Abstract
Pro-opiomelanocortin (POMC) is the archetypal polypeptide precursor of hormones and neuropeptides. In this review, we examine the variability in the individual peptides produced in different tissues and the impact of the simultaneous presence of their precursors or fragments. We also discuss the problems inherent in accurately measuring which of the precursors and their derived peptides are present in biological samples. We address how not being able to measure all the combinations of precursors and fragments quantitatively has affected our understanding of the pathophysiology associated with POMC processing. To understand how different ratios of peptides arise, we describe the role of the pro-hormone convertases (PCs) and their tissue specificities and consider the cellular processing pathways which enable regulated secretion of different peptides that play crucial roles in integrating a range of vital physiological functions. In the pituitary, correct processing of POMC peptides is essential to maintain the hypothalamic-pituitary-adrenal axis, and this processing can be disrupted in POMC-expressing tumors. In hypothalamic neurons expressing POMC, abnormalities in processing critically impact on the regulation of appetite, energy homeostasis, and body composition. More work is needed to understand whether expression of the POMC gene in a tissue equates to release of bioactive peptides. We suggest that this comprehensive view of POMC processing, with a focus on gaining a better understanding of the combination of peptides produced and their relative bioactivity, is a necessity for all involved in studying this fascinating physiological regulatory phenomenon.
I. INTRODUCTION
A. The Discovery of POMC as a Precursor
The phenomena of pro-opiomelanocortin (POMC) as a hormone precursor emerged gradually over time as observations slowly filled in pieces of the puzzle. Long before the concept of hormone precursors was realized, the bronzed skin color described by Addison in his patient with adrenal insufficiency (“melasma suprarenale”) gave perhaps the first hints of a connection between the hypothalamic, pituitary, adrenal (HPA) axis and skin color. A similar link between the pituitary and pigmentation came from the studies of Allen (5) and Smith (376) who both noted that immersing tadpoles in pituitary extract made their skins darker. In humans too, large doses of porcine pituitary extract also appeared to cause pigmentation (218), with this active extract of the pars intermedia of the pituitary henceforth termed “melanocyte stimulating hormone” or MSH.
In 1932, Cushing extended his clinical reports of a polyglandular syndrome caused by basophilic adenomas of the pituitary by linking this finding with adrenal hyperactivity. In the 1930s, work by Ingle and Kendall (177) showed that administration of large amounts of “cortin,” a purified adrenal extract, produced atrophy of the adrenal cortex in rats. Importantly, they found that administration of the “adrenotropic principle” of the anterior pituitary was effective in preventing adrenal cortical regression following treatment with cortin. The first hints of a behavioral angle to POMC biology came from studies by Ferrari in the 1950s, when “stretching-yawning syndrome,” a bizarre crisis of muscular tone, occurred following central administration of MSH. Many other studies assessing the effects of central α-MSH on motivational processes followed, but it was not until 1976 that Panskepp observed for the first time that this peptide decreased food intake (294).
Viewed from the comfort and assured knowledge of the modern molecular world, these observations and interventions could be considered overtly simplistic. However, we believe that these classic observations should be regarded as essential building blocks, not only for our understanding of POMC peptide processing, but also for the work which subsequently tied together these seemingly diverse peptides.
B. The Emergence of the Precursor Paradigm
It is likely that POMC arose over 500 million years ago by an insertion of the melanocortin sequences into a prepro-endorphin gene. Evidence for this comes from structural identities with other opioid precursors in both the NH2- and COOH-terminal regions of POMC (266). The common opioid gene was thought to arise during chordate evolution. There are four opioid genes that are on three chromosomes in the vertebrate genome. An intragenic duplication event in tetrapods is thought to have led to the presence of α-MSH, β-MSH, and γ-MSH (265). The γ-MSH sequence is not present in teleosts and is found as a vestige in non-teleosts, whereas an additional melanocortin peptide, termed δ-MSH, has been found in cartilaginous fish. This suggests a divergence in MSH sequences in cartilaginous, ray, and lobe-finned fish (266).
The golden age for the precursor paradigm came in the 1960s and 1970s particularly when the first evidence for a precursor of insulin was unearthed by Don Steiner and his team (382, 383). Sequencing confirmed the existence of pro-insulin in 1968 (60), and subsequently pro-insulin was shown to be relatively less active compared with insulin (202). This inspiring work by Don Steiner paved the way for a much greater understanding of a whole range of pro-hormones particularly in relation to their processing.
1. High-molecular-weight forms of ACTH and β-LPH
Although adrenocorticotropic hormone (ACTH) and β-lipotropin (β-LPH) had been characterized separately, the concept that they were produced as part of a common precursor had not been considered and only emerged after a number of different approaches suggested the sequences for these different peptides in the same molecule (FIGURE 1) (reviewed in Refs. 66, 280). Elegant studies by Yalow and Berson (433), using normal human pituitary extracts and an ectopic ACTH producing thymoma, indicated that ACTH was present in a high-molecular-weight form. These high-molecular-weight forms of ACTH were also identified in the mouse pituitary tumor cell line AtT20 (119, 232). Lowry et al. (230) went on to use human pituitary extracts and precipitated a single pro-hormone using antibodies to the different peptides (228). This was made possible because previous work by Chrétien and Li (65) had discovered that the γ-LPH sequence was found within β-LPH and that it had the β-MSH sequence at its COOH terminal. This led them to propose a pro-hormone theory (reviewed in Ref. 66). The presence of an opioid peptide at the COOH terminal of β-LPH was a serendipitous finding by Hughes et al. (173) when they identified the Met-enkephalin sequence at the NH2 terminal of β-endorphin in the β-LPH molecule. This was confirmed by the sequencing of β-endorphin (154).
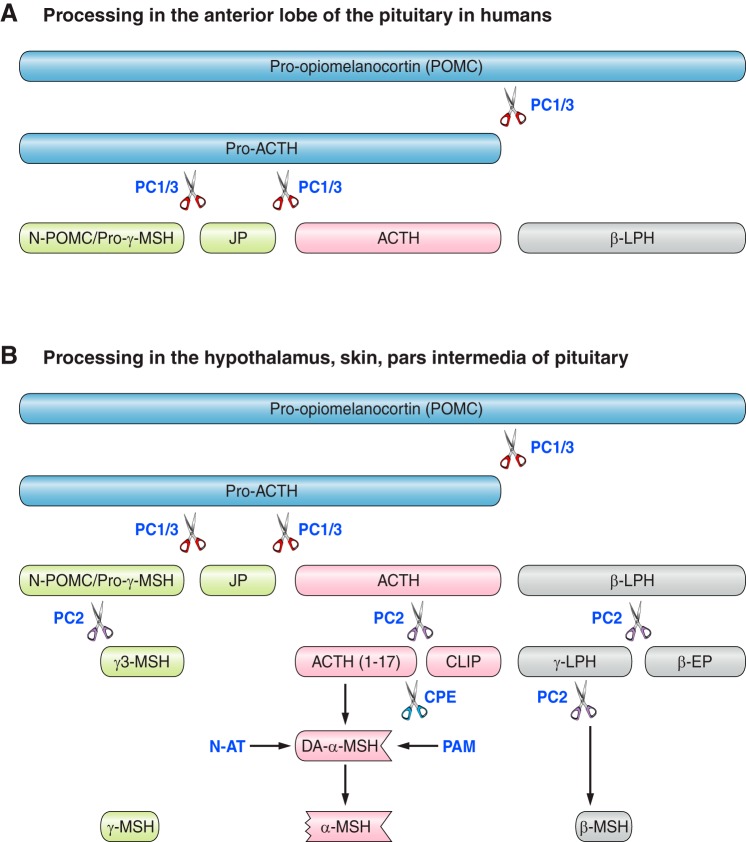
FIGURE 1.
Processing of human pro-opiomelanocortin (POMC) in different tissues. A: in the anterior lobe of the pituitary in humans. B: in the hypothalamus, skin, and pars intermedia of the pituitary. Pro-hormone convertase 1/3 (PC1/3) sequentially cleaves POMC → pro-ACTH → adrenocorticotropic hormone (ACTH). In hypothalamus, skin, and pars intermedia of the pituitary, ACTH is further cleaved by PC2 to produce ACTH (1–17) and corticotropin-like intermediate peptide (CLIP). Carboxypeptidase E (CPE) then cleaves basic amino acid residues from the COOH terminal, allowing amidation by peptidyl-glycine α-amidating monooxygenase (PAM) to form des-acetyl α-MSH (DA-α-MSH). N-acetyltransferase (N-AT) finally acetylates DA-α-MSH to produce α-MSH. PC2 cleaves β-lipotropic hormone (β-LPH) to β-endorphin (β-EP) and γ-LPH, which is further cleaved to β-MSH. The NH2-terminal peptide N-POMC has dibasic amino acids at the NH2 terminal of γ-MSH which are thought to be cleaved by PC2.
In 1978, the concept that POMC was a pro-hormone for ACTH and β-LPH was confirmed in studies with the ACTH-secreting AtT20 cell line. Mains and Eipper (234) radiolabeled amino acids in the cells and then used immunoprecipitation and SDS gel electrophoresis, enabling them to identify a 31-kDa peptide recognized by antibodies to both ACTH and β-LPH. Roberts and Herbert (329) utilized a similar approach but with cell-free translation and antisera to both peptides and reported similar results.
2. The emergence of the full structure of POMC
Not long after these studies, the precursor peptide was purified from rat pituitaries (335) and (rather strangely) from camel pituitaries (200). Michel Chrétien, Nabil Seidah, and co-workers (64) then named the precursor pro-opiomelanocortin to reflect the known roles of the peptides in the precursor. The same year, cloning cDNA from pituitary pars intermedia provided the gene sequence for bovine POMC (265) which was independently confirmed by protein sequencing (264). Similar approaches identified the sequences for the human (72, 391), mouse (407), rat (105), and pig (38) genes (69). Despite the sequence being highly conserved, there is some variation in the lengths of some of the peptides in different species (FIGURE 2). This led to confusion when numbering the amino acids from the NH2 terminal of POMC as the amino acids in the smaller peptides were given different nomenclatures depending on the species (FIGURE 2). Nevertheless, the structure of the gene itself is well conserved, especially in the regions covering the biologically active peptides including ACTH, α-MSH, and β-endorphin (165). Importantly, there are a few key species differences that affect the processing, and this is covered in section IIE after the details of processing have been described.
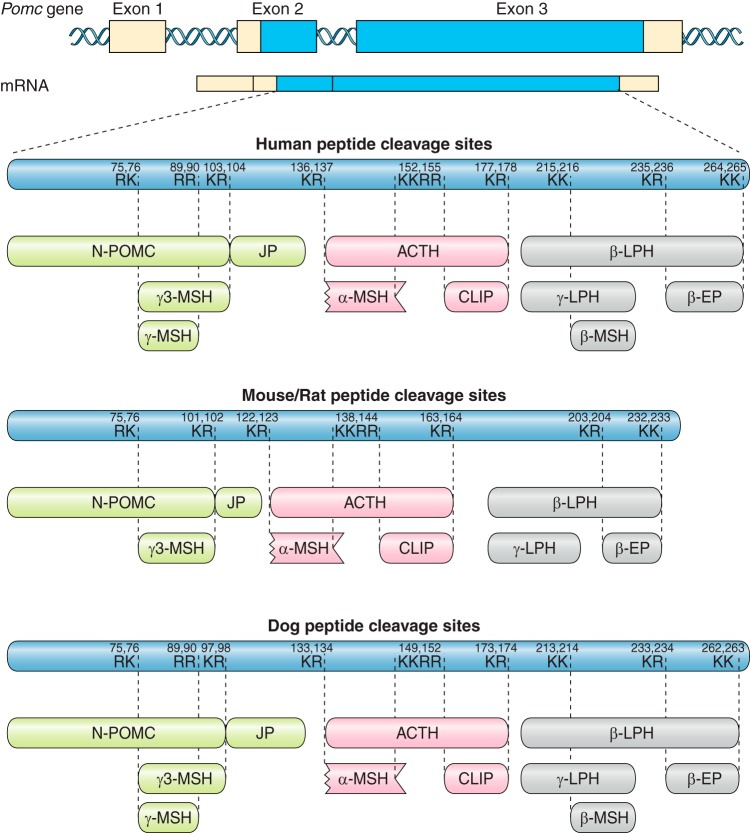
FIGURE 2.
Species differences in the cleavage sites of pro-opiomelanocortin (POMC). The POMC gene has three exons with the translation start site in exon 2. Pro-hormone convertases (PC) cleave at dibasic sites comprising lysine (K) and arginine (R). These sites are generally well conserved, but occur at different amino acid numbers in the human, mouse/rat, and dog sequences. The absence of pairs of dibasic amino acids at the relevant sites in the rat/mouse POMC sequence predicts that γ-melanocyte stimulating hormone (MSH) and β-MSH will not be produced. ACTH, adrenocorticotropic hormone; CLIP, corticotropin-like intermediate peptide; β-LPH, β-lipotropic hormone; β-EP, β-endorphin.
C. Tissue Localization of POMC
There is a wealth of evidence that in a few key tissues, where both the POMC gene is expressed and peptides derived from the POMC precursor protein are released, POMC has an important and biologically meaningful role. These tissues include the pituitary, the arcuate nucleus of the hypothalamus, the nucleus tractus solitarius, and the skin. However, the POMC gene has been reported to be widely expressed throughout the body including in the testis (94, 151, 211, 309), ovary (62, 94), placenta (62), spleen (94), lung (94, 183), liver (94), thymus (183, 211), thyroid (94, 183), heart (253), kidney (94), lymphocytes (275), duodenum (94, 183), colon (94), and adrenal gland (94, 183, 211). Many of these studies were carried out using techniques such as Northern blot and PCR and show expression, but not whether translation to the protein or processing occurs in these locations. In fact, it has been shown that many of these tissues contain a shorter mRNA transcript that would not be translated and therefore no peptide produced (69). Furthermore, in both humans and murine models lacking POMC, no obvious phenotypes relating to these diverse tissues have been reported. Therefore, even if active POMC peptides were made in these tissues, their functional significance would appear to be negligible.
The use of the POMC-Cre mouse line expressing a fluorescent protein has further confused our understanding of the expression patterns of POMC, especially in brain regions. POMC is widely expressed during development, but it becomes more restricted in adulthood. However, the Pomc-Cre manipulation will allow fluorescent protein to continue to be expressed in adulthood, even if POMC was only expressed in that particular region during a developmental period. This was first highlighted in the arcuate nucleus of the hypothalamus, where agouti-related peptide (AgRP)/neuropeptide Y (NPY) and POMC neurons are mutually exclusive in adulthood. However, the AgRP/NPY neurons expressed the Pomc-Cre lineage in adulthood, although they did not continue to express POMC at this time (283). The same group carried out a further study using the Pomc-Cre line examining other brain regions and found POMC recombination in regions including the hippocampus, regions of the cortex, and midbrain (284). Peripheral tissues have not been examined, but this same ectopic pattern may be true for POMC expression outside the brain. Furthermore, using the Pomc-Cre mouse line to excise genes in POMC-expressing tissues may lead to spurious deletion in other regions where it may not be truly relevant.
Expression the POMC gene is only one facet of a complex mechanism which requires coordinate release of POMC protein and processing enzymes to generate a biologically relevant effect. We have concentrated on the pituitary, the hypothalamus, and skin where there is evidence for all these processes and for the roles of the peptides produced from these tissues.
II. OVERVIEW OF POMC PROCESSING
POMC is cleaved by pro-hormone convertases (PCs) at well-defined dibasic amino acid sequences. The type of pro-hormone convertase in a particular tissue defines the specific peptides produced. There is no doubt that the processing of pro-hormones is a very specific mechanism but why this is necessary has not been addressed in detail in this review (FIGURE 1).
In the anterior pituitary, POMC is initially cleaved between the COOH terminal of ACTH and the NH2 terminal of β-LPH (119) to yield pro-ACTH and β-LPH. This cleavage is carried out by pro-hormone convertase 1/3 (PC1/3) which cleaves at sites where there are dibasic amino acids. In this case, the cleavage is at the Lys-Arg site at the COOH terminal of ACTH. There are other dibasic amino acid sequences in POMC, indicating that any preference for cleavage at one site over another is most likely due to neighboring amino acids or the resultant three-dimensional structure allowing easier access to the active site of the convertase.
The next stage in cleavage occurs between the COOH terminal of joining peptide and the NH2 terminal of ACTH. This releases ACTH and an NH2-terminal peptide containing N-POMC (also called pro-γ MSH) and joining peptide. The latter was discovered as the “missing fragment” in human POMC in 1981 (354). The human joining peptide is amidated and secreted as a homodimer joined by a cysteine bridge (25). In humans, it is thought that there is relatively little further processing in the anterior pituitary. This would result in N-POMC, joining peptide, ACTH, and β-LPH as the major POMC-derived peptides released from the anterior pituitary.
A. Generation of MSH Peptides
In the hypothalamus and pars intermedia of the anterior lobe of the pituitary (present in rodents and human fetal pituitaries, but rudimentary in adult humans), there is much more extensive processing of POMC. Again, the degree of processing is determined by which enzymes are expressed in the different tissues.
B. Generation of α-MSH From ACTH
Generation of α-MSH initially involves cleavage of ACTH by PC2 to give ACTH (1–17) and corticotrophin-like intermediate lobe peptide (CLIP), which represents ACTH (18–39) (FIGURE 1). To generate α-MSH from ACTH (1–17), COOH-terminal amino acids are removed in a stepwise fashion by carboxypeptidase E (CPE). Disruption to the activity of this enzyme has major consequences for processing (described in sect. VI). ACTH (1–13) is then amidated at the COOH terminal by peptidyl-glycine α-amidating monooxygenease (PAM) to give ACTH (1–13)-NH2, which is also known as des-acetyl α-MSH. This is then acetylated at the NH2 terminal by N-acetyltransferase (N-AT) to give α-MSH (152). The main effect of NH2-terminal acetylation is not obvious (261) as some functions are increased and others are blocked by this process. For instance, α-MSH is more potent in modulating pigmentation, memory, and attraction, whereas des-acetyl α-MSH is more effective in blocking opiate analgesia (49, 273).
C. Generation of β-MSH and β-Endorphin From β-LPH
β-LPH is processed initially by cleavage of the amino acids between the COOH terminal of γ-lipotropin (γ-LPH) and the NH2 terminal of β-endorphin (FIGURE 1). γ-LPH can then be processed at a Lys-Lys site to release β-MSH from its COOH terminal. This Lys-Lys site is present in the human POMC sequence but not in that of rats or mice, and therefore, it is thought that β-MSH does not exist as a separate peptide in rodents (114).
The sequence of β-endorphin is the 31 amino acids at the COOH terminal of POMC. The initial processing of POMC may only yield β-LPH; however, cleavage can continue to give β-endorphin within the secretory granules before release from some pituitary corticotropic cells (439). Several studies have shown that in addition to β-endorphin (1–31) some further processing can occur to give β-endorphin (1–27) and β-endorphin (1–26) which are also present in pituitary and brain.
D. Generation of γ-MSH From N-POMC
The NH2-terminal region of POMC contains the sequence for the third melanocortin peptide γ-MSH (FIGURE 1). Pro-γ-MSH is often called N-POMC or N-POC (1–76 in humans and 1–74 in rat and mouse). In the human N-POMC sequence, there is a pair of dibasic amino acids at 49/50 which would enable enzymatic cleavage to N-POMC (1–49) and γ3-MSH (also known as Lys-γ3-MSH) which has 27 amino acids. From the gene sequence, γ3-MSH was not expected to include the first lysine, but the cleavage takes place at the COOH-terminal side of the arginine residue leaving lysine as the first amino acid in γ3-MSH (29). As it is an extension to the predicted sequence, it is sometimes included in the nomenclature. Further processing occurs to produce the γ2-MSH sequence which is a dodecapeptide, and then this can be cleaved to the 11 amino acid γ1-MSH. However, this processing can be restricted by glycosylation at Asn16 in γ3-MSH (32).
E. Species Differences in POMC Processing
Many of the melanocortin peptides are conserved among mammalian species, although there are some exceptions, which have consequences for physiology (FIGURE 2). Neither rats nor mice are able to produce β-MSH, as they lack the dibasic residues required for cleavage at their NH2-terminal region (16). For guinea pigs, there is speculation that they may also have a shorter version of β-MSH, as they have two sets of dibasic residues in the COOH-terminal region, which could potentially give rise to two variations of β-MSH (113).
In mouse, rat, and guinea pig, γ1- or γ2-MSH may not exist because the COOH-terminal region does not have the dibasic amino acids to allow cleavage (113). This would suggest that rodents only have the extended γ3-MSH peptide, whereas in the human POMC sequence, the γ1-MSH peptide has flanking dibasic amino acids and therefore the potential for cleavage (FIGURE 2).
III. PROCESSING ENZYMES THAT GENERATE POMC PEPTIDES
The very specific processing pathway for peptide hormones enables enzymatic cleavage of the precursors in a defined environment. While a lot is known about the pro-hormone convertases and the cleavage of pro-insulin, many of the mechanisms involving these cleavage processes were identified by studying the processing of POMC. In addition, there are a number of other enzymatic modifications that occur in the processing pathways to prepare the hormones for their roles (FIGURE 1).
A. Pro-hormone Convertases
The PCs are a family of serine proteinases of the subtilisin/kexin type, and although PC1/3 and PC2 are the most important for POMC processing, studies on PC4, PACE4, PC5/6, PC7, S1P/SKI-1, and PCSK9 have informed our knowledge of the mechanisms of proprotein processing. Much of the early work on the convertases has been reviewed by Bergeron et al. (22), Seidah and Chrétien (351), Seidah (349), and Chrétien and Mbikay (66).
The subtilisin endoproteases are highly homologous to human furin. These proteases are calcium dependent and cleave at single or dibasic amino acids. The cleavage occurs at the COOH terminal of the pair of dibasic amino acids. In POMC, the Lys-Arg (KR) site at the COOH terminal of ACTH is cleaved first and then the Lys-Arg at the NH2 terminal of ACTH. The Lys-Lys-Arg-Arg site in ACTH, which is cleaved to give ACTH (1–17) in the processing to α-MSH, is not cleaved in human anterior pituitary corticotropes. This provides evidence that the adjacent amino acids influence the ability of the PCs to identify the cleavage sites. These types of cleavage sites are found in most peptide hormones and neuropeptides. It is thought that Arg-Lys and Lys-Lys sites are cleaved very slowly over days, and this cleavage occurs only in melanotropes and not in corticotropes.
1. PC1/3 and PC2: how they got their names
Although POMC was identified as the precursor of ACTH and β-LPH in 1977 (235, 329), it took 15 yr to discover the enzymes that cleave the peptides from POMC (reviewed in Ref. 66). It was the identification of the yeast protease Kex2 that led to the breakthrough. The kex2-like subtilisins have similar catalytic mechanisms to trypsin-like proteases. This led to the identification of a human insulinoma cDNA encoding a pro-hormone convertase subsequently named PC2 (375). At about the same time, a second group published the sequence of a mouse pro-hormone convertase which they referred to as PC1 (352). Smeekens and Steiner (374) then isolated cDNA from the human insulinoma encoding a similar convertase, which they named PC3. This turned out to be identical to PC1, such that the nomenclature is now PC1/3.
2. Active pro-hormone convertases are cleaved from inactive precursors
All pro-hormone convertases are themselves derived from precursors and are trafficked to the secretory granules where POMC processing occurs. The maturation of PC1/3 from its precursor is described by Stijnen et al. (387). PC1/3 has a signal peptide and an 80–90 amino acid prosegment at the NH2 terminal. The prosegment is thought to act as an intramolecular chaperone and a competitive inhibitor of the active site of the enzyme. In the endoplasmic reticulum (ER), the inhibitory prosegment is removed by an autocatalytic process. A similar mechanism occurs for PC1/3 (153).
The precursor protein seems to act as a competitive inhibitor at the active site of the processed PC. In particular, Pro-PC1/3, expressed in its trans-conformation, is able to act as an inhibitor of PC1/3 (215). The prosegments of PCs may have inhibitory actions that are distinct for each PC, as they are different in each PC precursor (40).
After the prosegment of PC1/3 is proteolytically removed, which takes several minutes (446), the resulting 84-kDa pro-hormone convertase moves to the trans-Golgi network (TGN) and then to immature secretory granules (ISGs) where a COOH-terminal inhibitory peptide (185) is removed. This leaves a 66-kDa form which is much more active than the 84-kDa form (447). This COOH-terminal peptide has to be cleaved by PC1/3 in the ISGs to stop its inhibitory action on the catalytic domain so that the mature 66-kDa form is fully active to cleave its target peptides. This suggests that the posttranslational processing of the PCs is regulated very precisely. Too much active PC1/3 in the ER would generate the fully active form, but without some autocatalytic activity the inhibitory forms would not be removed. The COOH-terminal domain is also important for directing PC1/3 into secretory granules; without this, the 66-kDa form would move to the constitutive pathway (350).
3. Activation of PC2: role of 7B2
PC2 is also synthesized as part of a precursor but is processed within the TGN and ISG. There is a very distinct mechanism for activation of PC2, which takes 1–2 h and provides the delay necessary for the correct stages of processing (reviewed in Ref. 393). PC2 has a specific binding protein, 7B2, which is required for transport, folding, and activation of PC2 (22). The NH2 terminal of 7B2 has a chaperone function, while the COOH terminal of 7B2 inhibits PC2 (136). 7B2 is thought to bind to the catalytic domain of PC2 and is required for the efficient transport and activation of the enzyme (350). 7B2 and pro-PC2 form a complex in the ER, and this enables trafficking to the TGN, where 7B2 is cleaved by furin. The COOH terminal of 7B2 then binds pro-PC2 and acts as an inhibitor. As the complex is trafficked into the immature secretory granules, the change in pH enables the autocatalytic processes to activate PC2. This in turn causes the cleavage of the COOH-terminal 7B2 peptide which releases the PC2 (244). Thus the biosynthesis and activation of PC2 is tightly linked with that of 7B2.
When 7B2 is knocked out in mice (422), the activity of PC2 in the pars intermedia of the pituitary is prevented. The mice fail to produce α-MSH and instead have dramatically increased ACTH levels and display a Cushing’s syndrome-like phenotype with central obesity. Mortality from the excess ACTH can be rescued by adrenalectomy (214). PC2 null mice have higher ACTH in the pars intermedia of the pituitary than 7B2 null mice, but the 7B2 null mice secrete more ACTH providing further evidence for the role of 7B2 in the regulated secretory process.
4. Role of proSAAS in inhibition of PC1/3
With the discovery of 7B2, there was a suggestion that endogenous inhibitors of PC1/3 might also exist. This led to the identification of proSAAS as a potential inhibitor of PC1/3. ProSAAS is expressed primarily in the brain and in other neuroendocrine tissues. Its overexpression in AtT20, mouse pituitary corticotroph adenoma cells, reduces POMC processing by inhibiting PC1/3, but PC2 is not affected (138).
5. Cellular site of action of PC1/3 and PC2
The subsequent identification of other members of this family of convertases, along with cellular localization studies has revealed that the majority of these endoproteases cleave peptides in the TGN or at the plasma membrane. In comparison, PC1/3 (238) and PC2 (238) cleave the peptides in dense-core secretory granules (100). This is very relevant as their targets are primarily hormones and neuropeptides, like POMC, and the regulation of the release of the active peptides is critical for the function of these hormones. Although it has been suggested that PC1/3 does not have a transmembrane domain (238), the endogenous 84- and 66-kDa forms of PC1/3 can associate with the secretory granule membranes in a lipid raft, with the NH2-terminal portion on the luminal side and the region 619–638 acting as a transmembrane domain. This leaves ~115 amino acids from the COOH terminus of PC1/3 in the cytoplasm, although an α-helical domain at the COOH terminus may associate with the cytoplasmic side of the secretory granule membrane. Therefore, the catalytic domain would be within the lumen of the secretory vesicle, and cleavage at Arg617-Arg618, adjacent to the membrane, would produce the mature PC1/3 (10). It has been suggested that the insertion into the membrane occurs in the rough ER cisternae and that PC1/3 is transported to the TGN in this form and subsequently packaged into secretory vesicles (10). Sorting PC1/3 and other enzymes to the regulated secretory pathway is an important mechanism, and PC2 and CPE may also associate with lipid rafts. However, an alternative suggestion for PC1/3 is that the pro-region associates with lipid rafts and this facilitates the sorting to the secretory pathway (35).
6. Tissue specificity of PC1/3 and PC2 in the processing of POMC
Further confirmation of the function of PC1/3 and PC2 came from their tissue specificity in the mouse pituitary (352, 353), where PC1/3 and PC2 mRNA were detected in the pars intermedia, but only PC1/3 mRNA in the anterior lobe. There was some controversy as studies on the rat pituitary revealed a slightly more complex picture based on in situ hybridization and colocalization (91). There were high levels of PC1/3 in the anterior pituitary but also lower but significant levels of PC2. However, this was clarified when colocalization experiments indicated that the PC2 was not present in the cells that express POMC. In comparison, the pars intermedia had much higher expression of PC2 than PC1/3 (91).
The presence of PC1/3 in the anterior pituitary enables the processing of POMC to ACTH, β-LPH, N-POMC (148), and presumably joining peptide, although there are very few studies that have focused on the molar ratios of each of the peptides. The lack of readily available assays for N-POMC and joining peptide makes it difficult to measure these peptides in human plasma and to predict if there is processing between N-POMC and joining peptide. The absence of PC2 from the anterior pituitary means that further processing of the peptides does not occur.
In comparison, the presence of PC2 in the hypothalamus and skin causes the further cleavage of ACTH, β-LPH, and N-POMC. This provides substrates for other enzymes to complete the processing to α-MSH, β-MSH, and γ-MSH.
PC2 is also found in the pars intermedia of the pituitary, which is present in rodents and the fetal human pituitary. This means that processing is more extensive and the melanocortin peptides are released under the control of regulatory mechanisms, which are distinct from those in the anterior pituitary. When POMC was coexpressed with PC1/3 and PC2, using vaccinia virus vectors in cells that exhibit regulated secretion, a very similar cleavage pattern of processing was observed to that seen in the pars intermedia of the pituitary (15, 398). However, such studies have to be viewed with caution, because of potential degradation of the cellular environment by the virus, and because of some observed ambiguities in that glucagon was not processed from pro-glucagon by PC2 using a similar method.
7. POMC converting enzyme (or Yapsin A)
Although many studies indicate that PC1/3 and PC2 are the major convertases, there are aspartyl-like proteases which may be involved in processing POMC in certain circumstances. A mammalian aspartyl protease was identified in 1985 called POMC converting enzyme (PCE) (224). This is immunologically related to Yapsin 1, which processes at paired basic residues in Kex2-deficient cell lines.
PCE cleaves POMC to give 21–23 kDa ACTH, 4.5 kDa ACTH, and 13 kDa ACTH (glycosylated), β-LPH, and β-endorphin. It also cleaves β-LPH to give β-MSH (222). The gene for PCE has not been cloned, and therefore, no in situ analysis has been undertaken (50).
B. Other Processing Enzymes Involved in Generating POMC-Derived Peptides
The further processing of POMC after the action of PC1/3 and PC2 involves multiple stages and many different enzymes (FIGURE 1). The production of α-, β-, and γ-MSH is particularly complex and occurs in the pars intermedia of the pituitary (in rodents) and in other tissues such as the arcuate nucleus of the hypothalamus and the skin.
1. Carboxypeptidase E
As stated above, the pro-hormone convertases process peptides usually at the carboxyl residue after the single or paired basic amino acid motif. After cleavage, the Lys and/or Arg residues are removed by CPE also known as carboxypeptidase H or encephalin convertase (reviewed in Ref. 50). Therefore, in the human anterior pituitary, once POMC has been processed by PC1/3 at the COOH terminal of ACTH, there is a Lys-Arg pair of amino acids that are then removed by a carboxypeptidase.
Similarly, in the production of α-MSH, CPE plays an important role in removing Lys and/or Arg residues from the COOH terminus of ACTH (1–17). Then there is further removal of glycine to generate the 13-amino acid peptide which is posttranslationally modified to generate α-MSH. This is described in more detail in section II.
However, there is more to the function of CPE than just its role in removal of basic amino acid residues, and it may well be that its secondary role is the more important one for POMC processing. In 1997, Peng Loh’s group (83) showed that CPE also acts as a pro-hormone sorting receptor for the regulated secretory pathway. This function is necessary for pro-hormones to move from the TGN into secretory granules (see below). The importance of this role is indicated by the results from the Cpe gene deletion which highlights the missorting of pro-hormones (83, 357).
2. PAM
PAM amidates the COOH terminal of ACTH (1–13) in the pathway creating α-MSH, but it can also amidate the COOH terminal of joining peptide (reviewed in Ref. 210). This process occurs when the POMC-derived peptides are in the secretory granules. It is difficult to find any evidence for a role for ACTH (1–13) without the subsequent modifications, so this suggests PAM is critical in the generation of α-MSH.
As the name implies, PAM amidates the COOH terminal of peptides after the basic amino acid residue has been cleaved by CPE, and primarily at glycine extended peptides such as is found in the processing to α-MSH. PAM is found in most large dense-core secretory vesicles (120) and exists as a bifunctional enzyme with a peptidylglycine α-hydroxylating monooxygenase (PHM) domain which catalyzes the first stage in the process and a peptidyl-α-hydroxyglycine α-amidating lyase (PAL) domain which catalyzes the second stage. The PAL domain is attached to a transmembrane domain and a cytosolic domain so both catalytic units are held at the membrane but project into the lumen of the large dense-core secretory vesicles (68). Secretory granule endoproteases cleave the two domains from the membrane so that they exist in the lumen of the granules. However, there is also a naturally occurring soluble form called PHM4, made up of only the PAL domain and generated by alternative splicing (68). There is also evidence that PAM alters the organization of the actin cytoskeleton which is important in the release of secretory vesicles from cells (121).
The role of PAM in POMC processing in the hypothalamus has received little attention, and there are currently no reports of mutations in humans that have resulted in obesity. If PAM is critical in the generation of α-MSH, in subjects carrying deleterious inactivating mutations, it may be there is a degree of redundancy in the system with other enzymes undertaking similar amidating activity to compensate.
3. N-acetyltransferase
Acetylation of the NH2-terminal amino acid residues of α-MSH and β-endorphin is important for the activity of these peptides. In general, it is a process thought to protect peptides from aminopeptidases and therefore increase their stability, although some peptides have NH2-terminal acetylation which targets them for degradation. NH2-terminal acetylation is generally restricted to intracellular proteins (429). Therefore, the N-acetylation of these two peptides, whose role is to act at distant sites within the brain and the skin, remains intriguing and not fully explained.
a) acetylation in different tissues.
The deacetylated form of α-MSH was identified in the pituitaries of a number of species as early as 1974 (see Ref. 273), but studies in the 1980s suggested that most of the α-MSH is in the acetylated form in the pars intermedia the pituitary (97).
In the human and rat hypothalamus, deacetylated α-MSH (subsequently termed des-acetyl α-MSH) was found to be a major component when assessed with HPLC techniques (273). Subsequently, the regional heterogeneity in the forms of α-MSH was investigated. In the arcuate nucleus of the hypothalamus, where this peptide has a major role, there was some α-MSH, but the majority was in the des-acetyl form. The amygdala and periaqueductal gray contained non-acetylated α-MSH and the nucleus accumbens had the mono- and di-acetyl (second acetyl group on the third amino acid) forms of α-MSH (97). In a separate study which showed the prevalence of des-acetyl α-MSH in the arcuate nucleus, it was also suggested that acetylation occurred in the nucleus tractus solitarius (NTS) because α-MSH was found there (113).
b) what does nh2-terminal acetylation do for α-msh?
In in vitro studies, the potencies of des-acetyl α-MSH and α-MSH appear similar at MC3R and MC4R (1). There is however some evidence that the two forms of α-MSH may activate intracellular signaling pathways differently, and this could vary depending on the type of tissues and the different melanocortin receptors (429).
That there is a difference in biological function between des-acetyl α-MSH and α-MSH has been recognized for some time in terms of behavioral effects (273). However, there are also several in vivo studies showing differences in the potencies of the N-acetylated and the des-acetyl forms of α-MSH, in terms of food intake (reviewed in Refs. 258, 429). These studies indicated that when des-acetyl and α-MSH are injected intracerebroventricularly at the same dose, des-acetyl α-MSH had a much smaller effect on food intake (1, 261, 404). However, a recent study in mice lacking endogenous α-MSH and des-acetyl α-MSH demonstrated that when these peptides were administered they could each equally decrease body weight (259) presumably by reducing food intake.
What may be most relevant is that the NH2-terminal acetylation of α-MSH confers stability on the peptide (47, 156, 272, 336). Des-acetyl α-MSH is readily degraded by aminopeptidases, whereas the NH2-terminal acetylation protects α-MSH from such degradation (156). Therefore, acetylation could be a mechanism by which the biological activities of POMC peptides are modulated, although further work needs to be carried out to fully understand the endogenous effects of the peptides.
There is also evidence that leptin induces an N-acetylase in mouse hypothalamus (156), so in addition to increasing the POMC gene expression, it was suggested that leptin could increase the biologically active α-MSH in relation to the less active des-acetyl α-MSH form. This suggests much greater subtlety in the control of POMC processing to melanocortin peptides. Some explanation is required, because the evidence points to very little of the active N-acetylated α-MSH relative to des-acetylated α-MSH in the arcuate nucleus (113), making it difficult to understand how α-MSH can have such a powerful role in regulating energy balance. There is speculation that the acetylation process occurs after the des-acetyl α-MSH has traveled along the neuron and just before secretion of the vesicles (258) in the paraventricular nucleus of the hypothalamus (PVN) (FIGURE 3). Therefore, the relative concentrations of α-MSH and des-acetyl α-MSH in the arcuate nucleus would be less relevant.
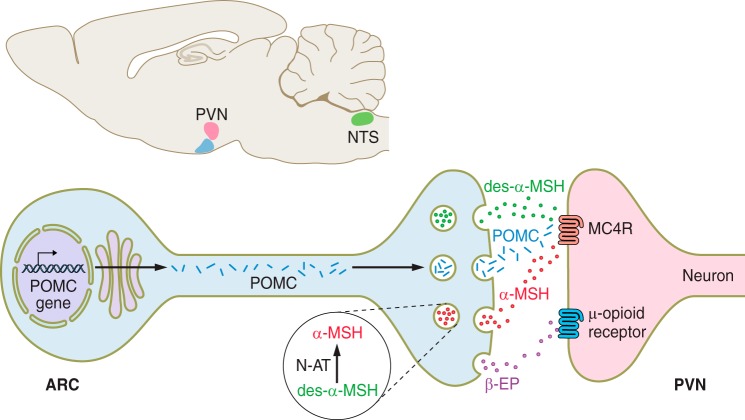
FIGURE 3.
Pro-opiomelanocortin (POMC) processing in neurons. POMC processing begins in the trans-Golgi network (TGN) which is based in the cell body in the arcuate nucleus (ARC). Very little is known about the sites of processing as the peptides move to the neuronal terminals in the paraventricular nucleus (PVN). There is some suggestion that N-acetyltransferase (N-AT) converts des-acetyl α-MSH (des-α-MSH) to α-MSH at the neuronal terminal such that α-MSH is released to activate melanocortin 4 receptor (MC4R) and decrease food intake (258, 312). POMC can also be processed in the nucleus tractus solitarius (NTS), where less is known about the processing of des-acetyl α-MSH and acetylated β-endorphin (β-EP) are the prominent peptides generated.
To add to the complexity, there is evidence that α-MSH is processed by prolylcarboxypeptidase (PRCP) to give α-MSH (1–12), which is inactive (see sect. IV).
c) acetylation of β-endorphin.
Nonacetylated β-endorphin is found in the arcuate nucleus of the hypothalamus, but acetylated β-endorphin was thought to be the main form in the NTS (113). This again raises issues about the role of these posttranslational modifications as acetylated endorphins do not bind to opioid receptors (3), and therefore, the process of acetylation prevents opioid activity (92). However, a more recent study has demonstrated opioid activity originating from POMC neurons in the NTS, indicating that nonacetylated β-endorphin may also be released from these neurons (54).
d) rationalization of acetylation function.
There is evidence to suggest that the acetylation of α-MSH and β-endorphin is tissue specific and differs between the hypothalamus and pituitary (258). The presence of N-acetyltransferase in the processing cascade would increase α-MSH, thus potentiating α-MSH activity and acetylate β-endorphin thus reducing its function. Therefore, this could be a mechanism to provide distinct melanotropic action and not opiate effects in the specific brain region.
4. PRCP
A further cleavage of α-MSH can be carried out by PRCP giving α-MSH (1–12), which has been demonstrated to occur both in vitro and in vivo (417). Additionally, there is evidence that prolyl endopeptidase (PREP, also known as prolyl oligopeptidase) can cleave the terminal amidated valine of α-MSH to also give α-MSH (1–12) (304). The function of α-MSH (1–12) is unclear as it does not activate MC4R and does not decrease food intake (417) and is therefore assumed to be inactive.
IV. CELLULAR PATHWAY TO SECRETION
Another critical arena that determines how POMC-derived peptides are released from cells in the correct spatial and temporal patterns is the pathway across the component parts of the intracellular secretory pathway. It is important to note that much of the work in this area has been carried out in the mouse pituitary adenoma (AtT20) cell line. It remains to be determined how this secretory pathway may differ from that in hypothalamic neurons where there are long projections between regional nuclei. Nevertheless, there are a lot of data which suggest POMC peptides follow at least two distinct pathways on their journey from translation to the extracellular space.
A. From the ER to the TGN
After translation, all pro-hormones are moved into the ER where the NH2-terminal recognition signal anchors them to the membrane. The ER then plays a role in removing the signal peptide at the NH2 terminal of POMC using a signalase enzyme (119). POMC has a specific “heart-shaped” conformation at its NH2 terminal which occurs by the formation of two disulfide bonds formed from Cys28/Cys50 and Cys34/Cys46 in the region upstream of γ-MSH, sometimes termed the 16K fragment (19, 80). As POMC passes out of the ER it will have had N-linked oligosaccharides added, which can influence processing or have no effect, depending on the region that is glycosylated (17).
B. From the Golgi to the Secretory Vesicle
In the Golgi apparatus, the pro-hormone is moved towards the ends of the cisternae where there is blebbing of the membranes to generate the secretory vesicles (399). During this process, the serine at amino acid 31 in ACTH is phosphorylated by casein kinase and sulfate groups are added to N-linked carbohydrate chains.
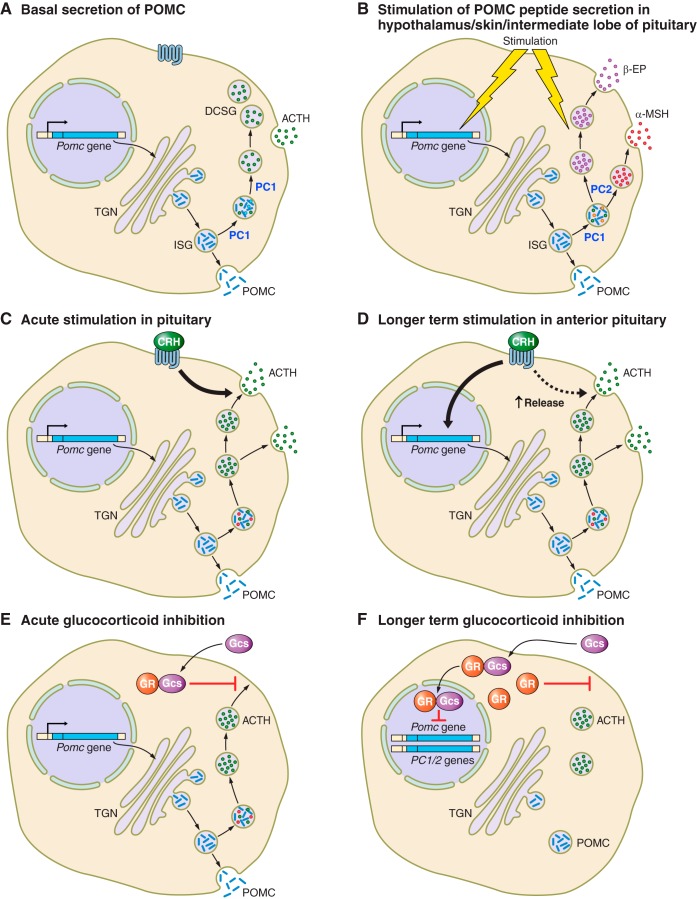
The sorting of pro-hormones for processing is dependent on a change in pH between the TGN and the secretory granules. Experiments using chloroquine, which neutralizes acidic compartments, resulted in a reduction of newly synthesized ACTH in mature granules (256). As POMC moves through the TGN and into granules, the pH changes from 6.8 (355) to 4.5–5.5 (225), which is coupled with changes in calcium concentrations. This environment provides the optimal conditions for activation of the pro-hormone convertases so that the initial phases of processing of the pro-hormone precursor can begin. There are data suggesting that POMC is primarily processed in secretory granules (134, 394), although other studies suggest it may begin in the TGN (249, 345, 445). Some of the evidence suggests that the initial cleavage of POMC at the COOH terminal of ACTH can occur in the Golgi apparatus, but subsequent modifications continue in the secretory vesicles (reviewed in Ref. 313). Therefore, the cleavage at the NH2 terminal of ACTH to generate mature ACTH (1–39) is likely to occur in the secretory vesicles (FIGURE 4).
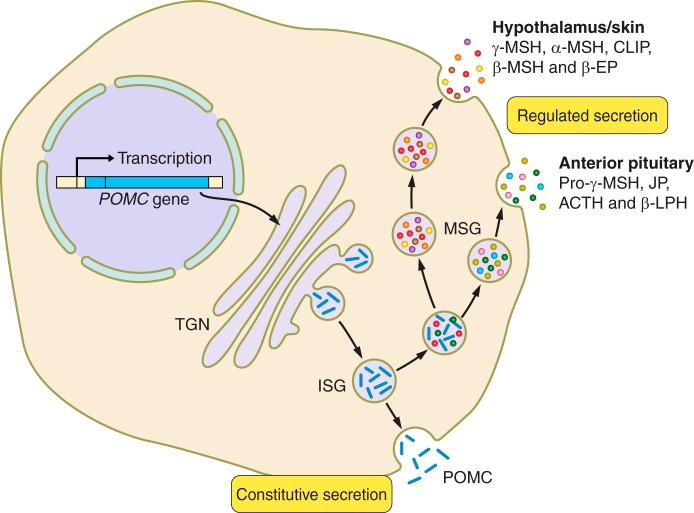
FIGURE 4.
Alternative secretory pathways for precursors and pro-opiomelanocortin (POMC)-derived peptides. POMC is either stored in immature secretory granules (ISG) and released by constitutive secretion or processed and peptides stored in mature secretory granules (MSG) before release by regulated secretion. The anterior pituitary has prohormone convertase (PC) 1/3 and therefore processing is more limited than in the hypothalamus and skin, which have both PC1/3 and PC2 as well as other enzymes. This combination of additional enzymes gives rise to further posttranslational processing that results in the melanocyte stimulating hormone (MSH) peptides. TGN, trans-Golgi network; ACTH, adrenocorticotropic hormone; CLIP, corticotropin-like intermediate peptide; β-LPH, β-lipotropic hormone; β-EP, β-endorphin.
If the initial cleavage between ACTH and β-LPH occurs in the Golgi apparatus, then it is likely that β-LPH (and therefore β-endorphin) could be found in different vesicles to ACTH and α-MSH. If all the processing occurs in the vesicle, then ACTH and β-endorphin will be present in the same vesicles. This is important for understanding whether α-MSH and β-endorphin peptides are released at the same time and at the same site, given that they may have opposing roles in the hypothalamus (see sect. VIE3).
Further processing of ACTH to α-MSH requires not only PC2 but also the enzymes CPE, PAM, and N-AT (see above) which are present in the secretory vesicles in a state ready to be activated. How activation is achieved is not fully understood (100). It is likely that these enzymes have recognition sequences that direct them to the TGN, but whether all secretory vesicles have this repertoire of enzymes is not clear.
C. What Is the Regulated Secretory Pathway?
Gumbiner and Kelly (155) in 1982 recognized that there are classical secretory cells such as those in the adrenal medulla, the exocrine pancreas, and the anterior pituitary which have large dense-core secretory granules. They defined the regulated secretory pathway (RSP) as one where secretagogues controlled the release of the contents of the secretory vesicles. In the absence of secretagogues, there is minimal exocytosis of secretory granule contents. The secretory vesicles have an electron dense-core and turn over is slow (half-life ~10 h), probably because these are the storage organelles for bioactive peptides. Biogenesis of secretory granules was initially thought to require chromogranin A (CGA), a member of the granin family which also includes chromogranin B (CGB, secretogranin I) and chromogranin C (CGC, secretogranin II) (199). However, targeted ablation of the chromogranin A (Chga) gene indicates that compensatory increases in the expression of other granin family members can compensate for CGA deficiency (163).
D. Pro-hormone Sorting
Sorting of peptides to the RSP is a prerequisite for processing of many pro-hormones. Although not fully clarified, it is reasonable to assume that this is also the case for pro-neuropeptides involved in energy balance. These are released from neurons that have their cell bodies in the arcuate nucleus but act at other sites within the hypothalamus. POMC neurons will release α-MSH primarily at the PVN. The molecular mechanisms for sorting pro-hormones to the RSP can involve aggregation of peptides in the presence of high calcium and low pH, as found in the TGN. There is evidence for aggregation of this type for chromogranins A and B (59); however, sorting can occur in the absence of aggregation (316), and other studies have suggested the importance of sorting signal motifs.
POMC has a sorting signal motif at its NH2-terminal region that is both necessary and sufficient for sorting to the RSP (80–82). This sorting signal in POMC was identified as a result of some of the early structural analysis of the NH2 terminus of POMC (18, 19). It is thought to involve two acidic residues, Asp10 and Glu14, and two amphipathic residues, Leu11 and Leu18, which are part of an amphipathic loop at POMC residues 8–20. This sequence was predicted to be a consensus sorting signal that could bind to a sorting receptor, and it has also been identified in pro-enkephalin (270) and pro-insulin (99).
For POMC, the sorting receptor was identified as CPE (81, 83). This has a ligand binding domain for the POMC sorting motif, which was originally identified by molecular modeling and then disruption of the receptor site by mutation (441). The binding site on CPE, which is distinct from the enzyme active site, also recognizes pro-insulin and pro-enkephalin (441). CPE is known to associate with membranes, and this appears to be necessary for its function in sorting pro-hormones to the RSP (98). This membrane association is with lipid rafts containing glycosphingolipids and cholesterol and is predominantly in the secretory granules, but also in the TGN. Depletion of cholesterol can reduce the association of CPE and its pro-hormone ligand with the membrane (98). Secretogranin III can also have a synergistic role with CPE in the trafficking of POMC and derived peptides (50). RNA silencing of secretogranin III decreases secretion through the RSP in AtT20 cells, suggesting that there are several pathways involved in regulated secretion (51).
Much of the work on the membrane association of CPE has used secretory granules, and it is not clear at what stage CPE binds to POMC. For CPE to be involved in sorting POMC from the ER to the TGN it would have to bind in the ER to transfer it into compartments within the TGN. However, as POMC moves through the TGN, CPE can enable POMC to be selected for immature granules that bud off the TGN. The interaction between CPE and POMC would then retain POMC in the granules and not allow it to move to the constitutive-like pathway (see below).
The relative importance of the roles of CPE in sorting of POMC to the RSP versus its true carboxypeptidase action has not been clearly delineated. Sorting seems to be critical because POMC is not processed but secreted in large amounts from the constitutive pathway in the pituitary of the Cpefat/fat mice (83, 357).
Another carboxypeptidase, CPD, is present in the TGN and cycles between the TGN and the cell surface. It appears to reside in immature secretory granules, but absent from the mature granules (411). Therefore, CPD may be responsible for removal of dibasic amino acids or sorting of pro-hormones in the absence of CPE (102).
E. Movement of Vesicles to the Cell Membrane
For POMC, one of the critical features is the very specific regulation of release of the processed peptides in response to defined signals. The cytoplasmic tail of CPE (i.e., the part of the molecule that remains outside the vesicle) also plays a role in transporting the vesicles containing POMC (or if POMC has been processed then the vesicles which contain ACTH and the other POMC-derived peptides). The secretory vesicles must be transported from the TGN to the cell membrane where they can be stored until there is a stimulus that orchestrates their release. The transport of the vesicles to the secretion sites in pituitary cells occurs along microtubules (205). This involves dynactin being recruited to the cytoplasmic tail of CPE, and dynactin then binding to kinesin 2 and kinesin 3 as part of the secretory process (50, 295). The very specific mechanisms involved in movement of vesicles to the cell membrane have been reviewed by Park and Loh (295).
F. What Happens to the Mature Peptides in the Secretory Vesicles?
The current working model is one in which the processing of POMC to the smaller peptides continues during the time when the vesicles are trafficking to the site of secretion. There is evidence that within dense-core secretory vesicles a large number of peptide and protein hormones aggregate into insoluble macromolecular complexes (237). These aggregates are crystalline or composed of amyloid fibrils that are cross-β-sheet structures. Interestingly, ACTH was one of the hormones that did not form amyloid-like aggregates on its own, but when mixed in vitro with β-endorphin, in the presence of heparin, the amyloid fibrils were formed. There is the caveat that β-endorphin does not seem to be processed from β-LPH in human pituitary cells so it is not clear if ACTH would form amyloid fibrils in this instance. Nevertheless, there is also evidence for amyloid aggregates in the mouse pituitary cell line AtT20, which is known to secrete ACTH and presumably β-LPH (237).
There is a suggestion that pro-hormones aggregate less than the hormones derived from them (438). Therefore, processing of the pro-hormone may be necessary before amyloid aggregation occurs. This would sort the hormone into the granule core and concentrate the molecules, excluding those hormones that do not aggregate which are then constitutively secreted (237). It is thought that the amyloid aggregation begins in the Golgi where the membrane surrounds the aggregates, although for POMC and ACTH this will depend on the degree of processing. The amyloid aggregates are stable and therefore they can be stored, but on stimulation there is a change in pH that is thought to trigger the dissociation of the monomeric hormone from the amyloid allowing its release from the cell (237). Whether this occurs in vivo and how it contributes to efficient processing is harder to determine.
G. Release of Secretory Vesicles From the Cell
Once the anterior pituitary cell is stimulated to release ACTH, the vesicles have to dock with the cell membrane. VAMP2, syntaxin 1, and SNAP-25 form a core complex (380) that interacts with NSF and SNAPs. These are termed SNARE proteins and together with synaptotagmin 1 are responsible for synaptic vesicle priming, docking, and fusion to the cell membrane. Each of the core complex proteins is related to other similar proteins in their class, which could give rise to specific combinations of these proteins in different complexes (20, 390). For the secretory vesicles to fuse with the plasma membrane, a complex process occurs involving actin and tubulin (399). This enables the secretory vesicles to exude their products into the extracellular space.
Exocytosis is coupled to specific extracellular stimuli, such as corticotropin releasing hormone (CRH) binding to its receptors on anterior pituitary cells and signaling to evoke secretion (111). How the receptor activation signals to the machinery for release of the secretory vesicles is very relevant, as the whole procedure must occur in milliseconds to release ACTH in times of stress. The release of ACTH from pituitary cells is also stimulated by arginine vasopressin (AVP) and inhibited by glucocorticoids, and this process has to synchronize with the mechanisms of release of the secretory vesicles. More details of the regulation of secretion of ACTH are found in section X.
H. Constitutive Versus Regulated Secretion
In addition to the regulated secretory pathway, there is also a constitutive pathway of secretion, which is a route allowing the release of peptides from cells which is not regulated by external factors (155); examples of peptides released in this way include lysosomal enzymes secreted by fibroblasts (166) and acetylcholinesterase released from muscle cells (332). This pathway can be inhibited by monensin, which is an ionophore that can inhibit the transport of secretory proteins through the TGN.
For secretory cells, there is evidence to suggest they have both constitutive and regulated secretory pathways. Moore et al. (257) stably transfected pro-insulin into AtT20 cells, which synthesize and process POMC to ACTH and therefore should have all the secretory components. They showed that AtT20 cells rapidly release newly synthesized pro-insulin from a constitutive pathway and store the processed insulin for release after stimulation by a secretagogue. There is evidence that the constitutive pathway releases peptides over ~40 min (155). If the regulated pathway is blocked by chloroquine, then newly synthesized ACTH is released from the constitutive pathway, which is further evidence that both pathways exist in secretory cells.
There are two mechanisms proposed for targeting peptides to vesicles. If the targeting occurs in the TGN, then it is termed “sorting by entry” (279, 400), but if it occurs in the ISGs, then it is termed “sorting by retention” (209). There is evidence for both mechanisms and despite much effort to unravel the processes that target peptides to granules, there are still many unanswered questions (100).
I. Release of POMC From the Constitutive-like Pathway
There is some early evidence to suggest that POMC is released from the constitutive pathway (256) (FIGURE 4). Analysis of how POMC is processed and how it is trafficked into secretory or constitutive granules utilized radiolabeling of the sulfates on carbohydrate chains linked to POMC in the TGN. POMC processing to convert POMC to ACTH began in the ISGs. However, incompletely processed POMC was also secreted in ISGs by a distinct pathway that has been termed the constitutive-like pathway (110).
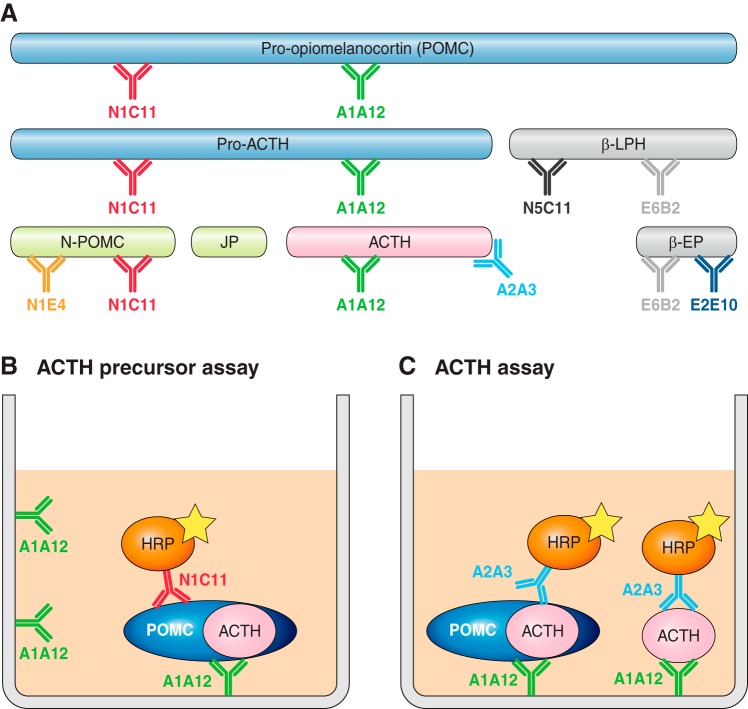
With the advent of a specific and sensitive two-site immunoassay for POMC (89), it has become possible to compare direct measurement of POMC and ACTH release from AtT20 cells. When cells are cultured under basal conditions, then much higher concentrations of POMC than ACTH are released. After stimulation with CRH for 2 h, there is a twofold increase in secreted ACTH with no change in secretion of ACTH precursors (384), suggesting ACTH is released from the regulated pathway but POMC is released from the constitutive-like pathway.
However, there are a number of caveats, the first being that the AtT20 cells release extremely high concentrations of ACTH-related peptides compared with normal mouse corticotrophs. Therefore, their secretory capacity may be different and the regulatory mechanisms may not reflect “normal” cells. A second caveat is that all ACTH assays recognize ACTH precursors to some degree (255), so the “ACTH” measured may in fact be ACTH precursors. We have calculated that ACTH precursors have <10% cross-reactivity in the ACTH assay we have developed (385). Therefore, the concentrations of precursors are contributing only a small amount to the ACTH concentrations measured.
V. RECEPTORS BINDING POMC-DERIVED PEPTIDES
The processed products of POMC bring about their biological actions through melanocortin receptors (MCRs) and the µ-opioid receptor. These will be briefly discussed below, but are reviewed in detail in Cone et al. (77) and Pasternak and Pan (298).
A. Melanocortin Receptors
The five melanocortin receptors (MC1R to MC5R) are differentiated by their tissue localization and ligand affinity (TABLE 1). They were named in the order they were discovered, rather than any association with their localization or ligands.
Table 1.
Melanocortin receptors and ligand selectivity
| Melanocortin Receptor | POMC-Derived Peptides |
|---|---|
| MC1R | α-MSH = ACTH > β-MSH > γ-MSH |
| MC2R | ACTH only |
| MC3R | α-MSH = β-MSH = γ-MSH = ACTH |
| MC4R | α-MSH = ACTH > β-MSH > γ-MSH |
| MC5R | α-MSH > ACTH > β-MSH > δ-MSH |
MSH, melanocyte stimulating hormone; ACTH, adrenocorticotropic hormone.
1. Melanocortin 1 receptor (MC1R)
MC1R is located primarily in melanocytes of skin and in hair follicles, but is also expressed in macrophages and adipocytes (169). The main role of melanocortin signaling through MC1R is in regulation of pigmentation in the skin and in hair follicles. Activation of MC1R by its ligand causes a switch from synthesis of the red and yellow, pheomelanin pigments, to the black and brown, eumelanin pigments. Mutations and variants of the MC1R have been found in patients with red hair and fair skin (409). There is also evidence that activation of MC1R can promote cell proliferation, DNA repair, and cell survival.
The primary ligand of MC1R is α-MSH, which is endogenously produced in the keratinocytes of skin and hair after exposure to ultraviolet light. Additionally, ACTH is also able to activate MC1R, and at high concentrations (such as when secreted from tumors), it can cause hyperpigmentation (406). There is also some evidence that MC1R can bind β-MSH and γ-MSH with lower affinity.
2. Melanocortin 2 receptor (MC2R)
The MC2R is also known as the ACTH receptor. It is unique among the MCR family as it only binds ACTH and is unable to bind any of the MSH peptides. The MC2R also has a much lower sequence homology with other melanocortin receptors, and in particular, it only has 38% homology with MC4R. This is primarily because MC2R has a different binding pocket compared with the other MCRs (436). MC2R is predominantly expressed in the adrenal cortex and requires the accessory protein MRAP to enable it to translocate to the cell surface so it can function. Binding of ACTH to the MC2R activates the cascade for synthesis of glucocorticoids as part of the HPA axis.
3. Melanocortin 3 receptor (MC3R)
The MC3R has a more minor role in energy homeostasis compared with MC4R and acts primarily as an inhibitory “auto-receptor” on POMC neurons in the arcuate nucleus, the region associated with energy balance. It binds α-, β-, and γ-MSH and ACTH equipotently (144). The MC3R is expressed in the hypothalamic region of the brain, but also in the limbic regions (331) and in peripheral tissues including the stomach, duodenum, pancreas, heart, testis, ovary, skeletal muscle, and kidney (78). However, the role of MC3R in these tissues is not as well defined.
4. Melanocortin 4 receptor (MC4R)
Many studies have elucidated the role of the MC4R in regulation of food intake and energy expenditure. MC4R is widely expressed throughout the central nervous system, but has a very high expression level in the PVN of the hypothalamus (260). Historically, the primary agonist for MC4R has been considered to be α-MSH, although as discussed in this review, and reviewed elsewhere (311), other POMC-derived peptide agonists such as des-acetyl α-MSH and β-MSH are likely to have similar physiological relevence. The primary agonist for MC4R is α-MSH, which is released from POMC neurons in the PVN. The antagonist for MC4R, agouti-related peptide (AgRP), is also released in the PVN, from the orexigenic AgRP/NPY neurons. The release of both the agonist and the antagonist at the receptor allows for a complex regulatory mechanism for signaling via MC4R in the PVN.
5. Melanocortin 5 receptor (MC5R)
The function of the MC5R is not as well understood as the other MCRs. It is highly expressed during embryogenesis and is known to be involved in exocrine gland function. Its expression pattern is different from the other MCRs in that it is widely expressed in a large variety of peripheral tissues; however, it is not expressed in the central nervous system (78). The primary ligand at MC5R is α-MSH, but ACTH, β-MSH, and γ-MSH are also able to bind.
B. µ-Opioid Receptors
Clearly, β-endorphin is different from other POMC-derived peptides in that it does not have the MSH sequence and therefore does not signal through a melanocortin receptor, but instead binds to the µ-opioid receptor. Although β-endorphin is the only POMC-derived peptide said to bind this type of receptor, it is not clear whether its immediate precursor, β-lipotropin, might also bind the receptor. It is thought that in adult humans, POMC is primarily processed to β-lipotropin and so β-endorphin would not be released from the pituitary to act on peripheral tissues. There is also the complexity as to how well other endorphins bind the receptors and the implications for morphine as a substrate.
µ-Opioid receptors are expressed centrally in regions including the cortex, hippocampus, hypothalamus, and brain stem (292, 293, 301) and are widely expressed in peripherally tissues including pancreas (421), testis (126, 432), ovary (432), and kidney (432). These receptors not only mediate analgesic effects, but can also play a role in the regulation of feeding behavior (as described below).
VI. ROLES OF POMC-DERIVED PEPTIDES
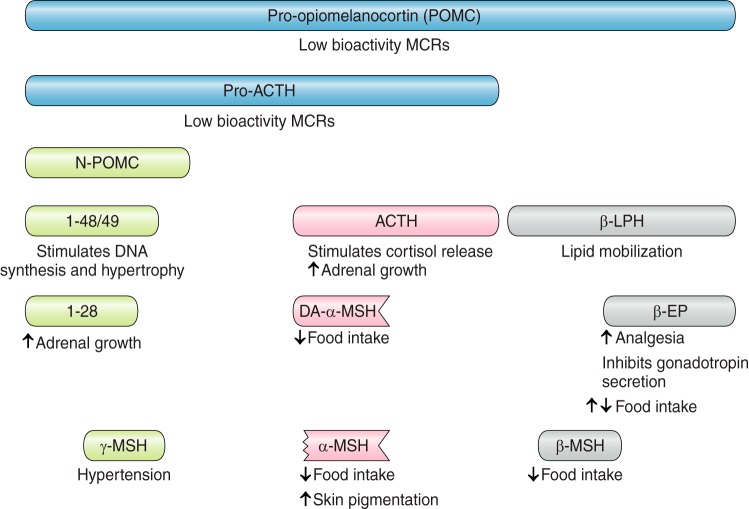
In trying to understand the importance of POMC as the precursor to a number of peptides, the inevitable question arises of why there are several bioactive peptides in one precursor molecule (FIGURE 5). Is there a survival advantage to having a single mechanism regulating the production of several peptides with different functions? Is it just serendipity that several bioactive peptides are present in the one precursor? If it is serendipitous evolution, then there are very complex events to provide ACTH for its role in the HPA axis and a very different set of mechanisms to generate α-MSH as the key peptide in the melanocortin regulation of energy balance. Alternatively, it may be that researchers working in different fields have focused on specific aspects and not put as much emphasis into investigating how other parts of the precursor may be involved.
FIGURE 5.
Pro-opiomelanocortin (POMC) processing generates numerous functional peptides. The primary roles of the different functional peptides cleaved from POMC are shown. ACTH, adrenocorticotropic hormone; β-LPH, β-lipotropic hormone; β-EP, β-endorphin; MSH, melanocyte stimulating hormone.
For researchers concentrating on the actions of α-MSH in regulation of food intake, it may not occur to them to question how POMC is processed to α-MSH and whether any of the other POMC-derived peptides could be contributing to the effect. For example, POMC is processed to ACTH and then to α-MSH, but processing may not be totally efficient in the hypothalamus, and if some ACTH is present, it could be acting at the MC4R. POMC itself has the amino acid sequence of α-MSH and could act at the MC4R, although because it is obviously a precursor molecule, we tend to think of it being efficiently processed and therefore not present outside the cell to act at receptors. However, POMC is found in relatively high concentrations in human cerebrospinal fluid (CSF) (286, 403) as well as in rat CSF (312), while α-MSH is at least 10-fold lower than POMC and 2-fold lower than ACTH in rat CSF. The much lower concentrations of α-MSH are most likely due to rapid degradation. Interestingly, in hypothalamic extracts, α-MSH is the most abundant of the three POMC peptides yet its concentration does not differ between lean and obese rats, while both POMC and ACTH are decreased in hypothalamic extracts in obese animals (312). There is no doubt that it is much harder to measure the different POMC peptides than it is to assess Pomc mRNA. The assays for the different peptides require slightly different extraction procedures especially when extracting them from hypothalamic tissue. These procedures can also affect the subsequent immunoassay, and careful optimization is required to ensure the molar ratios are not affected by these processes.
Given that these precursors are present, is it the relative affinity at the MC4R that makes α-MSH the only relevant ligand? However, α-MSH and ACTH have similar binding affinities at this receptor (311), although POMC is thought to have a lower affinity (White, unpublished data). Therefore, in understanding the dynamic roles of the different peptides, we need to address the relative importance of the processing pathway and the functionality of the different peptides.
A. Role of ACTH
1. ACTH as an integral part of the HPA axis
The central role of ACTH in the HPA axis is undisputed. Clearly the major function of ACTH in stressful situations is to increase the concentration of glucocorticoids in the blood, enabling them to have their pleiotropic actions. We think of stress being evoked by trauma and pain, but other stressors such as hemorrhage, infection, cold, hypoglycemia, inflammatory reactions, fear, emotional events, and exceptional exercise can all stimulate the HPA axis response.
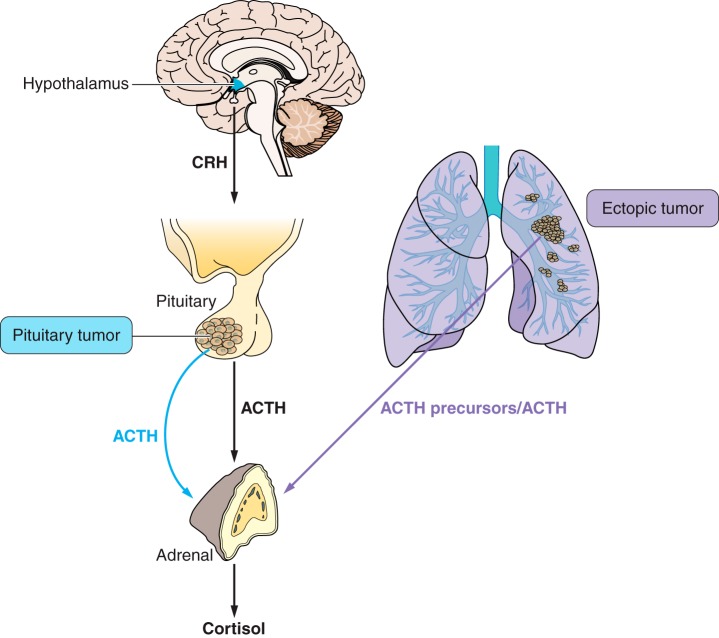
When the HPA axis is stimulated, ACTH is released from the anterior pituitary within minutes, to travel to the adrenal gland and increase glucocorticoids. The most compelling evidence for the rapidity of the release of ACTH in humans is where patients are investigated for a pituitary tumor by petrosal sinus sampling. In this investigation, patients are given CRH (peripherally) and the resulting increase in ACTH in the petrosal sinuses draining the pituitary occurs within 2–3 min (277). Therefore, this process must be stimulating release of preformed ACTH, and this ACTH must be in secretory vesicles, having been processed from POMC and then stored in readiness to respond to stressful stimuli.
ACTH travels in the circulation and acts on the adrenal gland to cause the release of cortisol in humans and corticosterone in rodents. This occurs in the zona fasciculata where ACTH binds to the MC2R. The acute effect of ACTH in the stress response occurs in the mitochondrion, where ACTH stimulates transcription and translation of steroidogenic acute regulatory (StAR) protein, which in turn increases translocation of cholesterol from the outer to the inner mitochondrial membrane (14, 388). Cholesterol is then converted to pregnenolone by the enzyme P450scc and the enzymatic cascade results in cortisol or corticosterone (162, 250). This must occur very rapidly in situations where stress stimulates the HPA axis.
After an initial stressor, there may be a need to respond to another stress in a relatively short timeframe. It has been suggested that one of the reasons for having a precursor molecule is that it can be synthesized and stored in immature secretory granules. Therefore, if there is a repeated stressor, it is possible to cleave POMC to ACTH quickly and release the bioactive molecule to provoke the stress response, without the need for stimulation of the POMC gene.
There is also a “basal” secretion of ACTH from the pituitary which has a diurnal rhythm, and this in turn evokes a circadian rhythm in cortisol. However, there is also a peripheral adrenal clock that modulates the diurnal rhythm of steroidogenesis, leading to the diurnal differences in cortisol release. Thus the basal ACTH secretion has an indirect role in modulating circadian biology, most obviously through initiating the cortisol rhythms (67).
2. Effects of ACTH on adrenal growth
The “non-stress” effects of ACTH on the adrenal gland include a role in increasing adrenal growth. This is somewhat controversial in that there are reports that this role is performed by a peptide from the NH2-terminal region of POMC (N-POMC 1–28) (see below). However, ACTH has a role in adrenal cortical development (187, 197), and ACTH replacement in POMC knockout mice is sufficient to cause normal adrenal development (75).
3. Role of ACTH in the skin
There are well-recognized extra-adrenal effects of ACTH in the skin. These are evidenced in some patients with excess secretion of ACTH-related peptides, e.g., Addison’s disease and some ACTH-secreting tumors, where there is marked excess skin pigmentation, which decreases when ACTH levels are returned to normal (426). This role of ACTH is described below in section IX.
4. Role of ACTH in adipocytes
Work in the 1970s suggested that ACTH had lipolytic activity in rat and rabbit adipocytes (320), and the effects of ACTH and MSH peptides on adipocytes have been reviewed by Boston (39). In addition to effects on lipolytic activity, ACTH and α-MSH can inhibit leptin expression and decrease insulin-induced glucose uptake, albeit mainly in murine 3T3-L1 cells (reviewed in Ref. 143). Given that α-MSH is not produced by the human anterior pituitary, the relevance of a role for circulating α-MSH in humans is difficult to interpret. However, MC2R is expressed in human mesenchymal cells undergoing differentiation into adipocytes (377), and therefore, circulating ACTH may be involved.
5. Role of ACTH in lymphocytes
The effect of ACTH synthesis and action in the immune system in an autocrine or paracrine manner is more questionable. It has been shown that POMC is synthesized by lymphocytes (34) and that ACTH is produced, suggesting that the processing of POMC follows a pattern similar to the anterior pituitary, requiring the coordinated expression of PC1/3 and the presence of a regulated secretory pathway. There is also evidence for ACTH receptors on lymphocytes (70), although the functional significance of this remains difficult to ascertain. More recently, it has been shown that ACTH controls growth of the thymus and that this is not via stimulation by glucocorticoids (392).
B. Role of α-MSH
1. α-MSH from the pars intermedia of the pituitary in rodents
While rodents have provided extremely valuable data in the understanding of POMC processing, there are some limitations that are often ignored. This is the case with POMC expression in the pituitary. In the adult human pituitary, which does not have a pars intermedia (228), POMC is only expressed in corticotroph cells in the anterior lobe. In contrast, rats and mice have a pars intermedia, comprised primarily of melanotrophs. Processing of POMC in the pars intermedia is similar to that in the hypothalamus, and this produces α-MSH and CLIP, rather than ACTH (233). This suggests that these smaller peptides are released into the circulation and must be in high concentrations in the blood of rodents. It is not clear what the functional significance of this is, as α-MSH does not bind with high affinity to the MC2R, so will not affect glucocorticoid release. An important corollary to this is that α-MSH is not produced by human pituitaries and so will not be released from the pituitary into the blood. It is also thought unlikely that α-MSH from the hypothalamus gets into the circulation, given it is not present in CSF in rats (312). However, there are reports of low levels of α-MSH in human blood (172, 190) which may be skin-derived (see sect. IX).
2. Role of α-MSH in other tissues
With the explosion of research into the role of the melanocortin system in the regulation of energy balance and its implications for obesity, there is no doubt that this is considered the most important function of α-MSH (FIGURE 4). However, as its name suggests, the role of α-melanocyte stimulating factor in darkening of frog skin was recognized many years earlier and this formed the basis of a bioassay for α-MSH (245). Subsequently, the role of melanocortin peptides in human skin has led to the suggestion that this evolution of POMC processing in skin is equivalent to a primeval stress axis (361).
3. Relative roles of α-MSH and its precursors: processing is key to function
As described above, there is a very well-defined set of intricate processing steps starting from the precursor peptide, POMC, and resulting in α-MSH (314). One could hypothesize that these processing steps have evolved to refine the regulation of energy balance. α-MSH certainly binds to the MC4R, but with affinity similar to des-acetyl α-MSH, β-MSH, and ACTH (144, 260, 311), and all four peptides have similar potency in stimulating cAMP which is required for MC4R signaling (311).
Central administration of α-MSH to POMC null mice reduced food intake to 35% of sham-treated animals and 3-day treatment reduced body weight (405). This confirmed earlier studies where administration of α-MSH to rodent brains reduced food intake (1, 247, 251, 310). However, other studies also showed that the α-MSH precursors, des-acetyl α-MSH (at high doses) and ACTH, had similar effects (4, 189). This is controversial as there is also evidence that des-acetyl α-MSH injected into the brain had no effect on food intake (1, 261, 404).
However, a recent study has generated a new mouse model where the cleavage site in ACTH, which is necessary to generate α-MSH, has been mutated. By treating these mice with either α-MSH or des-acetyl α-MSH, it has highlighted the importance of des-acetyl α-MSH, by showing it can have an equivalent effect to α-MSH in reducing body weight (259).
If ACTH can bind to the MC4R and inhibit food intake, what is the purpose of processing ACTH to α-MSH, given this involves cleavage of ACTH to ACTH (1–17), removal of amino acids 14–17, and then amidation and acetylation? Is it more that the key question is which peptides are stable in the POMC neurons in the hypothalamus and which peptides are presented to the MC4R? We have previously shown that ACTH and POMC were present in rat CSF and regulated by fasting, while α-MSH was undetectable (312). However, in hypothalamic extracts, we found that α-MSH was present at higher concentrations than POMC or ACTH and the ratios were altered depending on energy requirement (312). Early work suggested that α-MSH’s immediate precursor, des-acetyl α-MSH, was the major product in the arcuate nucleus with lesser amounts of α-MSH and ACTH, while α-MSH predominated in the NTS in the brain stem (113, 114). Other studies also suggest that des-acetyl α-MSH is more abundant than α-MSH in the arcuate nucleus (97, 156, 182, 201, 296, 328), but not in the brain stem (103). This seems at odds with reports that des-acetyl α-MSH is relatively unstable compared with other POMC-derived peptides (156, 272).
It is difficult to distill a coherent mechanism from the contradictory data. There is evidence that the acetylation of des-acetyl α-MSH to generate α-MSH is regulated by leptin (156) and may be regulated by dopamine (127, 252, 410, 412), although others suggest this is not the case (95). If the final stage in the processing pathway is important for the flux of peptides at the MC4R, this would imply that the N-AT acts on des-acetyl α-MSH at the synapse/bouton/neuronal extremity (FIGURE 3) (258). Given that the POMC neurons release their peptides in the PVN to act on the MC4R, it is tempting to speculate that future studies should focus on the regulation of whichever peptide is released in proximity to the MC4R.
Although much of the focus on the function of α-MSH relates to suppression of food intake, there is evidence of a role for MC4R in mediating increased energy expenditure (48), oxygen consumption, and fuel oxidation. Melanocortin regulation of these metabolic processes appears to occur via the sympathetic nervous system. There is some evidence for this from central injection of MT-II, a very potent synthetic melanocortin peptide analogue, which led to loss of body fat in rats. This was caused by enhanced thermogenesis mediated via sympathetic nervous system outflow to white and brown adipose tissue (359, 430, 444). However, the significance of this to in situ physiological mechanisms is not clear.
C. Role for β-LPH as a Precursor of β-Endorphin and β-MSH
Early work suggested that β-LPH had a role in mobilizing lipid, hence its name (326) and subsequently that it was the new aldosterone stimulating factor (240). However, over the years, it has become established that β-LPH functions primarily as a precursor for β-MSH and β-endorphin. In the human pituitary, β-LPH is unlikely to be further processed, as the cleavage sites require PC2 which is not present. Therefore, β-LPH should be released into the human circulation in molar equivalents to ACTH. We have previously identified β-LPH as the major COOH-terminal POMC peptide in blood from normal subjects (148, 149). However, several reports suggest that β-endorphin is increased in human plasma with exercise (196). While it may be that exercise specifically changes the processing to give β-endorphin, it could also be explained if it was primarily β-LPH present in the circulation and the β-endorphin immunoassay cross-reacted with β-LPH.
D. Role of β-MSH in Energy Balance
The impact of β-MSH is somewhat controversial. While β-MSH is present in the human brain, the NH2-terminal cleavage site to generate β-MSH is not found in rodent POMC (16, 258). Studies have shown that β-MSH binds MC4R with similar affinity to α-MSH and has a similar potency (311). In addition, β-MSH is able to reduce food intake in corticosterone-supplemented Pomc null mice, although to a lesser extent than α-MSH (405). Evidence for a role for β-MSH also comes from studies in humans with mutations in β-MSH. Our colleagues in Cambridge have described an obese child with a mutation in POMC that creates a fusion protein of β-MSH and β-endorphin, preventing cleavage of these peptides. One possible hypothesis to explain why α-MSH was not sufficient to prevent the obesity is that the fusion peptide had a dominant negative effect (57). Three subsequent papers (30, 216, 217) describe other mutations in β-MSH that contribute to the evidence that this peptide does have a role in energy balance, which should be considered alongside that of α-MSH. This is described in more detail in section VII. Intriguingly, some Labradors noted for their voracious appetites have loss of the β-MSH sequence. This is caused by a mutation that results in a truncated POMC which loses part of the β-LPH region encompassing β-MSH and β-endorphin (318).
E. Roles of β-Endorphin
1. The opiate activity of β-endorphin
The highest concentration of β-endorphin in the brain is found in the hypothalamus and specifically in the arcuate nucleus, median eminence, and ventromedial border of the third ventricle (440). β-Endorphin (1–31) is the major form and is active at µ-opioid receptors (see commentary by Loh, Ref. 223). As described above, β-LPH is cleaved by PC2 to give γ-LPH and β-endorphin which can be further cleaved by CPE to β-endorphin (1–27) and β-endorphin (1–26), which have much less analgesic activity (269). These enzymes act in secretory granules within cells, so this implies that the cleavage of β-endorphin to the COOH-terminally truncated β-endorphin peptides is a mechanism to reduce opioid activity in tissues where other POMC peptides are released for non-opioid functions. Acetylation of β-endorphin at its NH2 terminal is also a mechanism for reduction of opioid activity (92), and this occurs in the pars intermedia of the pituitary and in the brain stem (440). In the NTS, there are POMC expressing neurons that primarily produce β-endorphin, but there is also a considerable amount of α,N-acetyl β-endorphin (1–27), which would be much less bioactive (103, 440).
The first indication that β-endorphin acts at µ-opioid receptors was in 1976, when it was shown to be 100 times more potent than morphine (132). The sequence of Met-enkephalin at its NH2 terminal is obviously responsible for the opiate activity, but β-endorphin has much longer-lasting effects compared with the transient activity of the enkephalins. This has been attributed to its sequence, which confers resistance to degradation. There is also the suggestion that the more COOH-terminal region of β-endorphin acts in “an address function,” by presenting the peptide to the receptor to aid specificity and potency (378). This could be considered another advantage of the presence of a peptide within a larger pro-hormone structure.
Given that β-endorphin is produced in POMC neurons in the arcuate nucleus of the hypothalamus, it is difficult to rationalize how POMC is stimulated to specifically produce β-endorphin to have its analgesic function in a physiological setting. However, immunohistochemical staining for β-endorphin has demonstrated its presence in nerve terminals that extend dorsally and laterally, and it can be found in the amygdala, colliculi, and hippocampus. While there is evidence for the role of β-endorphin in energy homeostasis (see below), there are very few reports which link how stimulation of POMC expression specifically drives analgesia without releasing the melanocortin peptides, which should have an important role in increasing energy expenditure and inhibiting food intake. It is tempting to speculate that this is where a hormone precursor is providing different peptides with different roles but with a common theme of coordinating a response to pain as a self-preservation mechanism.
In a very elegant study, Rubinstein et al. (334) produced mice with a targeted mutation that inserted a premature stop codon in the POMC gene to prevent the synthesis of β-endorphin. These mice were not able to mount an analgesic response to a mild swim stress and had a compensatory upregulation of other pain inhibitory pathways. This does suggest that a stress activation of POMC would produce an analgesic response mediated by β-endorphin.
2. Role of β-endorphin in reproductive function
Early work on endogenous opioid peptides, including β-endorphin, indicated that they inhibited gonadotropin secretion (133) and the opioid antagonist naloxone stimulated luteinizing hormone release in men and women (122, 315). The mechanism was elucidated in studies in rats and involves the release of hypothalamic β-endorphin into hypophysial portal blood, which is stimulated by ovarian steroids (342) and inhibited by testosterone (418). An interesting aspect of the precursor role of POMC is that in producing both α-MSH and β-endorphin there is the potential to have two peptides which antagonize each other. α-MSH blocks both stress-induced and β-endorphin-stimulated release of prolactin in rats (267). In monkeys, α-MSH has a similar effect on β-endorphin-induced prolactin and blocks the β-endorphin-mediated decrease in luteinizing hormone (419).
3. Hypothalamic β-endorphin function and regulation of energy balance
Given that many of the component peptides of POMC have a role in energy balance, it is important to consider whether β-endorphin may also be involved in some capacity. Mechanisms regulating the release of melanocortin peptides from POMC in hypothalamic neurons will generate β-endorphin. However, it is important to consider whether the β-endorphin actions are synergistic with those of the melanocortins, or whether they are not commensurate, implying that they would oppose each other. If it is the latter, then there may be processing mechanisms to inactivate β-endorphin when melanocortins are activated, and vice versa. Although inactivation by acetylation of β-endorphin is not thought to occur in the hypothalamus (258), it may be that processing to β-endorphin (1–27) and β-endorphin (1–26) is a mechanism that at least reduces its activity (269). As early as the 1920s there were suggestions that the endogenous opioid system was involved in the regulation of food intake and body weight, with morphine causing a decrease in body weight but a “voracious” appetite. However, there is a lot of contradictory data in both animal and human studies (12). Nevertheless, more recent compelling data from a study of mice with deletion of β-endorphin showed that the male mice were obese and hyperphagic (9). This suggests that loss of β-endorphin results in hyperphagia, highlighting an unexpected anorexigenic effect of endogenous β-endorphin, which parallels the melanocortin actions of the other peptides derived from POMC. Nevertheless, β-endorphin is involved in a motivational reward behavior in nondeprived conditions (227), and other studies have found stimulatory effects on feeding (36), suggesting that there are two different roles for β-endorphin depending on the circumstances. This concept would support the finding that cannabinoid-induced feeding is dependent on β-endorphin (203).
In considering the endogenous POMC activity, it is difficult to rationalize the concept that several peptides are produced simultaneously which have opposing actions. This is nevertheless implied by the fact that α-MSH and β-MSH cause anorexigenic actions while β-endorphin stimulates feeding. It may be that the regulation of POMC processing events underpins how effective POMC peptides are in coordinately regulating energy balance. Dutia et al. (112) gave β-endorphin by intracerebroventricular injection and compared food intake and body weight gain in rats when an analogue of α-MSH (NDP-MSH) was coadministered. When β-endorphin was given over 2–6 h, it stimulated food intake, and it reversed the inhibitory effect of NDP-MSH on food intake. However, with more chronic dosing over 4–7 days, β-endorphin failed to antagonize the effects of NDP-MSH.
This still leaves several questions regarding the mechanisms that balance the effects of melanocortins and β-endorphin on energy balance. Given there are so many regulatory stages in the processing of POMC, it suggests that processing has evolved in such a way to provide subtle regulation of active peptides in the hypothalamic neurons.
4. Role of β-endorphin in skin
It has also been suggested that β-endorphin has very specific roles in the skin, and this is described in section IX.
F. Roles for the N-POMC Peptides
Compared with the other regions of POMC, there are fewer reports on functional roles for the POMC-derived peptides that are linked to the NH2 terminal of ACTH, at least in the human. It may be that there has been relatively less research in this area, rather than that the peptides do not have physiological roles. To our knowledge, there are relatively few mutations in this region that inform function, and in the human these are involved in obesity (128). However, many of the mutations in this region would also affect the translation of POMC.
1. Role of NH2-terminal POMC peptides in adrenal growth
There is a wide body of data from the 1980s, described in detail in a review by Bicknell (27), which suggests that a fragment of human N-POMC increases rat adrenal gland weight and mitotic index (124). Previous work had indicated that the full-length N-POMC peptide (1–76 in humans and 1–74 in rats) was not active (123). The most effective fragment was N-POMC (1–28), which had been isolated from human pituitaries as part of the purification of growth hormone (124) but was known to be a purification artifact so presumably did not exist normally in pituitaries (246). Subsequently in rats, N-POMC (1–28) partially regenerated adrenal glands which had been enucleated (125). Further work provided evidence that the N-POMC (1–28) peptide stimulated cell division in primary bovine adrenal cells, Y1 cells, and human adrenal tumor cells (NCI-H295-R) (131). However, when N-POMC (1–28) was given to mice with a null mutation in the Pomc gene, there was no effect on adrenal growth, and no change in adrenal morphology, in a setting where ACTH (1–24) caused adrenocortical hypertrophy (75).
Later work with Y1 adrenal cells has extended the analysis of how synthetic N-POMC (1–28) stimulates the pathways involved in cell proliferation (reviewed in Ref. 226). There is evidence that N-POMC (1–28) increases phosphorylation of ERK1/2 as well as activation of MEK and c-RAF (303). This has been complemented with studies in isolated rat adrenal cells showing activation of the ERK pathway by N-POMC (1–28) (241) and in vivo in rat adrenal cortex where synthetic N-POMC (1–28) upregulated proliferation and blocked apoptosis (401).
In parallel with the earlier studies described above, the group led by Phil Lowry (229) injected antisera raised to N-POMC (1–28) and to a synthetic γ3-MSH peptide into rats. They found different effects on compensatory adrenal growth in the contralateral gland, following the removal of the other adrenal gland (229). They suggested that N-POMC (1–48/49) stimulates DNA synthesis and mitogenesis, while a second region in N-POMC (i.e., γ3-MSH) increases RNA synthesis and hypertrophy (229).
In essence, the early work showed that full-length N-POMC was not active in stimulating adrenal growth, but that the shorter N-POMC (1–28) was able to stimulate adrenal gland mitogenesis. This led to the hypothesis that N-POMC had to be cleaved to have effects on adrenal growth. N-POMC (also called pro-γ-MSH) can be measured in human plasma (58, 148, 160) and is one of the main products secreted from rat pituitary corticotrope cells (117, 181), indicating that it is likely that this is the major NH2-terminal POMC peptide in the circulation. Therefore, any cleavage of N-POMC would occur at the target cells, i.e., at the adrenal cortex. The discovery of a rat adrenal gland-derived trypsin-like enzyme called adrenal secretory protein (AsP) is described in detail in Bicknell (27). This enzyme cleaves between valine and methionine and so would generate N-POMC (1–52) which can stimulate adrenal mitogenesis (28). However, there is evidence that the human equivalent of AsP does not have a physiological role in regulation of adrenocortical growth because of low expression of the enzyme in human adrenal tissue (158).
It is difficult to resolve some of the inconsistencies in the understanding of the role of N-POMC peptides, because the large body of data has used different peptides and various models, which are not always comparable. One possibility may be that N-POMC (1–28) is an extraction artifact and there is evidence that N-POMC (1–48/49) may not circulate to get to the adrenals because the O-glycosylation at Thr45 inhibits cleavage at Arg49-Lys50, which is a likely site for pro-hormone convertases (348). The presence of full-length N-POMC in human plasma substantiates the evidence of a lack of cleavage in human pituitary cells. Therefore, if fragments of the N-POMC peptides play a role in adrenal mitogenesis, it would have to be after cleavage at the adrenal gland. However, it seems that the human equivalent of AsP is not capable of this role.
It may be that stimulation of growth of the adult adrenal cortex by POMC peptides is more physiologically relevant than it is in the fetus. There is evidence for this in Pomc null mice where the adrenal glands undergo atrophy after birth. Transplantation of these adrenals into wild-type mice rescues growth and corticosterone production (188). This implies that some POMC-derived peptides restore growth and steroid secretion in mice. However, it is not possible to determine which POMC-derived peptides are responsible, although there is evidence for and against ACTH (reviewed in Refs. 27, 226).
2. Role of NH2-terminal POMC peptides in salt-sensitive hypertension
There is some intriguing evidence about the role of γ-MSH and its effects on natriuresis and control of blood pressure (reviewed in Ref. 174). Several studies have shown that γ-MSH can have a hypertensive effect, acting via a central mechanism. However, other studies have indicated the opposite effect. In PC2 knockout mice, where there is decreased γ-MSH, hypertension occurred on a high-salt diet, and treatment with γ-MSH prevented the increased mean arterial pressure. Absence of the Mc3r gene also caused a hypertensive effect (268). In addition, there is some suggestion that γ-MSH acts directly on MC3R in the kidney to play a role in natriuresis, while other evidence points to a central role acting via sympathetic outflow on the periphery.
Our understanding of these mechanisms is complicated by the fact that in mouse, rat, and guinea pig, γ-MSH may not exist as a separate peptide, because the COOH-terminal region does not have the dibasic amino acids to allow cleavage from the N-POMC region (114). In the studies described above on adrenal growth, it was presumed that the full-length N-POMC is released from the anterior pituitary and therefore γ-MSH would only be released from the pars intermedia of the pituitary, which is rudimentary in humans. Therefore, the relevance of these mechanisms in humans needs further clarification. It is tempting to speculate that if there is an enzyme which cleaves N-POMC at the adrenal to produce a peptide which promotes adrenal cortex mitogenesis, then the same enzyme may also be present in the kidney to generate peptides that stimulate natriuresis.
The evolution of POMC as a precursor of peptides with multiple actions leads to the question of whether it would be valuable to have a response that releases stress hormones and a natriuretic hormone that decreases blood pressure. Perhaps this overlooks the subtlety of the system and these two responses have evolved to respond to different stimuli in different tissues.
3. Role of γ-MSH in energy balance
While there is clear evidence that α-MSH plays a role in decreasing food intake, it is more difficult to determine the relative importance of γ-MSH peptides and the net effect of coordinated processing of POMC. It is predicted that γ3-MSH and γ2-MSH can be produced in the hypothalamus in humans, but that γ3-MSH cannot be processed to γ2-MSH or γ1-MSH in rats and mice because of the lack of suitable dibasic amino acids (114). It is also difficult to find direct evidence that indicates a role for these peptides in energy balance. γ2-MSH binds to the mouse MC3R (and MC5R) better than to other MCRs (184), and the MC3R is important for energy homeostasis (reviewed in Ref. 258), although it does not appear to have a role in food intake (1). However, α-MSH has comparable binding activity to γ-MSH at the human MC3R (reviewed in Ref. 198), so it is not clear which is the natural ligand at least in the hypothalamus.
G. Does Joining Peptide Have a Role?
There is very little evidence for a role for joining peptide. There was a suggestion that a peptide identical to joining peptide (1–18) stimulated production of dihydro-epiandrostenedione (DHEA) from adult human adrenal cells (297) and was therefore designated as the missing cortical androgen-stimulating hormone (CASH). However, other studies have failed to find evidence for this in adult (302) or fetal (330) adrenal cells.
VII. CHILDREN WITH MUTATIONS AFFECTING POMC PROCESSING
A. Mutations in POMC Lead to Obesity
The processing of human POMC is very different in the hypothalamus to that in the pituitary. In the hypothalamus it involves the sequential effects of two pro-hormone convertases and numerous posttranslational modifications to generate the melanocortin peptides α-, β-, and γ-MSH. Both α- and β-MSH are recognized to have important roles in the regulation of energy balance, and either loss of POMC or disruption of pro-hormone processing results in severe obesity. The following examples in children give insights into the importance of the pro-hormone and the requirements for the different melanocortin peptides.
1. Early studies linking mutations in POMC to obesity
The earliest reports suggesting mutations in the POMC gene were associated with obesity came from linkage studies in Mexican Americans. In this analysis, patients with increased leptin levels had a polymorphism that was mapped to chromosome 2p21, where POMC is located (76). Another linkage study showed that French subjects had a similar mutation in chromosome 2p21, demonstrating that the mutations were found in other ethnicities and cultural backgrounds (157). These studies were carried out in advance of the POMC gene deletion in mice and gave an initial association between mutations in POMC and increases in leptin and fat mass.
2. Mutations leading to global loss of POMC peptides
The strongest evidence for a link between mutations in POMC and obesity comes from children who have either homozygous or compound heterozygous mutations in the gene, leading to the absence of all melanocortin peptides. One of the first patients described had a homozygous C → T mutation at 3804 in exon 2, which is in an untranslated region. This created an additional out of frame start codon which abolished the translation of wild-type POMC. The clinical features observed in the patient are linked to the loss of binding of POMC-derived peptides to the MCRs in specific tissues. The patients had red hair, indicating lack of binding to MC1R, and they were hypocortisolemic due to absence of ACTH binding to MC2R and obese due to deficiency in MSH binding to MC3R and MC4R (207). The same group later described another patient with the same mutation and phenotype (208).
Heterozygous mutations in the noncoding region have also been described and are associated with obesity. By screening obese populations, two patients were found with different heterozygous mutations in exon 2 of the POMC gene. These have been implicated in disruption of POMC sorting to the regulatory secretory pathway. Examination of the processing in these patients indicated that the mutations had interfered with the entry of POMC into the normal regulated secretory pathway (86). This disruption to the processing of POMC and the reduction in processed peptides were associated with the development of obesity.
3. Mutations in the NH2-terminal region of POMC
In the initial paper describing children with a lack of POMC, one of the patients showed two separate mutations in exon 3 of POMC, giving a compound heterozygous mutation. The first mutation was a G → T substitution at nucleotide 7013, leading to a premature stop codon at codon 79. The second mutation was a single base pair deletion at nucleotide 7133, predicating a frame shift that would disrupt ACTH and α-MSH binding motifs as well as inserting a stop codon at 131. Similar to the patient with the homozygous mutation in the noncoding region of POMC, this patient also had red hair, decreased cortisol, and obesity (207). As both α- and β-MSH are disrupted by these mutations, it is difficult to clearly discern their relative importance.
The same research group later characterized two further children who also had mutations in the NH2-terminal region of POMC. They both had compound heterozygote mutations with a frame shift or a premature stop codon, preventing translation of the region with ACTH and the MSH peptides. These patients were obese and also had red hair due to the lack of melanocortin peptides (208).
The first patient to be described without red hair was a Turkish child with a novel homozygous frame shift mutation at nucleotide 6906, although he did have dark red follicles. This mutation would be predicted to lead to a loss of all POMC-derived peptides. As with the other patients, this child had severe hyperphagia leading to early-onset severe obesity (129). The association between heterozygous mutations in POMC and obesity was strengthened in this study. Of the 12 heterozygous relatives of the child, 11 were overweight or obese (129).
Other novel mutations in the ACTH region of POMC have been described, where the POMC-derived peptides are still immunoreactive, but have lower biological activity (339). A more recent study described a patient with red hair who had moderate obesity at an early age, with undetectable plasma ACTH and serum cortisol. This index case was a compound heterozygote with one mutation in the NH2-terminal region of POMC and the second mutation upstream from the coding domain. The latter affected a region involved in translation of the protein such that there was preserved, but markedly diminished, levels of wild-type POMC transcript (7).
Other patients have been described with mutations in this region. The hair color phenotype has not been observed in all patients, even when mutations were predicted to lead to an absence in α-, β-, and γ-MSH as well as β-endorphin (71, 248). However, all patients identified with deletions in the NH2 terminal of POMC have severe obesity (71, 167, 248, 254).
4. Mutations in the α-MSH region
There are very few reports of mutations in this region. Studies examining patients with severe obesity have occasionally identified heterozygous mutations in the α-MSH region of POMC. However, these mutations were rare in the obese population and were also found in the lean control population, indicating that the loss of one allele of α-MSH can be tolerated in the context of energy balance (109, 216), and/or that β-MSH plays a more important role. There is some evidence to substantiate this in the section below.
5. Defects in the β-MSH and β-endorphin regions of the POMC gene
Mutations in the β-MSH region of POMC have strong associations with obesity. During screening studies of patients with early-onset obesity, patients have been described with a heterozygous R236G mutation in the highly conserved dibasic processing site between β-MSH and β-endorphin (46, 57, 254). This mutation led to the formation of a fusion protein of the two peptides, which was able to bind MC4R, but was less functional (57). These patients have the characteristic hyperphagia and early-onset obesity associated with reduced binding to MCRs (57). Interestingly, there were relatives of the index patient who were also heterozygous for the mutation. Although they did not have the severity of obesity observed in the index case, they were more likely to be overweight than a relative without the mutation (57).
Another mutation identified in the β-MSH region is the Y221C mutation. This altered form of β-MSH was able to bind MC4R, but was unable to activate it. This mutation was strongly associated with obesity, as 11 of 13 relatives with the same heterozygous mutation were obese. However, some noncarriers were also found to be overweight (30, 216), indicating that at least in this kindred, the obesity phenotype cannot solely be as a result of the mutation in β-MSH.
6. Variants in the γ-MSH region of POMC
There have been multiple reports of many subjects with 6, 9, and 19 base pair insertions in the γ-MSH region of POMC, in screening studies investigating POMC mutations in obese patients from different ethnic backgrounds. Although these insertions have been found in the obese cohort, they have also been identified in the normal-weight participants making it difficult to associate these insertions with obesity (116, 167, 254, 340).
B. Mutations in PC1/3 Cause Obesity
Many of the early papers characterizing patients with mutations in PC1/3 predate the discovery of leptin and were seminal in defining novel monogenic causes of obesity. As with POMC mutations, homozygous or compound heterozygous mutations in PC1/3 cause severe hyperphagia leading to early-onset obesity (130, 179, 180, 239, 274). This is most likely related to the abnormal processing of POMC. These patients have been found to have high levels of POMC (130, 179, 180, 274), but with normal circulating ACTH or normal to low cortisol levels (130, 179, 239, 274). The ACTH has been shown to be authentic, bioactive ACTH (179), which was surprising since PC1/3 mutations would be expected to prevent cleavage of POMC to ACTH. This indicates that in some instances, other enzymes such as PC5A, furin, and PACE4 may be able to act in place of PC1/3.
As PC1/3 cleaves numerous pro-peptides, a plethora of other clinical phenotypes were noted in these patients. For example, with an impairment of the cleavage of pro-insulin to insulin, these patients also had abnormal glucose metabolism (130, 179, 274).
Initial analysis of heterozygous PC1/3 loss was not thought to have any metabolic sequelae, as heterozygous parents of index subjects without a functional copy of PC1/3 were not obese (130, 179, 180). However, further human genetic analysis, both of the PCSK1 gene (85) and SNPs in this gene (21), suggest this may not be the case. For example, a nonsense mutation in PCSK1 has been reported to cause dominantly inherited obesity (306) even though in vitro bioactivity predicts as little as 20% reduction in enzyme activity (306).
PC1/3 mutations appear to be the only mutations in POMC processing enzymes associated with obesity in humans. Surprisingly, to date, no obese patients have been described with mutations in PC2. A screening study of families with type 2 diabetes found a mutation in CPE, but the authors concluded that the mutation was not a significant cause of the diabetes (63).
VIII. MOUSE MODELS: DISORDERED PROCESSING IN THE HYPOTHALAMUS
A. Global Deletion of POMC
To investigate the role of POMC and its processed peptides, mice with a global knockout of POMC have been developed. There have been two separate approaches to removing POMC, both involving the deletion of exon 3. The original model left the possibility that the NH2-terminal fragments of POMC could still be transcribed (437), but the later version ensured this was not possible (56). Both strains experienced some embryonic lethality (56, 437), indicating the importance of POMC in development and maturation in utero.
Overall, the loss of POMC has a significant impact on the metabolic phenotype of the mouse with much concordance between reports. Pomc null mice develop obesity from around 2 mo of age and have increased fat and lean mass (56) as well as an increase in body length (437). The obesity seen in the original model persisted when the mutant allele was backcrossed onto a C57Bl/6 background (371). Both murine models were hyperphagic on low- and high-fat diets (56, 437) with administration of α-MSH to one of the Pomc null models able to ameliorate the hyperphagia and bring about weight loss (437). A lower resting oxygen consumption seen in one model (56) may have an additional role in the development of obesity. Pomc null mice have a normal glucose tolerance, but they have an increased sensitivity to insulin, likely due to their corticosterone deficiency (170).
Pomc null mouse models have also highlighted the importance of the POMC-derived peptides in the maintenance of adrenal gland development. There has been controversy around which peptides contribute to adrenal gland growth, with evidence for both ACTH and N-POMC peptides as key to the process. At birth, POMC knockout mice have adrenal glands that are morphologically indistinguishable from those of their wild-type littermates (188), but by adulthood, these mice have either no macroscopically determinable (437) or very small but identifiable (56, 371) adrenal glands. The lack of POMC in these mice results in no circulating ACTH, and consequently, they lack circulating corticosterone (56, 371, 437), even before atrophy of the adrenal glands (188). Acute administration of ACTH appeared insufficient to induce corticosterone production (188); however, a longer treatment period normalized adrenal weight and circulating corticosterone (74). In contrast, treatment with POMC (1–28) did not “rescue” the adrenal glands (75). Furthermore, when adrenal glands from knockout mice were transplanted into POMC-intact mice, they were able to produce corticosterone (188) showing the importance of POMC-derived peptides in maintenance of adrenal gland corticosterone production. Together, these results demonstrate that ACTH is required to maintain adrenal gland function.
In these Pomc null mouse models, the impact of loss of hypothalamic Pomc on body weight may have been tempered by lack of pituitary ACTH and therefore lack of corticosteroids. This has led to an interesting phenotype of obesity in the absence of circulating glucocorticoids. Therefore, to investigate the full impact of the loss of POMC in the presence of glucocorticoids, two approaches have been reported: 1) administration of corticosterone in drinking water and 2) restoration of POMC in the pituitary to enable ACTH production and then corticosterone synthesis. In the first approach, corticosterone supplementation normalized the circulating corticosterone in Pomc null mice, but significantly increased body fat and body weight further (73). This was associated with a significant increase in the expression of the MC4R antagonist AgRP (73). In the second approach, Malcolm Low’s laboratory (372) introduced a POMC transgene into the pituitary of POMC knockout mice to rescue POMC-derived peptides in the pituitary. In contrast to the global POMC knockout mice, this transgenic line had large adrenal glands, and while female mice had a normal corticosterone diurnal rhythm, males had both exaggerated peak and nadir levels leading to overall higher levels of corticosterone, in keeping with a Cushing’s type syndrome (373). Compared with POMC knockout mice, these pituitary rescued POMC knockout mice were even more hyperphagic and developed a greater degree of obesity. Additionally, they developed hyperglycaemia and insulin resistance with hepatic steatosis (372), likely to be due to the excess pituitary ACTH increasing glucocorticoids.
To elucidate the role of hypothalamic POMC, and in particular α-MSH, at different stages in the evolving obesity seen in global POMC deficiency, another murine model was established that allowed for reexpression of hypothalamic POMC at different ages in global Pomc null mice. As one might expect, restoration of neuronal POMC and α-MSH expression at all ages effectively normalized the hyperphagia in Pomc null mice. However, the effectiveness of this treatment to normalize body weight and diminish adipose mass declined progressively as the age at which Pomc was inducted increased, with a diminished impact on body fat reduction in older, and hence fatter, mice (45). Finally, in yet another mouse model, reexpression of Pomc solely in hypothalamic neurons expressing the leptin receptor was sufficient not only to normalize the increased body weight and food intake observed in the global Pomc null mice but also to correct alterations in glucose homeostasis and locomotor function (212).
Together, a range of mouse models with genetically altered POMC have helped to elucidate the roles of POMC in many aspects of adrenal development and metabolic homeostasis. Perhaps surprisingly however, a distinct coat color phenotype was only clearly seen in POMC null mice on a 129 background (56).
B. Loss of PC1/3: Implications for POMC
1. PC1/3 null mice
The importance of POMC as a precursor compared with the derived peptides should be determined by the knockout of PC1/3, as this has the potential to produce POMC in vivo without any of the peptides derived from it. However, the reality has proven much more complex, because of concomitant lack of processing of other peptides and the potential for cleavage of POMC by other peptidases.
The role of PC1/3 in the cleavage of POMC in vivo was first elucidated in PC1/3 null mice developed in 2002. These mice have increased unprocessed POMC in the pituitary and a lack of processing to ACTH (291, 449). Surprisingly, even though there was an absence of ACTH, there was no difference in corticosterone (449), suggesting that perhaps the higher levels of POMC could compensate for the lack of ACTH. However, this has not been confirmed by other studies (387). Relative levels of other POMC-derived peptides were not altered in PC1/3 null mice (291), indicating some adaptation or compensation. Either PC2 or another enzyme must be in place to maintain the levels of other POMC-derived peptides.
The first PC1/3 null mice were not obese, unlike patients with mutations in the gene (see sect. VII). This may not be as surprising as it first seems, because the mice have unaltered levels of POMC cleavage products including α-MSH. PC1/3 null mice also have other metabolic abnormalities, including undetectable levels of insulin in pancreatic islets as they are unable to cleave pro-insulin to insulin (448).Intriguingly, despite this marked hyperproinsulinemia, the mice appear not to have an impairment of glucose tolerance (449).
A second PC1/3 null mouse was developed by Seidah, Chrétien, and co-workers in 2007 (243). However, this mouse was embryonic lethal and therefore could not be used in further experiments (243).
2. PCSK1-N222D hypomorph mouse
Interestingly, a single point mutation in the Psck1 gene led to a mouse with an obese phenotype, similar to that seen in patients with these mutations. This Pcsk1-N222D hypomorph mouse had a 60% reduction in PC1/3 activity (221). Unlike the PC1/3 knockout mouse, this strain was a normal size due to its ability to process pro-growth hormone-releasing hormone (386). These mice developed obesity, and by 6 mo, males were 32% heavier and females 68% heavier then their wild-type littermates as a result of increased fat mass (221).
In the hypothalamus, the expression of the Pomc gene in Pcsk1-N222D hypomorph mice was similar to wild-type mice, but they had a 45% reduction in α-MSH, which may have a played a role in the observed hyperphagia and could have contributed to the obesity (221). There was also impaired processing in the pituitary, in that they had increased pro-ACTH levels compared with wild-type mice (386). Surprisingly, the Pcsk1-N222D hypomorph mice had a slight elevation in ACTH which supports the theory that processing of POMC to ACTH may not be completely dependent on PC1/3 (221).
C. PC2 Knockout Mice: Implications for POMC Processing
The PC2 knockout strain was developed in 1997 by deletion of exon 3. The mice appeared normal at birth, but grew at a slightly slower rate and had normal fat distribution and mass (140). In addition, PC2 null mice had high circulating ACTH, but normal circulating corticosterone (300). Abnormalities in the processing of POMC in both the pituitary and hypothalamus were noted. The pituitary had reduced Pomc mRNA levels, but both the glycosylated and unglycosylated forms of POMC protein were increased (213). This was accompanied by increased pituitary ACTH concentrations (213, 300) and higher numbers of secretory granules (213), which was consistent with the elevated POMC and ACTH concentrations. The pituitaries from PC2 knockout mice also contain increased amounts of β-LPH and reduced amounts of its cleavage products γ-LPH and β-endorphin due to the lack of processing (213). Depending on the method of detection, α-MSH was found to be either absent (213) or very diminished (161). Furthermore, des-acetyl α-MSH, di-acetyl α-MSH forms, and CLIP were also found at much lower levels than in wild-type mice (161).
In the hypothalamus, the levels of POMC were not altered. Again, similar to the pituitary, there was a large reduction in the cleavage of β-LPH to γ-LPH and β-endorphin, but with still about one-third of the normal conversion (6), indicating that although PC2 is the primary processing enzyme, other pathways are possible. Of the β-endorphin (1–31) present, there was a reduced amount of processing to β-endorphin (1–27) and β-endorphin (1–26) (6). Like the pituitary, α-MSH, des-acetyl α-MSH, and CLIP were all absent in the hypothalamus (290, 443). It would be interesting to investigate the effects of deletion of both PC1/3 and PC2 on POMC processing; however, the double knockout strain was lethal (420).
D. CPE Gene Deletion: Implications for POMC
CPEfat/fat mice have a missense mutation in the gene for CPE at Ser202. Investigation of this identified a problem with the translation of CPE, as the mRNA levels were normal, but the protein was absent (263). Phenotypically the mice had late-onset obesity and were hyperglycemic, but responded to exogenous insulin, demonstrating that pro-insulin processing was defective (263). Although CPE was completely absent in CPEfat/fat mice, there was other carboxypeptidase activity in some tissues. In the pituitary, it was at ~6%, in brain at 50–57%, but in heart and duodenum there was no reduction in activity. This was most likely due to the activity of CPD, CPN, and/or CPM (137).
The studies carried out on POMC in CPEfat/fat mice have helped elucidate the role of CPE as a sorting enzyme, controlling the release of POMC and its processed products between constitutive and regulated pathways. CPEfat/fat mice had increased constitutive POMC and ACTH release from their pituitaries (83, 356, 357). They also had very few of the small cleavage products of POMC and, as most of this processing occurs in regulated secretory granules, this is consistent with these mice not being able to sort POMC into these secretory granules. Therefore, in CPEfat/fat mice, due to the lack of CPE, POMC is missorted and mainly released constitutively.
Additionally, many studies have examined the processing of POMC in the CPEfat/fat mice. POMC accumulated in the pituitary in these mice at a level of 24-fold greater than wild-type controls (357), but it was poorly processed to ACTH, with only 30% of the expected amount (83, 357). There were also reductions in the levels of α-MSH, β-endorphin, β-LPH, and CLIP (23). This may be because there is missorting or because of altered levels of PC1/3 and PC2 in different brain regions of CPEfat/fat mice (23, 220). These changes in peptide levels in the hypothalamus had a functional effect on the body weight phenotype. There was a reduction in peptides like α-MSH, which could lead to an increase in body weight, but no change in the hypothalamic levels of those known to do the opposite, such as β-endorphin (1–31) (442). Overall, this may enhance the obesity phenotype.
More recently, the Loh group generated a CPE global knockout mouse. This strain is similar to the CPEfat/fat mice, in that they had late-onset obesity, hyperglycaemia, and higher levels of pro-insulin than insulin (53). Very little work has been carried out in these mice in relation to POMC processing. However, they were found to have reduced hypothalamic α-MSH. In addition, the pituitary levels of ACTH and α-MSH were also reduced, with higher levels of unprocessed POMC in the pituitary (52). Overall, it appears that the global deletion of CPE gives a very similar phenotype because of the same reduction in protein expression as seen with the single point mutation in the CPEfat/fat mice.
IX. POMC PROCESSING IN THE SKIN
Given that the MSH peptides were named melanocyte stimulating hormones after their role in skin (55, 358), it is not surprising that there is a long history concerning the production and the roles of POMC and constituent peptides. The POMC peptides were detected in the skin before it was obvious that they came from a common precursor (26, 364, 366, 397, 414), and some of the work underpinned the evolution of the links between the different peptides (228). Subsequently, the identification of children with loss of function of the POMC gene, who have red hair and pale skin, is clear evidence of the importance of POMC-derived peptides in skin and hair pigmentation (207).
Early work detected α-MSH and β-endorphin, in addition to ACTH, in cultured human keratinocytes (343, 431) and human epidermal melanocytes (191, 343, 362, 363, 414, 431). This suggests that POMC is processed in skin in a manner similar to the hypothalamus, rather than the human pituitary. The more extensive processing of POMC is substantiated by evidence that PC2 as well as PC1/3 is expressed in human and rodent skin (242, 305), in cultured epidermal melanocytes (305), and in human keratinocytes (333). Interestingly, even human dermal fibroblasts express the POMC processing enzymes (344). Ultraviolet irradiation increases POMC and α-MSH-like immunoreactivity (α-MSH-LI) in auricular skin from mice, but interestingly, the α-MSH-LI was found to be ACTH (1–8), formed by tryptase digestion in the extracellular space. The ACTH (1–8) was shown to stimulate melanin production via the MC1R (435). This is an unusual processing step that has not been reported in other tissues.
A. POMC-Derived Peptides and Melanogenesis
The action of α-MSH was shown dramatically in an in vitro assay using skin from frogs, because the α-MSH stimulated melanin production and therefore the darkening of the skin cells (55, 358). We and others have subsequently used the darkening of human melanoma cells, which is visible in the cell pellet, as a bioassay to show that although POMC is a precursor of α-MSH, it is still bioactive itself (333).
Early work provided evidence that α-MSH stimulated melanogenesis in human melanocytes (2, 175), but there was also evidence that the immediate precursor of α-MSH, i.e., ACTH, could also stimulate melanogenesis (175, 414). This is also evidenced by the pigmentation of some patients, particularly those with Nelson’s syndrome, who have excessively high concentrations of ACTH in their blood (323). However, there is always the question as to whether the tumors undergo abnormal processing of POMC and produce α-MSH. We identified one ectopic tumor where the patient had enhanced pigmentation that disappeared after removal of the tumor. There was elevated POMC and ACTH in the blood, but no excess α-MSH (426). This led us to question the relative release of POMC, ACTH, and α-MSH by normal human epidermal keratinocytes, melanocytes, and hair follicle cells and their relative bioactivity in skin (333). The subtlety lies in the concentrations of the respective peptides, as POMC has a low potency so will only be bioactive if present at the MC1R at high concentrations. In patients with tumors, which are secreting grossly elevated concentrations of POMC, it is likely that these precursors of α-MSH can cause pigmentation. However, under normal conditions, the processing of POMC and the regulation of release of the MSH peptides allow for paracrine (and maybe autocrine) activity at the melanocytes in skin.
It seems plausible that keratinocytes would secrete α-MSH-related peptides that act on cell surface MCRs on melanocytes to stimulate melanogenesis and proliferation (186). However, it is less clear why epidermal melanocytes secrete these peptides. It may be that there is a necessity for an autocrine pathway, or that they act on surrounding keratinocytes and dermal fibroblasts where they have a different role, perhaps in differentiation or proliferation.
Both β-endorphin and the μ-opiate receptor have been identified in human epidermal melanocytes, using immunohistochemistry, again suggesting that there is an autocrine mechanism operating in these cells (191). The role of β-endorphin in stimulating melanogenesis, mitogenesis, and dendrite outgrowth suggests its function is very similar to that of α-MSH (191).
B. The Skin Equivalent of the HPA Axis: Implications for Processing
It was somewhat surprising to learn that all the hormonal components of the HPA axis exist in the skin (360, 370). CRH is expressed in the skin (362, 365) and can act to stimulate POMC activity and corticosterone synthesis in dermal fibroblasts (367–369). Differentiation of human keratinocytes alters expression of the components of this skin “HPA axis,” indicating marked integration of the pathways (428). It has been suggested that the HPA axis represents an evolutionary development from the skin “HPA axis” (361). In the skin, the “HPA axis” interacts with the innate immune system to protect against pathogens and other stressors and then forms an inhibitory loop giving anti-inflammatory effects (361). In this model, CRH acts to stimulate POMC gene expression in situations where the POMC peptides have an immunoregulatory role (37) and where corticosteroids can act to suppress the skin-immune mechanisms.
C. Hair Follicles and POMC Processing
Hair follicles also produce POMC and process it in a manner analogous to the skin (194). This provides the POMC-derived peptides in the hair follicles which can modulate pigment formation, activate differentiation, and have an immunoregulatory role (37, 193). The hair follicles are also regulated by CRH (192) and have an equivalent to the HPA axis with synthesis of cortisol (178).
D. Assessment of POMC Peptides in Skin: Implications for Interpretation
The evidence is compelling for the presence of the POMC peptides and the importance of their role in skin. However, we must accept that while dispersing and culturing the cells allows better quantitation of specific peptides, it may mask the true endogenous peptide networks between the cells and cause abnormal function. On the other hand, analysis of the peptides in tissues by immunohistochemistry is only as good as the knowledge of the specificity of the antibodies. We know that antibodies produced with specificity for specific peptides such as ACTH may detect POMC or pro-ACTH as well as ACTH when used in immunohistochemistry. Others may be unaware of this, because they do not have purified forms of POMC and pro-ACTH to test on their antibodies. Another consideration is that some of our knowledge comes from rodents, and this might be difficult to extrapolate, for example, because they do not get the same exposure to sunlight (361).
The skin can be considered one of the largest organs in the body because of its surface area. Therefore, in extrapolating the concentrations of POMC-derived peptides in skin to what might appear in blood, some calculations suggest that the levels will be so high as to be compatible with causing Cushing’s syndrome. Clearly, this is not the case, and it is thought that the MSH and ACTH peptides are degraded locally (346).
X. PITUITARY POMC PROCESSING: A KEY FACET IN REGULATION OF THE HPA AXIS
The importance of this axis in managing the response to stress is well known. However, it is sometimes hard to believe that the intricacy of the production of POMC and its processing to ACTH is designed primarily to regulate the release of glucocorticoids. In studying the role of ACTH, it is apparent that its effects are mainly via glucocorticoids, which act on most tissues in the body to modulate homeostatic processes. This is evidenced by the many clinical features that occur as a result of glucocorticoid excess as in Cushing’s syndrome.
Secretion of a hormone is almost always regulated by a series of mechanisms to tightly control release into the circulation. When there is a hormone precursor this adds another layer of complexity. For POMC, the three key stages are regulation of 1) the gene, 2) the processing enzymes, and 3) the secretion from cells, as well as some cell-specific posttranslational processing, such as glycosylation. Unfortunately, we rarely consider all the stages together, so it is difficult to understand which of the regulatory mechanisms or which stage in the pathway dominates the outcome.
From the perspective of POMC processing, the different stages regulating the POMC gene, the enzymes for cleavage of POMC, and the secretory vesicle release of ACTH have to be coordinated. This is needed so that ACTH is secreted in a pulsatile manner, which underpins the circadian rhythm, creating the diurnal changes. This “basal” production of ACTH is distinct from the stress-related stimulation of ACTH secretion.
A. Ultradian Rhythm and Pulsatile Secretion
The complexity of the rhythms of the HPA axis is still unfolding as new techniques give us greater understanding. The pulsatile pattern of secretion of ACTH and cortisol has been difficult to assess in humans, because of the stress of repetitive blood collection. However, the use of automated sampling techniques (164) has uncovered the very dynamic nature of these hormones (338). The pulses of ACTH occur every 60–90 min and have a higher amplitude and greater frequency at the circadian peak. Pioneering studies by Stafford Lightman and colleagues used mathematical modeling (416) and subsequent in vivo experiments to show that the ultradian pattern of ACTH and cortisol derives from a feed-forward and a feedback system, involving the pituitary and adrenal (415). This rejects the long-held view that pulsatility is caused by a pulse-generator in a higher center such as the hypothalamus or hippocampus. Their research highlighted the timing of this loop: 1) ACTH secretion, 2) its action on the MC2R in the adrenal gland, 3) the de novo synthesis of cortisol, and 4) its rapid nongenomic negative feedback on ACTH secretion. Given the relatively short half-life of ACTH and cortisol, this loop continues with the degradation of cortisol, which then removes the glucocorticoid inhibition so that constant CRH stimulation can then increase the ACTH as part of the rising phase of the next pulse. The details of this system and the studies that underpin these hypotheses are amply described in the review by Russell et al. (338). The biological significance of the ultradian pulsatility is highlighted by studies showing that there are different effects on target genes if glucocorticoids are given constantly or in pulses (79, 381).
What is less clear is how POMC processing contributes to this pulsatility, if at all. POMC is released into the human circulation (89, 148) and in our study of HPA ultradian activity in humans, where POMC was measured in blood, there was no evidence for POMC pulsatility (337). Our hypothesis is that POMC is released from cells via a constitutive pathway (162, 314) which is separate from the regulated release of ACTH. Therefore, the rapid glucocorticoid feedback proposed for the ultradian pulses could act at secretory vesicles containing ACTH. This may occur via cortisol acting on membrane glucocorticoid receptors (389, 395), which would provide a nongenomic pathway of feedback inhibition. This mechanism is supported by early studies in rodents which showed that a rapid glucocorticoid inhibition of ACTH secretion is independent of protein synthesis (195) and of POMC processing (115).
B. Circadian Rhythm
The concentrations of ACTH in the human circulation show a distinct circadian rhythm, with highest levels just before wakening and then a decline throughout the day to a nadir between 11 p.m. and 3 a.m. Interestingly, the peak and trough values for ACTH differ by two- to threefold, while those for cortisol can be four- to sixfold in healthy individuals. This may be because the adrenal gland has sympathetic innervation that is regulated by the PVN or because it has an independent clock. Whichever mechanism prevails in the adrenal gland, it impacts on the cortisol circadian rhythm to generate the greater magnitude in cortisol diurnal rhythm (reviewed in Ref. 338).
This rhythm is under the control of the suprachiasmatic nucleus which stimulates release of CRH and AVP from the PVN in the hypothalamus (reviewed in Ref. 44). These neuropeptides can stimulate both synthesis of POMC and release of ACTH, and it is not entirely clear whether one or both mechanisms primarily generate the diurnal rhythm. These mechanisms are explored more fully in stress-related stimulation of POMC and ACTH below.
C. Acute Stress
In the physiological context, stress which stimulates the HPA axis encompasses acute illness, hemorrhage, hypoglycemia, and flight from predators requiring extreme activation of muscles.
1. CRH stimulation of ACTH release from secretory vesicles
The classical stressor requires that the hypothalamus releases CRH to cause very fast secretion of ACTH into the circulation to stimulate cortisol release. The mechanism by which CRH stimulates release of secretory vesicles containing ACTH involves extracellular calcium influx and release from intracellular pools (379). The immediate release of ACTH from secretory vesicles combined with the stimulation of POMC synthesis leads to a biphasic response in humans (93). It is difficult to assess the immediate release of ACTH and POMC after CRH in normal subjects, but this has been documented in patients who undergo petrosal sinus sampling for a suspected ACTH-secreting pituitary tumor. After CRH stimulation, ACTH released into the petrosal sinus capillaries can be measured within minutes. The stimulated release usually results in a peak of ACTH within 5–15 min. POMC release from the pituitary can be detected, but it does not mimic the marked increase in ACTH (FIGURE 6) (148). This has led us to hypothesize that POMC is released from the corticotropes via a different mechanism to ACTH. It could be that in the steady state when there are sufficient ACTH vesicles, the excess POMC exits the cells by a constitutive pathway representing an “overflow” mechanism (384). This is substantiated in normal subjects where CRH caused an increase in circulating ACTH but no change in ACTH precursors (337).
FIGURE 6.
Regulatory processes for secretion of pro-opiomelanocortin (POMC) and its peptides. A: POMC moves from the trans-Golgi network (TGN) to immature secretory granules (ISG) and is secreted from cells by constitutive secretion. Pro-hormone convertase (PC) 1 cleaves POMC to produce adrenocorticotropic hormone (ACTH) which is stored in dense-core secretory granules (DCSGs) before secretion is stimulated. B: on stimulation, α-melanocyte stimulating hormone (MSH) and possibly ACTH is released from the cells in the hypothalamus/skin/pars intermedia of the anterior lobe of the pituitary. C: acute corticotropin releasing hormone (CRH) stimulation in the anterior pituitary causes the release of ACTH. POMC is also released but not subject to stimulation. D: long-term CRH stimulation upregulates the POMC gene and release of ACTH. E: glucocorticoids (Gcs) can inhibit ACTH secretion in an acute, nongenomic manner in the anterior pituitary. F: chronic exposure to glucocorticoids inhibits POMC transcription and ACTH release. [Adapted from Stevens and White (384), with permission from Springer Nature.]
2. CRH stimulation of the POMC gene in the anterior pituitary
It has long been recognized that the binding of CRH to a corticotropic cell results in CRH activation of POMC transcription (142), presumably to replenish stores of ACTH peptide in the secretory vesicles. It is possible that stimulation of the POMC gene involves the same intermediary factors that act on the channels to cause ACTH secretion. CRH receptor activation seems to have effects on a number of pathways (overview in Ref. 162; reviewed in detail in Ref. 104). Early work defined the effect of CRH on cAMP pathways both in vitro and in vivo (231). CRH is known to increase cAMP, calcium, and mitogen-activated protein kinase (MAPK) in POMC expressing cells (204, 236). There is also evidence that CRH stimulates transcription of JunB, c-fos, and FosB, transcription factors that bind to the AP-1 transcription factor binding site in exon 1 of the POMC promoter, activating POMC transcription (13, 42).
More recent data have delineated a MAPK pathway that activates nuclear receptors related to NGFI-B (Nur77), and these bind to a Nur response element in a regulatory element at −404 bp of the rat Pomc promoter (reviewed in Ref. 104). MAPK signaling enables the Nur factors to bind SRC coactivators which enhance POMC transcription. CRH signaling also activates Tif1β which is synergistic with SRC2 action (321).
3. CRH regulation of processing
There is very little evidence in the literature to suggest that the processing of POMC to ACTH is regulated or that the pro-hormone convertase, PC1/3, is stimulated by CRH. However, there is always the caveat that “this is an experiment waiting to be done.”
4. Stimulation of ACTH by other factors acting on CRH
Many other factors have been reported to stimulate release of ACTH, such as catecholamines, angiotensin II, interleukins (24, 341), ghrelin, vasoactive intestinal polypeptide (VIP) (271), serotonin, and oxytocin. However, they mostly act via CRH, and there is little evidence for direct effects on POMC gene expression. Opioid peptides also affect the HPA axis, but in differing ways depending on the species studied and whether the actions are acute or chronic. It has also been suggested that opioids are acting at the level of CRH release (396, 402).
5. Stimulation of ACTH by arginine vasopressin
Early work outlined the potentiation of CRH-stimulated secretion of POMC-derived peptides by arginine vasopressin (AVP) (408), and this is often stated as a key regulatory role in the HPA axis. There seems to be a clear distinction, because while AVP can synergize with CRH to stimulate ACTH release, it does not appear to act on transcription of the gene. In fact, AVP alone decreases levels of the POMC primary transcript and does not act in a synergistic manner with CRH on POMC gene expression in rat anterior pituitary primary cultures (219).
6. Other factors that stimulate POMC gene expression
Given the physiological importance of the interactions between the immune system and the HPA axis, it is not surprising that leukemia inhibitory factor (LIF), which is a pro-inflammatory cytokine, activates POMC gene expression (41, 324, 325). LIF binding activates the Jak-STAT pathway, and there is a STAT binding site in the proximal POMC promoter close to the Nur response element (262). This suggests that the HPA axis role in toning down the cytokine response requires increased POMC gene expression, which may provide a greater or prolonged effect on glucocorticoid release compared with the stress response that is set up to deliver fast release of ACTH into the circulation.
D. Feedback Inhibition of the HPA Axis by Glucocorticoids
The classical hormonal axes involving the hypothalamus and pituitary have the ability to generate a loop, whereby stimulation at several stages leads to a hormone, which feeds back and switches off the cascade. Glucocorticoid inhibition of the HPA axis is one of the best examples of this tightly regulated feedback system. Stress activation of the HPA axis must incorporate a mechanism to switch off glucocorticoid release, which if unrestrained would lead to not only prolonged immune suppression, but also a very adverse profile of effects in many tissues. The inhibition by glucocorticoids is complex and some reports highlight the importance of regulation at the level of CRH in the hypothalamus, while others suggest that inhibition of POMC gene expression and ACTH release are the critical components (96). There have been some elegant studies addressing the issues of the effects of acute glucocorticoid feedback on stress-related activation of the HPA axis, considering the timing of glucocorticoid feedback particularly in in vivo paradigms (90, 282).
1. Glucocorticoid inhibition of ACTH release
After a stressor activates the HPA axis, the surge in cortisol feeds back at the pituitary and inhibits ACTH release. This is rapid and is thought to be a nongenomic mechanism, with glucocorticoids acting on secretory vesicle release (96). Early research suggested that glucocorticoids inhibit the calcium signaling, which triggers release of ACTH from the plasma membrane (8).
Another mechanism involves Annexin 1 (ANXA1) which was originally identified as an anti-inflammatory protein. ANXA1 is released from folliculostellate cells in the pituitary in response to glucocorticoid stimulation, and this causes translocation of ANXA1 to the outside of the cell by a mechanism that does not involve exocytosis. ANXA1 then inhibits CRH-stimulated ACTH secretion (43). Given the steps involved, it would seem that this feedback would take longer to have its effects on ACTH.
2. Glucocorticoid inhibition of the POMC gene
Over the longer term, glucocorticoids can inhibit POMC gene expression. They access the pituitary because it is outside the blood-brain barrier and then diffuse into the corticotropes to bind the intracellular glucocorticoid receptors (GRs). These receptors are part of the nuclear hormone receptor family, and on binding of ligand, they are released from heat shock proteins in the cytoplasm, allowing them to translocate to the nucleus. There they bind to negative glucocorticoid response elements (nGREs) in the promoter region of the POMC gene to inhibit transcription. The regulation of the POMC gene by glucocorticoids was first explored in the 1980s (33, 141). A region necessary for repression was identified at −77 to −50 relative to the transcription start site of POMC (107, 108), and a second site between −480 and −320 was subsequently mapped (327). This site involves the NurRE at −395 bp from the start site (307, 308). GRs interact with Nur factors by protein-protein interactions, and this requires BRG1, part of the SW1/SNF remodeling complex, and HDAC2, a histone deacetylase (31). Both these sites are necessary to effect transcriptional inhibition. What is unusual about the nGRE at −63 bp in the rat Pomc promoter is that it binds a GR homodimer as found in positive GREs but then a GR monomer binds to the opposite side of the helix in the promoter (106).
3. Glucocorticoid inhibition of POMC processing enzymes
It seems logical that the regulation of expression of the processing enzyme that cleaves POMC, i.e., PC1/3 parallels that of POMC in corticotropes. Indeed, studies in rats showed that adrenalectomy increased the mRNA levels of PC1/3 and that dexamethasone treatment of the adrenalectomized animals reversed this. This suggests that endogenous glucocorticoids would inhibit the expression of the processing enzymes in the corticotropic cells (101).
E. Stress Recovery Mechanisms: Role of Cannabinoids
The endocannabinoids are involved in stress recovery mechanisms and homeostasis; therefore, it is not surprising that they have effects on the HPA axis. However, the literature suggests that the effects are very dependent on the context. Several studies have reported that cannabinoid agonists increase circulating ACTH in animal models (reviewed in Ref. 288). There is some suggestion that these agonists act by increasing CRH, although the cannabinoid receptor CB1 is present on ACTH secreting cells (289), indicating that cannabinoids are having a direct effect at the level of the pituitary. Interestingly, mice with knockdown of CB1 have increased levels of corticosterone and a generalized upregulation of the HPA axis (84), which would indicate that endocannabinoids also have the potential to inhibit the HPA axis. This fits with the proposal that endocannabinoids can inhibit stress-induced HPA axis activation and therefore may be of value in treatment of anxiety-related disorders (299).
XI. POMC PROCESSING BY TUMORS
Cushing’s syndrome is defined by excess cortisol secretion which can be caused by pituitary tumors that secrete ACTH or nonpituitary or ectopic tumors, which we believe secrete predominantly ACTH precursors (423) (FIGURE 7). The latter is referred to as ectopic ACTH syndrome, but we have suggested it should be renamed ectopic ACTH precursor syndrome (385). The tumors are often small cell lung carcinomas (SCLC) but can also be pancreatic, thyroid, or carcinoid tumors or pheochromocytomas.
FIGURE 7.
Adrenocorticotropic hormone (ACTH) precursor secretion in ectopic ACTH syndrome. Pituitary tumors have excess production of ACTH, while ACTH precursors are released from ectopic (non-pituitary) tumors. The increased ACTH-related peptides lead to increased cortisol production. CRH, corticotropin releasing hormone.
Much of what we know now about POMC as the precursor of ACTH arose from observations of tumors producing “abnormal” ACTH molecules. This was often investigated because the patient would have symptoms of Cushing’s syndrome which were suggestive of high ACTH concentrations in the patient’s blood, but the results would be inconsistent. We now know this is most often because some of these tumors were producing ACTH precursors. These precursors seem to have a lower bioactivity, as only relatively high concentrations stimulate cortisol production and are associated with clinical symptoms of cortisol excess. In addition, although ACTH assays used in the clinic have some cross-reactivity for ACTH precursors, they only measure ~2% of the total precursors (255). This can result in a “normal” ACTH and only slightly elevated cortisol, but because there is no diurnal rhythm, this change in the HPA axis can give symptoms of Cushing’s syndrome. With more specific assays and more sensitive imaging techniques, it is now easier to get a diagnosis, although there are still some patients who present with a confusing set of diagnostic results, indicating we still have more to discover.
We developed a two-site immunoradiometric assay for ACTH precursors in 1988 (89), using a pair of monoclonal antibodies. One antibody binds to the ACTH region of POMC and the other to the γ-MSH region (see sect. XII). Binding of both antibodies is required to generate a signal, and this only occurs when POMC or pro-ACTH are present. Importantly, this assay for ACTH precursors does not detect ACTH. In contrast, an assay for ACTH will always recognize ACTH precursors to some degree, because the ACTH sequence is present in both pro-ACTH and POMC. This is very important in diagnostic ACTH assays, as they need to identify any peptides with ACTH-like activity. However, as the antibodies cross-react 100% with ACTH but only <5% with POMC (255, 385), this can lead to the discrepancies described above.
A. ACTH Precursor Secretion in Ectopic ACTH Syndrome
High-molecular-weight forms of ACTH were first found in an ectopic tumor extract using chromatographic separation and then radioimmunoassay (433). These high-molecular-weight forms were subsequently found in patients’ blood (159, 322), but it required large volumes of plasma for the procedure and took several days, so it was not a suitable approach to use routinely. Most reported chromatograms show a major elution peak at the position for POMC and a shoulder to the peak, suggesting some pro-ACTH, but the resolution is not usually specific enough to determine relative amounts.
Quantifying ACTH precursors, using the two-site immunoradiometric assay, revealed that most of the patients with ectopic ACTH syndrome had elevated circulating concentrations of ACTH precursors (385) (FIGURE 8). It is very difficult to prove whether the ACTH precursors or bona fide ACTH cause the clinical symptoms in these patients. Low concentrations of ACTH (~1.0 pM) secreted continuously from tumors, and therefore not subject to a diurnal rhythm, are thought to be able to cause elevated cortisol, which can result in Cushing’s syndrome. We did not detect ACTH in the chromatographed plasma from a patient with ectopic ACTH syndrome, where we measured high levels of ACTH precursors. This suggests that ACTH precursors were bioactive in this case, but we cannot completely rule out the possibility that the lack of detection of ACTH may be due to sensitivity limitations of the chromatography (385).
FIGURE 8.
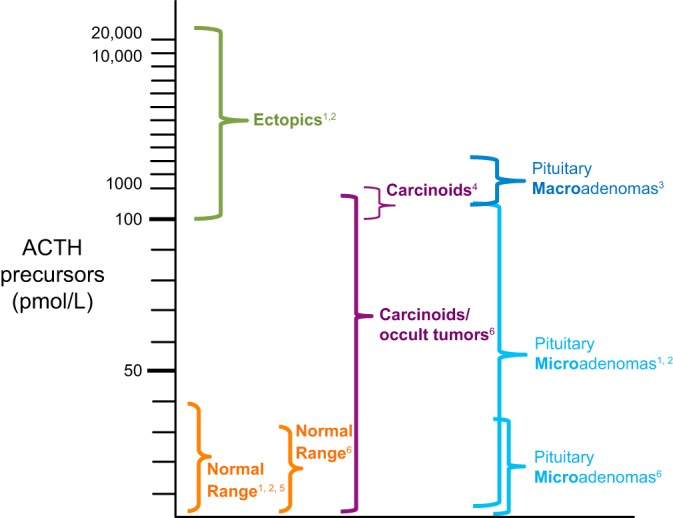
Concentrations of adrenocorticotropic hormone (ACTH) precursors in different patient groups. The ranges relate to concentrations of ACTH precursors in blood samples from different groups of patients. The superscript numbers indicate the following references: 1, Ref. 89; 2, Ref. 385; 3, Ref. 150; 4, Ref. 278; 5, Ref. 337; 6, Ref. 285.
Carcinoid tumors can also secrete ACTH precursors and produce features of the ectopic ACTH syndrome, even though they are often much smaller tumors (423). However, other work using an assay specific for POMC, which did not detect pro-ACTH, suggested POMC was not present in the bronchial carcinoids they studied (319). To add to the confusion, this group detected CLIP (the COOH-terminal fragment of ACTH) in four carcinoid tumor extracts (413). Unfortunately, the concentrations of precursors, ACTH and CLIP were not measured in the same patients in any of the studies, and it is not clear if the antibody to CLIP recognized POMC. If CLIP is present in a selection of tumors, this suggests that these tumors have the processing enzymes, PC1/3 and PC2, to cleave POMC to these smaller fragments. Indeed, PC2 has been detected in the majority of carcinoid tumors studied (347). However, more recently, it has become clear that patients with less aggressive Cushing’s syndrome caused by carcinoid tumors have elevated ACTH precursors (285) (FIGURE 8).
B. POMC Processing in Pituitary Microadenomas
Patients with small pituitary microadenomas causing Cushing’s disease process POMC to ACTH, seemingly in a similar way to normal subjects. Therefore, while ACTH precursors are detectable (as they are in normal subjects), the concentrations range from low normal to ~100 pM (278). However, patients with ectopic tumors causing Cushing’s syndrome have ACTH precursors in the range 100–20,000 pM (425). This gives virtually 100% discrimination between patients with pituitary-dependent Cushing’s syndrome and ectopic ACTH syndrome (FIGURE 8).
In addition to ACTH, the precursors can be detected in samples taken from the inferior petrosal sinuses, draining the pituitary, in patients with pituitary microadenomas. However, when CRH is given as part of this procedure, while the ACTH concentrations increase, the ACTH precursors do not seem to respond to CRH to the same degree, suggesting that the mechanisms for regulation of release of ACTH and ACTH precursors may differ (148).
C. POMC Processing in Large Invasive Pituitary Tumors
There is a small subset of patients who have much larger tumors, which tend to be invasive (11), and they may present with vague symptoms of Cushing’s syndrome and abnormally low ACTH results considering the clinical features. Investigating a small group of these tumors, we found that they also have high concentrations of ACTH precursors in the blood (150), and a POMC specific assay has identified elevated POMC in seven of eight patients (319).
There are also “silent” tumors in patients who do not appear to have elevated ACTH in the circulation, but their tumors stain positively for “ACTH.” In cases we have studied, this is because the tumors produce ACTH precursors, which can be detected by the ACTH antibody used in immunohistochemistry. If the tumors have not been completely removed at surgery and there is recurrence, the ACTH precursors can be detected in the circulation as a sensitive means of monitoring tumor progression.
There is also some evidence for large invasive pituitary adenomas producing α-MSH and most of these were immunopositive for PC2 (176). Unfortunately, ACTH precursors were not measured in these patients, so it is not possible to speculate on the molar ratios of the POMC peptides produced by these tumors.
D. POMC Processing in Nelson’s Syndrome
Nelson’s syndrome is relatively rare but has highlighted some interesting aspects of POMC processing that are still not fully understood. Nelson’s syndrome occurs after bilateral adrenalectomy which is used as a means of treating some cases of Cushing’s syndrome. Subsequently, a pituitary adenoma, usually not detected as the cause of the Cushing’s syndrome, then expands and secretes high concentrations of ACTH, often resulting in pigmentation.
These pituitary tumors are often invasive, and this led us to investigate whether they secreted ACTH precursors in a similar fashion to the group of large invasive pituitary tumors we had studied previously (150). The ACTH precursors were elevated in 11 of the 24 patients (median 97.5 pM, range 26–647 pM), compared with untreated Cushing’s disease where the range was 9–104 pM (FIGURE 7). However, the processing of POMC to ACTH appeared to be enhanced as evidenced by the ratio of precursors to ACTH (323). This seemed unusual, but suggests that the lack of endogenous cortisol and the presence of oral hydrocortisone for only part of the 24-h period may have affected the processing of POMC to ACTH.
E. Processing of POMC in Tumor Cells
There is ample evidence of POMC production and secretion by pituitary and ectopic tumors (276, 278, 285, 319). Why this occurs is still a matter of speculation (423). The processing pathway to produce ACTH is complex and requires the presence of PC1/3 and mature secretory vesicles, to provide the correct calcium and pH optimal for the processing. Therefore, it seems reasonable to predict that some tumors (particularly nonpituitary tumors) may not have differentiated sufficiently to generate this pathway. However, these ectopic tumors are often characterized by large dense-core secretory vesicles, which is the location for processing precursor hormones. It may be that these tumors are less differentiated and not able to synthesize PC1/3. This is supported by a study of 13 SCLC cell lines which had restricted expression of PC1/3 (87). We have also found high levels of precursors and undetectable ACTH in SCLC cell lines (88, 385). The fact that large invasive pituitary tumors can produce ACTH precursors at higher concentrations than ACTH also supports the suggestion that aggressive, less differentiated tumors may not fully process POMC to ACTH. However, the increased processing in those pituitary tumors associated with Nelson’s syndrome suggests that the lack of “natural” glucocorticoid feedback inhibition may be influencing processing.
XII. POMC PEPTIDE MEASUREMENT: WHAT DO YOU REALLY DETECT?
Measurement of ACTH initially involved a bioassay using rat adrenal cells that secreted corticosterone. This was very sensitive and could detect circulating ACTH but was also very variable due to the unpredictability in responsiveness of different adrenal preparations (61). Subsequently, a few research groups, largely based within hospital laboratories, began to produce polyclonal antisera and develop immunoassays to measure ACTH. With these assays it was possible to identify discordance between the clinical features in some patients with tumors and the low levels of ACTH detected. This led to the concept that some tumors might be producing abnormal molecules with ACTH activity (see sect. XI). This set the context for the development of methods to accurately assess the POMC peptides.
A. The Value of Pulse-Chase Analysis
In trying to understand the relevance of pro-hormone processing, we rely heavily on the methodology used to address the questions. Without pulse-chase analysis it may have been many years before pro-insulin was discovered. Similarly, much of the early work on POMC, as the precursor of ACTH, relied on pulse-chase analysis of POMC peptides from the AtT20 mouse pituitary adenoma cell line (119, 232). These cells were incubated with a radiolabeled amino acid and then after a set time “chased” with unlabeled amino acid. This provides a profile of labeled peptides over time. Antibodies are used to identify and concentrate the specific peptides and then SDS-gel electrophoresis determines the size of the peptides. This is a dynamic process studying the timing of the appearance and disappearance of the labeled peptides. The information on the relative amounts of each of the peptides in the processing pathway depends on the ability of the antibody to recognize a particular epitope in ACTH, pro-ACTH, and POMC. From immunoassay data, we are aware that antibodies may recognize ACTH to a greater degree than the precursors. There is also the possibility that the different conditions used in immunoprecipitation can affect the relative recognition of the three peptides, so it is important to consider antibody specificity for the different peptides under these conditions, to interpret the data.
Much of the data proving that ACTH and β-endorphin come from POMC were derived from extensive and very methodical pulse-chase analysis (119). This biochemical analysis preceded the identification of the gene structure and provided invaluable information on POMC processing.
B. Immunoassays for ACTH
The clinical need for measurement of ACTH became apparent with identification of tumors secreting ACTH as a cause of Cushing’s syndrome (281, 434). Many immunoassays for clinically relevant hormones were developed shortly after the initial immunoassay for insulin had been described. However, development of polyclonal antisera to ACTH proved difficult, as the ACTH peptide is not very immunogenic. In addition, there were problems with radiolabeling ACTH for the radioimmunoassays, as it was very labile. At this time, extraction of ACTH from plasma was necessary to improve the detection of low normal concentrations and laboratories used the knowledge that ACTH sticks to ground glass or silica for this purpose. This process concentrated the ACTH, to allow for the lack of sensitivity of the immunoassays, while at the same time removing the plasma, which interfered in many of the immunoassays.
The advent of a two-site immunoradiometric assay based on polyclonal antibodies to ACTH provided significant advantages (171). However, the ability to generate monoclonal antibodies to ACTH (424) (FIGURE 9) enabled the characterization of a monoclonal antibody-based immunoradiometric assay (427). Having hybrid cell lines secreting large quantities of monoclonal antibodies led the way for development of commercial diagnostic assays, which opened up ACTH measurement to a much wider clinical community. However, it was also known that some tumors produced high-molecular-weight forms of ACTH, while other tumors were thought to produce ACTH fragments. The immunoradiometric assays were considered very specific for ACTH (135), and the assay based on the paper by Hodgkinson et al. (171) was found not to detect the “big ACTH” produced by tumors (139). Similarly, ACTH fragments not detected in the immunoradiometric assays can cause problems (317). In essence, it is important that the ACTH assays do detect ACTH precursors or fragments produced by tumors, but only if the precursors or fragments are bioactive and responsible for the clinical symptoms.
FIGURE 9.
Monoclonal antibody-based assays to pro-opiomelanocortin (POMC)-derived peptides. A: the monoclonal antibodies (MAbs) bind to specific epitopes on the peptides. A pair of antibodies is required for a two-site assay, which gives specificity. B: the adrenocorticotropic hormone (ACTH) precursor assay has one MAb specific for the ACTH region and one within the N-POMC region. C: the ACTH assay uses a pair of MAbs which recognize the NH2 and COOH regions of ACTH. They can recognize these epitopes in POMC but only bind ~2% of the precursors. HRP, horseradish peroxidase.
C. Immunometric Assays for ACTH Precursors
Many immunoassays for ACTH can also detect the high-molecular-weight forms of ACTH, such as POMC and pro-ACTH. In this instance, the antibodies that bind ACTH can also recognize this sequence in POMC. However, the antibody may not recognize the ACTH sequence to the same degree in the larger precursor molecule. Therefore, the ACTH assay may underestimate the concentration of POMC, because of its low cross-reactivity with the antibodies. This has been accentuated in the two-site immunometric assays for ACTH, with many of them only detecting 2% of the POMC precursors present (255).
The development of an immunometric assay for POMC and pro-ACTH (89) provided the opportunity to specifically quantitate these precursors without the problem of trying to accurately measure them in the ACTH assay. In the precursor assay we developed, one antibody detects an epitope in ACTH and the other antibody detects an epitope in γ-MSH. Since both antibodies are required to generate a signal, only peptides containing both epitopes (i.e., POMC and pro-ACTH) are measured. Therefore, the smaller peptides, including ACTH, are not recognized by the assay (FIGURE 9). The second factor that made this assay possible was that we were able to use culture medium from a pituitary tumor growing in vitro as a source of POMC and subsequently to prepare standards by purifying POMC from a human small cell lung cancer cell line (89).
This assay for ACTH precursors confirmed the early work on high-molecular-weight ACTH, i.e., POMC and pro-ACTH, in tumors. It has enabled analysis of much larger numbers of patients and proved that high levels of ACTH precursors can be found in the blood of patients with ectopic ACTH syndrome (385). The greater sensitivity of this approach allows ACTH precursors to be measured directly, without the need for chromatography, greatly enhancing our understanding of the processing in ACTH-related disorders (150, 278, 285, 323). Another immunometric assay for POMC based on antibodies to epitopes in ACTH and β-endorphin showed greater heterogeneity, but still detected elevated POMC levels associated with more aggressive tumors in patients with ACTH-related disorders (319).
The immunometric assay technology enables lower concentrations to be detected. This has made it possible to measure POMC in plasma (148, 285, 337) and in CSF (286, 403) from normal subjects.
D. Immunometric Assays for β-LPH and β-Endorphin
By developing two-site assays similar to those for ACTH and ACTH precursors, it has been possible to generate assays that distinguish β-LPH and β-endorphin (149). This provided evidence that there is very little β-endorphin in the human circulation and its precursor, β-LPH, is more prevalent. It is likely that some of the original radioimmunoassays for β-endorphin actually detected its precursor, β-LPH.
E. Which POMC Peptide Are You Measuring by Immunohistochemistry?
Accurate detection of POMC and the smaller melanocortin peptides in tissues by immunohistochemistry remains complex. Antibodies raised to α-MSH, for example, may be wholly specific for that peptide or may recognize the amino acid sequence in ACTH, pro-ACTH, and/or POMC. It is challenging to use single antibodies in immunohistochemistry to detect fully processed peptides as they may be identifying the larger POMC precursor. This is made more relevant as the precursors are thought to be less biologically active and therefore a tissue or tumor may have a bioactive smaller peptide or a less bioactive precursor. The specificity of the antibody can be assessed by competing with increasing concentrations of the precursors, but these are not generally available.
We have an antibody that recognizes the COOH terminal of ACTH and therefore detects ACTH and pro-ACTH but not POMC. This has been proven in immunoassays and has also been used in immunohistochemistry to show that a tumor was producing POMC but not ACTH, which helped explain the clinical symptoms in relation to the POMC-derived peptides in the circulation (146).
XIII. BIOACTIVITY OF POMC PEPTIDES
A. Bioactivity of ACTH Precursors
The perceived role of a precursor in relation to its peptide products would suggest that the precursor is not biologically active and that the reason for regulating the processing steps is to provide bioactive end products. The situation is slightly more complex with POMC and ACTH, and much less is known about other POMC-derived peptides such as α-MSH and β-endorphin.
The relative bioactivity of POMC and ACTH was addressed in the 1970s using POMC (then called pro-ACTH/endorphin) isolated primarily from AtT20 mouse pituitary adenoma cells. The peptides were purified by gel chromatography and SDS gel electrophoresis, then measured using an ACTH radioimmunoassay and tested on rat adrenocortical cells, which produced corticosterone as the evidence of bioactivity. Initially this approach provided proof of the relative position of the different peptides within the POMC precursor, in that it had not previously been known that N-POMC was found beyond the NH2 terminal of ACTH and ACTH was next to the NH2 terminal of β-LPH. These studies also showed that POMC and pro-ACTH were two orders of magnitude less bioactive than ACTH (1–39) (145). These data do depend on the accuracy of the quantitation of the precursor peptides used in the bioassay. It is clear that this was recognized as an issue at that stage because Eipper and Mains (119) commented “it has been known for some time that radioimmunoassays for ACTH may only detect a few percent of the high-molecular-weight forms.” They indicate that this will bias the ratio of bioactive to immunoactive peptide measured.
This careful study of bioactivity provided evidence that the precursors of ACTH were able to stimulate corticosterone production without a time lag, indicating that it did not require proteolysis for the precursors to act at the MCRs on the rat adrenocortical cell membranes (145). Nevertheless, much higher concentrations of precursors were needed to have an effect.
This work underpinned the concept that POMC is a precursor with relatively low biological activity (119, 276). However, pro-ACTH has 8–33% the potency of ACTH in a cytochemical bioassay (322), suggesting that having the ACTH sequence at the COOH-terminal end of this precursor makes it more bioactive than when ACTH is flanked at both ends by other peptides. This makes the interpretation of which peptides are present in the circulation and how they are measured very relevant (423).
To analyze bioactivity, research groups primarily used tumor extracts as the source of POMC. The “big” ACTH in human nonpituitary tumor extracts was found to be relatively biologically inactive (168) or had <4% bioactivity (147). It was also shown that trypsin can convert the “big” ACTH peptide to a biologically active ACTH (147). Other groups isolated “big” ACTH, from a human pituitary tumor, and found it to have 30% of the bioactivity of ACTH (118, 206), suggesting that the peptides isolated may have been a mixture of POMC and pro-ACTH.
Using the ACTH precursor assay to quantitate POMC and pro-ACTH purified from the plasma of a patient with an ectopic tumor provided evidence that the ACTH precursors, rather than ACTH, might be responsible for the clinical symptoms (385). It was suggested that although they have low bioactivity, they may still act with low potency if they are present in the circulation at very high concentrations. Another option is that the high concentrations of ACTH precursors are cleaved at the adrenal cells and, even if this cleavage is very inefficient, the concentrations of ACTH generated may be sufficient to stimulate excess cortisol, especially at the diurnal nadir.
B. Relative Bioactivity of α-MSH and Its Precursors ACTH and POMC
For some reason, very little has been done to consider if the precursors of α-MSH act in the hypothalamus at MC4R or in the skin at MC1R, although we have tried to address this. ACTH is able to act at the MC4R, found in the hypothalamus, with a similar potency to α-MSH (311). POMC is less bioactive, but may still act if present at 100-fold higher concentrations, and it is intriguing that this level of excess is found in the CSF (287, 403). Similarly, ACTH can act at the MC1R found in skin with a similar potency to α-MSH (333) where again POMC has low bioactivity. Therefore, it is important to understand the relative concentrations of the precursors as well as their derived peptides in the vicinity of their receptors, particularly if acetylation of α-MSH is so tightly regulated (156).
XIV. CONUNDRUMS AND FUTURE PERSPECTIVES
The wealth of data presented in this review is testament to the essential roles played by POMC-derived peptides in a vast array of tissues and in many diverse physiological systems. In many research arenas there is an inevitable tendency to focus in on one particular tissue or cell type. Hopefully this review will encourage those working, for example, on hypothalamic signaling to also look at the complexity of regulation of POMC processing in the skin and vice versa. Only by grappling with the subtlety of homeostatic control mechanisms in all relevant tissues can one appreciate the full power of hormone processing vital for physiological processes in many biological systems.
In presenting POMC as the archetypal polypeptide precursor, this review has addressed processing to generate the smaller peptide fragments at the molecular and cellular level. Understanding the processing steps responsible for cleavage of POMC and how they differ in certain tissues sets the context for recognition of how mutations lead to such widespread phenotypes in both humans and mice. By considering the processing pathway from the TGN to the cell surface, it is possible to address questions about how the regulated secretion of ACTH from the pituitary occurs so rapidly in response to stress. This is obviously necessary to stimulate the glucocorticoid concentrations needed for metabolic support in the “fight and flight” mechanisms. However, a complete understanding of the regulation of the POMC gene in the hypothalamus remains more elusive, and much less is known about how processing in the secretory granules proceeds within relevant hypothalamic neurons. For example, this review has provided evidence for regulation of the many enzymes involved in generating bioactive α-MSH. Because this needs to occur in secretory vesicles rather than in the extracellular space, it remains to be determined how the length of the neuronal projections affects the various stages in acetylation and amidation of α-MSH.
Our gathering of evidence for the complexity of POMC processing in all of the many tissues where it is expressed has again raised issues about which POMC peptides have biological activity and highlighted continuing uncertainty of the unique versus overlapping roles of melanocortins. Where a block in the posttranslational mechanisms affects processing and there is a build up of POMC precursors, it is important to understand if the biological activity of these precursors is contributing, even in part, to changes in physiological processes normally ascribed to the smaller, more highly processed peptides like ACTH, α-MSH, or β-endorphin. This phenomenon has been accepted for many years by endocrinologists studying tumors secreting ACTH-related peptides, but the more recent identification of children with mutations in POMC or with mutations in the enzymes involved in processing POMC, has shone a light into another biological theater in which precursors could have important actions.
One of the major stumbling blocks to a more complete knowledge of which POMC peptides are involved in particular physiological processes is the problem of attempting to measure one peptide specifically without inadvertently also measuring its larger precursor or a smaller peptide derived from it. This is a particular issue for immunoassays that rely on the specificity of the antibody. For example, in the case of ACTH assays, their recognition of POMC or pro-ACTH is rarely understood. There is now evidence that most immunometric assays for ACTH only detect ~2% of the precursors present. If the aim is to use this approach to measure POMC and pro-ACTH, then it massively underestimates their true concentrations. This has undoubtedly hampered the understanding of some cell-based studies.
The sensitivity of the detection system is also critical. Some techniques to concentrate the various peptides before analysis are recognized to recover the larger precursors in different proportions to the smaller peptides, thereby introducing error. This is especially an issue for mass spectrometry or gel chromatography. Similarly, there is no tradition of checking the antibodies used in immunohistochemistry to determine if they are recognizing POMC precursors to the same degree as the peptide to which they have been raised. Much of the literature makes statements about the peptides detected, but rarely do reports qualify this with mention or measure of other POMC peptides that might also be present.
A. Future Perspectives on POMC Processing in the Hypothalamus
Given the significant amount of knowledge gained in the last 20 yr about the role of POMC in regulating energy balance, it may seem that there is little more to unravel. However, this review has highlighted the uncertainty around the respective roles of α- and β-MSH. There is no doubt that loss of the complete POMC gene is associated with obesity; however, it is more difficult to understand the contribution of α- and β-MSH in patients with mutations in these regions. To date, evidence indicates that loss of β-MSH rather than α-MSH is more likely to be associated with an increased risk of obesity, but the mutations reported have been in the heterozygous state and number of affected probands remains small. The loss of active β-MSH in ravenously hungry and overweight Labradors provides intriguing evidence for its function. On the other hand, the inability to produce β-MSH in rodents points to a redundancy. The comparable potency of the two peptides at the MC4R suggests that either peptide could regulate energy balance. Therefore, it may be that discrimination lies at the level of posttranslational modifications which might generate bioactive α-MSH but inactive β-MSH or vice versa. An ability to measure the relative concentrations of each of the bioactive peptides in the vicinity of the receptors in vivo would be invaluable, but to date, this remains a very technically challenging procedure.
In addition to the contention around which species of MSH may predominate at the MC4R, there still remains uncertainty around the acetylation of α-MSH. There are conflicting data about whether α-MSH or des-acetyl α-MSH represents the more relevant form in the hypothalamus. NH2-terminal acetylation is thought to increase the stability of peptides, although other reports indicate it depends on the type of NH2-terminal acetylase and may target peptides for degradation. Early literature suggests des-acetyl α-MSH is the major form in the arcuate nucleus of the hypothalamus. There is a suggestion that the des-acetyl α-MSH peptide travels within the POMC neurons from the arcuate nucleus to the PVN and then the N-acetyltransferase acts at the neuronal terminals in the PVN just before release from the neuron. Nevertheless, it is the acetylated form that is thought to be biologically active in terms of food intake although very recent data suggest that des-acetyl α-MSH may be as effective. However, there is also controversy about the relative concentrations of its precursors. While α-MSH is detected at higher concentrations than POMC and ACTH in rat hypothalamic extracts, its levels are not regulated by fasting, in contrast to those of its precursors. This again highlights some gaps in our understanding of POMC processing.
B. Why Is POMC Present in the Circulation?
It is often assumed that hormone precursor molecules will be efficiently processed within the cell before release of the small active peptide, but like pro-insulin, POMC is released into the circulation. There is now good evidence for POMC in the circulation, both from normal human subjects and particularly in patients with tumors secreting ACTH-related peptides. It is tempting to speculate that this is an “overflow” mechanism, occurring in the pituitary corticotrophs. As ACTH has to be present in secretory vesicles ready for release at times of acute stress, it may be that POMC is produced continuously to supply ACTH, but when there is sufficient ACTH, POMC is routed to immature secretory granules and released from the corticotrophs in an “overflow” pathway. This would explain the data suggesting that the precursors may not be regulated to the same degree as ACTH.
C. Are ACTH Precursors Responsible for the Clinical Symptoms of Ectopic ACTH Syndrome?
Increased ACTH precursors are frequently used in our laboratory as a diagnostic tool to identify patients with ectopic ACTH syndrome (and those with large pituitary corticotroph macroadenomas). In addition, as stated above, most ACTH assays detect the ACTH precursors, but underestimate their true concentrations. Therefore, it would seem tempting to speculate that the ACTH precursors are the cause, at least in part, of the clinical symptoms.
On the other hand, this review has highlighted several reasons why it is difficult to make these assumptions. The early studies assessing the bioactivity of POMC and pro-ACTH have suggested that these precursors may only have low bioactivity at the MC2R. However, with newer approaches to measuring the precursors and with the discovery of MRAP, it may be that this evidence needs to be revisited. It is also likely that the higher molecular weight precursors will have a longer half-life, and this might increase their ability to stimulate the receptors at the adrenal gland.
In contrast, it is difficult to completely rule out ACTH as the causative agent. This is particularly true in those cases of ectopic ACTH syndrome where ACTH precursors are high, but ACTH concentrations are normal or slightly elevated. It is known that these levels of ACTH, if continuously secreted by a tumor, so that they remain elevated at night, can give rise to Cushing’s syndrome. Therefore, given these reservations, it is important to continue to improve our understanding of the role of ACTH precursors and their processing in Cushing’s syndrome.
D. Summary
POMC represents a conundrum in many ways. Is it just an inactive precursor? Does it have a role as a relatively stable protein, binding with low affinity to receptors? Does the secretion of unprocessed POMC by tumors reflect a relative lack of differentiation of the malignant cells? How important is regulation of POMC gene expression when the enzymes generating the smaller bioactive peptides are also tightly regulated? POMC is certainly an archetypal hormone precursor, delivering exquisite physiological control to complex multiorgan processes, and we need to learn more about it.
GRANTS
This work was supported by the Barbara Mawer-Fitzgerald endowment fund, University of Manchester. A. P. Coll is supported by the Medical Research Council (MRC Metabolic Diseases Unit MRC_MC_UU_12012.1).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors. Monoclonal antibodies to ACTH have been licensed by A. White to a number of companies for diagnostic kits.
ACKNOWLEDGMENTS
We thank all the members of our research group for valuable discussions and helpful proofreading. Special thanks to Charlotte Sefton for help with the figures.
Address for reprint requests and other correspondence: A. White, Div. of Diabetes, Endocrinology and Gastroenterology, School of Medical Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester Academic Health Sciences Centre, 3.016 AV Hill Building, Manchester, M13 9PT, UK (e-mail: anne.white@manchester.ac.uk).
REFERENCES
- 1.Abbott CR, Rossi M, Kim M, AlAhmed SH, Taylor GM, Ghatei MA, Smith DM, Bloom SR. Investigation of the melanocyte stimulating hormones on food intake. Lack of evidence to support a role for the melanocortin-3-receptor. Brain Res 869: 203–210, 2000. doi: 10.1016/S0006-8993(00)02386-6. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Malek Z, Swope VB, Suzuki I, Akcali C, Harriger MD, Boyce ST, Urabe K, Hearing VJ. Mitogenic and melanogenic stimulation of normal human melanocytes by melanotropic peptides. Proc Natl Acad Sci USA 92: 1789–1793, 1995. doi: 10.1073/pnas.92.5.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akil H, Young E, Watson SJ, Coy DH. Opiate binding properties of naturally occurring N- and C-terminus modified beta-endorphins. Peptides 2: 289–292, 1981. doi: 10.1016/S0196-9781(81)80121-0. [DOI] [PubMed] [Google Scholar]
- 4.Al-Barazanji KA, Miller JE, Rice SQ, Arch JR, Chambers JK. C-terminal fragments of ACTH stimulate feeding in fasted rats. Horm Metab Res 33: 480–485, 2001. doi: 10.1055/s-2001-16941. [DOI] [PubMed] [Google Scholar]
- 5.Allen BM. Extirpation experiments in Rana pipiens larvae. Science 44: 755–757, 1916. doi: 10.1126/science.44.1143.755. [DOI] [PubMed] [Google Scholar]
- 6.Allen RG, Peng B, Pellegrino MJ, Miller ED, Grandy DK, Lundblad JR, Washburn CL, Pintar JE. Altered processing of pro-orphanin FQ/nociceptin and pro-opiomelanocortin-derived peptides in the brains of mice expressing defective prohormone convertase 2. J Neurosci 21: 5864–5870, 2001. doi: 10.1523/JNEUROSCI.21-16-05864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anisimova AS, Rubtsov PM, Akulich KA, Dmitriev SE, Frolova E, Tiulpakov A. Late Diagnosis of POMC Deficiency and In Vitro Evidence of Residual Translation From Allele With c.-11C>A Mutation. J Clin Endocrinol Metab 102: 359–362, 2017. doi: 10.1210/jc.2016-3318. [DOI] [PubMed] [Google Scholar]
- 8.Antoni FA. Mortyn Jones Memorial Lecture–1995. Calcium checks cyclic AMP–corticosteroid feedback in adenohypophysial corticotrophs. J Neuroendocrinol 8: 659–672, 1996. doi: 10.1111/j.1365-2826.1996.tb00703.x. [DOI] [PubMed] [Google Scholar]
- 9.Appleyard SM, Hayward M, Young JI, Butler AA, Cone RD, Rubinstein M, Low MJ. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology 144: 1753–1760, 2003. doi: 10.1210/en.2002-221096. [DOI] [PubMed] [Google Scholar]
- 10.Arnaoutova I, Smith AM, Coates LC, Sharpe JC, Dhanvantari S, Snell CR, Birch NP, Loh YP. The prohormone processing enzyme PC3 is a lipid raft-associated transmembrane protein. Biochemistry 42: 10445–10455, 2003. doi: 10.1021/bi034277y. [DOI] [PubMed] [Google Scholar]
- 11.Aron DC, Findling JW, Fitzgerald PA, Forsham PH, Wilson CB, Tyrrell JB. Cushing’s syndrome: problems in management. Endocr Rev 3: 229–244, 1982. doi: 10.1210/edrv-3-3-229. [DOI] [PubMed] [Google Scholar]
- 12.Atkinson RL. Opioid regulation of food intake and body weight in humans. Fed Proc 46: 178–182, 1987. [PubMed] [Google Scholar]
- 13.Autelitano DJ. Stress-induced stimulation of pituitary POMC gene expression is associated with activation of transcription factor AP-1 in hypothalamus and pituitary. Brain Res Bull 45: 75–82, 1998. doi: 10.1016/S0361-9230(97)00303-1. [DOI] [PubMed] [Google Scholar]
- 14.Begeot M, Saez JM. Melanocortins and Adrenocortical Funcion. In: The Melanocortin Receptors, edtied by Cone RD. Totowa, NJ: Humana, 2000, p. 75–107. [Google Scholar]
- 15.Benjannet S, Rondeau N, Day R, Chrétien M, Seidah NG. PC1 and PC2 are proprotein convertases capable of cleaving proopiomelanocortin at distinct pairs of basic residues. Proc Natl Acad Sci USA 88: 3564–3568, 1991. doi: 10.1073/pnas.88.9.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett HP. Biosynthetic fate of the amino-terminal fragment of pro-opiomelanocortin within the intermediate lobe of the mouse pituitary. Peptides 7: 615–622, 1986. doi: 10.1016/0196-9781(86)90036-7. [DOI] [PubMed] [Google Scholar]
- 17.Bennett HP. Glycosylation, phosphorylation and sulphation of peptide hormones and their precursors. In: Peptide Biosynthesis and Processing, edited by Fricker LD. Boca Raton, FL: CRC, 1991, p. 111–140. [Google Scholar]
- 18.Bennett HP. Isolation and characterization of the 1 to 49 amino-terminal sequence of pro-opiomelanocortin from bovine posterior pituitaries. Biochem Biophys Res Commun 125: 229–236, 1984. doi: 10.1016/S0006-291X(84)80358-7. [DOI] [PubMed] [Google Scholar]
- 19.Bennett HP, Seidah NG, Benjannet S, Solomon S, Chrétien M. Reinvestigation of the disulfide bridge arrangement in human pro-opiomelanocortin N-terminal segment (hNT 1-76). Int J Pept Protein Res 27: 306–313, 1986. doi: 10.1111/j.1399-3011.1986.tb01825.x. [DOI] [PubMed] [Google Scholar]
- 20.Bennett MK. SNAREs and the specificity of transport vesicle targeting. Curr Opin Cell Biol 7: 581–586, 1995. doi: 10.1016/0955-0674(95)80016-6. [DOI] [PubMed] [Google Scholar]
- 21.Benzinou M, Creemers JW, Choquet H, Lobbens S, Dina C, Durand E, Guerardel A, Boutin P, Jouret B, Heude B, Balkau B, Tichet J, Marre M, Potoczna N, Horber F, Le Stunff C, Czernichow S, Sandbaek A, Lauritzen T, Borch-Johnsen K, Andersen G, Kiess W, Körner A, Kovacs P, Jacobson P, Carlsson LM, Walley AJ, Jørgensen T, Hansen T, Pedersen O, Meyre D, Froguel P. Common nonsynonymous variants in PCSK1 confer risk of obesity. Nat Genet 40: 943–945, 2008. doi: 10.1038/ng.177. [DOI] [PubMed] [Google Scholar]
- 22.Bergeron F, Leduc R, Day R. Subtilase-like pro-protein convertases: from molecular specificity to therapeutic applications. J Mol Endocrinol 24: 1–22, 2000. doi: 10.1677/jme.0.0240001. [DOI] [PubMed] [Google Scholar]
- 23.Berman Y, Mzhavia N, Polonskaia A, Devi LA. Impaired prohormone convertases in Cpe(fat)/Cpe(fat) mice. J Biol Chem 276: 1466–1473, 2001. doi: 10.1074/jbc.M008499200. [DOI] [PubMed] [Google Scholar]
- 24.Bernton EW, Beach JE, Holaday JW, Smallridge RC, Fein HG. Release of multiple hormones by a direct action of interleukin-1 on pituitary cells. Science 238: 519–521, 1987. doi: 10.1126/science.2821620. [DOI] [PubMed] [Google Scholar]
- 25.Bertagna X, Camus F, Lenne F, Girard F, Luton JP. Human joining peptide: a proopiomelanocortin product secreted as a homodimer. Mol Endocrinol 2: 1108–1114, 1988. doi: 10.1210/mend-2-11-1108. [DOI] [PubMed] [Google Scholar]
- 26.Bhardwaj RS, Luger TA. Proopiomelanocortin production by epidermal cells: evidence for an immune neuroendocrine network in the epidermis. Arch Dermatol Res 287: 85–90, 1994. doi: 10.1007/BF00370724. [DOI] [PubMed] [Google Scholar]
- 27.Bicknell AB. 60 YEARS OF POMC: N-terminal POMC peptides and adrenal growth. J Mol Endocrinol 56: T39–T48, 2016. doi: 10.1530/JME-15-0269. [DOI] [PubMed] [Google Scholar]
- 28.Bicknell AB, Lomthaisong K, Woods RJ, Hutchinson EG, Bennett HP, Gladwell RT, Lowry PJ. Characterization of a serine protease that cleaves pro-gamma-melanotropin at the adrenal to stimulate growth. Cell 105: 903–912, 2001. doi: 10.1016/S0092-8674(01)00403-2. [DOI] [PubMed] [Google Scholar]
- 29.Bicknell KA, Harmer SC, Yiangson S, Lockwood W, Bicknell AB. Lys-gamma3-MSH: a global regulator of hormone sensitive lipase activity? Mol Cell Endocrinol 300: 71–76, 2009. doi: 10.1016/j.mce.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 30.Biebermann H, Castañeda TR, van Landeghem F, von Deimling A, Escher F, Brabant G, Hebebrand J, Hinney A, Tschöp MH, Grüters A, Krude H. A role for beta-melanocyte-stimulating hormone in human body-weight regulation. Cell Metab 3: 141–146, 2006. doi: 10.1016/j.cmet.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Bilodeau S, Vallette-Kasic S, Gauthier Y, Figarella-Branger D, Brue T, Berthelet F, Lacroix A, Batista D, Stratakis C, Hanson J, Meij B, Drouin J. Role of Brg1 and HDAC2 in GR trans-repression of the pituitary POMC gene and misexpression in Cushing disease. Genes Dev 20: 2871–2886, 2006. doi: 10.1101/gad.1444606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birch NP, Estivariz FE, Bennett HP, Loh YP. Differential glycosylation of N-POMC1-77 regulates the production of gamma 3-MSH by purified pro-opiomelanocortin converting enzyme. A possible mechanism for tissue-specific processing. FEBS Lett 290: 191–194, 1991. doi: 10.1016/0014-5793(91)81257-9. [DOI] [PubMed] [Google Scholar]
- 33.Birnberg NC, Lissitzky JC, Hinman M, Herbert E. Glucocorticoids regulate proopiomelanocortin gene expression in vivo at the levels of transcription and secretion. Proc Natl Acad Sci USA 80: 6982–6986, 1983. doi: 10.1073/pnas.80.22.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blalock JE. Proopiomelanocortin and the immune-neuroendocrine connection. Ann N Y Acad Sci 885: 161–172, 1999. doi: 10.1111/j.1749-6632.1999.tb08673.x. [DOI] [PubMed] [Google Scholar]
- 35.Blázquez M, Docherty K, Shennan KI. Association of prohormone convertase 3 with membrane lipid rafts. J Mol Endocrinol 27: 107–116, 2001. doi: 10.1677/jme.0.0270107. [DOI] [PubMed] [Google Scholar]
- 36.Bodnar RJ. Endogenous opioids and feeding behavior: a 30-year historical perspective. Peptides 25: 697–725, 2004. doi: 10.1016/j.peptides.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Böhm M, Eickelmann M, Li Z, Schneider SW, Oji V, Diederichs S, Barsh GS, Vogt A, Stieler K, Blume-Peytavi U, Luger TA. Detection of functionally active melanocortin receptors and evidence for an immunoregulatory activity of alpha-melanocyte-stimulating hormone in human dermal papilla cells. Endocrinology 146: 4635–4646, 2005. doi: 10.1210/en.2005-0665. [DOI] [PubMed] [Google Scholar]
- 38.Boileau G, Barbeau C, Jeannotte L, Chrétien M, Drouin J. Complete structure of the porcine pro-opiomelanocortin mRNA derived from the nucleotide sequence of cloned cDNA. Nucleic Acids Res 11: 8063–8071, 1983. doi: 10.1093/nar/11.22.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boston BA. The role of melanocortins in adipocyte function. Ann N Y Acad Sci 885: 75–84, 1999. doi: 10.1111/j.1749-6632.1999.tb08666.x. [DOI] [PubMed] [Google Scholar]
- 40.Boudreault A, Gauthier D, Lazure C. Proprotein convertase PC1/3-related peptides are potent slow tight-binding inhibitors of murine PC1/3 and Hfurin. J Biol Chem 273: 31574–31580, 1998. doi: 10.1074/jbc.273.47.31574. [DOI] [PubMed] [Google Scholar]
- 41.Bousquet C, Zatelli MC, Melmed S. Direct regulation of pituitary proopiomelanocortin by STAT3 provides a novel mechanism for immuno-neuroendocrine interfacing. J Clin Invest 106: 1417–1425, 2000. doi: 10.1172/JCI11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boutillier AL, Monnier D, Lorang D, Lundblad JR, Roberts JL, Loeffler JP. Corticotropin-releasing hormone stimulates proopiomelanocortin transcription by cFos-dependent and -independent pathways: characterization of an AP1 site in exon 1. Mol Endocrinol 9: 745–755, 1995. [DOI] [PubMed] [Google Scholar]
- 43.Buckingham JC. Glucocorticoids: exemplars of multi-tasking. Br J Pharmacol 147, Suppl 1: S258–S268, 2006. doi: 10.1038/sj.bjp.0706456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci 2: 521–526, 2001. doi: 10.1038/35081582. [DOI] [PubMed] [Google Scholar]
- 45.Bumaschny VF, Yamashita M, Casas-Cordero R, Otero-Corchón V, de Souza FS, Rubinstein M, Low MJ. Obesity-programmed mice are rescued by early genetic intervention. J Clin Invest 122: 4203–4212, 2012. doi: 10.1172/JCI62543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buono P, Pasanisi F, Nardelli C, Ieno L, Capone S, Liguori R, Finelli C, Oriani G, Contaldo F, Sacchetti L. Six novel mutations in the proopiomelanocortin and melanocortin receptor 4 genes in severely obese adults living in southern Italy. Clin Chem 51: 1358–1364, 2005. doi: 10.1373/clinchem.2005.047886. [DOI] [PubMed] [Google Scholar]
- 47.Burbach JP, Lebouille JL, Wang X. Metabolic conversion of pro-opiomelanocortin peptides by brain peptidases: ACTH, a-MSH and b-Endorphin. In: Central Actions of ACTH and Related Peptides, edited by De Wied D, Ferrari W. Padova: Liviana, 1986, p. 53–67. [Google Scholar]
- 48.Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci 4: 605–611, 2001. doi: 10.1038/88423. [DOI] [PubMed] [Google Scholar]
- 49.Cangemi L, Adage T, Morabito A, Portaleone P. N-Acetyltransferase mechanism for alpha-melanocyte stimulating hormone regulation in rat ageing. Neurosci Lett 201: 65–68, 1995. doi: 10.1016/0304-3940(95)12135-Q. [DOI] [PubMed] [Google Scholar]
- 50.Cawley NX, Li Z, Loh YP. 60 YEARS OF POMC: Biosynthesis, trafficking, and secretion of pro-opiomelanocortin-derived peptides. J Mol Endocrinol 56: T77–T97, 2016. doi: 10.1530/JME-15-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cawley NX, Rathod T, Young S, Lou H, Birch N, Loh YP. Carboxypeptidase E and Secretogranin III coordinately facilitate efficient sorting of pro-opiomelanocortin to the regulated secretory pathway in AtT20 cells. Mol Endocrinol 30: 37–47, 2016. doi: 10.1210/me.2015-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cawley NX, Yanik T, Woronowicz A, Chang W, Marini JC, Loh YP. Obese carboxypeptidase E knockout mice exhibit multiple defects in peptide hormone processing contributing to low bone mineral density. Am J Physiol Endocrinol Metab 299: E189–E197, 2010. doi: 10.1152/ajpendo.00516.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cawley NX, Zhou J, Hill JM, Abebe D, Romboz S, Yanik T, Rodriguiz RM, Wetsel WC, Loh YP. The carboxypeptidase E knockout mouse exhibits endocrinological and behavioral deficits. Endocrinology 145: 5807–5819, 2004. doi: 10.1210/en.2004-0847. [DOI] [PubMed] [Google Scholar]
- 54.Cerritelli S, Hirschberg S, Hill R, Balthasar N, Pickering AE. Activation of Brainstem Pro-opiomelanocortin Neurons Produces Opioidergic Analgesia, Bradycardia and Bradypnoea. PLoS One 11: e0153187, 2016. doi: 10.1371/journal.pone.0153187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chadwick A, Lowry PJ. In vitro assay of MSH using skin from the frog Hyla arborea. Gen Comp Endocrinol 15: 493–495, 1970. doi: 10.1016/0016-6480(70)90124-3. [DOI] [PubMed] [Google Scholar]
- 56.Challis BG, Coll AP, Yeo GS, Pinnock SB, Dickson SL, Thresher RR, Dixon J, Zahn D, Rochford JJ, White A, Oliver RL, Millington G, Aparicio SA, Colledge WH, Russ AP, Carlton MB, O’Rahilly S. Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY(3-36). Proc Natl Acad Sci USA 101: 4695–4700, 2004. doi: 10.1073/pnas.0306931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Challis BG, Pritchard LE, Creemers JW, Delplanque J, Keogh JM, Luan J, Wareham NJ, Yeo GS, Bhattacharyya S, Froguel P, White A, Farooqi IS, O’Rahilly S. A missense mutation disrupting a dibasic prohormone processing site in pro-opiomelanocortin (POMC) increases susceptibility to early-onset obesity through a novel molecular mechanism. Hum Mol Genet 11: 1997–2004, 2002. doi: 10.1093/hmg/11.17.1997. [DOI] [PubMed] [Google Scholar]
- 58.Chan JS, Seidah NG, Chrétien M. Measurement of N-terminal (1-76) of human proopiomelanocortin in human plasma: correlation with adrenocorticotropin. J Clin Endocrinol Metab 56: 791–796, 1983. doi: 10.1210/jcem-56-4-791. [DOI] [PubMed] [Google Scholar]
- 59.Chanat E, Huttner WB. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J Cell Biol 115: 1505–1519, 1991. doi: 10.1083/jcb.115.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chance RE, Ellis RM, Bromer WW. Porcine proinsulin: characterization and amino acid sequence. Science 161: 165–167, 1968. doi: 10.1126/science.161.3837.165. [DOI] [PubMed] [Google Scholar]
- 61.Chayen J, Loveridge N, Daly JR. A sensitive bioassay for adrenocorticotrophic hormone in human plasma. Clin Endocrinol (Oxf) 1: 219–233, 1972. doi: 10.1111/j.1365-2265.1972.tb00393.x. [DOI] [PubMed] [Google Scholar]
- 62.Chen CL, Chang CC, Krieger DT, Bardin CW. Expression and regulation of proopiomelanocortin-like gene in the ovary and placenta: comparison with the testis. Endocrinology 118: 2382–2389, 1986. doi: 10.1210/endo-118-6-2382. [DOI] [PubMed] [Google Scholar]
- 63.Chen H, Jawahar S, Qian Y, Duong Q, Chan G, Parker A, Meyer JM, Moore KJ, Chayen S, Gross DJ, Glaser B, Permutt MA, Fricker LD. Missense polymorphism in the human carboxypeptidase E gene alters enzymatic activity. Hum Mutat 18: 120–131, 2001. doi: 10.1002/humu.1161. [DOI] [PubMed] [Google Scholar]
- 64.Chrétien M, Benjannet S, Gossard F, Gianoulakis C, Crine P, Lis M, Seidah NG. From beta-lipotropin to beta-endorphin and ‘pro-opio-melanocortin.’ Can J Biochem 57: 1111–1121, 1979. doi: 10.1139/o79-143. [DOI] [PubMed] [Google Scholar]
- 65.Chrétien M, Li CH. Isolation, purification, and characterization of gamma-lipotropic hormone from sheep pituitary glands. Can J Biochem 45: 1163–1174, 1967. doi: 10.1139/o67-133. [DOI] [PubMed] [Google Scholar]
- 66.Chrétien M, Mbikay M. 60 YEARS OF POMC: From the prohormone theory to pro-opiomelanocortin and to proprotein convertases (PCSK1 to PCSK9). J Mol Endocrinol 56: T49–T62, 2016. doi: 10.1530/JME-15-0261. [DOI] [PubMed] [Google Scholar]
- 67.Chung S, Son GH, Kim K. Circadian rhythm of adrenal glucocorticoid: its regulation and clinical implications. Biochim Biophys Acta 1812: 581–591, 2011. doi: 10.1016/j.bbadis.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 68.Ciccotosto GD, Schiller MR, Eipper BA, Mains RE. Induction of integral membrane PAM expression in AtT-20 cells alters the storage and trafficking of POMC and PC1. J Cell Biol 144: 459–471, 1999. doi: 10.1083/jcb.144.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clark AJ. 60 YEARS OF POMC: The proopiomelanocortin gene: discovery, deletion and disease. J Mol Endocrinol 56: T27–T37, 2016. doi: 10.1530/JME-15-0268. [DOI] [PubMed] [Google Scholar]
- 70.Clarke BL, Bost KL. Differential expression of functional adrenocorticotropic hormone receptors by subpopulations of lymphocytes. J Immunol 143: 464–469, 1989. [PubMed] [Google Scholar]
- 71.Clément K, Dubern B, Mencarelli M, Czernichow P, Ito S, Wakamatsu K, Barsh GS, Vaisse C, Leger J. Unexpected endocrine features and normal pigmentation in a young adult patient carrying a novel homozygous mutation in the POMC gene. J Clin Endocrinol Metab 93: 4955–4962, 2008. doi: 10.1210/jc.2008-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cochet M, Chang AC, Cohen SN. Characterization of the structural gene and putative 5′-regulatory sequences for human proopiomelanocortin. Nature 297: 335–339, 1982. doi: 10.1038/297335a0. [DOI] [PubMed] [Google Scholar]
- 73.Coll AP, Challis BG, López M, Piper S, Yeo GS, O’Rahilly S. Proopiomelanocortin-deficient mice are hypersensitive to the adverse metabolic effects of glucocorticoids. Diabetes 54: 2269–2276, 2005. doi: 10.2337/diabetes.54.8.2269. [DOI] [PubMed] [Google Scholar]
- 74.Coll AP, Challis BG, Yeo GS, Snell K, Piper SJ, Halsall D, Thresher RR, O’Rahilly S. The effects of proopiomelanocortin deficiency on murine adrenal development and responsiveness to adrenocorticotropin. Endocrinology 145: 4721–4727, 2004. doi: 10.1210/en.2004-0491. [DOI] [PubMed] [Google Scholar]
- 75.Coll AP, Fassnacht M, Klammer S, Hahner S, Schulte DM, Piper S, Tung YC, Challis BG, Weinstein Y, Allolio B, O’Rahilly S, Beuschlein F. Peripheral administration of the N-terminal pro-opiomelanocortin fragment 1-28 to Pomc−/− mice reduces food intake and weight but does not affect adrenal growth or corticosterone production. J Endocrinol 190: 515–525, 2006. doi: 10.1677/joe.1.06749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Comuzzie AG, Hixson JE, Almasy L, Mitchell BD, Mahaney MC, Dyer TD, Stern MP, MacCluer JW, Blangero J. A major quantitative trait locus determining serum leptin levels and fat mass is located on human chromosome 2. Nat Genet 15: 273–276, 1997. doi: 10.1038/ng0397-273. [DOI] [PubMed] [Google Scholar]
- 77.Cone RD. The Melanocortin Receptors. Totowa, NJ: Humana, 2000. doi: 10.1385/1592590314 [DOI] [Google Scholar]
- 78.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev 27: 736–749, 2006. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 79.Conway-Campbell BL, Sarabdjitsingh RA, McKenna MA, Pooley JR, Kershaw YM, Meijer OC, de Kloet ER, Lightman SL. Glucocorticoid ultradian rhythmicity directs cyclical gene pulsing of the clock gene period 1 in rat hippocampus. J Neuroendocrinol 22: 1093–1100, 2010. doi: 10.1111/j.1365-2826.2010.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cool DR, Fenger M, Snell CR, Loh YP. Identification of the sorting signal motif within pro-opiomelanocortin for the regulated secretory pathway. J Biol Chem 270: 8723–8729, 1995. doi: 10.1074/jbc.270.15.8723. [DOI] [PubMed] [Google Scholar]
- 81.Cool DR, Loh YP. Carboxypeptidase E is a sorting receptor for prohormones: binding and kinetic studies. Mol Cell Endocrinol 139: 7–13, 1998. doi: 10.1016/S0303-7207(98)00081-1. [DOI] [PubMed] [Google Scholar]
- 82.Cool DR, Loh YP. Identification of a sorting signal for the regulated secretory pathway at the N-terminus of pro-opiomelanocortin. Biochimie 76: 265–270, 1994. doi: 10.1016/0300-9084(94)90156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cool DR, Normant E, Shen F, Chen HC, Pannell L, Zhang Y, Loh YP. Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell 88: 73–83, 1997. doi: 10.1016/S0092-8674(00)81860-7. [DOI] [PubMed] [Google Scholar]
- 84.Cota D, Steiner MA, Marsicano G, Cervino C, Herman JP, Grübler Y, Stalla J, Pasquali R, Lutz B, Stalla GK, Pagotto U. Requirement of cannabinoid receptor type 1 for the basal modulation of hypothalamic-pituitary-adrenal axis function. Endocrinology 148: 1574–1581, 2007. doi: 10.1210/en.2005-1649. [DOI] [PubMed] [Google Scholar]
- 85.Creemers JW, Choquet H, Stijnen P, Vatin V, Pigeyre M, Beckers S, Meulemans S, Than ME, Yengo L, Tauber M, Balkau B, Elliott P, Jarvelin MR, Van Hul W, Van Gaal L, Horber F, Pattou F, Froguel P, Meyre D. Heterozygous mutations causing partial prohormone convertase 1 deficiency contribute to human obesity. Diabetes 61: 383–390, 2012. doi: 10.2337/db11-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Creemers JW, Lee YS, Oliver RL, Bahceci M, Tuzcu A, Gokalp D, Keogh J, Herber S, White A, O’Rahilly S, Farooqi IS. Mutations in the amino-terminal region of proopiomelanocortin (POMC) in patients with early-onset obesity impair POMC sorting to the regulated secretory pathway. J Clin Endocrinol Metab 93: 4494–4499, 2008. doi: 10.1210/jc.2008-0954. [DOI] [PubMed] [Google Scholar]
- 87.Creemers JW, Roebroek AJ, Van de Ven WJ. Expression in human lung tumor cells of the proprotein processing enzyme PC1/PC3. Cloning and primary sequence of a 5 kb cDNA. FEBS Lett 300: 82–88, 1992. doi: 10.1016/0014-5793(92)80169-H. [DOI] [PubMed] [Google Scholar]
- 88.Crosby SR, Stewart MF, Farrell WE, Gibson S, White A. Comparison of ACTH and ACTH precursor peptides secreted by human pituitary and lung tumour cells in vitro. J Endocrinol 125: 147–152, 1990. doi: 10.1677/joe.0.1250147. [DOI] [PubMed] [Google Scholar]
- 89.Crosby SR, Stewart MF, Ratcliffe JG, White A. Direct measurement of the precursors of adrenocorticotropin in human plasma by two-site immunoradiometric assay. J Clin Endocrinol Metab 67: 1272–1277, 1988. doi: 10.1210/jcem-67-6-1272. [DOI] [PubMed] [Google Scholar]
- 90.Dallman MF, Akana SF, Scribner KA, Bradbury MJ, Walker CD, Strack AM, Cascio CS. Stress, feedback and facilitation in the hypothalamo-pituitary-adrenal axis. J Neuroendocrinol 4: 517–526, 1992. doi: 10.1111/j.1365-2826.1992.tb00200.x. [DOI] [PubMed] [Google Scholar]
- 91.Day R, Schafer MK, Watson SJ, Chrétien M, Seidah NG. Distribution and regulation of the prohormone convertases PC1 and PC2 in the rat pituitary. Mol Endocrinol 6: 485–497, 1992. [DOI] [PubMed] [Google Scholar]
- 92.Deakin JF, Doströvsky JO, Smyth DG. Influence of N-terminal acetylation and C-terminal proteolysis on the analgesic activity of beta-endorphin. Biochem J 189: 501–506, 1980. doi: 10.1042/bj1890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.DeBold CR, DeCherney GS, Jackson RV, Sheldon WR, Alexander AN, Island DP, Rivier J, Vale W, Orth DN. Effect of synthetic ovine corticotropin-releasing factor: prolonged duration of action and biphasic response of plasma adrenocorticotropin and cortisol. J Clin Endocrinol Metab 57: 294–298, 1983. doi: 10.1210/jcem-57-2-294. [DOI] [PubMed] [Google Scholar]
- 94.DeBold CR, Menefee JK, Nicholson WE, Orth DN. Proopiomelanocortin gene is expressed in many normal human tissues and in tumors not associated with ectopic adrenocorticotropin syndrome. Mol Endocrinol 2: 862–870, 1988. doi: 10.1210/mend-2-9-862. [DOI] [PubMed] [Google Scholar]
- 95.Delbende C, Jégou S, Tranchand-Bunel D, Pelletier G, Vaudry H. Hypothalamic alpha-melanocyte-stimulating hormone (alpha-MSH) is not under dopaminergic control. Brain Res 423: 203–212, 1987. doi: 10.1016/0006-8993(87)90841-9. [DOI] [PubMed] [Google Scholar]
- 96.Deng Q, Riquelme D, Trinh L, Low MJ, Tomić M, Stojilkovic S, Aguilera G. Rapid Glucocorticoid Feedback Inhibition of ACTH Secretion Involves Ligand-Dependent Membrane Association of Glucocorticoid Receptors. Endocrinology 156: 3215–3227, 2015. doi: 10.1210/EN.2015-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dennis M, Seidah NG, Chrétien M. Regional heterogeneity in the processing of pro-opiomelanocortin in rat brain. Life Sci 33, Suppl 1: 49–52, 1983. doi: 10.1016/0024-3205(83)90441-1. [DOI] [PubMed] [Google Scholar]
- 98.Dhanvantari S, Loh YP. Lipid raft association of carboxypeptidase E is necessary for its function as a regulated secretory pathway sorting receptor. J Biol Chem 275: 29887–29893, 2000. doi: 10.1074/jbc.M005364200. [DOI] [PubMed] [Google Scholar]
- 99.Dhanvantari S, Shen FS, Adams T, Snell CR, Zhang C, Mackin RB, Morris SJ, Loh YP. Disruption of a receptor-mediated mechanism for intracellular sorting of proinsulin in familial hyperproinsulinemia. Mol Endocrinol 17: 1856–1867, 2003. doi: 10.1210/me.2002-0380. [DOI] [PubMed] [Google Scholar]
- 100.Dikeakos JD, Reudelhuber TL. Sending proteins to dense core secretory granules: still a lot to sort out. J Cell Biol 177: 191–196, 2007. doi: 10.1083/jcb.200701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dong W, Day R. Gene expression of proprotein convertases in individual rat anterior pituitary cells and their regulation in corticotrophs mediated by glucocorticoids. Endocrinology 143: 254–262, 2002. doi: 10.1210/endo.143.1.8570. [DOI] [PubMed] [Google Scholar]
- 102.Dong W, Fricker LD, Day R. Carboxypeptidase D is a potential candidate to carry out redundant processing functions of carboxypeptidase E based on comparative distribution studies in the rat central nervous system. Neuroscience 89: 1301–1317, 1999. doi: 10.1016/S0306-4522(98)00381-9. [DOI] [PubMed] [Google Scholar]
- 103.Dores RM, Jain M, Akil H. Characterization of the forms of beta-endorphin and alpha-MSH in the caudal medulla of the rat and guinea pig. Brain Res 377: 251–260, 1986. doi: 10.1016/0006-8993(86)90866-8. [DOI] [PubMed] [Google Scholar]
- 104.Drouin J. 60 YEARS OF POMC: Transcriptional and epigenetic regulation of POMC gene expression. J Mol Endocrinol 56: T99–T112, 2016. doi: 10.1530/JME-15-0289. [DOI] [PubMed] [Google Scholar]
- 105.Drouin J, Goodman HM. Most of the coding region of rat ACTH beta–LPH precursor gene lacks intervening sequences. Nature 288: 610–613, 1980. doi: 10.1038/288610a0. [DOI] [PubMed] [Google Scholar]
- 106.Drouin J, Sun YL, Chamberland M, Gauthier Y, De Léan A, Nemer M, Schmidt TJ. Novel glucocorticoid receptor complex with DNA element of the hormone-repressed POMC gene. EMBO J 12: 145–156, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Drouin J, Sun YL, Nemer M. Glucocorticoid repression of pro-opiomelanocortin gene transcription. J Steroid Biochem 34: 63–69, 1989. doi: 10.1016/0022-4731(89)90066-6. [DOI] [PubMed] [Google Scholar]
- 108.Drouin J, Trifiro MA, Plante RK, Nemer M, Eriksson P, Wrange O. Glucocorticoid receptor binding to a specific DNA sequence is required for hormone-dependent repression of pro-opiomelanocortin gene transcription. Mol Cell Biol 9: 5305–5314, 1989. doi: 10.1128/MCB.9.12.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dubern B, Lubrano-Berthelier C, Mencarelli M, Ersoy B, Frelut ML, Bouglé D, Costes B, Simon C, Tounian P, Vaisse C, Clement K. Mutational analysis of the pro-opiomelanocortin gene in French obese children led to the identification of a novel deleterious heterozygous mutation located in the alpha-melanocyte stimulating hormone domain. Pediatr Res 63: 211–216, 2008. doi: 10.1203/PDR.0b013e31815ed62b. [DOI] [PubMed] [Google Scholar]
- 110.Dumermuth E, Moore HP. Analysis of constitutive and constitutive-like secretion in semi-intact pituitary cells. Methods 16: 188–197, 1998. doi: 10.1006/meth.1998.0666. [DOI] [PubMed] [Google Scholar]
- 111.Duncan PJ, Şengül S, Tabak J, Ruth P, Bertram R, Shipston MJ. Large conductance Ca2+-activated K+ (BK) channels promote secretagogue-induced transition from spiking to bursting in murine anterior pituitary corticotrophs. J Physiol 593: 1197–1211, 2015. doi: 10.1113/jphysiol.2015.284471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dutia R, Meece K, Dighe S, Kim AJ, Wardlaw SL. β-Endorphin antagonizes the effects of α-MSH on food intake and body weight. Endocrinology 153: 4246–4255, 2012. doi: 10.1210/en.2012-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eberle AN. The Melanotropins: Chemistry, Physiology and Mechanisms of Action. Basel: Karger, 1988. [Google Scholar]
- 114.Eberle AN. Proopiomelanocortin and the Melanocortin Peptides. In: The Melanocortin Receptors, edited by Cone RD. Totowa, NJ: Humana, 2000. [Google Scholar]
- 115.Eberwine JH, Roberts JL. Glucocorticoid regulation of pro-opiomelanocortin gene transcription in the rat pituitary. J Biol Chem 259: 2166–2170, 1984. [PubMed] [Google Scholar]
- 116.Echwald SM, Sørensen TI, Andersen T, Tybjaerg-Hansen A, Clausen JO, Pedersen O. Mutational analysis of the proopiomelanocortin gene in Caucasians with early onset obesity. Int J Obes Relat Metab Disord 23: 293–298, 1999. doi: 10.1038/sj.ijo.0800814. [DOI] [PubMed] [Google Scholar]
- 117.Eipper BA, Mains RE. Analysis of the common precursor to corticotropin and endorphin. J Biol Chem 253: 5732–5744, 1978. [PubMed] [Google Scholar]
- 118.Eipper BA, Mains RE. High molecular weight forms of adrenocorticotropic hormone in the mouse pituitary and in a mouse pituitary tumor cell line. Biochemistry 14: 3836–3844, 1975. doi: 10.1021/bi00688a016. [DOI] [PubMed] [Google Scholar]
- 119.Eipper BA, Mains RE. Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr Rev 1: 1–27, 1980. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- 120.Eipper BA, Milgram SL, Husten EJ, Yun HY, Mains RE. Peptidylglycine alpha-amidating monooxygenase: a multifunctional protein with catalytic, processing, and routing domains. Protein Sci 2: 489–497, 1993. doi: 10.1002/pro.5560020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.El Meskini R, Galano GJ, Marx R, Mains RE, Eipper BA. Targeting of membrane proteins to the regulated secretory pathway in anterior pituitary endocrine cells. J Biol Chem 276: 3384–3393, 2001. doi: 10.1074/jbc.M008062200. [DOI] [PubMed] [Google Scholar]
- 122.Ellingboe J, Veldhuis JD, Mendelson JH, Kuehnle JC, Mello NK, Holbrook PG. Effect of endogenous opioid blockade on the amplitude and frequency of pulsatile luteinizing hormone secretion in normal men. J Clin Endocrinol Metab 54: 854–857, 1982. doi: 10.1210/jcem-54-4-854. [DOI] [PubMed] [Google Scholar]
- 123.Estivariz FE, Hope J, McLean C, Lowry PJ. Purification and characterization of a gamma-melanotropin precursor from frozen human pituitary glands. Biochem J 191: 125–132, 1980. doi: 10.1042/bj1910125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Estivariz FE, Iturriza F, McLean C, Hope J, Lowry PJ. Stimulation of adrenal mitogenesis by N-terminal proopiocortin peptides. Nature 297: 419–422, 1982. doi: 10.1038/297419a0. [DOI] [PubMed] [Google Scholar]
- 125.Estivariz FE, Morano MI, Carino M, Jackson S, Lowry PJ. Adrenal regeneration in the rat is mediated by mitogenic N-terminal pro-opiomelanocortin peptides generated by changes in precursor processing in the anterior pituitary. J Endocrinol 116: 207–216, 1988. doi: 10.1677/joe.0.1160207. [DOI] [PubMed] [Google Scholar]
- 126.Estomba H, Muñoa-Hoyos I, Gianzo M, Urizar-Arenaza I, Casis L, Irazusta J, Subirán N. Expression and Localization of Opioid Receptors in Male Germ Cells and the Implication for Mouse Spermatogenesis. PLoS One 11: e0152162, 2016. doi: 10.1371/journal.pone.0152162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Facchinetti F, Perez-Fernandez R, Toma MO, Gaudiero GJ, Lechuga MJ, Devesa J, Genazzani AR. Dopamine acts on acetylation of proopiomelanocortin-derived products in dog pituitary. Acta Endocrinol (Copenh) 117: 33–38, 1988. [DOI] [PubMed] [Google Scholar]
- 128.Farooqi IS. Monogenic human obesity. Front Horm Res 36: 1–11, 2008. doi: 10.1159/0000115333. [DOI] [PubMed] [Google Scholar]
- 129.Farooqi IS, Drop S, Clements A, Keogh JM, Biernacka J, Lowenbein S, Challis BG, O’Rahilly S. Heterozygosity for a POMC-null mutation and increased obesity risk in humans. Diabetes 55: 2549–2553, 2006. doi: 10.2337/db06-0214. [DOI] [PubMed] [Google Scholar]
- 130.Farooqi IS, Volders K, Stanhope R, Heuschkel R, White A, Lank E, Keogh J, O’Rahilly S, Creemers JW. Hyperphagia and early-onset obesity due to a novel homozygous missense mutation in prohormone convertase 1/3. J Clin Endocrinol Metab 92: 3369–3373, 2007. doi: 10.1210/jc.2007-0687. [DOI] [PubMed] [Google Scholar]
- 131.Fassnacht M, Hahner S, Hansen IA, Kreutzberger T, Zink M, Adermann K, Jakob F, Troppmair J, Allolio B. N-terminal proopiomelanocortin acts as a mitogen in adrenocortical tumor cells and decreases adrenal steroidogenesis. J Clin Endocrinol Metab 88: 2171–2179, 2003. doi: 10.1210/jc.2002-021318. [DOI] [PubMed] [Google Scholar]
- 132.Feldberg W, Smyth DG. The C-fragment of lipotropin–a potent analgesic. J Physiol 260: 30P–31P, 1976. [PubMed] [Google Scholar]
- 133.Ferin M, Van Vugt D, Wardlaw S. The hypothalamic control of the menstrual cycle and the role of endogenous opioid peptides. Recent Prog Horm Res 40: 441–485, 1984. [DOI] [PubMed] [Google Scholar]
- 134.Fernandez CJ, Haugwitz M, Eaton B, Moore HP. Distinct molecular events during secretory granule biogenesis revealed by sensitivities to brefeldin A. Mol Biol Cell 8: 2171–2185, 1997. doi: 10.1091/mbc.8.11.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Findling JW, Engeland WC, Raff H. The use of immunoradiometric assay for the measurement of ACTH in human plasma. Trends Endocrinol Metab 1: 283–287, 1990. doi: 10.1016/1043-2760(90)90064-A. [DOI] [PubMed] [Google Scholar]
- 136.Fortenberry Y, Liu J, Lindberg I. The role of the 7B2 CT peptide in the inhibition of prohormone convertase 2 in endocrine cell lines. J Neurochem 73: 994–1003, 1999. doi: 10.1046/j.1471-4159.1999.0730994.x. [DOI] [PubMed] [Google Scholar]
- 137.Fricker LD, Berman YL, Leiter EH, Devi LA. Carboxypeptidase E activity is deficient in mice with the fat mutation. Effect on peptide processing. J Biol Chem 271: 30619–30624, 1996. doi: 10.1074/jbc.271.48.30619. [DOI] [PubMed] [Google Scholar]
- 138.Fricker LD, McKinzie AA, Sun J, Curran E, Qian Y, Yan L, Patterson SD, Courchesne PL, Richards B, Levin N, Mzhavia N, Devi LA, Douglass J. Identification and characterization of proSAAS, a granin-like neuroendocrine peptide precursor that inhibits prohormone processing. J Neurosci 20: 639–648, 2000. doi: 10.1523/JNEUROSCI.20-02-00639.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fukata J, Naitoh Y, Usui T, Nakaishi S, Nakai Y, Imura H. Two-site immunoradiometric assay for adrenocorticotrophin: a cautionary study about the reactivity to its precursor molecules. Endocrinol Jpn 36: 155–161, 1989. doi: 10.1507/endocrj1954.36.155. [DOI] [PubMed] [Google Scholar]
- 140.Furuta M, Yano H, Zhou A, Rouillé Y, Holst JJ, Carroll R, Ravazzola M, Orci L, Furuta H, Steiner DF. Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proc Natl Acad Sci USA 94: 6646–6651, 1997. doi: 10.1073/pnas.94.13.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gagner JP, Drouin J. Opposite regulation of pro-opiomelanocortin gene transcription by glucocorticoids and CRH. Mol Cell Endocrinol 40: 25–32, 1985. doi: 10.1016/0303-7207(85)90154-6. [DOI] [PubMed] [Google Scholar]
- 142.Gagner JP, Drouin J. Tissue-specific regulation of pituitary proopiomelanocortin gene transcription by corticotropin-releasing hormone, 3′,5′-cyclic adenosine monophosphate, and glucocorticoids. Mol Endocrinol 1: 677–682, 1987. doi: 10.1210/mend-1-10-677. [DOI] [PubMed] [Google Scholar]
- 143.Gallo-Payet N. 60 YEARS OF POMC: Adrenal and extra-adrenal functions of ACTH. J Mol Endocrinol 56: T135–T156, 2016. doi: 10.1530/JME-15-0257. [DOI] [PubMed] [Google Scholar]
- 144.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, DelValle J, Yamada T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem 268: 15174–15179, 1993. [PubMed] [Google Scholar]
- 145.Gasson JC. Steroidogenic activity of high molecular weight forms of corticotropin. Biochemistry 18: 4215–4224, 1979. doi: 10.1021/bi00586a028. [DOI] [PubMed] [Google Scholar]
- 146.Gevers EF, Meredith S, Shah P, Torpiano J, Peters C, Sebire NJ, Slater O, White A, Dattani MT. Cushing syndrome in a child due to pro-opiomelanocortin (POMC) secretion from a yolk sac tumor. Eur J Endocrinol 176: K1–K7, 2017. doi: 10.1530/EJE-16-0776. [DOI] [PubMed] [Google Scholar]
- 147.Gewirtz G, Schneider B, Krieger DT, Yalow RS. Big ACTH: conversion to biologically active ACTH by trypsin. J Clin Endocrinol Metab 38: 227–230, 1974. doi: 10.1210/jcem-38-2-227. [DOI] [PubMed] [Google Scholar]
- 148.Gibson S, Crosby SR, Stewart MF, Jennings AM, McCall E, White A. Differential release of proopiomelanocortin-derived peptides from the human pituitary: evidence from a panel of two-site immunoradiometric assays. J Clin Endocrinol Metab 78: 835–841, 1994. [DOI] [PubMed] [Google Scholar]
- 149.Gibson S, Crosby SR, White A. Discrimination between beta-endorphin and beta-lipotrophin in human plasma using two-site immunoradiometric assays. Clin Endocrinol (Oxf) 39: 445–453, 1993. doi: 10.1111/j.1365-2265.1993.tb02392.x. [DOI] [PubMed] [Google Scholar]
- 150.Gibson S, Ray DW, Crosby SR, Dornan TL, Jennings AM, Bevan JS, Davis JR, White A. Impaired processing of proopiomelanocortin in corticotroph macroadenomas. J Clin Endocrinol Metab 81: 497–502, 1996. [DOI] [PubMed] [Google Scholar]
- 151.Gizang-Ginsberg E, Wolgemuth DJ. Expression of the proopiomelanocortin gene is developmentally regulated and affected by germ cells in the male mouse reproductive system. Proc Natl Acad Sci USA 84: 1600–1604, 1987. doi: 10.1073/pnas.84.6.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Glauder J, Ragg H, Rauch J, Engels JW. Human peptidylglycine alpha-amidating monooxygenase: cDNA, cloning and functional expression of a truncated form in COS cells. Biochem Biophys Res Commun 169: 551–558, 1990. doi: 10.1016/0006-291X(90)90366-U. [DOI] [PubMed] [Google Scholar]
- 153.Goodman LJ, Gorman CM. Autoproteolytic activation of the mouse prohormone convertase mPC1. Biochem Biophys Res Commun 201: 795–804, 1994. doi: 10.1006/bbrc.1994.1771. [DOI] [PubMed] [Google Scholar]
- 154.Guillemin R. Peptides in the brain: the new endocrinology of the neuron. Science 202: 390–402, 1978. doi: 10.1126/science.212832. [DOI] [PubMed] [Google Scholar]
- 155.Gumbiner B, Kelly RB. Two distinct intracellular pathways transport secretory and membrane glycoproteins to the surface of pituitary tumor cells. Cell 28: 51–59, 1982. doi: 10.1016/0092-8674(82)90374-9. [DOI] [PubMed] [Google Scholar]
- 156.Guo L, Münzberg H, Stuart RC, Nillni EA, Bjørbaek C. N-acetylation of hypothalamic alpha-melanocyte-stimulating hormone and regulation by leptin. Proc Natl Acad Sci USA 101: 11797–11802, 2004. doi: 10.1073/pnas.0403165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hager J, Dina C, Francke S, Dubois S, Houari M, Vatin V, Vaillant E, Lorentz N, Basdevant A, Clement K, Guy-Grand B, Froguel P. A genome-wide scan for human obesity genes reveals a major susceptibility locus on chromosome 10. Nat Genet 20: 304–308, 1998. doi: 10.1038/3123. [DOI] [PubMed] [Google Scholar]
- 158.Hahner S, Fassnacht M, Hammer F, Schammann M, Weismann D, Hansen IA, Allolio B. Evidence against a role of human airway trypsin-like protease–the human analogue of the growth-promoting rat adrenal secretory protease–in adrenal tumourigenesis. Eur J Endocrinol 152: 143–153, 2005. doi: 10.1530/eje.1.01834. [DOI] [PubMed] [Google Scholar]
- 159.Hale AC, Besser GM, Rees LH. Characterization of pro-opiomelanocortin-derived peptides in pituitary and ectopic adrenocorticotrophin-secreting tumours. J Endocrinol 108: 49–56, 1986. doi: 10.1677/joe.0.1080049. [DOI] [PubMed] [Google Scholar]
- 160.Hale AC, Ratter SJ, Tomlin SJ, Lytras N, Besser GM, Rees LH. Measurement of immunoreactive gamma-MSH in human plasma. Clin Endocrinol (Oxf) 21: 139–148, 1984. doi: 10.1111/j.1365-2265.1984.tb03453.x. [DOI] [PubMed] [Google Scholar]
- 161.Hardiman A, Friedman TC, Grunwald WC Jr, Furuta M, Zhu Z, Steiner DF, Cool DR. Endocrinomic profile of neurointermediate lobe pituitary prohormone processing in PC1/3- and PC2-Null mice using SELDI-TOF mass spectrometry. J Mol Endocrinol 34: 739–751, 2005. doi: 10.1677/jme.1.01812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Harno E, White A. Adrenocorticotropic Hormone. In: Endocrinology: Adult and Pediatric, edited by Jameson JL, De Groot LJ, de Kretser DM, Giudice LC, Grossman AB, Melmed S, Potts JT Jr, Weir GC. Philadelphia, PA: Elsevier, 2015, p. 129–146. [Google Scholar]
- 163.Hendy GN, Li T, Girard M, Feldstein RC, Mulay S, Desjardins R, Day R, Karaplis AC, Tremblay ML, Canaff L. Targeted ablation of the chromogranin a (Chga) gene: normal neuroendocrine dense-core secretory granules and increased expression of other granins. Mol Endocrinol 20: 1935–1947, 2006. doi: 10.1210/me.2005-0398. [DOI] [PubMed] [Google Scholar]
- 164.Henley DE, Leendertz JA, Russell GM, Wood SA, Taheri S, Woltersdorf WW, Lightman SL. Development of an automated blood sampling system for use in humans. J Med Eng Technol 33: 199–208, 2009. doi: 10.1080/03091900802185970. [DOI] [PubMed] [Google Scholar]
- 165.Herbert E, Comb M, Thomas G, Liston D, Civelli O, Martin MG, Birnberg NC. Biosynthesis of ACTH and related peptides. In: Hormonal Proteins and Peptides, edited by Li CH. Orlando, FL: Academic, 1987, p. 59–87. [Google Scholar]
- 166.Hickman S, Shapiro LJ, Neufeld EF. A recognition marker required for uptake of a lysosomal enzyme by cultured fibroblasts. Biochem Biophys Res Commun 57: 55–61, 1974. doi: 10.1016/S0006-291X(74)80356-6. [DOI] [PubMed] [Google Scholar]
- 167.Hinney A, Becker I, Heibült O, Nottebom K, Schmidt A, Ziegler A, Mayer H, Siegfried W, Blum WF, Remschmidt H, Hebebrand J. Systematic mutation screening of the pro-opiomelanocortin gene: identification of several genetic variants including three different insertions, one nonsense and two missense point mutations in probands of different weight extremes. J Clin Endocrinol Metab 83: 3737–3741, 1998. doi: 10.1210/jcem.83.10.5298. [DOI] [PubMed] [Google Scholar]
- 168.Hirata Y, Yamamoto H, Matsukura S, Imura H. In vitro release and biosynthesis of tumor ACTH in ectopic ACTH producing tumors. J Clin Endocrinol Metab 41: 106–114, 1975. doi: 10.1210/jcem-41-1-106. [DOI] [PubMed] [Google Scholar]
- 169.Hoch M, Hirzel E, Lindinger P, Eberle AN, Linscheid P, Martin I, Peters T, Peterli R. Weak functional coupling of the melanocortin-1 receptor expressed in human adipocytes. J Recept Signal Transduct Res 28: 485–504, 2008. doi: 10.1080/10799890802442622. [DOI] [PubMed] [Google Scholar]
- 170.Hochgeschwender U, Costa JL, Reed P, Bui S, Brennan MB. Altered glucose homeostasis in proopiomelanocortin-null mouse mutants lacking central and peripheral melanocortin. Endocrinology 144: 5194–5202, 2003. doi: 10.1210/en.2003-1008. [DOI] [PubMed] [Google Scholar]
- 171.Hodgkinson SC, Allolio B, Landon J, Lowry PJ. Development of a non-extracted ‘two-site’ immunoradiometric assay for corticotropin utilizing extreme amino- and carboxy-terminally directed antibodies. Biochem J 218: 703–711, 1984. doi: 10.1042/bj2180703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hoggard N, Johnstone AM, Faber P, Gibney ER, Elia M, Lobley G, Rayner V, Horgan G, Hunter L, Bashir S, Stubbs RJ. Plasma concentrations of alpha-MSH, AgRP and leptin in lean and obese men and their relationship to differing states of energy balance perturbation. Clin Endocrinol (Oxf) 61: 31–39, 2004. doi: 10.1111/j.1365-2265.2004.02056.x. [DOI] [PubMed] [Google Scholar]
- 173.Hughes J, Smith TW, Kosterlitz HW, Fothergill LA, Morgan BA, Morris HR. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature 258: 577–579, 1975. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- 174.Humphreys MH. Gamma-MSH, sodium metabolism, and salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 286: R417–R430, 2004. doi: 10.1152/ajpregu.00365.2003. [DOI] [PubMed] [Google Scholar]
- 175.Hunt G, Todd C, Cresswell JE, Thody AJ. Alpha-melanocyte stimulating hormone and its analogue Nle4DPhe7 alpha-MSH affect morphology, tyrosinase activity and melanogenesis in cultured human melanocytes. J Cell Sci 107: 205–211, 1994. [DOI] [PubMed] [Google Scholar]
- 176.Iino K, Oki Y, Yamashita M, Matsushita F, Hayashi C, Yogo K, Nishizawa S, Yamada S, Maekawa M, Sasano H, Nakamura H. Possible relevance between prohormone convertase 2 expression and tumor growth in human adrenocorticotropin-producing pituitary adenoma. J Clin Endocrinol Metab 95: 4003–4011, 2010. doi: 10.1210/jc.2009-2716. [DOI] [PubMed] [Google Scholar]
- 177.Ingle DJ, Kendall EC. Atrophy of the Adrenal Cortex of the Rat Produced by the Administration of Large Amounts of Cortin. Science 86: 245, 1937. doi: 10.1126/science.86.2228.245. [DOI] [PubMed] [Google Scholar]
- 178.Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J 19: 1332–1334, 2005. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- 179.Jackson RS, Creemers JW, Farooqi IS, Raffin-Sanson ML, Varro A, Dockray GJ, Holst JJ, Brubaker PL, Corvol P, Polonsky KS, Ostrega D, Becker KL, Bertagna X, Hutton JC, White A, Dattani MT, Hussain K, Middleton SJ, Nicole TM, Milla PJ, Lindley KJ, O’Rahilly S. Small-intestinal dysfunction accompanies the complex endocrinopathy of human proprotein convertase 1 deficiency. J Clin Invest 112: 1550–1560, 2003. doi: 10.1172/JCI200318784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jackson RS, Creemers JW, Ohagi S, Raffin-Sanson ML, Sanders L, Montague CT, Hutton JC, O’Rahilly S. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet 16: 303–306, 1997. doi: 10.1038/ng0797-303. [DOI] [PubMed] [Google Scholar]
- 181.Jackson S, Salacinski P, Hope J, Lowry PJ. An investigation of N-terminal pro-opiocortin peptides in the rat pituitary. Peptides 4: 431–438, 1983. doi: 10.1016/0196-9781(83)90045-1. [DOI] [PubMed] [Google Scholar]
- 182.Jégou S, Tranchand-Bunel D, Delbende C, Blasquez C, Vaudry H. Characterization of alpha-MSH-related peptides released from rat hypothalamic neurons in vitro. Brain Res Mol Brain Res 5: 219–226, 1989. doi: 10.1016/0169-328X(89)90038-7. [DOI] [PubMed] [Google Scholar]
- 183.Jingami H, Nakanishi S, Imura H, Numa S. Tissue distribution of messenger RNAs coding for opioid peptide precursors and related RNA. Eur J Biochem 142: 441–447, 1984. doi: 10.1111/j.1432-1033.1984.tb08306.x. [DOI] [PubMed] [Google Scholar]
- 184.Joseph CG, Yao H, Scott JW, Sorensen NB, Marnane RN, Mountjoy KG, Haskell-Luevano C. γ2-Melanocyte stimulation hormone (γ2-MSH) truncation studies results in the cautionary note that γ2-MSH is not selective for the mouse MC3R over the mouse MC5R. Peptides 31: 2304–2313, 2010. doi: 10.1016/j.peptides.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jutras I, Seidah NG, Reudelhuber TL, Brechler V. Two activation states of the prohormone convertase PC1 in the secretory pathway. J Biol Chem 272: 15184–15188, 1997. doi: 10.1074/jbc.272.24.15184. [DOI] [PubMed] [Google Scholar]
- 186.Kadekaro AL, Kanto H, Kavanagh R, Abdel-Malek Z. Significance of the melanocortin 1 receptor in regulating human melanocyte pigmentation, proliferation, and survival. Ann N Y Acad Sci 994: 359–365, 2003. doi: 10.1111/j.1749-6632.2003.tb03200.x. [DOI] [PubMed] [Google Scholar]
- 187.Karpac J, Kern A, Hochgeschwender U. Pro-opiomelanocortin peptides and the adrenal gland. Mol Cell Endocrinol 265-266: 29–33, 2007. doi: 10.1016/j.mce.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 188.Karpac J, Ostwald D, Bui S, Hunnewell P, Shankar M, Hochgeschwender U. Development, maintenance, and function of the adrenal gland in early postnatal proopiomelanocortin-null mutant mice. Endocrinology 146: 2555–2562, 2005. doi: 10.1210/en.2004-1290. [DOI] [PubMed] [Google Scholar]
- 189.Kask A, Rägo L, Wikberg JE, Schiöth HB. Differential effects of melanocortin peptides on ingestive behaviour in rats: evidence against the involvement of MC(3) receptor in the regulation of food intake. Neurosci Lett 283: 1–4, 2000. doi: 10.1016/S0304-3940(00)00837-5. [DOI] [PubMed] [Google Scholar]
- 190.Katsuki A, Sumida Y, Murashima S, Furuta M, Araki-Sasaki R, Tsuchihashi K, Hori Y, Yano Y, Adachi Y. Elevated plasma levels of alpha-melanocyte stimulating hormone (alpha-MSH) are correlated with insulin resistance in obese men. Int J Obes Relat Metab Disord 24: 1260–1264, 2000. doi: 10.1038/sj.ijo.0801400. [DOI] [PubMed] [Google Scholar]
- 191.Kauser S, Schallreuter KU, Thody AJ, Gummer C, Tobin DJ. Regulation of human epidermal melanocyte biology by beta-endorphin. J Invest Dermatol 120: 1073–1080, 2003. doi: 10.1046/j.1523-1747.2003.12242.x. [DOI] [PubMed] [Google Scholar]
- 192.Kauser S, Slominski A, Wei ET, Tobin DJ. Modulation of the human hair follicle pigmentary unit by corticotropin-releasing hormone and urocortin peptides. FASEB J 20: 882–895, 2006. doi: 10.1096/fj.05-5257com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kauser S, Thody AJ, Schallreuter KU, Gummer CL, Tobin DJ. beta-Endorphin as a regulator of human hair follicle melanocyte biology. J Invest Dermatol 123: 184–195, 2004. doi: 10.1111/j.0022-202X.2004.22724.x. [DOI] [PubMed] [Google Scholar]
- 194.Kauser S, Thody AJ, Schallreuter KU, Gummer CL, Tobin DJ. A fully functional proopiomelanocortin/melanocortin-1 receptor system regulates the differentiation of human scalp hair follicle melanocytes. Endocrinology 146: 532–543, 2005. doi: 10.1210/en.2004-1145. [DOI] [PubMed] [Google Scholar]
- 195.Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev 5: 1–24, 1984. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- 196.Kelso TB, Herbert WG, Gwazdauskas FC, Goss FL, Hess JL. Exercise-thermoregulatory stress and increased plasma beta-endorphin/beta-lipotropin in humans. J Appl Physiol Respir Environ Exerc Physiol 57: 444–449, 1984. doi: 10.1152/jappl.1984.57.2.444. [DOI] [PubMed] [Google Scholar]
- 197.Kempná P, Flück CE. Adrenal gland development and defects. Best Pract Res Clin Endocrinol Metab 22: 77–93, 2008. doi: 10.1016/j.beem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 198.Kesterson RA. The Melanocortin-3 Receptor. In: The Melanocortin Receptors, edited by Cone RD. Totowa, NJ: Humana, 2000, p. 385–404. [Google Scholar]
- 199.Kim T, Tao-Cheng JH, Eiden LE, Loh YP. Chromogranin A, an “on/off” switch controlling dense-core secretory granule biogenesis. Cell 106: 499–509, 2001. doi: 10.1016/S0092-8674(01)00459-7. [DOI] [PubMed] [Google Scholar]
- 200.Kimura S, Lewis RV, Gerber LD, Brink L, Rubinstein M, Stein S, Udenfriend S. Purification to homogeneity of camel pituitary pro-opiocortin, the common precursor of opioid peptides and corticotropin. Proc Natl Acad Sci USA 76: 1756–1759, 1979. doi: 10.1073/pnas.76.4.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kishida M, Baker BI, Bird DJ. Localisation and identification of melanocyte-stimulating hormones in the fish brain. Gen Comp Endocrinol 71: 229–242, 1988. doi: 10.1016/0016-6480(88)90251-1. [DOI] [PubMed] [Google Scholar]
- 202.Kitabchi AE. The biological and immunological properties of pork and beef insulin, proinsulin, and connecting peptides. J Clin Invest 49: 979–987, 1970. doi: 10.1172/JCI106317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Koch M, Varela L, Kim JG, Kim JD, Hernández-Nuño F, Simonds SE, Castorena CM, Vianna CR, Elmquist JK, Morozov YM, Rakic P, Bechmann I, Cowley MA, Szigeti-Buck K, Dietrich MO, Gao XB, Diano S, Horvath TL. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature 519: 45–50, 2015. doi: 10.1038/nature14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kovalovsky D, Refojo D, Liberman AC, Hochbaum D, Pereda MP, Coso OA, Stalla GK, Holsboer F, Arzt E. Activation and induction of NUR77/NURR1 in corticotrophs by CRH/cAMP: involvement of calcium, protein kinase A, and MAPK pathways. Mol Endocrinol 16: 1638–1651, 2002. doi: 10.1210/mend.16.7.0863. [DOI] [PubMed] [Google Scholar]
- 205.Kreis TE, Matteoni R, Hollinshead M, Tooze J. Secretory granules and endosomes show saltatory movement biased to the anterograde and retrograde directions, respectively, along microtubules in AtT20 cells. Eur J Cell Biol 49: 128–139, 1989. [PubMed] [Google Scholar]
- 206.Krieger DT, Choi HS, Anderson PJ. The characterization of the ACTH produced by a primary pituitary tumour in a patient with Cushing’s disease. Clin Endocrinol (Oxf) 5: 455–472, 1976. doi: 10.1111/j.1365-2265.1976.tb01975.x. [DOI] [PubMed] [Google Scholar]
- 207.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet 19: 155–157, 1998. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 208.Krude H, Biebermann H, Schnabel D, Tansek MZ, Theunissen P, Mullis PE, Grüters A. Obesity due to proopiomelanocortin deficiency: three new cases and treatment trials with thyroid hormone and ACTH4-10. J Clin Endocrinol Metab 88: 4633–4640, 2003. doi: 10.1210/jc.2003-030502. [DOI] [PubMed] [Google Scholar]
- 209.Kuliawat R, Arvan P. Protein targeting via the “constitutive-like” secretory pathway in isolated pancreatic islets: passive sorting in the immature granule compartment. J Cell Biol 118: 521–529, 1992. doi: 10.1083/jcb.118.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kumar D, Mains RE, Eipper BA. 60 YEARS OF POMC: From POMC and α-MSH to PAM, molecular oxygen, copper, and vitamin C. J Mol Endocrinol 56: T63–T76, 2016. doi: 10.1530/JME-15-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lacaze-Masmonteil T, de Keyzer Y, Luton JP, Kahn A, Bertagna X. Characterization of proopiomelanocortin transcripts in human nonpituitary tissues. Proc Natl Acad Sci USA 84: 7261–7265, 1987. doi: 10.1073/pnas.84.20.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lam DD, Attard CA, Mercer AJ, Myers MG Jr, Rubinstein M, Low MJ. Conditional expression of Pomc in the Lepr-positive subpopulation of POMC neurons is sufficient for normal energy homeostasis and metabolism. Endocrinology 156: 1292–1302, 2015. doi: 10.1210/en.2014-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Laurent V, Jaubert-Miazza L, Desjardins R, Day R, Lindberg I. Biosynthesis of proopiomelanocortin-derived peptides in prohormone convertase 2 and 7B2 null mice. Endocrinology 145: 519–528, 2004. doi: 10.1210/en.2003-0829. [DOI] [PubMed] [Google Scholar]
- 214.Laurent V, Kimble A, Peng B, Zhu P, Pintar JE, Steiner DF, Lindberg I. Mortality in 7B2 null mice can be rescued by adrenalectomy: involvement of dopamine in ACTH hypersecretion. Proc Natl Acad Sci USA 99: 3087–3092, 2002. doi: 10.1073/pnas.261715099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lee SN, Prodhomme E, Lindberg I. Prohormone convertase 1 (PC1) processing and sorting: effect of PC1 propeptide and proSAAS. J Endocrinol 182: 353–364, 2004. doi: 10.1677/joe.0.1820353. [DOI] [PubMed] [Google Scholar]
- 216.Lee YS, Challis BG, Thompson DA, Yeo GS, Keogh JM, Madonna ME, Wraight V, Sims M, Vatin V, Meyre D, Shield J, Burren C, Ibrahim Z, Cheetham T, Swift P, Blackwood A, Hung CC, Wareham NJ, Froguel P, Millhauser GL, O’Rahilly S, Farooqi IS. A POMC variant implicates beta-melanocyte-stimulating hormone in the control of human energy balance. Cell Metab 3: 135–140, 2006. doi: 10.1016/j.cmet.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 217.Leibel RL. The molecular genetics of the melanocortin pathway and energy homeostasis. Cell Metab 3: 79–81, 2006. doi: 10.1016/j.cmet.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 218.Lerner AB, Shizume K, Bunding I. The mechanism of endocrine control of melanin pigmentation. J Clin Endocrinol Metab 14: 1463–1490, 1954. doi: 10.1210/jcem-14-12-1463. [DOI] [PubMed] [Google Scholar]
- 219.Levin N, Blum M, Roberts JL. Modulation of basal and corticotropin-releasing factor-stimulated proopiomelanocortin gene expression by vasopressin in rat anterior pituitary. Endocrinology 125: 2957–2966, 1989. doi: 10.1210/endo-125-6-2957. [DOI] [PubMed] [Google Scholar]
- 220.Lim J, Berezniuk I, Che FY, Parikh R, Biswas R, Pan H, Fricker LD. Altered neuropeptide processing in prefrontal cortex of Cpe (fat/fat) mice: implications for neuropeptide discovery. J Neurochem 96: 1169–1181, 2006. doi: 10.1111/j.1471-4159.2005.03614.x. [DOI] [PubMed] [Google Scholar]
- 221.Lloyd DJ, Bohan S, Gekakis N. Obesity, hyperphagia and increased metabolic efficiency in Pc1 mutant mice. Hum Mol Genet 15: 1884–1893, 2006. doi: 10.1093/hmg/ddl111. [DOI] [PubMed] [Google Scholar]
- 222.Loh YP. Kinetic studies on the processing of human beta-lipotropin by bovine pituitary intermediate lobe pro-opiomelanocortin-converting enzyme. J Biol Chem 261: 11949–11955, 1986. [PubMed] [Google Scholar]
- 223.Loh YP. Molecular mechanisms of beta-endorphin biosynthesis. Biochem Pharmacol 44: 843–849, 1992. doi: 10.1016/0006-2952(92)90114-X. [DOI] [PubMed] [Google Scholar]
- 224.Loh YP, Parish DC, Tuteja R. Purification and characterization of a paired basic residue-specific pro-opiomelanocortin converting enzyme from bovine pituitary intermediate lobe secretory vesicles. J Biol Chem 260: 7194–7205, 1985. [PubMed] [Google Scholar]
- 225.Loh YP, Tam WW, Russell JT. Measurement of delta pH and membrane potential in secretory vesicles isolated from bovine pituitary intermediate lobe. J Biol Chem 259: 8238–8245, 1984. [PubMed] [Google Scholar]
- 226.Lotfi CF, de Mendonca PO. Comparative Effect of ACTH and Related Peptides on Proliferation and Growth of Rat Adrenal Gland. Front Endocrinol (Lausanne) 7: 39, 2016. doi: 10.3389/fendo.2016.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Low MJ, Hayward MD, Appleyard SM, Rubinstein M. State-dependent modulation of feeding behavior by proopiomelanocortin-derived beta-endorphin. Ann N Y Acad Sci 994: 192–201, 2003. doi: 10.1111/j.1749-6632.2003.tb03180.x. [DOI] [PubMed] [Google Scholar]
- 228.Lowry P. 60 YEARS OF POMC: Purification and biological characterisation of melanotrophins and corticotrophins. J Mol Endocrinol 56: T1–T12, 2016. doi: 10.1530/JME-15-0260. [DOI] [PubMed] [Google Scholar]
- 229.Lowry PJ, Silas L, McLean C, Linton EA, Estivariz FE. Pro-gamma-melanocyte-stimulating hormone cleavage in adrenal gland undergoing compensatory growth. Nature 306: 70–73, 1983. doi: 10.1038/306070a0. [DOI] [PubMed] [Google Scholar]
- 230.Lowry PJ, Silman RE, Hope J, Scott AP. Structure and biosynthesis of peptides related to corticotropins and beta-melanotropins. Ann N Y Acad Sci 297: 49–62, 1977. doi: 10.1111/j.1749-6632.1977.tb41845.x. [DOI] [PubMed] [Google Scholar]
- 231.Lundblad JR, Roberts JL. Regulation of proopiomelanocortin gene expression in pituitary. Endocr Rev 9: 135–158, 1988. doi: 10.1210/edrv-9-1-135. [DOI] [PubMed] [Google Scholar]
- 232.Mains RE, Eipper BA. Biosynthesis of adrenocorticotropic hormone in mouse pituitary tumor cells. J Biol Chem 251: 4115–4120, 1976. [PubMed] [Google Scholar]
- 233.Mains RE, Eipper BA. Comparison of rat anterior and intermediate pituitary in tissue culture: corticotropin (ACTH) and beta-endorphin. Ciba Found Symp 81: 32–54, 1981. [DOI] [PubMed] [Google Scholar]
- 234.Mains RE, Eipper BA. Coordinate synthesis of corticotropins and endorphins by mouse pituitary tumor cells. J Biol Chem 253: 651–655, 1978. [PubMed] [Google Scholar]
- 235.Mains RE, Eipper BA, Ling N. Common precursor to corticotropins and endorphins. Proc Natl Acad Sci USA 74: 3014–3018, 1977. doi: 10.1073/pnas.74.7.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Maira M, Couture C, Le Martelot G, Pulichino AM, Bilodeau S, Drouin J. The T-box factor Tpit recruits SRC/p160 co-activators and mediates hormone action. J Biol Chem 278: 46523–46532, 2003. doi: 10.1074/jbc.M305626200. [DOI] [PubMed] [Google Scholar]
- 237.Maji SK, Perrin MH, Sawaya MR, Jessberger S, Vadodaria K, Rissman RA, Singru PS, Nilsson KP, Simon R, Schubert D, Eisenberg D, Rivier J, Sawchenko P, Vale W, Riek R. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science 325: 328–332, 2009. doi: 10.1126/science.1173155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Malide D, Seidah NG, Chrétien M, Bendayan M. Electron microscopic immunocytochemical evidence for the involvement of the convertases PC1 and PC2 in the processing of proinsulin in pancreatic beta-cells. J Histochem Cytochem 43: 11–19, 1995. doi: 10.1177/43.1.7822759. [DOI] [PubMed] [Google Scholar]
- 239.Martín MG, Lindberg I, Solorzano-Vargas RS, Wang J, Avitzur Y, Bandsma R, Sokollik C, Lawrence S, Pickett LA, Chen Z, Egritas O, Dalgic B, Albornoz V, de Ridder L, Hulst J, Gok F, Aydoğan A, Al-Hussaini A, Gok DE, Yourshaw M, Wu SV, Cortina G, Stanford S, Georgia S. Congenital proprotein convertase 1/3 deficiency causes malabsorptive diarrhea and other endocrinopathies in a pediatric cohort. Gastroenterology 145: 138–148, 2013. doi: 10.1053/j.gastro.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Matsuoka H, Mulrow PJ, Li CH. Beta-lipotropin: a new aldosterone-stimulating factor. Science 209: 307–308, 1980. doi: 10.1126/science.6247763. [DOI] [PubMed] [Google Scholar]
- 241.Mattos GE, Jacysyn JF, Amarante-Mendes GP, Lotfi CF. Comparative effect of FGF2, synthetic peptides 1-28 N-POMC and ACTH on proliferation in rat adrenal cell primary cultures. Cell Tissue Res 345: 343–356, 2011. doi: 10.1007/s00441-011-1220-8. [DOI] [PubMed] [Google Scholar]
- 242.Mazurkiewicz JE, Corliss D, Slominski A. Spatiotemporal expression, distribution, and processing of POMC and POMC-derived peptides in murine skin. J Histochem Cytochem 48: 905–914, 2000. doi: 10.1177/002215540004800703. [DOI] [PubMed] [Google Scholar]
- 243.Mbikay M, Croissandeau G, Sirois F, Anini Y, Mayne J, Seidah NG, Chrétien M. A targeted deletion/insertion in the mouse Pcsk1 locus is associated with homozygous embryo preimplantation lethality, mutant allele preferential transmission and heterozygous female susceptibility to dietary fat. Dev Biol 306: 584–598, 2007. doi: 10.1016/j.ydbio.2007.03.523. [DOI] [PubMed] [Google Scholar]
- 244.Mbikay M, Seidah NG, Chrétien M. Neuroendocrine secretory protein 7B2: structure, expression and functions. Biochem J 357: 329–342, 2001. doi: 10.1042/bj3570329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.McGuire J. Melanin granule dispersion in epidermal melanocytes. J Invest Dermatol 45: 547–548, 1965. doi: 10.1038/jid.1965.171. [DOI] [PubMed] [Google Scholar]
- 246.McLean C, Hope J, Salacinski P, Estivariz F, Lowry PJ. Purification and characterization of amino-terminal pro-opiocortin peptides from human pituitary glands. Biosci Rep 1: 843–849, 1981. doi: 10.1007/BF01114817. [DOI] [PubMed] [Google Scholar]
- 247.McMinn JE, Wilkinson CW, Havel PJ, Woods SC, Schwartz MW. Effect of intracerebroventricular alpha-MSH on food intake, adiposity, c-Fos induction, and neuropeptide expression. Am J Physiol Regul Integr Comp Physiol 279: R695–R703, 2000. doi: 10.1152/ajpregu.2000.279.2.R695. [DOI] [PubMed] [Google Scholar]
- 248.Mendiratta MS, Yang Y, Balazs AE, Willis AS, Eng CM, Karaviti LP, Potocki L. Early onset obesity and adrenal insufficiency associated with a homozygous POMC mutation. Int J Pediatr Endocrinol 2011: 5, 2011. doi: 10.1186/1687-9856-2011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Milgram SL, Mains RE. Differential effects of temperature blockade on the proteolytic processing of three secretory granule-associated proteins. J Cell Sci 107: 737–745, 1994. [DOI] [PubMed] [Google Scholar]
- 250.Miller WL. Molecular biology of steroid hormone synthesis. Endocr Rev 9: 295–318, 1988. doi: 10.1210/edrv-9-3-295. [DOI] [PubMed] [Google Scholar]
- 251.Millington GW, Tung YC, Hewson AK, O’Rahilly S, Dickson SL. Differential effects of alpha-, beta- and gamma(2)-melanocyte-stimulating hormones on hypothalamic neuronal activation and feeding in the fasted rat. Neuroscience 108: 437–445, 2001. doi: 10.1016/S0306-4522(01)00428-6. [DOI] [PubMed] [Google Scholar]
- 252.Millington WR, O’Donohue TL, Chappell MC, Roberts JL, Mueller GP. Coordinate regulation of peptide acetyltransferase activity and proopiomelanocortin gene expression in the intermediate lobe of the rat pituitary. Endocrinology 118: 2024–2033, 1986. doi: 10.1210/endo-118-5-2024. [DOI] [PubMed] [Google Scholar]
- 253.Millington WR, Rosenthal DW, Unal CB, Nyquist-Battie C. Localization of pro-opiomelanocortin mRNA transcripts and peptide immunoreactivity in rat heart. Cardiovasc Res 43: 107–116, 1999. doi: 10.1016/S0008-6363(99)00076-0. [DOI] [PubMed] [Google Scholar]
- 254.Miraglia del Giudice E, Cirillo G, Santoro N, D’Urso L, Carbone MT, Di Toro R, Perrone L. Molecular screening of the proopiomelanocortin (POMC) gene in Italian obese children: report of three new mutations. Int J Obes Relat Metab Disord 25: 61–67, 2001. doi: 10.1038/sj.ijo.0801485. [DOI] [PubMed] [Google Scholar]
- 255.Monaghan PJ, Kyriacou A, Sturgeon C, Davies A, Trainer PJ, White A, Higham CE. Proopiomelanocortin interference in the measurement of adrenocorticotrophic hormone: a United Kingdom National External Quality Assessment Service study. Clin Endocrinol (Oxf) 85: 569–574, 2016. doi: 10.1111/cen.13118. [DOI] [PubMed] [Google Scholar]
- 256.Moore HP, Gumbiner B, Kelly RB. Chloroquine diverts ACTH from a regulated to a constitutive secretory pathway in AtT-20 cells. Nature 302: 434–436, 1983. doi: 10.1038/302434a0. [DOI] [PubMed] [Google Scholar]
- 257.Moore HP, Walker MD, Lee F, Kelly RB. Expressing a human proinsulin cDNA in a mouse ACTH-secreting cell. Intracellular storage, proteolytic processing, and secretion on stimulation. Cell 35: 531–538, 1983. doi: 10.1016/0092-8674(83)90187-3. [DOI] [PubMed] [Google Scholar]
- 258.Mountjoy KG. Functions for pro-opiomelanocortin-derived peptides in obesity and diabetes. Biochem J 428: 305–324, 2010. doi: 10.1042/BJ20091957. [DOI] [PubMed] [Google Scholar]
- 259.Mountjoy KG, Caron A, Hubbard K, Shome A, Grey AC, Sun B, Bould S, Middleditch M, Pontré B, McGregor A, Harris PWR, Kowalczyk R, Brimble MA, Botha R, Tan KML, Piper SJ, Buchanan C, Lee S, Coll AP, Elmquist JK. Desacetyl-α-melanocyte stimulating hormone and α-melanocyte stimulating hormone are required to regulate energy balance. Mol Metab 9: 207–216, 2018. doi: 10.1016/j.molmet.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol 8: 1298–1308, 1994. [DOI] [PubMed] [Google Scholar]
- 261.Mountjoy KG, Wu CS, Cornish J, Callon KE. Alpha-MSH and desacetyl-alpha-MSH signaling through melanocortin receptors. Ann N Y Acad Sci 994: 58–65, 2003. doi: 10.1111/j.1749-6632.2003.tb03162.x. [DOI] [PubMed] [Google Scholar]
- 262.Mynard V, Guignat L, Devin-Leclerc J, Bertagna X, Catelli MG. Different mechanisms for leukemia inhibitory factor-dependent activation of two proopiomelanocortin promoter regions. Endocrinology 143: 3916–3924, 2002. doi: 10.1210/en.2002-220323. [DOI] [PubMed] [Google Scholar]
- 263.Naggert JK, Fricker LD, Varlamov O, Nishina PM, Rouille Y, Steiner DF, Carroll RJ, Paigen BJ, Leiter EH. Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nat Genet 10: 135–142, 1995. doi: 10.1038/ng0695-135. [DOI] [PubMed] [Google Scholar]
- 264.Nakamura M, Inoue A, Nakanishi S, Numa S. Partial amino-terminal sequence of cell-free translation product encoded by bovine corticotropin-beta-lipotropin precursor messenger RNA. FEBS Lett 105: 357–359, 1979. doi: 10.1016/0014-5793(79)80648-1. [DOI] [PubMed] [Google Scholar]
- 265.Nakanishi S, Inoue A, Kita T, Inoue A, Nakamura M, Chang AC, Cohen SN, Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature 278: 423–427, 1979. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- 266.Navarro S, Soletto L, Puchol S, Rotllant J, Soengas JL, Cerdá-Reverter JM. 60 YEARS OF POMC: POMC: an evolutionary perspective. J Mol Endocrinol 56: T113–T118, 2016. doi: 10.1530/JME-15-0288. [DOI] [PubMed] [Google Scholar]
- 267.Newman CB, Wardlaw SL, Frantz AG. Suppression of basal and stress-induced prolactin release and stimulation of luteinizing hormone secretion by alpha-melanocyte-stimulating hormone. Life Sci 36: 1661–1668, 1985. doi: 10.1016/0024-3205(85)90369-8. [DOI] [PubMed] [Google Scholar]
- 268.Ni XP, Pearce D, Butler AA, Cone RD, Humphreys MH. Genetic disruption of gamma-melanocyte-stimulating hormone signaling leads to salt-sensitive hypertension in the mouse. J Clin Invest 111: 1251–1258, 2003. doi: 10.1172/JCI200316993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Nicolas P, Li CH. Beta-endorphin-(1-27) is a naturally occurring antagonist to etorphine-induced analgesia. Proc Natl Acad Sci USA 82: 3178–3181, 1985. doi: 10.1073/pnas.82.10.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Normant E, Loh YP. Depletion of carboxypeptidase E, a regulated secretory pathway sorting receptor, causes misrouting and constitutive secretion of proinsulin and proenkephalin, but not chromogranin A. Endocrinology 139: 2137–2145, 1998. doi: 10.1210/endo.139.4.5951. [DOI] [PubMed] [Google Scholar]
- 271.Nussdorfer GG, Malendowicz LK. Role of VIP, PACAP, and related peptides in the regulation of the hypothalamo-pituitary-adrenal axis. Peptides 19: 1443–1467, 1998. doi: 10.1016/S0196-9781(98)00102-8. [DOI] [PubMed] [Google Scholar]
- 272.O’Donohue TL, Handelmann GE, Chaconas T, Miller RL, Jacobowitz DM. Evidence that N-acetylation regulates the behavioral activity of alpha-MSH in the rat and human central nervous system. Peptides 2: 333–344, 1981. doi: 10.1016/S0196-9781(81)80126-X. [DOI] [PubMed] [Google Scholar]
- 273.O’Donohye TL, Handelmann GE, Miller RL, Jacobowitz DM. N-acetylation regulates the behavioral activity of alpha-melanotropin in a multineurotransmitter neuron. Science 215: 1125–1127, 1982. doi: 10.1126/science.7063845. [DOI] [PubMed] [Google Scholar]
- 274.O’Rahilly S, Gray H, Humphreys PJ, Krook A, Polonsky KS, White A, Gibson S, Taylor K, Carr C. Brief report: impaired processing of prohormones associated with abnormalities of glucose homeostasis and adrenal function. N Engl J Med 333: 1386–1391, 1995. doi: 10.1056/NEJM199511233332104. [DOI] [PubMed] [Google Scholar]
- 275.Oates EL, Allaway GP, Prabhakar BS. A potential for mutual regulation of proopiomelanocortin gene and Epstein-Barr virus expression in human lymphocytes. Ann N Y Acad Sci 594: 60–65, 1990. doi: 10.1111/j.1749-6632.1990.tb40467.x. [DOI] [PubMed] [Google Scholar]
- 276.Odell WD. Ectopic ACTH secretion. A misnomer. Endocrinol Metab Clin North Am 20: 371–379, 1991. [PubMed] [Google Scholar]
- 277.Oldfield EH, Doppman JL, Nieman LK, Chrousos GP, Miller DL, Katz DA, Cutler GB Jr, Loriaux DL. Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing’s syndrome. N Engl J Med 325: 897–905, 1991. doi: 10.1056/NEJM199109263251301. [DOI] [PubMed] [Google Scholar]
- 278.Oliver RL, Davis JR, White A. Characterisation of ACTH related peptides in ectopic Cushing’s syndrome. Pituitary 6: 119–126, 2003. doi: 10.1023/B:PITU.0000011172.26649.df. [DOI] [PubMed] [Google Scholar]
- 279.Orci L, Ravazzola M, Amherdt M, Perrelet A, Powell SK, Quinn DL, Moore HP. The trans-most cisternae of the Golgi complex: a compartment for sorting of secretory and plasma membrane proteins. Cell 51: 1039–1051, 1987. doi: 10.1016/0092-8674(87)90590-3. [DOI] [PubMed] [Google Scholar]
- 280.Orth D, Nicholson W, Shapiro M, Byyny R. Adrenocorticotropic hormone (ACTH) and malanocyte-stimulating hormone (MSH) production by a single cell. Program Endocrine Society 52nd Meeting St. Louis, MO, 1970. p. 140. [Google Scholar]
- 281.Orth DN, Nicholson WE, Mitchell WM, Island DP, Shapiro M, Byyny RL. ACTH and MSH production by a single cloned mouse pituitary tumor cell line. Endocrinology 92: 385–393, 1973. doi: 10.1210/endo-92-2-385. [DOI] [PubMed] [Google Scholar]
- 282.Osterlund CD, Rodriguez-Santiago M, Woodruff ER, Newsom RJ, Chadayammuri AP, Spencer RL. Glucocorticoid Fast Feedback Inhibition of Stress-Induced ACTH Secretion in the Male Rat: Rate Independence and Stress-State Resistance. Endocrinology 157: 2785–2798, 2016. doi: 10.1210/en.2016-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Padilla SL, Carmody JS, Zeltser LM. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat Med 16: 403–405, 2010. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Padilla SL, Reef D, Zeltser LM. Defining POMC neurons using transgenic reagents: impact of transient Pomc expression in diverse immature neuronal populations. Endocrinology 153: 1219–1231, 2012. doi: 10.1210/en.2011-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Page-Wilson G, Freda PU, Jacobs TP, Khandji AG, Bruce JN, Foo ST, Meece K, White A, Wardlaw SL. Clinical utility of plasma POMC and AgRP measurements in the differential diagnosis of ACTH-dependent Cushing’s syndrome. J Clin Endocrinol Metab 99: E1838–E1845, 2014. doi: 10.1210/jc.2014-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Page-Wilson G, Meece K, White A, Rosenbaum M, Leibel RL, Smiley R, Wardlaw SL. Proopiomelanocortin, agouti-related protein, and leptin in human cerebrospinal fluid: correlations with body weight and adiposity. Am J Physiol Endocrinol Metab 309: E458–E465, 2015. doi: 10.1152/ajpendo.00206.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Page-Wilson G, Reitman-Ivashkov E, Meece K, White A, Rosenbaum M, Smiley RM, Wardlaw SL. Cerebrospinal fluid levels of leptin, proopiomelanocortin, and agouti-related protein in human pregnancy: evidence for leptin resistance. J Clin Endocrinol Metab 98: 264–271, 2013. doi: 10.1210/jc.2012-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev 27: 73–100, 2006. doi: 10.1210/er.2005-0009. [DOI] [PubMed] [Google Scholar]
- 289.Pagotto U, Marsicano G, Fezza F, Theodoropoulou M, Grübler Y, Stalla J, Arzberger T, Milone A, Losa M, Di Marzo V, Lutz B, Stalla GK. Normal human pituitary gland and pituitary adenomas express cannabinoid receptor type 1 and synthesize endogenous cannabinoids: first evidence for a direct role of cannabinoids on hormone modulation at the human pituitary level. J Clin Endocrinol Metab 86: 2687–2696, 2001. doi: 10.1210/jcem.86.6.7565. [DOI] [PubMed] [Google Scholar]
- 290.Pan H, Che FY, Peng B, Steiner DF, Pintar JE, Fricker LD. The role of prohormone convertase-2 in hypothalamic neuropeptide processing: a quantitative neuropeptidomic study. J Neurochem 98: 1763–1777, 2006. doi: 10.1111/j.1471-4159.2006.04067.x. [DOI] [PubMed] [Google Scholar]
- 291.Pan H, Nanno D, Che FY, Zhu X, Salton SR, Steiner DF, Fricker LD, Devi LA. Neuropeptide processing profile in mice lacking prohormone convertase-1. Biochemistry 44: 4939–4948, 2005. doi: 10.1021/bi047852m. [DOI] [PubMed] [Google Scholar]
- 292.Pan YX, Xu J, Bolan E, Abbadie C, Chang A, Zuckerman A, Rossi G, Pasternak GW. Identification and characterization of three new alternatively spliced mu-opioid receptor isoforms. Mol Pharmacol 56: 396–403, 1999. doi: 10.1124/mol.56.2.396. [DOI] [PubMed] [Google Scholar]
- 293.Pan YX, Xu J, Mahurter L, Bolan E, Xu M, Pasternak GW. Generation of the mu opioid receptor (MOR-1) protein by three new splice variants of the Oprm gene. Proc Natl Acad Sci USA 98: 14084–14089, 2001. doi: 10.1073/pnas.241296098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Panskepp J, Reilly P, Bishop P, Meeker RB, Vilberg TR, Kastin AJ. Effects of alpha-MSH on motivation, vigilance and brain respiration. Pharmacol Biochem Behav 5, Suppl 1: 59–64, 1976. doi: 10.1016/0091-3057(76)90329-4. [DOI] [PubMed] [Google Scholar]
- 295.Park JJ, Cawley NX, Loh YP. Carboxypeptidase E cytoplasmic tail-driven vesicle transport is key for activity-dependent secretion of peptide hormones. Mol Endocrinol 22: 989–1005, 2008. doi: 10.1210/me.2007-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Parker CR Jr, Barnea A, Tilders FJ, Porter JC. Characterization of immunoreactive alpha-melanocyte stimulating hormone (alpha-MSHi) in human brain tissue. Brain Res Bull 6: 275–280, 1981. doi: 10.1016/S0361-9230(81)80058-5. [DOI] [PubMed] [Google Scholar]
- 297.Parker LN, Odell WD. Evidence for existence of cortical androgen-stimulating hormone. Am J Physiol Endocrinol Metab 236: E616–E620, 1979. [DOI] [PubMed] [Google Scholar]
- 298.Pasternak GW, Pan YX. Mu opioids and their receptors: evolution of a concept. Pharmacol Rev 65: 1257–1317, 2013. doi: 10.1124/pr.112.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology 145: 5431–5438, 2004. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- 300.Peinado JR, Laurent V, Lee SN, Peng BW, Pintar JE, Steiner DF, Lindberg I. Strain-dependent influences on the hypothalamo-pituitary-adrenal axis profoundly affect the 7B2 and PC2 null phenotypes. Endocrinology 146: 3438–3444, 2005. doi: 10.1210/en.2004-1289. [DOI] [PubMed] [Google Scholar]
- 301.Peng J, Sarkar S, Chang SL. Opioid receptor expression in human brain and peripheral tissues using absolute quantitative real-time RT-PCR. Drug Alcohol Depend 124: 223–228, 2012. doi: 10.1016/j.drugalcdep.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Penhoat A, Sanchez P, Jaillard C, Langlois D, Bégeot M, Saez JM. Human proopiomelanocortin-(79-96), a proposed cortical androgen-stimulating hormone, does not affect steroidogenesis in cultured human adult adrenal cells. J Clin Endocrinol Metab 72: 23–26, 1991. doi: 10.1210/jcem-72-1-23. [DOI] [PubMed] [Google Scholar]
- 303.Pepper DJ, Bicknell AB. The stimulation of mitogenic signaling pathways by N-POMC peptides. Mol Cell Endocrinol 300: 77–82, 2009. doi: 10.1016/j.mce.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Perroud B, Alvarado RJ, Espinal GM, Morado AR, Phinney BS, Warden CH. In vivo multiplex quantitative analysis of 3 forms of alpha melanocyte stimulating hormone in pituitary of prolyl endopeptidase deficient mice. Mol Brain 2: 14, 2009. doi: 10.1186/1756-6606-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Peters EMJ, Tobin DJ, Seidah NG, Schallreuter KU. Pro-opiomelanocortin-related peptides, prohormone convertases 1 and 2 and the regulatory peptide 7B2 are present in melanosomes of human melanocytes. J Invest Dermatol 114: 430–437, 2000. doi: 10.1046/j.1523-1747.2000.00913.x. [DOI] [PubMed] [Google Scholar]
- 306.Philippe J, Stijnen P, Meyre D, De Graeve F, Thuillier D, Delplanque J, Gyapay G, Sand O, Creemers JW, Froguel P, Bonnefond A. A nonsense loss-of-function mutation in PCSK1 contributes to dominantly inherited human obesity. Int J Obes 39: 295–302, 2015. doi: 10.1038/ijo.2014.96. [DOI] [PubMed] [Google Scholar]
- 307.Philips A, Lesage S, Gingras R, Maira MH, Gauthier Y, Hugo P, Drouin J. Novel dimeric Nur77 signaling mechanism in endocrine and lymphoid cells. Mol Cell Biol 17: 5946–5951, 1997. doi: 10.1128/MCB.17.10.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Philips A, Maira M, Mullick A, Chamberland M, Lesage S, Hugo P, Drouin J. Antagonism between Nur77 and glucocorticoid receptor for control of transcription. Mol Cell Biol 17: 5952–5959, 1997. doi: 10.1128/MCB.17.10.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Pintar JE, Schachter BS, Herman AB, Durgerian S, Krieger DT. Characterization and localization of proopiomelanocortin messenger RNA in the adult rat testis. Science 225: 632–634, 1984. doi: 10.1126/science.6740329. [DOI] [PubMed] [Google Scholar]
- 310.Poggioli R, Vergoni AV, Bertolini A. ACTH-(1-24) and alpha-MSH antagonize feeding behavior stimulated by kappa opiate agonists. Peptides 7: 843–848, 1986. doi: 10.1016/0196-9781(86)90104-X. [DOI] [PubMed] [Google Scholar]
- 311.Pritchard LE, Armstrong D, Davies N, Oliver RL, Schmitz CA, Brennand JC, Wilkinson GF, White A. Agouti-related protein (83-132) is a competitive antagonist at the human melanocortin-4 receptor: no evidence for differential interactions with pro-opiomelanocortin-derived ligands. J Endocrinol 180: 183–191, 2004. doi: 10.1677/joe.0.1800183. [DOI] [PubMed] [Google Scholar]
- 312.Pritchard LE, Oliver RL, McLoughlin JD, Birtles S, Lawrence CB, Turnbull AV, White A. Proopiomelanocortin-derived peptides in rat cerebrospinal fluid and hypothalamic extracts: evidence that secretion is regulated with respect to energy balance. Endocrinology 144: 760–766, 2003. doi: 10.1210/en.2002-220866. [DOI] [PubMed] [Google Scholar]
- 313.Pritchard LE, Turnbull AV, White A. Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signalling and obesity. J Endocrinol 172: 411–421, 2002. doi: 10.1677/joe.0.1720411. [DOI] [PubMed] [Google Scholar]
- 314.Pritchard LE, White A. Neuropeptide processing and its impact on melanocortin pathways. Endocrinology 148: 4201–4207, 2007. doi: 10.1210/en.2006-1686. [DOI] [PubMed] [Google Scholar]
- 315.Quigley ME, Yen SS. The role of endogenous opiates in LH secretion during the menstrual cycle. J Clin Endocrinol Metab 51: 179–181, 1980. doi: 10.1210/jcem-51-1-179. [DOI] [PubMed] [Google Scholar]
- 316.Quinn D, Orci L, Ravazzola M, Moore HP. Intracellular transport and sorting of mutant human proinsulins that fail to form hexamers. J Cell Biol 113: 987–996, 1991. doi: 10.1083/jcb.113.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Raff H, Findling JW. A new immunoradiometric assay for corticotropin evaluated in normal subjects and patients with Cushing’s syndrome. Clin Chem 35: 596–600, 1989. [PubMed] [Google Scholar]
- 318.Raffan E, Dennis RJ, O’Donovan CJ, Becker JM, Scott RA, Smith SP, Withers DJ, Wood CJ, Conci E, Clements DN, Summers KM, German AJ, Mellersh CS, Arendt ML, Iyemere VP, Withers E, Söder J, Wernersson S, Andersson G, Lindblad-Toh K, Yeo GS, O’Rahilly S. A Deletion in the Canine POMC Gene Is Associated with Weight and Appetite in Obesity-Prone Labrador Retriever Dogs. Cell Metab 23: 893–900, 2016. doi: 10.1016/j.cmet.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Raffin-Sanson ML, Massias JF, Dumont C, Raux-Demay MC, Proeschel MF, Luton JP, Bertagna X. High plasma proopiomelanocortin in aggressive adrenocorticotropin-secreting tumors. J Clin Endocrinol Metab 81: 4272–4277, 1996. [DOI] [PubMed] [Google Scholar]
- 320.Ramachandran J, Farmer SW, Liles S, Li CH. Comparison of the steroidogenic and melanotropic activities of corticotropin, alpha-melanotropin and analogs with their lipolytic activities in rat and rabbit adipocytes. Biochim Biophys Acta 428: 347–354, 1976. doi: 10.1016/0304-4165(76)90042-8. [DOI] [PubMed] [Google Scholar]
- 321.Rambaud J, Desroches J, Balsalobre A, Drouin J. TIF1beta/KAP-1 is a coactivator of the orphan nuclear receptor NGFI-B/Nur77. J Biol Chem 284: 14147–14156, 2009. doi: 10.1074/jbc.M809023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Ratter SJ, Gillies G, Hope J, Hale AC, Grossman A, Gaillard R, Cook D, Edwards CR, Rees LH. Pro-opiocortin related peptides in human pituitary and ectopic ACTH secreting tumours. Clin Endocrinol (Oxf) 18: 211–218, 1983. doi: 10.1111/j.1365-2265.1983.tb03205.x. [DOI] [PubMed] [Google Scholar]
- 323.Ray DW, Gibson S, Crosby SR, Davies D, Davis JR, White A. Elevated levels of adrenocorticotropin (ACTH) precursors in post-adrenalectomy Cushing’s disease and their regulation by glucocorticoids. J Clin Endocrinol Metab 80: 2430–2436, 1995. [DOI] [PubMed] [Google Scholar]
- 324.Ray DW, Ren SG, Melmed S. Leukemia inhibitory factor (LIF) stimulates proopiomelanocortin (POMC) expression in a corticotroph cell line. Role of STAT pathway. J Clin Invest 97: 1852–1859, 1996. doi: 10.1172/JCI118615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Ray DW, Ren SG, Melmed S. Leukemia inhibitory factor regulates proopiomelanocortin transcription. Ann N Y Acad Sci 840: 162–173, 1998. doi: 10.1111/j.1749-6632.1998.tb09560.x. [DOI] [PubMed] [Google Scholar]
- 326.Richter WO, Schwandt P. Physiologic concentrations of beta-lipotropin stimulate lipolysis in rabbit adipocytes. Metabolism 34: 539–543, 1985. doi: 10.1016/0026-0495(85)90191-X. [DOI] [PubMed] [Google Scholar]
- 327.Riegel AT, Lu Y, Remenick J, Wolford RG, Berard DS, Hager GL. Proopiomelanocortin gene promoter elements required for constitutive and glucocorticoid-repressed transcription. Mol Endocrinol 5: 1973–1982, 1991. doi: 10.1210/mend-5-12-1973. [DOI] [PubMed] [Google Scholar]
- 328.Rius RA, Chikuma T, Loh YP. Prenatal processing of pro-opiomelanocortin in the brain and pituitary of mouse embryos. Brain Res Dev Brain Res 60: 179–185, 1991. doi: 10.1016/0165-3806(91)90046-L. [DOI] [PubMed] [Google Scholar]
- 329.Roberts JL, Herbert E. Characterization of a common precursor to corticotropin and beta-lipotropin: cell-free synthesis of the precursor and identification of corticotropin peptides in the molecule. Proc Natl Acad Sci USA 74: 4826–4830, 1977. doi: 10.1073/pnas.74.11.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Robinson P, Bateman A, Mulay S, Spencer SJ, Jaffe RB, Solomon S, Bennett HP. Isolation and characterization of three forms of joining peptide from adult human pituitaries: lack of adrenal androgen-stimulating activity. Endocrinology 129: 859–867, 1991. doi: 10.1210/endo-129-2-859. [DOI] [PubMed] [Google Scholar]
- 331.Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB, Cone RD. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci USA 90: 8856–8860, 1993. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Rotundo RL, Fambrough DM. Secretion of acetylcholinesterase: relation to acetylcholine receptor metabolism. Cell 22: 595–602, 1980. doi: 10.1016/0092-8674(80)90369-4. [DOI] [PubMed] [Google Scholar]
- 333.Rousseau K, Kauser S, Pritchard LE, Warhurst A, Oliver RL, Slominski A, Wei ET, Thody AJ, Tobin DJ, White A. Proopiomelanocortin (POMC), the ACTH/melanocortin precursor, is secreted by human epidermal keratinocytes and melanocytes and stimulates melanogenesis. FASEB J 21: 1844–1856, 2007. doi: 10.1096/fj.06-7398com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Rubinstein M, Mogil JS, Japón M, Chan EC, Allen RG, Low MJ. Absence of opioid stress-induced analgesia in mice lacking beta-endorphin by site-directed mutagenesis. Proc Natl Acad Sci USA 93: 3995–4000, 1996. doi: 10.1073/pnas.93.9.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Rubinstein M, Stein S, Udenfriend S. Characterization of pro-opiocortin, a precursor to opioid peptides and corticotropin. Proc Natl Acad Sci USA 75: 669–671, 1978. doi: 10.1073/pnas.75.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Rudman D, Hollins BM, Kutner MH, Moffitt SD, Lynn MJ. Three types of alpha-melanocyte-stimulating hormone: bioactivities and half-lives. Am J Physiol Endocrinol Metab 245: E47–E54, 1983. [DOI] [PubMed] [Google Scholar]
- 337.Russell GM, Henley DE, Leendertz J, Douthwaite JA, Wood SA, Stevens A, Woltersdorf WW, Peeters BW, Ruigt GS, White A, Veldhuis JD, Lightman SL. Rapid glucocorticoid receptor-mediated inhibition of hypothalamic-pituitary-adrenal ultradian activity in healthy males. J Neurosci 30: 6106–6115, 2010. doi: 10.1523/JNEUROSCI.5332-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Russell GM, Kalafatakis K, Lightman SL. The importance of biological oscillators for hypothalamic-pituitary-adrenal activity and tissue glucocorticoid response: coordinating stress and neurobehavioural adaptation. J Neuroendocrinol 27: 378–388, 2015. doi: 10.1111/jne.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Samuels ME, Gallo-Payet N, Pinard S, Hasselmann C, Magne F, Patry L, Chouinard L, Schwartzentruber J, René P, Sawyer N, Bouvier M, Djemli A, Delvin E, Huot C, Eugene D, Deal CL, Van Vliet G, Majewski J, Deladoëy J; FORGE Canada Consortium . Bioinactive ACTH causing glucocorticoid deficiency. J Clin Endocrinol Metab 98: 736–742, 2013. doi: 10.1210/jc.2012-3199. [DOI] [PubMed] [Google Scholar]
- 340.Santoro N, del Giudice EM, Cirillo G, Raimondo P, Corsi I, Amato A, Grandone A, Perrone L. An insertional polymorphism of the proopiomelanocortin gene is associated with fasting insulin levels in childhood obesity. J Clin Endocrinol Metab 89: 4846–4849, 2004. doi: 10.1210/jc.2004-0333. [DOI] [PubMed] [Google Scholar]
- 341.Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science 238: 522–524, 1987. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- 342.Sarkar DK, Yen SS. Changes in beta-endorphin-like immunoreactivity in pituitary portal blood during the estrous cycle and after ovariectomy in rats. Endocrinology 116: 2075–2079, 1985. doi: 10.1210/endo-116-5-2075. [DOI] [PubMed] [Google Scholar]
- 343.Schauer E, Trautinger F, Köck A, Schwarz A, Bhardwaj R, Simon M, Ansel JC, Schwarz T, Luger TA. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J Clin Invest 93: 2258–2262, 1994. doi: 10.1172/JCI117224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Schiller M, Raghunath M, Kubitscheck U, Scholzen TE, Fisbeck T, Metze D, Luger TA, Böhm M. Human dermal fibroblasts express prohormone convertases 1 and 2 and produce proopiomelanocortin-derived peptides. J Invest Dermatol 117: 227–235, 2001. doi: 10.1046/j.0022-202x.2001.01412.x. [DOI] [PubMed] [Google Scholar]
- 345.Schnabel E, Mains RE, Farquhar MG. Proteolytic processing of pro-ACTH/endorphin begins in the Golgi complex of pituitary corticotropes and AtT-20 cells. Mol Endocrinol 3: 1223–1235, 1989. doi: 10.1210/mend-3-8-1223. [DOI] [PubMed] [Google Scholar]
- 346.Scholzen TE, König S, Fastrich M, Böhm M, Luger TA. Terminating the stress: peripheral peptidolysis of proopiomelanocortin-derived regulatory hormones by the dermal microvascular endothelial cell extracellular peptidases neprilysin and angiotensin-converting enzyme. Endocrinology 148: 2793–2805, 2007. doi: 10.1210/en.2006-1765. [DOI] [PubMed] [Google Scholar]
- 347.Scopsi L, Gullo M, Rilke F, Martin S, Steiner DF. Proprotein convertases (PC1/PC3 and PC2) in normal and neoplastic human tissues: their use as markers of neuroendocrine differentiation. J Clin Endocrinol Metab 80: 294–301, 1995. [DOI] [PubMed] [Google Scholar]
- 348.Seger MA, Bennett HP. Structure and bioactivity of the amino-terminal fragment of pro-opiomelanocortin. J Steroid Biochem 25: 703–710, 1986. doi: 10.1016/0022-4731(86)90298-0. [DOI] [PubMed] [Google Scholar]
- 349.Seidah NG. The proprotein convertases, 20 years later. Methods Mol Biol 768: 23–57, 2011. doi: 10.1007/978-1-61779-204-5_3. [DOI] [PubMed] [Google Scholar]
- 350.Seidah NG, Benjannet S, Hamelin J, Mamarbachi AM, Basak A, Marcinkiewicz J, Mbikay M, Chrétien M, Marcinkiewicz M. The subtilisin/kexin family of precursor convertases. Emphasis on PC1, PC2/7B2, POMC and the novel enzyme SKI-1. Ann N Y Acad Sci 885: 57–74, 1999. doi: 10.1111/j.1749-6632.1999.tb08665.x. [DOI] [PubMed] [Google Scholar]
- 351.Seidah NG, Chrétien M. Proprotein and prohormone convertases: a family of subtilases generating diverse bioactive polypeptides. Brain Res 848: 45–62, 1999. doi: 10.1016/S0006-8993(99)01909-5. [DOI] [PubMed] [Google Scholar]
- 352.Seidah NG, Gaspar L, Mion P, Marcinkiewicz M, Mbikay M, Chrétien M. cDNA sequence of two distinct pituitary proteins homologous to Kex2 and furin gene products: tissue-specific mRNAs encoding candidates for pro-hormone processing proteinases. DNA Cell Biol 9: 415–424, 1990. doi: 10.1089/dna.1990.9.415. [DOI] [PubMed] [Google Scholar]
- 353.Seidah NG, Marcinkiewicz M, Benjannet S, Gaspar L, Beaubien G, Mattei MG, Lazure C, Mbikay M, Chrétien M. Cloning and primary sequence of a mouse candidate prohormone convertase PC1 homologous to PC2, Furin, and Kex2: distinct chromosomal localization and messenger RNA distribution in brain and pituitary compared to PC2. Mol Endocrinol 5: 111–122, 1991. doi: 10.1210/mend-5-1-111. [DOI] [PubMed] [Google Scholar]
- 354.Seidah NG, Rochemont J, Hamelin J, Benjannet S, Chrétien M. The missing fragment of the pro-sequence of human pro-opiomelanocortin: sequence and evidence for C-terminal amidation. Biochem Biophys Res Commun 102: 710–716, 1981. doi: 10.1016/S0006-291X(81)80190-8. [DOI] [PubMed] [Google Scholar]
- 355.Seksek O, Biwersi J, Verkman AS. Direct measurement of trans-Golgi pH in living cells and regulation by second messengers. J Biol Chem 270: 4967–4970, 1995. doi: 10.1074/jbc.270.10.4967. [DOI] [PubMed] [Google Scholar]
- 356.Shen FS, Aguilera G, Loh YP. Altered biosynthesis and secretion of pro-opiomelanocortin in the intermediate and anterior pituitary of carboxypeptidase E-deficient, Cpe(fat)/Cpe(fat)mice. Neuropeptides 33: 276–280, 1999. doi: 10.1054/npep.1999.0045. [DOI] [PubMed] [Google Scholar]
- 357.Shen FS, Loh YP. Intracellular misrouting and abnormal secretion of adrenocorticotropin and growth hormone in cpefat mice associated with a carboxypeptidase E mutation. Proc Natl Acad Sci USA 94: 5314–5319, 1997. doi: 10.1073/pnas.94.10.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Shizume K, Lerner AB, Fitzpatrick TB. In vitro bioassay for the melanocyte stimulating hormone. Endocrinology 54: 553–560, 1954. doi: 10.1210/endo-54-5-553. [DOI] [PubMed] [Google Scholar]
- 359.Skibicka KP, Grill HJ. Energetic responses are triggered by caudal brainstem melanocortin receptor stimulation and mediated by local sympathetic effector circuits. Endocrinology 149: 3605–3616, 2008. doi: 10.1210/en.2007-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC, Slominski A. Cutaneous hypothalamic-pituitary-adrenal axis homolog: regulation by ultraviolet radiation. Am J Physiol Endocrinol Metab 301: E484–E493, 2011. doi: 10.1152/ajpendo.00217.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Slominski A. A nervous breakdown in the skin: stress and the epidermal barrier. J Clin Invest 117: 3166–3169, 2007. doi: 10.1172/JCI33508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Slominski A, Ermak G, Mazurkiewicz JE, Baker J, Wortsman J. Characterization of corticotropin-releasing hormone (CRH) in human skin. J Clin Endocrinol Metab 83: 1020–1024, 1998. [DOI] [PubMed] [Google Scholar]
- 363.Slominski A, Heasley D, Mazurkiewicz JE, Ermak G, Baker J, Carlson JA. Expression of proopiomelanocortin (POMC)-derived melanocyte-stimulating hormone (MSH) and adrenocorticotropic hormone (ACTH) peptides in skin of basal cell carcinoma patients. Hum Pathol 30: 208–215, 1999. doi: 10.1016/S0046-8177(99)90278-2. [DOI] [PubMed] [Google Scholar]
- 364.Slominski A, Paus R, Wortsman J. On the potential role of proopiomelanocortin in skin physiology and pathology. Mol Cell Endocrinol 93: C1–C6, 1993. doi: 10.1016/0303-7207(93)90131-3. [DOI] [PubMed] [Google Scholar]
- 365.Slominski A, Pisarchik A, Tobin DJ, Mazurkiewicz JE, Wortsman J. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology 145: 941–950, 2004. doi: 10.1210/en.2003-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev 80: 979–1020, 2000. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 367.Slominski A, Zbytek B, Nikolakis G, Manna PR, Skobowiat C, Zmijewski M, Li W, Janjetovic Z, Postlethwaite A, Zouboulis CC, Tuckey RC. Steroidogenesis in the skin: implications for local immune functions. J Steroid Biochem Mol Biol 137: 107–123, 2013. doi: 10.1016/j.jsbmb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Slominski A, Zbytek B, Semak I, Sweatman T, Wortsman J. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J Neuroimmunol 162: 97–102, 2005. doi: 10.1016/j.jneuroim.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 369.Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, Wortsman J. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab 288: E701–E706, 2005. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- 370.Slominski A, Zbytek B, Zmijewski M, Slominski RM, Kauser S, Wortsman J, Tobin DJ. Corticotropin releasing hormone and the skin. Front Biosci 11: 2230–2248, 2006. doi: 10.2741/1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Smart JL, Low MJ. Lack of proopiomelanocortin peptides results in obesity and defective adrenal function but normal melanocyte pigmentation in the murine C57BL/6 genetic background. Ann N Y Acad Sci 994: 202–210, 2003. doi: 10.1111/j.1749-6632.2003.tb03181.x. [DOI] [PubMed] [Google Scholar]
- 372.Smart JL, Tolle V, Low MJ. Glucocorticoids exacerbate obesity and insulin resistance in neuron-specific proopiomelanocortin-deficient mice. J Clin Invest 116: 495–505, 2006. doi: 10.1172/JCI25243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Smart JL, Tolle V, Otero-Corchon V, Low MJ. Central dysregulation of the hypothalamic-pituitary-adrenal axis in neuron-specific proopiomelanocortin-deficient mice. Endocrinology 148: 647–659, 2007. doi: 10.1210/en.2006-0990. [DOI] [PubMed] [Google Scholar]
- 374.Smeekens SP, Avruch AS, LaMendola J, Chan SJ, Steiner DF. Identification of a cDNA encoding a second putative prohormone convertase related to PC2 in AtT20 cells and islets of Langerhans. Proc Natl Acad Sci USA 88: 340–344, 1991. doi: 10.1073/pnas.88.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Smeekens SP, Steiner DF. Identification of a human insulinoma cDNA encoding a novel mammalian protein structurally related to the yeast dibasic processing protease Kex2. J Biol Chem 265: 2997–3000, 1990. [PubMed] [Google Scholar]
- 376.Smith PE. Experimental Ablation of the Hypophysis in the Frog Embryo. Science 44: 280–282, 1916. doi: 10.1126/science.44.1130.280. [DOI] [PubMed] [Google Scholar]
- 377.Smith SR, Gawronska-Kozak B, Janderová L, Nguyen T, Murrell A, Stephens JM, Mynatt RL. Agouti expression in human adipose tissue: functional consequences and increased expression in type 2 diabetes. Diabetes 52: 2914–2922, 2003. doi: 10.2337/diabetes.52.12.2914. [DOI] [PubMed] [Google Scholar]
- 378.Smyth DG. 60 YEARS OF POMC: Lipotropin and beta-endorphin: a perspective. J Mol Endocrinol 56: T13–T25, 2016. doi: 10.1530/JME-16-0033. [DOI] [PubMed] [Google Scholar]
- 379.Soares SM, Thompson M, Chini EN. Role of the second-messenger cyclic-adenosine 5′-diphosphate-ribose on adrenocorticotropin secretion from pituitary cells. Endocrinology 146: 2186–2192, 2005. doi: 10.1210/en.2004-1298. [DOI] [PubMed] [Google Scholar]
- 380.Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature 362: 318–324, 1993. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 381.Stavreva DA, Wiench M, John S, Conway-Campbell BL, McKenna MA, Pooley JR, Johnson TA, Voss TC, Lightman SL, Hager GL. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol 11: 1093–1102, 2009. doi: 10.1038/ncb1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Steiner DF, Cunningham D, Spigelman L, Aten B. Insulin biosynthesis: evidence for a precursor. Science 157: 697–700, 1967. doi: 10.1126/science.157.3789.697. [DOI] [PubMed] [Google Scholar]
- 383.Steiner DF, Oyer PE. The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma. Proc Natl Acad Sci USA 57: 473–480, 1967. doi: 10.1073/pnas.57.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Stevens A, White A. ACTH: cellular peptide hormone synthesis and secretory pathways. Results Probl Cell Differ 50: 63–84, 2010. doi: 10.1007/400_2009_30. [DOI] [PubMed] [Google Scholar]
- 385.Stewart PM, Gibson S, Crosby SR, Penn R, Holder R, Ferry D, Thatcher N, Phillips P, London DR, White A. ACTH precursors characterize the ectopic ACTH syndrome. Clin Endocrinol (Oxf) 40: 199–204, 1994. doi: 10.1111/j.1365-2265.1994.tb02468.x. [DOI] [PubMed] [Google Scholar]
- 386.Stijnen P, Brouwers B, Dirkx E, Ramos-Molina B, Van Lommel L, Schuit F, Thorrez L, Declercq J, Creemers JW. Endoplasmic reticulum-associated degradation of the mouse PC1/3-N222D hypomorph and human PCSK1 mutations contributes to obesity. Int J Obes 40: 973–981, 2016. doi: 10.1038/ijo.2016.3. [DOI] [PubMed] [Google Scholar]
- 387.Stijnen P, Ramos-Molina B, O’Rahilly S, Creemers JW. PCSK1 Mutations and Human Endocrinopathies: From Obesity to Gastrointestinal Disorders. Endocr Rev 37: 347–371, 2016. doi: 10.1210/er.2015-1117. [DOI] [PubMed] [Google Scholar]
- 388.Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev 17: 221–244, 1996. [DOI] [PubMed] [Google Scholar]
- 389.Strehl C, Buttgereit F. Unraveling the functions of the membrane-bound glucocorticoid receptors: first clues on origin and functional activity. Ann N Y Acad Sci 1318: 1–6, 2014. doi: 10.1111/nyas.12364. [DOI] [PubMed] [Google Scholar]
- 390.Südhof TC, Rizo J. Synaptotagmins: C2-domain proteins that regulate membrane traffic. Neuron 17: 379–388, 1996. doi: 10.1016/S0896-6273(00)80171-3. [DOI] [PubMed] [Google Scholar]
- 391.Takahashi H, Hakamata Y, Watanabe Y, Kikuno R, Miyata T, Numa S. Complete nucleotide sequence of the human corticotropin-beta-lipotropin precursor gene. Nucleic Acids Res 11: 6847–6858, 1983. doi: 10.1093/nar/11.19.6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Talaber G, Tuckermann JP, Okret S. ACTH controls thymocyte homeostasis independent of glucocorticoids. FASEB J 29: 2526–2534, 2015. doi: 10.1096/fj.14-268508. [DOI] [PubMed] [Google Scholar]
- 393.Tanaka S. Comparative aspects of intracellular proteolytic processing of peptide hormone precursors: studies of proopiomelanocortin processing. Zoolog Sci 20: 1183–1198, 2003. doi: 10.2108/zsj.20.1183. [DOI] [PubMed] [Google Scholar]
- 394.Tanaka S, Yora T, Nakayama K, Inoue K, Kurosumi K. Proteolytic processing of pro-opiomelanocortin occurs in acidifying secretory granules of AtT-20 cells. J Histochem Cytochem 45: 425–436, 1997. doi: 10.1177/002215549704500310. [DOI] [PubMed] [Google Scholar]
- 395.Tasker JG, Di S, Malcher-Lopes R. Minireview: rapid glucocorticoid signaling via membrane-associated receptors. Endocrinology 147: 5549–5556, 2006. doi: 10.1210/en.2006-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Taylor T, Dluhy RG, Williams GH. Beta-endorphin suppresses adrenocorticotropin and cortisol levels in normal human subjects. J Clin Endocrinol Metab 57: 592–596, 1983. doi: 10.1210/jcem-57-3-592. [DOI] [PubMed] [Google Scholar]
- 397.Thody AJ, Ridley K, Penny RJ, Chalmers R, Fisher C, Shuster S. MSH peptides are present in mammalian skin. Peptides 4: 813–816, 1983. doi: 10.1016/0196-9781(83)90072-4. [DOI] [PubMed] [Google Scholar]
- 398.Thomas L, Leduc R, Thorne BA, Smeekens SP, Steiner DF, Thomas G. Kex2-like endoproteases PC2 and PC3 accurately cleave a model prohormone in mammalian cells: evidence for a common core of neuroendocrine processing enzymes. Proc Natl Acad Sci USA 88: 5297–5301, 1991. doi: 10.1073/pnas.88.12.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Tooze J, Tooze SA. Clathrin-coated vesicular transport of secretory proteins during the formation of ACTH-containing secretory granules in AtT20 cells. J Cell Biol 103: 839–850, 1986. doi: 10.1083/jcb.103.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Tooze J, Tooze SA, Fuller SD. Sorting of progeny coronavirus from condensed secretory proteins at the exit from the trans-Golgi network of AtT20 cells. J Cell Biol 105: 1215–1226, 1987. doi: 10.1083/jcb.105.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Torres TE, de Mendonça PO, Lotfi CF. Synthetic modified N-POMC(1-28) controls in vivo proliferation and blocks apoptosis in rat adrenal cortex. Cell Tissue Res 341: 239–250, 2010. doi: 10.1007/s00441-010-0998-0. [DOI] [PubMed] [Google Scholar]
- 402.Tsagarakis S, Navarra P, Rees LH, Besser M, Grossman A, Navara P. Morphine directly modulates the release of stimulated corticotrophin-releasing factor-41 from rat hypothalamus in vitro. Endocrinology 124: 2330–2335, 1989. doi: 10.1210/endo-124-5-2330. [DOI] [PubMed] [Google Scholar]
- 403.Tsigos C, Crosby SR, Gibson S, Young RJ, White A. Proopiomelanocortin is the predominant adrenocorticotropin-related peptide in human cerebrospinal fluid. J Clin Endocrinol Metab 76: 620–624, 1993. [DOI] [PubMed] [Google Scholar]
- 404.Tsujii S, Bray GA. Acetylation alters the feeding response to MSH and beta-endorphin. Brain Res Bull 23: 165–169, 1989. doi: 10.1016/0361-9230(89)90142-1. [DOI] [PubMed] [Google Scholar]
- 405.Tung YC, Piper SJ, Yeung D, O’Rahilly S, Coll AP. A comparative study of the central effects of specific proopiomelancortin (POMC)-derived melanocortin peptides on food intake and body weight in pomc null mice. Endocrinology 147: 5940–5947, 2006. doi: 10.1210/en.2006-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Turan S, Hughes C, Atay Z, Guran T, Haliloglu B, Clark AJ, Bereket A, Metherell LA. An atypical case of familial glucocorticoid deficiency without pigmentation caused by coexistent homozygous mutations in MC2R (T152K) and MC1R (R160W). J Clin Endocrinol Metab 97: E771–E774, 2012. doi: 10.1210/jc.2011-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Uhler M, Herbert E. Complete amino acid sequence of mouse pro-opiomelanocortin derived from the nucleotide sequence of pro-opiomelanocortin cDNA. J Biol Chem 258: 257–261, 1983. [PubMed] [Google Scholar]
- 408.Vale W, Vaughan J, Smith M, Yamamoto G. Effects of synthetic ovine corticotropin-releasing factor, glucocorticoids, catecholamines, neurohypophysial peptides, and other substances on cultured corticotropic cells. Endocrinology 113: 1121–1131, 1983. doi: 10.1210/endo-113-3-1121. [DOI] [PubMed] [Google Scholar]
- 409.Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet 11: 328–330, 1995. doi: 10.1038/ng1195-328. [DOI] [PubMed] [Google Scholar]
- 410.Van der Salm AL, Pavlidis M, Flik G, Wendelaar Bonga SE. Differential release of alpha-melanophore stimulating hormone isoforms by the pituitary gland of red porgy, Pagrus pagrus. Gen Comp Endocrinol 135: 126–133, 2004. doi: 10.1016/j.ygcen.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 411.Varlamov O, Eng FJ, Novikova EG, Fricker LD. Localization of metallocarboxypeptidase D in AtT-20 cells. Potential role in prohormone processing. J Biol Chem 274: 14759–14767, 1999. doi: 10.1074/jbc.274.21.14759. [DOI] [PubMed] [Google Scholar]
- 412.Verburg-Van Kemenade BM, Jenks BG, Driessen AG. GABA and dopamine act directly on melanotropes of Xenopus to inhibit MSH secretion. Brain Res Bull 17: 697–704, 1986. doi: 10.1016/0361-9230(86)90203-0. [DOI] [PubMed] [Google Scholar]
- 413.Vieau D, Massias JF, Girard F, Luton JP, Bertagna X. Corticotrophin-like intermediary lobe peptide as a marker of alternate pro-opiomelanocortin processing in ACTH-producing non-pituitary tumours. Clin Endocrinol (Oxf) 31: 691–700, 1989. doi: 10.1111/j.1365-2265.1989.tb01294.x. [DOI] [PubMed] [Google Scholar]
- 414.Wakamatsu K, Graham A, Cook D, Thody AJ. Characterisation of ACTH peptides in human skin and their activation of the melanocortin-1 receptor. Pigment Cell Res 10: 288–297, 1997. doi: 10.1111/j.1600-0749.1997.tb00688.x. [DOI] [PubMed] [Google Scholar]
- 415.Walker JJ, Spiga F, Waite E, Zhao Z, Kershaw Y, Terry JR, Lightman SL. The origin of glucocorticoid hormone oscillations. PLoS Biol 10: e1001341, 2012. doi: 10.1371/journal.pbio.1001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Walker JJ, Terry JR, Lightman SL. Origin of ultradian pulsatility in the hypothalamic-pituitary-adrenal axis. Proc Biol Sci 277: 1627–1633, 2010. doi: 10.1098/rspb.2009.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Wallingford N, Perroud B, Gao Q, Coppola A, Gyengesi E, Liu ZW, Gao XB, Diament A, Haus KA, Shariat-Madar Z, Mahdi F, Wardlaw SL, Schmaier AH, Warden CH, Diano S. Prolylcarboxypeptidase regulates food intake by inactivating alpha-MSH in rodents. J Clin Invest 119: 2291–2303, 2009. doi: 10.1172/JCI37209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Wardlaw SL. Regulation of beta-endorphin, corticotropin-like intermediate lobe peptide, and alpha-melanotropin-stimulating hormone in the hypothalamus by testosterone. Endocrinology 119: 19–24, 1986. doi: 10.1210/endo-119-1-19. [DOI] [PubMed] [Google Scholar]
- 419.Wardlaw SL, Ferin M. Interaction between beta-endorphin and alpha-melanocyte-stimulating hormone in the control of prolactin and luteinizing hormone secretion in the primate. Endocrinology 126: 2035–2040, 1990. doi: 10.1210/endo-126-4-2035. [DOI] [PubMed] [Google Scholar]
- 420.Wardman JH, Zhang X, Gagnon S, Castro LM, Zhu X, Steiner DF, Day R, Fricker LD. Analysis of peptides in prohormone convertase 1/3 null mouse brain using quantitative peptidomics. J Neurochem 114: 215–225, 2010. doi: 10.1111/j.1471-4159.2010.06760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Wen T, Peng B, Pintar JE. The MOR-1 opioid receptor regulates glucose homeostasis by modulating insulin secretion. Mol Endocrinol 23: 671–678, 2009. doi: 10.1210/me.2008-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Westphal CH, Muller L, Zhou A, Zhu X, Bonner-Weir S, Schambelan M, Steiner DF, Lindberg I, Leder P. The neuroendocrine protein 7B2 is required for peptide hormone processing in vivo and provides a novel mechanism for pituitary Cushing’s disease. Cell 96: 689–700, 1999. doi: 10.1016/S0092-8674(00)80579-6. [DOI] [PubMed] [Google Scholar]
- 423.White A, Gibson S. ACTH precursors: biological significance and clinical relevance. Clin Endocrinol (Oxf) 48: 251–255, 1998. doi: 10.1046/j.1365-2265.1998.00451.x. [DOI] [PubMed] [Google Scholar]
- 424.White A, Gray C, Ratcliffe JG. Characterisation of monoclonal antibodies to adrenocorticotrophin. J Immunol Methods 79: 185–194, 1985. doi: 10.1016/0022-1759(85)90098-5. [DOI] [PubMed] [Google Scholar]
- 425.White A, Ray DW. ACTH precursors in different pathalogical conditions. In: The ACTH axis: Pathogenesis, Diagnosis and Treatment, edited by Gaillard R. The Netherlands: Kluwer Academic, 2003, p. 85–107. doi: 10.1007/978-1-4615-0501-3_5. [DOI] [Google Scholar]
- 426.White A, Ray DW, Talbot A, Abraham P, Thody AJ, Bevan JS. Cushing’s syndrome due to phaeochromocytoma secreting the precursors of adrenocorticotropin. J Clin Endocrinol Metab 85: 4771–4775, 2000. doi: 10.1210/jcem.85.12.7047. [DOI] [PubMed] [Google Scholar]
- 427.White A, Smith H, Hoadley M, Dobson SH, Ratcliffe JG. Clinical evaluation of a two-site immunoradiometric assay for adrenocorticotrophin in unextracted human plasma using monoclonal antibodies. Clin Endocrinol (Oxf) 26: 41–52, 1987. doi: 10.1111/j.1365-2265.1987.tb03637.x. [DOI] [PubMed] [Google Scholar]
- 428.Wierzbicka JM, Żmijewski MA, Antoniewicz J, Sobjanek M, Slominski AT. Differentiation of Keratinocytes Modulates Skin HPA Analog. J Cell Physiol 232: 154–166, 2017. doi: 10.1002/jcp.25400. [DOI] [PubMed] [Google Scholar]
- 429.Wilkinson CW. Roles of acetylation and other post-translational modifications in melanocortin function and interactions with endorphins. Peptides 27: 453–471, 2006. doi: 10.1016/j.peptides.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 430.Williams DL, Bowers RR, Bartness TJ, Kaplan JM, Grill HJ. Brainstem melanocortin 3/4 receptor stimulation increases uncoupling protein gene expression in brown fat. Endocrinology 144: 4692–4697, 2003. doi: 10.1210/en.2003-0440. [DOI] [PubMed] [Google Scholar]
- 431.Wintzen M, Yaar M, Burbach JP, Gilchrest BA. Proopiomelanocortin gene product regulation in keratinocytes. J Invest Dermatol 106: 673–678, 1996. doi: 10.1111/1523-1747.ep12345496. [DOI] [PubMed] [Google Scholar]
- 432.Wittert G, Hope P, Pyle D. Tissue distribution of opioid receptor gene expression in the rat. Biochem Biophys Res Commun 218: 877–881, 1996. doi: 10.1006/bbrc.1996.0156. [DOI] [PubMed] [Google Scholar]
- 433.Yalow RS, Berson SA. Size heterogeneity of immunoreactive human ACTH in plasma and in extracts of pituitary glands and ACTH-producing thymoma. Biochem Biophys Res Commun 44: 439–445, 1971. doi: 10.1016/0006-291X(71)90620-6. [DOI] [PubMed] [Google Scholar]
- 434.Yalow RS, Glick SM, Roth J, Berson SA. Radioimmunoassay of Human Plasma ACTH. J Clin Endocrinol Metab 24: 1219–1225, 1964. doi: 10.1210/jcem-24-11-1219. [DOI] [PubMed] [Google Scholar]
- 435.Yamamoto H, Yamane T, Iguchi K, Tanaka K, Iddamalgoda A, Unno K, Hoshino M, Takeda A. Melanin production through novel processing of proopiomelanocortin in the extracellular compartment of the auricular skin of C57BL/6 mice after UV-irradiation. Sci Rep 5: 14579, 2015. doi: 10.1038/srep14579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Yang Y. Structure, function and regulation of the melanocortin receptors. Eur J Pharmacol 660: 125–130, 2011. doi: 10.1016/j.ejphar.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med 5: 1066–1070, 1999. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- 438.Yonemoto IT, Kroon GJ, Dyson HJ, Balch WE, Kelly JW. Amylin proprotein processing generates progressively more amyloidogenic peptides that initially sample the helical state. Biochemistry 47: 9900–9910, 2008. doi: 10.1021/bi800828u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Young EA, Lewis J, Akil H. The preferential release of beta-endorphin from the anterior pituitary lobe by corticotropin releasing factor (CRF). Peptides 7: 603–607, 1986. doi: 10.1016/0196-9781(86)90034-3. [DOI] [PubMed] [Google Scholar]
- 440.Zakarian S, Smyth DG. beta-Endorphin is processed differently in specific regions of rat pituitary and brain. Nature 296: 250–252, 1982. doi: 10.1038/296250a0. [DOI] [PubMed] [Google Scholar]
- 441.Zhang C-F, Snell CR, Loh YP. Identification of a novel prohormone sorting signal-binding site on carboxypeptidase E, a regulated secretory pathway-sorting receptor. Mol Endocrinol 13: 527–536, 1999. doi: 10.1210/mend.13.4.0267. [DOI] [PubMed] [Google Scholar]
- 442.Zhang X, Che FY, Berezniuk I, Sonmez K, Toll L, Fricker LD. Peptidomics of Cpe(fat/fat) mouse brain regions: implications for neuropeptide processing. J Neurochem 107: 1596–1613, 2008. doi: 10.1111/j.1471-4159.2008.05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Zhang X, Pan H, Peng B, Steiner DF, Pintar JE, Fricker LD. Neuropeptidomic analysis establishes a major role for prohormone convertase-2 in neuropeptide biosynthesis. J Neurochem 112: 1168–1179, 2010. doi: 10.1111/j.1471-4159.2009.06530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Zhang ZH, Felder RB. Melanocortin receptors mediate the excitatory effects of blood-borne murine leptin on hypothalamic paraventricular neurons in rat. Am J Physiol Regul Integr Comp Physiol 286: R303–R310, 2004. doi: 10.1152/ajpregu.00504.2003. [DOI] [PubMed] [Google Scholar]
- 445.Zhou A, Bloomquist BT, Mains RE. The prohormone convertases PC1 and PC2 mediate distinct endoproteolytic cleavages in a strict temporal order during proopiomelanocortin biosynthetic processing. J Biol Chem 268: 1763–1769, 1993. [PubMed] [Google Scholar]
- 446.Zhou A, Mains RE. Endoproteolytic processing of proopiomelanocortin and prohormone convertases 1 and 2 in neuroendocrine cells overexpressing prohormone convertases 1 or 2. J Biol Chem 269: 17440–17447, 1994. [PubMed] [Google Scholar]
- 447.Zhou Y, Lindberg I. Enzymatic properties of carboxyl-terminally truncated prohormone convertase 1 (PC1/SPC3) and evidence for autocatalytic conversion. J Biol Chem 269: 18408–18413, 1994. [PubMed] [Google Scholar]
- 448.Zhu X, Orci L, Carroll R, Norrbom C, Ravazzola M, Steiner DF. Severe block in processing of proinsulin to insulin accompanied by elevation of des-64,65 proinsulin intermediates in islets of mice lacking prohormone convertase 1/3. Proc Natl Acad Sci USA 99: 10299–10304, 2002. doi: 10.1073/pnas.162352799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Zhu X, Zhou A, Dey A, Norrbom C, Carroll R, Zhang C, Laurent V, Lindberg I, Ugleholdt R, Holst JJ, Steiner DF. Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proc Natl Acad Sci USA 99: 10293–10298, 2002. doi: 10.1073/pnas.162352599. [DOI] [PMC free article] [PubMed] [Google Scholar]