PubMed] [Google Scholar]
- 11. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005; 352: 997–1003. [DOI] [PubMed] [Google Scholar]
- 12. Sottoriva A, Spiteri I, Piccirillo SGM, et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci U S A 2013; 110: 4009–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel AP, Tirosh I, Trombetta JJ, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014; 344: 1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Furnari FB, Cloughesy TF, Cavenee WK, et al. Heterogeneity of epidermal growth factor receptor signaling networks in glioblastoma. Nat Rev Cancer 2015; 15: 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qazi MA, Vora P, Venugopal C, et al. Intratumoral heterogeneity: pathways to treatment resistance and relapse in human glioblastoma. Ann Oncol 2017; 28: 1448–1456. [DOI] [PubMed] [Google Scholar]
- 16. Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res 2004; 64: 7011–7021. [DOI] [PubMed] [Google Scholar]
- 17. Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature 2004; 432: 396–401. [DOI] [PubMed] [Google Scholar]
- 18. Lathia JD, Mack SC, Mulkearns-Hubert EE, et al. Cancer stem cells in glioblastoma. Genes Dev 2015; 29: 1203–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ishikawa H, Marshall WF. Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol 2011; 12: 222–234. [DOI] [PubMed] [Google Scholar]
- 20. Elliott KH, Brugmann SA. Sending mixed signals: cilia-dependent signaling during development and disease. Dev Biol. Epub ahead of print 13 March 2018. DOI: 10.1016/j.ydbio.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol 2010; 26: 59–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garcia-Gonzalo FR, Reiter JF. Open sesame: how transition fibers and the transition zone control ciliary composition. Cold Spring Harb Perspect Biol 2017; 9: a028134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pedersen LB, Veland IR, Schrøder JM, et al. Assembly of primary cilia. Dev Dyn 2008; 237: 1993–2006. [DOI] [PubMed] [Google Scholar]
- 24. Vertii A, Hehnly H, Doxsey S. The centrosome, a multitalented renaissance organelle. Cold Spring Harb Perspect Biol 2016; 8: a025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lechtreck KF. IFT-cargo interactions and protein transport in cilia. Trends Biochem Sci 2015; 40: 765–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nachury MV. The molecular machines that traffic signaling receptors into and out of cilia. Curr Opin Cell Biol 2018; 51: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Christensen ST, Morthorst SK, Mogensen JB, et al. Primary cilia and coordination of receptor tyrosine kinase (RTK) and transforming growth factor b (TGF-b) signaling. Cold Spring Harb Perspect Biol 2017; 9: a028167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wheway G, Nazlamova L, Hancock JT. Signaling through the primary cilium. Front Cell Dev Biol 2018; 6: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Han YG, Alvarez-Buylla A. Role of primary cilia in brain development and cancer. Curr Opin Neurobiol 2010; 20: 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Youn YH, Han YG. Primary cilia in brain development and diseases. Am J Pathol 2018; 188: 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Green JA, Mykytyn K. Neuronal ciliary signaling in homeostasis and disease. Cell Mol Life Sci 2010; 67: 3287–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mitchison HM, Valente EM. Motile and non-motile cilia in human pathology: from function to phenotypes. J Pathol 2017; 241: 294–309. [DOI] [PubMed] [Google Scholar]
- 33. Reiter JF, Leroux MR. Genes and molecular pathways underpinning ciliopathies. Nat Rev Mol Cell Biol 2017; 18: 533–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Valente EM, Rosti RO, Gibbs E, et al. Primary cilia in neurodevelopmental disorders. Nat Rev Neurol 2014; 10: 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vaisse C, Reiter JF, Berbari NF. Cilia and obesity. Cold Spring Harb Perspect Biol 2017; 9: a028217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hassounah NB, Bunch TA, McDermott KM. Molecular pathways: the role of primary cilia in cancer progression and therapeutics with a focus on Hedgehog signaling. Clin Cancer Res 2012; 18: 2429–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seeger-Nukpezah T, Little JL, Serzhanova V, et al. Cilia and cilia-associated proteins in cancer. Drug Discov Today Dis Mech 2013; 10: e135–e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gradilone SA, Pisarello MJL, LaRusso NF. Primary cilia in tumor biology: the primary cilium as a therapeutic target in cholangiocarcinoma. Curr Drug Targets 2017; 18: 958–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Izawa I, Goto H, Kasahara K, et al. Current topics of functional links between primary cilia and cell cycle. Cilia 2015; 4: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Briscoe J, Thérond PP. The mechanisms of Hedgehog signaling and its roles in development and disease. Nat Rev Mol Cell Biol 2013; 14: 416–429. [DOI] [PubMed] [Google Scholar]
- 41. Bangs F, Anderson KV. Primary cilia and mammalian Hedgehog signaling. Cold Spring Harb Perspect Biol 2017; 9: a028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pampliega O, Orhon I, Patel B, et al. Functional interaction between autophagy and ciliogenesis. Nature 2013; 502: 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cao M, Zhong Q. Cilia in autophagy and cancer. Cilia 2016; 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berbari NF, Bishop GA, Askwith CC, et al. Hippocampal neurons possess primary cilia in culture. J Neurosci Res 2007; 85: 1095–1100. [DOI] [PubMed] [Google Scholar]
- 45. Bishop GA, Berbari NF, Lewis J, et al. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol 2007; 505: 562–571. [DOI] [PubMed] [Google Scholar]
- 46. Yoshimura K, Kawate T, Takeda S. Signaling through the primary cilium affects glial cell survival under a stressed environment. Glia 2011; 59: 333–344. [DOI] [PubMed] [Google Scholar]
- 47. Kasahara K, Miyoshi K, Murakami S, et al. Visualization of astrocytic primary cilia in the mouse brain by immunofluorescent analysis using the cilia marker Arl13b. Acta Med Okayama 2014; 68: 317–322. [DOI] [PubMed] [Google Scholar]
- 48. Sipos É, Komoly S, Ács P. Quantitative comparison of primary cilia marker expression and length in the mouse brain. J Mol Neurosci 2018; 64: 397–409. [DOI] [PubMed] [Google Scholar]
- 49. Zhang Q, Hu J, Ling K. Molecular views of Arf-like small GTPases in cilia and ciliopathies. Exp Cell Res 2013; 319: 2316–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Breunig JJ, Sarkisian MR, Arellano JI, et al. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci U S A 2008; 105: 13127–13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Han YG, Spassky N, Romaguera-Ros M, et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci 2008; 11: 277–284. [DOI] [PubMed] [Google Scholar]
- 52. Tong CK, Han YG, Shah JK, et al. Primary cilia are required in a unique subpopulation of neural progenitors. Proc Natl Acad Sci U S A 2014; 111: 12438–12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tani E, Ametani T. Ciliated human astrocytoma cells. Acta Neuropathol 1970; 15: 208–219. [DOI] [PubMed] [Google Scholar]
- 54. Moser JJ, Fritzler MJ, Rattner JB. Primary ciliogenesis defects are associated with human astrocytoma/glioblastoma cells. BMC Cancer 2009; 9: 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moser JJ, Fritzler MJ, Rattner JB. Ultrastructural characterization of primary cilia in pathologically characterized human glioblastoma multiforme (GBM) tumors. BMC Clin Pathol 2014; 14: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sarkisian MR, Siebzehnrubl D, Hoang-Minh L, et al. Detection of primary cilia in human glioblastoma. J Neurooncol 2014; 117: 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shpak M, Goldberg MM, Cowperthwaite MC. Cilia gene expression patterns in cancer. Cancer Genomics Proteomics 2014; 11: 13–24. [PubMed] [Google Scholar]
- 58. Han YG, Kim HJ, Dlugosz AA, et al. Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med 2009; 15: 1062–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wong SY, Seol AD, So PL, et al. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med 2009; 15: 1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang Y, Roine N, Mäkelä TP. CCRK depletion inhibits glioblastoma cell proliferation in a cilium-dependent manner. EMBO Rep 2013; 14: 741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ng SS, Cheung YT, An XM, et al. Cell cycle-related kinase: a novel candidate oncogene in human glioblastoma. J Natl Cancer Inst 2007; 99: 936–948. [DOI] [PubMed] [Google Scholar]
- 62. Loskutov YV, Griffin CL, Marinak KM, et al. LPA signaling is regulated through the primary cilium: a novel target in glioblastoma. Oncogene 2018; 37: 1457–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Seeley ES, Carrière C, Goetze T, et al. Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia. Cancer Res 2009; 69: 422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Barakat MT, Humke EW, Scott MP. Kif3a is necessary for initiation and maintenance of medulloblastoma. Carcinogenesis 2013; 34: 1382–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hoang-Minh LB, Deleyrolle LP, Siebzehnrubl D, et al. Disruption of KIF3A in patient-derived glioblastoma cells: effects on ciliogenesis, hedgehog sensitivity, and tumorigenesis. Oncotarget 2016; 7: 7029–7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hoang-Minh LB, Deleyrolle LP, Nakamura NS, et al. PCM1 depletion inhibits glioblastoma cell ciliogenesis and increases cell death and sensitivity to temozolomide. Transl Oncol 2016; 9: 392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang G, Liu M, Wang H, et al. Centrosomal protein of 55 regulates glucose metabolism, proliferation and apoptosis of glioma cells via the Akt/mTOR signaling pathway. J Cancer 2016; 7: 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhu H, Chen D, Tang J, et al. Overexpression of centrosomal protein 55 regulates the proliferation of glioma cell and mediates proliferation promoted by EGFRvIII in glioblastoma U251 cells. Oncol Lett 2018; 15: 2700–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Siebzehnrubl FA, Silver DJ, Tugertimur B, et al. The ZEB1 pathway links glioblastoma initiation, invasion and chemoresistance. EMBO Mol Med 2013; 5: 1196–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Higginbotham H, Eom TY, Mariani LE, et al. Arl13b in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex. Dev Cell 2012; 23: 925–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Piasecki BP, Burghoorn J, Swoboda P. Regulatory factor X (RFX)-mediated transcriptional rewiring of ciliary genes in animals. Proc Natl Acad Sci U S A 2010; 107: 12969–12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Choksi SP, Lauter G, Swoboda P, et al. Switching on cilia: transcriptional networks regulating ciliogenesis. Development 2014; 141: 1427–1441. [DOI] [PubMed] [Google Scholar]
- 73. Ohashi Y, Ueda M, Kawase T, et al. Identification of an epigenetically silenced gene, RFX1, in human glioma cells using restriction landmark genomic scanning. Oncogene 2004; 23: 7772–7779. [DOI] [PubMed] [Google Scholar]
- 74. Feng C, Zhang Y, Yin J, et al. Regulatory factor X1 is a new tumor suppressive transcription factor that acts via direct downregulation of CD44 in glioblastoma. Neuro Oncol 2014; 16: 1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hsu YC, Liao WC, Kao CY, et al. Regulation of FGF1 gene promoter through transcription factor RFX1. J Biol Chem 2010; 285: 13885–13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hsu YC, Kao CY, Chung YF, et al. Ciliogenic RFX transcription factors regulate FGF1 gene promoter. J Cell Biochem 2012; 113: 2511–2522. [DOI] [PubMed] [Google Scholar]
- 77. Purvis TL, Hearn T, Spalluto C, et al. Transcriptional regulation of the Alström syndrome gene ALMS1 by members of the RFX family and Sp1. Gene 2010; 460: 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chang N, Ahn SH, Kong DS, et al. The role of STAT3 in glioblastoma progression through dual influences on tumor cells and the immune microenvironment. Mol Cell Endocrinol 2017; 451: 53–65. [DOI] [PubMed] [Google Scholar]
- 79. Oh EC, Vasanth S, Katsanis N. Metabolic regulation and energy homeostasis through the primary cilium. Cell Metab 2015; 21: 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. De la Rocha AM, Sampron N, Alonso MM, et al. Role of SOX family of transcription factors in central nervous system tumors. Am J Cancer Res 2014; 4: 312–324. [PMC free article] [PubMed] [Google Scholar]
- 81. Poncy A, Antoniou A, Cordi S, et al. Transcription factors SOX4 and SOX9 cooperatively control development of bile ducts. Dev Biol 2015; 404: 136–148. [DOI] [PubMed] [Google Scholar]
- 82. Christensen ST, Clement CA, Satir P, et al. Primary cilia and coordination of receptor tyrosine kinase (RTK) signaling. J Pathol 2012; 226: 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev 2011; 25: 201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Clement CA, Ajbro KD, Koefoed K, et al. TGF-β signaling is associated with endocytosis at the pocket region of the primary cilium. Cell Rep 2013; 3: 1806–1814. [DOI] [PubMed] [Google Scholar]
- 85. Ezratty EJ, Stokes N, Chai S, et al. A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell 2011; 145: 1129–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Leitch CC, Lodh S, Prieto-Echagüe V, et al. Basal body proteins regulate Notch signaling through endosomal trafficking. J Cell Sci 2014; 127(Pt 11): 2407–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Danilov AI, Gomes-Leal W, Ahlenius H, et al. Ultrastructural and antigenic properties of neural stem cells and their progeny in adult rat subventricular zone. Glia 2009; 57: 136–152. [DOI] [PubMed] [Google Scholar]
- 88. Benmerah A. The ciliary pocket. Curr Opin Cell Biol 2013; 25: 78–84. [DOI] [PubMed] [Google Scholar]
- 89. Pedersen LB, Mogensen JB, Christensen ST. Endocytic control of cellular signaling at the primary cilium. Trends Biochem Sci 2016; 41: 784–797. [DOI] [PubMed] [Google Scholar]
- 90. Sorkin A, von Zastrow M. Endocytosis and signaling: intertwining molecular networks. Nat Rev Mol Cell Biol 2009; 10: 609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kasahara K, Aoki H, Kiyono T, et al. EGF receptor kinase suppresses ciliogenesis through activation of USP8 deubiquitinase. Nat Commun 2018; 9: 758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Valadez JG, Grover VK, Carter MD, et al. Identification of Hedgehog pathway responsive glioblastomas by isocitrate dehydrogenase mutation. Cancer Lett 2013; 328: 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tabuchi S. The autotaxin–lysophosphatidic acid–lysophosphatidic acid receptor cascade: proposal of a novel potential therapeutic target for treating glioblastoma multiforme. Lipids Health Dis 2015; 14: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Schou KB, Pedersen LB, Christensen ST. Ins and outs of GPCR signaling in primary cilia. EMBO Rep 2015; 16: 1099–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mykytyn K, Askwith C. G-protein-coupled receptor signaling in cilia. Cold Spring Harb Perspect Biol 2017; 9: a028183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Guen VJ, Chavarria TE, Kröger C, et al. EMT programs promote basal mammary stem cell and tumor-initiating cell stemness by inducing primary ciliogenesis and Hedgehog signaling. Proc Natl Acad Sci U S A 2017; 114: E10532–E10539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ocbina PJR, Eggenschwiler JT, Moskowitz IP, et al. Complex interactions between genes controlling trafficking in primary cilia. Nat Genet 2011; 43: 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dummer A, Poelma C, DeRuiter MC, et al. Measuring the primary cilium length: improved method for unbiased high-throughput analysis. Cilia 2016; 5: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hilgendorf KI, Johnson CT, Jackson PK. The primary cilium as a cellular receiver: organizing ciliary GPCR signaling. Curr Opin Cell Biol 2016; 39: 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Jenks AD, Vyse S, Wong JP, et al. Primary cilia mediate diverse kinase inhibitor resistance mechanisms in cancer. Cell Rep 2018; 23: 3042–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nager AR, Goldstein JS, Herranz-Pérez V, et al. An actin network dispatches ciliary GPCRs into extracellular vesicles to modulate signaling. Cell 2017; 168: 252–263.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wang J, Barr MM. Ciliary extracellular vesicles: txt msg organelles. Cell Mol Neurobiol 2016; 36: 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Xu R, Rai A, Chen M, et al. Extracellular vesicles in cancer: implications for future improvements in cancer care. Nat Rev Clin Oncol. Epub ahead of print 23 May 2018. DOI: 10.1038/s41571-018-0036-9. [DOI] [PubMed] [Google Scholar]
- 104. Oushy S, Hellwinkel JE, Wang M, et al. Glioblastoma multiforme-derived extracellular vesicles drive normal astrocytes towards a tumour-enhancing phenotype. Philos Trans R Soc Lond B Biol Sci 2018; 373: 20160477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gourlay J, Morokoff AP, Luwor RB, et al. The emergent role of exosomes in glioma. J Clin Neurosci 2017; 35: 13–23. [DOI] [PubMed] [Google Scholar]
- 106. Quezada C, Torres Á, Niechi I, et al. Role of extracellular vesicles in glioma progression. Mol Aspects Med 2018; 60: 38–51. [DOI] [PubMed] [Google Scholar]
- 107. Khan NA, Willemarck N, Talebi A, et al. Identification of drugs that restore primary cilium expression in cancer cells. Oncotarget 2016; 7: 9975–9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Arrizabalaga O, Moreno-Cugnon L, Auzmendi-Iriarte J, et al. High expression of MKP1/DUSP1 counteracts glioma stem cell activity and mediates HDAC inhibitor response. Oncogenesis 2017; 6: 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bezecny P. Histone deacetylase inhibitors in glioblastoma: pre-clinical and clinical experience. Med Oncol 2014; 31: 985. [DOI] [PubMed] [Google Scholar]