Abstract
The central nervous system relies on a continual supply of glucose, and must be able to detect glucose levels and regulate peripheral organ functions to ensure that its energy requirements are met. Specialized glucose-sensing neurons, first described half a century ago, use glucose as a signal and modulate their firing rates as glucose levels change. Glucose-excited neurons are activated by increasing glucose concentrations, while glucose-inhibited neurons increase their firing rate as glucose concentrations fall and decrease their firing rate as glucose concentrations rise. Glucose-sensing neurons are present in multiple brain regions and are highly expressed in hypothalamic regions, where they are involved in functions related to glucose homeostasis. However, the roles of glucose-sensing neurons in healthy and disease states remain poorly understood. Technologies that can rapidly and reversibly activate or inhibit defined neural populations provide invaluable tools to investigate how specific neural populations regulate metabolism and other physiological roles. Optogenetics has high temporal and spatial resolutions, requires implants for neural stimulation, and is suitable for modulating local neural populations. Chemogenetics, which requires injection of a synthetic ligand, can target both local and widespread populations. Radio- and magnetogenetics offer rapid neural activation in localized or widespread neural populations without the need for implants or injections. These tools will allow us to better understand glucose-sensing neurons and their metabolism-regulating circuits.
Keywords: glucose, magnetogenetics, neuromodulation, radiogenetics
INTRODUCTION
Studies done over 50 years ago demonstrated that the brain is a primary user of the body’s energy. Despite comprising <2% of human body weight, the brain uses >20% of the body’s daily energy production (69, 144). Except under rare circumstances, such as fasting or in newborns fed maternal milk, where ketones can provide energy (133), the central nervous system (CNS) uses glucose as its fuel and requires a constant supply to maintain its processes (147). To ensure that this glucose supply is maintained, the CNS must be able to detect glucose levels and regulate peripheral organs to provide sufficient energy for bodily processes, including neural function. Glucose sensors, which react to changes in glucose availability, are present in the periphery and in the CNS, where they serve to initiate peripheral responses to maintain glucose homeostasis.
GLUCOSE UPTAKE INTO THE BRAIN
Glucose entry into the CNS appears to be a regulated process (78, 122). The blood-brain barrier, composed of tight junctions between brain microvascular endothelial cells, prevents free diffusion of glucose into the CNS. Glucose enters the CNS through high-affinity glucose transporter (GLUT) type 1 (GLUT1), which is expressed on the luminal and outer membranes of CNS endothelial cells (32, 107). GLUT1 is expressed in glia, the choroid plexus, and most ependymal cells (165). Under physiological conditions, GLUT1 expression in neurons is minimal. Under most conditions, glucose transport does not limit fuel availability to the CNS, but CNS glucose levels are markedly lower than circulating glucose concentrations (20). Microdialysis studies in the hippocampus (67), hypothalamus (5), and other brain regions (102) have demonstrated that interstitial brain glucose levels in awake animals range from 0.7 to 2.5 mM (from fed to fasted state) under normal conditions and from 0.2 to 4.5 mM in pathological states such as hypoglycemia and hyperglycemia, respectively. However, the circumventricular organs (including the median eminence and area postrema), which do not have a blood-brain barrier, are likely to be exposed to glucose levels closer to circulating concentrations (40). In addition, it appears that there is differential diffusion of glucose from cerebrospinal fluid into specific brain regions, such as the arcuate nucleus (ARC) (127). Interestingly, previous work has shown that fasting, hypoglycemia, and hyperglycemia can alter glucose transport into the brain (28, 86).
Once glucose enters the brain, additional transporters move glucose into cells (144). Most neurons express GLUT3, a GLUT with even higher affinity than GLUT1, which transports glucose into the cell (112). Low expression levels of other GLUTs have also been reported (101). GLUT2 is expressed in astrocytes, tanycytes, and neurons, and has been implicated in glucose sensing (90). Intracellular GLUT6 is found in neurons and may transport hexose molecules across organelle membranes. Similarly, low levels of GLUT8 have been described in defined brain regions, including the hippocampus and amygdala (112).
Once within neurons, glucose undergoes oxidative metabolism (3); in glia, however, glucose can undergo anaerobic glycolysis into lactate (55). Recent work indicates that glucose can also undergo glycolysis in activated neurons (33). The primary step in both of these processes is conversion of glucose to glucose-6-phosphate, which is predominantly carried out by hexokinase I, a very high-affinity kinase (26). This sequence of high-affinity transporters and kinases ensures that CNS glucose supplies are maintained over a wide range of physiological glucose concentrations.
GLUCOSE-SENSING POPULATIONS AND MECHANISMS IN THE CNS
Specialized groups of neurons, glucose-sensing neurons, use glucose as a signal as well as a fuel (51, 159). These neurons alter their firing rate with changes in glucose levels (143). Glucose-sensing neurons can be broadly categorized as glucose-inhibited (GI) or glucose-excited (GE) neurons (35). As glucose levels rise, the firing rate of GE neurons also rises and the activity of GI neurons is reduced. As glucose concentrations decrease, GI neurons are excited, whereas GE neurons decrease their activity. Glucose-sensing neurons are present in multiple brain regions but are found at high levels in hypothalamic regions, such as the ARC (25, 124), ventromedial nucleus (VMH) (77), lateral hypothalamus (LH) (16, 17), paraventricular nucleus of the hypothalamus (PVH) (104), and supraoptic nucleus (148). Interestingly, most regions are reported to harbor both GE and GI neurons (35, 177). In some areas, such as the VMH, there may be some anatomic segregation with higher expression of GE neurons in the dorsomedial VMH but an enrichment of GI neurons in the ventrolateral VMH (139, 170). There is also some anatomic division of glucose-sensing neurons in the nucleus accumbens (NAc) (123).
Glucose-sensing neurons have been identified in regions outside the hypothalamus, notably in several nuclei of the reward system, including the NAc (123), amygdala, paraventricular thalamus (89), prefrontal cortex (110), and hippocampus (160). Many of these regions form part of the mesolimbic and mesocortical dopaminergic pathways and are integral for motivation and reward processing, including addiction and different aspects of motivated food intake. Approximately 25% of neurons in the NAc have been reported to be glucose-sensing (123), with the NAc shell predominantly expressing GI neurons and the NAc core predominantly expressing GE neurons. However, these experiments used glucose at 500 mM, which is higher than the expected physiological concentration (123). In the rat, 6% of tested medial amygdala neurons increased their firing with increased glucose (from 0.5 to 2.5 mM), while 7.5% were GI neurons (177). Hippocampal neurons may also be glucose responsive. They express glucokinase (GK) (91), ATP-dependent K+ (KATP) channels (70), and GLUT1 (23), as well as the metabolism-independent heterodimeric sweet taste receptor T1R2/T1R3, in the CA fields and dentate gyrus (130).
Glucose-sensing neurons are also found in hindbrain regions. The nucleus of the solitary tract (NTS) contains glucose-sensing populations (12, 90), with 35% of NTS neurons excited by 5 mM glucose and 21% inhibited by 5 mM glucose (108). Many of these neurons also respond to melanocortin signaling (108), which can act in the NTS to modulate food intake and body weight (36). In the lateral parabrachial nucleus (PBN) of the pons, cholecystokinin (CCK)-expressing neurons are glucose responsive, showing a significant increase in activity as glucose levels fall from 5 to 0.5 mM (39, 53). Lateral PBN neurons are also known to play a role in appetite suppression (39, 53).
Multiple mechanisms have been reported to contribute to neuronal glucose sensing, as summarized in Fig. 1. The best-characterized glucose-sensing mechanism parallels that in pancreatic beta cells. GE neurons in the ARC and VMH use GK (hexokinase IV), rather than hexokinase I, for the initial step of glucose metabolism, catalyzing phosphorylation of glucose to glucose-6-phosphate (35, 76). Unlike hexokinase I, GK is a low-affinity kinase with a high Km for glucose and without end-product inhibition (4). GK activity is, therefore, proportional to glucose concentrations. Increasing glucose concentrations increase glucose metabolism and the intracellular ATP-to-ADP ratio (97), which, in some GE neurons, leads to closure of the KATP channel, resulting in depolarization and activation (2). Additional K+ and cation channels have also been described to mediate glucose sensing in some populations of GE neurons, including GE neurons of the PVH (104) and gonadotropin-releasing hormone neurons (134), but sensing mechanisms remain unknown in other cell types (16, 104, 134). GK is also expressed in GI neurons in the VMH and other regions (149). In VMH GI neurons, reduced glucose levels decreased cystic fibrosis transmembrane conductance regulator Cl− currents, which in turn depolarized and activated the cells (116). Pathways involving AMP-activated protein kinase and nitric oxide are likely to be involved. In addition, AMP-activated protein kinase has been postulated to be a glucose sensor in some ARC and anterior hypothalamic neurons (117, 134).
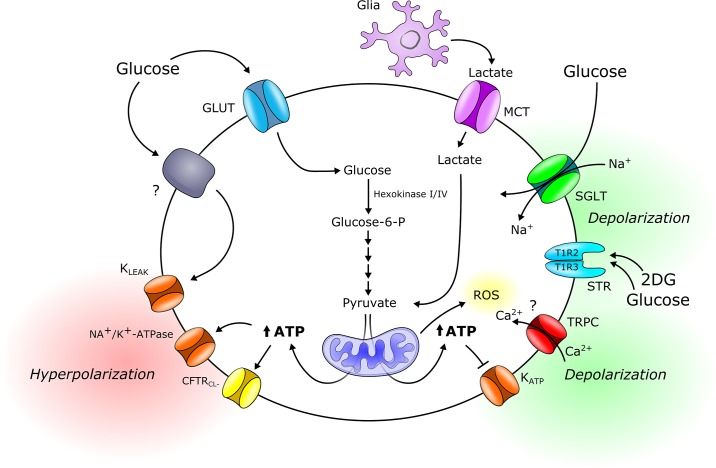
Fig. 1.
Schematic drawing of proposed neural glucose-sensing mechanisms. Glucose can be transported into the neuron by glucose transporters (GLUTs) or cotransported with Na+ by sodium-glucose transport proteins (SGLTs). The latter mechanism of entry would lead to depolarization and increased excitability of the neuron by generation of an inward current. Inside the neuron, glucose phosphorylation by glucokinase (hexokinase IV) acts as a rate-limiting step proportional to extracellular glucose concentrations. Lactate can be taken up by the neuron via monocarboxylate transporters (MCTs) to serve as a glycolytic substrate and metabolized into pyruvate. Mitochondrial uptake and metabolism of pyruvate lead to an increased ATP-to-ADP ratio and inhibition of ATP-dependent K+ (KATP) channels, resulting in plasma membrane depolarization and neurotransmitter release. Alternatively, an increased ATP-to-ADP ratio could increase Na+/K+-ATPase activity or trigger cystic fibrosis transmembrane conductance regulator (CTFRCl) channel opening, resulting in plasma membrane hyperpolarization. In orexin-expressing glucose-inhibited neurons, both glucose and 2-deoxy-d-glucose (2DG) interact with an unknown protein to trigger K+ leak (Kleak) channels, resulting in plasma membrane hyperpolarization. The metabolism-independent sweet taste receptor (STR) T1R2/T1R3 has been implicated in the glucose response of non-proopiomelanocortin (POMC) glucose-excited neurons in the arcuate nucleus (ARC). Transient receptor potential canonical (TRPC) type 3 cation channels are implicated in the glucose response of glucose-excited neurons of the mediobasal hypothalamus via a mechanism dependent on reactive oxygen species (ROS)/H2O2 production, rather than KATP channels. However, the exact mechanism in which ROS activates TRPC channels remains elusive. Glucose-6-P, glucose-6-phosphate.
Some glucose-sensing neurons do not require glucose metabolism for sensing. Both glucose (5 mM) and 2-deoxy-d-glucose (2DG), a nonmetabolizable glucose analog (1 and 5 mM), can inhibit orexin-expressing GI neurons in the LH (59). Glucose and 2DG interact with an unknown molecular player to initiate events that lead to the opening of a K+ leak channel and hyperpolarization. Sodium-glucose cotransporters (SGLTs) are present in some glucose-sensing neurons. SGLT1, -3a, and -3b are present in subpopulations of GE neurons, where expression would link inward transport of Na+ with glucose to produce depolarization (77, 119). A small number of VMH GI neurons have also been reported to express SGLT1 (77). A number of hypothalamic glucose-sensing populations also express additional GLUTs, including the lower-affinity GLUT2 and insulin-dependent GLUT4, both of which are expressed in some GE- and GI-sensing neurons (77).
The anorexigenic proopiomelanocortin (POMC) neurons of the ARC have been proposed to be glucose-sensing; however, the glucose-sensing mechanism in this population is unclear (42). The Kir6.2 subunit of the KATP channel has been reported to mediate the glucose response of ARC POMC GE neurons at between 3−5 mM and 5−10 mM glucose (71, 124), whereas the mitochondrial fission regulator dynamin-related protein 1 has been implicated at 0.2−2.5 mM glucose (140). However, other studies have found that POMC neurons do not respond to glucose or respond via an indirect, rather than a direct, mechanism (41, 42, 167). On the other hand, metabolism-independent sweet taste receptors have been implicated in the glucose-sensing properties of ARC non-POMC GE neurons at high (10 mM) glucose concentrations (82). The ARC also harbors neuropeptide Y/agouti-related protein-expressing (NPY/AgRP) neurons, a subset of which is inhibited by an increase in glucose from 1 to 10 mM (42, 115). NPY/AgRP GI neurons appear to be directly activated by decreased glucose levels (from 2.5 mM to 0.1 mM) via K+ channel closure (66), similar to LH NPY neurons (98). Both ARC POMC neurons and NPY/AgRP neurons have been reported to express GK in rats (35) and mice (149).
Orexin/hypocretin neurons of the LH are inhibited by increasing glucose levels (from 0.2 to 4.5 mM) via tandem-pore K+ (K2P) channels (17, 59). However, while TASK1 and TASK3 channels of the K2P channel superfamily increase membrane excitability of orexin neurons, they do not appear to be essential for the glucose response of orexin neurons, as revealed by studies using 0.2−5 mM glucose in TASK1 knockout mice, TASK3 knockout mice, or TASK1/3 double-knockout mice (58, 63).
A majority of putative PVH parvocellular neurons are GE and GI neurons, responding to 0.2−10 mM glucose (104). Although the glucose-sensing mechanism is not fully established, the response of PVH GE neurons to glucose does not appear to require KATP channels (104).
Transient receptor potential (TRP) canonical type 3 (TRPC3) channels were recently shown to be involved in glucose detection in vivo and required for the glucose response of isolated GE neurons of the mediobasal hypothalamus (including the ARC and VMH) in vitro (identified by a response to an increase from 2.5 to 10 mM glucose) (24). However, it remains to be determined which specific neurons within the mediobasal hypothalamus express TRPC3 (24).
Studies have identified neuronal populations that respond to glucose fluctuations >5 mM, usually referred to as high-GE or high-GI neurons. The firing rate of isolated orexin neurons of the LH was shown to be inhibited at 10−30 mM glucose (18, 171). High-GE and high-GI neurons that respond to >5 mM changes in glucose concentration have also been identified in the mediobasal hypothalamus (24) and, specifically, in the ARC (42, 43, 71), and these high-GE ARC neurons appear to sense glucose via a KATP channel-independent mechanism (43, 167).
Glucose sensing is not limited to neurons. Tanycytes lining the third ventricle express several proteins that may be involved in glucose sensing, such as GK, KATP channels, and GLUT2. These cells respond to elevated glucose (tested at 0.5−8 mM) by increasing intracellular Ca2+ in vitro (37), although these cells also respond to nonmetabolizable 2DG (49). Astrocytes have also been reported to be involved in glucose sensing, and GLUT2 expression has been described in astrocytic populations in the hypothalamus (62) and brain stem (100). Furthermore, astrocytes take up glucose, a large part of which enters the glycolytic pathway and is released as lactate via monocarboxylate transporters into the extracellular space (10, 125). Lactate and glucose exist in similar concentrations in the extracellular space, and there is growing evidence that lactate may be an energy source for neurons (52, 141). Astrocytes also play essential roles in the neurovascular unit, and they may regulate cerebral blood flow based on the metabolic state of neurons (60).
EVIDENCE FOR NEURAL REGULATION OF METABOLISM
The brain not only detects changes in glucose levels, but animal and human studies suggest that the CNS plays a role in glucose regulation. Peripheral and intraventricular injections of 2DG, which mimics hypoglycemia, increase expression of c-fos, an early-immediate gene and a marker of neural activation, in many brain regions expressing glucose-sensing populations, including the ARC (14, 109), VMH (48), and LH (15). Central 2DG administration in rats suppresses pancreatic insulin release (50, 113), increases plasma glucagon, enhances hepatic glucose, induces lipolysis (27), and increases food intake (114). Central injection of glucose suppresses plasma glucose (120). In humans, glucose infusion has been shown to alter CNS activity in hypothalamic regions, including the VMH and LH (121). Additional studies have examined the effects of hypothalamic lesions and electrical stimulation on glucose metabolism. VMH lesioning in mice and rats can modulate pancreatic hormone release (61), peripheral glucose uptake (29), and glucose production by the liver (126). The neural and hormonal responses to restore glucose levels after hypoglycemia are also diminished. ARC lesions with monosodium glutamate prevented hypoglycemia-induced feeding (94), while PVH lesions increased feeding and body weight but only altered insulin secondary to obesity (145). In rodents, VMH, PVH, and anterior hypothalamus stimulation using electrodes increased blood glucose (142). LH stimulation had the opposite effect, decreasing blood glucose in rats (31) and rabbits (142) and increasing glycogenesis (7). Similarly, posterior hypothalamic stimulation reduced blood glucose (154). Lesions and stimulation outside the hypothalamus also regulate blood glucose. Electrical stimulation of the dorsal or median raphe nuclei (75) or the rostral ventrolateral medulla (84) increased blood glucose, while stimulation of the nucleus ambiguus (6), NTS, and dorsal motor nucleus of the vagus increased plasma insulin (73). Lesioning of the medial amygdala was shown to impair the counterregulatory hormonal response to hypoglycemia (177).
More recently, neuron-specific drivers have been used to modulate the expression of specific factors, such as receptors, channels, and neurotransmitters, in distinct cell populations in the hypothalamus and elsewhere to determine how they contribute to glucose regulation. These studies are summarized in Table 1. However, lesioning and electrical stimulation studies affect multiple neural cell types as well as fibers passing through the regions involved. The consequences of transgenic expression or deletion of specific proteins can be masked or blunted by compensatory effects. So technologies that can rapidly and reversibly activate or inhibit defined neural populations provide invaluable tools to investigate how specific neural populations regulate blood glucose.
Table 1.
Summary of studies examining effects of manipulation of anatomically or molecularly defined neural populations on glucose metabolism and appetite
| Area | Cell Type | Intervention | Effect on Metabolism | Feeding Effect | Reference |
|---|---|---|---|---|---|
| ARC | All | Overexpression of hexokinase I | Increased fasting glucose, impaired glucose tolerance | ? | 135 |
| All | Leptin injection | Increased glucose uptake into BAT | ? | 157 | |
| AgRP + POMC | Leptin receptor KO | Increased insulin, normal glucose tolerance | Increased | 164 | |
| AgRP | Ribosomal S6K1 KO | Muscle insulin resistance | Normal | 146 | |
| AgRP | Postembryonic deletion | Decreased insulin | Decreased | 8 | |
| AgRP | Postembryonic deletion | Normal glucoprivic feeding | Decreased | 95 | |
| AgRP | Insulin receptor KO | Loss of insulin suppression of hepatic glucose production | Normal | 85 | |
| AgRP | AMPK2α KO | Loss of glucose sensing in subpopulation of AgRP neurons, no effect on insulin | Unchanged | 25 | |
| AgRP | Postnatal ablation by diphtheria toxin | Increased lipid utilization, decreased sympathetic outflow | Blunted refeeding | 74 | |
| AgRP | TXNIP deletion | Improved fasting glucose and glucose tolerance | Decreased weight | 11 | |
| AgRP | Glutamaterigc GluN2B receptor KO | Correction of hyperglycemia in ob/ob mice | Unchanged | 162 | |
| AgRP | Leptin receptor replacement in ob/ob mice | Correction of hyperglycemia | Normal | 57 | |
| AgRP | GHSR restoration in KO mice | Correction of hypoglycemia with calorie restriction | Normal | 166 | |
| AgRP | Carnitine acetyltransferase deletion | Reduced glucose disposal and suppression of glucose production | Decreased | 129 | |
| AgRP | CRF receptor 1 KO | Reduced hepatic glucose production in fasting in females | Normal | 88 | |
| AgRP | Loss of GABA signaling | Glucose intolerance in young mice | Reduced | 106 | |
| AgRP | Antagonism of AP1 transcription factor | Improved glucose clearance and insulin sensitivity | ? | 72 | |
| POMC | Ribosomal S6K1 KO | Hepatic insulin resistance | ? | 146 | |
| POMC | AMPK2α KO | Loss of glucose sensing in POMC neurons | Increased | 25 | |
| POMC | ATP-insensitive KATP channel | Impaired glucose sensing in POMC neurons, impaired glucose tolerance | ? | 124 | |
| POMC | Inositol-requiring enzyme 1 deficiency | Impaired glucose and insulin tolerance, reduced hepatic insulin sensitivity | Increased | 172 | |
| POMC | Antagonism of AP1 transcription factor | Improved glucose clearance and insulin sensitivity | 72 | ||
| POMC | Arcuate POMC KO | Improved glucose tolerance | 22 | ||
| PVH | All | MT-II injection | Increased glucose uptake into BAT | ? | 157 |
| SIM1 | CB1 receptor KO | Increased insulin sensitivity on high-fat diet | Unchanged | 19 | |
| DMH | All | NPY knockdown | Increased hepatic insulin sensitivity | ? | 93 |
| All | CCK receptor replacement in OLETF rats | Improved glucose homeostasis | Decreased | 178 | |
| LH | All | MC4R reexpression | Improved glucose tolerance | Unchanged | 111 |
| MCH | Insulin receptor KO | Improved glucose tolerance on high-fat diet | Normal | 68 | |
| MCH | ATP-insensitive KATP channel | Impaired glucose tolerance | Normal | 83 | |
| Orexin | Orexin KO | Loss of diurnal glucose rhythm | Unchanged | 161 | |
| VMH | All | Leptin injection | Increased glucose uptake into skeletal muscle, BAT | ? | 157 |
| All | MT-II | Increased glucose uptake into skeletal muscle, BAT | ? | 157 | |
| All | LIN28a viral overexpression | Improved glucose tolerance and insulin sensitivity on high-fat diet | Unchanged | 79 | |
| SF1 | Leptin receptor KO | Glucose intolerance, hyperinsulinemia | Increased | 9 | |
| SF1 | VGLUT KO | Hypoglycemia during fasting | ? | 158 | |
| SF1 | Insulin receptor KO | Protected against insulin resistance on high-fat diet | Reduced | 81 | |
| SF1 | FOXO1 KO | Improved glucose tolerance and insulin sensitivity in muscle | Unchanged | 80 | |
| SF1 | SOCS3 KO | Improved glucose tolerance | Reduced | 174 | |
| MPO | All | Norepinephrine injection | Increased plasma glucose and insulin | ? | 47 |
| VLM | CA | CA neuron ablation | Abolished hyperglycemic response to stress | ? | 176 |
| PBN | CCK | Leptin receptor KO | Enhanced counterregulatory response to hypoglycemia | Unchanged | 45 |
| PAG | Leptin receptor | Optogenetic activation | Increased blood glucose | Unchanged | 44 |
AgRP, agouti related peptide; AMPK2α, AMP-activated protein kinase α-subunit; AP1, activator protein 1; ARC, arcuate nucleus of the hypothalamus; BAT, brown adipose tissue; CA, catecholaminergic; CB1, cannabinoid type 1; CCK, cholecystokinin; CRF, corticotropin-releasing factor; DMH, dorsomedial nucleus of the hypothalamus; FOXO1, forkhead box protein O1; GABA, γ-aminobutyric acid; GHSR, growth hormone secretagogue receptor; GluN2B, glutamate ionotropic receptor NMDA type 2B; KATP, ATP-dependent K+ channel; KO, knockout; LH, lateral hypothalamus; LIN28a, RNA binding protein; MCH, melanin-concentrating hormone; MC4R, melanocortin 4 receptor; MPO, medial preoptic area; MT-II, melanotan II; NPY, neuropeptide Y; OLETF, Otsuka Long Evans Tokushima fatty rat; PAG, periaqueductal gray; PBN, parabrachial nucleus; PVH, paraventricular nucleus of the hypothalamus; POMC, proopiomelanocortin; SF1, steroidogenic factor 1; SIM-1, single-minded 1; SOCS3, suppressor of cytokine signaling 3; S6K1, ribosomal S6 protein kinase 1; TXNIP, thioredoxin-interacting protein; VGLUT, vesicular glutamate transporter; VLM, ventrolateral medulla; VMH, ventromedial hypothalamic nucleus.
TRANSGENIC ANIMAL MODELS FOR STUDIES OF GLUCOSE METABOLISM
Transgenic mouse models have emerged as invaluable tools for targeting specific neuronal populations such as subsets of glucose-sensing neurons, particularly in combination with viral tools for optogenetics, chemogenetics, and magneto-/radiogenetics. However, because of the heterogeneous nature of glucose-sensing neurons and the wide variety and low expression of glucose-sensing markers, specific glucose-sensing populations remain difficult to genetically pinpoint. GK is a marker for some glucose-sensing populations but is not expressed in all glucose-sensing neurons, and some GK-expressing neurons do not respond to glucose. For instance, in the VMH, GK is expressed in 64% of GE neurons, 43% of GI neurons, and 8% of non-glucose-sensing neurons (77). Another marker of some glucose-sensing neurons, the Kir6.2 subunit of the KATP channel, is expressed in 42% of GE and GI neurons and 15% of non-glucose-sensing neurons (77). Viral tools can also be targeted to neural populations with cre expression driven by specific neuropeptide promoters such as CCK, POMC, or AgRP. However, often only a portion of these neuropeptide-expressing neurons are glucose-sensing. Targeted mutations in putative glucose-sensing mechanisms can be valuable to address the role of specific neurons. For instance, a mouse model with transgenic expression of mutant Kir6.2 subunits preventing ATP-mediated closure of KATP channels was used to disrupt glucose sensing in POMC neurons (124). Moreover, TASK1/3 knockout mice have been used to address the glucose-sensing mechanisms of orexin neurons (58, 63). Advanced Cre-LoxP transgenic mouse models allowing targeting of more defined subtypes of neurons will be invaluable tools for targeting glucose-sensing neurons in the future.
NEUROMODULATORY TOOLS TO ASSESS METABOLIC REGULATION
Several techniques for acute neuromodulation use light, chemicals, or electromagnetic fields to gate ion channels or activate receptors. The most widely used technologies today are optogenetics and chemogenetics. These technologies allow acute activation or silencing of anatomically and/or genetically defined neural populations. When applied to glucose-sensing neural populations, they allow us to examine the effects of modulating these neural populations on metabolic parameters such as blood glucose, hormone release, and sensitivity. Unlike life-long genetic manipulations, compensatory mechanisms that may blunt or hide essential roles of specific populations are unlikely. In addition, the precise temporal control and reversibility may allow better correlation between the activity of specific neurons and physiological changes. These tools, in combination with existing technologies such as transgenic mice, allow unprecedented investigation of the neural populations and circuits regulating glucose metabolism.
Optogenetics
Optogenetic tools use the targeted expression of light-responsive opsins to activate or inhibit specific neural populations. Channelrhodopsins are robustly and rapidly (on the order of milliseconds) opened by light and can be used to deliver a specific pattern of neural activity to the target neural population (13). A wide array of channels, with multiple temporal patterns and other characteristics, can be applied to investigate the roles of neural populations. Because light spread in the CNS is relatively limited (105), optogenetics can be used to modulate cell bodies or specific projection sites with precision (128). However, the limited light spread can be a drawback in some studies. For most optogenetic studies, a fiber-optic implant is needed to deliver light stimuli to the target region, especially for regions deep in the CNS, such as the hypothalamus. Only populations immediately adjacent to the fiber will receive sufficient light delivery to be activated or inhibited. Thus, target populations that are spread throughout a CNS region or structure can be difficult to modulate by optogenetics. In addition, optogenetic studies require light delivery to the implant itself through a head-mounted light source or an optical cable. Animals need to be restrained for attachment of the light source or cable, and this can be stressful and alter glucose metabolism even in well-handled animals. Tethering by the light cable during studies can also be a stressor or interfere with the behaviors being investigated.
Optogenetics has been used extensively for mapping of neuronal networks underlying the control of feeding (see Ref. 136 for review), but far fewer studies have examined the cell populations and circuits controlling glucose metabolism. Photoactivation of VMH steroidogenic factor 1 (SF1) neurons was shown to induce hyperglycemia, whereas photoinhibition had no effect on glucose levels under basal conditions but severely impaired recovery from hypoglycemia (103). Photostimulation of LH neurons expressing melanin-concentrating hormone (MCH) highlighted their role in sensing the nutrient value of sucrose and their potential roles in nutrient preference (34). Optogenetic tools have also been used to modulate GLUT2-expressing neurons. Activation of glucose-inhibited, GLUT2-expressing neurons in the paraventricular thalamus increased sucrose intake (89). Other studies that examined photostimulation of a population of glucose-inhibited, GLUT2-expressing neurons in the NTS showed that activation significantly increased vagal activity and blood glucagon levels (90).
Chemogenetics
Chemogenetics offers an alternative approach for neuromodulation based on engineered molecules and ligands. These systems use G-protein coupled receptors (1, 118) or ion channels (92, 96) engineered to be unresponsive to their natural ligand but sensitive to an inert (or largely inert) reagent. The modified channel or receptor is expressed in the target neural population and stimulated by peripheral injection of its ligand, leading to channel opening or receptor activation and neuromodulation (155). Unlike optogenetics, chemogenetic tools can be used to activate or silence cells in multiple regions or across large structures. The animals are not tethered and can move freely during experiments. However, some of the chemogenetic technologies are relatively slow (of the order of minutes) (137), and animals still need to be handled to administer the ligand, which can act as a stressor to perturb some metabolic studies. Off-target effects from the ligand or its metabolites may also require consideration (56). Chemogenetics has been used to characterize the metabolic effects of activating glucose-sensing neurons (163, 168). The effects of activating VMH SF1 neurons on glucose uptake and insulin sensitivity in skeletal muscle, brown adipose tissue, and heart were tested using the designer receptor exclusively activated by designer drugs (DREADD) hM3Dq (30). Activation of SF1 neurons increased hepatic glucose production and glucose uptake into skeletal muscle. In the ARC, chemogenetic activation of AgRP neurons, but not POMC neurons, impaired glucose tolerance and insulin sensitivity of brown adipose tissue (153). Interestingly, the same study found that optogenetic activation of AgRP neurons only impaired insulin sensitivity, a discrepancy possibly related to methodological differences (153). Chemogenetic stimulation of neurons expressing preproglucagon peptide (GCG neurons) suppressed glucose production without affecting glucose uptake and reduced metabolic rate and food intake in the fed and fasted states of lean GCG-cre mice (54). In GCG-cre mice with diet-induced obesity, the effect of chemogenetic GCG neuronal stimulation on glucose production was abolished, but the effect of reduced food intake persisted (54). Chemogenetic experiments have also been used to assess the effects of activating LH MCH neurons but showed that excitation of MCH neurons had no effect on glucose tolerance or insulin sensitivity (68).
Chemogenetic tools have also been used to examine the roles of glucose-sensing neurons outside the hypothalamus. DREADD modulation of glucose-inhibited CCK neurons in the lateral PBN increased blood glucose, suggesting that these neurons are likely to be involved in the counterregulatory response to hypoglycemia (45). Similarly, DREADD activation of leptin receptor-expressing neurons in the PBN or periaqueductal gray increased blood glucose and adrenal sympathetic nerve activity (44). Chemogenetic activation of an additional glucose-sensing population, catecholaminergic neurons in the ventrolateral medulla, also increased blood glucose (176).
Radio- and Magnetogenetics
Remote neural modulation using electromagnetic fields, which has been termed “magnetogenetic” or “radiogenetic” modulation, allows minimally invasive neural regulation. Low-frequency, alternating magnetic fields are already used clinically to program pacemakers (64) or neural stimulation devices. Unlike tissue, which does not absorb these fields to any significant extent (173), metal oxide nanoparticles absorb energy from electromagnetic fields to a degree that depends on the size, shape, and material of the nanoparticle and the strength of the applied field (46, 65, 99, 132). The energy captured by nanoparticles from electromagnetic fields can be used to open a modified ion channel, which, in turn, regulates neural activity. A number of technologies using either injected or genetically encoded nanoparticles have been developed for this purpose. Iron oxide nanoparticles modified to prevent cell uptake can be injected into the CNS (21) and will then act on target cells that have been altered to express a genetically modified multimodal ion channel, TRP vanilloid 1 (TRPV1). An alternative approach is to express a modified TRPV1 channel with an epitope tag inserted into the first extracellular loop in target cells. This is combined with injection of iron oxide nanoparticles coated with a tag-specific antibody that then bind to the channel (150). When alternating magnetic fields are applied, the nanoparticles absorb this energy and transduce it into TRPV1 channel activation, leading to Na+ and Ca2+ entry into the cell and depolarization. However, injected nanoparticles can only target a limited area to modulate cell activity. In addition, the particles can be cleared from the injection site by endocytosis (138), so multiple nanoparticle injections may be needed for repeated neural activation.
Modified versions of this technology have been developed to overcome the need for nanoparticle injection. These systems use genetically encoded nanoparticles, rather than extracellular particles, to activate ion channels. Genetically encoded nanoparticles in these technologies are produced intracellularly by a modified form of ferritin. Ferritin is an almost ubiquitous iron storage protein consisting of 24 subunits that self-aggregate to form a protein shell. Once assembled, iron ions from the cytoplasm are taken up into the ferritin shell and form an iron oxide nanoparticle (156, 175). In the first system, outlined in Fig. 2, two components are required: 1) ferritin, which has been modified by addition of green fluorescent protein (GFP), and 2) a modified TRPV1 channel with the addition of a small anti-GFP domain. When these components are expressed in the target cells, the GFP-tagged ferritin self-assembles and iron oxide nanoparticles form within the shell, while the modified TRPV1 channel is expressed at the cell surface and tethers the GFP-ferritin through its anti-GFP domain (151, 152). In the second system, the alternative multimodal ion channel TRPV4 is expressed as a fusion protein with ferritin and targeted to defined cell populations (169). In both cases, when the target cells are treated with alternating or static magnetic fields (radio- or magnetogenetic systems, respectively), energy absorbed by nanoparticles in the ferritin lead to TRP channel activation, depolarization, and neural activation. The mechanism(s) linking energy absorption by the nanoparticles to channel opening remain unknown. One possibility is that the applied magnetic field leads to a change in entropy and a very localized temperature change, which, in turn, activates the associated ion channel.
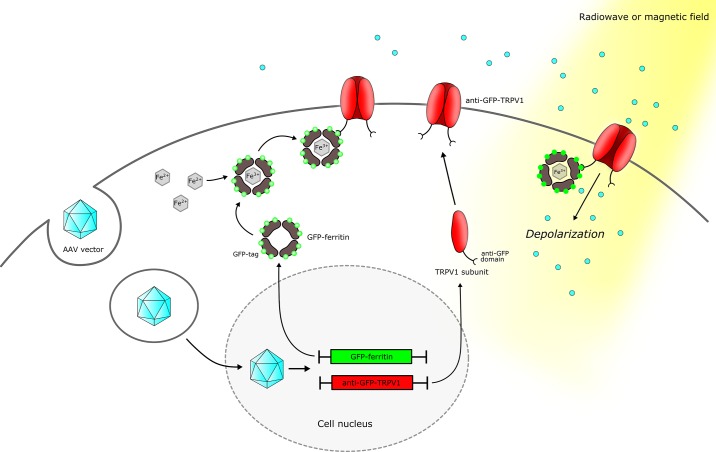
Fig. 2.
Neuronal modulation using radio- or magnetogenetics. A viral vector can be used to deliver the 2 components required for radio- or magnetogenetics, GFP-ferritin and anti-GFP-TRPV1, to the target cell. GFP-ferritin is a ubiquitous iron storage protein that has been modified by addition of green fluorescent protein (GFP), whereas anti-GFP-TRPV1 is a transient receptor potential vanilloid 1 (TRPV1) cation channel modified by addition of a small anti-GFP domain. Once expressed by the cell, the GFP-ferritin self-assembles into a shell and sequesters iron ions in a nanoparticle. TRPV1 subunits self-assemble on the cell membrane. The GFP tag of GFP-ferritin will bind to the anti-GFP domain of the first extracellular loop of the modified TRPV1 channel, thus tethering GFP-ferritin to the modified TRPV1 channel. Application of a radio frequency or a magnetic field will transfer energy to the iron oxide nanoparticles within the GFP-ferritin and trigger ion channel opening, Na+ and Ca2+ entry into the cell, and depolarization.
Electromagnetic treatment leads to rapid (on the order of <1 s) neural activation to assess the function of defined neural populations. This technology has been further modified to develop a tool for remote neural inhibition. Mutation of an amino acid residue in the pore region of the TRPV1 channel leads to a change in its ion conductance from cations (predominantly Ca2+ and Na+) to anions (Cl−) (87). When this mutant TRPV1 (TRPV1mutant) channel is fused to the anti-GFP domain and expressed in cells with GFP-ferritin, electromagnetic field treatment opens the TRPV1mutant channel, leading to Cl− entry, hyperpolarization, and silencing of neural activity. The components required for radio- or magnetogenetics can be delivered by virus injection into defined anatomic regions and/or targeted to defined cell populations with use of cre-lox technology (151, 169).
Radio- and magnetogenetic tools have a number of advantages. They can be applied to activate or silence populations in multiple areas or across a large CNS region. There is no need to handle the experimental animal to connect the optical cable or inject a ligand before the study, and the animal can move freely during the experiment. The range of tools is currently smaller than those for optogenetics or chemogenetics, but these techniques allow fast, wireless neuromodulation in a freely moving animal without an implant and without the need for injection. These characteristics make these technologies particularly useful for studying physiological functions that can be disrupted by stress, such as glucose regulation.
RADIO- AND MAGNETOGENETIC NEURAL MODULATION TO ASSESS GLUCOSE METABOLISM
The roles of a population of GK-expressing VMH glucose-sensing neurons in glucose metabolism and feeding have been examined using electromagnetic modulation. GK-expressing neurons were targeted by injection of the cre-dependent activating construct (adeno-associated virus containing anti-GFP-TRPV1 and GFP-ferritin) into the VMH in GK-cre and wild-type (WT) mice (151). In fed GK-cre mice, both alternating magnetic field (radio waves, 31 mT, 30 min) and gradient magnetic field treatment used to activate VMH GK neurons significantly increased blood glucose. This effect was proportional to field strength and similar in time course and magnitude to optogenetic stimulation of VMH GK neurons (15-ms pulse width for 30 min, 5 Hz) (151). Plasma glucagon and growth hormone levels were significantly increased, as was expression of hepatic glucose-6-phosphatase, a gluconeogenic enzyme. Magnetic field activation of VMH GK neurons also significantly increased feeding. Magnetogenetic activation in WT mice or in the dorsal striatum, a brain area not known to express glucose-sensing neurons, did not alter blood glucose. The endocrine and behavioral responses generated by activation of VMH GK neurons mirror many of the responses to hypoglycemia, suggesting that GI neurons are the functionally dominant population in this region (151).
The effects of magnetogenetic silencing of VMH GK neurons were also assessed using cre-dependent expression of anti-GFP-TRPV1mutant/GFP-ferritin in GK-cre and WT mice (151). In contrast to activation, inhibition of GK-expressing neurons in GK-cre mice led to a significant decrease in blood glucose in the fasted state with increased plasma insulin, reduced hepatic expression of glucose-6-phosphatase, and decreased feeding after a 4-h fast compared with WT mice. In addition, silencing VMH GK neurons diminished the counterregulatory response to neuroglycopenia following 2DG injection (151).
In combination, these studies demonstrate the utility of remote neural activation and silencing using electromagnetic fields. In addition, they suggest that GK-expressing VMH neurons may acutely modulate food intake and glucose metabolism and, therefore, may contribute to maintaining blood glucose during fasting and hypoglycemia.
SUMMARY AND FUTURE DIRECTIONS
Studies using targeted neuromodulatory tools such as optogenetics, chemogenetics, and radio-/magnetogenetics have already transformed our knowledge of the cells and circuits underlying many neurological diseases. These tools are now being used to assess how specific glucose-sensing neural populations regulate metabolism, including plasma glucose. When combined with in vivo imaging techniques to assess neural activity, tissue clearing to define circuits in whole brains (38, 131), and technologies to examine gene expression in defined neural populations, these tools may allow us to better understand how defined cells and their projections contribute to metabolic regulation. Future studies will determine if similar cells and circuits play a role in human metabolic regulation and how these are disrupted in metabolic disease.
GRANTS
A. Alvarsson is supported by a postdoctoral fellowship from the Swedish Society for Medical Research. Support for this work was also provided by National Institutes of Health Grants MH-105941 and 1R01 NS-097184, American Diabetes Association Grant 1-17-ACE-31, an Einstein-Mt. Sinai Diabetes Research Center Pilot and Feasibility Award (National Institute of Diabetes and Digestive and Kidney Diseases Grant P30 DK-020541), and an Alexander and Alexandrine Sinsheimer Scholar Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.A. and S.A.S. prepared figures; A.A. and S.A.S. drafted manuscript; A.A. and S.A.S. edited and revised manuscript; S.A.S. approved final version of manuscript.
REFERENCES
- 1.Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63: 27–39, 2009. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashford ML, Boden PR, Treherne JM. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflügers Arch 415: 479–483, 1990. doi: 10.1007/BF00373626. [DOI] [PubMed] [Google Scholar]
- 3.Ashrafi G, Ryan TA. Glucose metabolism in nerve terminals. Curr Opin Neurobiol 45: 156–161, 2017. doi: 10.1016/j.conb.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachelard HS. Glucose-phosphorylating enzyme with high Km in human brain. Nature 215: 959–960, 1967. doi: 10.1038/215959a0. [DOI] [PubMed] [Google Scholar]
- 5.Barnes MB, Lawson MA, Beverly JL. Rate of fall in blood glucose and recurrent hypoglycemia affect glucose dynamics and noradrenergic activation in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol 301: R1815–R1820, 2011. doi: 10.1152/ajpregu.00171.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bereiter DA, Berthoud HR, Brunsmann M, Jeanrenaud B. Nucleus ambiguus stimulation increases plasma insulin levels in the rat. Am J Physiol Endocrinol Metab 241: E22–E27, 1981. doi: 10.1152/ajpendo.1981.241.1.E22. [DOI] [PubMed] [Google Scholar]
- 7.Bernardis LL, Bellinger LL. The lateral hypothalamic area revisited: neuroanatomy, body weight regulation, neuroendocrinology and metabolism. Neurosci Biobehav Rev 17: 141–193, 1993. doi: 10.1016/S0149-7634(05)80149-6. [DOI] [PubMed] [Google Scholar]
- 8.Bewick GA, Gardiner JV, Dhillo WS, Kent AS, White NE, Webster Z, Ghatei MA, Bloom SR. Post-embryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. FASEB J 19: 1680–1682, 2005. doi: 10.1096/fj.04-3434fje. [DOI] [PubMed] [Google Scholar]
- 9.Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology 149: 2138–2148, 2008. doi: 10.1210/en.2007-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binkofski F, Loebig M, Jauch-Chara K, Bergmann S, Melchert UH, Scholand-Engler HG, Schweiger U, Pellerin L, Oltmanns KM. Brain energy consumption induced by electrical stimulation promotes systemic glucose uptake. Biol Psychiatry 70: 690–695, 2011. doi: 10.1016/j.biopsych.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Blouet C, Liu SM, Jo YH, Chua S, Schwartz GJ. TXNIP in Agrp neurons regulates adiposity, energy expenditure, and central leptin sensitivity. J Neurosci 32: 9870–9877, 2012. doi: 10.1523/JNEUROSCI.0353-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boychuk CR, Gyarmati P, Xu H, Smith BN. Glucose sensing by GABAergic neurons in the mouse nucleus tractus solitarii. J Neurophysiol 114: 999–1007, 2015. doi: 10.1152/jn.00310.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8: 1263–1268, 2005. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 14.Briski KP. Glucoprivic induction of Fos immunoreactivity in hypothalamic dopaminergic neurons. Neuroreport 9: 289–295, 1998. doi: 10.1097/00001756-199801260-00021. [DOI] [PubMed] [Google Scholar]
- 15.Briski KP, Sylvester PW. Hypothalamic orexin-A-immunpositive neurons express Fos in response to central glucopenia. Neuroreport 12: 531–534, 2001. doi: 10.1097/00001756-200103050-00020. [DOI] [PubMed] [Google Scholar]
- 16.Burdakov D, Gerasimenko O, Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J Neurosci 25: 2429–2433, 2005. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O’Kelly I, Gerasimenko O, Fugger L, Verkhratsky A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron 50: 711–722, 2006. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 18.Burdakov D, Luckman SM, Verkhratsky A. Glucose-sensing neurons of the hypothalamus. Philos Trans R Soc Lond B Biol Sci 360: 2227–2235, 2005. doi: 10.1098/rstb.2005.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardinal P, Bellocchio L, Guzmán-Quevedo O, André C, Clark S, Elie M, Leste-Lasserre T, Gonzales D, Cannich A, Marsicano G, Cota D. Cannabinoid type 1 (CB1) receptors on Sim1-expressing neurons regulate energy expenditure in male mice. Endocrinology 156: 411–418, 2015. doi: 10.1210/en.2014-1437. [DOI] [PubMed] [Google Scholar]
- 20.Chen LQ, Cheung LS, Feng L, Tanner W, Frommer WB. Transport of sugars. Annu Rev Biochem 84: 865–894, 2015. doi: 10.1146/annurev-biochem-060614-033904. [DOI] [PubMed] [Google Scholar]
- 21.Chen R, Romero G, Christiansen MG, Mohr A, Anikeeva P. Wireless magnetothermal deep brain stimulation. Science 347: 1477–1480, 2015. doi: 10.1126/science.1261821. [DOI] [PubMed] [Google Scholar]
- 22.Chhabra KH, Morgan DA, Tooke BP, Adams JM, Rahmouni K, Low MJ. Reduced renal sympathetic nerve activity contributes to elevated glycosuria and improved glucose tolerance in hypothalamus-specific Pomc knockout mice. Mol Metab 6: 1274–1285, 2017. doi: 10.1016/j.molmet.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choeiri C, Staines W, Miki T, Seino S, Messier C. Glucose transporter plasticity during memory processing. Neuroscience 130: 591–600, 2005. doi: 10.1016/j.neuroscience.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Chrétien C, Fenech C, Liénard F, Grall S, Chevalier C, Chaudy S, Brenachot X, Berges R, Louche K, Stark R, Nédélec E, Laderrière A, Andrews ZB, Benani A, Flockerzi V, Gascuel J, Hartmann J, Moro C, Birnbaumer L, Leloup C, Pénicaud L, Fioramonti X. Transient receptor potential canonical 3 (trpc3) channels are required for hypothalamic glucose detection and energy homeostasis. Diabetes 66: 314–324, 2017. doi: 10.2337/db16-1114. [DOI] [PubMed] [Google Scholar]
- 25.Claret M, Smith MA, Knauf C, Al-Qassab H, Woods A, Heslegrave A, Piipari K, Emmanuel JJ, Colom A, Valet P, Cani PD, Begum G, White A, Mucket P, Peters M, Mizuno K, Batterham RL, Giese KP, Ashworth A, Burcelin R, Ashford ML, Carling D, Withers DJ. Deletion of Lkb1 in pro-opiomelanocortin neurons impairs peripheral glucose homeostasis in mice. Diabetes 60: 735–745, 2011. doi: 10.2337/db10-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coerver KA, Gray SM, Barnes JE, Armstrong DL, McCabe ER. Developmental expression of hexokinase 1 and 3 in rats. Histochem Cell Biol 109: 75–86, 1998. doi: 10.1007/s004180050204. [DOI] [PubMed] [Google Scholar]
- 27.Coimbra CC, Gross JL, Migliorini RH. Intraventricular 2-deoxyglucose, glucose, insulin, and free fatty acid mobilization. Am J Physiol Endocrinol Metab 236: E317–E327, 1979. doi: 10.1152/ajpendo.1979.236.4.E317. [DOI] [PubMed] [Google Scholar]
- 28.Cornford EM, Hyman S, Cornford ME, Clare-Salzler M. Down-regulation of blood-brain glucose transport in the hyperglycemic nonobese diabetic mouse. Neurochem Res 20: 869–873, 1995. doi: 10.1007/BF00969700. [DOI] [PubMed] [Google Scholar]
- 29.Cousin B, Agou K, Leturque A, Ferre P, Girard J, Pénicaud L. Molecular and metabolic changes in white adipose tissue of the rat during development of ventromedial hypothalamic obesity. Eur J Biochem 207: 377–382, 1992. doi: 10.1111/j.1432-1033.1992.tb17060.x. [DOI] [PubMed] [Google Scholar]
- 30.Coutinho EA, Okamoto S, Ishikawa AW, Yokota S, Wada N, Hirabayashi T, Saito K, Sato T, Takagi K, Wang CC, Kobayashi K, Ogawa Y, Shioda S, Yoshimura Y, Minokoshi Y. Activation of SF1 neurons in the ventromedial hypothalamus by DREADD technology increases insulin sensitivity in peripheral tissues. Diabetes 66: 2372–2386, 2017. doi: 10.2337/db16-1344. [DOI] [PubMed] [Google Scholar]
- 31.Curry DL, Safarik RH, Joy RM, Stern JS. Direct neural effect of lateral hypothalamic stimulation on insulin secretion by pancreases of normal and obese rats. Horm Metab Res 22: 129–135, 1990. doi: 10.1055/s-2007-1004869. [DOI] [PubMed] [Google Scholar]
- 32.Devraj K, Klinger ME, Myers RL, Mokashi A, Hawkins RA, Simpson IA. GLUT-1 glucose transporters in the blood-brain barrier: differential phosphorylation. J Neurosci Res 89: 1913–1925, 2011. doi: 10.1002/jnr.22738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Díaz-García CM, Mongeon R, Lahmann C, Koveal D, Zucker H, Yellen G. Neuronal stimulation triggers neuronal glycolysis and not lactate uptake. Cell Metab 26: 361–374.e4, 2017. doi: 10.1016/j.cmet.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Domingos AI, Sordillo A, Dietrich MO, Liu ZW, Tellez LA, Vaynshteyn J, Ferreira JG, Ekstrand MI, Horvath TL, de Araujo IE, Friedman JM. Hypothalamic melanin concentrating hormone neurons communicate the nutrient value of sugar (Abstract). eLife 2: e01462, 2013. doi: 10.7554/eLife.01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE. Glucokinase is the likely mediator of glucosensing in both glucose-excited and glucose-inhibited central neurons. Diabetes 51: 2056–2065, 2002. doi: 10.2337/diabetes.51.7.2056. [DOI] [PubMed] [Google Scholar]
- 36.Eerola K, Rinne P, Penttinen AM, Vähätalo L, Savontaus M, Savontaus E. α-MSH overexpression in the nucleus tractus solitarius decreases fat mass and elevates heart rate. J Endocrinol 222: 123–136, 2014. doi: 10.1530/JOE-14-0064. [DOI] [PubMed] [Google Scholar]
- 37.Elizondo-Vega R, Cortes-Campos C, Barahona MJ, Oyarce KA, Carril CA, García-Robles MA. The role of tanycytes in hypothalamic glucosensing. J Cell Mol Med 19: 1471–1482, 2015. doi: 10.1111/jcmm.12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Epp JR, Niibori Y, Liz Hsiang HL, Mercaldo V, Deisseroth K, Josselyn SA, Frankland PW. Optimization of CLARITY for clearing whole-brain and other intact organs. eNeuro 2, 2015. doi: 10.1523/ENEURO.0022-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Essner RA, Smith AG, Jamnik AA, Ryba AR, Trutner ZD, Carter ME. AgRP neurons can increase food intake during conditions of appetite suppression and inhibit anorexigenic parabrachial neurons. J Neurosci 37: 8678–8687, 2017. doi: 10.1523/JNEUROSCI.0798-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferguson AV. Circumventricular organs: integrators of circulating signals controlling hydration, energy balance, and immune function. In: Neurobiology of Body Fluid Homeostasis: Transduction and Integration, edited by De Luca LA Jr, Menani JV, Johnson AK. Boca Raton, FL: CRC, 2014. [PubMed] [Google Scholar]
- 41.Fioramonti X, Chrétien C, Leloup C, Pénicaud L. Recent advances in the cellular and molecular mechanisms of hypothalamic neuronal glucose detection. Front Physiol 8: 875, 2017. doi: 10.3389/fphys.2017.00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fioramonti X, Contié S, Song Z, Routh VH, Lorsignol A, Pénicaud L. Characterization of glucosensing neuron subpopulations in the arcuate nucleus: integration in neuropeptide Y and pro-opio melanocortin networks? Diabetes 56: 1219–1227, 2007. doi: 10.2337/db06-0567. [DOI] [PubMed] [Google Scholar]
- 43.Fioramonti X, Lorsignol A, Taupignon A, Pénicaud L. A new ATP-sensitive K+ channel-independent mechanism is involved in glucose-excited neurons of mouse arcuate nucleus. Diabetes 53: 2767–2775, 2004. doi: 10.2337/diabetes.53.11.2767. [DOI] [PubMed] [Google Scholar]
- 44.Flak JN, Arble D, Pan W, Patterson C, Lanigan T, Goforth PB, Sacksner J, Joosten M, Morgan DA, Allison MB, Hayes J, Feldman E, Seeley RJ, Olson DP, Rahmouni K, Myers MG Jr. A leptin-regulated circuit controls glucose mobilization during noxious stimuli. J Clin Invest 127: 3103–3113, 2017. doi: 10.1172/JCI90147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flak JN, Patterson CM, Garfield AS, D’Agostino G, Goforth PB, Sutton AK, Malec PA, Wong JT, Germani M, Jones JC, Rajala M, Satin L, Rhodes CJ, Olson DP, Kennedy RT, Heisler LK, Myers MG Jr. Leptin-inhibited PBN neurons enhance responses to hypoglycemia in negative energy balance. Nat Neurosci 17: 1744–1750, 2014. doi: 10.1038/nn.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fortin JP, Wilhelm C, Servais J, Ménager C, Bacri JC, Gazeau F. Size-sorted anionic iron oxide nanomagnets as colloidal mediators for magnetic hyperthermia. J Am Chem Soc 129: 2628–2635, 2007. doi: 10.1021/ja067457e. [DOI] [PubMed] [Google Scholar]
- 47.Fóscolo RB, de Castro MG, Marubayashi U, dos Reis AM, Coimbra CC. Medial preoptic area adrenergic receptors modulate glycemia and insulinemia in freely moving rats. Brain Res 985: 56–64, 2003. doi: 10.1016/S0006-8993(03)03158-5. [DOI] [PubMed] [Google Scholar]
- 48.Foster NN, Azam S, Watts AG. Rapid-onset hypoglycemia suppresses Fos expression in discrete parts of the ventromedial nucleus of the hypothalamus. Am J Physiol Regul Integr Comp Physiol 310: R1177–R1185, 2016. doi: 10.1152/ajpregu.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frayling C, Britton R, Dale N. ATP-mediated glucosensing by hypothalamic tanycytes. J Physiol 589: 2275–2286, 2011. doi: 10.1113/jphysiol.2010.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frohman LA, Muller EE, Cocchi D. Central nervous system mediated inhibition of insulin secretion due to 2-deoxyglucose. Horm Metab Res 5: 21–26, 1973. doi: 10.1055/s-0028-1093995. [DOI] [PubMed] [Google Scholar]
- 51.Fukuda M, Ono T, Nishino H, Sasaki K. Independent glucose effects on rat hypothalamic neurons: an in vitro study. J Auton Nerv Syst 10: 373–381, 1984. doi: 10.1016/0165-1838(84)90034-1. [DOI] [PubMed] [Google Scholar]
- 52.Gallagher CN, Carpenter KL, Grice P, Howe DJ, Mason A, Timofeev I, Menon DK, Kirkpatrick PJ, Pickard JD, Sutherland GR, Hutchinson PJ. The human brain utilizes lactate via the tricarboxylic acid cycle: a 13C-labelled microdialysis and high-resolution nuclear magnetic resonance study. Brain 132: 2839–2849, 2009. doi: 10.1093/brain/awp202. [DOI] [PubMed] [Google Scholar]
- 53.Garfield AS, Shah BP, Madara JC, Burke LK, Patterson CM, Flak J, Neve RL, Evans ML, Lowell BB, Myers MG Jr, Heisler LK. A parabrachial-hypothalamic cholecystokinin neurocircuit controls counterregulatory responses to hypoglycemia. Cell Metab 20: 1030–1037, 2014. doi: 10.1016/j.cmet.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gaykema RP, Newmyer BA, Ottolini M, Raje V, Warthen DM, Lambeth PS, Niccum M, Yao T, Huang Y, Schulman IG, Harris TE, Patel MK, Williams KW, Scott MM. Activation of murine pre-proglucagon-producing neurons reduces food intake and body weight. J Clin Invest 127: 1031–1045, 2017. doi: 10.1172/JCI81335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghosh S, Castillo E, Frias ES, Swanson RA. Bioenergetic regulation of microglia. Glia 66:1200–1212, 2018. doi: 10.1002/glia.23271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A, Michaelides M. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357: 503–507, 2017. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonçalves GH, Li W, Garcia AV, Figueiredo MS, Bjørbæk C. Hypothalamic agouti-related peptide neurons and the central melanocortin system are crucial mediators of leptin’s antidiabetic actions. Cell Reports 7: 1093–1103, 2014. doi: 10.1016/j.celrep.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.González JA, Jensen LT, Doyle SE, Miranda-Anaya M, Menaker M, Fugger L, Bayliss DA, Burdakov D. Deletion of TASK1 and TASK3 channels disrupts intrinsic excitability but does not abolish glucose or pH responses of orexin/hypocretin neurons. Eur J Neurosci 30: 57–64, 2009. doi: 10.1111/j.1460-9568.2009.06789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.González JA, Jensen LT, Fugger L, Burdakov D. Metabolism-independent sugar sensing in central orexin neurons. Diabetes 57: 2569–2576, 2008. doi: 10.2337/db08-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 456: 745–749, 2008. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goto Y, Carpenter RG, Berelowitz M, Frohman LA. Effect of ventromedial hypothalamic lesions on the secretion of somatostatin, insulin, and glucagon by the perfused rat pancreas. Metabolism 29: 986–990, 1980. doi: 10.1016/0026-0495(80)90044-X. [DOI] [PubMed] [Google Scholar]
- 62.Guillod-Maximin E, Lorsignol A, Alquier T, Pénicaud L. Acute intracarotid glucose injection towards the brain induces specific c-fos activation in hypothalamic nuclei: involvement of astrocytes in cerebral glucose-sensing in rats. J Neuroendocrinol 16: 464–471, 2004. doi: 10.1111/j.1365-2826.2004.01185.x. [DOI] [PubMed] [Google Scholar]
- 63.Guyon A, Tardy MP, Rovère C, Nahon JL, Barhanin J, Lesage F. Glucose inhibition persists in hypothalamic neurons lacking tandem-pore K+ channels. J Neurosci 29: 2528–2533, 2009. doi: 10.1523/JNEUROSCI.5764-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Halperin D, Clark SS, Fu K, Heydt-Benjamin TS, Defend B, Kohno T, Ransford B, Morgan W, Maisel WH. Pacemakers and implantable cardiac defibrillators: software radio attacks and zero-power defenses. Conf Proc IEEE Symp Security and Privacy 2008: 129–142, 2008. [Google Scholar]
- 65.Hamad-Schifferli K, Schwartz JJ, Santos AT, Zhang S, Jacobson JM. Remote electronic control of DNA hybridization through inductive coupling to an attached metal nanocrystal antenna. Nature 415: 152–155, 2002. doi: 10.1038/415152a. [DOI] [PubMed] [Google Scholar]
- 66.Hao L, Sheng Z, Potian J, Deak A, Rohowsky-Kochan C, Routh VH. Lipopolysaccharide (LPS) and tumor necrosis factor-α (TNFα) blunt the response of neuropeptide Y/agouti-related peptide (NPY/AgRP) glucose inhibited (GI) neurons to decreased glucose. Brain Res 1648, Pt A: 181–192, 2016. doi: 10.1016/j.brainres.2016.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harada M, Sawa T, Okuda C, Matsuda T, Tanaka Y. Effects of glucose load on brain extracellular lactate concentration in conscious rats using a microdialysis technique. Horm Metab Res 25: 560–563, 1993. doi: 10.1055/s-2007-1002177. [DOI] [PubMed] [Google Scholar]
- 68.Hausen AC, Ruud J, Jiang H, Hess S, Varbanov H, Kloppenburg P, Brüning JC. Insulin-dependent activation of MCH neurons impairs locomotor activity and insulin sensitivity in obesity. Cell Reports 17: 2512–2521, 2016. doi: 10.1016/j.celrep.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 69.Howarth C, Gleeson P, Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab 32: 1222–1232, 2012. doi: 10.1038/jcbfm.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang CW, Huang CC, Cheng JT, Tsai JJ, Wu SN. Glucose and hippocampal neuronal excitability: role of ATP-sensitive potassium channels. J Neurosci Res 85: 1468–1477, 2007. doi: 10.1002/jnr.21284. [DOI] [PubMed] [Google Scholar]
- 71.Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Rønnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose responsive and express KATP channels. Endocrinology 144: 1331–1340, 2003. doi: 10.1210/en.2002-221033. [DOI] [PubMed] [Google Scholar]
- 72.Idelevich A, Sato K, Nagano K, Rowe G, Gori F, Baron R. Neuronal hypothalamic regulation of body metabolism and bone density is galanin dependent. J Clin Invest 128: 2626–2641, 2018. doi: 10.1172/JCI99350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ionescu E, Rohner-Jeanrenaud F, Berthoud HR, Jeanrenaud B. Increases in plasma insulin levels in response to electrical stimulation of the dorsal motor nucleus of the vagus nerve. Endocrinology 112: 904–910, 1983. doi: 10.1210/endo-112-3-904. [DOI] [PubMed] [Google Scholar]
- 74.Joly-Amado A, Denis RG, Castel J, Lacombe A, Cansell C, Rouch C, Kassis N, Dairou J, Cani PD, Ventura-Clapier R, Prola A, Flamment M, Foufelle F, Magnan C, Luquet S. Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning. EMBO J 31: 4276–4288, 2012. doi: 10.1038/emboj.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kai Y, Oomura Y, Shimizu N. Responses of rat lateral hypothalamic neuron activity to dorsal raphe nuclei stimulation. J Neurophysiol 60: 524–535, 1988. doi: 10.1152/jn.1988.60.2.524. [DOI] [PubMed] [Google Scholar]
- 76.Kang L, Dunn-Meynell AA, Routh VH, Gaspers LD, Nagata Y, Nishimura T, Eiki J, Zhang BB, Levin BE. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes 55: 412–420, 2006. doi: 10.2337/diabetes.55.02.06.db05-1229. [DOI] [PubMed] [Google Scholar]
- 77.Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 53: 549–559, 2004. doi: 10.2337/diabetes.53.3.549. [DOI] [PubMed] [Google Scholar]
- 78.Keaney J, Campbell M. The dynamic blood-brain barrier. FEBS J 282: 4067–4079, 2015. doi: 10.1111/febs.13412. [DOI] [PubMed] [Google Scholar]
- 79.Kim JD, Toda C, Ramírez CM, Fernández-Hernando C, Diano S. Hypothalamic ventromedial Lin28a enhances glucose metabolism in diet-induced obesity. Diabetes 66: 2102–2111, 2017. doi: 10.2337/db16-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim KW, Donato J Jr, Berglund ED, Choi YH, Kohno D, Elias CF, Depinho RA, Elmquist JK. FOXO1 in the ventromedial hypothalamus regulates energy balance. J Clin Invest 122: 2578–2589, 2012. doi: 10.1172/JCI62848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klöckener T, Hess S, Belgardt BF, Paeger L, Verhagen LA, Husch A, Sohn JW, Hampel B, Dhillon H, Zigman JM, Lowell BB, Williams KW, Elmquist JK, Horvath TL, Kloppenburg P, Brüning JC. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nat Neurosci 14: 911–918, 2011. doi: 10.1038/nn.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kohno D, Koike M, Ninomiya Y, Kojima I, Kitamura T, Yada T. Sweet taste receptor serves to activate glucose- and leptin-responsive neurons in the hypothalamic arcuate nucleus and participates in glucose responsiveness. Front Neurosci 10: 502, 2016. doi: 10.3389/fnins.2016.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kong D, Vong L, Parton LE, Ye C, Tong Q, Hu X, Choi B, Brüning JC, Lowell BB. Glucose stimulation of hypothalamic MCH neurons involves KATP channels, is modulated by UCP2, and regulates peripheral glucose homeostasis. Cell Metab 12: 545–552, 2010. doi: 10.1016/j.cmet.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.König SA, Kochen W, Czachurski J, Seller H. Stimulation of the rostroventrolateral medulla of the cat increases blood glucose. Horm Metab Res 24: 136–137, 1992. doi: 10.1055/s-2007-1003277. [DOI] [PubMed] [Google Scholar]
- 85.Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Brüning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 5: 438–449, 2007. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 86.Koranyi L, Bourey RE, James D, Mueckler M, Fiedorek FT Jr, Permutt MA. Glucose transporter gene expression in rat brain: pretranslational changes associated with chronic insulin-induced hypoglycemia, fasting, and diabetes. Mol Cell Neurosci 2: 244–252, 1991. doi: 10.1016/1044-7431(91)90051-O. [DOI] [PubMed] [Google Scholar]
- 87.Kühn FJ, Knop G, Lückhoff A. The transmembrane segment S6 determines cation versus anion selectivity of TRPM2 and TRPM8. J Biol Chem 282: 27598–27609, 2007. doi: 10.1074/jbc.M702247200. [DOI] [PubMed] [Google Scholar]
- 88.Kuperman Y, Weiss M, Dine J, Staikin K, Golani O, Ramot A, Nahum T, Kühne C, Shemesh Y, Wurst W, Harmelin A, Deussing JM, Eder M, Chen A. CRFR1 in AgRP neurons modulates sympathetic nervous system activity to adapt to cold stress and fasting. Cell Metab 23: 1185–1199, 2016. doi: 10.1016/j.cmet.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Labouèbe G, Boutrel B, Tarussio D, Thorens B. Glucose-responsive neurons of the paraventricular thalamus control sucrose-seeking behavior. Nat Neurosci 19: 999–1002, 2016. doi: 10.1038/nn.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lamy CM, Sanno H, Labouèbe G, Picard A, Magnan C, Chatton JY, Thorens B. Hypoglycemia-activated GLUT2 neurons of the nucleus tractus solitarius stimulate vagal activity and glucagon secretion. Cell Metab 19: 527–538, 2014. doi: 10.1016/j.cmet.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 91.Lee CH, Park JH, Won MH. Decreased glucokinase protein expression in the aged gerbil hippocampus. Cell Mol Neurobiol 33: 477–481, 2013. doi: 10.1007/s10571-013-9928-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lerchner W, Xiao C, Nashmi R, Slimko EM, van Trigt L, Lester HA, Anderson DJ. Reversible silencing of neuronal excitability in behaving mice by a genetically targeted, ivermectin-gated Cl− channel. Neuron 54: 35–49, 2007. doi: 10.1016/j.neuron.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 93.Li L, de La Serre CB, Zhang N, Yang L, Li H, Bi S. Knockdown of neuropeptide Y in the dorsomedial hypothalamus promotes hepatic insulin sensitivity in male rats. Endocrinology 157: 4842–4852, 2016. doi: 10.1210/en.2016-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lorden JF, Sims JS. Monosodium l-glutamate lesions reduce susceptibility to hypoglycemic feeding and convulsions. Behav Brain Res 24: 139–146, 1987. doi: 10.1016/0166-4328(87)90251-8. [DOI] [PubMed] [Google Scholar]
- 95.Luquet S, Phillips CT, Palmiter RD. NPY/AgRP neurons are not essential for feeding responses to glucoprivation. Peptides 28: 214–225, 2007. doi: 10.1016/j.peptides.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 96.Magnus CJ, Lee PH, Atasoy D, Su HH, Looger LL, Sternson SM. Chemical and genetic engineering of selective ion channel-ligand interactions. Science 333: 1292–1296, 2011. doi: 10.1126/science.1206606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Malaisse WJ, Sener A. Glucose-induced changes in cytosolic ATP content in pancreatic islets. Biochim Biophys Acta 927: 190–195, 1987. doi: 10.1016/0167-4889(87)90134-0. [DOI] [PubMed] [Google Scholar]
- 98.Marston OJ, Hurst P, Evans ML, Burdakov DI, Heisler LK. Neuropeptide Y cells represent a distinct glucose-sensing population in the lateral hypothalamus. Endocrinology 152: 4046–4052, 2011. doi: 10.1210/en.2011-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martinez-Boubeta C, Simeonidis K, Makridis A, Angelakeris M, Iglesias O, Guardia P, Cabot A, Yedra L, Estradé S, Peiró F, Saghi Z, Midgley PA, Conde-Leborán I, Serantes D, Baldomir D. Learning from nature to improve the heat generation of iron-oxide nanoparticles for magnetic hyperthermia applications. Sci Rep 3: 1652, 2013. doi: 10.1038/srep01652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marty N, Dallaporta M, Foretz M, Emery M, Tarussio D, Bady I, Binnert C, Beermann F, Thorens B. Regulation of glucagon secretion by glucose transporter type 2 (glut2) and astrocyte-dependent glucose sensors. J Clin Invest 115: 3545–3553, 2005. doi: 10.1172/JCI26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McEwen BS, Reagan LP. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur J Pharmacol 490: 13–24, 2004. doi: 10.1016/j.ejphar.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 102.McNay EC, McCarty RC, Gold PE. Fluctuations in brain glucose concentration during behavioral testing: dissociations between brain areas and between brain and blood. Neurobiol Learn Mem 75: 325–337, 2001. doi: 10.1006/nlme.2000.3976. [DOI] [PubMed] [Google Scholar]
- 103.Meek TH, Nelson JT, Matsen ME, Dorfman MD, Guyenet SJ, Damian V, Allison MB, Scarlett JM, Nguyen HT, Thaler JP, Olson DP, Myers MG Jr, Schwartz MW, Morton GJ. Functional identification of a neurocircuit regulating blood glucose. Proc Natl Acad Sci USA 113: E2073–E2082, 2016. doi: 10.1073/pnas.1521160113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Melnick IV, Price CJ, Colmers WF. Glucosensing in parvocellular neurons of the rat hypothalamic paraventricular nucleus. Eur J Neurosci 34: 272–282, 2011. doi: 10.1111/j.1460-9568.2011.07742.x. [DOI] [PubMed] [Google Scholar]
- 105.Melo CA, Lima AL, Brasil IR, Castro e Silva O Jr, Magalhães DV, Marcassa LG, Bagnato VS. Characterization of light penetration in rat tissues. J Clin Laser Med Surg 19: 175–179, 2001. doi: 10.1089/104454701316918925. [DOI] [PubMed] [Google Scholar]
- 106.Meng F, Han Y, Srisai D, Belakhov V, Farias M, Xu Y, Palmiter RD, Baasov T, Wu Q. New inducible genetic method reveals critical roles of GABA in the control of feeding and metabolism. Proc Natl Acad Sci USA 113: 3645–3650, 2016. doi: 10.1073/pnas.1602049113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci 36: 587–597, 2013. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mimee A, Ferguson AV. Glycemic state regulates melanocortin, but not nesfatin-1, responsiveness of glucose-sensing neurons in the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 308: R690–R699, 2015. doi: 10.1152/ajpregu.00477.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Minami S, Kamegai J, Sugihara H, Suzuki N, Higuchi H, Wakabayashi I. Central glucoprivation evoked by administration of 2-deoxy-d-glucose induces expression of the c-fos gene in a subpopulation of neuropeptide Y neurons in the rat hypothalamus. Brain Res Mol Brain Res 33: 305–310, 1995. doi: 10.1016/0169-328X(95)00151-H. [DOI] [PubMed] [Google Scholar]
- 110.Mobbs CV, Kow LM, Yang XJ. Brain glucose-sensing mechanisms: ubiquitous silencing by aglycemia vs. hypothalamic neuroendocrine responses. Am J Physiol Endocrinol Metab 281: E649–E654, 2001. doi: 10.1152/ajpendo.2001.281.4.E649. [DOI] [PubMed] [Google Scholar]
- 111.Morgan DA, McDaniel LN, Yin T, Khan M, Jiang J, Acevedo MR, Walsh SA, Ponto LL, Norris AW, Lutter M, Rahmouni K, Cui H. Regulation of glucose tolerance and sympathetic activity by MC4R signaling in the lateral hypothalamus. Diabetes 64: 1976–1987, 2015. doi: 10.2337/db14-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mueckler M. Facilitative glucose transporters. Eur J Biochem 219: 713–725, 1994. doi: 10.1111/j.1432-1033.1994.tb18550.x. [DOI] [PubMed] [Google Scholar]
- 113.Müller EE, Panerai A, Cocchi D, Frohman LA, Mantegazza P. Central glucoprivation: some physiological effects induced by the intraventricular administration of 2-deoxy-d-glucose. Experientia 29: 874–876, 1973. doi: 10.1007/BF01946338. [DOI] [PubMed] [Google Scholar]
- 114.Müller EE, Pecile A, Cocchi D, Olgiati VR. Hyperglycemic or feeding response to glucoprivation and hypothalamic glucoreceptors. Am J Physiol 226: 1100–1109, 1974. doi: 10.1152/ajplegacy.1974.226.5.1100. [DOI] [PubMed] [Google Scholar]
- 115.Muroya S, Yada T, Shioda S, Takigawa M. Glucose-sensitive neurons in the rat arcuate nucleus contain neuropeptide Y. Neurosci Lett 264: 113–116, 1999. doi: 10.1016/S0304-3940(99)00185-8. [DOI] [PubMed] [Google Scholar]
- 116.Murphy BA, Fakira KA, Song Z, Beuve A, Routh VH. AMP-activated protein kinase and nitric oxide regulate the glucose sensitivity of ventromedial hypothalamic glucose-inhibited neurons. Am J Physiol Cell Physiol 297: C750–C758, 2009. doi: 10.1152/ajpcell.00127.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Murphy BA, Fioramonti X, Jochnowitz N, Fakira K, Gagen K, Contie S, Lorsignol A, Penicaud L, Martin WJ, Routh VH. Fasting enhances the response of arcuate neuropeptide Y-glucose-inhibited neurons to decreased extracellular glucose. Am J Physiol Cell Physiol 296: C746–C756, 2009. doi: 10.1152/ajpcell.00641.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nawaratne V, Leach K, Suratman N, Loiacono RE, Felder CC, Armbruster BN, Roth BL, Sexton PM, Christopoulos A. New insights into the function of M4 muscarinic acetylcholine receptors gained using a novel allosteric modulator and a DREADD (designer receptor exclusively activated by a designer drug). Mol Pharmacol 74: 1119–1131, 2008. doi: 10.1124/mol.108.049353. [DOI] [PubMed] [Google Scholar]
- 119.O’Malley D, Reimann F, Simpson AK, Gribble FM. Sodium-coupled glucose cotransporters contribute to hypothalamic glucose sensing. Diabetes 55: 3381–3386, 2006. doi: 10.2337/db06-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ono T, Steffens AB, Sasaki K. Influence of peripheral and intracerebroventricular glucose and insulin infusions on peripheral and cerebrospinal fluid glucose and insulin levels. Physiol Behav 30: 301–306, 1983. doi: 10.1016/0031-9384(83)90023-9. [DOI] [PubMed] [Google Scholar]
- 121.Osada T, Suzuki R, Ogawa A, Tanaka M, Hori M, Aoki S, Tamura Y, Watada H, Kawamori R, Konishi S. Functional subdivisions of the hypothalamus using areal parcellation and their signal changes related to glucose metabolism. Neuroimage 162: 1–12, 2017. doi: 10.1016/j.neuroimage.2017.08.056. [DOI] [PubMed] [Google Scholar]
- 122.Pan W, Kastin AJ. The blood-brain barrier: regulatory roles in wakefulness and sleep. Neuroscientist 23: 124–136, 2017. doi: 10.1177/1073858416639005. [DOI] [PubMed] [Google Scholar]
- 123.Papp S, Lukáts B, Takács G, Szalay C, Karádi Z. Glucose-monitoring neurons in the nucleus accumbens. Neuroreport 18: 1561–1565, 2007. doi: 10.1097/WNR.0b013e3281667eca. [DOI] [PubMed] [Google Scholar]
- 124.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 449: 228–232, 2007. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 125.Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 55: 1251–1262, 2007. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- 126.Pénicaud L, Rohner-Jeanrenaud F, Jeanrenaud B. In vivo metabolic changes as studied longitudinally after ventromedial hypothalamic lesions. Am J Physiol Endocrinol Metab 250: E662–E668, 1986. doi: 10.1152/ajpendo.1986.250.6.E662. [DOI] [PubMed] [Google Scholar]
- 127.Peruzzo B, Pastor FE, Blázquez JL, Schöbitz K, Peláez B, Amat P, Rodríguez EM. A second look at the barriers of the medial basal hypothalamus. Exp Brain Res 132: 10–26, 2000. doi: 10.1007/s002219900289. [DOI] [PubMed] [Google Scholar]
- 128.Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci 10: 663–668, 2007. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- 129.Reichenbach A, Stark R, Mequinion M, Denis RRG, Goularte JF, Clarke RE, Lockie SH, Lemus MB, Kowalski GM, Bruce CR, Huang C, Schittenhelm RB, Mynatt RL, Oldfield BJ, Watt MJ, Luquet S, Andrews ZB. AgRP neurons require carnitine acetyltransferase to regulate metabolic flexibility and peripheral nutrient partitioning. Cell Reports 22: 1745–1759, 2018. doi: 10.1016/j.celrep.2018.01.067. [DOI] [PubMed] [Google Scholar]
- 130.Ren X, Zhou L, Terwilliger R, Newton SS, de Araujo IE. Sweet taste signaling functions as a hypothalamic glucose sensor. Front Integr Neurosci 3: 12, 2009. doi: 10.3389/neuro.07.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Renier N, Wu Z, Simon DJ, Yang J, Ariel P, Tessier-Lavigne M. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159: 896–910, 2014. doi: 10.1016/j.cell.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 132.Richardson HH, Hickman ZN, Govorov AO, Thomas AC, Zhang W, Kordesch ME. Thermooptical properties of gold nanoparticles embedded in ice: characterization of heat generation and melting. Nano Lett 6: 783–788, 2006. doi: 10.1021/nl060105l. [DOI] [PubMed] [Google Scholar]
- 133.Robinson AM, Williamson DH. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev 60: 143–187, 1980. doi: 10.1152/physrev.1980.60.1.143. [DOI] [PubMed] [Google Scholar]
- 134.Roland AV, Moenter SM. Glucosensing by GnRH neurons: inhibition by androgens and involvement of AMP-activated protein kinase. Mol Endocrinol 25: 847–858, 2011. doi: 10.1210/me.2010-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rosario W, Singh I, Wautlet A, Patterson C, Flak J, Becker TC, Ali A, Tamarina N, Philipson LH, Enquist LW, Myers MG Jr, Rhodes CJ. The brain-to-pancreatic islet neuronal map reveals differential glucose regulation from distinct hypothalamic regions. Diabetes 65: 2711–2723, 2016. doi: 10.2337/db15-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rossi MA, Stuber GD. Overlapping brain circuits for homeostatic and hedonic feeding. Cell Metab 27: 42–56, 2018. doi: 10.1016/j.cmet.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roth BL. DREADDs for neuroscientists. Neuron 89: 683–694, 2016. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Salatin S, Maleki Dizaj S, Yari Khosroushahi A. Effect of the surface modification, size, and shape on cellular uptake of nanoparticles. Cell Biol Int 39: 881–890, 2015. doi: 10.1002/cbin.10459. [DOI] [PubMed] [Google Scholar]
- 139.Santiago AM, Clegg DJ, Routh VH. Estrogens modulate ventrolateral ventromedial hypothalamic glucose-inhibited neurons. Mol Metab 5: 823–833, 2016. doi: 10.1016/j.molmet.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Santoro A, Campolo M, Liu C, Sesaki H, Meli R, Liu ZW, Kim JD, Diano S. DRP1 suppresses leptin and glucose sensing of POMC neurons. Cell Metab 25: 647–660, 2017. doi: 10.1016/j.cmet.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schurr A, Miller JJ, Payne RS, Rigor BM. An increase in lactate output by brain tissue serves to meet the energy needs of glutamate-activated neurons. J Neurosci 19: 34–39, 1999. doi: 10.1523/JNEUROSCI.19-01-00034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shimazu T, Fukuda A, Ban T. Reciprocal influences of the ventromedial and lateral hypothalamic nuclei on blood glucose level and liver glycogen content. Nature 210: 1178–1179, 1966. doi: 10.1038/2101178a0. [DOI] [PubMed] [Google Scholar]
- 143.Silver IA, Erecińska M. Glucose-induced intracellular ion changes in sugar-sensitive hypothalamic neurons. J Neurophysiol 79: 1733–1745, 1998. doi: 10.1152/jn.1998.79.4.1733. [DOI] [PubMed] [Google Scholar]
- 144.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab 27: 1766–1791, 2007. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sims JS, Lorden JF. Effect of paraventricular nucleus lesions on body weight, food intake and insulin levels. Behav Brain Res 22: 265–281, 1986. doi: 10.1016/0166-4328(86)90071-9. [DOI] [PubMed] [Google Scholar]
- 146.Smith MA, Katsouri L, Irvine EE, Hankir MK, Pedroni SM, Voshol PJ, Gordon MW, Choudhury AI, Woods A, Vidal-Puig A, Carling D, Withers DJ. Ribosomal S6K1 in POMC and AgRP neurons regulates glucose homeostasis but not feeding behavior in mice. Cell Reports 11: 335–343, 2015. doi: 10.1016/j.celrep.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sokoloff L. Metabolism of ketone bodies by the brain. Annu Rev Med 24: 271–280, 1973. doi: 10.1146/annurev.me.24.020173.001415. [DOI] [PubMed] [Google Scholar]
- 148.Song Z, Levin BE, Stevens W, Sladek CD. Supraoptic oxytocin and vasopressin neurons function as glucose and metabolic sensors. Am J Physiol Regul Integr Comp Physiol 306: R447–R456, 2014. doi: 10.1152/ajpregu.00520.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stanley S, Domingos AI, Kelly L, Garfield A, Damanpour S, Heisler L, Friedman J. Profiling of glucose-sensing neurons reveals that GHRH neurons are activated by hypoglycemia. Cell Metab 18: 596–607, 2013. doi: 10.1016/j.cmet.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 150.Stanley SA, Gagner JE, Damanpour S, Yoshida M, Dordick JS, Friedman JM. Radio-wave heating of iron oxide nanoparticles can regulate plasma glucose in mice. Science 336: 604–608, 2012. doi: 10.1126/science.1216753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stanley SA, Kelly L, Latcha KN, Schmidt SF, Yu X, Nectow AR, Sauer J, Dyke JP, Dordick JS, Friedman JM. Bidirectional electromagnetic control of the hypothalamus regulates feeding and metabolism. Nature 531: 647–650, 2016. doi: 10.1038/nature17183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Stanley SA, Sauer J, Kane RS, Dordick JS, Friedman JM. Remote regulation of glucose homeostasis in mice using genetically encoded nanoparticles. Nat Med 21: 92–98, 2015. doi: 10.1038/nm.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Steculorum SM, Ruud J, Karakasilioti I, Backes H, Engström Ruud L, Timper K, Hess ME, Tsaousidou E, Mauer J, Vogt MC, Paeger L, Bremser S, Klein AC, Morgan DA, Frommolt P, Brinkkötter PT, Hammerschmidt P, Benzing T, Rahmouni K, Wunderlich FT, Kloppenburg P, Brüning JC. AgRP neurons control systemic insulin sensitivity via myostatin expression in brown adipose tissue. Cell 165: 125–138, 2016. doi: 10.1016/j.cell.2016.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stephens DN, Morrissey SM. Hypothalamic stimulation induces acid secretion, hypoglycemia, and hyperinsulinemia. Am J Physiol 228: 1206–1209, 1975. doi: 10.1152/ajplegacy.1975.228.4.1206. [DOI] [PubMed] [Google Scholar]
- 155.Sternson SM, Roth BL. Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci 37: 387–407, 2014. doi: 10.1146/annurev-neuro-071013-014048. [DOI] [PubMed] [Google Scholar]
- 156.Theil EC. Ferritin: the protein nanocage and iron biomineral in health and in disease. Inorg Chem 52: 12223–12233, 2013. doi: 10.1021/ic400484n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Toda C, Shiuchi T, Lee S, Yamato-Esaki M, Fujino Y, Suzuki A, Okamoto S, Minokoshi Y. Distinct effects of leptin and a melanocortin receptor agonist injected into medial hypothalamic nuclei on glucose uptake in peripheral tissues. Diabetes 58: 2757–2765, 2009. doi: 10.2337/db09-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab 5: 383–393, 2007. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Treherne JM, Ashford ML. Calcium-activated potassium channels in rat dissociated ventromedial hypothalamic neurons. J Neuroendocrinol 3: 323–329, 1991. doi: 10.1111/j.1365-2826.1991.tb00282.x. [DOI] [PubMed] [Google Scholar]
- 160.Tromba C, Salvaggio A, Racagni G, Volterra A. Hypoglycemia-activated K+ channels in hippocampal neurons. Neurosci Lett 143: 185–189, 1992. doi: 10.1016/0304-3940(92)90262-6. [DOI] [PubMed] [Google Scholar]
- 161.Tsuneki H, Tokai E, Nakamura Y, Takahashi K, Fujita M, Asaoka T, Kon K, Anzawa Y, Wada T, Takasaki I, Kimura K, Inoue H, Yanagisawa M, Sakurai T, Sasaoka T. Hypothalamic orexin prevents hepatic insulin resistance via daily bidirectional regulation of autonomic nervous system in mice. Diabetes 64: 459–470, 2015. doi: 10.2337/db14-0695. [DOI] [PubMed] [Google Scholar]
- 162.Üner A, Gonçalves GH, Li W, Porceban M, Caron N, Schönke M, Delpire E, Sakimura K, Bjørbæk C. The role of GluN2A and GluN2B NMDA receptor subunits in AgRP and POMC neurons on body weight and glucose homeostasis. Mol Metab 4: 678–691, 2015. doi: 10.1016/j.molmet.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Urban DJ, Roth BL. DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu Rev Pharmacol Toxicol 55: 399–417, 2015. doi: 10.1146/annurev-pharmtox-010814-124803. [DOI] [PubMed] [Google Scholar]
- 164.van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, Elmquist J, Lowell BB, Barsh GS, de Luca C, Myers MG Jr, Schwartz GJ, Chua SC Jr. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology 149: 1773–1785, 2008. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vannucci SJ, Maher F, Simpson IA. Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia 21: 2–21, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 166.Wang Q, Liu C, Uchida A, Chuang JC, Walker A, Liu T, Osborne-Lawrence S, Mason BL, Mosher C, Berglund ED, Elmquist JK, Zigman JM. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol Metab 3: 64–72, 2013. doi: 10.1016/j.molmet.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang R, Liu X, Hentges ST, Dunn-Meynell AA, Levin BE, Wang W, Routh VH. The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes 53: 1959–1965, 2004. doi: 10.2337/diabetes.53.8.1959. [DOI] [PubMed] [Google Scholar]
- 168.Wess J. Use of designer G protein-coupled receptors to dissect metabolic pathways. Trends Endocrinol Metab 27: 600–603, 2016. doi: 10.1016/j.tem.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wheeler MA, Smith CJ, Ottolini M, Barker BS, Purohit AM, Grippo RM, Gaykema RP, Spano AJ, Beenhakker MP, Kucenas S, Patel MK, Deppmann CD, Güler AD. Genetically targeted magnetic control of the nervous system. Nat Neurosci 19: 756–761, 2016. doi: 10.1038/nn.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Williams RH, Burdakov D. Silencing of ventromedial hypothalamic neurons by glucose-stimulated K+ currents. Pflügers Arch 458: 777–783, 2009. doi: 10.1007/s00424-009-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, Yanagisawa M, Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 38: 701–713, 2003. doi: 10.1016/S0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 172.Yao T, Deng Z, Gao Y, Sun J, Kong X, Huang Y, He Z, Xu Y, Chang Y, Yu KJ, Findley BG, Berglund ED, Wang RT, Guo H, Chen H, Li X, Kaufman RJ, Yan J, Liu T, Williams KW. Ire1α in Pomc neurons is required for thermogenesis and glycemia. Diabetes 66: 663–673, 2017. doi: 10.2337/db16-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Young JH, Wang MT, Brezovich I. Frequency/depth-penetration considerations in hyperthermia by magnetically induced currents. Electron Lett 16: 358–359, 1980. doi: 10.1049/el:19800255. [DOI] [Google Scholar]
- 174.Zhang R, Dhillon H, Yin H, Yoshimura A, Lowell BB, Maratos-Flier E, Flier JS. Selective inactivation of Socs3 in SF1 neurons improves glucose homeostasis without affecting body weight. Endocrinology 149: 5654–5661, 2008. doi: 10.1210/en.2008-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang Y, Orner BP. Self-assembly in the ferritin nano-cage protein superfamily. Int J Mol Sci 12: 5406–5421, 2011. doi: 10.3390/ijms12085406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhao Z, Wang L, Gao W, Hu F, Zhang J, Ren Y, Lin R, Feng Q, Cheng M, Ju D, Chi Q, Wang D, Song S, Luo M, Zhan C. A central catecholaminergic circuit controls blood glucose levels during stress. Neuron 95: 138–152.e5, 2017. doi: 10.1016/j.neuron.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 177.Zhou L, Podolsky N, Sang Z, Ding Y, Fan X, Tong Q, Levin BE, McCrimmon RJ. The medial amygdalar nucleus: a novel glucose-sensing region that modulates the counterregulatory response to hypoglycemia. Diabetes 59: 2646–2652, 2010. doi: 10.2337/db09-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhu G, Yan J, Smith WW, Moran TH, Bi S. Roles of dorsomedial hypothalamic cholecystokinin signaling in the controls of meal patterns and glucose homeostasis. Physiol Behav 105: 234–241, 2012. doi: 10.1016/j.physbeh.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]