Abstract
We conducted a phase I study investigating the efficacy, safety, and tolerability of ONO-2160, a newly developed levodopa pro-drug, and carbidopa compared with levodopa and carbidopa to stabilize levodopa plasma concentration fluctuations in Japanese patients with Parkinson's disease. In an open-label two-period design, patients (n = 12) with Parkinson's disease received levodopa and carbidopa for 3 days before 7 days of treatment with ONO-2160 and carbidopa. Patients were primarily evaluated using the Unified Parkinson's Disease Rating Scale Part III, a Parkinson's disease symptom diary, and analysis of adverse events. Pharmacokinetic analysis of plasma levodopa concentration was also performed.
ONO-2160 and carbidopa therapy stabilized effective plasma levodopa concentration. No adverse events with safety concerns were observed. The combination of ONO-2160 and carbidopa produced a prolonged and stable plasma levodopa concentration with a reduction in Unified Parkinson's Disease Rating Scale Part III total scores. The combination was well tolerated, with no safety concerns, when administered to Japanese patients with Parkinson's disease.
Keywords: ONO-2160, Levodopa, Parkinson's disease, Motor fluctuations
Abbreviations: AE, adverse event; ADR, adverse drug reactions; CD, carbidopa; COMT, catechol-O-methyltransferase; DDCI, dopa-decarboxylase inhibitor; MMSE, Mini-Mental State Examination; PD, Parkinson's disease; SD, standard deviation; SE, standard error; UPDRS, Unified Parkinson's Disease Rating Scale
1. Introduction
Parkinson's disease (PD) is the most common form of parkinsonism and is characterized by tremors, muscle rigidity, postural instability, and bradykinesia. These motor deficits are a result of progressive neurodegeneration of dopaminergic neurons in the substantia nigra. Although there is no cure for PD, there are treatments that can effectively manage the symptoms. Levodopa is a dopamine precursor and is a first-line treatment that can restore motor function in PD patients [1]. The combination with levodopa and a dopa-decarboxylase inhibitor (DDCI), such as carbidopa or benserazide, reduces the peripheral DDC breakdown of levodopa and improves the proportion of peripheral levodopa crossing the blood–brain barrier. Appropriate treatment strategies can offer effective symptomatic relief for a few years; however, after several years of therapy, motor fluctuations emerge.
There are some levodopa modification strategies available to patients who begin to show symptoms of wearing-off [[2], [3], [4]]. Some of these strategies include using lower and more frequent doses of levodopa, changing to a treatment formulation that provides a more controlled release of levodopa, or adding in a dopamine agonist [[5], [6], [7]]. Alternatively, the addition of a catechol-O-methyltransferase (COMT) inhibitor, such as entacapone or tolcapone, can also be used. This combination also prevents the degradation of levodopa in the periphery. In some patients, the administration of carbidopa and entacapone with levodopa results in a significant increase in the duration of levodopa's therapeutic activity [8,9].
However, these strategies have limitations. Patients with moderate-to-severe motor fluctuations have a poor predictability of response with inconsistent reductions in symptom OFF-time [[10], [11], [12]]. The extended release formulation delays the onset of effects and increases dyskinesias at peak dose [13,14]. Therefore, there is a need for better formulations that provide a more consistent delivery of levodopa that improves symptomatic relief and prevents motor complications.
ONO-2160 is a newly developed pro-drug of levodopa that has been designed to minimize fluctuations of plasma levodopa concentrations and to prolong its efficacy. In vivo rat data suggest that ONO-2160 is passively and slowly absorbed throughout the gastrointestinal tract into the blood, where it is efficiently hydrolyzed by esterase enzymes into levodopa before crossing the blood–brain barrier and conversion to dopamine in the brain (data not shown), increasing dopamine stores. This non-randomized, open-label phase I study aimed to evaluate the safety, pharmacokinetic profile, and efficacy of ONO-2160 and carbidopa (ONO-2160/CD) combination therapy, and to compare it against an active comparator, the immediate-release formulation of levodopa and carbidopa (levodopa/CD) combination therapy, in Japanese patients with PD.
2. Methods
2.1. Ethics
This study complied with the ethical principles based on the Declaration of Helsinki, the standards stipulated in Article 14 - Paragraph 3 and Article 80-2 of the Pharmaceutical Affairs Law (or the “Law on Securing Quality, Efficacy and Safety of Pharmaceuticals and Medical devices” since November 25, 2014), and the “Ministerial Ordinance on Good Clinical Practice (GCP)” (MHW Ordinance No. 28).
The study protocol was approved by the institutional review board of the Graduate School of Medicine at Ehime University. Trial registration number: JapicCTI-142,702.
2.2. Study design and interventions
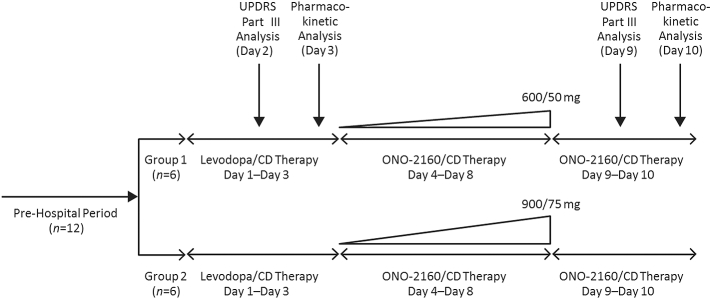
This open-label, phase I study was carried out in Japanese patients with Parkinson's disease who exhibited motor fluctuations and who were currently on levodopa therapy. The study started with a 3-day observation period (days 1–3) with patients remaining on their established levodopa/CD dose followed by an equivalent ONO-2160/CD dosage (ONO-2160/CD 300/25 mg is approximately equal to levodopa/CD at 100/10 mg; Fig. 1). The dose was then steadily increased to a maximum dose of either ONO-2160/CD 600/50 mg (group 1) or ONO-2160/CD 900/75 mg (group 2). The patients received the doses three times daily at 5-h intervals over 5 days (days 4–8). This was done by increments of 150/12.5 mg until efficacy and pharmacokinetic analysis on day 9 and day 10, respectively.
Fig. 1.
Study design.
2.3. Patients
This open-label study involved 12 Japanese patients based on the following key inclusion criteria: male or female patient aged ≥20 to <80 years and was given a diagnosis of PD on the basis of the Clinical Diagnostic Criteria of the UK PD Society Brain Bank; a Modified Hoehn and Yahr Scale stage 1 to 3; and ≥24 points in Mini-Mental State Examination (MMSE) at screening [[15], [16], [17]].
Patients also had to have ≥2 h of OFF-time per day on average in the previous 7 consecutive days; be receiving levodopa products (levodopa/CD) at a consistent dose and dosage frequency for the previous 7 days before the start of the study; and be judged capable of accurately recording symptom variations in a PD symptom diary.
The key exclusion criteria included the presence of any of the following: parkinsonism other than PD; received or due to receive surgical treatment for PD; psychiatric symptoms related to PD; concurrent angle closure glaucoma; stomach or duodenum ulcers; diabetes mellitus; heart or lung disease; underwent ≥400-ml blood collection within 90 days or ≥200-ml blood collection within 30 days; history of serious drug or food allergies; alcohol or drug abuse; or judged ineligible to be a study subject by the investigators as a result of clinical observation, laboratory test, physical examination, ECG, and ophthalmological examination.
2.4. Pharmacokinetic analysis
Pharmacokinetic analysis involved venous blood sampling throughout the study for evaluating plasma concentrations of levodopa as measured by the LC/MS/MS facility at Sumika Chemical Analysis Service, Ltd., Osaka, Japan.
2.5. Motor function evaluation
The Unified Parkinson's Disease Rating Scale (UPDRS) Part III (motor evaluation) was carried out on PD patients given a diagnosis of motor fluctuations, before each dose and every hour for 10 h after each dose.
2.6. Safety and tolerability
For adverse events (AEs) and adverse drug reactions (ADRs), incidences and number of events were calculated. A physical examination (blood pressure, pulse rate, respiratory rate, body temperature, and body weight) was carried out at baseline and at regular time points throughout the study, and the changes over time were recorded. Quantitative analysis of common laboratory tests, including blood biochemistry, hematology, coagulation, and urinalysis, was carried out at baseline and at regular time points throughout the study; the changes over time were also recorded.
2.7. Statistical analysis
The analysis set for safety and pharmacodynamics included patients who had received the study drug at least once. The motor function and pharmacokinetic analysis sets included patients who met the inclusion criteria and none of the exclusion criteria, who had received the study drug at least once and also had their data recorded at least once after administration of the study drug in the ONO-2160/CD period. For drug plasma concentrations, summary statistics (number of patients, and mean and standard deviation [SD]) were calculated. For the pharmacokinetic parameters, summary statistics (number of patients, mean, SD, coefficient of variation, maximum, minimum, median, and geometric mean) were also calculated. For the total scores of UPDRS Part III, summary statistics (the actual values and the changes from baseline) were calculated, and figures showing the courses of means ± standard errors (SE) of the actual values were created at each time point in each treatment period.
For the results of the physical examination (blood pressure, pulse rate, respiratory rate, body temperature, and body weight), summary statistics (the actual values and the changes from baseline) were calculated and figures showing the courses of the mean ± SD of the actual values were created. For quantitative values among the common laboratory tests (blood biochemistry, hematology, coagulation, and urinalysis), summary statistics (the actual values and the changes from baseline) were calculated.
3. Results
3.1. Patients
Between May 2015 and October 2015, written informed consent was obtained from 12 patients who were enrolled and observed for ≥3 days prior to hospitalization and assignment to ONO-2160/CD treatment based on the dose and dosage frequency of current levodopa therapy. All 12 patients were screened against inclusion and exclusion criteria and treated with the study drug. There were no deviations from protocol and no patients were withdrawn from the study. The mean (± SD) age was 68.1 ± 6.0 years.
Patients were evenly divided into two dose groups (group 1: ONO-2160/CD 600/50; and group 2: ONO-2160/CD 900/75 mg; n = 6 per group) on the basis of their current levodopa therapy. The demographic and clinical characteristics of both groups were shown to be similar (Table 1). In brief, group 1 patients had a mean MMSE score, Hoehn & Yahr Stage, and UPDRS Part III of 28.3 (range: 27–30), 2.5 (range: 2–3), and 28.0 (range: 13–51), respectively. Group 2 patients had a mean MMSE score, Hoehn & Yahr Stage, and UPDRS Part III of 28.8 (range: 27–30), 2.9 (range: 2.5–3), and 30.3 (range: 22–41), respectively.
Table 1.
Demographic and clinical characteristics of the study population.
| Demographic or clinical characteristic | Group 1 mean (range) | Group 2 mean (range) |
|---|---|---|
| Age, years | 67.2 (57–73) | 69.0 (63–79) |
| Sex: Male (n) | 3 | 4 |
| Sex: Female (n) | 3 | 2 |
| Duration of PD (months) | 95.5 (34–186) | 77.0 (40–139) |
| MMSE Score | 28.3 (27–30) | 28.8 (27–30) |
| Hoehn & Yahr Stage (ON-time) | 2.5 (2–3) | 2.9 (2.5–3) |
| Levodopa daily dose | 491.7 (300–650) | 600.0 (300–900) |
| Levodopa daily dosing frequency | 3.5 (3–5) | 4.2 (3–7) |
| Off time (hours) | 4.65 (2.0–8.1) | 5.15 (1.9–8.9) |
| UPDRS score: Part III | 28.0 (13–51) | 30.3 (22–41) |
| UPDRS score: total, when ON | 39.5 (18–69) | 45.5 (30–59) |
| UPDRS score: total, when OFF | 43.0 (23–75) | 55.8 (39–72) |
MMSE, Mini-Mental State Examination; PD, Parkinson's disease; UPDRS, Unified Parkinson's Disease Rating Scale.
In both groups, the current mean levodopa daily dose and mean daily dosing frequency were recorded. Group 1 patients were on a mean daily dose of 491.7 mg (range: 300–650 mg) levodopa with a mean daily dosing frequency of 3.5 (range: 3–5). Group 2 patients were on a mean daily dose of 600.0 mg (range: 300–900 mg) levodopa with a mean daily dosing frequency of 4.2 (range: 3–7).
3.2. Pharmacokinetics
Levodopa/CD was administered 3–7 times daily for 3 days at the same dosing frequency as the pre-hospital phase for each patient (n = 12). To compare the pharmacokinetic parameters of ONO-2160/CD and levodopa/CD precisely when administered the same dosing intervals, pharmacokinetic data from day 3 were expressed as mean (±SD) plasma levodopa concentrations for patients who were dosed three times daily (n = 8) (Fig. 2). Four patients receiving more than three doses a day were excluded from this analysis. Data from day 10 were expressed as mean (± SD) plasma levodopa concentration in each group (Fig. 2).
Fig. 2.
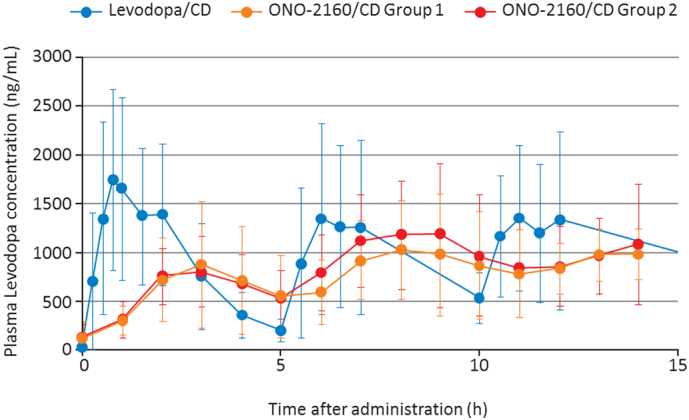
Plasma levodopa concentration following repeated multiple dosing with levodopa/CD for 3 days or ONO-2160/CD for 7 days in Parkinson's disease patients. Data values are mean values with SD error bars.
ONO-2160/CD therapy resulted in a sustained plasma levodopa concentration with smaller peak-to-trough fluctuations when compared with levodopa/CD therapy. This was evidenced when calculating the Cmax/Cmin ratio, which uses the highest peak and lowest trough of plasma levodopa concentrations, to show stabilization of the active drug. After levodopa/CD treatment, the mean (± SD) Cmax/Cmin was 14.27 (±8.41) (data not shown), whereas the Cmax/Cmin of ONO-2160/CD 600/50 mg and ONO-2160/CD 900/75 mg treatments were 4.43 (± 3.71) and 2.98 (± 1.05), respectively (Table 2).
Table 2.
Summary of plasma levodopa pharmacokinetic parameters on day 10.
| Group 1 |
Group 2 |
|||||
|---|---|---|---|---|---|---|
| Day 10 First dose | Day 10 Second dose | Day 10 Third dose | Day 10 First dose | Day 10 Second dose | Day 10 Third dose | |
| Patient (n) | 6 | 6 | 6 | 6 | 6 | 6 |
| Cmax (ng/mL) | 906 ± 621 | 1120 ± 502 | 1170 ± 243 | 906 ± 331 | 1330 ± 623 | 1220 ± 600 |
| Tmax (h) | 3.00 (2.00–4.00) | 3.51 (1.00–4.00) | 1.50 (0.00–4.00) | 2.50 (2.00–4.00) | 3.00 (2.00–4.00) | 3.50 (0.00–4.08) |
| AUC5h (ng·h/mL) | 2830 ± 1870 | 4050 ± 2120 | – | 2760 ± 1040 | 4820 ± 2380 | – |
| AUC24h (ng·h/mL) | 13,700 ± 6030 | 15,000 ± 6960 | ||||
| Cmax/Cmin | 4.43 ± 3.71 | 2.98 ± 1.05 | ||||
All values are means ± SD except for Tmax, which is the median (min - max).
Cmax, maximum plasma concentration; Tmax, time to maximum plasma concentration; AUC, area under the concentration–time curve; Cmax/Cmin, highest Cmax of the day / lowest plasma concentration among those obtained from the Tmax after the first dose until the Tmax after the third dose. The dash indicates data not determined.
Pharmacokinetics of plasma levodopa were evaluated in patients who had initially received levodopa/CD for 3 days and 7 days of ONO-2160/CD at either 600/50 mg or 900/75 mg (Table 2). The Cmax after each dose on day 10 for patients receiving either ONO-2160/CD 600/50 mg or ONO-2160/CD 900/75 mg was 906 ng/mL and 906 ng/mL after the first dose, 1120 ng/mL and 1330 ng/mL after the second dose, and 1170 ng/mL and 1220 ng/mL after the third dose, respectively (Table 2). The AUC5h on day 10 after receiving ONO-2160/CD 600/50 mg or ONO-2160/CD 900/75 mg was 2830 ng·h/mL and 2760 ng·h/mL after the first dose, and 4050 ng·h/mL and 4820 ng·h/mL after the second dose, respectively. The AUC24h on day 10 was 13,700 ng·h/mL after three doses of ONO-2160/CD 600/50 mg and 15,000 ng·h/mL after three doses of ONO-2160/CD 900/75 mg (Table 2).
3.3. Motor function response
The analysis set included all 12 patients for the levodopa/CD period and the ONO-2160/CD period (in which six patients had received 600/50 mg and six patients had received 900/75 mg). Fig. 3A and B show the mean UPDRS Part III scores for the 10 h after dosing during each period. For the total scores at each time point, summary statistics (actual score, change from baseline, and change between predefined corresponding times in the levodopa/CD period and the ONO-2160/CD period) were calculated by treatment period. During ONO-2160/CD therapy, UPDRS Part III scores were maintained at a low level from baseline to 10 h and tended to be more stable than those for levodopa/CD treatment.
Fig. 3.
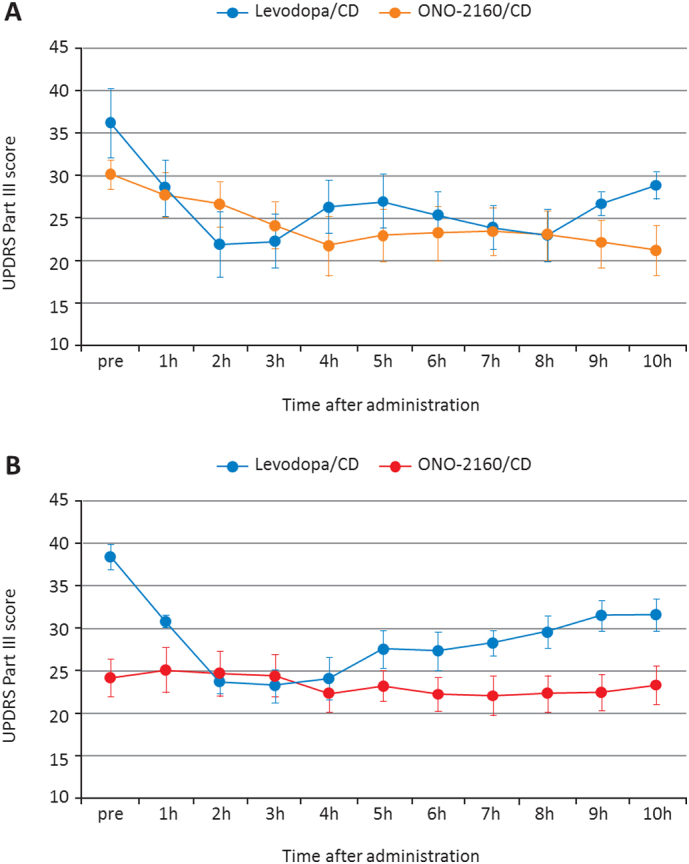
A. Mean UPDRS Part III score for the 10-hour period after dosing and increasing the maximum dose of ONO-2160/CD 600/50 mg (group 1) vs. levodopa/CD (n = 6). B. Mean UPDRS Part III score for the 10-h period after dosing and increasing the maximum dose of ONO-2160/CD 900/75 mg (group 2) vs. levodopa/CD (n = 6). Data points are the mean values with SE error bars.
3.4. Adverse events
AEs occurred in no patients in the levodopa/CD period and 58.3% of patients in the ONO-2160/CD period. Serious AEs occurred in 16.7% of patients in the ONO-2160/CD period, which included one event of perforated appendix and one event of constipation.
ADRs occurred in 25.0% (low serum potassium level in one patient, worsened constipation in two patients) of patients in the ONO-2160/CD period. One serious ADR occurred in the ONO-2160/CD period, which was one event of constipation. No deaths occurred during the study and overall ONO-2160/CD was well tolerated at all the investigated doses and no clinically relevant trends were seen in vital signs, clinical laboratory parameters, physical examinations, or electrocardiograms (data not shown).
4. Discussion
This phase I study investigated the efficacy, safety, and tolerability of a novel levodopa pro-drug, ONO-2160. ONO-2160 administered three times daily with CD resulted in sustained plasma levodopa concentrations, with smaller peak-to-trough fluctuations in plasma levodopa levels compared with levodopa/CD. Levodopa has been the gold standard antiparkinsonian agent for the treatment of PD for decades; however, the value of long-term therapy is questionable due to its poor bioavailability, reduced efficacy with chronic treatment, and peripheral side effects [[18], [19], [20]].
Traditionally, this is managed with the addition of compounds that inhibit levodopa metabolism in the periphery and improve the pharmacokinetic profile compared with therapy with levodopa alone [21,22]. An improved pharmacokinetic profile then translates into significant reductions in OFF-time and significant increases in ON-time, which then correlates with better symptomatic control and better quality of life scores. However, even in the presence of CD, plasma concentrations of levodopa are erratic and dyskinesia and motor fluctuations can emerge as early as 6 months after initiation of levodopa therapy [23].
Attempts to develop a controlled-release formulation of levodopa that provides a more continuous plasma level of levodopa have not been successful and have been prone to significant delays to ON-time as well as equally unpredictable absorption rates [[24], [25], [26]]. Therefore, the development of pro-drugs such as ONO-2160 that improve the pharmacological and pharmacokinetic profiles is essential to overcome the shortcomings of levodopa therapy in PD patients.
The efficacy of ONO-2160 to reduce and stabilize motor fluctuations was analyzed by determining the change in the patients' total UPDRS Part III score throughout the dosing period over three days. The results showed that ONO-2160/CD therapy produced a UPDRS Part III score that was maintained at a low level from baseline to 10 h and that this was more stable in comparison with levodopa/CD treatment. Furthermore, a comparison of the UPDRS Part III scores in patients treated with the maximum dose of ONO-2160/CD of 900/75 mg, with those in patients treated with a maximum dose of ONO-2160/CD of 600/50 mg, suggests that this increased dose of ONO-2160/CD further improves motor function in PD patients.
Prior to the first dose on day 9, the reduction in UPDRS Part III scores of patients treated with ONO-2160/CD was greater than that seen prior to the first dose on day 2 in those treated with levodopa/CD, suggesting that multiple administration of ONO-2160/CD may ameliorate the motor dysfunction of patients in the early morning. At 3 h after the first treatment on day 2, the UPDRS Part III scores in patients treated with levodopa/CD were lower than those in patients receiving ONO-2160/CD treatment on day 9, suggesting that the time to maximum plasma concentration (Tmax) of plasma levodopa with levodopa/CD treatment is short compared with that for ONO-2160/CD.
This phase I study involved multiple dosing with ONO-2160/CD, three times per day for 7 days. The ONO-2160/CD combination was well tolerated at both doses investigated in Japanese PD patients. No patients withdrew from the study and there were no clinically relevant trends observed in vital signs, clinical laboratory parameters, physical examinations, or electrocardiograms. In comparison, a common strategy in clinical practice to combat long-term motor complications of levodopa treatment is to begin adjuvant therapy that combines other classes of antiparkinsonian drugs, such as dopamine agonists, COMT inhibitors, or monoamine oxidase B inhibitors. A systematic review of the literature, including 45 clinical trials and nearly 9000 patients, showed that although adjuvant therapy significantly improves symptoms there is an increase in AEs, including dyskinesia, across all three adjuvant therapies [27].
Although the present trial is limited by the short duration of treatment, it is reassuring that no drug-related AEs were observed. Therefore, future studies should contain longer treatment durations to determine the presence of any side effects of treatment. Furthermore, this trial was also limited by the small study population, the lack of blinding, and the open-label study design. However, this is a phase I study that aimed to evaluate the safety, tolerability, and pharmacokinetic profile of ONO-2160/CD in comparison to levodopa/CD, and the data generated here can be used in the design of a phase II study.
5. Conclusion
ONO-2160/CD treatment produced a prolonged and stable plasma levodopa concentration with a reduction in UPDRS Part III total scores. Larger, randomized studies are needed; however, the stabilization of plasma levodopa concentration, with smaller peak-to-trough fluctuations, may help to reduce AEs associated with levodopa.
Acknowledgments
Acknowledgements
The authors thank all participating patients, their families, and health care professionals who made this study possible. The authors would like to thank James Graham, PhD, of Edanz Medical Writing, on behalf of inScience Communications, Springer Healthcare, for providing medical writing assistance, which was funded by Ono Pharmaceutical Co., Ltd.
Funding
This study was supported by Ono Pharmaceutical Co., Ltd. Medical writing assistance was funded by Ono Pharmaceutical Co., Ltd.
Authors' contributions
M. Nomoto, M. Nagai, N.N., and R.A. participated in data collection. Y.K., K.Y., and S.S. organized the study. A.T. played advisory roles in this study. All authors contributed to manuscript writing by providing scientific contributions and finalizing the draft that was prepared by a medical writing service. All data were collected by investigators independently from the sponsor and all data were accessible to authors.
Data statement
The datasets generated during the current study are not publicly available and remain proprietary.
Relevant conflicts of interest/financial disclosures
M. Nomoto has received grants from Ono Pharmaceutical Co. Ltd., financial support for investigator-initiated trials from Shionogi Pharmaceutical Co. Ltd. and Morinaga & Co. Ltd., and speaker honoraria from Sumitomo Dainippon Pharma Co. Ltd., Hisamitsu Pharmaceutical Co. Inc., Ono Pharmaceutical Co. Ltd., and Kyowa Kirin. M. Nagai has received grants from Ono Pharmaceutical Co. Ltd., consultancy fees from Takeda Pharmaceutical Co. Ltd., and honoraria from Novartis. N.N. has received research expenses from Kisyu Hosokawa. Y.K., K.Y., and S.S. are employees of Ono Pharmaceutical Co., Ltd. A.T. has received consultancy fees from Ono Pharmaceutical Co. Ltd., grants from Meiji Seika Pharma Co., Ltd., Hisamitsu Pharmaceutical Co., Inc., Pfizer Inc., Sumitomo Dainippon Pharma Co., Ltd., and Kyowa Hakko Kirin Co., Ltd., and honoraria from Sumitomo Dainippon Pharma Co., Ltd., Kyowa Hakko Kirin Co., Ltd., and AbbVie GKR. R.A. has no conflict of interest to disclose.
References
- 1.Lewitt P.A. Levodopa for the treatment of Parkinson's disease. N Engl J Med. 2008;359:2468–2476. doi: 10.1056/NEJMct0800326. [DOI] [PubMed] [Google Scholar]
- 2.Rascol O., Brooks D.J., Korczyn A.D. A five-year study of the incidence of dyskinesia in patients with early Parkinson's disease who were treated with ropinirole or levodopa. N Engl J Med. 2000;342:1484–1491. doi: 10.1056/NEJM200005183422004. [DOI] [PubMed] [Google Scholar]
- 3.Parkinson Study Group Pramipexole vs levodopa as initial treatment for Parkinson disease: a randomized controlled trial. Parkinson Study Group. JAMA. 2000;284:1931–1938. doi: 10.1001/jama.284.15.1931. [DOI] [PubMed] [Google Scholar]
- 4.Oertel W.H., Wolters E., Sampaio C. Pergolide versus levodopa monotherapy in early Parkinson's disease patients: the PELMOPET study. Mov Disord. 2006;21:343–353. doi: 10.1002/mds.20724. [DOI] [PubMed] [Google Scholar]
- 5.Olanow C.W., Obeso J.A., Stocchi F. Continuous dopamine-receptor treatment of Parkinson's disease: scientific rationale and clinical implications. Lancet Neurol. 2006;5:677–687. doi: 10.1016/S1474-4422(06)70521-X. [DOI] [PubMed] [Google Scholar]
- 6.Olanow C.W., Obeso J.A., Stocchi F. Drug insight: continuous dopaminergic stimulation in the treatment of Parkinson's disease. Nat Clin Pract Neurol. 2006;2:382–392. doi: 10.1038/ncpneuro0222. [DOI] [PubMed] [Google Scholar]
- 7.Wright B.A., Waters C.H. Continuous dopaminergic delivery to minimize motor complications in Parkinson's disease. Expert Rev Neurother. 2013;13:719–729. doi: 10.1586/ern.13.47. [DOI] [PubMed] [Google Scholar]
- 8.Brooks D.J., Leinonen M., Kuoppamaki M. Five-year efficacy and safety of levodopa/DDCI and entacapone in patients with Parkinson's disease. J Neural Transm (Vienna) 2008;115:843–849. doi: 10.1007/s00702-008-0025-8. [DOI] [PubMed] [Google Scholar]
- 9.Damier P., Viallet F., Ziegler M. Levodopa/DDCI and entacapone is the preferred treatment for Parkinson's disease patients with motor fluctuations in routine practice: a retrospective, observational analysis of a large French cohort. Eur J Neurol. 2008;15:643–648. doi: 10.1111/j.1468-1331.2008.02165.x. [DOI] [PubMed] [Google Scholar]
- 10.Lieberman A., Gopinathan G., Miller E. Randomized double-blind cross-over study of Sinemet-controlled release (CR4 50/200) versus Sinemet 25/100 in Parkinson's disease. Eur Neurol. 1990;30:75–78. doi: 10.1159/000117314. [DOI] [PubMed] [Google Scholar]
- 11.Jankovic J., Schwartz K., Vander Linden C. Comparison of Sinemet CR4 and standard Sinemet: double blind and long-term open trial in parkinsonian patients with fluctuations. Mov Disord. 1989;4:303–309. doi: 10.1002/mds.870040403. [DOI] [PubMed] [Google Scholar]
- 12.Pahwa R., Factor S.A., Lyons K.E. Practice Parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66:983–995. doi: 10.1212/01.wnl.0000215250.82576.87. [DOI] [PubMed] [Google Scholar]
- 13.Deleu D., Jacques M., Michotte Y. Controlled-release carbidopa/levodopa (CR) in parkinsonian patients with response fluctuations on standard levodopa treatment: clinical and pharmacokinetic observations. Neurology. 1989;39:88–92. [PubMed] [Google Scholar]
- 14.Stocchi F., Quinn N.P., Barbato L. Comparison between a fast and a slow release preparation of levodopa and a combination of the two: a clinical and pharmacokinetic study. Clin Neuropharmacol. 1994;17:38–44. doi: 10.1097/00002826-199402000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Hughes A.J., Daniel S.E., Kilford L. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoehn M.M., Yahr M.D. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 17.Folstein M.F., Folstein S.E., McHugh P.R. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Chapuis S., Ouchchane L., Metz O. Impact of the motor complications of Parkinson's disease on the quality of life. Mov Disord. 2005;20:224–230. doi: 10.1002/mds.20279. [DOI] [PubMed] [Google Scholar]
- 19.Nutt J.G., Fellman J.H. Pharmacokinetics of levodopa. Clin Neuropharmacol. 1984;7:35–49. doi: 10.1097/00002826-198403000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Hagan J.J., Middlemiss D.N., Sharpe P.C. G.H, Parkinson's disease: prospects for improved drug therapy. Trends Pharmacol Sci. 1997;18:156–163. doi: 10.1016/s0165-6147(97)01050-x. [DOI] [PubMed] [Google Scholar]
- 21.Sasahara K., Nitanai T., Habara T. Dosage form design for improvement of bioavailability of levodopa III: Influence of dose on pharmacokinetic behavior of levodopa in dogs and Parkinsonian patients. J Pharm Sci. 1980;69:1374–1378. doi: 10.1002/jps.2600691205. [DOI] [PubMed] [Google Scholar]
- 22.Nutt J.G., Woodward W.R. Levodopa pharmacokinetics and pharmacodynamics in fluctuating parkinsonian patients. Neurology. 1986;36:739–744. doi: 10.1212/wnl.36.6.739. [DOI] [PubMed] [Google Scholar]
- 23.Fahn S., Parkinson Study Group Does levodopa slow or hasten the rate of progression of Parkinson's disease? J Neurol. 2005;252(Suppl. 4):IV37–IV42. doi: 10.1007/s00415-005-4008-5. [DOI] [PubMed] [Google Scholar]
- 24.Hutton J.T., Morris J.L., Roman G.C. Treatment of chronic Parkinson's disease with controlled-release carbidopa/levodopa. Arch Neurol. 1988;45:861–864. doi: 10.1001/archneur.1988.00520320047014. [DOI] [PubMed] [Google Scholar]
- 25.Goole J., Vanderbist F., Amighi K. Development and evaluation of new multiple-unit levodopa sustained-release floating dosage forms. Int J Pharm. 2007;334:35–41. doi: 10.1016/j.ijpharm.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Nyholm D., Askmark H., Gomes-Trolin C. Optimizing levodopa pharmacokinetics: intestinal infusion versus oral sustained-release tablets. Clin Neuropharmacol. 2003;26:156–163. doi: 10.1097/00002826-200305000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Stowe R., Ives N., Clarke C.E. Meta-analysis of the comparative efficacy and safety of adjuvant treatment to levodopa in later Parkinson's disease. Mov Disord. 2011;26:587–598. doi: 10.1002/mds.23517. [DOI] [PubMed] [Google Scholar]