Abstract
Few Orthopaedics and Traumatology journals from Latin America and Spain are indexed in major databases; controlled clinical trials published in these journals cannot be exhaustively retrieved using electronic literature searches. We aimed to identify, describe and assess the quality of controlled clinical trials published in Orthopaedics and Traumatology journals from Latin America and Spain through handsearching and evidence mapping methods. We identified controlled clinical trials published in eligible Orthopaedics/Traumatology journals in Spanish until July 2017 by handsearching. Data were extracted for controlled clinical trials main characteristics and the Cochrane risk of bias tool was used to assess the controlled clinical trials methodological quality. In addition, we mapped the main findings of these trials. As a result, we assessed 5631 references in 29 eligible journals of which 57 were controlled clinical trials (1.0%). Controlled clinical trials were published between 1995 and 2017 at a rate of 2.5 per year. Journals from Spain and Mexico published around 63% of the controlled clinical trials identified. The median sample size of patients enrolled was 60 (range = 30–300 participants). About conditions assessed, 38.5% of controlled clinical trials assessed issues related to knee conditions, 15.7% about hip and 10.5% about trauma or spine. The risk of bias domains most affected was selective reporting bias and random sequence generation. In addition, only two and seven trials had low risk of bias in all items related to participant/personnel and outcome assessment blindings, respectively. More than 40% of studies did not report differences on benefits/harms between the interventions assessed. As a conclusion, the number of controlled clinical trials published in Orthopaedics/Traumatology journals from Latin America and Spain is low. These controlled clinical trials had important methodological shortcomings and were judged as unclear or high risk of bias. These trials are now available in CENTRAL for their potential inclusion in systematic reviews and other documents of synthesis.
Keywords: Orthopaedics and Traumatology, systematic review, handsearching, controlled clinical trials, evidence mapping
Introduction
The global burden of musculoskeletal conditions, such as hip fractures, osteoporosis and anterior cruciate ligament injuries, is related to high morbidity and mortality, as well as to an increase in the use of resources in worldwide healthcare systems.1–6 There is a growing need of high-quality evidence in this field in order to inform patients, healthcare professionals and policymakers about the best alternatives for the management of bone and joint–related injuries and disorders.7
Controlled clinical trials (CCTs) continue to be the cornerstone of evidence-based medicine. High-quality CCTs provide the best evidence of the effectiveness and safety of interventions in healthcare by implementing methodology aimed at diminishing confounding and other associated biases.8 CCTs in Orthopaedics and Traumatology are scarce and have been widely criticized because of their lack of internal and external validity as well as deficiencies in reporting.9–13 Bhandari et al.14 assessed CCTs published in The Journal of Bone and Joint Surgery, finding that over 50% had issues regarding the allocation concealment, sample size calculation and blinding of outcome assessors, among others. Likewise, Little et al.15 reported in 2015 that over 50% of articles published in the top ten Orthopaedics and Traumatology journals belonged to the fourth level of evidence in the Oxford Centre for Evidence-Based Medicine classification.
Integrative studies, such as systematic reviews, rely on electronic strategies to exhaustively identify CCTs in a variety of sources.16,17 However, electronic strategies are limited by several reasons. First, the term CCT was only indexed in major databases in the 1990s. Second, poor reporting of methods hampers the indexing process. Finally, articles published in journals not indexed in any database simply cannot be retrieved. As a result, integrative studies may be limited by dissemination bias. Cochrane proposes a handsearching approach involving a page-by-page examination of all issues for a selected journal in order to identify potential studies that address the research question.18,19
Several studies have researched the gap between the electronic search findings and those provided by handsearching.18–21 Hopewell et al.18 in 2007 located 34 studies comparing the number of trials identified by handsearching versus those identified by electronic searches, finding that individual electronic databases only identified from 49% to 67% of trials. In addition, they found that the retrieval rate for an electronic search was lower when the search was restricted to languages other than English (39% vs 62%). The impact of handsearching of clinical trials in Spanish has been assessed for topics such as gynaecology and obstetrics,22 dermatology23 and physiotherapy,24 among others, finding similar results.
Few Orthopaedics and Traumatology journals from Latin America and Spain are indexed in major databases. CCTs published in these journals cannot be exhaustively retrieved using electronic literature searches. In order to overcome this shortcoming, we aimed to identify CCTs published in Orthopaedics and Traumatology journals from Latin America and Spain. In addition, we assessed their methodological quality and developed an evidence map in order to obtain a clear picture of the distribution of the information in this field. Finally, this body of evidence was submitted to CENTRAL, the Cochrane Collaboration global repository of CCTs.
Methodology
We performed a systematic review following a common methodology previously reported in other publications22,24–26 and published in our website (http://es.cochrane.org/handsearching-clinical-trials-project), as well as PRISMA guidelines for reporting.27,28 First, we identified and listed all journals in Orthopaedics and Traumatology through the Spanish Medical Index, the National Catalogue of Spanish Publications in Health Sciences Libraries C-17, Latindex, LILACS, SCIELO and MEDLINE/PubMed. The content of all eligible journals up to July 2017, including original articles, letters to the editor, abstracts and conference proceedings, was included in our research. Handsearching may take place in four stages: (a) reading the document’s table of contents; (b) locating keywords in the title of each article (randomized, random, fortuitous, blind etc.); (c) reading the summary of each article; and (d) reading the materials and methods section. The process should be completed retrospectively (i.e. backwards from the last year of publication). If no CCTs were found in a period of 5 consecutive years, handsearching for the corresponding journal was stopped. Three researchers conducted this process and disagreements were solved by consensus.
After selection of potential CCTs, the three authors independently considered the following eligibility criteria:
The study compared interventions in humans.
The study was conducted in a prospective way.
The study compared two or more interventions.
Allocations of interventions should be reported as randomized or quasi-randomized.
Once the CCTs were selected, authors collected qualitative information including topic, population and sample size. In addition, we extracted research questions framed in a PICO (specifying four key components: population, intervention, comparison and outcomes) format.
The Cochrane risk of bias (RoB) tool was applied to included trials by two researchers independently.29 We assessed the following domains as having a low, unclear or high RoB, as follows:
Generation of the allocation sequence. Low risk: if methods of randomization include, for example, a random number table, computer-generated lists or similar methods. Unclear risk: if the trial is described as randomized, but no description of the methods used to allocate participants to treatment group was given. High risk: if methods of randomization include, for example, alternation, the use of case record numbers, dates of birth or day of the week and any procedure that is entirely transparent before allocation, such as an open list of random numbers.29
Allocation concealment. Low risk: if the allocation of participants involved, for example, a central independent unit, on-site locked computer, identically appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator, or sealed opaque envelopes. Unclear risk: if the method used to conceal the allocation was not described. High risk: if the allocation sequence was known to or could be deciphered by the investigators who assigned participants.29
Blinding of participants and providers of care. Low risk: no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding, or blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. Unclear risk: insufficient information to permit judgement of low or high risk. High risk: no blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding or blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding.29
Blinding of outcome assessors. Low risk: no blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding, or blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. Unclear risk: insufficient information to permit judgement of low or high risk. High risk: no blinding of outcome assessment and the outcome measurement is likely to be influenced by lack of blinding, or blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding.29
Incomplete outcome data. Low risk: no missing outcome data, reasons for missing outcome data unlikely to be related to true outcome, missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate for dichotomous outcome data), or missing data have been imputed using appropriate methods. Unclear risk: insufficient information to permit judgement of low or high risk. High risk: reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate, ‘as-treated’ analysis done with substantial departure of the intervention received from that assigned at randomization, or potentially inappropriate application of simple imputation. In addition, trials with a total attrition of more than 30% were considered as with high RoB for this domain.29
Selective outcome reporting. Low risk: if the trial protocol is available and all of the trial’s pre-specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre-specified way, or the trial protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre-specified. Unclear risk: if there is insufficient information to permit judgement of low or high risk. High risk: if, for example, not all of the study’s pre-specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre-specified.29
Other sources of bias. Low risk: if the study appears to be free of other sources of bias. Unclear risk: if there is insufficient information to assess whether an important RoB exists. High risk: if, for example, the trial has a potential source of bias related to the specific study design used or was stopped early due to some data-dependent process.29
We considered CCTs as ‘high risk’ or ‘unclear risk’ if at least one of the assessed domains had a high or unclear RoB judgement, respectively.
For descriptive purposes, we categorized the conclusions on the effectiveness of those assessed interventions reported in included CCTs into six categories:
‘Inconclusive’: authors did not provide a clear conclusion of this research, including insufficient statistical methods to compare the assessed groups.
‘No effect conclusion’: evidence for no difference between intervention and comparator.
‘Harmful conclusion’: the reporting of the conclusions was clearly indicative of a harmful effect.
‘Probably harmful conclusion’: insufficient results to draw firm conclusions despite the negative treatment effect.
‘Probably beneficial conclusion’: insufficient results to draw firm conclusions despite the positive treatment effect, mostly based in physiological/laboratory results.
‘Beneficial conclusion’: authors used a language clearly indicative of a beneficial effect, based on patient-reported outcomes.
These categories were assigned by two of the researchers and were checked by another one. A descriptive analysis of this information was performed using STATA 14.0 (College Station, TX, USA).
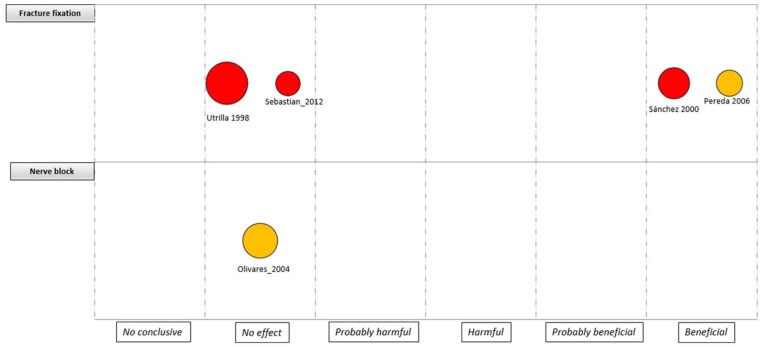
In addition, we mapped the main findings of the identified CCTs. To this effect, we created bubble plots according to the topic of the PICOs, where each bubble represented one CCT. This chart displays information in three dimensions: (a) the rating of conclusions in the x-axis, (b) the interventions in the y-axis and (c) bubble size according to the number of patients included in each CCT.
Results
We identified 5631 studies published 29 Orthopaedics/Traumatology journals between 1995 and 2017. A total of 116 references from 11 journals from six countries (Argentina, Chile, Colombia, Cuba, Spain and Mexico) were reviewed in full text, and 57 (1%) were included after consideration of eligibility criteria (Supplemental files 1 and 2; Figure 1).
Figure 1.
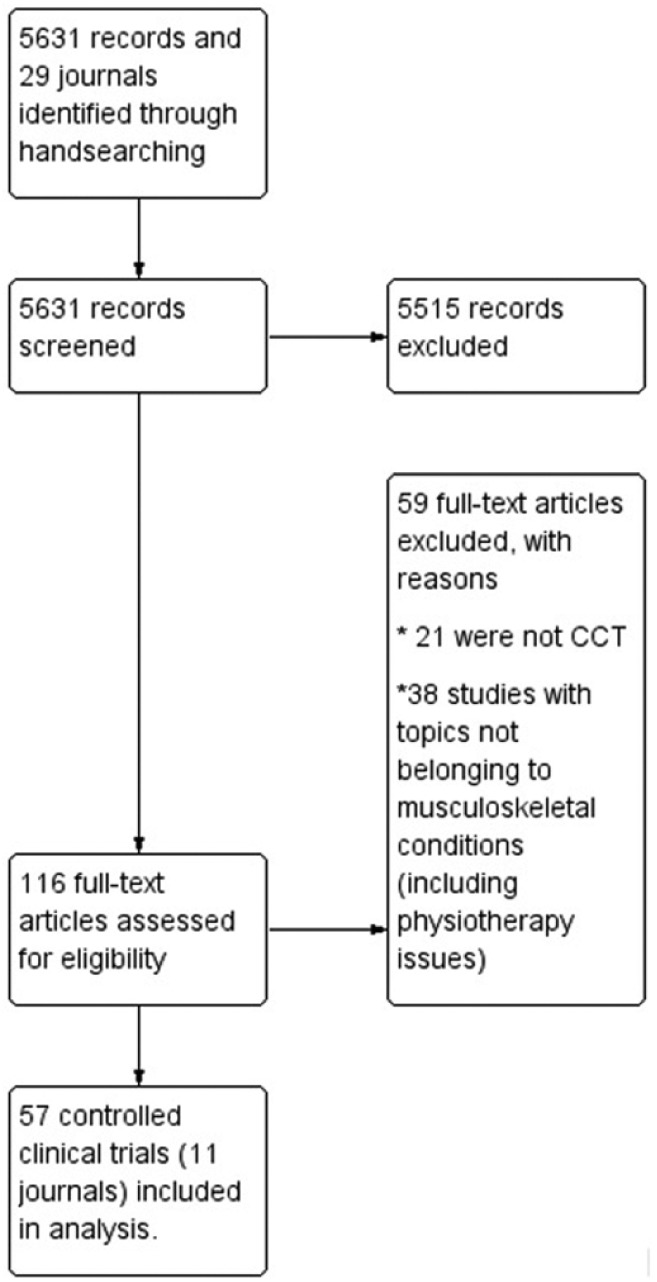
Flow chart for identification of CCT – Orthopaedics and Traumatology journals in Spanish.
Journals from Spain and Mexico published over 63% of trials identified. About 33% of trials were published in Acta Ortopédica Mexicana (19/57). Most of these trials (8/57) were published in 2015 (Supplemental file 1).
The median patient sample size was 60 (range = 30–300 participants). Most CCTs involved a comparison between a single intervention and a control group (89.5%). In all, 27 out of 57 studies did not find differences regarding benefits/harms between the interventions assessed (47.4%). Seven CCTs (12.3%) found benefits, but these were based on non-patient reported outcomes (such as hospital stay, biomechanical angles or costs). About 22 studies (38.5%) found benefits with the use of an assessed intervention.
Regarding the topic of included studies, 22 trials assessed issues related to knee conditions (38.5%), followed by 9 on hip (15.7%), and 6 on trauma or spine (10.5% for both conditions). Only one study (1.75%) involved paediatric populations. Knee issues assessed anterior cruciate ligament management, knee arthroscopy, knee replacement and patellofemoral syndrome, among others. Hip issues assessed included intertrochanteric fracture management, hip arthroplasty and replacement. Only one study assessed antimicrobial prophylaxis regimens. The intervention most frequently assessed was fracture fixation (8/57; 14.0%), followed by surgical haemostasis (6/57; 10.5%). Likewise, the most frequent patient-reported outcome assessed was pain (12.5%; 32/255 outcomes reported), followed by pre- and post-operative complications and surgical time (7.0% and 3.9%, respectively). A full analysis of these trials, in terms of population, intervention assessed and outcomes evaluated are available in Supplemental file 2.
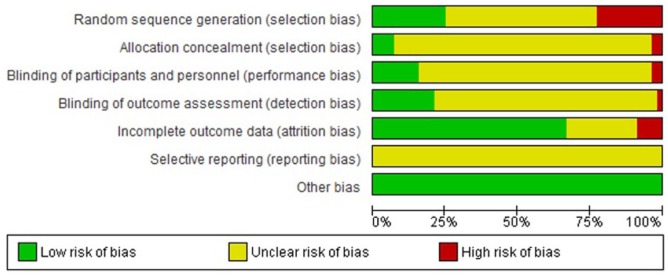
Regarding the quality of trials, all trials were considered as unclear or high risk in at least one assessed domain (Figure 2 and Supplemental file 3). All trials were judged as unclear in the selective reporting domain as the study protocol was not available for the reader. The second domain most affected was random sequence generation concealment (30 with unclear risk and 13 with high RoB). Only two trials had low RoB in all items related to selection bias.30,31 Blinding of participants and personnel was the most affected domain of all assessed blindings (46 with unclear RoB and 2 with high RoB). Blinding of outcome assessment was judged as low risk for 12 trials (21%). Seven trials were judged as low risk in all assessed blindings.32–38 In total, 19 trials were considered as unclear or high risk of incomplete outcome data (34%). Only five studies (8.8%) reported whether there was financial support for the developing of the research. Likewise, seven trials (12.3%) reported the absence of conflicts of interest. No trials reported the use of CONSORT guidelines to improve the quality of reporting.
Figure 2.
Risk of bias graph – Orthopaedics and Traumatology journals in Spanish.
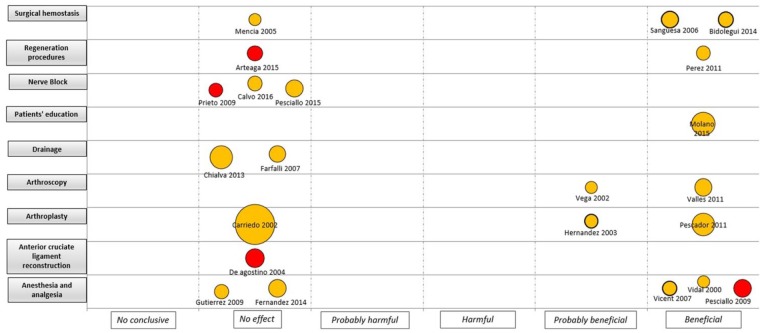
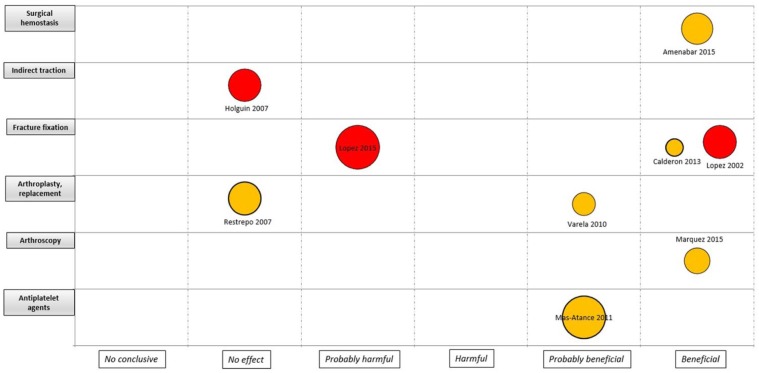
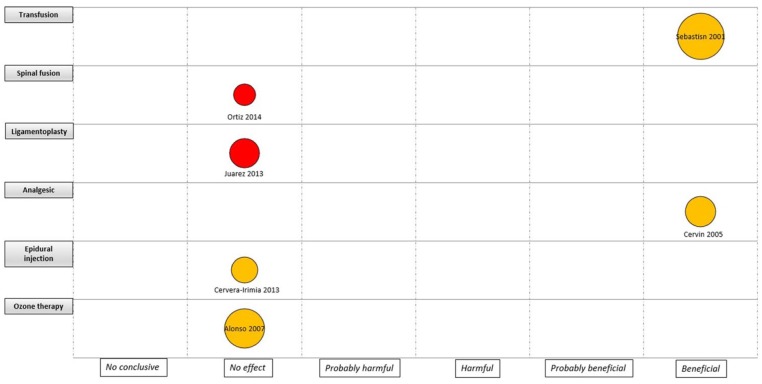
An evidence map was developed for the four major areas (i.e. knee, hip, trauma and spine), considering the quality of the evidence, the assessed intervention and the sample size by each study (Figures 3 –6). For interventions in the knee, most of the trials were considered as unclear RoB (18 trials) and without effect (11 trials). Anaesthesia and analgesic management was the most frequently assessed interventions (five trials). Two CCTs based on laboratory/physiological findings reported benefits for arthroscopy and arthroplasty interventions. No CCTs on nerve block, drainage and anterior cruciate ligament reconstruction showed benefits (Figure 3). For interventions in the hip, one study with unclear RoB reported benefits in surgical haemostasis (tranexamic acid use). In addition, one trial reported potential hams with the use of gamma-3 nail plus distal locking, but it was assessed as high RoB. Other fields with reported benefits were fracture fixation and arthroscopy, but with unclear or high RoB (Figure 4). For the spine area, only six trials were found, and two reported benefits with the intervention assessed (transfusion procedures and percutaneous vertebroplasty), but with unclear RoB. The remaining trials did not report effects for fields such as spinal fusion, ligamentoplasty, epidural injection and ozone therapy (Figure 5). Finally, regarding trauma, most of the identified trials were high or unclear RoB. Interventions related to fracture fixation showed benefits in two cases: closed reduction plus stabilization with Norian SRS in distal radius fracture and use of HAP-200 coraline hydroxyapatite plus open reduction of closed tibial diaphyseal fractures. One trial related to nerve block did not find effect with the use of supraclavicular blocking in patients with upper limb injuries (Figure 6).
Figure 3.
Mapping of controlled clinical trials on knee; Y-axis: Assessed intervention; X-axis: rating of conclusions; bubble size: number of patient included in each CCT; bubble color: Risk of bias (RoB) assessment (Green= low RoB; Yellow= unclear RoB; Red= high RoB).
Figure 4.
Mapping of controlled clinical trials on hip; Y-axis: Assessed intervention; X-axis: rating of conclusions; bubble size: number of patient included in each CCT; bubble color: Risk of bias (RoB) assessment (Green= low RoB; Yellow= unclear RoB; Red= high RoB).
Figure 5.
Mapping of controlled clinical trials on spine; Y-axis: Assessed intervention; X-axis: rating of conclusions; bubble size: number of patient included in each CCT; bubble color: Risk of bias (RoB) assessment (Green= low RoB; Yellow= unclear RoB; Red= high RoB).
Figure 6.
Mapping of controlled clinical trials on trauma; Y-axis: Assessed intervention; X-axis: rating of conclusions; bubble size: number of patient included in each CCT; bubble color: Risk of bias (RoB) assessment (Green= low RoB; Yellow= unclear RoB; Red= high RoB).
Discussion
Orthopaedics and Traumatology is one of the largest surgical fields, thus its relevance is increasing due to the growth of the incidence and prevalence of musculoskeletal conditions.39 To face all the challenges related to the management of these patients, constant research in evidence-based interventions is still needed.39
CCTs are preferred to assess the effectiveness and safety of healthcare interventions, including surgical interventions.40,41 In our review, we found 57 trials published over 22 years related to the management of orthopaedics and traumatology issues (1995–2017), with an estimated rate of publication of 2.5 trials per year. An important number of these trials was judged as unclear or high RoB (96%) using the RoB tool developed by the Cochrane Collaboration. It is critical to improving the adherence of standardized guidelines, such as CONSORT, which help to assure the adequate assessment of RoB in clinical trials.42 We checked the authors’ instructions for the two journals with the highest number of CCTs identified in this review, and we noticed that these guidelines are currently included as mandatory since 2012–2014.
Limitations in the methodological quality of clinical trials in surgery fields had been assessed before with similar results.14,15 Information about the basal characteristic of patients, the consistency in the reporting of primary and secondary outcomes and the clear report of missing data are essential to assess the validity of trials. Recently, Zhang et al.43 evaluated a sample of 299 trials published in high-impact-factor journals in terms of consistency between outcomes reported in protocols and those reported in final manuscripts. They found that 8.4% of assessed trials presented selective outcome reporting, including changes of primary outcomes, the omission of primary outcomes declared in the protocol as well as changes in timing for outcome measurement. In our review, we did not have the opportunity to access the protocols of CCTs identified due to none of them have reported a previously published protocol or a registration in a public trial database. As such, we cannot draw any conclusions about selective outcome reporting in these CCTs.
A considerable number of trials had no-effect results. From our perspective, we consider that these trials could have been rejected by English-language journals with higher impact factors. At present, only two of the reviewed journals are currently indexed in MEDLINE: Revista Española de Cirugía Ortopédica y Traumatología and Acta Ortopedica Mexicana. Around 37% of trials published in Spanish for orthopaedics and traumatology would not have been identified without handsearching. It is important to remark that the use of varied sources not only reduces the RoB but also increases the time and resources required to reach a conclusion.16
As a limitation of our review, we were unable to obtain access in all the sources we considered to the full-text articles of five journals: three published in Latin America and two in Spain (Supplemental file 1). However, the number of potential trials missing could be minimal, taking into account the total frequency of trials found by our study. In addition, we were unable to identify CCTs on Orthopaedic and Traumatology conducted in Spain and Latin America but published in international journals. Presumably, this body of work can be of higher quality. We are currently completing a study aimed to identify these CCTs and to compare their main characteristics with those identified in this study. In addition, we summarized the overall RoB by study level in order to classify and describe the methodological quality of identified trials. Despite that the Cochrane Handbook for Systematic reviews of interventions does not recommend an overall summary of RoB for a study across outcomes,29 we adopt this approach taking into account the multiplicity of both interventions and outcomes retrieved in this review, and the consequent limitations to prioritize specific RoB domains in this heterogeneous setting.
In conclusion, the number of CCTs published in Orthopaedic and Traumatology journals in Spanish is low. In addition, these CCTs have serious methodological limitations. Most of the CCTs identified in this study would not have been retrieved using an electronic search strategy; therefore, handsearching of CCTs is an invaluable aid to comprehensively identifying all available evidence. These studies are now available in CENTRAL for potential inclusion in systematic reviews and other documents of synthesis.
Supplemental Material
Supplemental material, SAGE_Medicine_Supplemental_files_1_to_3_revised_130818 for Quality assessment of controlled clinical trials published in Orthopaedics and Traumatology journals in Spanish: An observational study through handsearching and evidence mapping by Ingrid Arevalo-Rodriguez, Edgar Muñoz, Diana Buitrago-Garcia, Solange Nuñez-González, Nadia Montero-Oleas, Vanessa Garzón, Hector Pardo-Hernandez and Xavier Bonfill in SAGE Open Medicine
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: I.A-R. is funded by a Sara Borrell contract from the Instituto de Salud Carlos III (CD17/00219; Acción Estrategica en Salud 2013–2016, co-funded by European Social Fund 2014–2020, ‘Investing in your future’).
ORCID iD: Ingrid Arevalo-Rodriguez  https://orcid.org/0000-0002-7326-4504
https://orcid.org/0000-0002-7326-4504
References
- 1. Johnell O, Kanis JA. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos Int 2004; 15(11): 897–902. [DOI] [PubMed] [Google Scholar]
- 2. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006; 17(12): 1726–1733. [DOI] [PubMed] [Google Scholar]
- 3. Konnopka A, Jerusel N, Konig HH. The health and economic consequences of osteopenia- and osteoporosis-attributable hip fractures in Germany: estimation for 2002 and projection until 2050. Osteoporos Int 2009; 20(7): 1117–1129. [DOI] [PubMed] [Google Scholar]
- 4. Morales-Torres J, Gutierrez-Urena S. The burden of osteoporosis in Latin America. Osteoporos Int 2004; 15(8): 625–632. [DOI] [PubMed] [Google Scholar]
- 5. Sanders TL, Maradit Kremers H, Bryan AJ, et al. Incidence of anterior cruciate ligament tears and reconstruction: a 21-year population-based study. Am J Sports Med 2016; 44(6): 1502–1507. [DOI] [PubMed] [Google Scholar]
- 6. Gornitzky AL, Lott A, Yellin JL, et al. Sport-specific yearly risk and incidence of anterior cruciate ligament tears in high school athletes: a systematic review and meta-analysis. Am J Sports Med 2016; 44(10): 2716–2723. [DOI] [PubMed] [Google Scholar]
- 7. Fernandez MA, Griffin XL, Costa ML. Hip fracture surgery: improving the quality of the evidence base. Bone Joint J 2015; 97-B(7): 875–879. [DOI] [PubMed] [Google Scholar]
- 8. Bhandari M, Guyatt GH, Swiontkowski MF. User’s guide to the orthopaedic literature: how to use an article about a surgical therapy. J Bone Joint Surg Am 2001; 83-A(6): 916–926. [DOI] [PubMed] [Google Scholar]
- 9. Poolman RW, Struijs PA, Krips R, et al. Does a ‘Level I Evidence’ rating imply high quality of reporting in orthopaedic randomised controlled trials? BMC Med Res Methodol 2006; 6: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dulai SK, Slobogean BL, Beauchamp RD, et al. A quality assessment of randomized clinical trials in pediatric orthopaedics. J Pediatr Orthop 2007; 27(5): 573–581. [DOI] [PubMed] [Google Scholar]
- 11. Boutron I, Tubach F, Giraudeau B, et al. Methodological differences in clinical trials evaluating nonpharmacological and pharmacological treatments of hip and knee osteoarthritis. JAMA 2003; 290(8): 1062–1070. [DOI] [PubMed] [Google Scholar]
- 12. Chan S, Bhandari M. The quality of reporting of orthopaedic randomized trials with use of a checklist for nonpharmacological therapies. J Bone Joint Surg Am 2007; 89(9): 1970–1978. [DOI] [PubMed] [Google Scholar]
- 13. Zhang J, Chen X, Zhu Q, et al. Methodological reporting quality of randomized controlled trials: a survey of seven core journals of orthopaedics from Mainland China over 5 years following the CONSORT statement. Orthop Traumatol Surg Res 2016; 102(7): 933–938. [DOI] [PubMed] [Google Scholar]
- 14. Bhandari M, Richards RR, Sprague S, et al. The quality of reporting of randomized trials in the Journal of Bone and Joint Surgery from 1988 through 2000. J Bone Joint Surg Am 2002; 84-A(3): 388–396. [DOI] [PubMed] [Google Scholar]
- 15. Little Z, Newman S, Dodds A, et al. Increase in quality and quantity of orthopaedic studies from 2002 to 2012. J Orthop Surg 2015; 23(3): 375–378. [DOI] [PubMed] [Google Scholar]
- 16. Crumley ET, Wiebe N, Cramer K, et al. Which resources should be used to identify RCT/CCTs for systematic reviews: a systematic review. BMC Med Res Methodol 2005; 5: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bhandari M, Guyatt GH, Montori V, et al. User’s guide to the orthopaedic literature: how to use a systematic literature review. J Bone Joint Surg Am 2002; 84-A(9): 1672–1682. [PubMed] [Google Scholar]
- 18. Hopewell S, Clarke M, Lefebvre C, et al. Handsearching versus electronic searching to identify reports of randomized trials. Cochrane Database Syst Rev 2007(2): MR000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hopewell S, Clarke M, Lusher A, et al. A comparison of handsearching versus MEDLINE searching to identify reports of randomized controlled trials. Stat Med 2002; 21(11): 1625–1634. [DOI] [PubMed] [Google Scholar]
- 20. Blumle A, Antes G. Handsearching for randomized controlled clinical trials in German medical journals. Dtsch Med Wochenschr 2008; 133(6): 230–234. [DOI] [PubMed] [Google Scholar]
- 21. Mattioli S, Farioli A, Cooke RM, et al. Hidden effectiveness? Results of hand-searching Italian language journals for occupational health interventions. Occup Environ Med 2012; 69(7): 522–524. [DOI] [PubMed] [Google Scholar]
- 22. Gutarra-Vilchez RB, Pardo-Hernandez H, Arevalo-Rodriguez I, et al. Identification and description of controlled clinical trials published in Spanish Gynaecology and Obstetrics journals and risk of bias assessment of trials on assisted reproductive techniques. Eur J Obstet Gynecol Reprod Biol 2016; 203: 5–11. [DOI] [PubMed] [Google Scholar]
- 23. Sanclemente G, Pardo H, Sanchez S, et al. Identifying randomized clinical trials in Spanish-language dermatology journals. Actas Dermosifiliogr 2015; 106(5): 415–422. [DOI] [PubMed] [Google Scholar]
- 24. Turrillas M, Sitja-Rabert M, Pardo H, et al. Identification and description of controlled clinical trials published in Physiotherapy journals in Spain. J Eval Clin Pract 2017; 23(1): 29–36. [DOI] [PubMed] [Google Scholar]
- 25. Bonfill X, Osorio D, Posso M, et al. Identification of biomedical journals in Spain and Latin America. Health Info Libr J 2015; 32(4): 276–286. [DOI] [PubMed] [Google Scholar]
- 26. Sanclemente G, Pardo H, Sanchez S, et al. Analysis of the quality of clinical trials published in Spanish-language dermatology journals between 1997 and 2012. Actas Dermosifiliogr 2016; 107(1): 44–54. [DOI] [PubMed] [Google Scholar]
- 27. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151(4): W65–W94. [DOI] [PubMed] [Google Scholar]
- 28. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151(4): 264W64–264W69. [DOI] [PubMed] [Google Scholar]
- 29. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011, https://handbook-5-1.cochrane.org/ [Google Scholar]
- 30. Arismendi Montoya A, Jaramillo Fernandez JC, Fernandez Lopera F, et al. Luxación acromioclavicular aguda: placa gancho vs. suturas coracoclaviculares. Rev Colomb Ortop Traumatol 2011; 25(4): 337–344. [Google Scholar]
- 31. De Napoli G, Ottolenghi J, Melo L. Comparación de sangrado y transfusiones en artroplastias primarias de cadera y rodilla con monodosis de ácido tranexámico frente a placebo en un hospital universitario. Estudio prospectivo. Rev Colomb Ortop Traumatol 2016; 30(3): 101–106. [Google Scholar]
- 32. Amenabar T, Jorge D, Charnay P, et al. Acido tranexámico para disminuir sangrado y necesidad de transfusión en artroplastia total de cadera: ¿importa el esquema de administración? Estudio prospectivo, randomizado, doble ciego. Rev Chil Ortop Traumatol 2015; 56(1): 7–15. [Google Scholar]
- 33. Hernandez-Vaquero D, Suarez A, Perez-Hernandez P, et al. Cirugía asistida con ordenador en las artroplastias de rodilla. Estudio prospectivo. Rev Exp Cir Ortop Traumatol 2003; 47: 328–335. [Google Scholar]
- 34. Palma Carpitiero E, Barros A, Zurbano X, et al. Tratamiento mediante hilera única versus transóseo doble hilera en las roturas del manguito de los rotadores de tamaño pequeño y medio. Estudio prospectivo y aleatorizado. Rev Esp Artrosc Cir Artic 2015; 22(3): 126–133. [Google Scholar]
- 35. Restrepo-Giraldo M. Mini-incisión en artroplastia total de cadera con vs. sin exovac: estudio experimental controlado, aleatorizado doble ciego. Rev Colomb Ortop Traumatol 2007; 21(2): 83–93. [Google Scholar]
- 36. Vargas-Mena R, Arrerondo-Gomez E, Pavia-Carrillo E. Efecto de un esquema corto de profilaxis antimicrobiana sobre la prevalencia de infecciones postoperatorias en cirugía electiva de traumatología y ortopedia. Acta Ortop Mex 2012; 26(6): 369–374. [PubMed] [Google Scholar]
- 37. Vicent-Vera J, Palomo J, Diaz-Almodovar J. Morfina intraarticular tras artroscopia de rodilla. Estudio prospectivo, aleatorizado y doble ciego. Rev Esp Cir Ortop Traumatol 2007; 51: 188–193. [Google Scholar]
- 38. Vidal-Rodriguez F, Anguiano-Garcia M. Anestesia intra-articular en la artroscopía de la rodilla. Estudio experimental comparativo doble ciego. Acta Ortop Mex 2000; 14(2): 196–199. [Google Scholar]
- 39. Rankin KS, Sprowson AP, McNamara I, et al. The orthopaedic research scene and strategies to improve it. Bone Joint J 2014; 96-B(12): 1578–1585. [DOI] [PubMed] [Google Scholar]
- 40. Dijkman BG, Kooistra BW, Pemberton J, et al. Can orthopedic trials change practice? Acta Orthop 2010; 81(1): 122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khan H, Hussain N, Bhandari M. The influence of large clinical trials in orthopedic trauma: do they change practice? J Orthop Trauma 2013; 27(12): e268–e274. [DOI] [PubMed] [Google Scholar]
- 42. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 2010; 63(8): 834–840. [DOI] [PubMed] [Google Scholar]
- 43. Zhang S, Liang F, Li W. Comparison between publicly accessible publications, registries, and protocols of phase III trials indicated persistence of selective outcome reporting. J Clin Epidemiol 2017; 91: 87–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, SAGE_Medicine_Supplemental_files_1_to_3_revised_130818 for Quality assessment of controlled clinical trials published in Orthopaedics and Traumatology journals in Spanish: An observational study through handsearching and evidence mapping by Ingrid Arevalo-Rodriguez, Edgar Muñoz, Diana Buitrago-Garcia, Solange Nuñez-González, Nadia Montero-Oleas, Vanessa Garzón, Hector Pardo-Hernandez and Xavier Bonfill in SAGE Open Medicine