Abstract
Osteoarthritis (OA) is characterized by synovitis and synovial fibrosis. Synoviocytes are fibroblast-like resident cells of the synovium that are activated by transforming growth factor (TGF)-β to proliferate, migrate, and produce extracellular matrix. Synoviocytes secrete hyaluronan (HA) and proteoglycan-4 (PRG4). HA reduces synovial fibrosis in vivo, and the Prg4−/− mouse exhibits synovial hyperplasia. We investigated the antifibrotic effects of increased intracellular cAMP in TGF-β-stimulated human OA synoviocytes. TGF-β1 stimulated collagen I (COL1A1), α-smooth muscle actin (α-SMA), tissue inhibitor of metalloproteinase (TIMP)-1, and procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2 (PLOD2) expression, and procollagen I, α-SMA, HA, and PRG4 production, migration, and proliferation of OA synoviocytes were measured. Treatment of OA synoviocytes with forskolin (10 μM) increased intracellular cAMP levels and reduced TGF-β1-stimulated COL1A1, α-SMA, and TIMP-1 expression, with no change in PLOD2 expression. Forskolin also reduced TGF-β1-stimulated procollagen I and α-SMA content as well as synoviocyte migration and proliferation. Forskolin (10 μM) increased HA secretion and PRG4 expression and production. A cell-permeant cAMP analog reduced COL1A1 and α-SMA expression and enhanced HA and PRG4 secretion by OA synoviocytes. HA and PRG4 reduced α-SMA expression and content, and PRG4 reduced COL1A1 expression and procollagen I content in OA synoviocytes. Prg4−/− synovium exhibited increased α-SMA, COL1A1, and TIMP-1 expression compared with Prg4+/+ synovium. Prg4−/− synoviocytes demonstrated strong α-SMA and collagen type I staining, whereas these were undetected in Prg4+/+ synoviocytes and were reduced with PRG4 treatment. We conclude that increasing intracellular cAMP levels in synoviocytes mitigates synovial fibrosis through enhanced production of HA and PRG4, possibly representing a novel approach for treatment of OA synovial fibrosis.
Keywords: cAMP, fibrosis, hyaluronan, osteoarthritis, proteoglycan-4
INTRODUCTION
Hallmarks of osteoarthritis (OA) include cartilage degeneration, subchondral bone remodeling, and synovitis (26, 27, 41). Major abnormalities in the OA synovium include synovial hyperplasia, inflammatory cell infiltration, angiogenesis, and fibrosis (4, 45, 47, 55). Synovial fibrosis is a common feature in advanced OA that contributes to joint pain and stiffness (13, 24). The transforming growth factor (TGF)-β1 family and its associated signaling pathways play an essential role in maintaining homeostasis in healthy joints (52). However, TGF-β1 switches to a pathological role in OA joints that drives synovial fibrosis (36). TGF-β1 upregulates the expression of synovial collagen type 1, tissue inhibitor of metalloproteinase 1 (TIMP-1), and procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2 (PLOD2) (35, 36). The net result is an increase in collagen I formation, an increase in collagen cross-links, and a reduction in collagen turnover (35, 36).
The normal synovium contains two types of intimal cells: type A macrophages and type B fibroblasts, or synoviocytes (48). In synoviocytes, TGF-β1 induces fibrotic changes characterized by cell proliferation and collagen type I accumulation (46). Additionally, TGF-β1 promotes the differentiation of OA synoviocytes into a myofibroblast-like phenotype, characterized by the expression of α-smooth muscle actin (α-SMA) (28). Synoviocytes produce hyaluronan (HA), a glycosaminoglycan synthesized by membrane-bound hyaluronan synthase (HAS), with three isoforms identified to date (HAS1, HAS2, and HAS3) (54). Synoviocytes also produce proteoglycan-4 (PRG4), a heavily glycosylated mucinous glycoprotein (9, 17). HA and PRG4 play important roles in joint lubrication (25). HA and PRG4 may also play a role in joint fibrosis, as HA treatment reduced synovial fibrosis in vivo and findings in synovial tissues from Prg4−/− mice include increased synovial thickening and a proliferative capacity for Prg4−/− synoviocytes under basal and cytokine-stimulated conditions (2, 32, 37).
Adenosine 3′,5′-cyclic monophosphate (cAMP) is a pleiotropic intracellular second messenger generated by adenylyl cyclase (AC) enzymes in response to activation (16). The antifibrotic effect of cAMP has been described in fibroblasts from multiple origins and includes inhibition of fibroblast proliferation, reduction in fibroblast migration, and reduced synthesis of extracellular matrix components (12, 38, 44, 56). The role of cAMP in regulating TGF-β1’s fibrotic response in synoviocytes is unknown. Our aim was to study the impact of forskolin, an AC activator, on the expression and production of α-SMA and collagen type I, expression of TIMP-1, PLOD2, HAS isoforms, and PRG4, and production of HA and PRG4 in a model of TGF-β1-stimulated OA synoviocytes. Given the potential involvement of HA and PRG4 in synovial fibrosis, we also studied the antifibrotic effect of HA and PRG4 in human and murine synoviocytes. We hypothesized that increasing intracellular cAMP exerts an antifibrotic effect in OA synoviocytes and promotes HA and PRG4 production.
MATERIALS AND METHODS
Ethical Approvals
Animal breeding and tissue harvest were approved by the Institutional Animal Care and Use Committee at Rhode Island Hospital.
Patient Characteristics and Experimental Approach
OA synoviocytes (500,000 cells per vial; Cell Applications) were isolated from synovial tissues from deidentified OA patients undergoing knee replacement (n = 10; median age = 63 yr, range: 54–69 yr). Six patients were women. Five patients were Caucasian, and the other five were unspecified. Synoviocytes were received in their second passage. OA synoviocytes were cultured in 75-cm2 culture flasks in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS and were used between the third and sixth passages to avoid alterations in patterns of gene expression (10, 30). Experimental data are represented as means ± SD of three to six biological replicates.
We initially studied cAMP generation in OA synoviocytes with a 0.01–10 μM forskolin concentration range; 10 μM was selected in light of previous studies (21, 50). After confirmation of cAMP accumulation by forskolin, we determined its effect on α-SMA, collagen type I, TIMP-1, and PLOD2 expression in TGF-β1-stimulated OA synoviocytes. The antifibrotic effect of intracellular cAMP accumulation in OA synoviocytes was further confirmed with a cell-permeant cAMP analog, 8-bromoadenosine 3′,5′-cyclic monophosphate (8-BrcAMP). To further appreciate the antifibrotic effect of forskolin, we evaluated the impact of cAMP generation on HAS isoform expression and HA production as well as PRG4 expression and production. In light of forskolin’s observed effect on HA and PRG4, we studied the antifibrotic effect of HA and PRG4 in human OA synoviocytes and murine Prg4−/− synoviocytes as well as fibrosis markers in the Prg4−/− synovium. Finally, we studied the efficacy of forskolin in mitigating TGF-β1-stimulated OA synoviocyte migration and proliferation.
cAMP Generation in OA Synoviocytes by Forskolin
cAMP levels were measured in OA synoviocytes with the cADDis assay (Montana Molecular). The assay utilizes a fluorescent cAMP sensor that measures changes in intracellular cAMP levels. An increase in intracellular cAMP levels results in a reduction in green fluorescence. OA synoviocytes (10,000 cells/well) were cultured overnight in sterile 96-well clear-bottom black plates in the presence of a recombinant mammalianized baclovirus expressing the cAMP sensor according to manufacturer’s recommendations. Subsequently, medium supernatants were replaced with Dulbecco’s PBS (Thermo Fisher Scientific; 200 μl/well). Forskolin (Sigma-Aldrich) at final concentrations of 0.01, 0.1, 1, and 10 μM, vehicle, and a positive control were added, and fluorescence intensity with 494-/522-nm wavelengths was measured every 30 s over 30 min. Data are presented as the ratio of fluorescence intensity reduction at each time point to fluorescence intensity at baseline.
Gene Expression Studies
OA synoviocytes (300,000 cells/well) were treated with TGF-β1 (1 ng/ml; R&D Systems) in serum-free DMEM ± forskolin (0.1, 1, or 10 μM), 8-BrcAMP (Sigma-Aldrich; 100 and 500 μM), human synoviocyte PRG4 (apparent mol mass 280 kDa as a monomer; 100 μg/ml) (18), and/or high-molecular-mass HA (mol mass >950 kDa; R&D Systems; 100 μg/ml) for 24 h, followed by RNA extraction, cDNA synthesis, and quantitative polymerase chain reaction (qPCR) as previously described (1). The cycle threshold (Ct) values of target genes were normalized to the Ct value of GAPDH in the same sample, and the relative expression was calculated with the 2−ΔΔCt method (23). Target genes included α-SMA (ACTA2), collagen type I (COL1A1), TIMP-1, PLOD2, HAS1, HAS2, HAS3, and PRG4 (primers and probes were obtained from Thermo Fisher Scientific). Data are presented as fold expression of target genes in the different experimental groups compared with untreated controls.
α-SMA and Procollagen Type I Quantitation
OA synoviocytes were seeded in cell culture dishes (20.8 cm2) at 1.0 × 106 cells/dish until confluence. OA synoviocytes in serum-free DMEM medium (5 ml/dish) were treated with TGF-β1 (1 ng/ml) ± forskolin (10 µM), PRG4 (100 µg/ml), or HA (100 µg/ml) for 24 h. Cell protein extraction was performed with M-PER reagent supplemented with protease and phosphatase cocktail inhibitor (Thermo Fisher Scientific) and quantified with a Micro BCA assay (Thermo Fisher Scientific).
Gel electrophoresis was performed with 10% PAGE gels (Bio-Rad) and 10 μg protein/well. After transfer, membranes were blocked with 5% nonfat dry milk for 2 h at room temperature. Membranes were probed with anti-α-SMA (1:1,000 dilution; ab5694, Abcam) and anti-GAPDH (1:5,000 dilution; ab9485, Abcam) antibodies overnight in Tris-buffered saline + 0.05% Tween 20 (TBS-T). After washing with TBS-T, membranes were incubated with horseradish peroxidase-conjugated anti-rabbit antibody (1:5,000 dilution; ab6721, Abcam) for 1 h at room temperature. Protein bands were developed with Lumigen ECL Ultra reagent (Lumigen) and visualized with the Bio-Rad ChemiDoc XRS+ system (Bio-Rad). Bands of interest were selected and quantified with ImageJ software. The ratio of α-SMA band intensities to corresponding GAPDH band intensities of the different experimental groups were calculated and normalized to controls.
Procollagen I content in OA synoviocytes was determined with an ELISA (Abcam and R&D Systems). A total of 5 μg of protein in 100 µl of buffer was used in each experimental group, and procollagen I concentrations (pg/ml) were divided by 50 and expressed as procollagen I protein (pg) per microgram of protein.
Immunocytostaining of α-SMA in OA Synoviocytes
OA synoviocytes (200,000 cells/well) were cultured on collagen type I-coated 12-mm glass coverslips for 48 h in DMEM supplemented with 10% FBS. Subsequently, cells were treated with TGF-β1 (1 ng/ml) ± forskolin (10 μM) for 48 h in serum-free DMEM. Synoviocytes were fixed in 10% neutral buffered formalin for 10 min, followed by washing twice with PBS. Cells were permeabilized for 5 min with 0.01% Triton X-100 in PBS and blocked with 2% BSA for 1 h at room temperature. Probing was performed with FITC-conjugated anti-α-SMA antibody (1:100; ab8211, Abcam) and Alexa Fluor 594-conjugated anti-α-tubulin antibody (1:500; ab195889, Abcam) overnight at 4°C. After washing with PBS, cells were mounted with DAPI mounting medium (Abcam) for 1 h and viewed under a confocal microscope
HA, PRG4, and HAS1 Quantitation
HA concentrations.
OA synoviocytes (300,000 cells/well) in serum-free DMEM were treated with TGF-β1 (1 ng/ml) ± forskolin (10 μM) for 24 h. Medium supernatants were collected and assayed for HA with a quantitative assay kit (R&D Systems). In a separate set of experiments, OA synoviocytes (300,000 cells/well) in serum-free DMEM were treated with TGF-β1 (1 ng/ml) ± 8-BrcAMP (100 and 500 μM) for 24 h. HA concentrations were determined as described above.
PRG4 concentrations.
OA synoviocytes (20,000 cells/well) were seeded in sterile 96-well plates for 48 h, followed by treatment with TGF-β1 (1 ng/ml) ± forskolin (10 μM) for 48 h. PRG4 concentrations, normalized to cell density, were determined in medium supernatants as previously described (1). In a separate set of experiments, OA synoviocytes (300,000 cells/well) in serum-free DMEM were treated with TGF-β1 (1 ng/ml) ± 8-BrcAMP (100 and 500 μM) for 24 h. PRG4 concentrations were determined as described above.
HAS1 content.
OA synoviocytes were seeded and treated as described for α-SMA and procollagen type I. HAS1 content in protein isolates were determined with an ELISA (MyBioSource). A total of 1 μg of total protein in a 100-μl volume was added to the wells of the ELISA plate. HAS1 concentrations (ng/ml) were divided by 10 and expressed as HAS1 content (ng) per microgram of total protein.
HAS1 Knockdown and Its Impact on Forskolin-Induced HA Secretion in OA Synoviocytes
OA synoviocytes (300,000 cells/well) in Opti-MEM reduced-serum medium (Thermo Fisher Scientific) were treated with a HAS1 small interfering RNA (siRNA) (Thermo Fisher Scientific) (25 pmol/well) or a nontargeted negative control siRNA (25 pmol/well) (Thermo Fisher Scientific) for 48 h. Transfection was performed with Lipofectamine RNAiMAX (Thermo Fisher Scientific) per manufacturer’s recommendations. To confirm HAS1 knockdown, HAS1 expression was determined as described above. In a separate set of experiments, HAS1 knockdown in OA synoviocytes was performed, followed by medium change to serum-free DMEM and stimulation with TGF-β1 (1 ng/ml) ± forskolin (10 μM) for 24 h. Subsequently, HA concentrations were determined in medium supernatants as described above.
Gene Expression Studies in Prg4+/+ and Prg4−/− Synovial Tissues and Immunocytostaining of Murine Synoviocytes
The phenotype of the Prg4−/− mouse has been reported previously (37) and is characterized by cartilage degeneration and a hyperplastic synovium contributing to joint failure (37). The Prg4−/− and Prg4+/+ mouse colonies are maintained by Dr. Jay at Rhode Island Hospital. The Prg4−/− mouse is also commercially available (stock no. 025737; The Jackson Laboratory). Synovial tissues were isolated from male Prg4−/− and Prg4+/+ mice (8–10 wk old). The skin and surrounding tissues of the knee joints were removed. The joint capsule was cut open along both sides of the patella under a stereomicroscope, and the synovium from the lateral and medial sides was carefully isolated. A total of 15 Prg4+/+ and 15 Prg4−/− mice were used in this study. Synovial tissues were harvested from every mouse, and tissues from three mice were randomly pooled into one sample, generating five pooled samples in each genotype. RNA isolation, cDNA synthesis, and qPCR were performed as previously described (53). Genes of interest included ACTA2, COL1A1, TIMP-1, and PLOD2, with GAPDH as an internal reference gene (Thermo Fisher Scientific).
Prg4+/+ and Prg4−/− synoviocytes were isolated as previously described (2). Synovial tissues from male Prg4+/+ and Prg4−/− mice (15 animals/genotype) were used to isolate the synoviocytes. Synoviocytes were plated onto sterile chamber slides (Thermo Fisher Scientific) at a density of 1.0 × 106 cells/well and allowed to adhere for 24 h. Synoviocytes were incubated with human synoviocyte PRG4 (100 μg/ml) in serum-free DMEM for 24 h, followed by washing with PBS and cell fixation with 4% formalin. Probing was performed with anti-α-SMA antibody (1:100 dilution; ab5694, Abcam) or anti-collagen type I antibody (1:200 dilution; ab34710, Abcam) at 4°C overnight. After washing with PBS, cells were incubated with Cy3 goat anti-rabbit IgG antibody (1:200 dilution; A10520, Thermo Fisher Scientific) for 1 h at room temperature in the dark. After washing with PBS, Alexa Fluor 488-conjugated phalloidin, a filamentous actin (F-actin) probe (1:125 dilution; A12379, Thermo Fisher Scientific), was added for 20 min in the dark. Cells were subsequently mounted with DAPI mounting medium (Vector Labs) and viewed under a fluorescent microscope (Nikon E 800).
Basal and TGF-β1-Induced OA Synoviocyte Proliferation and Migration
In sterile 96-well plates, OA synoviocytes (10,000 cells/well) were cultured in serum-free DMEM medium and incubated with forskolin (3, 10, and 30 μM) ± TGF-β1 (1 ng/ml) for 48 h at 37°C. Cell proliferation was determined with MTT reagent [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide; Sigma]. OA synoviocytes (100,000 cells/well) were seeded in 24-well culture plates in DMEM + 10% FBS for 72 h. A 1,000-μl pipette tip was used to perform a scratch in the confluent synoviocyte monolayer. TGF-β1 (1 ng/ml) stimulation was performed in serum-free DMEM ± forskolin (10 μM) for 48 h. Subsequently, medium was aspirated and cells were stained (Cell Biolabs), followed by imaging with an all-in-one fluorescence microscope (Keyence). A region of interest was defined, and scratch width was measured at multiple locations in the region of interest. The mean scratch width was calculated and used to estimate the mean scratch area. Data are presented as the ratio of the scratch areas of the different experimental groups to the scratch area at baseline.
Statistical Analyses
Variables were initially tested for normality. Normally distributed variables were compared with Student’s t-test for two groups or analysis of variance (ANOVA) with Tukey’s post hoc test for more than two groups. Variables that did not satisfy the normality assumption were tested by ANOVA on the ranks. Statistical analysis of gene expression data was performed with ΔCt values (Ct target gene − Ct GAPDH) for each gene of interest. Significance level was set at 0.05.
RESULTS
Forskolin Treatment Increased Intracellular cAMP, Reduced ACTA2, COL1A1, and TIMP-1 Expression, and Reduced α-SMA and Procollagen Type I in TGF-β1-Stimulated OA Synoviocytes
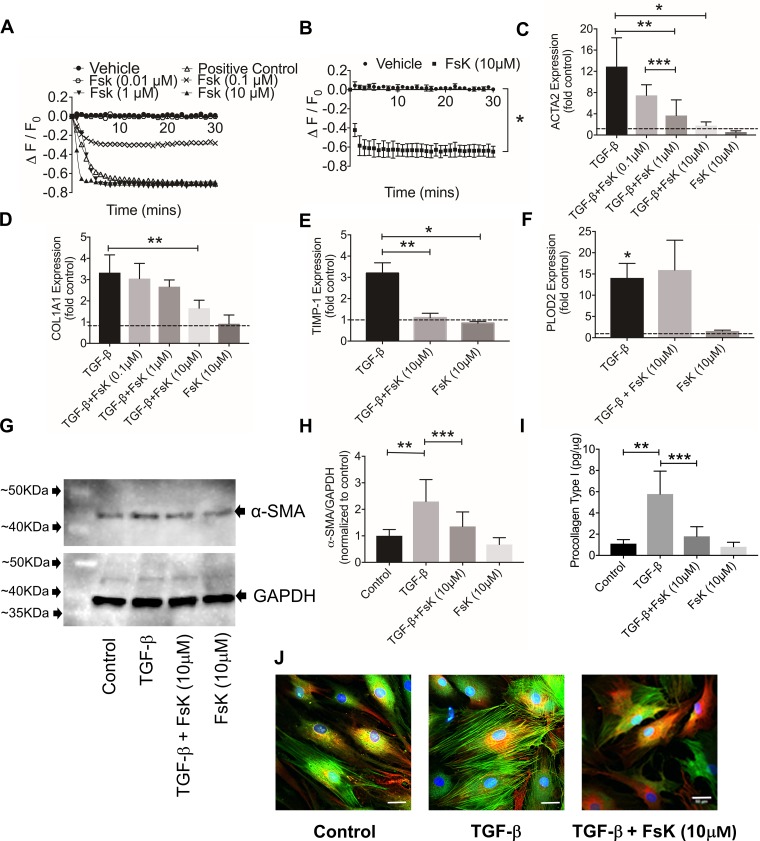
A representative dose response of forskolin is shown in Fig. 1A. Treatment with 0.01 μM forskolin did not increase intracellular cAMP, whereas the 0.1, 1, and 10 μM forskolin treatments resulted in detectable increases in cAMP. Forskolin (10 μM) increased intracellular cAMP compared with vehicle (Fig. 1B; P < 0.001, n = 3 patients). TGF-β1 induced ACTA2, COL1A1, TIMP-1, and PLOD2 expression (Fig. 1, C–F; P < 0.001 vs. control for the 4 genes, n = 4 patients). Forskolin treatment reduced ACTA2 (Fig. 1C; P < 0.001), COL1A1 (Fig. 1D; P < 0.01), and TIMP-1 (Fig. 1E; P < 0.001) expression compared with TGF-β1 alone. Forskolin treatment did not alter TGF-β1-stimulated PLOD2 expression (Fig. 1F; P = 0.833). Forskolin did not alter basal COL1A1 (P = 0.623), TIMP-1 (P = 0.802), or PLOD2 (P = 0.752) expression. In contrast, forskolin reduced basal ACTA2 expression (P = 0.018).
Fig. 1.
Impact of forskolin (FsK) treatment on intracellular cAMP levels, basal and transforming growth factor(TGF)-β1-induced α-smooth muscle actin (ACTA2), collagen I (COL1A1), tissue inhibitor of metalloproteinase 1 (TIMP-1), and procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2 (PLOD2) gene expression and α-smooth muscle action (α-SMA) and procollagen type I production in osteoarthritic (OA) synoviocytes. TGF-β1 (1 ng/ml) stimulation of OA fibroblast-like synoviocytes was performed for 24 h in all experiments except α-SMA immunocytostaining (stimulation was performed with 1 ng/ml TGF-β1 for 48 h). Data are means ± SD of experiments utilizing OA synoviocytes from different patients. A: representative dynamic change in intracellular cAMP levels in OA synoviocytes after treatment with FsK (0.01, 0.1, 1, and 10 μM). FsK treatment at 0.1, 1, and 10 μM resulted in detectable cAMP levels in OA synoviocytes. cAMP signal was detected with a cAMP-specific sensor. ΔF/F0, ratio of fluorescence intensity reduction at each time point to fluorescence intensity at baseline. B: cAMP levels were elevated in FsK (10 μM)-treated OA synoviocytes (n = 3 patients). C: FsK treatment at 1 and 10 μM reduced TGF-β1-induced ACTA2 expression (n = 4 patients). D: FsK treatment at 10 μM reduced TGF-β1-induced COL1A1 expression (n = 4 patients). E: FsK treatment at 10 μM reduced TGF-β1-induced TIMP-1 expression (n = 4 patients). F: FsK treatment (10 μM) did not alter TGF-β1-induced PLOD2 expression (n = 4 patients). G: Western blot of α-SMA (predicted mol mass: 42 kDa) in control and TGF-β1-, TGF-β1 + FsK-, and FsK-treated OA synoviocytes. GAPDH (predicted mol mass: 40 kDa) was used as loading control. H: semiquantitative densitometry analysis of α-SMA normalized to GAPDH and expressed as ratio to control in cell extracts of control and TGF-β1-, TGF-β1 + FsK-, and FsK-treated OA synoviocytes. FsK (10 μM) treatment reduced TGF-β1-linked increase in α-SMA in OA synoviocytes (n = 6 patients). I: procollagen type I content in cell extracts of control and TGF-β1-, TGF-β1 + FsK-, and FsK-treated OA synoviocytes. Data were normalized to total protein content. FsK (10 μM) treatment reduced TGF-β1-linked increase in procollagen type I content in OA synoviocytes (n = 3 patients). J: FsK (10 μM) treatment reduced α-SMA staining and myofibroblast-like phenotype in TGF-β1-stimulated OA synoviocytes. *P < 0.001, **P < 0.01, ***P < 0.05.
A Western blot and semiquantitative analysis of α-SMA using GAPDH as a loading control are shown in Fig. 1, G and H, respectively. TGF-β1 increased α-SMA protein in OA synoviocytes compared with control (Fig. 1H; P < 0.001, n = 6 patients). Forskolin reduced TGF-β1-stimulated α-SMA production (P = 0.013). Forskolin alone did not alter basal α-SMA content (P = 0.660). Representative confocal images of TGF-β1-treated OA synoviocytes ± forskolin are shown in Fig. 1J. Control OA synoviocytes exhibited a positive α-SMA staining and the appearance of a myofibroblast-like phenotype in a number of cells. TGF-β1 treatment resulted in stronger α-SMA staining and the appearance of myofibrils, and this was markedly reduced with forskolin cotreatment. TGF-β1 increased procollagen type I protein compared with control (Fig. 1I; P < 0.01, n = 3 patients). Forskolin reduced TGF-β1-linked procollagen type I production (P = 0.015) and did not alter basal procollagen type I (P = 0.991).
Forskolin Treatment Enhanced HA Secretion and Modulated HAS Isoform Gene Expression, and HAS1 Knockdown Attenuated Forskolin’s Effect on HA Production in TGF-β1-Stimulated OA Synoviocytes
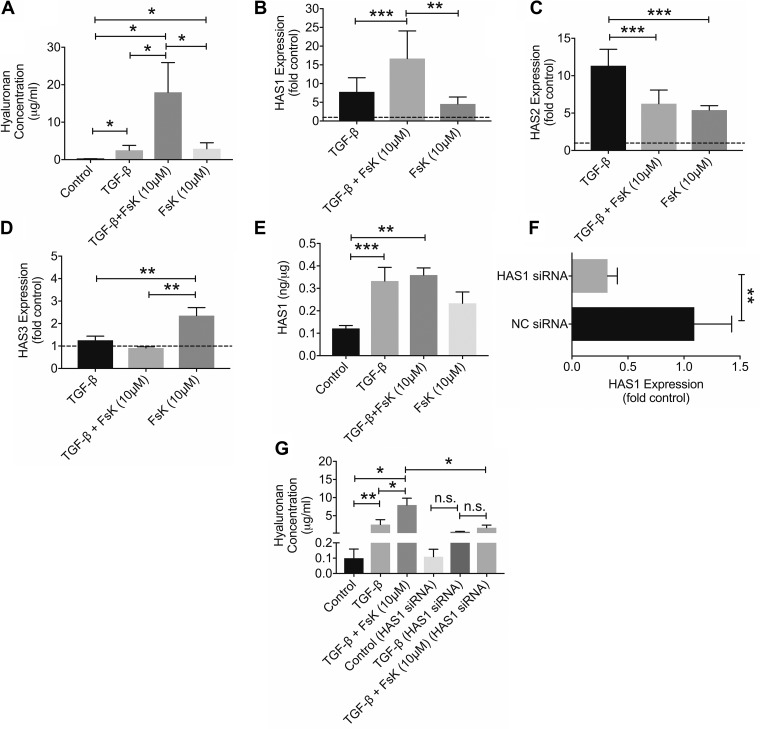
HA concentrations were higher in TGF-β1-treated OA synoviocytes and forskolin-treated OA synoviocytes compared with untreated controls (Fig. 2A; P < 0.001 for both comparisons, n = 4 patients). HA concentrations in the TGF-β1 + forskolin group were higher than HA concentrations in the TGF-β1-alone and forskolin-alone groups (P < 0.001 for both comparisons). TGF-β1 induced HAS1 (Fig. 2B; P < 0.001) and HAS2 (Fig. 2C; P < 0.001) with no effect on HAS3 (Fig. 2D; P = 0.719) expression in OA synoviocytes (n = 4 patients). Forskolin treatment upregulated basal HAS1, HAS2, and HAS3 expression (P < 0.001 for all comparisons). Combined treatment of TGF-β1 and forskolin increased HAS1 expression over TGF-β1 alone (Fig. 2B; P = 0.026). In contrast, HAS2 expression in the TGF-β1 + forskolin group was lower than HAS2 expression in the TGF-β1 group (Fig. 2C; P = 0.024).
Fig. 2.
Impact of forskolin (FsK; 10 μM) treatment on basal and transforming growth factor (TGF)-β1-induced hyaluronan (HA) production, expression of hyaluronan synthase isoforms 1, 2, and 3 (HAS1, HAS2, and HAS3) and the role of HAS1 in mediating TGF-β1- and FsK-linked HA production in osteoarthritic (OA) synoviocytes. Data are means ± SD of experiments utilizing OA synoviocytes from 4 patients. A: HA concentrations in control and TGF-β1-, TGF-β1 + FsK-, and FsK-treated OA synoviocytes. B: FsK treatment enhanced TGF-β1-induced HAS1 expression. C: FsK treatment reduced TGF-β1-induced HAS2 expression. D: FsK treatment increased basal HAS3 expression in OA synoviocytes. E: HAS1 protein content in cell extracts of control and TGF-β1-, TGF-β1 + FsK-, and FsK-treated OA synoviocytes. Data were normalized to total protein content. There was no difference in HAS1 protein between TGF-β1 and TGF-β1 + FsK treatments. F: HAS1 expression was reduced in HAS1 small interfering (siRNA)-treated OA synoviocytes compared with untreated control and negative control (NC) siRNA-treated OA synoviocytes. G: FsK and/or TGF-β1 treatments did not significantly change HA production after HAS1 knockdown in OA synoviocytes. *P < 0.001, **P < 0.01, ***P < 0.05. n.s., Nonsignificant.
Total cellular HAS1 content was higher with TGF-β1 (P = 0.021) and TGF-β1 + forskolin (P < 0.01) treatments compared with control (Fig. 2E; n = 4 patients). HAS1 content was not different between TGF-β1 + forskolin and TGF-β1-alone groups (P = 0.971). Similarly, there was no difference in HAS1 between forskolin and control groups (P = 0.303). HAS1 expression was reduced by ~68% in OA synoviocytes transfected with HAS1 siRNA (Fig. 2F; P < 0.01, n = 4 OA patients). HA concentrations in TGF-β1-stimulated HAS1-knockdown OA synoviocytes were not different from unstimulated HAS1-knockdown OA synoviocytes (Fig. 2G; P = 0.962, n = 4 patients). Similarly, HA concentrations in TGF-β1 + forskolin-treated HAS1-knockdown OA synoviocytes were not different from HA concentrations in TGF-β1-treated HAS1-knockdown OA synoviocytes (P = 0.514). Finally, HA concentrations in TGF-β1 + forskolin-treated OA synoviocytes were higher than HA concentrations in TGF-β1 + forskolin treated HAS1-knockdown OA synoviocytes (P < 0.001).
Forskolin Treatment Enhanced PRG4 Expression and Secretion in TGF-β1-Stimulated OA Synoviocytes
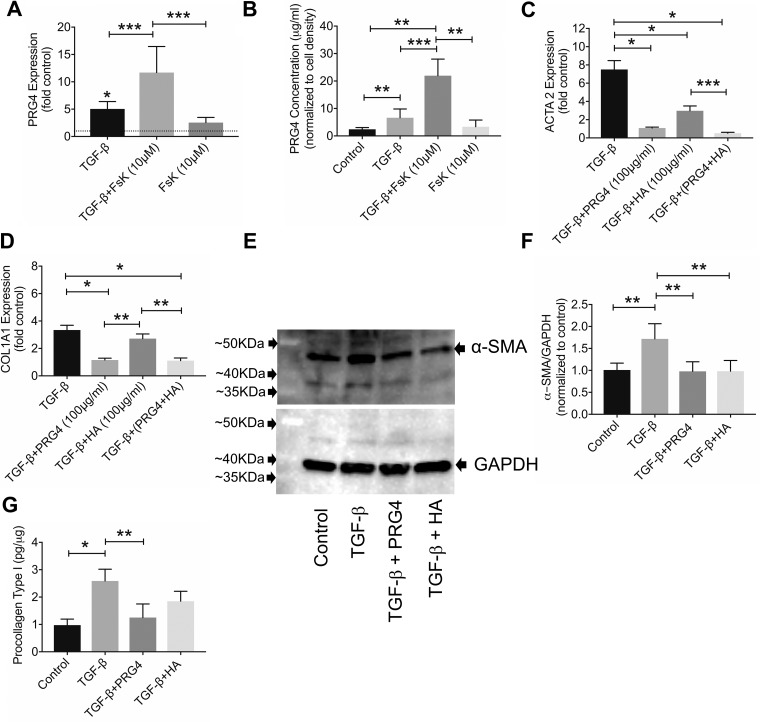
TGF-β1 induced PRG4 expression (Fig. 3A; P < 0.001) and increased PRG4 production by OA synoviocytes (Fig. 3B; P < 0.01, n = 4 patients). Forskolin did not alter basal PRG4 expression (Fig. 3A; P = 0.063) or PRG4 production (Fig. 3B; P = 0.996) in OA synoviocytes. PRG4 expression in the TGF-β1 + forskolin group was higher than with TGF-β1 alone (Fig. 3A; P = 0.037). Correspondingly, PRG4 concentrations were higher in the TGF-β1 + forskolin group compared with the TGF-β1 group (Fig. 3B; P = 0.031).
Fig. 3.
Impact of forskolin (FsK; 10 μM) treatment on basal and transforming growth factor (TGF)-β1-induced proteoglycan-4 (PRG4) gene expression and production by osteoarthritic (OA) synoviocytes and efficacy of human synoviocyte PRG4 (100 μg/ml) and hyaluronan (HA) (100 μg/ml) in modulating TGF-β1-induced expression and production of α-smooth muscle actin and collagen type I in OA synoviocytes. Data are means ± SD of experiments utilizing OA synoviocytes from 4 patients. A: TGF-β1 increased PRG4 expression, and FsK treatment enhanced TGF-β1’s effect. B: FsK treatment enhanced TGF-β1-linked PRG4 production by OA synoviocytes. C: PRG4 and/or HA treatments reduced TGF-β1-induced α-smooth muscle actin gene (ACTA2) expression in OA synoviocytes. D: PRG4 treatment reduced TGF-β1-induced collagen type I gene (COL1A1) expression in OA synoviocytes. E: Western blot of α-smooth muscle action (α-SMA) (predicted mol mass: 42 kDa) in control and TGF-β1-, TGF-β1 + PRG4 (100 μg/ml)- and TGF-β1 + HA (100 μg/ml)-treated OA synoviocytes. GAPDH (predicted mol mass: 40 kDa) was used as loading control. F: semiquantitative densitometry analysis of α-SMA normalized to GAPDH and expressed as ratio to control in cell extracts of control and TGF-β1-, TGF-β1 + PRG4-, and TGF-β1 + HA-treated OA synoviocytes. PRG4 and HA treatments reduced TGF-β1-linked increase in α-SMA in OA synoviocytes. G: procollagen type I content in cell extracts of control and TGF-β1-, TGF-β1 + PRG4-, and TGF-β1 + HA-treated OA synoviocytes. Data were normalized to total protein content. PRG4 treatment reduced TGF-β1-linked increase in procollagen type I content in OA synoviocytes. *P < 0.001, **P < 0.01, ***P < 0.05.
Impact of PRG4 and HA Treatments on ACTA2 and COL1A1 Expression and α-SMA and Procollagen Type I in TGF-β1-Stimulated OA Synoviocytes
ACTA2 expression was lower in the TGF-β1 + PRG4 group compared with TGF-β1 alone (Fig. 3C; P < 0.001, n = 4 patients). Similarly, ACTA2 expression in the TGF-β1+ HA and TGF-β1 + PRG4 + HA groups was lower than ACTA2 expression in TGF-β1 alone (P < 0.001 for both comparisons). COL1A1 expression was lower in the TGF-β1 + PRG4 group compared with TGF-β1 + HA (P < 0.01) or TGF-β1 alone (P < 0.001; Fig. 3D; n = 4 OA patients). In contrast, HA treatment did not alter TGF-β1-induced COL1A1 expression (P = 0.897). COL1A1 expression in the TGF-β1 + PRG4 + HA group was lower than in TGF-β1 (P < 0.001) and TGF- β1 + HA (P < 0.01) groups.
A Western blot and semiquantitative analysis of α-SMA using GAPDH as a loading control are shown in Fig. 3, E and F, respectively. α-SMA content was lower in TGF-β1 + PRG4 (Fig. 3F; P < 0.01) and in TGF-β1 + HA (P < 0.01) compared with TGF-β1 alone (n = 4 patients). Procollagen type I content was lower in TGF-β1 + PRG4 compared with TGF-β1 alone (Fig. 3G; P < 0.01, n = 4 patients). There was no difference in procollagen type I content between TGF-β1 + HA and TGF-β1 groups (P = 0.059).
Cell-Permeant cAMP Analog Treatment Reduced ACTA2 and COL1A1 Expression and Enhanced HA and PRG4 Secretion in TGF-β1-Stimulated OA Synoviocytes
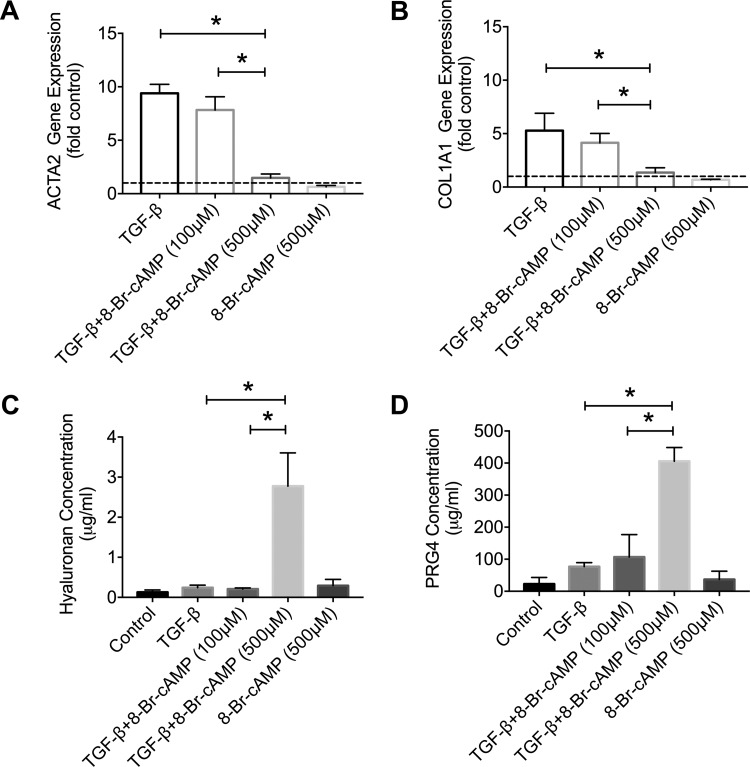
TGF-β1 induced ACTA2 and COL1A1 expression in OA synoviocytes (Fig. 4, A and B; P < 0.001 against control for both genes). 8-BrcAMP (100 μM) treatment did not significantly alter TGF-β1-induced ACTA2 and COL1A1 expression (P > 0.05 for both comparisons). In contrast, 8-BrcAMP (500 μM) treatment reduced ACTA2 (P < 0.001) and COL1A1 (P < 0.001) expression in TGF-β1-stimulated OA synoviocytes (n = 3 OA patients). HA and PRG4 medium concentrations in the TGF-β1 + 8-BrcAMP (500 μM) group were significantly higher than corresponding concentrations in the TGF-β1-only group (Fig. 4, C and D; P < 0.001 for both comparisons, n = 3 OA patients).
Fig. 4.
Impact of 8-bromoadenosine 3′,5′-cyclic monophosphate (8-BrcAMP; 100 and 500 μM) treatment on basal and transforming growth factor (TGF)-β1-induced α-alpha smooth muscle actin gene (ACTA2) and collagen I gene (COL1A1) expression and hyaluronan (HA) and proteoglycan-4 (PRG4) secretion in osteoarthritic (OA) synoviocytes. Data are means ± SD of experiments utilizing OA synoviocytes from 3 patients. A: 8-BrcAMP at 500 μM reduced TGF-β1-linked ACTA2 expression in OA synoviocytes. B: 8-BrcAMP at 500 μM reduced TGF-β1-linked COL1A1 expression in OA synoviocytes. C: 8-BrcAMP at 500 μM) increased HA secretion in TGF-β1-stimulated OA synoviocytes. D: 8-BrcAMP at 500 μM increased PRG4 secretion in TGF-β1-stimulated OA synoviocytes. *P < 0.001.
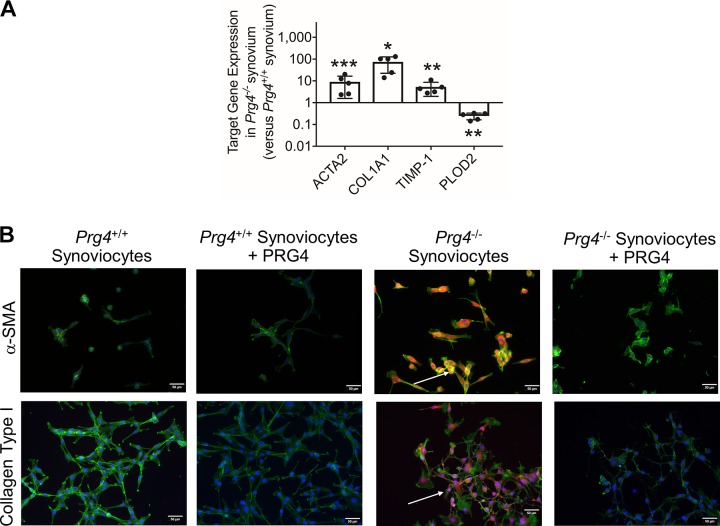
ACTA2, COL1A1, and TIMP-1 Expression Was Higher in Prg4−/− Synovial Tissues, and Human Synoviocyte PRG4 Treatment Reduced α-SMA and Collagen Type I Staining in Prg4−/− Synoviocytes
Expression of ACTA2 (P = 0.021), COL1A1 (P < 0.001), and TIMP-1 (P < 0.01) was higher in Prg4−/− synovia compared with Prg4+/+ synovia (Fig. 5A). In contrast, PLOD2 expression was lower in Prg4−/− tissues compared with Prg4+/+ tissues (P < 0.01).
Fig. 5.
Gene expression of α-smooth muscle actin (ACTA2), collagen type I (COL1A1), tissue inhibitor of metalloproteinase-1 (TIMP-1), and procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2 (PLOD2) in synovial tissues isolated from Prg4+/+ and Prg4−/− mice and immunocytostaining of α-smooth muscle actin (α-SMA) and collagen type I in Prg4+/+ and Prg4−/− synoviocytes and impact of human synoviocyte PRG4 treatment. A: ACTA2, COL1A1, and TIMP-1 expression in Prg4−/− synovial tissues was higher than in Prg4+/+ synovial tissues. PLOD2 expression in Prg4−/− synovial tissues was lower than in Prg4+/+ synovial tissues. Each group contained 5 samples, with each sample generated by pooling synovial tissues from 3 mice. B: merged images depicting α-SMA and collagen type I protein immunostaining in isolated Prg4+/+ synoviocytes and Prg4−/− synoviocytes (bright orange) and counterstained with F-actin (green) and DAPI (blue). α-SMA and collagen type I staining was detected in Prg4−/− synoviocytes (white arrows), and no staining was detected in Prg4+/+ synoviocytes. α-SMA and collagen type I staining intensities were reduced by human synoviocyte PRG4 treatment for 24 h. *P < 0.001, **P < 0.01, ***P < 0.05.
Merged images of α-SMA- and collagen type I-stained Prg4+/+ and Prg4−/− synoviocytes are shown in Fig. 5B. We observed strong α-SMA and collagen type I staining in Prg4−/− synoviocytes. α-SMA staining colocalized with F-actin staining. In contrast, no α-SMA or collagen type I staining was detected in Prg4+/+ synoviocytes. PRG4 treatment reduced α-SMA and collagen type I staining in Prg4−/− synoviocytes.
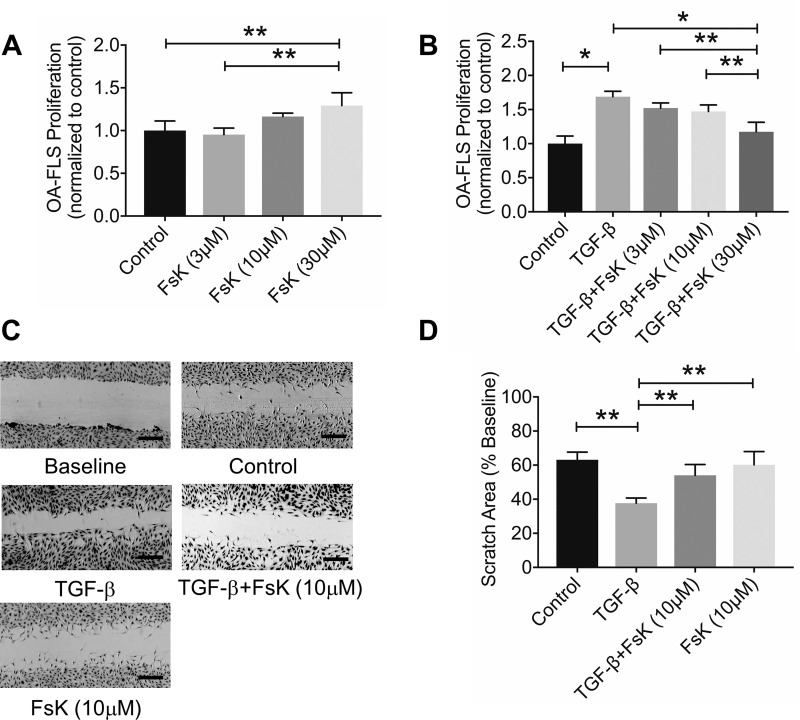
Forskolin Reduced TGF-β1-Induced OA Synoviocyte Proliferation and Migration
Forskolin 3 μM and 10 μM treatments did not alter basal OA synoviocyte proliferation (Fig. 6A; P = 0.891 and P = 0.117, n = 4 patients). In contrast, the 30 μM treatment increased basal OA synoviocyte proliferation compared with untreated control (P < 0.01). TGF-β1 stimulated OA synoviocyte proliferation (Fig. 6B; P < 0.001, n = 4 patients). OA synoviocyte proliferation in the TGF-β1 + forskolin (30 μM) group was lower than TGF-β1-alone (P < 0.001), TGF-β1 + forskolin (3 μM) (P < 0.01), or TGF-β1 + forskolin (10 μM) (P < 0.01) groups. There was no difference in cell proliferation between TGF-β1 + forskolin (10 μM) and TGF-β1 alone (P = 0.063). Representative wound scratch images are shown in Fig. 6C. TGF-β1 enhanced OA synoviocyte migration (Fig. 6D; P < 0.01, n = 4 patients). OA synoviocyte migration in the TGF-β1 + forskolin (10 μM) group was lower than in the TGF-β1-only group (P < 0.01). There was no difference in cell migration between forskolin-treated and untreated OA synoviocytes (P = 0.887).
Fig. 6.
Impact of forskolin (FsK) treatment on basal and transforming growth factor (TGF)-β1-induced proliferation and migration of osteoarthritic (OA) synoviocytes. Data are means ± SD of experiments utilizing OA synoviocytes from 4 patients. A: FsK treatment at 30 μM enhanced basal OA synoviocyte proliferation. B: FsK treatment at 30 μM reduced TGF-β1-induced OA synoviocyte proliferation. C: representative images showing baseline and 48-h basal, TGF-β1-, TGF-β1 + FsK-, and FsK alone-induced OA synoviocyte migration with an in vitro scratch assay. D: FSK (10 μM) treatment reduced TGF-β1-stimulated OA synoviocyte migration. Scale bars, 1,000 μm. *P < 0.001, **P < 0.01. FLS, fibroblast-like synoviocyte.
DISCUSSION
In this report, we show that TGF- β1 resulted in excess collagen type I production, induction of TIMP-1 and PLOD2 expression, and α-SMA upregulation together with stimulating OA synoviocyte migration and proliferation. Forskolin, by virtue of its ability to generate cAMP, reduced collagen production and blunted TIMP-1 expression while inhibiting synoviocyte proliferation and migration. PLOD2 induction in OA synoviocytes is consistent with its established role in mediating synovial collagen cross-linking (3, 29). Forskolin did not alter PLOD2 expression, which may be related to the TGF-β1 signaling pathways. Remst et al. have shown that in OA synoviocytes an anaplastic lymphoma kinase 1/2/3/6 inhibitor completely blocked TGF-β1-induced collagen type I expression, whereas TGF-β1-induced PLOD2 expression was only slightly reduced (34).
OA synoviocytes proliferate in response to various mitogenic stimuli (1, 5, 15). In our experiments, TGF-β1 induced cell proliferation with a magnitude comparable to what has been previously reported (5). Forskolin, at the treatment level that inhibited migration, exhibited a marginal antiproliferative effect, and a higher concentration was needed to observe significant antagonism of TGF-β1’s mitogenic effect. This might be due to a low proliferative capacity of OA synoviocytes. In the absence of TGF-β1, forskolin acted as a mitogen to produce a low, yet significant, stimulation of proliferation. Forskolin directly binds to AC and generates cAMP from ATP (43). The increase in cAMP results in activation of protein kinase A (PKA)-dependent and -independent pathways (39, 43). In the presence of growth factors, forskolin activates cAMP-dependent PKA, which interferes with Raf-1 activation and signaling to blunt cell proliferation (11). PKA also activates CREB, which can compete for cofactors with SMAD-mediated transcription stimulated by TGF-β1 (22). In the absence of growth factor, PKA-dependent and -independent pathways stimulate cAMP-mediated cell proliferation (6).
We measured α-SMA in OA synoviocytes from human patients, and forskolin addition consistently attenuated TGF-β1-stimulated α-SMA expression. α-SMA is a specific marker of myofibroblasts (7, 8). Myofibroblasts are effector cells in fibrosis that possess enhanced ability to produce collagen, proliferate, and migrate (7, 8). We have shown that forskolin treatment appeared to markedly reduce TGF-β1’s induced myofibroblast-like phenotype in OA synoviocytes. The causal role that myofibroblasts may play in synovial fibrosis is understudied and unclear. Steenvoorden et al. reported that α-SMA staining was only found in blood vessels in synovia from healthy individuals (49). Interestingly, TGF- β1 stimulation of normal synoviocytes increased collagen type I expression with no effect on α-SMA expression or production (49). Mattey et al. have shown that TGF-β1 or IL-4 treatments trigger differentiation of OA synoviocytes into myofibroblast-like cells, characterized by α-SMA expression in vitro (28). Evidence relating myofibroblasts to changes occurring in joint fibrosis was reported by Sasabe et al. (40). With a rat knee contracture model, myofibroblasts expressing α-SMA were detected as early as 1 wk from joint immobilization, and this was associated with increased collagen type I expression and joint capsule fibrosis (40). We have also detected α-SMA protein in Prg4−/− synoviocytes with no α-SMA signal in normal murine synoviocytes. The positive α-SMA signal in Prg4−/− knockout synoviocytes is associated with phenotypical changes in the synovium including synovial lining thickening and enhanced synoviocyte proliferation (37).
TGF-β1 induced HAS1 and HAS2 expression, with no effect on HAS3, and enhanced HA secretion by OA synoviocytes. Synoviocytes contain higher levels of HAS1 message compared with HAS2, with HAS3 being the least abundant (33). Earlier reports are in agreement with our finding that TGF-β1 stimulation of arthritic synoviocytes resulted in a higher HAS1-to-HAS2 transcript ratio above control level and that in turn resulted in higher extracellular HA levels (5, 33). Our data suggest that the majority of TGF-β1-linked HA secretion is mediated by HAS1, as HAS1 knockdown diminished extracellular HA concentrations. Cotreatment with forskolin increased the HAS1-to-HAS2 transcript ratio above the corresponding TGF-β1 ratio with greater HA secretion over 24 h. The increase in HAS1 mRNA in forskolin-treated synoviocytes did not translate to an increased HAS1 cellular pool. This might be related to the rate at which the message is being translated. Furthermore, the majority of the HAS1 cellular fraction is inactive and is found in the cytoplasm either diffused or partially colocalized with the Golgi apparatus, whereas the plasma membrane-bound fraction is small and is catalytically active (51). Therefore, the possibility that the newly synthesized HAS1 enzyme, in response to forskolin treatment, could have been trafficked to the membrane, resulting in increasing the rate of HA synthesis, could not be ruled out. Assessing the impact of forskolin on membrane-bound HAS1 level and activity was technically challenging and was not feasible to perform. Our data should also be considered in the context that other factors, e.g., posttranslational modifications, availability of precursors, and regulation of HAS activity, will likely contribute to the amount of HA secreted by the OA synoviocytes (33).
Increasing intracellular cAMP resulted in increasing PRG4 expression and production by OA synoviocytes in the setting of TGF-β1 stimulation. This contextual effect is due to CREB stimulation, which was previously shown to enhance PRG4 production by superficial zone articular chondrocytes (31). PRG4 is a mucinlike glycoprotein synthesized by synoviocytes and superficial zone articular chondrocytes with a heavily glycosylated central domain and NH2 and COOH termini (19). The mouse Prg4 gene is highly homologous to the human Prg4 gene (14). Human and mouse Prg4 genes each consist of 12 exons, and the NH2 and COOH termini are highly conserved across species (14). PRG4 exists in the synovial fluid in monomeric or multimeric forms and functions as a boundary lubricant (19, 42). PRG4 binds to CD44, the HA receptor, and exerts an anti-inflammatory effect in OA synoviocytes (1, 2). PRG4 also acts in an autocrine manner to regulate OA synoviocyte proliferation (1). We have found that PRG4 and HA had equivalent efficacy in reducing α-SMA content in OA synoviocytes. However, PRG4, at a physiologically relevant concentration (20), was more efficacious than HA in reducing collagen I expression and production, indicative of a potential role in antagonizing profibrotic alterations in synovial tissues. This function is likely related to its interaction with the CD44 receptor, given that HA reduced synovial fibrosis in a CD44-mediated manner (32). PRG4 reduced collagen I and α-SMA staining in Prg4−/− synoviocytes. The link between PRG4 expression and synovial fibrosis is further illustrated by the upregulation of collagen type I, TIMP-1, and α-SMA in the Prg4−/− synovium. As laying down excess collagen type I is a prominent feature in synovial fibrosis, the strong immunocytostaining for collagen type I in Prg4−/− synoviocytes, coupled with other synovial changes, supports a fibrotic Prg4−/− synovium. We did not include human normal synoviocytes in our study design. Furthermore, we did not examine the efficacy of forskolin or PRG4 in an in vivo model of synovial fibrosis.
In summary, our data demonstrate that forskolin, a diterpene produced by the roots of the Indian plant Coleus forskohlii (43), increases intracellular cAMP levels and produces an antifibrotic effect in OA synoviocytes. Increasing intracellular cAMP levels directly via treatment with a cell-permeant cAMP analog recapitulated the antifibrotic effect of forskolin. Forskolin reduces collagen type I expression and procollagen type I production and inhibits TGF-β1-linked fibroblast migration and proliferation. Forskolin also increased HA and PRG4 secretion by OA synoviocytes, an effect that may contribute to its overall antifibrotic efficacy. Approaches that increase cAMP levels in synoviocytes can promote an antifibrotic phenotype and may be a novel approach for slowing the progression of synovial fibrosis in OA.
GRANTS
This work is supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R01 AR-067748 to K. A. Elsaid and G. D. Jay.
DISCLOSURES
G. D. Jay authored patents related to use of recombinant PRG4 and holds equity in Lubris LLC. K. A. Elsaid coauthored patent applications related to use of recombinant PRG4. M. M. Qadri, L. X. Zhang, and R. S. Ostrom have nothing to disclose.
AUTHOR CONTRIBUTIONS
G.D.J., R.S.O., and K.A.E. conceived and designed research; M.M.Q., L.X.Z., and K.A.E. performed experiments; M.M.Q., G.D.J., R.S.O., L.X.Z., and K.A.E. analyzed data; M.M.Q., G.D.J., R.S.O., L.X.Z., and K.A.E. interpreted results of experiments; M.M.Q., L.X.Z., and K.A.E. prepared figures; M.M.Q., G.D.J., and K.A.E. drafted manuscript; M.M.Q., G.D.J., R.S.O., L.X.Z., and K.A.E. edited and revised manuscript; M.M.Q., G.D.J., R.S.O., L.X.Z., and K.A.E. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Austin Kazarian for help with the cAMP assays.
REFERENCES
- 1.Alquraini A, Jamal M, Zhang L, Schmidt T, Jay GD, Elsaid KA. The autocrine role of proteoglycan-4 (PRG4) in modulating osteoarthritic synoviocyte proliferation and expression of matrix degrading enzymes. Arthritis Res Ther 19: 89, 2017. doi: 10.1186/s13075-017-1301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Sharif A, Jamal M, Zhang LX, Larson K, Schmidt TA, Jay GD, Elsaid KA. Lubricin/proteoglycan 4 binding to CD44 receptor: a mechanism of the suppression of proinflammatory cytokine-induced synoviocyte proliferation by lubricin. Arthritis Rheumatol 67: 1503–1513, 2015. doi: 10.1002/art.39087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastiaansen-Jenniskens YM, Wei W, Feijt C, Waarsing JH, Verhaar JA, Zuurmond AM, Hanemaaijer R, Stoop R, van Osch GJ. Stimulation of fibrotic processes by the infrapatellar fat pad in cultured synoviocytes from patients with osteoarthritis: a possible role for prostaglandin f2α. Arthritis Rheum 65: 2070–2080, 2013. doi: 10.1002/art.37996. [DOI] [PubMed] [Google Scholar]
- 4.Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis 64: 1263–1267, 2005. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blewis ME, Lao BJ, Schumacher BL, Bugbee WD, Sah RL, Firestein GS. Interactive cytokine regulation of synoviocyte lubricant secretion. Tissue Eng Part A 16: 1329–1337, 2010. doi: 10.1089/ten.tea.2009.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cass LA, Summers SA, Prendergast GV, Backer JM, Birnbaum MJ, Meinkoth JL. Protein kinase A-dependent and -independent signaling pathways contribute to cyclic AMP-stimulated proliferation. Mol Cell Biol 19: 5882–5891, 1999. doi: 10.1128/MCB.19.9.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaponnier C, Gabbiani G. Pathological situations characterized by altered actin isoform expression. J Pathol 204: 386–395, 2004. doi: 10.1002/path.1635. [DOI] [PubMed] [Google Scholar]
- 8.Ehrlich HP, Allison GM, Leggett M. The myofibroblast, cadherin, alpha smooth muscle actin and the collagen effect. Cell Biochem Funct 24: 63–70, 2006. doi: 10.1002/cbf.1188. [DOI] [PubMed] [Google Scholar]
- 9.Flannery CR, Hughes CE, Schumacher BL, Tudor D, Aydelotte MB, Kuettner KE, Caterson B. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun 254: 535–541, 1999. doi: 10.1006/bbrc.1998.0104. [DOI] [PubMed] [Google Scholar]
- 10.Haas S, Straub RH. Disruption of rhythms of molecular clocks in primary synovial fibroblasts of patients with osteoarthritis and rheumatoid arthritis, role of IL-1β/TNF. Arthritis Res Ther 14: R122, 2012. doi: 10.1186/ar3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Häfner S, Adler HS, Mischak H, Janosch P, Heidecker G, Wolfman A, Pippig S, Lohse M, Ueffing M, Kolch W. Mechanism of inhibition of Raf-1 by protein kinase A. Mol Cell Biol 14: 6696–6703, 1994. doi: 10.1128/MCB.14.10.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heusinger-Ribeiro J, Eberlein M, Wahab NA, Goppelt-Struebe M. Expression of connective tissue growth factor in human renal fibroblasts: regulatory roles of RhoA and cAMP. J Am Soc Nephrol 12: 1853–1861, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, Gale D, Grainger A, Conaghan P, Felson DT. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis 66: 1599–1603, 2007. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikegawa S, Sano M, Koshizuka Y, Nakamura Y. Isolation, characterization and mapping of the mouse and human PRG4 (proteoglycan 4) genes. Cytogenet Cell Genet 90: 291–297, 2000. doi: 10.1159/000056791. [DOI] [PubMed] [Google Scholar]
- 15.Inoue H, Takamori M, Nagata N, Nishikawa T, Oda H, Yamamoto S, Koshihara Y. An investigation of cell proliferation and soluble mediators induced by interleukin 1beta in human synovial fibroblasts: comparative response in osteoarthritis and rheumatoid arthritis. Inflamm Res 50: 65–72, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Insel PA, Murray F, Yokoyama U, Romano S, Yun H, Brown L, Snead A, Lu D, Aroonsakool N. cAMP and Epac in the regulation of tissue fibrosis. Br J Pharmacol 166: 447–456, 2012. doi: 10.1111/j.1476-5381.2012.01847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jay GD, Britt DE, Cha CJ. Lubricin is a product of megakaryocyte stimulating factor gene expression by human synovial fibroblasts. J Rheumatol 27: 594–600, 2000. [PubMed] [Google Scholar]
- 18.Jay GD, Fleming BC, Watkins BA, McHugh KA, Anderson SC, Zhang LX, Teeple E, Waller KA, Elsaid KA. Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum 62: 2382–2391, 2010. doi: 10.1002/art.27550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jay GD, Waller KA. The biology of lubricin: near frictionless joint motion. Matrix Biol 39: 17–24, 2014. doi: 10.1016/j.matbio.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Kosinska MK, Ludwig TE, Liebisch G, Zhang R, Siebert HC, Wilhelm J, Kaesser U, Dettmeyer RB, Klein H, Ishaque B, Rickert M, Schmitz G, Schmidt TA, Steinmeyer J. Articular joint lubricants during osteoarthritis and rheumatoid arthritis display altered levels and molecular species. PLoS One 10: e0125192, 2015. doi: 10.1371/journal.pone.0125192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Sun SQ, Ostrom RS. Fibrotic lung fibroblasts show blunted inhibition by cAMP due to deficient cAMP response element-binding protein phosphorylation. J Pharmacol Exp Ther 315: 678–687, 2005. doi: 10.1124/jpet.105.090324. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Sun SQ, Hassid A, Ostrom RS. cAMP inhibits transforming growth factor-beta-stimulated collagen synthesis via inhibition of extracellular signal-regulated kinase 1/2 and Smad signaling in cardiac fibroblasts. Mol Pharmacol 70: 1992–2003, 2006. doi: 10.1124/mol.106.028951. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Loeuille D, Chary-Valckenaere I, Champigneulle J, Rat AC, Toussaint F, Pinzano-Watrin A, Goebel JC, Mainard D, Blum A, Pourel J, Netter P, Gillet P. Macroscopic and microscopic features of synovial membrane inflammation in the osteoarthritic knee: correlating magnetic resonance imaging findings with disease severity. Arthritis Rheum 52: 3492–3501, 2005. doi: 10.1002/art.21373. [DOI] [PubMed] [Google Scholar]
- 25.Ludwig TE, Hunter MM, Schmidt TA. Cartilage boundary lubrication synergism is mediated by hyaluronan concentration and PRG4 concentration and structure. BMC Musculoskelet Disord 16: 386, 2015. doi: 10.1186/s12891-015-0842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, Goldring SR, Jones G, Teichtahl AJ, Pelletier JP. Osteoarthritis. Nat Rev Dis Primers 2: 16072, 2016. doi: 10.1038/nrdp.2016.72. [DOI] [PubMed] [Google Scholar]
- 27.Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther 19: 18, 2017. doi: 10.1186/s13075-017-1229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattey DL, Dawes PT, Nixon NB, Slater H. Transforming growth factor β1 and interleukin 4 induced α smooth muscle actin expression and myofibroblast-like differentiation in human synovial fibroblasts in vitro: modulation by basic fibroblast growth factor. Ann Rheum Dis 56: 426–431, 1997. doi: 10.1136/ard.56.7.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murakami S, Muneta T, Ezura Y, Furuya K, Yamamoto H. Quantitative analysis of synovial fibrosis in the infrapatellar fat pad before and after anterior cruciate ligament reconstruction. Am J Sports Med 25: 29–34, 1997. doi: 10.1177/036354659702500106. [DOI] [PubMed] [Google Scholar]
- 30.Neumann E, Riepl B, Knedla A, Lefèvre S, Tarner IH, Grifka J, Steinmeyer J, Schölmerich J, Gay S, Müller-Ladner U. Cell culture and passaging alters gene expression pattern and proliferation rate in rheumatoid arthritis synovial fibroblasts. Arthritis Res Ther 12: R83, 2010. doi: 10.1186/ar3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogawa H, Kozhemyakina E, Hung HH, Grodzinsky AJ, Lassar AB. Mechanical motion promotes expression of Prg4 in articular cartilage via multiple CREB-dependent, fluid flow shear stress-induced signaling pathways. Genes Dev 28: 127–139, 2014. doi: 10.1101/gad.231969.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plaas A, Li J, Riesco J, Das R, Sandy JD, Harrison A. Intraarticular injection of hyaluronan prevents cartilage erosion, periarticular fibrosis and mechanical allodynia and normalizes stance time in murine knee osteoarthritis. Arthritis Res Ther 13: R46, 2011. doi: 10.1186/ar3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Recklies AD, White C, Melching L, Roughley PJ. Differential regulation and expression of hyaluronan synthases in human articular chondrocytes, synovial cells and osteosarcoma cells. Biochem J 354: 17–24, 2001. doi: 10.1042/bj3540017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Remst DF, Blaney Davidson EN, Vitters EL, Bank RA, van den Berg WB, van der Kraan PM. TGF-β induces lysyl hydroxylase 2b in human synovial osteoarthritic fibroblasts through ALK5 signaling. Cell Tissue Res 355: 163–171, 2014. doi: 10.1007/s00441-013-1740-5. [DOI] [PubMed] [Google Scholar]
- 35.Remst DF, Blom AB, Vitters EL, Bank RA, van den Berg WB, Blaney Davidson EN, van der Kraan PM. Gene expression analysis of murine and human osteoarthritis synovium reveals elevation of transforming growth factor β-responsive genes in osteoarthritis-related fibrosis. Arthritis Rheumatol 66: 647–656, 2014. doi: 10.1002/art.38266. [DOI] [PubMed] [Google Scholar]
- 36.Remst DF, Blaney Davidson EN, Vitters EL, Blom AB, Stoop R, Snabel JM, Bank RA, van den Berg WB, van der Kraan PM. Osteoarthritis-related fibrosis is associated with both elevated pyridinoline cross-link formation and lysyl hydroxylase 2b expression. Osteoarthritis Cartilage 21: 157–164, 2013. doi: 10.1016/j.joca.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Rhee DK, Marcelino J, Baker M, Gong Y, Smits P, Lefebvre V, Jay GD, Stewart M, Wang H, Warman ML, Carpten JD. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest 115: 622–631, 2005. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sachs BD, Baillie GS, McCall JR, Passino MA, Schachtrup C, Wallace DA, Dunlop AJ, MacKenzie KF, Klussmann E, Lynch MJ, Sikorski SL, Nuriel T, Tsigelny I, Zhang J, Houslay MD, Chao MV, Akassoglou K. p75 neurotrophin receptor regulates tissue fibrosis through inhibition of plasminogen activation via a PDE4/cAMP/PKA pathway. J Cell Biol 177: 1119–1132, 2007. doi: 10.1083/jcb.200701040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sapio L, Gallo M, Illiano M, Chiosi E, Naviglio D, Spina A, Naviglio S. The natural cAMP elevating compound forskolin in cancer therapy: is it time? J Cell Physiol 232: 922–927, 2017. doi: 10.1002/jcp.25650. [DOI] [PubMed] [Google Scholar]
- 40.Sasabe R, Sakamoto J, Goto K, Honda Y, Kataoka H, Nakano J, Origuchi T, Endo D, Koji T, Okita M. Effects of joint immobilization on changes in myofibroblasts and collagen in the rat knee contracture model. J Orthop Res 35: 1998–2006, 2017. doi: 10.1002/jor.23498. [DOI] [PubMed] [Google Scholar]
- 41.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone 51: 249–257, 2012. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt TA, Plaas AH, Sandy JD. Disulfide-bonded multimers of proteoglycan 4 PRG4 are present in normal synovial fluids. Biochim Biophys Acta 1790: 375–384, 2009. doi: 10.1016/j.bbagen.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 43.Seamon KB, Padgett W, Daly JW. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci USA 78: 3363–3367, 1981. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selige J, Tenor H, Hatzelmann A, Dunkern T. Cytokine-dependent balance of mitogenic effects in primary human lung fibroblasts related to cyclic AMP signaling and phosphodiesterase 4 inhibition. J Cell Physiol 223: 317–326, 2010. doi: 10.1002/jcp.22037. [DOI] [PubMed] [Google Scholar]
- 45.Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol 6: 625–635, 2010. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 46.Shen J, Li S, Chen D. TGF-β signaling and the development of osteoarthritis. Bone Res 2: 14002, 2014. doi: 10.1038/boneres.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith MD, Triantafillou S, Parker A, Youssef PP, Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol 24: 365–371, 1997. [PubMed] [Google Scholar]
- 48.Smith MD. The normal synovium. Open Rheumatol J 5: 100–106, 2011. doi: 10.2174/1874312901105010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steenvoorden MM, Tolboom TC, van der Pluijm G, Löwik C, Visser CP, DeGroot J, Gittenberger-DeGroot AC, DeRuiter MC, Wisse BJ, Huizinga TW, Toes RE. Transition of healthy to diseased synovial tissue in rheumatoid arthritis is associated with gain of mesenchymal/fibrotic characteristics. Arthritis Res Ther 8: R165, 2006. doi: 10.1186/ar2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc Natl Acad Sci USA 102: 437–442, 2005. doi: 10.1073/pnas.0408704102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Törrönen K, Nikunen K, Kärnä R, Tammi M, Tammi R, Rilla K. Tissue distribution and subcellular localization of hyaluronan synthase isoenzymes. Histochem Cell Biol 141: 17–31, 2014. doi: 10.1007/s00418-013-1143-4. [DOI] [PubMed] [Google Scholar]
- 52.van der Kraan PM. The changing role of TGFβ in healthy, ageing and osteoarthritic joints. Nat Rev Rheumatol 13: 155–163, 2017. doi: 10.1038/nrrheum.2016.219. [DOI] [PubMed] [Google Scholar]
- 53.Waller KA, Zhang LX, Jay GD. Friction-induced mitochondrial dysregulation contributes to joint deterioration in Prg4 knockout mice. Int J Mol Sci 18: E1252, 2017. doi: 10.3390/ijms18061252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weigel PH, DeAngelis PL. Hyaluronan synthases: a decade-plus of novel glycosyltransferases. J Biol Chem 282: 36777–36781, 2007. doi: 10.1074/jbc.R700036200. [DOI] [PubMed] [Google Scholar]
- 55.Wenham CY, Conaghan PG. The role of synovitis in osteoarthritis. Ther Adv Musculoskelet Dis 2: 349–359, 2010. doi: 10.1177/1759720X10378373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yokoyama U, Patel HH, Lai NC, Aroonsakool N, Roth DM, Insel PA. The cyclic AMP effector Epac integrates pro- and anti-fibrotic signals. Proc Natl Acad Sci USA 105: 6386–6391, 2008. [Erratum in Proc Natl Acad Sci USA 105: 8160, 2008.] doi: 10.1073/pnas.0801490105. [DOI] [PMC free article] [PubMed] [Google Scholar]