Abstract
Hydrogen peroxide (H2O2) increases paracellular permeability of Madin-Darby canine kidney (MDCK) cells, but the mechanism mediating this effect remains unclear. Treatment of MDCK cells with H2O2 activated ERK 1/2. Inhibition of ERK 1/2 activation blocked the ability of H2O2 to increase paracellular permeability. Knockdown of zonula occludens-1 (ZO-1) protein but not occludin eliminated the ability of H2O2 to increase paracellular permeability. H2O2 treatment did not, however, affect the total cell content or contents of the Triton X-100-soluble and -insoluble fractions for occludin, ZO-1, or ZO-2. H2O2 treatment decreased the number of F-actin stress fibers in the basal portion of the cells. Similar to wild-type MDCK cells, H2O2 increased ERK 1/2 activation in ZO-1 knockdown and occludin knockdown cells. Inhibition of ERK 1/2 activation blocked the increase in paracellular permeability in occludin knockdown cells. ZO-1 knockdown cell paracellular permeability was regulated by PP1, an src inhibitor, indicating that the loss of response to H2O2 was not a general loss of the ability to regulate the paracellular barrier. Inhibition of myosin ATPase activity with blebbistatin increased paracellular permeability in ZO-1 knockdown cells but not in wild-type MDCK cells. H2O2 treatment sensitized wild-type MDCK cells to inhibition of myosin ATPase. Knockdown of TOCA-1 protein, which promotes formation of local branched actin networks, reproduced the effects of ZO-1 protein knockdown. These results demonstrate that H2O2 increases MDCK cell paracellular permeability through activation of ERK 1/2. This H2O2 action requires ZO-1 protein and TOCA-1 protein, suggesting involvement of the actin cytoskeleton.
Keywords: hydrogen peroxide, permeability, tight junction
INTRODUCTION
A primary function of epithelial cells is the formation of a permeability barrier separating body compartments enabling differential modification of the body compartments. The permeability barrier for movement of solutes between adjacent epithelial cells (paracellular permeability) is accomplished primarily through the formation of circumferential tight junction structures at the apicolateral border of adjoining epithelial cells (26). Recent studies have demonstrated that the tight junction is a dynamic structure capable of modulating separately its permeability to small ions/solutes (pore pathway) and larger solutes (leak pathway) (6, 26). The molecular mechanism(s) by which various stimuli modulate paracellular permeability is an active area of investigation. Studies have implicated involvement of different tight junction proteins, including occludin, zonula occludens-1 (ZO-1), and ZO-2 (see, e.g., 2, 5, 31, 32), and different signaling pathways (see, e.g., 4, 5, 35) in the modulation of epithelial and endothelial paracellular permeability.
Many previous studies have examined the regulation of intestinal epithelial cell paracellular permeability by hydrogen peroxide (H2O2), a model for intestinal ischemia/reperfusion injury among other pathological conditions. These studies demonstrated that treatment of intestinal epithelial cells with H2O2 produced an activation of src family kinases (SFKs) and increased paracellular permeability through both the pore pathway and the leak pathway (4). Inhibition of SFK activity blocked the ability of H2O2 to increase paracellular permeability. Prior activation of the ERK 1/2 signaling pathway inhibited the ability of subsequent H2O2 treatment to increase intestinal cell paracellular permeability (5), leading to the hypothesis that tight junction protein tyrosine phosphorylation increased paracellular permeability, whereas serine-threonine phosphorylation decreased paracellular permeability. Studies implicated occludin protein in mediating these effects (5). Al-Sadi et al. (2) reported data implicating occludin protein content in regulation of basal paracellular permeability to large macromolecules across intestinal cell monolayers.
H2O2 treatment of intestinal epithelial cells, however, produced a modest activation of ERK 1/2 and its upstream activator, MEK 1/2 (5), suggesting a complexity to the role of ERK 1/2 in modulating paracellular permeability. Several studies have implicated ERK 1/2 activity in the regulation of paracellular permeability of renal epithelial cells, although the effect of ERK 1/2 activation on permeability is controversial (7, 11, 13, 22, 33). It has also been reported that ERK 1/2 activation is required for induction of renal damage following renal ischemia/reperfusion, although its role in specifically increasing tubular filtrate back leak is unclear (3, 36). We previously reported that treatment of renal epithelial cells with SFK inhibitors increased selectively the leak pathway component of paracellular permeability (6). This was not affected by occludin protein content. In contrast, occludin protein content was inversely correlated with the ability of H2O2 to increase paracellular movement of large molecules across renal epithelial cell monolayers (12). In addition to occludin, studies have implicated other tight junction proteins, e.g., ZO-1, in regulation of paracellular permeability (see, e.g., 30).
These results suggest that renal epithelial cells may exhibit kinase-mediated regulation of paracellular permeability distinct from that demonstrated in intestinal epithelial cells. In this study, we examine directly the role of ERK 1/2 activation in mediating the H2O2-induced increase in paracellular permeability across renal epithelial cell monolayers and the roles of specific tight junction proteins in mediating this effect.
MATERIALS AND METHODS
Reagents.
H2O2 (3% solution) was obtained from Acros Organics. Calcein was obtained from Invitrogen. U0126, blebbistatin, and PP1 were obtained from Enzo Life Sciences. Trypan Blue solution was obtained from HyClone. α-Modification Minimal Essential Medium was obtained from Corning-CellGro. Heat-inactivated fetal bovine serum was obtained from Atlanta Biologicals. Penicillin/streptomycin solution (100×) was obtained from MP Biomedicals. l-Glutamine solution (200 mM) was obtained from GIBCO Life Technologies. Trypsin/EDTA Solution (0.25%) was obtained from HyClone. Antibodies used in the studies presented here are as follows: rabbit anti-ERK 1/2 protein antibody (Invitrogen, catalog no. 44-654G), rabbit anti-ERK 1/2 [pT185pY187] antibody (Invitrogen, catalog no. 44-680G), rabbit anti-occludin (C-term) antibody (Life Technologies, catalog no. 404700), rabbit anti-ZO-1 antibody (Invitrogen, catalog no. 40-2200), rabbit anti-ZO-2 antibody (Life Technologies, catalog no. 711400), rabbit anti-TOCA-1 antibody (Bethyl Laboratories, catalog no. A303-469A), rabbit anti-actin antibody (Cell Signaling Technologies, catalog no. 4967S), mouse anti-β-actin antibody (R&D Systems, catalog no. MAB8929), mouse anti-β-tubulin antibody (R&D Systems, catalog no. MAB1195), mouse horseradish peroxidase (HRP)-conjugated anti-β-tubulin antibody (Invitrogen, catalog no. MA5–16308-HRP), HRP-conjugated goat anti-rabbit F(abʹ)2 fragment antibody (Jackson ImmunoResearch Laboratories, catalog no. 111-036-003), HRP-conjugated goat anti-rabbit F(abʹ)2 fragment antibody (Invitrogen, catalog no. 31461), HRP-conjugated goat anti-mouse F(abʹ)2 fragment antibody (Invitrogen, catalog no. A24524), and Alexa488-conjugated goat anti-rabbit Fc fragment antibody (Life Technologies, catalog no. A11034). Rhodamine phalloidin was obtained from Invitrogen.
Cell lines.
Wild-type Madin-Darby canine kidney (MDCK) type II cell line and TOCA-1 knockout MDCK type II cell line were a gift from Dr. C. M. Van Itallie (National Heart, Lung, and Blood Institute, Bethesda, MD). The occludin knockdown MDCK Type II cell line was a gift from Dr. E. E. Schneeberger (Massachusetts General Hospital and Harvard Medical School). The ZO-1 knockdown MDCK Type II cell line was a kind gift from Dr. Alan Fanning (University of North Carolina). All MDCK Type II cell lines were obtained from the same parental MDCK Type II cell line. Characterization of the occludin knockdown MDCK II cell line is described by Yu et al. (34). Characterization of the ZO-1 knockdown MDCK II cell line is described by Medina et al. (19) and Van Itallie et al. (30). Creation and characterization of the TOCA-1 knockout MDCK II cell line is described by Van Itallie et al. (32). Cell cultures were checked for mycoplasma contamination upon thaw of a new stock vial and periodically thereafter (MycoAlert Mycoplasma Detection Kit, Lonza). All tests were uniformly negative. New stock cell populations of each cell line were thawed after 15 passages. Cell line authentication was not performed.
Cell populations were grown as stock cultures maintained at a subconfluent density in tissue culture-treated flasks in Complete Medium (αMEM supplemented with 10% fetal bovine serum plus 2 mM l-glutamine plus penicillin-streptomycin) at 37°C in a humidified 5% CO2 atmosphere. Cells were passaged every 3–4 days by detaching cells with trypsin/EDTA solution and replating at a 1:10–1:20 dilution onto tissue culture-treated flasks. For flux experiments, detached cells were seeded onto permeable membrane filters (BD Biosciences; 25-mm diameter, 0.4-μm pore diameter) in six-well tissue culture plates containing 2 ml Complete Medium in both the upper and lower compartments. Medium was replenished every 2–3 days. Twelve to thirteen days after seeding, medium was replenished with serum-free αMEM supplemented with 2 mM l-glutamine plus penicillin/streptomycin. Cell populations were incubated overnight and then used for flux assays as described previously (6).
Paracellular permeability.
The paracellular permeability of MDCK cell populations to large solutes (leak pathway) was determined using calcein as the solute as described previously (6). Flux assay data are presented as means (SD) of triplicate independent samples. The presented results are representative of at least three independent experiments. Cell death following the flux assay (a total of 5–6 h incubation with reagents) was monitored by quantitation of Trypan Blue-stained nuclei as described previously (6, 12). Apparent permeability (Papp) was defined as (dQ/dt)/AC0 as described by Van Itallie et al. (29). Papp for each cell line under control conditions was calculated from the average flux rate for each flux curve. The mean (SD) of at least three independent experiments for each cell line was calculated.
Western blot analysis.
Cell lysates were prepared from cell populations maintained under the conditions used for measurement of paracellular permeability. Following a 2 h incubation without or with 55 μM H2O2, a time point at which permeability was increased, lysates of total cell protein and Triton X-100-soluble and Triton X-100-insoluble fractions were prepared as previously described (6). Western blotting was performed as previously described (6). Primary antibody dilutions for Western blotting were as follows: ERK 1/2 protein, 1:250–1:1,000; ERK 1/2 [pT185pY187], 1:500–1:1,000; occludin, 1:500–1:2,000; ZO-1, 1:500–1:2,000; ZO-2, 1:500–1:2,000; TOCA-1, 1:2,000–1:10,000; β-actin, 1:5,000; β-tubulin, 1:10,000. HRP-conjugated anti-rabbit Fc fragment antibodies were used at a dilution of 1:10,000–1:20,000. Presented blots are representative of at least four separate blots obtained from at least four independent sets of samples. Contents of tight junction proteins in each cell line were normalized to the content of either β-actin or β-tubulin in the same lysate sample. The normalized content of each tight junction protein in the knockdown cell lines was then expressed as a fraction of the normalized content of the same tight junction protein in lysates of wild-type MDCK cells. Relative tight junction protein content was measured in at least four independent lysate samples for each cell line. Data are expressed as means (SD) of at least four independent measurements.
Immunofluorescence microscopy.
Cell populations grown on permeable membrane filters were treated without or with 55 μM H2O2 for 2 h and then fixed with 4% paraformaldehyde. Samples were processed for immunofluorescence microscopy as described previously (6, 12). All primary antibodies were used at a 1:10–1:50 dilution. Rhodamine phalloidin was used at a 1:50–1:250 dilution. Alexa488-conjugated anti-rabbit Fc fragment antibody was used at a 1:100–1:500 dilution. Images were captured and processed as described previously (6). Actin filament quantitation was performed using Fiji software. Data are presented as means (SD) of number of actin fibers/cell. Data are representative of quantitation of at least 15 cells per condition from four independent experiments.
Statistics.
Data for flux assays were calculated as means (SD) of triplicate independent samples. Trypan Blue-staining nuclei were calculated as means (SD) of triplicate independent samples. Significance was determined by two-tailed t-test with a value of 0.05 as cutoff.
RESULTS
H2O2 treatment activates ERK 1/2 in MDCK cells.
We reported that treatment of multiple renal epithelial cell lines with micromolar concentrations of H2O2 increased paracellular permeability to large molecules (leak pathway; 18). It was previously reported that treatment of a renal epithelial cell line, MDCK, with millimolar H2O2 concentrations rapidly increased ERK 1/2 activation (11). We confirmed that treatment of MDCK cells with H2O2 using our experimental protocol (55 μM H2O2) increased ERK 1/2 activation by an average of 8.28 (SD 4.74)-fold (Fig. 1; P < 0.001, n = 5). The activation exhibited both time and concentration dependence. Signal strength peaked at 10–30 min after addition and declined to near basal levels by 2 h after H2O2 addition (data not shown).
Fig. 1.
Effect of treatment of postconfluent wild-type Madin-Darby canine kidney (MDCK) cell populations with 0 μM H2O2, 55 μM H2O2, and 110 μM H2O2 on ERK 1/2 phosphorylation/activation. Cell populations were treated with or without H2O2 for either 10 min or 30 min and then harvested for preparation of cell lysates. Solubilized proteins were separated by PAGE, transferred to nitrocellulose, and blotted for either ERK 1/2 protein (bottom) or ERK 1/2 [pT185pY187] (top). This is a representative experiment of 10 separate experiments using 4 independent lysate samples.
H2O2-induced increase in renal epithelial cell paracellular permeability requires ERK 1/2 activation.
Because H2O2 treatment increased both paracellular permeability and ERK 1/2 activation in MDCK cells, we asked if inhibition of ERK 1/2 activation could prevent the H2O2-induced increase in renal epithelial cell paracellular permeability. We previously reported that H2O2 treatment of renal epithelial cells using our experimental conditions did not affect pore pathway permeability (12). We therefore did not monitor transepithelial resistance in the experiments presented here. MDCK cell populations were pretreated without or with the ERK 1/2 activation inhibitor U0126, followed by pretreatment without or with H2O2 in the continued absence or presence of U0126. Leak pathway permeability was assessed by measuring the transepithelial movement of the fluorescent dye calcein. We have previously shown that transepithelial calcein movement is a valid measure of leak pathway permeability in renal epithelial cell lines (6).
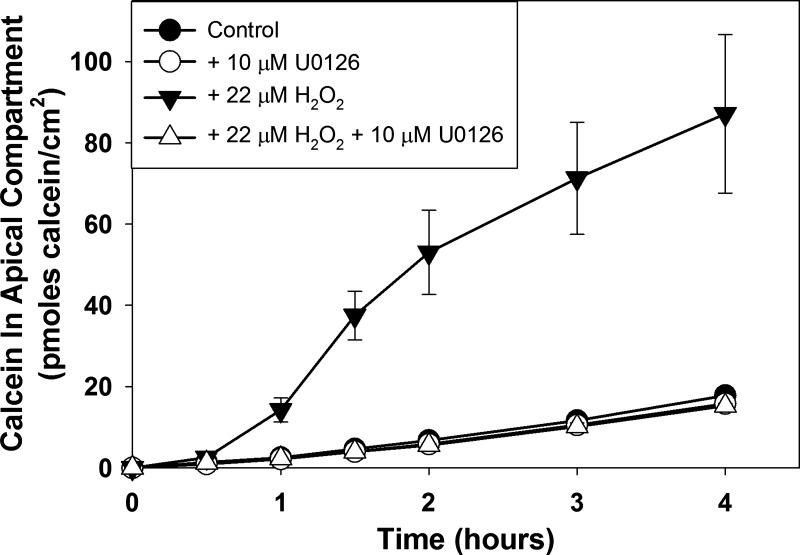
Treatment of MDCK cell populations with U0126 alone did not affect paracellular calcein flux rate (Fig. 2; P = 0.056 compared with control flux rate). As expected, treatment of renal epithelial cell populations with H2O2 increased paracellular calcein flux rate (P = 0.0016 compared with control flux rate). Pretreatment of cell populations with U0126 followed by treatment with U0126 plus H2O2, however, decreased the paracellular calcein flux rate to a level slightly below the control flux rate (P = 0.046 compared with control flux rate). Neither U0126 nor H2O2 was cytotoxic using these experimental conditions (Table 1). A similar, although weaker, effect was observed with another ERK 1/2 activation inhibitor, PD98059 (data not shown). Interestingly, we found that when 0.1% DMSO (final concentration of solvent used to make stock inhibitor solutions) is included in the experimental solutions, the H2O2 concentration required to increase paracellular calcein flux in MDCK cell populations (22 μM) is substantially lower than previously observed (44–55 μM) (see Fig. 3, Ref. 12).
Fig. 2.
Effect of treatment of postconfluent wild-type Madin-Darby canine kidney (MDCK) cell populations without or with 10 μM U0126 and/or 22 μM H2O2 on paracellular calcein flux. Cells were pretreated without or with U0126 for 1 h and then without or with U0126 and without or with H2O2 for 1 h before initiation of the calcein flux assay. Calcein flux was measured periodically over the next 4 h. Data are presented as means (SD) of triplicate independent samples. This is a representative experiment of at least 4 separate experiments.
Table 1.
Effect of treatment with H2O2 and U0126 on cell death of postconfluent wild-type MDCK cell populations
| Condition | Mean ± SD | P Value (vs. Control) |
|---|---|---|
| Control | 0.083 (SD 0.289) (n = 12) | |
| +22 μM H2O2 | 0.333 (SD 0.888) (n = 12) | 0.364 |
| +10 μM U0126 | 0.000 (SD 0.000) (n = 12) | 0.328 |
| +22 μM H2O2 +10 μM U0126 | 0.083 (SD 0.289) (n = 12) | 1.000 |
Data are means (SD); number of samples (n) is shown in parentheses. Postconfluent wild-type Madin-Darby canine kidney (MDCK) cells were treated without or with 22 μM H2O2 and 10 μM U0126 for 6 h total. Cell death was quantitated as number of Trypan Blue-stained nuclei per high power (×40 objective) microscopic field.
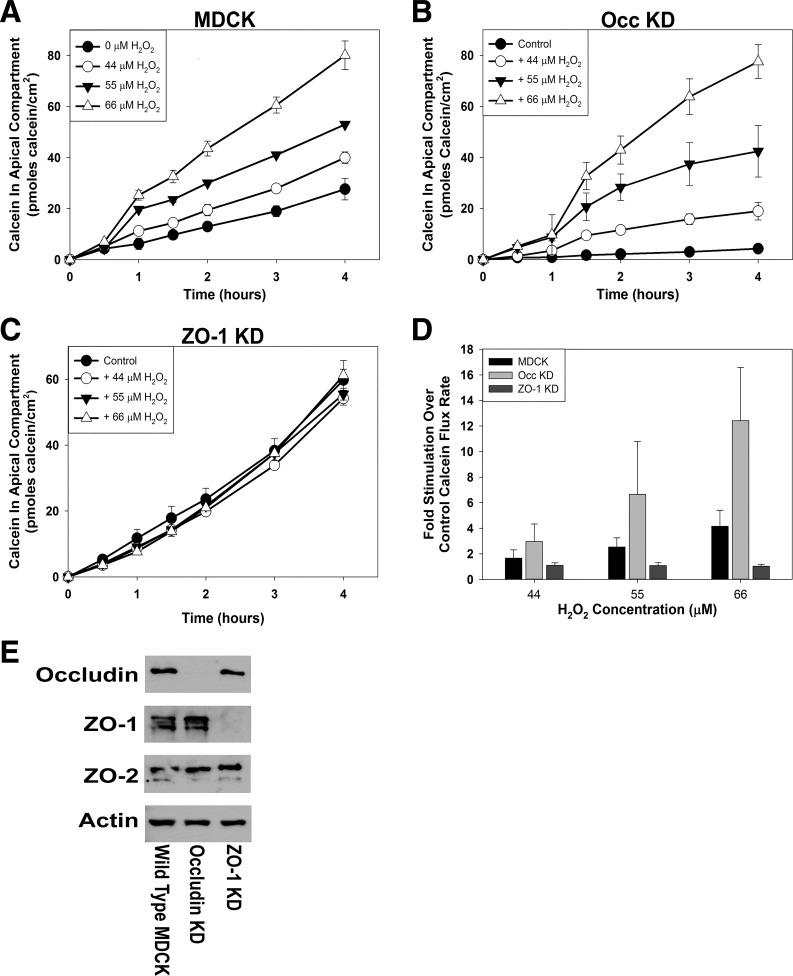
Fig. 3.
Effect of treatment of postconfluent populations of wild-type Madin-Darby canine kidney (MDCK) cells (A), occludin knockdown (Occ KD) MDCK cells (B), or zonula occludens-1 knockdown (ZO-1 KD) MDCK cells (C) with various H2O2 concentrations on paracellular calcein flux. Average flux rates for each condition were calculated and normalized to the control flux rate for each cell line (D). Cells were pretreated with 0 μM, 44 μM, 55 μM, or 66 μM H2O2 for 1 h before initiation of the calcein flux assay. Calcein flux was measured periodically over the next 4 h. Data are presented as means (SD) of triplicate independent samples. These are representative experiments of at least 3 separate experiments for each cell line. Content of tight junction proteins, occludin, ZO-1, and ZO-2 was determined by Western blotting (E) and was normalized to the content of β-actin in the same sample. Images shown are representative of the results obtained from at least 3 separate lysates.
ZO-1 protein but not occludin protein is required for H2O2-induced increase in renal epithelial cell paracellular permeability.
To investigate the roles of specific tight junction proteins in mediating the H2O2 action, the H2O2 concentration dependence for increasing paracellular calcein flux rate was compared in wild-type MDCK cell populations and in MDCK cell populations in which the content of individual tight junction proteins was knocked down [occludin knockdown (Occ KD; 31, 34); ZO-1 knockdown (ZO-1 KD; 30)]. Confirming previous reports (6, 30, 31), knockdown of the targeted protein was demonstrated for each cell line (Fig. 3E). In ZO-1 KD cells, normalized ZO-1 protein expression was 9% ± 11% of the normalized ZO-1 protein content in wild-type MDCK cells (P < 0.0001, n = 8). In Occ KD cells, normalized occludin protein content was 2% ± 1% of the normalized occludin protein content in wild-type MDCK cells (P < 0.0001, n = 5). In the ZO-1 KD and Occ KD cell lines, the normalized contents of the other examined tight junction proteins, occludin, ZO-1, and ZO-2, were similar to the contents in wild-type MDCK cells (P > 0.05).
As reported previously, wild-type MDCK cell populations exhibited an H2O2 concentration-dependent increase in paracellular calcein flux rate (Fig. 3A; P < 0.01 for each H2O2 concentration compared with control). Occ KD cell populations also exhibited an H2O2 concentration-dependent increase in paracellular calcein flux rate (Fig. 3B; P < 0.005 for each H2O2 concentration compared with control). In contrast, ZO-1 KD cell populations did not exhibit an increase in paracellular calcein flux rate with increasing H2O2 concentration (Fig. 3C; P > 0.10 for all H2O2 concentrations compared with control). As reported previously (12), higher H2O2 concentrations were cytotoxic to these renal epithelial cells under our experimental conditions. We did not, therefore, pursue the effects of higher H2O2 concentrations in these experiments.
Plotting average paracellular calcein flux rates as a function of the control flux rate (0 μM H2O2) for each cell line provides a direct comparison of the responsiveness of each cell line to H2O2 (Fig. 3D). This presentation indicates that Occ KD cell populations exhibit an enhanced response to H2O2 compared with wild-type MDCK cell populations (P < 0.01 at all H2O2 concentrations compared with wild-type MDCK fold increase), as reported previously (12). The response of ZO-1 KD cell populations is minimal throughout this H2O2 concentration range. The fold change was not significantly different from wild-type MDCK cells at 44 μM H2O2 (P = 0.073) but was significantly less than was observed for wild-type MDCK cells at 55 μM and 66 μM H2O2 (P < 0.01). Calculation of Papp for each cell line under control conditions confirms the results of Van Itallie et al. (30) that the ZO-1 KD MDCK cell line exhibits a higher paracellular flux rate for large solutes compared with either wild type or Occ KD MDCK cell lines (Table 2).
Table 2.
Calculated Papp for wild-type and knockdown/knockout MDCK cell populations
| Cell Line | Papp, (cm/s) × 106 | P Value vs. MDCK |
|---|---|---|
| MDCK | 0.029847 (SD 0.008143) (n = 17) | |
| Occludin knockdown | 0.028890 (SD 0.007807) (n = 3) | 0.858954 |
| ZO-1 knockdown | 0.091359 (SD 0.025161) (n = 8) | 0.000173 |
| TOCA-1 knockout | 0.057063 (SD 0.015859) (n = 3) | 0.009557 |
Data are means (SD) of multiple independent experiments (numbers in parentheses). Calcein flux rates for individual experiments were averaged for each cell line. Apparent permeability (Papp) was calculated according to Van Itallie et al. (29). Significance (P values) was determined using two-tailed Student’s t-test compared with the flux rate for wild-type Madin-Darby canine kidney (MDCK) cell populations. ZO-1, zonula occludens-1.
H2O2 treatment does not affect tight junction protein content or subcellular localization.
Previous studies have reported a correlation between increased paracellular permeability and changes to the content and/or distribution of one or more tight junction proteins (see, e.g., 2, 5, 30, 31). We therefore asked if H2O2 treatment of wild-type MDCK cells produced changes in the total content of occludin, ZO-1, or ZO-2 proteins or their distributions between the Triton X-100-soluble and -insoluble cellular pools. Postconfluent cell populations were treated without or with 55 μM H2O2 for 2 h. Cells were lysed and either total cellular protein or Triton X-100-soluble and -insoluble cell fractions were collected. The contents of occludin protein, ZO-1 protein, and ZO-2 protein were determined by Western blot analysis. Using our treatment conditions, H2O2 treatment did not produce a change in either the total content of any of these tight junction proteins or their relative distribution between the two cell fractions (data not shown). Likewise, no major changes in the subcellular localization of occludin, ZO-1, or ZO-2 proteins were observed following H2O2 treatment (data not shown). A decrease in the density of basal F-actin stress fibers was routinely observed following H2O2 treatment (0 μM H2O2, 21.5 (SD 6.0) actin fibers/cell; 55 μM H2O2, 16.9 (SD 5.8) actin fibers/cell; P = 0.023, n = 17).
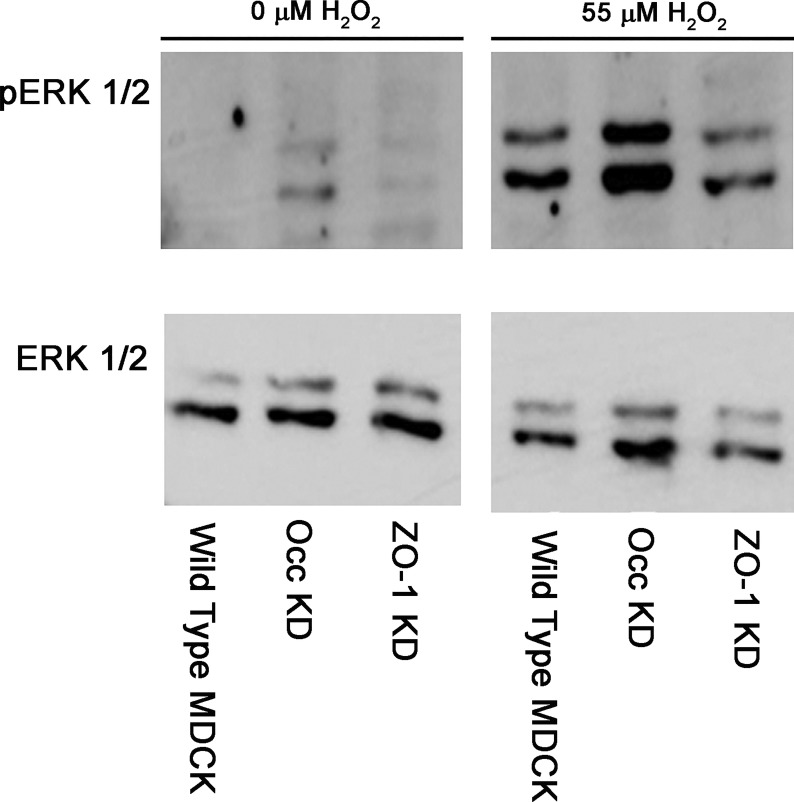
H2O2 treatment activates ERK 1/2 in knockdown MDCK cell lines.
Our results indicate that ERK 1/2 activation is required for the H2O2-induced increase in wild-type MDCK cells. Occludin KD MDCK cells, but not ZO-1 KD MDCK cells, also increased leak pathway permeability in response to H2O2 treatment. One possible explanation for this difference is that H2O2 treatment produces activation of ERK 1/2 in wild-type MDCK cells and occludin KD MDCK cells but not in ZO-1 KD MDCK cells. We therefore examined the ability of H2O2 treatment to activate ERK 1/2 in the knockdown MDCK cell lines. Similar to wild-type MDCK cells (8.36 (SD 4.57)-fold stimulation, n = 9), treatment with H2O2 at concentrations that increased paracellular calcein flux also increased activation of ERK 1/2 in both ZO-1 KD (17.05 (SD 10.88)-fold stimulation, n = 5; P = 0.1515 compared with wild-type MDCK) and occludin KD (16.51 (SD 15.58)-fold stimulation, n = 5; P = 0.3753 compared with wild-type MDCK) MDCK cell lines (Fig. 4). Absolute signal strength for phosphorylated ERK 1/2 appeared to be lower in the ZO-1 KD cell populations than in the wild-type MDCK and Occ KD cell populations under both control and H2O2-treated conditions, but the fold stimulation produced by H2O2 was similar.
Fig. 4.
Effect of treatment of postconfluent wild type Madin-Darby canine kidney (Wild Type MDCK) cell populations, occludin knockdown MDCK (Occ KD) cell populations, and zonula occludens-1 knockdown MDCK (ZO-1 KD) cell populations with 0 μM H2O2 or 55 μM H2O2 on ERK 1/2 phosphorylation/activation. Cell populations were treated with or without H2O2 for 10 min and then harvested for preparation of cell lysates. Solubilized proteins were separated by PAGE, transferred to nitrocellulose, and blotted for either ERK 1/2 protein (ERK, bottom) or ERK 1/2 [pT185pY187] (pERK, top). This is a representative experiment of at least three separate experiments using at least 2 independent lysate samples.
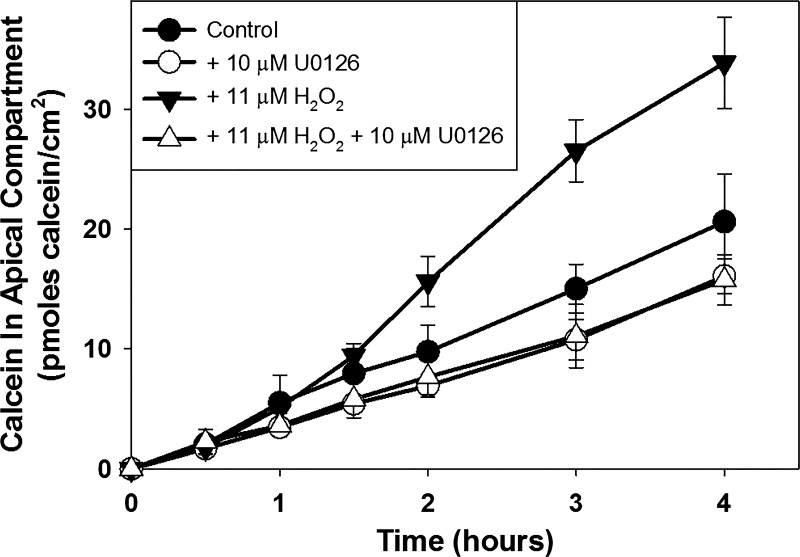
Inhibition of ERK 1/2 activation blocks H2O2-induced increase in paracellular permeability in occludin KD cell line.
Because H2O2 treatment activated ERK 1/2 in the knockdown cell lines, we asked if inhibiting ERK 1/2 activation would block the ability of H2O2 to increase paracellular calcein flux in the occludin knockdown MDCK cell populations, as it does in wild-type MDCK cell populations. In the experiment shown, pretreatment of Occ KD cell populations with U0126 produced a small but statistically significant decrease in paracellular calcein flux rate (Fig. 5; P < 0.001 compared with control flux rate), but this was not a consistent observation. H2O2 treatment increased paracellular calcein flux rate in Occ KD cell populations (P = 0.006 compared with control flux rate). Pretreatment with U0126 blocked the H2O2-induced increase in paracellular calcein flux rate (P = 0.203 compared with U0126 flux rate). Because of its increased sensitivity to H2O2 (see above), a lower H2O2 concentration was used to treat the Occ KD cells (Occ KD, 11 μM; MDCK, 22 μM). Treatment of ZO-1 KD cells with U0126 or H2O2, either alone or in combination, did not produce a statistically significant change in paracellular calcein flux (data not shown; P > 0.05 compared with control flux rate for each condition).
Fig. 5.
Effect of treatment of postconfluent occludin knockdown Madin-Darby canine kidney (MDCK) cell populations without or with 10 μM U0126 and/or 11 μM H2O2 on paracellular calcein flux. Cells were pretreated without or with U0126 for 1 h and then without or with U0126 and without or with H2O2 for 1 h before initiation of the calcein flux assay. Calcein flux was measured periodically over the next 4 h. Data are presented as means (SD) of triplicate independent samples. This is a representative experiment of at least 3 separate experiments.
SFK inhibition increases paracellular calcein flux in ZO-1 KD MDCK cell line.
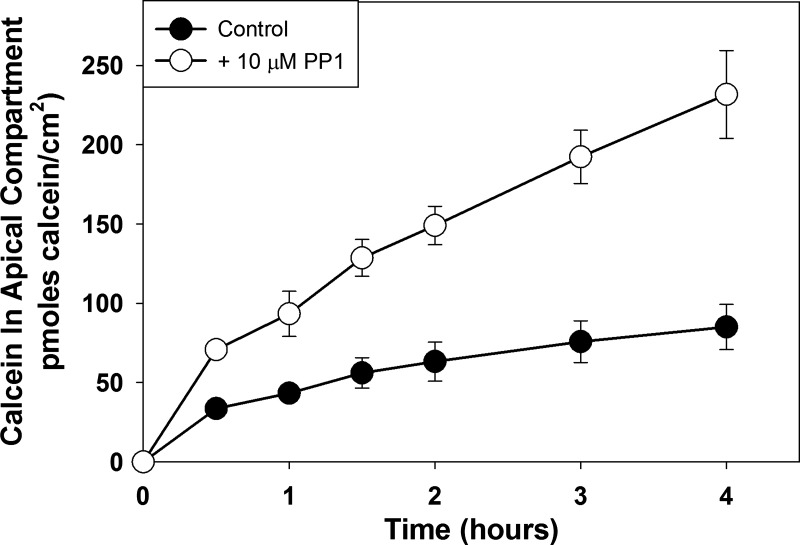
The results shown above demonstrate that ZO-1 KD MDCK cells do not increase their paracellular calcein permeability in response to H2O2 treatment. One explanation for this result is that knockdown of ZO-1 protein renders the MDCK cells unable to increase paracellular permeability in response to any stimulus. To determine if ZO-1 KD cell populations could increase paracellular calcein permeability in response to a different stimulus, we examined the ability of PP1, a src family kinase inhibitor, to increase paracellular calcein permeability. We have previously shown that PP1 increases paracellular calcein permeability in wild-type MDCK cells and in MDCK cells in which occludin protein was either knocked down or overexpressed (6). As shown in Fig. 6, treatment with PP1 also increased paracellular calcein flux rate in ZO-1 KD MDCK cells (P = 0.0023 compared with control flux rate), indicating that ZO-1 KD cells are capable of increasing their paracellular calcein permeability in response to some stimuli.
Fig. 6.
Effect of treatment of postconfluent populations of zonula occludens-1 knockdown Madin-Darby canine kidney cells with 10 μM PP1 on paracellular calcein flux. Cells were pretreated with 10 μM PP1 1 h before initiation of the calcein flux assay. Calcein flux was measured periodically over the next 4 h. Data are presented as means (SD) of triplicate independent samples. These are representative experiments of at least 3 separate experiments.
TOCA-1 knockout MDCK cells do not increase paracellular calcein flux rate in response to H2O2.
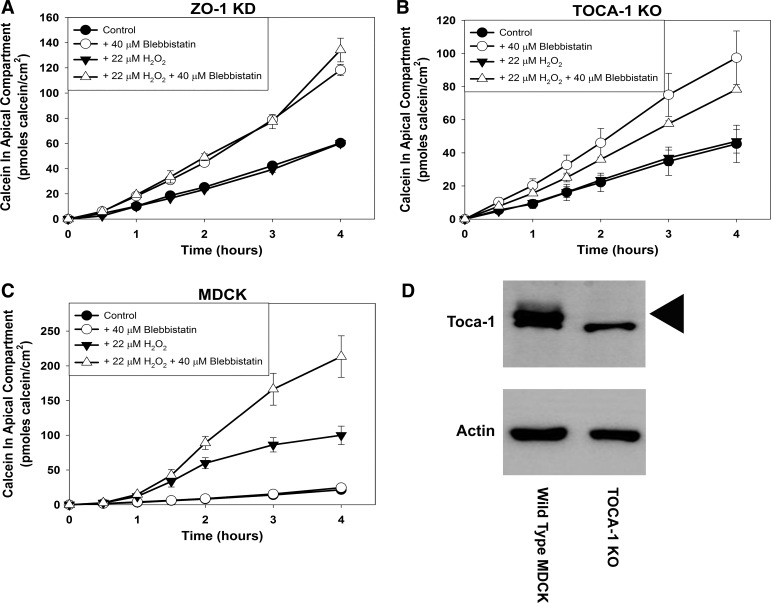
It was recently reported that ZO-1 protein bound to and targeted TOCA-1 protein, an F-BAR protein which promotes formation of localized branched actin networks (16), to the tight junction region of MDCK cells (32). MDCK cells in which the TOCA-1 gene was knocked out (TOCA-1 KO) exhibited increased paracellular flux to large molecules and delayed tight junction formation (32), similar to what was observed in ZO-1 KD MDCK cells (30). We asked if, as observed in ZO-1 KD MDCK cells, TOCA-1 KO MDCK cell populations would fail to increase paracellular calcein flux rate in response to H2O2 treatment. Knockout of TOCA-1 protein was confirmed by Western blot analysis (arrowhead, Fig. 7D). The TOCA-1 protein antibody used in these blots cross-reacted with a protein of a slightly lower molecular weight in both wild-type MDCK and TOCA-1 KO cell lysates. Consistent with previous reports (32), TOCA-1 KO MDCK cells exhibited an approximately twofold greater basal calcein flux rate compared with wild-type MDCK cells (Table 2). As was observed with ZO-1 KD cell populations, H2O2 treatment did not increase paracellular calcein flux rate in TOCA-1 KO cell populations (Fig. 7B; P = 0.414).
Fig. 7.
Effect of treatment of postconfluent zonula occludens-1 knockdown (ZO-1 KD) Madin-Darby canine kidney (MDCK) cell populations (A), TOCA-1 knockout (KO) MDCK cell populations (B), and wild-type MDCK cell populations (C) without or with 40 μM blebbistatin and/or 22 μM H2O2 on paracellular calcein flux. Western blot analysis of TOCA-1 protein content in wild-type MDCK cell lysates and in TOCA-1 knockout MDCK cell lysates (D). Cells were pretreated without or with blebbistatin for 1 h and then without or with blebbistatin and without or with H2O2 for 1 h before initiation of the calcein flux assay. Calcein flux was measured periodically over the next 4 h. Data are presented as means (SD) of triplicate independent samples. These are representative experiments of at least 3 separate experiments for each cell line.
TOCA-1 knockout MDCK cells increase paracellular calcein flux rate in response to blebbistatin.
Blebbistatin, a myosin ATPase inhibitor, increased paracellular permeability to large solutes in ZO-1 KD cell populations but not in wild-type MDCK cell populations (30). To further investigate the similarity between ZO-1 KD and TOCA-1 KO MDCK cells, we asked whether blebbistatin would also increase paracellular leak pathway permeability in TOCA-1 KO MDCK cell populations. We first confirmed that treatment with blebbistatin alone increased paracellular calcein flux rate in ZO-1 KD MDCK cell populations (Fig. 7A; P < 0.001). As expected, treatment with H2O2 alone did not increase paracellular calcein flux rate in these cells (P = 0.373 compared with control flux rate). The combination of H2O2 and blebbistatin did not increase paracellular calcein flux rate beyond that obtained with blebbistatin alone (P = 0.665 compared with blebbistatin alone).
Treatment of TOCA-1 KO MDCK cell populations with blebbistatin alone increased paracellular calcein flux rate approximately twofold (Fig. 7B; P = 0.017 compared with control flux rate). The paracellular calcein flux rate of TOCA-1 KO cell populations treated with both H2O2 and blebbistatin was not significantly different from the calcein flux rate of TOCA-1 KO cell populations treated with blebbistatin alone (P = 0.176).
H2O2 treatment sensitizes wild-type MDCK cells to treatment with blebbistatin.
One explanation for the lack of effect of H2O2 on ZO-1 KD and TOCA-1 KO MDCK cell populations is that H2O2 treatment produces a functionally similar change to MDCK cell physiology as ZO-1 KD or TOCA-1 KO, at least as it relates to regulation of leak pathway permeability. As shown above and previously (30), both ZO-1 KD cell populations and TOCA-1 KO cell populations increased paracellular calcein flux rate in response to blebbistatin treatment. If H2O2 treatment produces a change in cell physiology similar to that of ZO-1 KD or TOCA-1 KO, we would predict that blebbistatin will only increase paracellular calcein flux in wild-type MDCK cell populations when they are also treated with H2O2. As previously reported (30), treatment of wild-type MDCK cell populations with blebbistatin alone did not increase paracellular calcein flux rate (Fig. 7C; P = 0.42). As expected, treatment of MDCK cells with H2O2 significantly increased paracellular calcein flux rate (P < 0.001). Consistent with our hypothesis that H2O2 treatment and ZO-1 KD or TOCA-1 KO produce similar functional changes in MDCK cells, treatment of wild-type MDCK cells with both H2O2 and blebbistatin further augmented the increased paracellular calcein flux rate compared with treatment with H2O2 alone (P = 0.012).
DISCUSSION
We and others have previously reported that H2O2 treatment of renal epithelial cells increases paracellular permeability (8, 11, 12, 20) at concentrations in the range of those observed during renal ischemia/reperfusion injury (see, e.g., 14). In this study, we demonstrate that H2O2 treatment of renal epithelial cells activates ERK 1/2 and that ERK 1/2 activation is required for H2O2 to increase renal epithelial paracellular permeability. In addition, we show that the H2O2-induced increase in paracellular calcein flux across MDCK cell monolayers requires normal levels of ZO-1 protein and TOCA-1 protein expression. Finally, we present data suggesting that H2O2 induces a change in MDCK cell physiology that is, at least with respect to regulation of paracellular permeability, functionally similar to that produced by ZO-1 protein knockdown and TOCA-1 protein knockout.
Our results indicating that H2O2 treatment activates ERK 1/2 are in agreement with previous studies in multiple cell types, including multiple epithelial cell types (5, 9, 11, 15; this study), endothelial cells (21), and neuroblastoma cells (25). These results have led to the hypothesis that mitogen-activated protein kinase activation is a common feature of the H2O2 effect on mammalian cells (27).
The role of ERK 1/2 activity in regulation of epithelial paracellular permeability is more controversial. In Calu-3 airway epithelial cells, cytokine-induced activation of ERK 1/2 produced an increase in transepithelial conductance (pore pathway; 23). In contrast, ERK 1/2 activation in Caco-2 intestinal epithelial cells protected these cells against the H2O2-induced increase in permeability via both the pore pathway and the leak pathway (5). This group later showed that ERK 1/2 activation had different and even opposite effects on Caco-2 cell paracellular permeability in “underdifferentiated” versus “well-differentiated” Caco-2 intestinal cell monolayers (1), suggesting a complexity to the role of ERK 1/2 activity in regulating intestinal, and perhaps other, epithelial cell paracellular permeability.
Previous studies have yielded complex results regarding the role of ERK 1/2 in regulating renal epithelial paracellular permeability. Cyclosporine A treatment of MDCK cell monolayers and LLC-PK1 cell monolayers activated ERK 1/2, leading to a decrease in transepithelial resistance, i.e., an increase in pore pathway permeability (13, 17). Cytokine treatment of MDCK cells decreased pore pathway permeability but increased leak pathway permeability (22). Both effects were blocked by inhibition of ERK 1/2 activation. Inhibition of ERK 1/2 activation reversed the effects of ras transformation in MDCK cells leading to formation of tight junctions and a paracellular permeability barrier (7). Membrane depolarization both induced ERK 1/2 activation and increased leak pathway permeability in MDCK cell and LLC-PK1 cell monolayers (33). Although the effect of inhibition of ERK 1/2 activation on the paracellular permeability change was not examined directly in this study, inhibition of ERK 1/2 activation blocked several of the intervening steps leading to increased leak pathway permeability. Gonzalez et al. (11) reported that H2O2 treatment of MDCK cells increased both ERK 1/2 activation and pore pathway permeability within 2–6 h. Blocking ERK 1/2 activation, however, did not inhibit the increase in pore pathway permeability in this study. The Gonzalez et al. study (11) used millimolar H2O2 concentrations which, using our experimental conditions, were highly cytotoxic to renal epithelial cells (12; data not shown). Our results indicate that activation of ERK 1/2 is required for H2O2 to increase wild-type MDCK cell leak pathway permeability. The basis for these varying results on the role of ERK 1/2 activation on regulation of paracellular permeability in renal epithelial cells and other cell types is currently unclear. It may reflect differences in experimental design and/or cell behavior. The findings of Aggarwal et al. (1) demonstrating changes in the role of ERK 1/2 in regulation of paracellular permeability in a single cell type, Caco-2, depending on growth conditions might be directly relevant here.
The involvement of different tight junction proteins in regulating tight junction permeability under basal conditions and in response to various stimuli appears complex. We reported previously (12) and confirmed here that knockdown of occludin protein in MDCK cells enhanced the ability of H2O2 to increase leak pathway permeability. Occludin overexpression diminished the ability of H2O2 to increase leak pathway permeability (12). In contrast, occludin knockdown diminished and occludin overexpression enhanced the changes in MDCK cell paracellular permeability produced by cytokines (31).
McNeil et al. (18) reported that knockdown of ZO-1 protein expression in MDCK cells produced a delay in tight junction formation following a Ca-switch approach. It also eliminated the transient overshoot in development of transepithelial resistance, but the tight junctions that eventually formed appeared normal and exhibited a typical transepithelial resistance. In a separate study, knockdown of ZO-1 protein expression in MDCK cells selectively increased the permeability to large solutes (30). TALEN-mediated knockout of the ZO-1 gene in MDCK cells also produced an increase in leak pathway permeability (28). Our results indicate that, under basal conditions, the ZO-1 knockdown MDCK cells exhibited an increased calcein flux rate compared with wild-type MDCK cells or occludin knockdown MDCK cells, consistent with previous reports (30). Interestingly, knockout of the TOCA-1 protein produced a similar increase in basal calcein flux rate in MDCK cells.
A major finding of our study is that knockdown of ZO-1 protein and knockout of TOCA-1 protein, but not knockdown of occludin protein, diminishes or eliminates the ability of H2O2 to increase leak pathway permeability in MDCK cells. ZO-1 protein was also required for the changes in MDCK cell paracellular permeability produced by inhibition of Rho-associated protein kinase and inhibition of myosin ATPase activity (30). Raleigh et al. (24) reported that ZO-1 protein, but also occludin protein and claudin-1 or claudin-2 protein, were required for a CK2 inhibitor to increase transepithelial resistance of Caco-2 cells. As reported here, H2O2 also produced a weaker ERK 1/2 phosphorylation in ZO-1 KD cell populations than in either wild-type MDCK cell populations or Occ KD cell populations. Although this could provide a mechanism to explain the lack of effect of H2O2 on ZO-1 KD cell leak pathway permeability, two pieces of evidence argue against this. First, although the absolute magnitudes of induced ERK 1/2 phosphorylation appeared to be weaker in ZO-1 KD cell populations, the fold stimulation of ERK 1/2 phosphorylation was similar in all three cell lines. Second, we have observed a similar weak induction of ERK 1/2 phosphorylation by H2O2 in ZO-2 knockdown MDCK cell populations, but these cell populations still responded to H2O2 by increasing leak pathway permeability (data not shown).
In our study, we have confirmed the report of Van Itallie et al. (30) that the myosin ATPase inhibitor blebbistatin produces a substantially greater increase in leak pathway permeability in ZO-1 knockdown MDCK cell populations than in wild-type MDCK cell populations. We show here that TOCA-1 knockout MDCK cells also increase calcein flux rate in response to blebbistatin treatment alone, suggesting that ZO-1 knockdown and TOCA-1 knockout produce a functionally similar physiological state in MDCK cells, at least with regard to regulation of leak pathway permeability. H2O2 treatment of wild-type MDCK cell populations also sensitizes the cell populations to blebbistatin, resulting in a blebbistatin-induced increase in paracellular calcein flux. Thus, ZO-1 KD, TOCA-1 KO, and H2O2 treatment sensitize MDCK cell populations to modulation of paracellular permeability by a myosin ATPase inhibitor. It is interesting to note that depletion of ZO-1 protein and knockout of TOCA-1 protein produced enhanced MDCK cell junctional/lateral F-actin staining (30). We demonstrate here that H2O2 treatment decreased the density of basal F-actin stress fibers. These results suggest ZO-1 knockdown, TOCA-1 knockout, and H2O2 treatment may act through a common mechanism of affecting F-actin organization of MDCK cells.
The similarity in the effects of ZO-1 protein knockdown and TOCA-1 protein knockout on regulation of leak pathway permeability in MDCK cells suggests that these proteins are components of a single regulatory mechanism. ZO-1 protein binds to and targets TOCA-1 protein to the tight junction structure (32). TOCA-1 protein and ZO-1 protein bind multiple proteins involved in regulation of actin dynamics (see, e.g., 10, 16). TOCA-1 protein facilitates formation of a localized branching actin network. ZO-1 protein binds to actin filaments. Based on our results, we speculate that two components of the actin cytoskeleton, branching actin networks and actomyosin cables, regulate leak pathway permeability in distinct ways. Under control conditions, the local branching actin network plays a primary role in regulation of leak pathway permeability through its association with the tight junction. Actomyosin cables and the tension they would impose on the tight junction structure have, at most, a minor role. Disruption of the tight junction association with the branching actin network, for example, through ZO-1 knockdown or TOCA-1 knockout, increases leak pathway permeability and exposes a secondary regulation by actomyosin contractility, as evidenced by the responsiveness to blebbistatin. Because H2O2 treatment induced in wild-type MDCK cells a similar responsiveness to blebbistatin, it is tempting to speculate that H2O2 treatment also disrupts tight junction association with the local branching actin network, thereby exposing this secondary regulation by actomyosin cables. Experiments are underway to investigate these proposed regulatory mechanisms.
One possible mechanism by which ERK 1/2 could mediate such an action of H2O2 is through phosphorylation of a critical protein involved in association of the branching actin network with the tight junction structure. Mammalian ZO-1 proteins contain a conserved (among human, dog, rat, and mouse) potential ERK 1/2 phosphorylation site (-pXsp-) in the U3 domain between the PDZ2 and PDZ3 domains (data not shown). We have been unable to date, however, to demonstrate increased serine/threonine phosphorylation of ZO-1 protein in response to H2O2 treatment of MDCK cells (data not shown). It is possible that our assay is not sufficiently sensitive to detect a modest increase in ZO-1 protein phosphorylation, particularly if it involves only a subset of total cellular ZO-1 protein. Alternatively, ERK 1/2 may phosphorylate and modulate the function of another relevant protein. One potential candidate is TOCA-1. It is currently unknown if ERK 1/2 can phosphorylate TOCA-1 protein. We are pursuing these possibilities.
Conceptually, there are at least two ways to explain the effects of ZO-1 KD, TOCA-1 knockout, and H2O2 treatment. The first potential mechanism proposes that ZO-1 protein and TOCA-1 protein act as positive regulators of leak pathway permeability. H2O2 and ERK 1/2 activity produce an “activated state” which actively increases MDCK cell leak pathway permeability. The second potential mechanism proposes that ZO-1 protein and TOCA-1 protein are negative regulators of paracellular permeability. ZO-1 protein and TOCA-1 protein act as brakes on paracellular permeability, maintaining it at a basal level. ZO-1 knockdown, TOCA-1 knockout, or H2O2 “release” this brake, leading to increased leak pathway permeability. In either mechanism, knockdown of ZO-1 protein or knockout of TOCA-1 protein would eliminate the ability of H2O2 to increase paracellular permeability. At present we are unable to distinguish between these two models.
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant R15-DK-091749-01A1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.A. conceived and designed research; S.B., S.J., D.J., N.S., S.T., A.V., J.W., J.A., and K.A. performed experiments; S.B., S.J., D.J., N.S., S.T., A.V., J.W., J.A., and K.A. analyzed data; S.B., S.J., D.J., N.S., S.T., A.V., J.W., J.A., and K.A. interpreted results of experiments; K.A. prepared figures; K.A. drafted manuscript; S.B., S.J., D.J., N.S., S.T., A.V., J.W., J.A., and K.A. edited and revised manuscript; S.B., S.J., D.J., N.S., S.T., A.V., J.W., J.A., and K.A. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. A. Fanning (University of North Carolina) for the kind gift of the ZO-1 knockdown MDCK cell line. The authors thank Dr. E.E. Schneeberger (Massachusetts General Hospital and Harvard Medical School) and Dr. C.M. Van Itallie (National Heart, Lung, and Blood Institute) for the kind gift of the occludin knockdown MDCK cell line. The authors thank Dr. C.M. Van Itallie for the kind gift of the TOCA-1 knockout MDCK cell line.
REFERENCES
- 1.Aggarwal S, Suzuki T, Taylor WL, Bhargava A, Rao RK. Contrasting effects of ERK on tight junction integrity in differentiated and under-differentiated Caco-2 cell monolayers. Biochem J 433: 51–63, 2011. doi: 10.1042/BJ20100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Sadi R, Khatib K, Guo S, Ye D, Youssef M, Ma T. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol 300: G1054–G1064, 2011. doi: 10.1152/ajpgi.00055.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alderliesten M, de Graauw M, Oldenampsen J, Qin Y, Pont C, van Buren L, van de Water B. Extracellular signal-regulated kinase activation during renal ischemia/reperfusion mediates focal adhesion dissolution and renal injury. Am J Pathol 171: 452–462, 2007. doi: 10.2353/ajpath.2007.060805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basuroy S, Sheth P, Kuppuswamy D, Balasubramanian S, Ray RM, Rao RK. Expression of kinase-inactive c-Src delays oxidative stress-induced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J Biol Chem 278: 11916–11924, 2003. doi: 10.1074/jbc.M211710200. [DOI] [PubMed] [Google Scholar]
- 5.Basuroy S, Seth A, Elias B, Naren AP, Rao R. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem J 393: 69–77, 2006. doi: 10.1042/BJ20050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caswell D, Jaggi S, Axis J, Amsler K. src family kinases regulate renal epithelial paracellular permeability barrier through an occludin-independent mechanism. J Cell Physiol 228: 1210–1220, 2013. doi: 10.1002/jcp.24274. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Lu Q, Schneeberger EE, Goodenough DA. Restoration of tight junction structure and barrier function by down-regulation of the mitogen-activated protein kinase pathway in ras-transformed Madin-Darby canine kidney cells. Mol Biol Cell 11: 849–862, 2000. doi: 10.1091/mbc.11.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collares-Buzato CB, Jepson MA, Simmons NL, Hirst BH. Increased tyrosine phosphorylation causes redistribution of adherens junction and tight junction proteins and perturbs paracellular barrier function in MDCK epithelia. Eur J Cell Biol 76: 85–92, 1998. doi: 10.1016/S0171-9335(98)80020-4. [DOI] [PubMed] [Google Scholar]
- 9.Dabrowski A, Boguslowicz C, Dabrowska M, Tribillo I, Gabryelewicz A. Reactive oxygen species activate mitogen-activated protein kinases in pancreatic acinar cells. Pancreas 21: 376–384, 2000. doi: 10.1097/00006676-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Fanning AS, Anderson JM. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann NY Acad Sci 1165: 113–120, 2009. doi: 10.1111/j.1749-6632.2009.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez JE, DiGeronimo RJ, Arthur DE, King JM. Remodeling of the tight junction during recovery from exposure to hydrogen peroxide in kidney epithelial cells. Free Radic Biol Med 47: 1561–1569, 2009. doi: 10.1016/j.freeradbiomed.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janosevic D, Axis J, Bacallao RL, Amsler K. Occludin modulates hydrogen peroxide-induced increase in renal epithelial paracellular permeability. J Cell Biochem 117: 769–779, 2016. doi: 10.1002/jcb.25362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiely B, Feldman G, Ryan MP. Modulation of renal epithelial barrier function by mitogen-activated protein kinases (MAPKs): mechanism of cyclosporine A-induced increase in transepithelial resistance. Kidney Int 63: 908–916, 2003. doi: 10.1046/j.1523-1755.2003.00804.x. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Seok YM, Jung K-J, Park KM. Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am J Physiol Renal Physiol 297: F461–F470, 2009. doi: 10.1152/ajprenal.90735.2008. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Ma Z, Ma J, Li X, Xu Q, Duan W, Chen X, Lv Y, Zhou S, Wu E, Ma Q, Huo X. Hydrogen peroxide mediates hyperglycemia-induced invasive activity via ERK and p38 MAPK in human pancreatic cancer. Oncotarget 6: 31119–31133, 2015. doi: 10.18632/oncotarget.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S, Xiong X, Zhao X, Yang X, Wang H. F-BAR family proteins, emerging regulators for cell membrane dynamic changes—from structure to human diseases. J Hematol Oncol 8: 47, 2015. doi: 10.1186/s13045-015-0144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin-Martin N, Ryan G, McMorrow T, Ryan MP. Sirolimus and cyclosporine A alter barrier function in renal proximal tubular cells through stimulation of ERK1/2 signaling and claudin-1 expression. Am J Physiol Renal Physiol 298: F672–F682, 2010. doi: 10.1152/ajprenal.00199.2009. [DOI] [PubMed] [Google Scholar]
- 18.McNeil E, Capaldo CT, Macara IG. Zonula occludens-1 function in the assembly of tight junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell 17: 1922–1932, 2006. doi: 10.1091/mbc.e05-07-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medina R, Rahner C, Mitic LL, Anderson JM, Van Itallie CM. Occludin localization at the tight junction requires the second extracellular loop. J Membr Biol 178: 235–247, 2000. doi: 10.1007/s002320010031. [DOI] [PubMed] [Google Scholar]
- 20.Meyer TN, Schwesinger C, Ye J, Denker BM, Nigam SK. Reassembly of the tight junction after oxidative stress depends on tyrosine kinase activity. J Biol Chem 276: 22048–22055, 2001. doi: 10.1074/jbc.M011477200. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen A, Chen P, Cai H. Role of CaMKII in hydrogen peroxide activation of ERK1/2, p38 MAPK, HSP27 and actin reorganization in endothelial cells. FEBS Lett 572: 307–313, 2004. doi: 10.1016/j.febslet.2004.06.061. [DOI] [PubMed] [Google Scholar]
- 22.Patrick DM, Leone AK, Shellenberger JJ, Dudowicz KA, King JM. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma modulate epithelial barrier function in Madin-Darby canine kidney cells through mitogen activated protein kinase signaling. BMC Physiol 6: 2, 2006. doi: 10.1186/1472-6793-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petecchia L, Sabatini F, Usai C, Caci E, Varesio L, Rossi GA. Cytokines induce tight junction disassembly in airway cells via an EGFR-dependent MAPK/ERK1/2-pathway. Lab Invest 92: 1140–1148, 2012. doi: 10.1038/labinvest.2012.67. [DOI] [PubMed] [Google Scholar]
- 24.Raleigh DR, Boe DM, Yu D, Weber CR, Marchiando AM, Bradford EM, Wang Y, Wu L, Schneeberger EE, Shen L, Turner JR. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J Cell Biol 193: 565–582, 2011. doi: 10.1083/jcb.201010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruffels J, Griffin M, Dickenson JM. Activation of ERK1/2, JNK and PKB by hydrogen peroxide in human SH-SY5Y neuroblastoma cells: role of ERK1/2 in H2O2-induced cell death. Eur J Pharmacol 483: 163–173, 2004. doi: 10.1016/j.ejphar.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 26.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol 73: 283–309, 2011. doi: 10.1146/annurev-physiol-012110-142150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO. Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J Signal Transduct 2011: 792639, 2011. doi: 10.1155/2011/792639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tokuda S, Higashi T, Furuse M. ZO-1 knockout by TALEN-mediated gene targeting in MDCK cells: involvement of ZO-1 in the regulation of cytoskeleton and cell shape. PLoS One 9: e104994, 2014. doi: 10.1371/journal.pone.0104994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci 121: 298–305, 2008. doi: 10.1242/jcs.021485. [DOI] [PubMed] [Google Scholar]
- 30.Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell 20: 3930–3940, 2009. doi: 10.1091/mbc.e09-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Itallie CM, Fanning AS, Holmes J, Anderson JM. Occludin is required for cytokine-induced regulation of tight junction barriers. J Cell Sci 123: 2844–2852, 2010. doi: 10.1242/jcs.065581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Itallie CM, Tietgens AJ, Krystofiak E, Kachar B, Anderson JM. A complex of ZO-1 and the BAR-domain protein TOCA-1 regulates actin assembly at the tight junction. Mol Biol Cell 26: 2769–2787, 2015. doi: 10.1091/mbc.e15-04-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waheed F, Speight P, Kawai G, Dan Q, Kapus A, Szászi K. Extracellular signal-regulated kinase and GEF-H1 mediate depolarization-induced Rho activation and paracellular permeability increase. Am J Physiol Cell Physiol 298: C1376–C1387, 2010. doi: 10.1152/ajpcell.00408.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol 288: C1231–C1241, 2005. doi: 10.1152/ajpcell.00581.2004. [DOI] [PubMed] [Google Scholar]
- 35.Yu W, Beaudry S, Negoro H, Boucher I, Tran M, Kong T, Denker BMH. H2O2 activates G protein, α12 to disrupt the junctional complex and enhance ischemia reperfusion injury. Proc Natl Acad Sci USA 109: 6680–6685, 2012. doi: 10.1073/pnas.1116800109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhuang S, Kinsey GR, Yan Y, Han J, Schnellmann RG. Extracellular signal-regulated kinase activation mediates mitochondrial dysfunction and necrosis induced by hydrogen peroxide in renal proximal tubular cells. J Pharmacol Exp Ther 325: 732–740, 2008. doi: 10.1124/jpet.108.136358. [DOI] [PubMed] [Google Scholar]