Abstract
Background:
Linaclotide, a guanylate cyclase C agonist, has been shown in clinical trials to improve symptoms of irritable bowel syndrome with constipation (IBS-C). Here we report data from a real-world study of linaclotide in the UK.
Methods:
This 1-year, multicentre, prospective, observational study in the UK enrolled patients aged 18 years and over initiating linaclotide for IBS-C. The primary assessment was change from baseline in IBS Symptom Severity Scale (IBS-SSS) score at 12 weeks, assessed in patients with paired baseline and 12-week data. Change from baseline in IBS-SSS score at 52 weeks was a secondary assessment. Adverse events were recorded.
Results:
In total, 202 patients were enrolled: 185 (91.6%) were female, median age was 44.9 years (range 18.1–77.2) and 84 (41.6%) reported baseline laxative use. Mean (standard deviation) baseline IBS-SSS score was 339 (92), with most patients (n = 129; 66.8%) classified as having severe disease (score ⩾300). In patients with paired data, there was a significant mean (95% confidence interval) decrease in IBS-SSS score from baseline to 12 weeks [−77.0 (−96.3, −57.7); p < 0.001; n = 124] and baseline to 52 weeks [−70.7 (−95.0, −46.5); p < 0.001; n = 76]. Overall, 174 adverse events were reported in 77 (38.1%) patients, most commonly diarrhoea (n = 54; 26.7%), abdominal pain (n = 21; 10.4%) and abdominal distension (n = 13; 6.4%).
Conclusion:
Linaclotide significantly improved IBS-SSS score at 12 and 52 weeks. These results provide insights into outcomes with linaclotide treatment over 1 year in patients with IBS-C in real-world clinical practice.
Keywords: irritable bowel syndrome with constipation, linaclotide, observational study
Introduction
Irritable bowel syndrome (IBS) is a common gastrointestinal disorder characterised by abdominal pain, bloating and an alteration in bowel habits.1,2 These criteria have been codified by the Rome Foundation and allow subtyping of IBS based on the predominant stool pattern, with subtypes including IBS with constipation (IBS-C), IBS with diarrhoea (IBS-D), IBS with a mixed stool pattern (IBS-M) and unclassified IBS (IBS-U), when the patient’s bowel habits cannot be accurately categorised.2
According to the Rome III criteria, approximately 11.5% of the European population is estimated to be affected by IBS; IBS-C represents approximately one third of cases, although estimates vary.3–5 Prevalence estimates with the Rome IV criteria appear to be lower, suggesting that 7.5% of women and 3.6% of men in the UK are affected.6 IBS is associated with a substantial reduction in quality of life, greater health care resource use and increased costs.7 In the UK, patients with IBS-C had significantly greater health care resource use, greater work productivity impairment and poorer physical and mental health status versus matched controls.7,8
Patients with IBS-C are often recommended, and make use of, lifestyle modifications or over-the-counter medications, such as fibre, nonprescription laxatives or stool softeners, to manage their symptoms.9,10 However, these therapies are primarily targeted at relief of a single symptom and are often associated with patient dissatisfaction, potentially resulting in the use of multiple therapies as well as repeated switching of medications.10,11 Failure of existing treatment options to adequately control the symptoms of IBS-C has been shown to result in increased treatment costs and health care resource use.12
Linaclotide is a minimally absorbed, 14-amino-acid peptide guanylate cyclase C agonist approved for the treatment of adults with moderate to severe IBS-C in the European Union,13,14 based on the results of two large phase III clinical trials demonstrating significant improvements in abdominal pain and bowel symptoms with linaclotide treatment versus placebo.15,16 However, data from the clinical trial setting may not accurately reflect real-world experience in clinical practice.
We therefore aimed to assess the impact of linaclotide treatment in patients with IBS-C in a 1-year, prospective, real-world study in the UK. Here we describe the results of this study at 12 and 52 weeks from initiation of linaclotide treatment.
Methods
Study design
We undertook a 1-year, multicentre, prospective, observational, single-arm study conducted in the gastroenterology and specialist surgical departments of eight secondary or tertiary care centres in the UK, with recruitment occurring from 16 January 2015 to 16 October 2015. There were no changes to patient management for the purposes of any part of this study and patients were not required to attend any visits in addition to their routine care, but were required to complete questionnaires during routine visits. The study observation period was 52 weeks in duration following the initiation of linaclotide for each patient. The use of concomitant treatments for IBS-C during the study was permitted, and patients withdrawing from linaclotide treatment may have been offered other treatment options, which were not recorded as part of the study.
The study received independent NHS Research Ethics Committee (REC) approval from the East of England, Hatfield REC (reference 14-EE-1221), and also received NHS Trust/Health Board Research and Development department approval at each study centre. All participants provided written, informed consent. Data can be obtained by contacting IR-medcom@allergan.com.
Study population
Eligible patients were men and women aged 18 years and over who had been initiated on linaclotide therapy, at a standard dose of 290 μg orally once daily, at a participating research centre as part of their usual care, and who were able and willing to complete questionnaires and consented to their medical records being used for research purposes. The study aimed to achieve a high level of recruitment of patients initiating linaclotide therapy at participating centres.
Study assessments
Efficacy assessments and endpoints
The primary objective of this study was to assess change in IBS Symptom Severity Scale (IBS-SSS) score from baseline to 12 weeks after linaclotide treatment initiation. Secondary objectives included change in IBS-SSS score from baseline to 52 weeks after linaclotide initiation, the proportion of patients responding to linaclotide treatment and change in quality of life, assessed with the IBS Quality of Life measure (IBS-QOL) and the EuroQoL, five-item, three-level questionnaire (EQ-5D-3L), from baseline to 12 and 52 weeks.
The IBS-SSS has five domains scored from 0 to 100, with the overall score reported on a scale of 0–500. Higher scores indicate more severe symptoms, and a score reduction of 50 points is considered adequate to reliably indicate improvement.17 Disease severity is stratified by IBS-SSS score as follows: normal: <75; mild: 75 to <175; moderate: 175 to <300; severe: ⩾300.17 The IBS-QOL is a disease-specific measure with an overall score ranging from 0 to 100 and eight subscale scores, with lower scores indicating lower quality of life. An increase in IBS-QOL score of 14 points is considered to represent a clinically meaningful improvement.18,19 The EQ-5D-3L is a standardised measure of general health status with two components: the EQ-5D index score, reported on a scale of 0–1, with higher scores indicating better health state, and the EQ visual analogue scale (VAS), reported on a scale of 0–100, with higher scores indicating better health.20
Participants were requested to self-complete the validated IBS-SSS questionnaire17 at baseline (up to a maximum of 3 days following initiation of linaclotide treatment) and at 4 (±1), 12 (±4) and 52 (±4) weeks after treatment initiation with linaclotide, and to complete the validated IBS-QOL19 and the EQ-5D-3L21 at baseline and at 12 (±4) and 52 (±4) weeks after linaclotide initiation. At each time point when study questionnaires were completed, participants also completed a questionnaire on laxative use, whether they were still taking linaclotide treatment and, if relevant, the date and reason for linaclotide discontinuation. A patient was recorded as having discontinued linaclotide treatment if they indicated that they were taking linaclotide for fewer than 4 days per week.
Safety assessments
All adverse events (AEs) and adverse drug reactions that occurred during the study period were documented by participating physicians or by enrolled patients via patient questionnaires. AEs reported by physicians were assessed for seriousness and severity and whether a causal relationship to linaclotide could not be excluded; patient-reported AEs were not assessed for severity.
Physician-reported AEs were defined as serious if they met any of the following criteria: results in death; is life-threatening; requires inpatient hospitalisation or prolongs existing hospitalisation results in persistent or significant disability or incapacity; is a congenital anomaly or birth defect; is any other medically important event that may jeopardise the patient or may require intervention to prevent one of the other preceding outcomes; or any suspected transmission via a medicinal product of an infectious agent.
Statistical analyses
A sample size of approximately 150–200 patients was considered adequate to detect a statistically significant reduction of ⩾50 points in the IBS-SSS score.17 The primary endpoint was assessed at 12 weeks, and so a sample size of 190–250 participants was planned to account for the possibility of up to 20% of participants discontinuing within the first 12 weeks and not completing the 12-week questionnaire, based on discontinuation rates observed in previous studies.22
Data were summarised using descriptive statistics and the significance of within-patient changes from baseline in IBS-SSS score and other quantitative outcomes were assessed using paired t tests. A response to linaclotide was defined as a reduction in overall IBS-SSS score of ⩾50, or a reduction in score to less than 150 for patients with a baseline score of ⩾150.23 Data from patients without paired questionnaires at both baseline and a given time point (4 weeks, 12 weeks or 52 weeks) were excluded from the analysis. Data were analysed using Microsoft Excel 2010© and Stata 14.2®.
Results
Baseline patient demographics and clinical characteristics
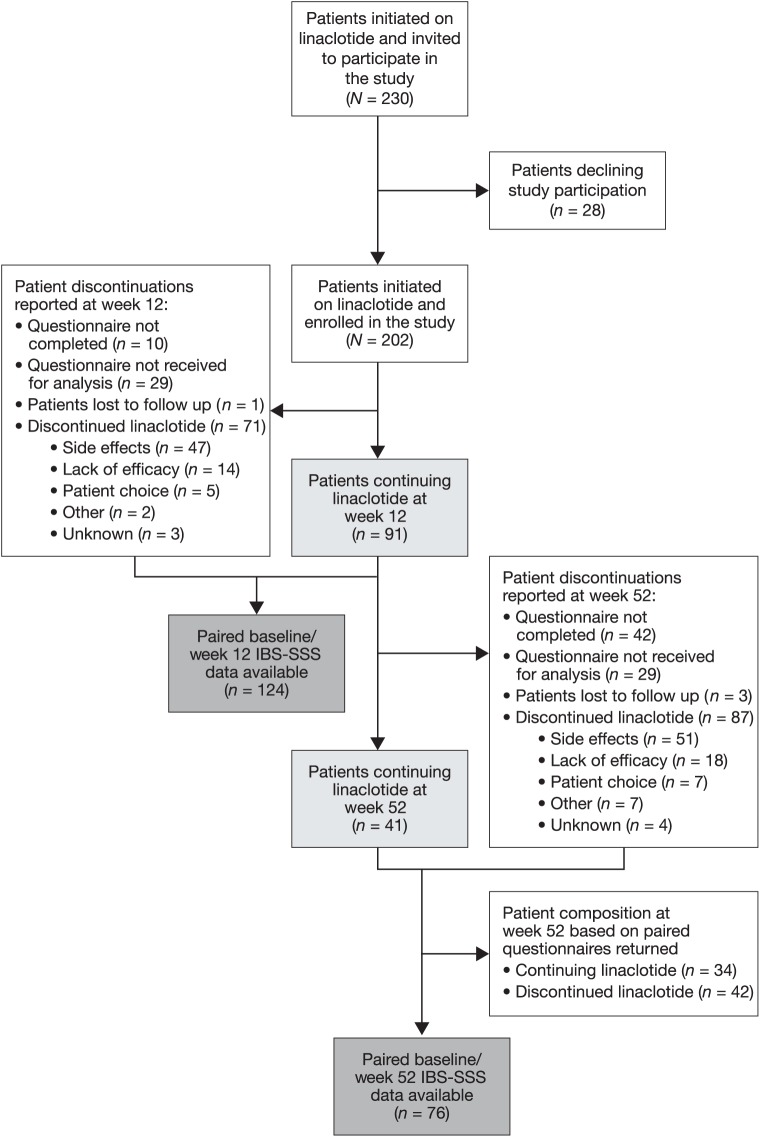
Overall, the study invited 230 patients with IBS-C to participate across eight centres. Of these invited patients, 202 consented to participate, with 151 (74.7%) patients meeting the Rome III diagnostic criteria for IBS. Of the 202 patients, 41 (20.3%) reported continuing on linaclotide treatment at 52 weeks (Figure 1). A further 87 (43.1%) patients reported discontinuing linaclotide treatment before 52 weeks. The remaining 74 patients did not provide a complete 52-week questionnaire (n = 42), did not return a 52-week questionnaire for analysis (n = 29) or were lost to follow up (n = 3).
Figure 1.
Participant flow.
IBS-SSS, Irritable Bowel Syndrome Symptom Severity Scale.
Among the 87 patients discontinuing before 52 weeks, the primary reason was AEs in 51 (25.2%) patients, followed by lack of efficacy in 18 (8.9%) patients. Thirty-eight patients had discontinued by 4 weeks, with 33 discontinuing between 4 and 12 weeks, and 16 discontinuing after 12 weeks of treatment.
Of the 202 patients enrolled, 124 had paired IBS-SSS data for the completed questionnaires at both baseline and 12 weeks, with 76 having baseline and 52-week paired IBS-SSS data (Figure 1). Of the 76 patients with paired data at 52 weeks, 34 continued on linaclotide treatment and 42 reported they had discontinued linaclotide treatment.
The median (range) age at baseline was 44.9 (18.1–77.2) years, and the majority of participants (n = 185; 91.6%) were female (Table 1). The median time since diagnosis was 0.21 years. The mean (standard deviation) IBS-SSS score at baseline was 339 (92), and the majority of patients (n = 129; 66.8%) had severe IBS-C, based on a baseline IBS-SSS score of ⩾300. The most common medication being taken for IBS at the time of linaclotide treatment initiation and continued during the treatment period was laxatives, reported by 84 (41.6%) patients. Antispasmodics and tricyclic antidepressants were continued at linaclotide initiation by 7.9% and 5.9% of patients, respectively.
Table 1.
Baseline demographics and clinical characteristics.
| Total (N = 202) |
|
|---|---|
| Female, n (%) | 185 (91.6) |
| Median age (range), years | 44.9 (18.1–77.2) |
| Median time since diagnosis of IBS-C (range), years | 0.21 (0–67.7) |
| Abdominal symptoms, n (%)a | |
| Constipation | 198 (98.0) |
| Abdominal pain | 169 (83.7) |
| Bloating | 167 (82.7) |
| Diarrhoea | 27 (13.4) |
| Comorbidities (occurring in ⩾4% of patients), n (%)a | |
| Gastroenterology and hepatology | 35 (17.3) |
| Obstetrics and gynaecology | 12 (5.9) |
| Cardiovascular | 9 (4.5) |
| Medications for IBS taken in the 12 months prior to linaclotide initiation (reported by ⩾5% of patients), n (%)a | |
| Laxatives | 158 (78.2) |
| Antispasmodics | 34 (16.8) |
| Tricyclic antidepressants | 24 (11.9) |
| Medications for IBS continued at linaclotide initiation (reported by ⩾5% of patients), n (%)a | |
| Laxatives | 84 (41.6) |
| Antispasmodics | 16 (7.9) |
| Tricyclic antidepressants | 12 (5.9) |
| Mean IBS-SSS score (SD)b | 339 (92) |
| IBS severity based on IBS-SSS score at baseline, n (%)b | |
| In remission (IBS-SSS score <75) | 1 (0.5) |
| Mild (IBS-SSS score 75 to <175) | 9 (4.7) |
| Moderate (IBS-SSS score 175 to <300) | 54 (28.0) |
| Severe (IBS-SSS score ⩾300) | 129 (66.8) |
Not mutually exclusive.
Data available in 193 patients.
IBS, irritable bowel syndrome; IBS-C, irritable bowel syndrome with constipation; IBS-SSS, Irritable Bowel Syndrome Symptom Severity Scale; SD, standard deviation.
Effect of linaclotide treatment on IBS-SSS score
Change from baseline in IBS-SSS score at week 12 and week 52
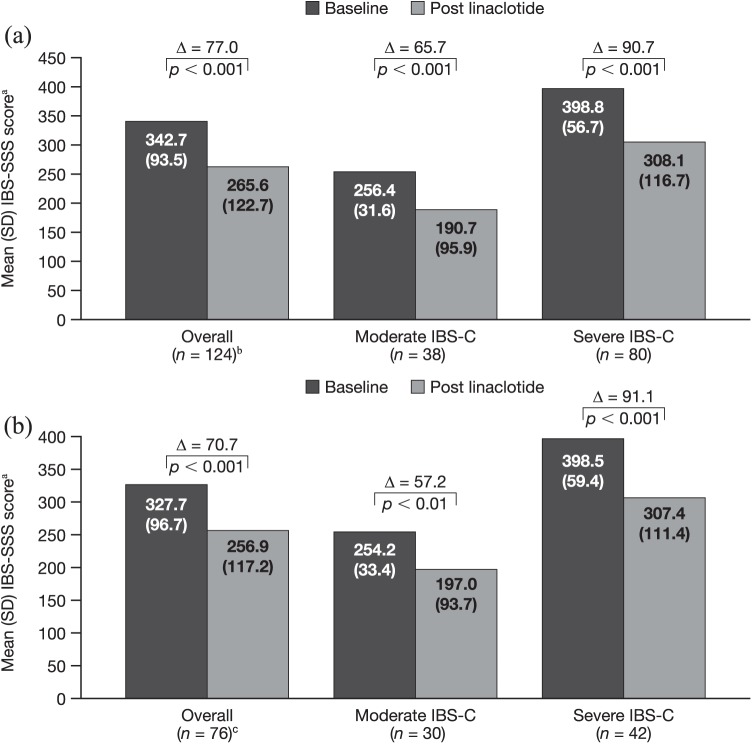
Among the 124 patients with paired baseline and week 12 data, including those who had discontinued linaclotide prior to week 12 and those still continuing on treatment, the mean IBS-SSS score was significantly reduced from baseline to 12 weeks after linaclotide initiation, with a mean [95% confidence interval (CI)] change of −77.0 (−96.3, −57.7; p < 0.001) [Figure 2(a)]. A similar reduction was seen among patients with paired data at week 52: mean IBS-SSS score was significantly reduced from baseline to 52 weeks, with a mean (95% CI) change of −70.7 (−95.0, −46.5; p < 0.001) [Figure 2(b)].
Figure 2.
Change in IBS-SSS score in the overall population and by baseline severity at 12 weeks (a) and 52 weeks (b).
aScored from 0 to 500; bincludes six patients with IBS-C classified as mild at baseline; cincludes four patients with IBS-C classified as mild at baseline.
IBS-C, irritable bowel syndrome with constipation; IBS-SSS, Irritable Bowel Syndrome Symptom Severity Scale; SD, standard deviation.
Of the patients with paired IBS-SSS data at week 12, 91 had a reduced IBS-SSS score at week 12 versus baseline, with 66 meeting the threshold of a reduction of ⩾50 points. Four patients had no change in score, and 29 had an increased score, indicating worsening symptoms. Of the 76 patients with paired IBS-SSS data at week 52, 55 experienced a decrease in IBS-SSS score versus baseline, with 40 experiencing a decrease of ⩾50 points. One patient had no change in score and 20 patients had an increase in score.
At week 12, a greater mean (95% CI) change from baseline in IBS-SSS score was observed in patients with severe IBS-C [−90.7 (−115.8, −65.7); p < 0.001] compared with patients with moderate IBS-C [−65.7 (−96.4, −35.0); p < 0.001] and the overall population [Figure 2(a)]. Similar results were seen at week 52, with a greater mean (95% CI) change in patients with severe IBS-C [−91.1 (−125.8, −56.5); p < 0.001] versus patients with moderate IBS-C [−57.2 (−90.7, −23.8); p < 0.01] and the overall population [Figure 2(b)].
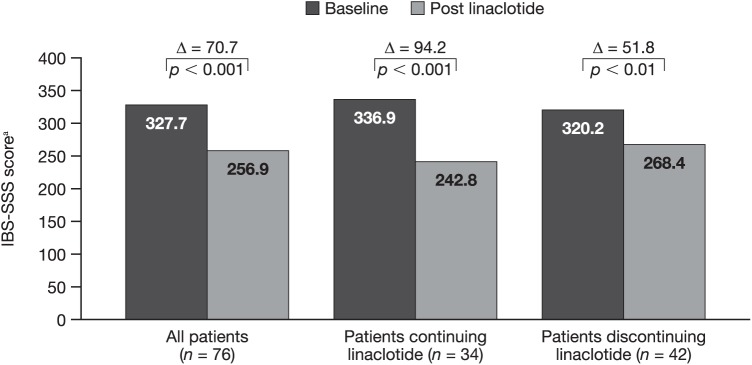
When patients with paired 52-week data were stratified according to whether they continued on linaclotide treatment or had discontinued before 52 weeks, the mean (95% CI) change in IBS-SSS score from baseline to week 52 was significant both in patients continuing on linaclotide treatment [−94.2 (−129.8, −58.5); p < 0.001] and in patients who had discontinued linaclotide [−51.8 (−85.1, −18.5); p < 0.01] (Figure 3).
Figure 3.
Change in IBS-SSS score at 52 weeks in patients continuing or discontinuing linaclotide.
aScored from 0 to 500.
IBS-SSS, Irritable Bowel Syndrome Symptom Severity Scale.
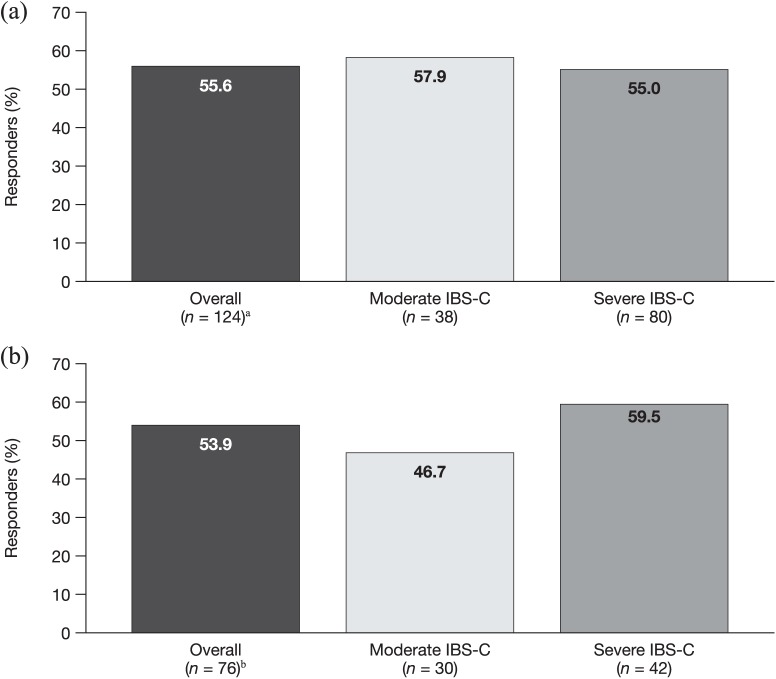
Response to linaclotide treatment
At 12 and 52 weeks, 55.6% and 53.9% of patients, respectively, with paired data displayed a response to linaclotide treatment (Figure 4). The proportion of responders at week 12 and week 52 was broadly similar to the overall population, both in patients reporting moderate IBS-C symptoms at baseline (57.9% and 46.7%, respectively) and in patients reporting severe symptoms at baseline (55.0% and 59.5%, respectively).
Figure 4.
Response to linaclotide in the overall population and by baseline severity at 12 weeks (a) and 52 weeks (b). Response to linaclotide was defined as a reduction in IBS-SSS overall score of ⩾50, or a reduction in score to <150 for patients with a baseline score of ⩾150.
aIncludes six patients with IBS-C classified as mild at baseline; bincludes four patients with IBS-C classified as mild at baseline.
IBS-C, irritable bowel syndrome with constipation; IBS-SSS, Irritable Bowel Syndrome Symptom Severity Scale.
Effect of linaclotide treatment on quality of life
Changes in IBS-QOL score at 12 and 52 weeks
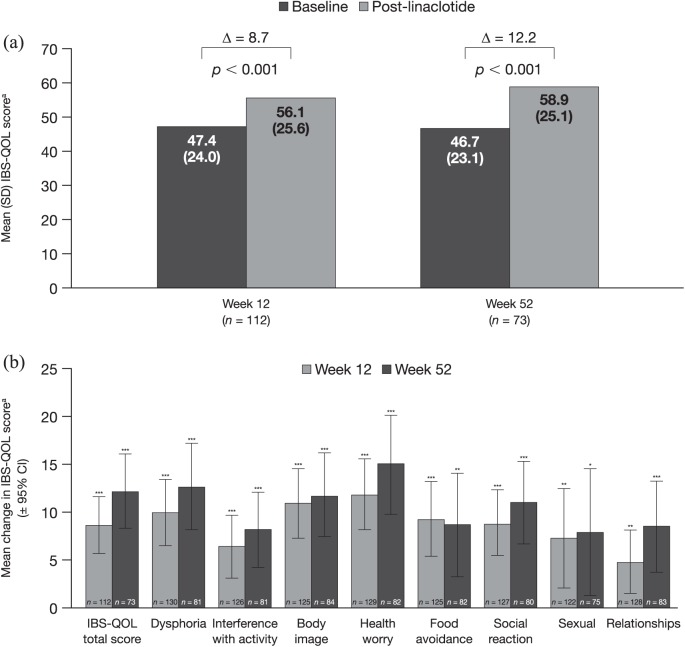
Linaclotide treatment significantly improved IBS-QOL total scores, with a mean (95% CI) change from baseline of 8.7 (5.8, 11.6) points (p < 0.001) at 12 weeks and 12.2 (8.3, 16.0) points (p < 0.001) at 52 weeks [Figure 5(a)]. A significant improvement of approximately 10–15 points versus baseline was seen at both 12 and 52 weeks after linaclotide treatment initiation across all subscales of the IBS-QOL (p < 0.05 for all comparisons) [Figure 5(b)]. The greatest mean (95% CI) changes at week 52 were seen for the health worry and dysphoria subscales, with improvements of 15.1 (10.0, 20.3) and 12.8 (8.3, 17.3) points, respectively.
Figure 5.
IBS-QOL overall score at 12 and 52 weeks (a) and change from baseline in IBS-QOL overall score and domain scores at 12 and 52 weeks (b).
aScored from 0 to 100 (higher scores indicate better functioning).
*p < 0.05; **p < 0.01; ***p < 0.001.
CI, confidence interval; IBS-QOL, Irritable Bowel Syndrome Quality of Life; SD, standard deviation.
Changes in EQ-5D-3L at 12 and 52 weeks
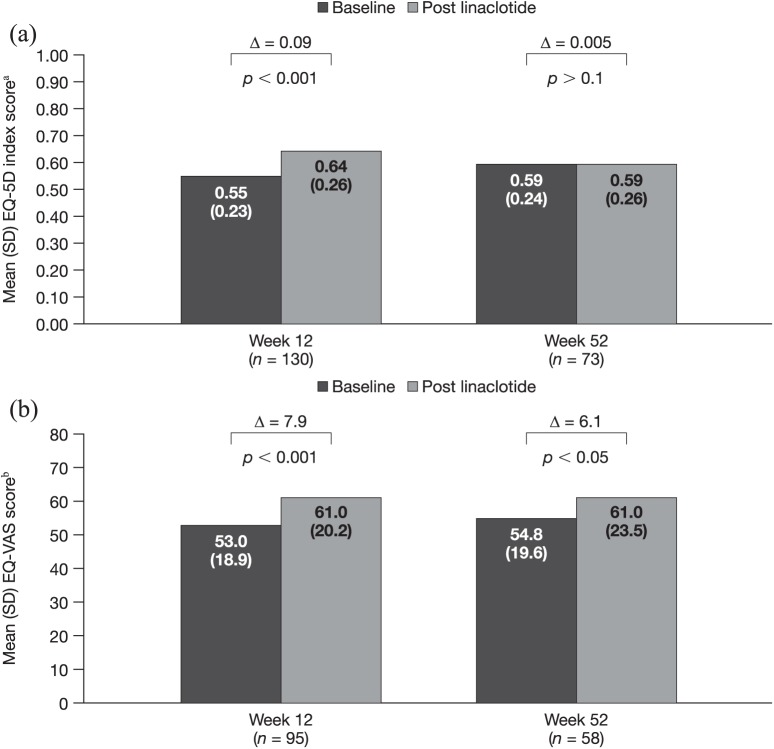
Mean EQ-5D index overall scores were significantly increased from baseline to week 12, with a mean (95% CI) change of 0.09 (0.05, 0.13) points (p < 0.001), but were similar from baseline to week 52, with a mean (95% CI) change of −0.005 (−0.05, 0.06; p > 0.1) [Figure 6(a)]. EQ-VAS overall scores were also significantly improved following linaclotide treatment initiation, with a mean (95% CI) change from baseline of 7.9 (4.0, 11.8) points (p < 0.001) at week 12 and 6.1 (0.03, 12.2) points (p < 0.05) at week 52 [Figure 6(b)].
Figure 6.
EQ-5D index overall score at 12 and 52 weeks (a) and EQ-VAS score at 12 and 52 weeks (b).
aScored from 0 to 1 (1 indicates full health); bscored from 0 to 100 (higher scores indicate better health state).
EQ-5D, EuroQol five dimension; EQ-VAS, EuroQol visual analogue scale; SD, standard deviation.
Safety
Overall, 77 (38.1%) patients reported 174 AEs during the 52-week study (Table 2). The most commonly reported AEs were diarrhoea (26.7%), abdominal pain (10.4%) and abdominal distention (6.4%). By week 12, 47 (23%) patients had discontinued the study due to AEs, and by 52 weeks, this number had increased to 51 patients (26%).
Table 2.
Summary of AEs, regardless of relationship to study drug.
| Total (N = 202) |
|
|---|---|
| Total number of AEs | 174 |
| Patients with ⩾1 AE, n (%)a | 77 (38.1) |
| Not seriousb | 73 (42.0) |
| Seriousb | 8 (4.6) |
| No assessment of seriousness recordedb | 93 (53.4) |
| AEs occurring in ⩾1.5% of patients, n (%)c | |
| Diarrhoead | 54 (26.7) |
| Abdominal pain | 21 (10.4) |
| Abdominal distension | 13 (6.4) |
| Anal incontinence | 8 (4.0) |
| Nausea | 8 (4.0) |
| Defecation urgency | 5 (2.5) |
| Dyspepsia | 5 (2.5) |
| Headache | 5 (2.5) |
| Vomiting | 4 (2.0) |
| Condition aggravated | 3 (1.5) |
| Constipation | 3 (1.5) |
| Gastrointestinal sounds abnormal | 3 (1.5) |
| Malaise | 3 (1.5) |
| Muscle spasms | 3 (1.5) |
Includes AEs where the relationship to study drug is related, probable, possible or unknown.
Expressed as a proportion of 174 events reported.
Expressed as number of events (percentage of patients).
Includes events of loose stools coded as diarrhoea.
AE, adverse event.
Of the AEs reported, 73 (42.0%) were assessed as not serious, 8 (4.6%) were serious and the remainder (93; 53.4%) had no assessment of seriousness recorded. The eight serious AEs were as follows: two events of spontaneous abortion occurring in one patient (1.1%; relationship to study drug unknown); one event of diarrhoea occurring in each of two patients (1.1%; both considered drug related); and one event in one patient each of abdominal discomfort (considered drug related), abdominal pain (considered drug related), constipation (relationship to study drug unknown) and rectal bleeding (considered probably drug related) [0.6% each].
A post hoc analysis demonstrated that 42 (50%) patients with baseline laxative use reported at least one AE during the study period, compared with 35 (30%) among patients not reporting baseline laxative use. AEs of diarrhoea were also more common in patients with baseline laxative use (32%) versus patients without baseline laxative use (20%). However, a χ2 test showed that the association between baseline laxative use and experiencing AEs of diarrhoea within 12 weeks of treatment initiation was not significant (odds ratio: 1.86; p = 0.057).
Discussion
The efficacy and safety of linaclotide were assessed in patients with moderate to severe IBS-C in the real-world setting in this 1-year, prospective, observational, open-label study conducted in gastroenterology and specialist surgical departments of secondary and tertiary care centres in the UK. Baseline patient characteristics, including severity scores and prior medication use, suggest that the patient cohort in this study experienced IBS-C symptoms towards the more severe end of the spectrum and may have been refractory to prior treatment. Symptom severity assessed with the IBS-SSS was significantly reduced compared with baseline at 12 and 52 weeks of linaclotide treatment among patients with paired data. These reductions were observed in patients with moderate IBS-C and with severe IBS-C, with a slightly greater change from baseline to week 12 and week 52 seen in patients with severe IBS-C versus the moderate subgroup and the overall population. Patients withdrawing from linaclotide therapy were offered other therapy options, although these were not recorded as part of the study, which may have been responsible for the reduction in severity at 52 weeks in the overall population, observed irrespective of whether patients had reported that they discontinued linaclotide treatment prior to 52 weeks or continued on treatment.
Current guidance from the UK National Institute for Health and Care Excellence for the diagnosis and management of IBS states that linaclotide should be considered if optimal or maximal tolerated doses of previous laxatives from different classes have not helped and the patient has had constipation for at least 12 months.24 In line with these guidelines, nearly 80% of patients in this study had used laxatives within the 12 months prior to linaclotide initiation, with approximately 40% still taking laxatives at the time of linaclotide initiation. As treatment failure in patients with IBS-C is known to result in increased health care resource use and costs,12 the finding from this study that linaclotide treatment is able to significantly reduce symptom severity is important.
The present data support the previously reported findings of two large phase III clinical trials of linaclotide in over 1500 patients with moderate to severe IBS-C, which demonstrated significant reductions in IBS severity with linaclotide treatment versus placebo.15,16 These data are also in line with the results from an observational study of linaclotide conducted in clinical practice in Germany (N = 375), where linaclotide treatment led to significant reductions in intensity of abdominal pain and bloating and improved bowel movement frequency versus baseline.25
The threshold for improvement in IBS-SSS overall score in this study was a reduction of ⩾50 points, in line with numerous studies making use of the IBS-SSS and the original description of this measure.17,26–30 However, a single study based on a cohort of 277 patients with IBS has suggested that a change of 95 points might represent a minimal clinically important difference.31 A 50% improvement from baseline in IBS-SSS score has also been proposed as a responder definition; however, this criterion requires further validation.32–34
Linaclotide treatment also led to significant improvements in quality of life. Pooled analysis of the linaclotide phase III trials demonstrated that linaclotide led to significant improvements versus placebo in quality of life, as assessed with the IBS-QOL,35 with over half of linaclotide-treated patients displaying a response after 12 weeks of treatment. These findings are supported by the significant improvements seen in IBS-QOL total score and across all subscales in the present study at both 12 and 52 weeks.
The most common AEs reported with linaclotide treatment in the present study were diarrhoea and abdominal pain. The safety profile observed with linaclotide in this 1-year study is similar to that seen in the phase III trials, with no signs of additional adverse events developing as a result of prolonged use.15,16 AEs of diarrhoea that were related, probably or possibly related, or unrelated to the study drug were reported in 26.7% of patients in the present study, and were reported (regardless of relatedness) in 19.5% and 19.7% of patients in the two phase III studies, respectively.15,16 The higher incidence of diarrhoea observed in this study may be attributable to approximately 40% of patients continuing laxative use after linaclotide initiation. In the present study, AEs of diarrhoea were more common among patients with baseline laxative use versus patients without. These findings are in line with the recommendation that linaclotide should not be coadministered with laxatives at the start of treatment.14
At 52 weeks, a high proportion of patients had discontinued treatment with linaclotide. The majority of patients discontinuing linaclotide treatment did so within the first 4 weeks, with only a small proportion of patients who continued on linaclotide after 12 weeks subsequently discontinuing. The most common reason for discontinuation was AEs, occurring in 25.2% of patients. In the two phase III studies, 7.9% and 10.2% of patients in the linaclotide treatment group discontinued due to a treatment-emergent AE.15,16 The higher rate of discontinuations due to AEs reported in the present study may be attributable to the manner in which the data were collected. Patients indicated whether they had taken linaclotide for fewer than 4 days over the past week; if they answered ‘yes’, they were considered to have discontinued. However, this could have been a temporary interruption of treatment and not a permanent discontinuation. Furthermore, this was a low-intensity, observational study with limited face-to-face study visits, and higher discontinuation rates may therefore be expected compared to those seen in a phase III study with more intensive patient follow up and more frequent patient contact. These results may therefore represent a more ‘real-world’ outcome.
The findings of this study should also be interpreted in the light of other limitations. First, the study was observational in nature and did not have a comparator group. The study also enrolled a number of patients who did not meet Rome III criteria and a small number of patients with IBS that was mild or in remission at baseline; such patients would typically be excluded from a phase III trial, but are reflective of the population with IBS seen in clinical practice. Second, the findings may have been influenced by confounding factors, such as changes in concomitant medications, including laxatives, or initiation of alternative medications after linaclotide discontinuation, which were not recorded during the study. For instance, patients were not required to cease laxative use at baseline, and the types of laxatives used were not collected from the trial population. Furthermore, patients discontinuing linaclotide treatment and initiating an alternative treatment option may have continued to return study questionnaires after stopping linaclotide. Third, completed patient questionnaires were not available for every patient at every time point, meaning that analyses are based only on data from those patients who had paired questionnaires. Finally, the study sample size was calculated to ensure sufficient power for the primary analysis. However, due to patient discontinuations and paired data not being available for every patient, other statistical analyses may not have been appropriately powered and should be considered descriptive only.
Overall, the results of this 1-year, prospective, observational, open-label study represent the first report of a 1-year, real-world study of linaclotide in the UK, and demonstrate that linaclotide treatment is effective in improving the severity of IBS-C with a favourable and consistent safety profile. There was a high rate of discontinuations in this study, partly related to the prevalence of AEs of diarrhoea, with patients not showing a response typically discontinuing early, and those continuing on treatment maintaining a response. These data support the findings of the linaclotide phase III clinical trials and show that the findings translate into the real-world setting of secondary and tertiary care centres in the UK.
Acknowledgments
In memory of Claire Shaw, Research Nurse at County Durham and Darlington NHS Foundation Trust, who contributed so much to this study. Editorial assistance in the writing and revision of the draft manuscript on the basis of detailed discussion and feedback from all the authors was provided by Helen Woodroof PhD of Complete HealthVizion, Inc., Chicago, IL, USA and funded by Allergan plc. All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship.
Footnotes
Funding: This study was sponsored by Allergan plc. It received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: Financial arrangements of the authors with companies whose products may be related to the present report are listed below, as declared by the authors. Y. Yiannakou has received funding from Accretio, Allergan plc, Almirall, Coloplast, Enteromed, Kyowa Kirin, Medtronic and Shire. A. Agrawal has no conflict of interest to declare. P.B. Allen has participated in advisory boards for Allergan plc. N. Arebi has received funding from Allergan plc and Almirall. S.R. Brown has received conference funding from Allergan plc. M.P. Eugenicos has participated in advisory boards for Allergan plc, Almirall, Dr Falk Pharma, Kyowa Kirin and Shire. A.D. Farmer has participated in advisory boards or given lectures for Allergan plc, Almirall, Kyowa Kirin and Shire. S. McLain-Smith is an employee of pH Associates Ltd and has carried out data analysis and provided medical writing support. J. McLaughlin received an award to act as a keynote speaker at a meeting organised by Almirall. D.S. Sanders has participated in advisory boards or given lectures for Allergan plc and Almirall. D. Lawrance is an employee of Allergan plc. A. Emmanuel has participated in advisory boards for Allergan plc, Almirall, Shire and Takeda.
Contributor Information
Yan Yiannakou, University Hospital of North Durham, County Durham and Darlington NHS Foundation Trust, North Road, Durham DH1 5TW, UK.
Anu Agrawal, Doncaster Royal Infirmary, Doncaster and Bassetlaw Hospitals NHS Foundation Trust, Doncaster, UK.
Patrick B. Allen, The Ulster Hospital, South Eastern Health and Social Care Trust, Belfast, UK
Naila Arebi, St Mark’s Hospital, London North West University Healthcare NHS Trust and Imperial College, London, UK.
Steven R. Brown, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK
Maria P. Eugenicos, Western General Hospital, NHS Lothian, Edinburgh, UK
Adam D. Farmer, University Hospitals of North Midlands NHS Trust, Stoke-on-Trent, UK and Institute of Applied Clinical Sciences, University of Keele, Keele, UK
Su McLain-Smith, pH Associates Ltd, Marlow, UK.
John McLaughlin, The University of Manchester, Manchester, UK, Salford Royal NHS Foundation Trust, Salford, UK and Manchester Academic Health Sciences Centre, Manchester, UK.
David S. Sanders, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK
Dominic Lawrance, Allergan plc, Marlow, UK.
Anton Emmanuel, University College Hospital, University College London Hospitals NHS Foundation Trust, London, UK.
References
- 1. Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA 2015; 313: 949–958. [DOI] [PubMed] [Google Scholar]
- 2. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology 2016; 150: 1393–1407. [DOI] [PubMed] [Google Scholar]
- 3. Drossman DA, Camilleri M, Mayer EA, et al. AGA technical review on irritable bowel syndrome. Gastroenterology 2002; 123: 2108–2131. [DOI] [PubMed] [Google Scholar]
- 4. Hungin APS, Whorwell PJ, Tack J, et al. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40 000 subjects. Aliment Pharmacol Ther 2003; 17: 643–650. [DOI] [PubMed] [Google Scholar]
- 5. Nellesen D, Yee K, Chawla A, et al. A systematic review of the economic and humanistic burden of illness in irritable bowel syndrome and chronic constipation. J Manag Care Pharm 2013; 19: 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Palsson OS, van Tilburg MA, Simren M, et al. Population prevalence of Rome IV and Rome III irritable bowel syndrome (IBS) in the United States (US), Canada and the United Kingdom (UK). Gastroenterology 2016; 150: S739–S740. [Google Scholar]
- 7. Canavan C, West J, Card T. Review article: the economic impact of the irritable bowel syndrome. Aliment Pharmacol Ther 2014; 40: 1023–1034. [DOI] [PubMed] [Google Scholar]
- 8. DiBonaventura MD, Prior M, Prieto P, et al. Burden of constipation-predominant irritable bowel syndrome (IBS-C) in France, Italy, and the United Kingdom. Clin Exp Gastroenterol 2012; 5: 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Gastroenterological Association. IBS in America: survey summary findings, http://ibsinamerica.gastro.org/files/IBS_in_America_Survey_Report_2015–12–16.pdf (2015, accessed 5 October 2016).
- 10. Hulisz D. The burden of illness of irritable bowel syndrome: current challenges and hope for the future. J Manag Care Pharm 2004; 10: 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moayyedi P, Mearin F, Azpiroz F, et al. Irritable bowel syndrome diagnosis and management: a simplified algorithm for clinical practice. United European Gastroenterol J 2017; 5: 773–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guerin A, Carson RT, Lewis B, et al. The economic burden of treatment failure amongst patients with irritable bowel syndrome with constipation or chronic constipation: a retrospective analysis of a Medicaid population. J Med Econ 2014; 17: 577–586. [DOI] [PubMed] [Google Scholar]
- 13. European Medicines Agency. Summary of product characteristics: Constella, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002490/WC500135622.pdf (2016, accessed 2 February 2017).
- 14. Rey E, Mearin F, Alcedo J, et al. Optimizing the use of linaclotide in patients with constipation-predominant irritable bowel syndrome: an expert consensus report. Adv Ther 2017; 34: 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol 2012; 107: 1702–1712. [DOI] [PubMed] [Google Scholar]
- 16. Rao S, Lembo AJ, Shiff SJ, et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol 2012; 107: 1714–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997; 11: 395–402. [DOI] [PubMed] [Google Scholar]
- 18. Drossman DA, Patrick DL, Whitehead WE, et al. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am J Gastroenterol 2000; 95: 999–1007. [DOI] [PubMed] [Google Scholar]
- 19. Patrick DL, Drossman DA, Frederick IO, et al. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci 1998; 43: 400–411. [DOI] [PubMed] [Google Scholar]
- 20. van Reenen M, Oppe M. EQ-5D-3L user guide, https://euroqol.org/wp-content/uploads/2016/09/EQ-5D-3L_UserGuide_2015.pdf (2015, accessed 16 August 2017).
- 21. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Annals of Medicine 2001; 33: 337–343. [DOI] [PubMed] [Google Scholar]
- 22. Quigley EMM, Tack J, Choi HS, et al. Randomised clinical trials: linaclotide phase 3 studies in IBS-C – a prespecified further analysis based on European Medicines Agency-specified endpoints. Aliment Pharmacol Ther 2013; 37: 49–61. [DOI] [PubMed] [Google Scholar]
- 23. Emmanuel A, McLaughlin J, McLain-Smith S, et al. UK clinical experience at 12 weeks with linaclotide for irritable bowel syndrome with constipation. United European Gastroenterol J 2016; 4: A295–A296. [Google Scholar]
- 24. National Institute for Health and Care Excellence. Clinical guideline 61: irritable bowel syndrome in adults: diagnosis and management of irritable bowel syndrome in primary care, https://www.nice.org.uk/guidance/cg61 (2017, accessed 7 November 2017). [PubMed]
- 25. Andresen V, Miehlke S, Beck E, et al. Efficacy and tolerability of linaclotide in the treatment of irritable bowel syndrome with constipation in a realworld setting - results from a German noninterventional study. Z Gastroenterol 2018; 56: 738–744. [DOI] [PubMed] [Google Scholar]
- 26. Garsed K, Chernova J, Hastings M, et al. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut 2014; 63: 1617–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lackner J, Jaccard J, Baum C, et al. Patient-reported outcomes for irritable bowel syndrome are associated with patients’ severity ratings of gastrointestinal symptoms and psychological factors. Clin Gastroenterol Hepatol 2011; 9: 957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pedersen N, Andersen NN, Végh Z, et al. Ehealth: low FODMAP diet vs Lactobacillus rhamnosus GG in irritable bowel syndrome. World J Gastroenterol 2014; 20: 16215–16226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harvie RM, Chisholm AW, Bisanz JE, et al. Long-term irritable bowel syndrome symptom control with reintroduction of selected FODMAPs. World J Gastroenterol 2017; 23: 4632–4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. MacPherson H, Tilbrook H, Bland JM, et al. Acupuncture for irritable bowel syndrome: primary care based pragmatic randomised controlled trial. BMC Gastroenterol 2012; 12: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Spiegel B, Bolus R, Harris LA, et al. Measuring irritable bowel syndrome patient-reported outcomes with an abdominal pain numeric rating scale. Aliment Pharmacol Ther 2009; 30: 1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Whitehead WE, Palsson OS, Levy RL, et al. Reports of “satisfactory relief” by IBS patients receiving usual medical care are confounded by baseline symptom severity and do not accurately reflect symptom improvement. Am J Gastroenterol 2006; 101: 1057–1065. [DOI] [PubMed] [Google Scholar]
- 33. Corazziari E, Bytzer P, Delvaux M, et al. Clinical trial guidelines for pharmacological treatment of irritable bowel syndrome. Aliment Pharmacol Ther 2003; 18: 569–580. [DOI] [PubMed] [Google Scholar]
- 34. Irvine EJ, Whitehead WE, Chey WD, et al. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology 2006; 130: 1538–1551. [DOI] [PubMed] [Google Scholar]
- 35. Rao SSC, Quigley EMM, Shiff SJ, et al. Effect of linaclotide on severe abdominal symptoms in patients with irritable bowel syndrome with constipation. Clin Gastroenterol Hepatol 2014; 12: 616–623. [DOI] [PubMed] [Google Scholar]